Abstract
The globus pallidus consists of the external (GPe) and the internal (GPi) segments. The GPe and GPi have different functional roles. The GPe is located centrally within multiple basal ganglia feedforward and feedback connections. The GPi is an output nucleus of the basal ganglia. A complex interplay between intrinsic pacemaking conductances and the balance of glutamatergic and GABAergic input largely determines the rate and pattern of firing of pallidal neurons. The initial part of this article introduces recent findings made in vivo that are related to the roles of glutamatergic and GABAergic inputs in the control of pallidal activity. The latter part describes the roles of intrinsic mechanisms of GPe neurons in the integration of the synaptic inputs. The presence of dendritic voltage-gated sodium channels may allow the initiation of dendritic spikes, giving distal inputs on the long and thin GPe dendrites an opportunity to strongly shape spiking activity. Basal ganglia disorders including Parkinson's disease, hemiballismus, and dystonias are accompanied by increased irregularity and synchronized bursts of pallidal activity. These changes may be in part due to changes in the GABA release in the GPe and GPi, but also involve intrinsic cellular changes in pallidal neurons.
Keywords: Pallidum, GABA, glutamate, basal ganglia, spontaneous firing, modeling
Network connections of Globus Pallidus
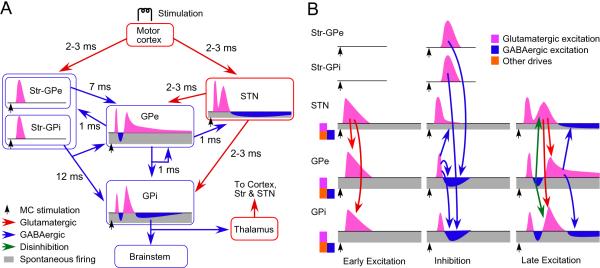
The globus pallidus consists of the external (GPe) and the internal (GPi) segments. In rodents and carnivores, the GPi is encapsulated in the internal capsule, hence it is also called the entopeduncular nucleus. Though the GPe and GPi receive similar inputs, GPe and GPi project to different sites and thus have different functional roles in the basal ganglia information processing. The GPe is centrally located within the multiple feedback loops of basal ganglia circuits (Figure 1A). The output of GPe is GABAergic, and inhibitory on its targets (Jessell et al., 1978). Attesting to the complexity of basal ganglia networks, GPe output directly connects to virtually every other basal ganglia nucleus, including itself (Figure 1A), though the inhibition of GPi and subthalamic nucleus (STN) are frequently considered to be its primary modes of action. The GPi receives inputs from striatum (Str), GPe, and STN and sends outputs outside the basal ganglia. The GPi neurons do not have extensive local axon collaterals (Nakanishi et al., 1991, Parent et al., 2001), though a few thin collaterals with poor arborization within GPi are sometimes present (Parent and Parent, 2004). Inputs to GPe/GPi can arrive from cerebral cortex via two major distinct pathways, one passing through the Str and the other through the STN. Other inputs to GPe/GPi originate from the intralaminar thalamic nuclei and brainstem nuclei including the pedunculopontine tegmentum (Edley and Graybiel, 1983, Deschenes et al., 1996). Thus, cortical inputs may arrive to GPe/GPi through these brain areas. The Str contains two groups of projection neurons; one projects to GPe only and other to GPe, GPi, and the Substantia Nigra pars reticulata (SNr). The Str-GPe neurons contain GABA, dynorphin, and enkephalin and express D2-like dopamine receptors (D2L-Rs). The Str-GPe/GPi/SNr neurons contain GABA, substance-P and express D1-like dopamine receptors (D1L-Rs). The striatal input to GPe/GPi is GABAergic, and inhibits GPe/GPi neurons (Kita, 1992). In contrast, input from STN is glutamatergic, and excitatory to GPe/GPi neurons (Kita, 1992). Since large areas of cortex (Cx), notably motor cortex, project to both Str and STN, activity in these areas has potentially two opposing effects on GPe/GPi activity: Disynaptic excitation via the STN and disynaptic inhibition via the Str. The levels and patterns of the firing activity of basal ganglia neurons changes with the development of basal ganglia diseases including Parkinson's disease, dystonia, and hemiballism (Tremblay and Filion, 1989, Nini et al., 1995, Wichmann and DeLong, 2006). Thus, understanding the synaptic and intrinsic mechanisms controlling the firing activity of GPe and GPi neurons in vivo is a crucial initial step for understanding basal ganglia functions in normal and pathological conditions.
Figure 1.
A: Diagrams show major synaptic connections form the motor cortex to GPi. B: Diagrams show major synaptic driving forces evoking an early excitation, an inhibition, and a late excitation in GPe and GPi after cortical stimulation. The diagrams are based on the suggestions obtained from previous studies (Nambu et al., 2000, Kita et al., 2004, Kita, 2007, Tachibana et al., 2008). The level of background activity of pallidal and STN neurons are controlled by glutamatergic, GABAergic, and other driving forces including autonomous firing and slow acting neuroactive substances. The motor cortex provides strong inputs to Str and STN. Stimulation of the motor cortex induces a shorter latency excitation in STN than Str. The difference in the excitation latencies in Str and STN produces an early excitation-inhibition sequence in many pallidal neurons. The early excitation in STN evokes an early excitation in GPe and GPi. The Cx-STN-GPe and Cx-Str pathways provide the main sources for the inhibition in STN, GPe, and GPi. The late excitation in STN, which may be due to various mechanisms including the disinhibition from GPe, provides the main source for the late excitation in GPe and GPi.
Motor cortex stimulation-induced responses in pallidal neurons in vivo
The responses of GPe and GPi neurons to stimulation of motor cortex typically consist of an early excitation, an inhibition, and a late excitation (Figure 1B). The early excitation is mediated mostly by AMPA-Rs and evoked through the Cx-STN-GPe/GPi pathways. The conduction time of these disynaptic pathways is short, 5–8 ms in rats and 8–11 ms in monkeys (Ryan and Clark, 1991, Kita, 1992, Nambu et al., 2000, Kita et al., 2004, Tachibana et al., 2008). The early excitation is followed by a GABAA-receptor (GABAA-R) mediated inhibition evoked by the Cx-Str-GPe/GPi pathways (Nambu et al., 2000, Kita et al., 2004, Tachibana et al., 2008). The early excitation of the GPe may also contribute to evoke the inhibition in the GPe/GPi neurons postsynaptic to GPe because stimulation of the GPe evokes short latency IPSPs/IPSCs in GPe and GPi neurons (Cooper and Stanford, 2001, Kita, 2001, Tachibana et al., 2008). The conduction times of these disynaptic inhibitory pathways is much longer, 8–17 ms in rats and 16–21 ms in monkeys (Ryan and Clark, 1991, Kita, 1992, Nambu et al., 2000, Kita et al., 2004, Tachibana et al., 2008). The difference in the conduction times between the STN and Str mediated pathways is due to two main factors: 1) Although cortical stimulation can induce short latency, 2–3 ms, monosynaptic EPSPs in both Str and STN neurons (Kitai and Deniau, 1981, Wilson, 1986), STN neurons have unique membrane properties that allow an excitatory input to trigger spikes with much shorter latency than in Str neurons (Farries et al., 2010). 2) The conduction velocities of Str axons are much slower than of STN axons (Tremblay and Filion, 1989, Kita, 1994). It is also possible that cortical neurons projecting bilaterally to Str, called intratelencephalically projecting cortico-Str neurons, may have a longer conduction time than pyramidal tract neurons that emit collaterals into the Str and STN (Cowan and Wilson, 1994, Mallet et al., 2006). The late excitation may be driven by several forces of which the most prominent force in normal conditions originate from STN because blockade of the STN by local injection of the GABAA-R agonist muscimol greatly diminished or completely abolished both the early and late response in pallidal neurons (Ryan and Clark, 1991, Kita, 1992, Nambu et al., 2000, Kita et al., 2004, Tachibana et al., 2008).
