Abstract
Huntington’s disease (HD) is a progressive, fatal neurological condition caused by an expansion of CAG (glutamine) repeats in the coding region of the Huntington gene. To date, there is no cure but great strides have been made to understand pathophysiological mechanisms. In particular, genetic animal models of HD have been instrumental in elucidating the progression of behavioral and physiological alterations, which had not been possible using classic neurotoxin models. Our groups have pioneered the use of transgenic HD mice to examine the excitotoxicity hypothesis of striatal neuronal dysfunction and degeneration, as well as alterations in excitation and inhibition in striatum and cerebral cortex. In this review, we focus on synaptic and receptor alterations of striatal medium-sized spiny (MSNs) and cortical pyramidal neurons in genetic HD mouse models. We demonstrate a complex series of alterations that are region-specific and time-dependent. In particular, many changes are bidirectional depending on the degree of disease progression, i.e., early versus late, and also on the region examined. Early synaptic dysfunction is manifested by dysregulated glutamate release in striatum followed by progressive disconnection between cortex and striatum. The differential effects of altered glutamate release on MSNs originating the direct and indirect pathways is also elucidated, with the unexpected finding that cells of the direct striatal pathway are involved early in the course of the disease. In addition, we review evidence for early N-methyl-D-aspartate receptor (NMDAR) dysfunction leading to enhanced sensitivity of extrasynaptic receptors and a critical role of GluN2B subunits. Some of the alterations in late HD could be compensatory mechanisms designed to cope with early synaptic and receptor dysfunctions. The main findings indicate that HD treatments need to be designed according to the stage of disease progression and should consider regional differences.
Keywords: Huntington’s disease, animal models, electrophysiology, synaptic activity, extrasynaptic NMDA receptors, direct and indirect pathways
1 Introduction: What is Huntington’s Disease?
Huntington’s disease (HD) is a dominantly inherited neurodegenerative disorder caused by an unstable expansion of a CAG repeat within the coding region of the IT15 gene on the short arm of chromosome 4 (The Huntington's Disease Collaborative Research Group, 1993). The gene encodes a protein called huntingtin (htt), and the mutation results in an elongated stretch of glutamine (polyQ>36) near its amino terminus (The Huntington's Disease Collaborative Research Group, 1993). Htt is a large protein (~350 kDa) that is highly conserved and expressed ubiquitously throughout the body (Strong et al., 1993). Prevalence of the mutation is approximately 4–10 cases per 100,000 in western European populations. However, many more are at risk of having inherited the mutant gene.
The symptoms of HD include abnormal dance-like movements (chorea), cognitive disturbances, and disorders of mood, particularly depression, which often precede the onset of motor abnormalities (Harper and Jones, 2002; Paulsen et al., 2008). HD is typically a late onset disease although juvenile onset occurs, when CAG repeats exceed 60. In young children, HD symptoms can include epileptic seizures (Rasmussen et al., 2000; Gencik et al., 2002). In both adult and juvenile onset HD the symptoms worsen progressively and invariably lead to death. At present, there is no cure for HD but palliative drugs have been used, mostly to alleviate chorea.
Our respective laboratories have published several specialized reviews on different aspects of HD, particularly based on genetic animal models (Cepeda et al., 2007; Fan and Raymond, 2007; André et al., 2010b; Milnerwood and Raymond, 2010). The purpose of the present review is to summarize the pathophysiology of HD and to concentrate, using primarily results from electrophysiological studies, on providing insights into understanding the physiological progression of the disorder at the cellular and circuit levels and to elucidate new targets for therapies.
2 Neuropathology of HD
Neuropathologically, HD is primarily characterized by neuronal loss in the striatum and cortex (Vonsattel and DiFiglia, 1998). However, many other nuclei including the globus pallidus (GP), thalamus, hypothalamus, subthalamic nucleus, substantia nigra (SN), and cerebellum also are affected (Kremer et al., 1990; Heinsen et al., 1996; Petersén et al., 2002; Kassubek et al., 2004; Petersén et al., 2005). In addition, diffusion tensor imaging has demonstrated pathology of the white matter in pre- and early symptomatic patients (Rosas et al., 2006). In this review we will restrict our analysis to the striatum and cortex.