A majority of the GPe and GPi neurons that showed the early excitation also showed the inhibition while only small fractions of neurons had responses without the early excitation or without the inhibition. This indicates that the somatotopically organized Cx-Str-GPe/GPi projections overlaps with the Cx-STN-GPe/GPi projections. These results further indicate that for a transient cortical signal the fastest signal processing pathway is mediated by the Cx-STN-GPe/GPi pathway (Kita, 1994).
However, besides transient signal activation that may be thought to derive from discrete cortical activations such as sensory events or fast limb movements, GPe and GPi show a considerable baseline activity rate. In awake monkeys, a pioneering study by DeLong (1971) showed that the most frequently encountered type of neuron (85%) in the GPe is high frequency firing (mean rate of 55 Hz) with pauses, whereas the GPi shows more continuous-irregular-high frequency firing with a mean rate of 63 Hz. Although the rates in GPe in awake rats are quite a bit lower at 31–36 Hz (Ruskin et al., 1999, Urbain et al., 2000), they are still surprisingly high given the large number of inhibitory Str inputs to individual GPe neurons. Are these high sustained activity rates then the result of intrinsic cellular properties, or a balance of excitatory inputs that exceeds inhibitory ones?
Intrinsic cellular properties of GPe neurons from brain slices
Brain slice experiments to study intrinsic GPe neuron properties have been predominantly performed using rodents. Therefore the intrinsic properties of primate GPe neurons are still not known in detail, though in most respects they are likely to be similar to rodent ones. In vitro studies on GPi neurons are scarce and details of their intrinsic properties remain to be studied. An intracellular recording study in slices from adult rats showed that most of GPi neurons have strong hyperpolarization-activated cyclic nucleotide-gated (HCN) current which can support a high frequency autonomous firing (Nakanishi et al., 1990). In rodents, GPe neurons recorded with whole cell recordings in brain slices when excitatory and inhibitory synaptic blockers are applied frequently still show regular autonomous spiking. A range of 0 – 26 Hz with a mean of 5.5 Hz was observed in a sample of 146 recordings (Gunay et al., 2008). The source of depolarization responsible for autonomous spiking has been traced to a combination of HCN and persistent sodium current (Chan et al., 2004, Mercer et al., 2007, Chan et al., 2011). While GPe neuron spiking in vitro is quite regular at high frequencies, they can fire intrinsically irregularly when the firing frequency is low. Regularity of firing was shown to be enhanced due to the properties of the SK-type calcium activated potassium conductance (Deister et al., 2009), but HCN channels also are likely to make an important contribution (Chan et al., 2011). Overall, more than 10 voltage-gated channel types are present in GPe neurons (Surmeier et al., 1994, Baranauskas et al., 1999, Chan et al., 2004, Mercer et al., 2007) that together determine their complex intrinsic properties (Gunay et al., 2008). Therefore, there is certainly a rich potential for intrinsic properties being responsible for important dynamical aspects of action potential firing in vivo. However, large synaptic input conductances would act to shunt the currents generated through intrinsic channels, and for this reason a neuron in vivo might instead be dominated by synaptic input patterns. Indeed, it is this rich interplay between synaptic and intrinsic dynamics that still needs to be resolved more fully in future studies. Nevertheless, some studies applying synaptic blockers in waking primates are beginning to shed light on the contribution of synaptic input to resting firing patterns, as outlined below.
The nature of background activity of pallidal neurons in waking primates
The role of excitatory input
The GPe and GPi receive their main excitatory inputs from the STN. STN neurons in quietly resting awake animals are spontaneously active. Thus the activity of GPe and GPi neurons, which are capable of generating autonomous firing, is modified by background glutamatergic inputs from STN. The contributions of the STN inputs to the basal firing activity of GPe and GPi neurons were examined by chemical blockade of STN with local injection of the potent and long acting GABAA-R agonist muscimol. The muscimol blockade of STN in awake monkeys resulted in dramatic changes in the firing pattern in the GPe. Initially, the STN blockade greatly decreased the firing rate, to complete silence in some neurons. This finding indicates that a tonic level of excitatory input plays an important role in the basal firing rate of GPe neurons in vivo. Interestingly, 5–10 min after the muscimol injection into STN, GPe activity began to increase with repeated occurrences of short clustered spike discharges. As time progressed, the activity further increased and developed into repeated occurrences of two to twelve seconds of a very high-frequency active phase and then two to twelve seconds of a completely silent period (Nambu et al., 2000, Kita et al., 2004). Alternating active and silent phases in GPe neurons were also induced with the injection of the AMPA/kainate blocker NBQX (0.2–1 mM in saline, 0.2 μl) into the vicinity of the GPe neurons through the electrode assembly that was designed so that injected drugs would cover a large somatodendritic extent of recorded neurons (Kita et al., 2004). The effects of NBQX on the recorded neurons were verified by observing the reduction of cortical stimulation-induced excitations in the neurons. The mechanisms underling the generations of long active and silent phases remain speculative. The abovementioned pacemaker mechanisms found in slice neurons are likely to play a role, but do not fully explain high-frequency firing episodes.
The removal of glutamatergic inputs may alter not only excitatory drives and membrane potential dependent ionic currents but also other synaptic inputs. For instance, the STN blockade may remove the tonic presynaptic suppression of GABA release by group III metabotropic glutamate receptors and makes GPe-GPe/GPi inhibitions more effective (Matsui and Kita, 2003). This possible trans-synaptic modulation also has significant functional implications. Parkinson's disease and hemiballismus have been associated with an increase and a decrease of STN activity, respectively (Bergman et al., 1990, Hassani et al., 1996). These changes in the STN inputs might trans-synaptically control the information flow of the Str-pallidal projections.
In the GPi, the STN blockade reduced the average firing rate of some but resulted in no change in other neurons. The STN blockade often regularized firing of GPi neurons while it induced pauses and clustering of firing in some GPi neurons (Nambu et al., 2000, Tachibana et al., 2008). These observations imply that the disynaptic STN-GPe-GPi inhibitory input is competing with monosynaptic STN-GPi excitatory input (Kita et al., 2005). The balance of the two inputs may change with conditions of the neurons involved. For instance, when STN neurons in normal animals fire with strong burst, the STN-GPe-GPi inhibition may become stronger than the STN-GPi excitation (Kita et al., 2005).
The role of GABAA-R mediated inhibition
The GPe and GPi receive their main inhibitory inputs from the Str and GPe, in which the GPe-GPe inputs are through recurrent-collateral axons. The roles of GABAergic inputs were examined by injections of the specific blocker of GABAA-R gabazine into the vicinity of the GPe or GPi neurons (Kita et al., 2004). During the experiments, the effect of gabazine was verified by observing a significant decrease of the cortical stimulation-induced short inhibition. The local gabazine greatly increased the firing rate and regularized firing of most of the GPe and GPi neurons examined. Local gabazine eliminated the spontaneous pauses of some GPe neurons while they became more apparent in others as described in more detail below. These findings indicate that GABAA-R mediated inhibition is continuously suppressing the firing of GPe and GPi neurons. In quietly resting awake animals, GPe neurons have high frequency (average ≈55 Hz) activity while Str projection neurons have very low or zero spontaneous activity. Injection of bicuculline into a small area of GPe in monkey increases firing rate of GPe neurons in the injection site but decreased in neurons in surrounding area (Matumura et al., 1995), and also muscimol blockade of GPe in monkey increased firing rate of GPi neurons (Tachibana et al., 2008). These results indicate that GPe exerts tonic inhibition in GPe and GPi. Although, the activity of Str projection neurons is very low, the numbers of Str synapses are much greater than GPe synapses in both GPe and GPi. Thus, it is possible that a large number of infrequently activated Str synapse may also contribute to the tonic inhibition. The GABA released from GPe and Str axon terminals may activate not only its postsynaptic GABAA-Rs but also extrasynaptic ones. Microdialysis studies reported that the ambient level of GABA in GPe is relatively high, approximately 0.5μM (Robertson et al., 1991, Galvan et al., 2005), and may be sufficient to stimulate extrasynaptic GABAA-Rs that exist on the dendrites of monkey GPe neurons (Charara et al., 2005).
Co-application of NBQX and gabazine greatly regularized the firing and made GPe and GPi neurons to fire in a clock-like manner, indicating that both AMPA and GABA inputs are contributing to the generation of irregular spontaneous activity seen in GPe and GPi. It should be noted that the remaining spiking activity of these neurons treated by NBQX and gabazine may be under the control of long acting transmitters and receptors as well as intrinsic pacemaker currents.
The role of GABAB-R mediated inhibition
Immunohistochemical and in situ hybridization studies showed expression of GABAB-R1 and GABAB-R2 subunits in the GPe and GPi (Smith et al., 2000, Chen et al., 2004, Charara et al., 2005). Electron microscopic studies localized GABAB-Rs mainly on extrasynaptic membranes and some on presynaptic membranes of GABAergic and glutamatergic boutons (Chen et al., 2004, Charara et al., 2005). Injections of the specific GABAB-R blocker CGP55845 into the vicinity of the monkey GPe or GPi neurons increased spontaneous firing rate and regularized firing of GPe and GPi neurons, indicating that GABAB-R activation controls the spontaneous firing activity of GPe and GPi neurons (Kita et al., 2006). Single stimulation of Str in rat slice preparations or in monkeys does not evoke detectable GABAB-R mediated inhibition in GPe or GPi neurons. The GABAB-R mediated inhibitions were detected in GPe or GPi after repetitive stimulation of the Str or GPe (Kita et al., 2006, Kaneda et al., 2007). This suggests that a relatively large amount or a prolonged release of GABA is necessary to effectively activate -Rs on extrasynaptic membranes. It is still uncertain whether both Str and GPe axon terminals can release enough GABA for the activation of GABAB-Rs. An in vitro study showed that a 50 or 100 Hz Str burst stimulation (which stimulate GPe-Str axons in addition to Str-GPe axons) effectively evokes GABAB-R-mediated inhibition in GPe (Kaneda et al., 2007). The recording in monkeys showed that repetitive activation of Str axons alone was not capable to evoke the GABAB responses in both GPe and GPi. An activation of GPe neurons was required to evoke the GABAB responses in both GPe and GPi (Kita et al., 2006). These observations suggest that GABAB-Rs in the GPe and GPi are continuously activated in some degree by the relatively high level of ambient GABA, as mentioned earlier, and that additional activation of GABAB-Rs may occur with bursting activities of GPe neurons that are associated with movements and with increased bursting activity seen in Parkinson's disease and dystonias.
Pauses of the GPe neurons
The most prominent difference in the spontaneous firing of GPe and GPi neurons in normal animals is the occurrence of pauses in former but not in latter neurons (DeLong, 1971). The durations of pauses in primate GPe are often long, over 300 ms (DeLong, 1971; Elias et al., 2007), while those in rats are short, mostly <200 ms (Gardiner and Kitai, 1992). An interesting unsolved question is how the pauses are generated only in GPe despite both neurons receiving very similar set of inputs. Spontaneously occurring pauses are not preceded by bursts rejecting involvements of rebound properties such as calcium-activated potassium current (Elias et al., 2007). One of the questions may be whether GABAergic inputs can generate the pauses.
After local gabazine application, the spontaneous pauses of GPe neurons disappeared in some neurons while they became more apparent in others, suggesting that at least in some cells the pauses were not due to GABAA-R-mediated inhibition. Local application of CGP55845 did not alter firing patterns such as pauses and bursting of GPe neurons, although the duration of pauses was decreased as the firing frequency increased (Galvan et al., 2005, Kita et al., 2006). The long silent periods in the GPe observed after muscimol blockade of the STN were also not abolished by CGP55845. It has been documented that rat GPe neurons in slice preparations can generate pauses when their somata were hyperpolarized and their dendrites were depolarized (Hashimoto and Kita, 2006). Thus, it is likely that background GABAergic inhibitory and glutamatergic excitatory inputs may create a condition in GPe neurons such that timely arrival of GABAergic or glutamatergic inputs can flip the neurons between hyperpolarized and depolarized states (Hashimoto and Kita, 2006). For instance, a burst activation of Str inputs in the slice preparation can induce a long inhibition in GPe (Kaneda and Kita, 2005) and single stimulation of Str in monkey can evoke a long, ≈400 ms, inhibition of GPe after muscimol blockade of STN (Kita et al., 2004, Kita et al., 2005).
In animals with parkinsonism and dystonias, pauses in many GPe neurons may by synchronized and generate strong bursts in the STN and GPi due to disinhibition. Pauses occur only rarely in GPi of normal animals probably due to differences in the ionic conductances involved in the generation of autonomous firing. However, in dopamine (DA)-depleted parkinsonian animals, GPi neurons fire with frequent pauses and bursts. It is possible that the strength of GPe-STN and GPe-GPi GABAergic inhibition may be increased after DA depletion by the removal of presynaptic DA-R mediated suppression of GABA release, as mentioned earlier. Under the increased background inhibition condition, the synchronized pauses in the GPe monosynaptically disinhibit GPi and at the same time disynaptic GPe-STN-GPi input excite GPi. When synchronized bursts occur in GPe, opposite actions will generate pauses in GPi. Although not discussed here, altered synaptic properties and membrane conductances of basal ganglia neurons after DA-depletion are also involved in the generations of pauses and bursts of these neurons. These abnormal activity patterns may cause abnormal and/or diminished capability of data processing in the basal ganglia and lead to psychomotor disabilities.
DA and 5-HT modulation of synaptic inputs
The GPe and GPi also receive DAergic and 5-HTergic innervations (Charara and Parent, 1994, Wallman et al., 2011). Recent data suggest that these amines strongly modulate glutamatergic and GABAergic inputs in the GPe and GPi (Kita et al., 2007, Hashimoto and Kita, 2008, Rav-Acha et al., 2008, Kliem et al., 2010). Str neurons projecting only to GPe and some of GPe neurons express high levels of D2L-Rs and Str neurons projecting to GPe/GPi/SNr neurons express high levels of D1L-Rs (Gerfen et al., 1990, Meador-Woodruff and Mansour, 1991). Thus axons of these neurons in the pallidum might express the same DA-Rs as do their cell bodies.
Local injection of D2L-R agonist suppresses Str stimulation-induced inhibitions in rodent GPe (Bergstrom and Walters, 1984). In vitro studies revealed that dopamine suppresses Str-GPe inhibitions and the suppressions were due to D2-R-mediated presynaptic and D4-R-mediated postsynaptic suppressions (Shin et al., 2003, Watanabe et al., 2009, Chuhma et al., 2011). The D2-R-mediated presynaptic modulation of GPe-GPe synapses has not been directly demonstrated. In vitro studies showed that D2-R agonist reduced frequency of mIPSC in GPe (Cooper and Stanford, 2001; Watanabe et al., 2007). Many of the mIPSPs recorded with holding membrane potential of −60mV are large, over 50nA. We think those large mIPSPs are evoked by GPe axons (Watanabe et al., 2009). Baufreton and Bevan (2008) showed that D2-R agonists suppress GABA release from GPe-STN synapses. GPe neurons projecting to the STN have local collaterals (Kita and Kitai, 1994; Sato et al., 2000; Sadek et al., 2007). Those results suggest that at least some population of GPe-GPe synapses is controlled by D2 presynaptic receptors. However, local stimulation induced IPSCs in coronal slices were only slightly reduced by D2-R agonist (Cooper and Stanford, 2001). Further studies are required to clarify presynaptic DA modulation of GPe synapses. Local injection of D1L-R agonist into GPi decreases firing activity in both rodent and monkey GPi probably due to an increase in inhibition mediated by the Str-GPi GABAergic pathway (Ferre et al., 1996, Kliem et al., 2010). A similar presynaptic D1L-R mediated enhancement of GABA release occurs in SNr (Radnikow and Misgeld, 1998, Chuhma et al., 2011). Thus, DA depletion or systemic DA supplement therapy would likely have a pronounced effect on GABAergic transmission in GPe as well as GPi.