2.1 Striatum
Medium-sized spiny neurons (MSNs) constitute 90–95% of all striatal neurons and utilize γ-aminobutyric acid (GABA) as their principal neurotransmitter (Kita and Kitai, 1988), as well as co-localizing specific neuropeptides. The dorsal striatum receives parallel sets of diffuse glutamatergic inputs from almost all neocortical areas (Wilson et al., 1990) and several thalamic nuclei (Bolam et al., 2000; Smith et al., 2004). These inputs primarily synapse onto spines of MSNs (Kemp, 1970). The striatum also contains a number of modulatory components including dopamine (DA) projections from the SN pars compacta (SNc) (Graybiel, 1990) and cholinergic or GABAergic inputs from striatal interneurons (Pisani et al., 2007; Tepper et al., 2008). These elements constitute the basic striatal microcircuit (Tepper et al., 2004), a circuit that is severely disrupted in HD (Figure 1). In the striatum, GABAergic MSNs are most affected and degeneration of these neurons occurs progressively (Vonsattel et al., 1985). The large aspiny cholinergic interneurons and the numerous other interneuronal GABAergic populations are relatively spared from degeneration (Ferrante et al., 1985; Vonsattel et al., 1985; Ferrante et al., 1987; Cicchetti et al., 2000).
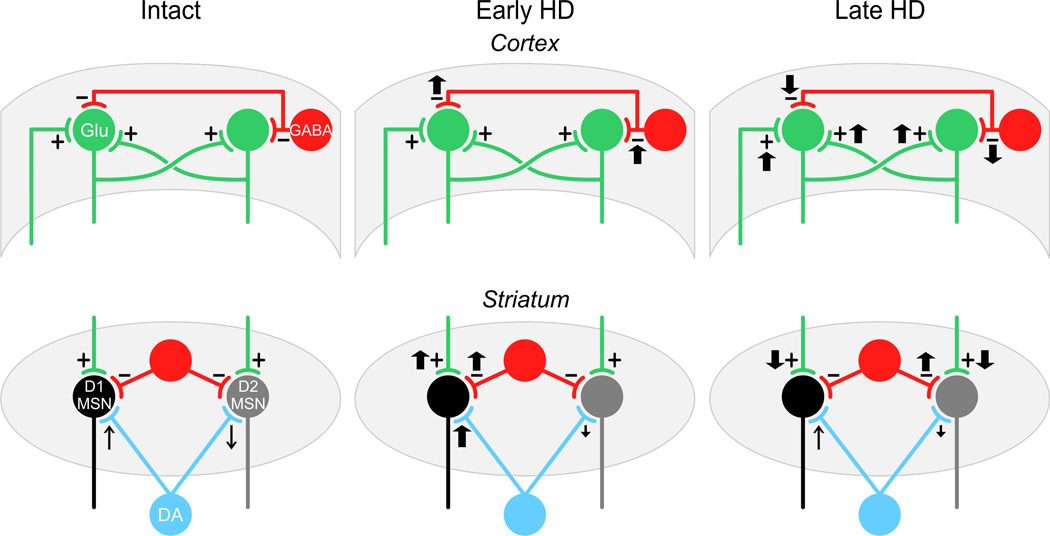
Figure 1.
Diagrammatic summary of time- and regional-dependent changes in excitation and inhibition in mouse models of HD. The left panels show the basic simplified microcircuits in the cortex (top) and striatum (bottom). The middle panels show changes in excitation and inhibition in early HD for cortex and striatum and the right panels show changes in excitation and inhibition in late HD. The basic microcircuit for the cortex (left top panel) shows excitatory input to pyramidal neurons from external inputs (primarily thalamus), intracortical excitatory connections from other pyramidal neurons and inhibitory input from interneurons. In early HD (top middle panel) there is an increase in inhibitory input with little change in excitatory input to pyramidal neurons. As indicated in the text, if inhibitory input is blocked there is a release of excitation (Cummings et al., 2009). In late HD there is a marked decrease in inhibitory inputs to pyramidal neurons and a concomitant increase in excitation. The basic circuit for the striatum (left bottom panel) shows MSNs of the direct (D1) and indirect (D2) pathways, their excitatory inputs (from cortex and thalamus), their inhibitory inputs from striatal interneurons and DA inputs from SNc (blue). The arrows in the left panel indicate that DA effects on D1 MSNs are facilitating, increasing inputs while those on D2 MSNs are attenuating, decreasing inputs. In early HD the DA inputs to D1 MSNs increase both excitatory and inhibitory inputs via a presynaptic effect. In contrast there is a decrease in D2 receptor function on indirect pathway MSNs, but no net presynaptic change. An increase in evoked EPSCs occurs that is probably mediated postsynaptically. In late HD there is a decrease in excitation to both D1 and D2 MSNs which is probably due to a disconnection from excitatory synaptic inputs. There also is an increase in inhibitory input, but only to D2 MSNs. These effects are explained further in the text.