Both segments of the pallidum receive abundant 5-HT innervation from the dorsal raphe (Charara and Parent, 1994) and express several 5-HT-Rs. The main effects of local injection of 5-HT into GPe and GPi of monkeys were strong suppression of cortical stimulation-induced excitation and inhibition (Kita et al., 2007). The suppression of GABAergic inhibition is probably through 5-HT1B-Rs and suppression of glutamatergic excitation is probably through 5-HT1A-Rs (Kita et al., 2007, Hashimoto and Kita, 2008, Rav-Acha et al., 2008). The 5-HT axon bundle originating from the dorsal raphe travels through the medial forebrain bundle located dorsomedial to the SN and STN and innervates all basal ganglia nuclei (Di Matteo et al., 2008, Wallman et al., 2011). DA depletion results in hyperinnervation by the 5-HT axons (Di Matteo et al., 2008). It is possible that stimulation of STN using a clinical deep brain stimulation electrode with large contacts activates the 5-HT axon bundle innervating the basal ganglia. In both GPe and GPi, 5-HT reduces GABAergic inputs and exerts therapeutic effects. Stimulation of 5-HT axons can also exert therapeutic effects by the conversion of L-DOPA to DA in the Str. Thus, after L-DOPA application, activation of the 5-HT axon bundle will also activate DA-Rs in the basal ganglia. It has been reported that improvement in parkinsonian motor signs during STN deep brain stimulation can occur without changes in rate and bursting activity in GPi (Hahn et al., 2008). It is tempting to speculate that the effect may be mediated by 5-HT. However, it has been also suggested that DA synthesized by 5-HT neurons and/or 5-HT released from newly sprouted 5-HT axons may plays major role in the development of L-DOPA-induced dyskinesias (Carta et al., 2007 and 2010; Rylander et al., 2010; Nevalainen et al., 2011).
Synaptic integration in GPe neurons
GPe projection neurons have extended thin dendrites (Difiglia et al., 1982, Park et al., 1982, Yelnik et al., 1984). In rats these dendrites extend 1 mm in the dorso-ventral axis (Park et al., 1982) and thus span about 1/3rd to 1/2 of the whole nucleus. While the somatotopic organization of the Str is preserved in the Str-GPe projection (Alexander et al., 1986), this large extent of dendrites would allow individual GPe neurons to sample input from multiple somatotopic regions, for example in the motor loop of the basal ganglia, and possibly even integrate information across major parallel functional loops coursing through the GPe (Alexander et al., 1986). In fact such integration was hypothesized for pallidal activity quite early on based on anatomical grounds (Percheron et al., 1984), but to date has not been functionally established in vivo. These considerations lead to the important question on how synaptic input along the length of the extended dendrites of GPe neurons is integrated and controls spike initiation. Anatomically, both inhibitory input from Str and excitatory input from STN is distributed along the entire length, and in fact little excitatory input is found close to the soma (Shink and Smith, 1995). In contrast, GPe collaterals onto GPe neurons are primarily located on the soma (Shink and Smith, 1995), suggesting that they may provide a powerful source of inhibition, especially since GPe firing rates are so high.
Dendritic spike initiation
Thin long dendrites result in severe electrotonic attenuation of passive current flow inside the dendrite (Rall, 1969, Rall and Rinzel, 1973). For this reason without additional active dendritic mechanisms, synaptic input more distal than approximately 250 βm in a submicron diameter GPe dendrite will barely cause any voltage deflection at the soma (Hanson et al., 2004). Therefore active dendritic mechanisms seem to be required if distal dendritic input to GPe neurons were to have a significant functional effect on spiking.
A dendritic localization of voltage gated sodium channels isoforms NaV1.1, NaV1.2 and NaV1.6 was found with electron microscopy after rat GPe tissue was processed with isoform specific antibodies (Hanson et al., 2004). In fact, NaV channels were 6-fold more concentrated near excitatory synapses, suggesting a form of synaptic boosting mechanism triggered by depolarizing synaptic potentials. Subsequent slice electrophysiology indeed found that activation of distal dendritic glutamatergic synapses could lead to spiking events apparently initiated in the distal dendrite as there was no subthreshold depolarization preceding each spike in the soma, and somatic hyperpolarization could not suppress such spikes (Hanson et al., 2004). These results then suggest that at least in some GPe neurons, dendritic NaV channel density is high enough to allow local spike initiation upon receiving excitatory input. Such spikes would allow an efficient transmission of distal dendritic synaptic events to the soma. The same mechanism may also act in GPi, but the presence and action of dendritic NaV channels have not been determined in this cell type.
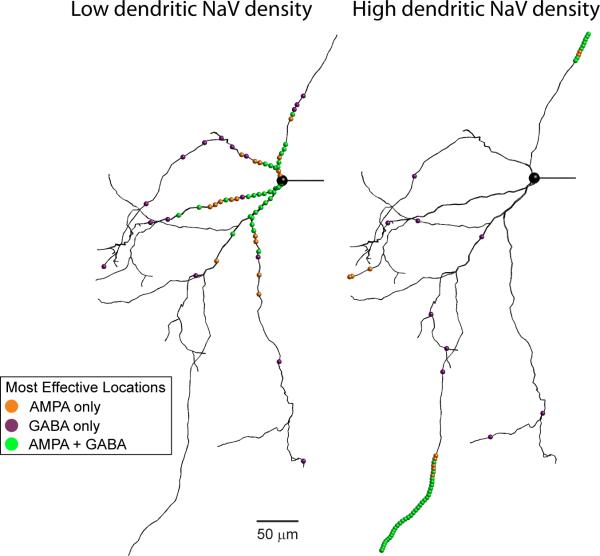
A computer model of a GPe neuron was constructed from the morphology of a neuron that was recorded from a rat brain slice and filled with biocytin to be subsequently reconstructed with the Neurolucida (MicroBrightField, Inc.) software (Gunay et al., 2008). Using this model, the effect of dendritic spike initiation with excitatory input was investigated for different synaptic STN and Str input patterns mimicking in vivo activity rates (Edgerton et al., 2010). The same model was tuned to match spiking properties with somatic current injection to slice recordings while incorporating varying gradients of dendritic NaV channel densities. High dendritic NaV channel densities led to dendritic spike initiation following excitatory inputs, but the summation of more than one input was needed to trigger a spike. Local dendritic inhibition on the other hand could suppress dendritic spike initiation. In models with dendritic spike initiation distal synapses were most effective in contributing to axonal spiking via propagated dendritic spikes (Figure 2, right panel). In contrast, the control of axonal spiking of models without or only little dendritic NaV was dominated by proximal synapses (Figure 2, left panel). Therefore, dendritic spike initiation would have important consequences for network integration with basal ganglia functional loops, as it would tend to support convergence across somatotopic zones.
Figure 2.
Rat GPe neuron morphology and location of effective synapses. Effectiveness of synapses was determined in a modeling study by measuring the mutual information between synaptic input at each location and output spikes (Edgerton et al., 2010). When spike initiation was entirely axonal (left), only synapses close to the soma were effective. In contrast, when spike initiation was entirely dendritic (right) only distal synapses were effective. With intermediate dendritic NaV channel densities the locations of effective synapses was more evenly distributed. Adapted from (Edgerton et al., 2010)
Besides resulting in a different distribution of effective synapses, dendritic spike initiation also lead to an overall increase in spike frequency and irregularity. Most notably, it lead to the property of giving synchronous inputs in clusters of excitatory synapses a particularly high transmission efficacy, which would allow GPe dendrites to operate as coincidence detectors. However, computer modeling cannot demonstrate the actual presence of this mechanism in vivo, and unfortunately dendritic spike initiation cannot be traced with currently existing recording methods in vivo. Potentially, future single-cell depth-imaging techniques that are currently under development (Chia and Levene, 2009, Engelbrecht et al., 2010) could be used to observe dendritic spikes in vivo, however.