Striatal output is largely segregated into two populations of MSNs (the direct and indirect pathways) with distinct projections, as well as DA receptor and neuropeptide expression (Kawaguchi et al., 1990), although some overlap exists (Surmeier et al., 1996; Shuen et al., 2008; André et al., 2010a). The direct pathway consists of MSNs that predominantly express D1 DA receptors (Gerfen et al., 1990) as well as substance P (SP) (Haber and Nauta, 1983) and dynorphin (Vincent et al., 1982) and project to the SN and the internal segment of the GP (GPi) (Albin et al., 1989; Gerfen et al., 1990). The indirect pathway is comprised of MSNs that express predominantly D2 receptors (Gerfen et al., 1990), met-enkephalin or neurotensin (Haber and Nauta, 1983; Steiner and Gerfen, 1999) and project to the external segment of the GP (GPe) (Kawaguchi et al., 1990).
This segregation is important in the context of HD, as there is evidence during disease progression for a time-dependent, differential loss of the two populations of striatal projection neurons. MSNs of the indirect pathway appear to be particularly vulnerable in HD and markers for these neurons and their projections, such as enkephalin, are lost in postmortem brains of fully symptomatic patients, in early symptomatic and presymptomatic brains and in genetic mouse models (Reiner et al., 1988; Albin et al., 1992; Richfield et al., 1995; Sapp et al., 1995; Menalled et al., 2000). In contrast, MSNs of the direct pathway are relatively spared in the early stages, although the SP-containing projections to the SN pars reticulata (SNr) are more severely affected than the SP-containing projections to the GPi and SNc (Deng et al., 2004). These results are consistent with the hypothesis that chorea results from preferential dysfunction and ultimate loss of indirect pathway MSNs and possibly that akinesia and dystonia which occur later in HD are a consequence of the additional dysfunction and loss of direct pathway MSNs (Albin et al., 1990a).
Another organizational feature of the striatum is the separation into a patch (striosome) and a matrix compartment, based on neurochemical markers such as opioid receptors and acetylcholinesterase staining, as well as input-output organization (Gerfen, 1992a, 1992b). Corticostriatal neurons in deep layer V and VI project to the striatal patch compartment, whereas superficial layer V and layers II–III neurons project to the matrix. Afferents to the striosomes originate in the SNc, prefrontal cortex and limbic system, whereas afferents to the matrix originate in the motor and somatosensory cortices, and in the parietal, occipital and frontal cortices (Gerfen et al., 1987). In terms of striatal outputs, neurons from the patch compartment provide inputs to the DA neurons of the SNc, whereas matrix neurons provide inputs to the GABAergic neurons of the SNr (Gerfen, 1992a; 1992b). During the early phase of HD (grades 0 and 1), discrete islands of neuronal loss and astrocytosis appear selective mostly to the patch compartment, whereas in later stages cell loss increasingly involves the matrix compartment (Hedreen and Folstein, 1995). As MSNs from patches project to the SNc it may be that early degeneration of these neurons produces hyperactivity of the nigrostriatal DA pathway, contributing to chorea and other early clinical manifestations of HD. These observations indicate an alternative explanation for the causes of chorea in HD and are discussed in more detail in section 7.
2.2 Cerebral Cortex
In the cortex, pyramidal neurons of layers III, V, and VI ultimately degenerate. Whereas striatal neuronal death may underlie many symptoms in late stage HD (Vonsattel and DiFiglia, 1998), early deficits, which are apparent years before the overt movement disorder, are more likely associated with cellular and synaptic dysfunction in the cortex (Backman and Farde, 2001; Berrios et al., 2001; Rosas et al., 2005; Paulsen et al., 2008; Rosas et al., 2008a; b). Advances in neuroimaging techniques have contributed greatly contributed to a better understanding of HD pathology, providing correlations between morphological brain changes and the development of cognitive deficits in attention, working memory, and executive functions (Rosas et al., 2004; Bohanna et al., 2008; Rosas et al., 2008b; Paulsen, 2009). Magnetic resonance imaging (MRI)-based morphometric analyses have shown that subjects carrying the HD mutation have significant volume reductions in the cortex (Rosas et al., 2003) and such changes occur before the onset of motor symptoms (Rosas et al., 2005; Rosas et al., 2006). In manifest HD, differential involvement of specific cortical areas might help explain much of the clinical heterogeneity and complexity of the disease, as specific regional cortical pathology correlates well with the nature of symptoms (Rosas et al., 2008a). For example, motor dysfunction correlates with the extent of cell loss in the primary motor cortex whereas mood changes are associated with cell loss in the cingulate cortex (Thu et al., 2010).