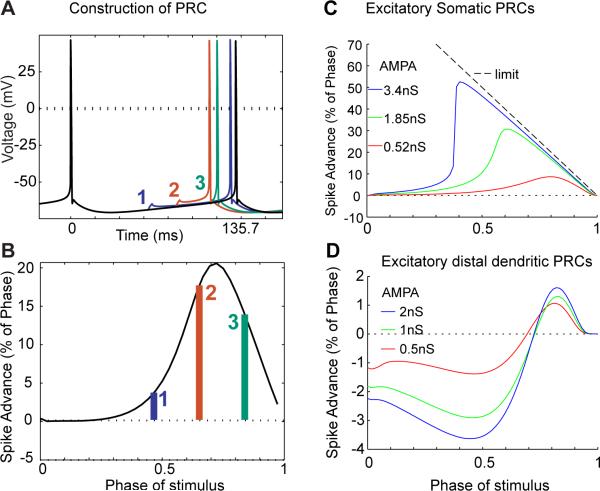
Phase response curves
The effect of a synaptic input on spiking output is generally dependent on the phase of the spike cycle that the synaptic input is received. A plot of how much an input advances or delays the next spike is called a phase response curve (PRC, Figure 3A,B). Neurons can have type I PRCs, in which excitatory input always advances the next spike, but they can also have type II PRCs, in which an excitatory input causes a delay in the next spike when an input is received early in the spike cycle, but an advance of the next spike when the input is received later in the spike cycle. Inputs to dendrites may show different PRC properties than inputs to the soma because of dendritic voltage-gated inward or outward currents (Goldberg et al., 2007). A recent modeling study of PRCs in full GPe neurons suggests that somatic input to GPe neurons shows a type I PRC (Figure 3C), whereas distal dendritic input - in the absence of directly triggering a dendritic spike - leads to a type II PRC (Figure 3D) (Schultheiss et al., 2010). The delay of the subsequent spike by excitatory input in this case was due to activation of dendritic calcium gated SK potassium conductance, which creates an outward current far outlasting the original excitatory post-synaptic current. The calcium inflow itself was triggered by voltage-gated calcium channels that are activated upon the highly depolarizing postsynaptic potentials in high input resistance regions of the dendrite. These findings give further evidence to the importance of dendritic active processing in GPe neurons. In particular, theoretical analyses have shown that type II PRCs favor synchronized modes of activity in connected networks of neurons (Hansel et al., 1995, Ermentrout, 1996, Achuthan and Canavier, 2009). This may be of particular interest in the GPe as normal activity in this structure is asynchronous between neurons, but becomes synchronized in parkinsonian conditions, as described in the next paragraph. Phase response analysis therefore suggests that alterations in dendritic input patterns in conjunction with specific active properties of GPe dendrites may be important in the generation of synchronized activity modes.
Figure 3.
Phase response curve (PRC) analysis of GPe neuron synaptic responses. A: An excitatory AMPA-type synaptic input applied at the soma results in an advance of the next spike that depends on the time at which the input is applied. The regular pacemaking spike cycle without input is shown in black. B: By stimulating the neuron with AMPA input at varying times during the spike cycle, the amount of spike advance vs. time of stimulus in spike cycle is plotted to generate the PRC. C: The PRCs for somatic AMPA inputs to our GPe neuron model are shown for 3 different synaptic conductance amplitudes. The dashed line shows the limit that would be reached if the neuron fired a spike immediately after the input. D: The PRCs for distal dendritic input are shown for 3 different AMPA conductance amplitudes. In this case excitatory inputs delivered during the first 2/3rds of the spike cycle result in a delay of the subsequent spike due to SK conductance activation. Adapted from (Schultheiss et al., 2010)
Altered GPe firing patterns in animal models of Parkinson's disease
In normal awake primates, GPe neurons generally show no spike correlation with each other (Nini et al., 1995, Bar-Gad et al., 2003), but in MPTP treated animals a strong synchronization of bursty activity between GPe neurons appears at a frequency of 7–13 Hz (Nini et al., 1995, Raz et al., 2000). Similarly, in rats GPe neurons are desynchronized in normal animals, while a strong synchronization appears in 6-OHDA treated animals, however at a somewhat higher frequency around 20 Hz within the 15–30 Hz beta band than seen in the MPTP primate and typically only with a single spike or a doublet at each period of the oscillation (Mallet et al., 2008). A slower 1–3 Hz global rhythm develops in anesthetized rats (Magill et al., 2000, Goldberg et al., 2003, Walters et al., 2007), in which GPe neurons are active in strong correlation to cerebral cortical up-states. The origin of beta rhythms in awake animals is not entirely clear to date. A direct feedback loop between STN and GPe likely plays an important role in this rhythm (Plenz and Kitai, 1999, Bevan et al., 2002, Mallet et al., 2008), but rhythmic cortical inputs to STN may also be essential (Sharott et al., 2005).
Ultimately pathological network activity in disease states is dependent on cellular changes. While undoubtedly many cellular changes in Parkinson's disease occur at the level of Str, changes at the level of GPe have also recently been discovered (Chan et al., 2011). This study showed that in a mouse model of Parkinson's disease a downregulation of HCN channels enhances burst firing through a reduction of regular pacemaking current. Given the rich repertoire of pallidal synaptic integrative properties and their implications for network integration described above, future work will likely show further implications of altered pallidal cellular properties in parkinsonian states.
Highlights
-
>
We review network connections, cellular properties, and control of spiking of pallidal neurons.
-
>
We examine the control of spiking by excitatory and inhibitory inputs in vivo.
-
>
We describe intrinsic neural mechanisms of excitability
-
>
We summarize mechanisms of synaptic intgration
-
>
We highlight relations to Parkinsonian activity patterns.
Acknowledgements
Supported by NIH/ NINDS Grants NS-57236 (HK) and NS1P50NS071669-01 (DJ)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achuthan S, Canavier CC. Phase-Resetting Curves Determine Synchronization, Phase Locking, and Clustering in Networks of Neural Oscillators. J Neurosci. 2009;29:5218–5233. doi: 10.1523/JNEUROSCI.0426-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. AnnRevNeurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Bar-Gad I, Heimer G, Ritov Y, Bergman H. Functional correlations between neighboring neurons in the primate globus pallidus are weak or nonexistent. J Neurosci. 2003;23:4012–4016. doi: 10.1523/JNEUROSCI.23-10-04012.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranauskas G, Tkatch T, Surmeier DJ. Delayed rectifier currents in rat globus pallidus neurons are attributable to Kv2.1 and Kv3.1/3.2 K+ channels. J Neurosci. 1999;19:6394–6404. doi: 10.1523/JNEUROSCI.19-15-06394.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baufreton J, Bevan MD. D2-like dopamine receptor-mediated modulation of activity-dependent plasticity at GABAergic synapses in the subthalamic nucleus. J Physiol. 2008;586:2121–2142. doi: 10.1113/jphysiol.2008.151118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- Bergstrom DA, Walters JR. Dopamine attenuates the effects of GABA on single unit activity in the globus pallidus. Brain Res. 1984;310:23–33. doi: 10.1016/0006-8993(84)90006-4. [DOI] [PubMed] [Google Scholar]
- Bevan MD, Magill PJ, Terman D, Bolam JP, Wilson CJ. Move to the rhythm: oscillations in the subthalamic nucleus-external globus pallidus network. Trends in Neurosciences. 2002;25:525–531. doi: 10.1016/s0166-2236(02)02235-x. [DOI] [PubMed] [Google Scholar]
- Carta M, Carlsson T, Kirik D, Bjorklund A. Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain. 2007;130:1819–1833. doi: 10.1093/brain/awm082. [DOI] [PubMed] [Google Scholar]
- Chan CS, Glajch KE, Gertler TS, Guzman JN, Mercer JN, Lewis AS, Goldberg AB, Tkatch T, Shigemoto R, Fleming SM, Chetkovich DM, Osten P, Kita H, Surmeier DJ. HCN channelopathy in external globus pallidus neurons in models of Parkinson's disease. Nat Neurosci. 2011;14:85–92. doi: 10.1038/nn.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CS, Shigemoto R, Mercer JN, Surmeier DJ. HCN2 and HCN1 channels govern the regularity of autonomous pacemaking and synaptic resetting in globus pallidus neurons. J Neurosci. 2004;24:9921–9932. doi: 10.1523/JNEUROSCI.2162-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charara A, Pare JF, Levey AI, Smith Y. Synaptic and extrasynaptic GABA-A and GABA-B receptors in the globus pallidus: an electron microscopic immunogold analysis in monkeys. Neuroscience. 2005;131:917–933. doi: 10.1016/j.neuroscience.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Charara A, Parent A. Brainstem dopaminergic, cholinergic and serotoninergic afferents to the pallidum in the squirrel monkey. Brain Res. 1994;640:155–170. doi: 10.1016/0006-8993(94)91870-8. [DOI] [PubMed] [Google Scholar]
- Chen L, Boyes J, Yung WH, Bolam JP. Subcellular localization of GABAB receptor subunits in rat globus pallidus. J Comp Neurol. 2004;474:340–352. doi: 10.1002/cne.20143. [DOI] [PubMed] [Google Scholar]
- Chia TH, Levene MJ. Microprisms for In Vivo Multilayer Cortical Imaging. J Neurophysiol. 2009;102:1310–1314. doi: 10.1152/jn.91208.2008. [DOI] [PubMed] [Google Scholar]
- Chuhma N, Tanaka KF, Hen R, Rayport S. Functional connectome of the striatal medium spiny neuron. J Neurosci. 2011;31:1183–1192. doi: 10.1523/JNEUROSCI.3833-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AJ, Stanford IM. Dopamine D2 receptor mediated presynaptic inhibition of striatopallidal GABA(A) IPSCs in vitro. Neuropharmacology. 2001;41:62–71. doi: 10.1016/s0028-3908(01)00038-7. [DOI] [PubMed] [Google Scholar]
- Cowan RL, Wilson CJ. Spontaneous firing patterns and axonal projections of single corticostriatal neurons in the rat medial agranular cortex. J Neurophysiol. 1994;71:17–32. doi: 10.1152/jn.1994.71.1.17. [DOI] [PubMed] [Google Scholar]
- Deister CA, Chan CS, Surmeier DJ, Wilson CJ. Calcium-Activated SK Channels Influence Voltage-Gated Ion Channels to Determine the Precision of Firing in Globus Pallidus Neurons. J Neurosci. 2009;29:8452–8461. doi: 10.1523/JNEUROSCI.0576-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR. Activity of pallidal neurons during movement. J Neurophysiol. 1971;34:414–427. doi: 10.1152/jn.1971.34.3.414. [DOI] [PubMed] [Google Scholar]
- Deschenes M, Bourassa J, Doan VD, Parent A. A single-cell study of the axonal projections arising from the posterior intralaminar thalamic nuclei in the rat. Eur J Neurosci. 1996;8:329–343. doi: 10.1111/j.1460-9568.1996.tb01217.x. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Pierucci M, Esposito E, Crescimanno G, Benigno A, Di Giovanni G. Serotonin modulation of the basal ganglia circuitry: therapeutic implication for Parkinson's disease and other motor disorders. Prog Brain Res. 2008;172:423–463. doi: 10.1016/S0079-6123(08)00921-7. [DOI] [PubMed] [Google Scholar]
- Difiglia M, Pasik P, Pasik T. A Golgi and ultrastructural study of the monkey globus pallidus. J Comp Neurol. 1982;212:53–75. doi: 10.1002/cne.902120105. [DOI] [PubMed] [Google Scholar]
- Edgerton JR, Hanson JE, Gunay C, Jaeger D. Dendritic Sodium Channels Regulate Network Integration in Globus Pallidus Neurons: A Modeling Study. J Neurosci. 2010;30:15146–15159. doi: 10.1523/JNEUROSCI.2662-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edley SM, Graybiel AM. The afferent and efferent connections of the feline nucleus tegmenti pedunculopontinus, pars compacta. J Comp Neurol. 1983;217:187–215. doi: 10.1002/cne.902170207. [DOI] [PubMed] [Google Scholar]
- Elias S, Joshua M, Goldberg JA, Heimer G, Arkadir D, Morris G, Bergman H. Statistical properties of pauses of the high-frequency discharge neurons in the external segment of the globus pallidus. J Neurosci. 2007;27:2525–2538. doi: 10.1523/JNEUROSCI.4156-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelbrecht CJ, Voigt F, Helmchen F. Miniaturized selective plane illumination microscopy for high-contrast in vivo fluorescence imaging. Opt Lett. 2010;35:1413–1415. doi: 10.1364/OL.35.001413. [DOI] [PubMed] [Google Scholar]
- Ermentrout B. Type I membranes, phase resetting curves, and synchrony. Neural Comput. 1996;8:979–1001. doi: 10.1162/neco.1996.8.5.979. [DOI] [PubMed] [Google Scholar]
- Farries MA, Kita H, Wilson CJ. Dynamic spike threshold and zero membrane slope conductance shape the response of subthalamic neurons to cortical input. J Neurosci. 2010;30:13180–13191. doi: 10.1523/JNEUROSCI.1909-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, O'Connor WT, Svenningsson P, Bjorklund L, Lindberg J, Tinner B, Stromberg I, Goldstein M, Ogren SO, Ungerstedt U, Fredholm BB, Fuxe K. Dopamine D1 receptor-mediated facilitation of GABAergic neurotransmission in the rat strioentopenduncular pathway and its modulation by adenosine A1 receptor-mediated mechanisms. Eur J Neurosci. 1996;8:1545–1553. doi: 10.1111/j.1460-9568.1996.tb01617.x. [DOI] [PubMed] [Google Scholar]
- Galvan A, Villalba RM, West SM, Maidment NT, Ackerson LC, Smith Y, Wichmann T. GABAergic modulation of the activity of globus pallidus neurons in primates: in vivo analysis of the functions of GABA receptors and GABA transporters. J Neurophysiol. 2005;94:990–1000. doi: 10.1152/jn.00068.2005. [DOI] [PubMed] [Google Scholar]
- Gardiner TW, Kitai ST. Single-unit activity in the globus pallidus and neostriatum of the rat during performance of a trained head movement. Exp Brain Res. 1992;88:517–530. doi: 10.1007/BF00228181. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr., Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Goldberg JA, Deister CA, Wilson CJ. Response Properties and Synchronization of Rhythmically Firing Dendritic Neurons. J Neurophysiol. 2007;97:208–219. doi: 10.1152/jn.00810.2006. [DOI] [PubMed] [Google Scholar]
- Goldberg JA, Kats SS, Jaeger D. Globus pallidus discharge is coincident with striatal activity during global slow wave activity in the rat. J Neurosci. 2003;23:10058–10063. doi: 10.1523/JNEUROSCI.23-31-10058.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunay C, Edgerton JR, Jaeger D. Channel Density Distributions Explain Spiking Variability in the Globus Pallidus: A Combined Physiology and Computer Simulation Database Approach. J Neurosci. 2008;28:7476–7491. doi: 10.1523/JNEUROSCI.4198-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn PJ, Russo GS, Hashimoto T, Miocinovic S, Xu W, McIntyre CC, Vitek JL. Pallidal burst activity during therapeutic deep brain stimulation. Exp Neurol. 2008;211:243–251. doi: 10.1016/j.expneurol.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel D, Mato G, Meunier C. Synchrony in excitatory neural networks. Neural Comput. 1995;7:307–337. doi: 10.1162/neco.1995.7.2.307. [DOI] [PubMed] [Google Scholar]
- Hanson JE, Smith Y, Jaeger D. Sodium channels and dendritic spike initiation at excitatory synapses in globus pallidus neurons. J Neurosci. 2004;24:329–340. doi: 10.1523/JNEUROSCI.3937-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Kita H. Slow oscillatory activity of rat globus pallidus neurons in vitro. Eur J Neurosci. 2006;23:443–453. doi: 10.1111/j.1460-9568.2005.04582.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Kita H. Serotonin activates presynaptic and postsynaptic receptors in rat globus pallidus. J Neurophysiol. 2008;99:1723–1732. doi: 10.1152/jn.01143.2007. [DOI] [PubMed] [Google Scholar]