Among the earliest prodromal changes in HD are alterations in cognitive function (Lawrence et al., 1998; Beglinger et al., 2010) and sensory integration (Boecker et al., 1999). In sensory tests that do not involve motor components, sensory-evoked brain activation is reduced in cortical (somatosensory and frontal) and subcortical (basal ganglia) areas (Boecker et al., 1999). Cognitive deficits have been shown in HD mutation carriers decades before motor diagnosis (Paulsen et al., 2008). These cognitive changes affect functional skills and work performance (Beglinger et al., 2010), and include deficits in attentional set shifting and semantic verbal fluency (Lawrence et al., 1998). Performance on the self-timed finger-tapping task, reflecting skilled motor learning, begins to decline more than 20 years prior to predicted age of HD onset (Paulsen et al., 2008). Moreover, the decline in a variety of other cognitive and motor tasks begins approximately 10–15 years prior to HD onset and correlates well with a sharply progressive reduction in tissue volume in the striatum and cortical white matter as assessed by MRI (Paulsen et al., 2008).
3 Animal Models of HD
A central question in understanding the pathogenesis of HD is to determine the molecular, cellular, and synaptic signaling alterations that underlie the behavioral and neuropathological changes. Although a number of molecular dysfunctions have been elucidated and may contribute to the early deterioration of MSNs, the exact mechanisms whereby mutation in htt causes the observed neuronal degeneration, despite a ubiquitous expression, are still unclear. Evidence shows that the pathophysiology of HD may arise both from cell autonomous processes within vulnerable neurons and dysfunction of cell-cell interactions, specifically at the level of the corticostriatal afferents (Gu et al., 2005; Cepeda et al., 2007; Fan and Raymond, 2007; Gu et al., 2007; Zuccato and Cattaneo, 2007; Brown et al., 2008; Imarisio et al., 2008; Kim et al., 2011). In order to tease apart these possible mechanisms, a large number of animal models of HD have been created.
Animal models of HD have been available for mechanistic studies for more than 30 years (Brouillet et al., 1999). Although early neurotoxin-based models (see section 8) were instrumental in the understanding of some of the mechanisms involved in cell death (DiFiglia, 1990), they were limited because it was not possible to study the progression of the disease or to replicate the widespread neuropathology observed in the human condition, not to mention the differential vulnerability of MSNs of the direct and indirect pathways. This fact is important because although it was generally believed that the progression of symptoms in HD is due to neurodegeneration of MSNs, recent studies in genetic animal models have demonstrated that severe neuronal dysfunction precedes degeneration and is probably the major cause of many symptoms (Tobin and Signer, 2000; Levine et al., 2004). After the discovery of the gene responsible for HD in 1993, a number of genetic models [fragment and full-length transgenic, knock in and conditional mouse models (Yamamoto et al., 2000) and transgenic rat models (von Hörsten et al., 2003)] were added to the research armamentarium and for the first time these models provided a glimpse into mechanisms of disease onset and progression (reviewed in Cepeda et al., 2010; Milnerwood and Raymond, 2010). Each model differs in terms of CAG repeat length and transgene expression levels (which depend on copy number), factors that contribute to the severity of the resulting HD phenotype. In this review we will concentrate on the mouse models and those we describe do not represent an exhaustive or complete list.
3.1 Fragment Transgenic Models
The R6 line of transgenic mice (Mangiarini et al., 1996) remains one of the most widely used models, not only because it was the first model generated, but because it offers many advantages. In particular, R6/2 mice (expressing exon 1 with about 150 CAG repeats) manifest a very aggressive, rapidly progressing phenotype, similar to the juvenile form of HD in humans. They display overt behavioral symptoms as early as 5–6 weeks of age, show hindlimb clasping and weight loss and die at about 15 weeks. Pathophysiological alterations include the formation of nuclear inclusions (Davies et al., 1997), which were later also shown to be present in human HD brains (DiFiglia et al., 1997). Such inclusions can be observed in the presymptomatic stage, particularly in the striatum and CA1 region of the hippocampus (Morton et al., 2000). There also are changes in neurotransmitter receptor expression (Cha et al., 1998; Ariano et al., 2002) and altered signaling mechanisms (Bibb et al., 2000; Luthi-Carter et al., 2000; Menalled et al., 2000). Many of these alterations are correlated with motor (Carter et al., 1999) and learning impairments on a number of cognitive tasks (Lione et al., 1999), as well as deficits in synaptic plasticity in the hippocampus before an overt phenotype appears (Murphy et al., 2000) and in the striatum of symptomatic animals (Picconi et al., 2006; Kung et al., 2007).
Another line, the R6/1 (with about 110 CAG repeats and decreased mutant htt expression compared to the R6/2), presents with similar phenotypic alterations as the R6/2 but in a more protracted form (Mangiarini et al., 1996). Weight loss and clasping can be observed at 19–23 weeks and become more pronounced with age. In addition, aberrant synaptic plasticity has been described in these mice (Cummings et al., 2006; Milnerwood et al., 2006; Cummings et al., 2007).
3.2 Full-Length Transgenic Models
The most widely studied full-length model uses the yeast artificial chromosome (YAC) expressing normal (YAC18) and mutant (YAC46, YAC72, and YAC128) human htt (Hodgson et al., 1999; Slow et al., 2003). YAC72 mice display abnormal behavior around 7 months of age, as well as selective degeneration of MSNs in the lateral striatum by 12 months. Although aggregates ultimately form, subsequent experiments in these mice showed that their formation may not be essential to initiate neuronal death (Hodgson et al., 1999; Slow et al., 2003). YAC128 mice display alterations similar to YAC72 mice, but these alterations are more severe and occur earlier (Slow et al., 2003). These mice exhibit increased open field activity at about 3 months, and rotarod performance deficits starting at 6 months (Slow et al., 2003). By 12 months, open field activity is diminished significantly compared to controls. Similar to human HD, cognitive dysfunction and mood disturbance, evident by 2–3 months of age in YAC128 mice, precede motor abnormalities, and cognitive dysfunction progresses with age (Van Raamsdonk et al., 2005b; Pouladi et al., 2009). In addition, significant and selective atrophy and neuronal loss (about 10%) occur in the striatum and cortex of YAC128 mice (Slow et al., 2003; Van Raamsdonk et al., 2005a).
A more recently described full-length model of HD uses a bacterial artificial chromosome (BAC) expressing mutant htt with 97 glutamine repeats (Gray et al., 2008b). These mice exhibit progressive motor deficits, neuronal synaptic dysfunction, and late-onset selective neuropathology in both the striatum and cortex. Interestingly, studies in BACHD mice revealed that the pathogenic process can occur without early and diffuse nuclear accumulation of aggregated mutant htt (Gray et al., 2008b). BAC models also have been instrumental in demonstrating an important differential role of cell-cell interactions and cell-autonomous processes in HD pathogenesis (Gu et al., 2005; Gu et al., 2007; Gray et al., 2008a).
3.3 Knock-In Models
Knock-in models also contributed to our understanding of HD. The major advantage of these models is that they express full-length mutant htt in its native genomic context. Several models that differ mainly in the number of CAG repeats (from 48–200) have been generated (White et al., 1997; Levine et al., 1999; Shelbourne et al., 1999; Wheeler et al., 2000; Lin et al., 2001; Heng et al., 2007; Heng et al., 2009). In the knock-in models early overt behavioral changes are often subtle, but sensitive and careful testing demonstrate abnormalities as early as 1–2 months of age (Menalled et al., 2002; Menalled et al., 2003). A more severe phenotype develops as the mice age beyond one year (Hickey et al., 2008). A consistent feature of knock-in mice is the presence of nuclear staining and microaggregates very early in the course of the disease (Hickey et al., 2008). By contrast, nuclear inclusions are observed only in older mice (10–18 months) (Menalled et al., 2002), and loss of MSNs occurs at about 2 years (Hickey et al., 2008).