- Hassani OK, Mouroux M, Feger J. Increased subthalamic neuronal activity after nigral dopaminergic lesion independent of disinhibition via the globus pallidus. Neuroscience. 1996;72:105–115. doi: 10.1016/0306-4522(95)00535-8. [DOI] [PubMed] [Google Scholar]
- Jessell TM, Emson PC, Paxinos G, Cuello AC. Topographic projections of substance P and GABA pathways in the striato- and pallido-nigral system: a biochemical and immunohistochemical study. Brain Res. 1978;152:487–498. doi: 10.1016/0006-8993(78)91104-6. [DOI] [PubMed] [Google Scholar]
- Kaneda K, Kita H. Synaptically released GABA activates both pre- and postsynaptic GABA(B) receptors in the rat globus pallidus. J Neurophysiol. 2005;94:1104–1114. doi: 10.1152/jn.00255.2005. [DOI] [PubMed] [Google Scholar]
- Kaneda K, Kita T, Kita H. Repetitive activation of glutamatergic inputs evokes a long-lasting excitation in rat globus pallidus neurons in vitro. J Neurophysiol. 2007;97:121–133. doi: 10.1152/jn.00010.2006. [DOI] [PubMed] [Google Scholar]
- Kita H. Responses of globus pallidus neurons to cortical stimulation: intracellular study in the rat. Brain Res. 1992;589:84–90. doi: 10.1016/0006-8993(92)91164-a. [DOI] [PubMed] [Google Scholar]
- Kita H. Physiology of two disynaptic pathways from the sensorimotor cortex to the basal ganglia output nuclei. In: Percheron G, McKenzie JS, Feger J, editors. The Basal Ganglia IV. Plenum; New York: 1994. pp. 263–276. [Google Scholar]
- Kita H. Neostriatal and globus pallidus stimulation induced inhibitory postsynaptic potentials in entopeduncular neurons in rat brain slice preparations. Neuroscience. 2001;105:871–879. doi: 10.1016/s0306-4522(01)00231-7. [DOI] [PubMed] [Google Scholar]
- Kita H. Globus pallidus external segment. Prog Brain Res. 2007;160:111–133. doi: 10.1016/S0079-6123(06)60007-1. [DOI] [PubMed] [Google Scholar]
- Kita H, Chiken S, Tachibana Y, Nambu A. Origins of GABA(A) and GABA(B) receptor-mediated responses of globus pallidus induced after stimulation of the putamen in the monkey. J Neurosci. 2006;26:6554–6562. doi: 10.1523/JNEUROSCI.1543-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Chiken S, Tachibana Y, Nambu A. Serotonin modulates pallidal neuronal activity in the awake monkey. J Neurosci. 2007;27:75–83. doi: 10.1523/JNEUROSCI.4058-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Kitai ST. The morphology of globus pallidus projection neurons in the rat: an intracellular staining study. Brain Research. 1994;636:308–319. doi: 10.1016/0006-8993(94)91030-8. [DOI] [PubMed] [Google Scholar]
- Kita H, Nambu A, Kaneda K, Tachibana Y, Takada M. Role of ionotropic glutamatergic and GABAergic inputs on the firing activity of neurons in the external pallidum in awake monkeys. J Neurophysiol. 2004;92:3069–3084. doi: 10.1152/jn.00346.2004. [DOI] [PubMed] [Google Scholar]
- Kita H, Tachibana Y, Nambu A, Chiken S. Balance of monosynaptic excitatory and disynaptic inhibitory responses of the globus pallidus induced after stimulation of the subthalamic nucleus in the monkey. J Neurosci. 2005;25:8611–8619. doi: 10.1523/JNEUROSCI.1719-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitai ST, Deniau JM. Cortical inputs to the subthalamus: intracellular analysis. Brain Res. 1981;214:411–415. doi: 10.1016/0006-8993(81)91204-x. [DOI] [PubMed] [Google Scholar]
- Kliem MA, Pare JF, Khan ZU, Wichmann T, Smith Y. Ultrastructural localization and function of dopamine D1-like receptors in the substantia nigra pars reticulata and the internal segment of the globus pallidus of parkinsonian monkeys. Eur J Neurosci. 2010;31:836–851. doi: 10.1111/j.1460-9568.2010.07109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magill PJ, Bolam JP, Bevan MD. Relationship of activity in the subthalamic nucleus-globus pallidus network to cortical electroencephalogram. J Neurosci. 2000;20:820–833. doi: 10.1523/JNEUROSCI.20-02-00820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Ballion B, Le Moine C, Gonon F. Cortical inputs and GABA interneurons imbalance projection neurons in the striatum of parkinsonian rats. J Neurosci. 2006;26:3875–3884. doi: 10.1523/JNEUROSCI.4439-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Pogosyan A, Marton LF, Bolam JP, Brown P, Magill PJ. Parkinsonian Beta Oscillations in the External Globus Pallidus and Their Relationship with Subthalamic Nucleus Activity. J Neurosci. 2008;28:14245–14258. doi: 10.1523/JNEUROSCI.4199-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Kita H. Activation of group III metabotropic glutamate receptors presynaptically reduces both GABAergic and glutamatergic transmission in the rat globus pallidus. Neuroscience. 2003;122:727–737. doi: 10.1016/j.neuroscience.2003.08.032. [DOI] [PubMed] [Google Scholar]
- Matsumura M, Tremblay L, Richard H, Filion M. Activity of pallidal neurons in the monkey during dyskinesia induced by injection of bicuculline in the external pallidum. Neuroscience. 1995;65:59–70. doi: 10.1016/0306-4522(94)00484-m. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Mansour A. A. E. Bennett Award paper. Expression of the dopamine D2 receptor gene in brain. Biol Psychiatry. 1991;30:985–1007. doi: 10.1016/0006-3223(91)90120-b. [DOI] [PubMed] [Google Scholar]
- Mercer JN, Chan CS, Tkatch T, Held J, Surmeier DJ. Nav1.6 sodium channels are critical to pacemaking and fast spiking in globus pallidus neurons. J Neurosci. 2007;27:13552–13566. doi: 10.1523/JNEUROSCI.3430-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi H, Kita H, Kitai ST. Intracellular study of rat entopeduncular nucleus neurons in an in vitro slice preparation: electrical membrane properties. Brain Res. 1990;527:81–88. doi: 10.1016/0006-8993(90)91063-m. [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Kita H, Kitai ST. Intracellular study of rat entopeduncular nucleus neurons in an in vitro slice preparation: response to subthalamic stimulation. Brain Res. 1991;549:285–291. doi: 10.1016/0006-8993(91)90469-c. [DOI] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Hamada I, Kita H, Imanishi M, Akazawa T, Ikeuchi Y, Hasegawa N. Excitatory cortical inputs to pallidal neurons via the subthalamic nucleus in the monkey. J Neurophysiol. 2000;84:289–300. doi: 10.1152/jn.2000.84.1.289. [DOI] [PubMed] [Google Scholar]
- Nevalainen N, Af Bjerken S, Lundblad M, Gerhardt GA, Stromberg I. Dopamine release from serotonergic nerve fibers is reduced in L-DOPA-induced dyskinesia. J Neurochem. 2011;118:12–23. doi: 10.1111/j.1471-4159.2011.07292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nini A, Feingold A, Slovin H, Bergman H. Neurons in the globus pallidus do not show correlated activity in the normal monkey, but phase-locked oscillations appear in the MPTP model of parkinsonism. J Neurophysiol. 1995;74:1800–1805. doi: 10.1152/jn.1995.74.4.1800. [DOI] [PubMed] [Google Scholar]
- Parent M, Levesque M, Parent A. Two types of projection neurons in the internal pallidum of primates: single-axon tracing and three-dimensional reconstruction. J Comp Neurol. 2001;439:162–175. doi: 10.1002/cne.1340. [DOI] [PubMed] [Google Scholar]