4 Role of DA in HD
We have recently reviewed in detail the role of DA and its interaction with glutamate in HD (André et al., 2010b). We will briefly summarize the most relevant information to put the role of DA in perspective when we describe our findings (section 7). In patients with HD, the guiding hypothesis has been that presynaptic activation of the nigrostriatal DA pathway induces chorea while loss of DA inputs induces akinesia (Bird, 1980; Spokes, 1981). This would indicate that in HD biphasic changes in DA function may occur, with early increases followed by late decreases. Overactivity in the nigrostriatal system might arise from a deficiency in GABA which normally inhibits DA release by activating GABA receptors on nigrostriatal somata and terminals (Bolam and Smith, 1990: Paladini et al., 1999). As mentioned in section 2.1, during the early phase of HD neuronal loss appears more selective to the patch compartment (Hedreen and Folstein, 1995). As MSNs in the patch project to the SNc, early degeneration of these neurons produces hyperactivity of the nigrostriatal DA pathway, contributing to chorea. Indeed, neurochemical studies in early HD have provided some evidence for increases in DA (Garrett and Soares-da-Silva, 1992). In addition, early work showed that DA levels and activity of tyrosine hydroxylase were increased in the striatum of postmortem HD brains (Spokes, 1981) and binding for the presynaptic DA transporter was reduced in the caudate nucleus of HD patients (Backman et al., 1997; Ginovart et al., 1997). Interestingly, in the transgenic rat model of HD, one of the few animal models that display chorea, evidence of increased DA levels in striatum was recently provided (Jahanshahi et al., 2010). Most mouse models exhibit a progressive reduction in DA levels. Microdialysis studies showed that DA release was progressively reduced in R6/2 mice between 6 and 14 weeks (Hickey et al., 2002; Johnson et al., 2006). Thus, in contrast to human and rat studies, in mouse models of HD, striatal DA release, and nigrostriatal terminals are reduced. However, it should be pointed out that most of these studies were performed in symptomatic mice, late in the progression of the phenotype. Thus, mouse studies of DA dynamics in early HD are necessary.
Changes in DA receptors also occur in HD. Imaging studies provide evidence supporting reduced DA receptor function in HD patients. In prodromal and manifest HD patients, positron emission tomography for D1 and D2 ligands found that both striatal D1 and D2 receptor levels were reduced and that the loss progressed each year (Andrews et al., 1999; Van Oostrom et al., 2009). Decreases in DA receptors also correlated with the duration of the disease, and with a decrease in GABA content, reflecting neuronal loss in the putamen (Ginovart et al., 1997). The R6/2 mouse model displays decreases in D1 and D2 receptor binding early (Cha et al., 1998), as well as decreases in functional D1 receptor-dependent modulation of AMPA currents (Bibb et al., 2000). At later ages D1 receptor-dependent striatal long-term potentiation is reduced as well (Kung et al., 2007). Surprisingly, DA receptor density was unchanged in 12-month old YAC128 mice (Benn et al., 2007).
5 Morphological Changes in Mouse Models
As in the human disease, most mouse models of HD display significant reductions in brain volume. While some mouse models (e.g., YAC128 and CAG140) show significant striatal neuronal loss at 12 and 24 months of age, respectively, that is commensurate with the extent of volume reduction (Slow et al., 2003; Hickey et al., 2008), other models show no neuronal loss in association with reduced brain volume, even in the more advanced stages of phenotype progression (Turmaine et al., 2000; Levine et al., 2004). More than cell death, reduced somatic size, thinning of dendrites, and spine loss, along with white matter pathology in striatum and cortex, are the main contributors to reduced brain volume in some mouse models (Klapstein et al., 2001; Laforet et al., 2001). The minimal cell loss could be related to the resiliency of neurons in mice compared with the human brain, and/or differences in the age of onset and the duration of disease progression (Van Raamsdonk et al., 2005a). Regardless of whether brain atrophy in HD mouse models is a result of reduction in neuronal size and dendritic complexity, or due to frank neuronal loss, the volume loss is small (10–15%) compared with that seen in human HD. This discrepancy suggests that neuronal dysfunction more than structural changes may contribute importantly to behavioral changes in these mouse models. At this point it remains unknown whether morphological changes are produced by initial functional alterations or if these morphological alterations are the consequence of gene-induced structural abnormalities.
6 Electrophysiological Alterations in Mouse Models of HD
As discussed, cellular dysfunction precedes cell loss in HD, and it appears sufficient to generate the initial symptoms. Using electrophysiological methods, functional alterations have been examined at the single cell and circuit levels. Most studies have concentrated on assessing electrophysiological changes in striatal MSNs and cortical pyramidal neurons (see Table1 and Figure 1 for summaries).
Table 1. Electrophysiological Alterations in Genetic Mouse Models of HD.
Data from R6/2, R6/1, YAC128, CAG140 and BACHD models and based primarily on slice or dissociated cell preparations. Cell and correlated firing are from in vivo studies.
| Presymptomatic | Symptomatic | |||||||
|---|---|---|---|---|---|---|---|---|
| Pyramidal | MSN* | D1MSN | D2MSN | Pyramidal | MSN* | D1MSN | D2MSN | |
| Basic Membrane Properties | ||||||||
| RMP | -- | -- | -- | -- | ↓ | ↓ | ||
| Cm | -- | -- | -- | -- | ↓ | ↓ | ↓ | ↓ |
| Ri | -- | -- | -- | -- | ↑ | ↑ | ↑ | -- |
| Voltage-gated Currents | ||||||||
| Ca2+ | -- | -- | ↑ | ↓ | ||||
| K+ | -- | -- | ↓ | ↓ | ||||
| Cell Firing | -- | -- | ↑ | ↑ | ||||
| Correlated Firing | -- | -- | ↓ | ↓ | ||||
| AMPA Synaptic Activity | ||||||||
| EPSC Frequency | -- | -- | ↑ | -- | ↑ | ↓ | ↓ | ↓ |
| mEPSC Frequency | -- | -- | ↑ | -- | ↑ | ↓ | ||
| eEPSCs /EPSPs | -- | ↑ | -- | ↑ | ↑ | ↓ | ↓ | -- |
| Release Probability | -- | ↑ | ↑ | -- | ↓ | ↓ | ↓ | -- |
| Receptor Sensitivity | ↓ | ↑ | -- | -- | ||||
| NMDA Receptors | ||||||||
| Synaptic | -- | ↓ | ||||||
| Extrasynaptic | ↑ | ↑ | ||||||
| Receptor Sensitivity | ↓ | ↑ | -- | ↓ | ||||
| GABA Synaptic Activity | ||||||||
| IPSC Frequency | ↑ | -- | ↑ | -- | ↓ | ↑ | -- | ↑ |
| mIPSC Frequency | -- | ↓ | -- | |||||
| eIPSCs | -- | ↓ | ||||||
| Release Probability | -- | |||||||
| Receptor Sensitivity | -- | -- | -- | ↑ | ||||
| Synaptic Plasticity | ||||||||
| LTD | ↑ | -- | ↓ | |||||
| LTP | ↓ | |||||||
unidentified MSNs,
no change.
Blanks=no information.
RMP=resting membrane potential, Cm=cell membrane capacitance, Ri=membrane input resistance. D1MSNs=MSNs expressing D1 receptors (direct pathway), D2MSNs=MSNs expressing D2 receptors (indirect pathway). EPSC/EPSP/IPSC=excitatory, inhibitory postsynaptic currents or potentials, m=miniature, e=evoked. LTD=long-term depression, LTP=long-term potentiation.
Data summarized from references: André et al., 2006, 2011a, b; Ariano et al., 2005; Centonze et al., 2005; Cepeda et al., 2001, 2003, 2004; Cherry, 2009; Cowan et al., 2008; Cummings et al., 2006, 2009, 2010; Graham et al., 2009; Grey et al., 2009; Joshi et al., 2009; Klapstein et al., 2001; Kung et al., 2007; Levine et al., 1999; Miller et al., 2008; Milnerwood et al., 2010; Rebec et al., 2006; Spampanato et al., 2008; Starling et al., 2005; Stern, 2011; Walker et al., 2008.
6.1 Basic Membrane Properties and Intrinsic Conductances
In the striatum, the primary alterations in basic membrane properties observed in MSNs of HD mice are increases in cell membrane input resistance and depolarized resting membrane potentials (Levine et al., 1999; Klapstein et al., 2001; Cummings et al., 2010). In some models there also is a decrease in membrane capacitance (Cepeda et al., 2003). Determinants of these biophysical parameters are primarily cell size (reductions in somatic cross-sectional areas and dendritic fields) and K+ conductances (which also are reduced) (Levine et al., 1999; Klapstein et al., 2001; Ariano et al., 2005; Cummings et al., 2010). There is a significant reduction in inward rectification in MSNs concomitant to the onset of overt behavioral symptoms; this change correlates well with immunofluorescence studies demonstrating decreases in the expression of the specific K+ channel subunit proteins, Kir2.1 and Kir2.3 (Ariano et al., 2005). High voltage-activated Ca2+ currents appear reduced in MSNs in fully symptomatic R6/2 HD mice (Cepeda et al., 2001a). One of the consequences of increases in membrane input resistance is an enhancement of cell excitability which, coupled with the depolarized membrane potentials, could lead to amplification of synaptic inputs and increased action potential firing in MSNs. Such changes also make MSNs more vulnerable to glutamate-induced NMDAR activation (see section 8).
Similar to MSNs, cortical pyramidal neurons in symptomatic mice also display depolarized resting membrane potentials, increased input resistance and decreased capacitance (Cummings et al., 2009; Stern 2011). In contrast to MSNs, in cortical pyramidal neurons Ca2+ currents are increased in late symptomatic R6/2 animals (André et al., 2006; Cherry, 2009) but unchanged (André et al., 2006) or even reduced (Cherry, 2009) in pre- or early symptomatic animals.