- Parent M, Parent A. The pallidofugal motor fiber system in primates. Parkinsonism Relat Disord. 2004;10:203–211. doi: 10.1016/j.parkreldis.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Park MR, Falls WM, Kitai ST. An intracellular HRP study of the rat globus pallidus. I. Responses and light microscopic analysis. J Comp Neurol. 1982;211:284–294. doi: 10.1002/cne.902110307. [DOI] [PubMed] [Google Scholar]
- Percheron G, Yelnik J, Francois C. A Golgi analysis of the primate globus pallidus. III. Spatial organization of the striato-pallidal complex. J Comp Neurol. 1984;227:214–227. doi: 10.1002/cne.902270207. [DOI] [PubMed] [Google Scholar]
- Plenz D, Kitai ST. A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus. Nature. 1999;400:677–682. doi: 10.1038/23281. [DOI] [PubMed] [Google Scholar]
- Radnikow G, Misgeld U. Dopamine D1 receptors facilitate GABAA synaptic currents in the rat substantia nigra pars reticulata. J Neurosci. 1998;18:2009–2016. doi: 10.1523/JNEUROSCI.18-06-02009.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall W. Time constants and electrotonic length of membrane cylinders and neurons. BiophysJ. 1969;9:1483–1508. doi: 10.1016/S0006-3495(69)86467-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall W, Rinzel J. Branch input resistance and steady attenuation for input to one branch of a dendritic neuron model. BiophysJ. 1973;13:648–687. doi: 10.1016/S0006-3495(73)86014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rav-Acha M, Bergman H, Yarom Y. Pre- and postsynaptic serotoninergic excitation of globus pallidus neurons. J Neurophysiol. 2008;100:1053–1066. doi: 10.1152/jn.00845.2007. [DOI] [PubMed] [Google Scholar]
- Raz A, Vaadia E, Bergman H. Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine vervet model of parkinsonism. J Neurosci. 2000;20:8559–8571. doi: 10.1523/JNEUROSCI.20-22-08559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson RG, Graham WC, Sambrook MA, Crossman AR. Further investigations into the pathophysiology of MPTP-induced parkinsonism in the primate: an intracerebral microdialysis study of gamma-aminobutyric acid in the lateral segment of the globus pallidus. Brain Res. 1991;563:278–280. doi: 10.1016/0006-8993(91)91545-c. [DOI] [PubMed] [Google Scholar]
- Ruskin DN, Bergstrom DA, Kaneoke Y, Patel BN, Twery MJ, Walters JR. Multisecond oscillations in firing rate in the basal ganglia: Robust modulation by dopamine receptor activation and anesthesia. J Neurophysiol. 1999;81:2046–2055. doi: 10.1152/jn.1999.81.5.2046. [DOI] [PubMed] [Google Scholar]
- Ryan LJ, Clark KB. The role of the subthalamic nucleus in the response of globus pallidus neurons to stimulation of the prelimbic and agranular frontal cortices in rats. Exp Brain Res. 1991;86:641–651. doi: 10.1007/BF00230538. [DOI] [PubMed] [Google Scholar]
- Rylander D, Parent M, O'Sullivan SS, Dovero S, Lees AJ, Bezard E, Descarries L, Cenci MA. Maladaptive plasticity of serotonin axon terminals in levodopa-induced dyskinesia. Ann Neurol. 2010;68:619–628. doi: 10.1002/ana.22097. [DOI] [PubMed] [Google Scholar]
- Sadek AR, Magill PJ, Bolam JP. A single-cell analysis of intrinsic connectivity in the rat globus pallidus. J Neurosci. 2007;27:6352–6362. doi: 10.1523/JNEUROSCI.0953-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato F, Lavallee P, Levesque M, Parent A. Single-axon tracing study of neurons of the external segment of the globus pallidus in primate. J Comp Neurol. 2000;417:17–31. [PubMed] [Google Scholar]
- Schultheiss NW, Edgerton JR, Jaeger D. Phase Response Curve Analysis of a Full Morphological Globus Pallidus Neuron Model Reveals Distinct Perisomatic and Dendritic Modes of Synaptic Integration. J Neurosci. 2010;30:2767–2782. doi: 10.1523/JNEUROSCI.3959-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharott A, Magill PJ, Harnack D, Kupsch A, Meissner W, Brown P. Dopamine depletion increases the power and coherence of beta-oscillations in the cerebral cortex and subthalamic nucleus of the awake rat. European J Neurosci. 2005;21:1413–1422. doi: 10.1111/j.1460-9568.2005.03973.x. [DOI] [PubMed] [Google Scholar]
- Shin RM, Masuda M, Miura M, Sano H, Shirasawa T, Song WJ, Kobayashi K, Aosaki T. Dopamine D4 receptor-induced postsynaptic inhibition of GABAergic currents in mouse globus pallidus neurons. J Neurosci. 2003;23:11662–11672. doi: 10.1523/JNEUROSCI.23-37-11662.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shink E, Smith Y. Differential synaptic innervation of neurons in the internal and external segments of the globus pallidus by the GABA- and glutamate-containing terminals in the squirrel monkey. J Comp Neurol. 1995;358:119–141. doi: 10.1002/cne.903580108. [DOI] [PubMed] [Google Scholar]
- Smith Y, Charara A, Hanson JE, Paquet M, Levey AI. GABA(B) and group I metabotropic glutamate receptors in the striatopallidal complex in primates. J Anat. 2000;196(Pt 4):555–576. doi: 10.1046/j.1469-7580.2000.19640555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Seno N, Kitai ST. Acutely isolated neurons of the rat globus pallidus exhibit four types of high-voltage-activated Ca2+ current. JNeurophysiol. 1994;71:1272–1280. doi: 10.1152/jn.1994.71.3.1272. [DOI] [PubMed] [Google Scholar]
- Tachibana Y, Kita H, Chiken S, Takada M, Nambu A. Motor cortical control of internal pallidal activity through glutamatergic and GABAergic inputs in awake monkeys. Eur J Neurosci. 2008;27:238–253. doi: 10.1111/j.1460-9568.2007.05990.x. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Filion M. Responses of pallidal neurons to striatal stimulation in intact waking monkeys. Brain Res. 1989;498:1–16. doi: 10.1016/0006-8993(89)90394-6. [DOI] [PubMed] [Google Scholar]
- Urbain N, Gervasoni D, Souliere F, Lobo L, Rentero N, Windels F, Astier B, Savasta M, Fort P, Renaud B, Luppi PH, Chouvet G. Unrelated course of subthalamic nucleus and globus pallidus neuronal activities across vigilance states in the rat. European J Neurosci. 2000;12:3361–3374. doi: 10.1046/j.1460-9568.2000.00199.x. [DOI] [PubMed] [Google Scholar]
- Wallman MJ, Gagnon D, Parent M. Serotonin innervation of human basal ganglia. Eur J Neurosci. 2011;33:1519–1532. doi: 10.1111/j.1460-9568.2011.07621.x. [DOI] [PubMed] [Google Scholar]
- Walters JR, Hu D, Itoga CA, Parr-Brownlie LC, Bergstrom DA. Phase relationships support a role for coordinated activity in the indirect pathway in organizing slow oscillations in basal ganglia output after loss of dopamine. Neuroscience. 2007;144:762–776. doi: 10.1016/j.neuroscience.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Kita T, Kita H. Presynaptic actions of d2-like receptors in the rat cortico-striato-globus pallidus disynaptic connection in vitro. J Neurophysiol. 2009;101:665–671. doi: 10.1152/jn.90806.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Basal ganglia discharge abnormalities in Parkinson's disease. J Neural Transm Suppl. 2006:21–25. doi: 10.1007/978-3-211-45295-0_5. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. Postsynaptic potentials evoked in spiny neostriatal projection neurons by stimulation of ipsilateral and contralateral neocortex. Brain Res. 1986;367:201–213. doi: 10.1016/0006-8993(86)91593-3. [DOI] [PubMed] [Google Scholar]
- Yelnik J, Percheron G, Francois C. A Golgi analysis of the primate globus pallidus. II. Quantitative morphology and spatial orientation of dendritic arborizations. J Comp Neurol. 1984;227:200–213. doi: 10.1002/cne.902270206. [DOI] [PubMed] [Google Scholar]