6.2 Synaptic Transmission in the Striatum
In neurons the htt protein distribution is similar to that of synaptophysin (Wood et al., 1996) and it associates with various proteins involved in synaptic function (Wood et al., 1996). Mutant htt produces specific impairment in exocytosis and endocytosis, potentially causing abnormal synaptic transmission, thus leading to the hypothesis that HD is a synaptopathology (Li et al., 2003b). In several genetic mouse models of HD the progression of synaptic alterations along the corticostriatal pathway, as well as synaptic changes in cortical pyramidal neurons, have been examined. The outcomes have shown that changes in synaptic transmission in the striatum and cortex are uniquely time- and region-dependent.
In R6/2 and YAC128 HD mice, glutamatergic function in the corticostriatal pathway changes dynamically in a biphasic manner. Dysregulation of glutamatergic input to MSNs occurs early and is manifested by the presence of large-amplitude and complex spontaneous synaptic events that peak at approximately 5–7 weeks of age in the R6/2 model (Cepeda et al., 2003) (Figure 1). In YAC128 mice there is a marked increase in frequency of spontaneous excitatory postsynaptic currents (EPSCs) in MSNs at 1–1.5 months of age (André et al., 2011a; Joshi et al., 2009). The large events as well as the increase in frequency of EPSCs could reflect increased cortical excitability and/or a possible reduction in presynaptic inhibitory receptor function that would typically decrease glutamate release from corticostriatal endings. Such changes could include decreases in function of DA D2, metabotropic glutamate (mGluR2/3) and/or endocannabinoid CB1 receptors (Cha et al., 1998; Luthi-Carter et al., 2000; Ariano et al., 2002).
As striatal neuronal action potential generation is highly dependent on cortical inputs, the presence of large-amplitude synaptic events in the striatum of R6/2 mice at 5–7 weeks, in conjunction with higher membrane input resistance, and depolarized resting membrane potentials predicts increased activity along the corticostriatal pathway. Consistent with this hypothesis, in vivo recordings of striatal neurons have demonstrated that cell firing is elevated in R6/2 transgenic relative to wildtype (WT) mice at 6–9 weeks of age (Rebec et al., 2006), an effect that can be reversed by upregulation of a glutamate transporter (Miller et al., 2008a). These effects also occur in other models (Miller et al., 2008b). In addition, correlated firing and coincident bursts between pairs of MSNs were prominent in cells from WT animals, but reduced in R6/2 and knock-in models (Miller et al., 2008b) suggesting that information processing at both the single-neuron and population level is compromised in the striatum of symptomatic HD mice (Miller et al., 2008b). Similar changes were also observed from in vivo recordings in cortical pyramidal neurons from symptomatic mice (Miller et al., 2008b).
A later marked change is a reduction in excitatory synaptic activity displayed by MSNs (Cepeda et al., 2003). While this reduction begins at the same age as the appearance of overt behavioral alterations, it becomes more severe as the behavioral phenotype progresses (Klapstein et al., 2001; Cepeda et al., 2003; Graham et al., 2009; Cummings et al., 2010) (Figure 1). The reduction correlates with alterations in synaptic markers (e.g., synaptophysin and PSD95) and suggests, in conjunction with the loss of dendritic spines, that MSNs become disconnected from many of their synaptic inputs in the later stages of the disease (Cepeda et al., 2003). Indeed, a recent study using electron microscopy demonstrates a significant reduction in excitatory synapses onto striatal neurons in 12 month-old YAC128 mice (Singaraja et al., 2011). In view of the progressive loss of synaptic inputs, the observed in vivo increased firing frequency of MSNs may be explained by findings that MSNs in the HD models are more depolarized, have smaller cross-sectional areas and higher input resistances. Thus, they are more excitable and will need less input to evoke action potentials. One of the outcomes of excitatory activity in the corticostriatal pathway is the concomitant release of brain-derived neurotrophic factor (BDNF), a trophic factor essential for postsynaptic neuronal support that is reduced in HD (Zuccato and Cattaneo, 2007). The progressive reduction in glutamate-mediated synaptic activity also will reduce the release of this trophic factor and potentially facilitate cellular dysfunction and subsequent degeneration.
Glutamate release in the corticostriatal pathway of YAC128 mice also has been examined at different stages of disease progression (1, 7 and 12 months), using combined optical and electrophysiological methods (Joshi et al., 2009). Similar to results from R6/2 mice, the results in YAC128 mice demonstrated biphasic age-dependent changes in corticostriatal function. At 1 month, before the behavioral phenotype develops, glutamate release and α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptor-mediated synaptic currents evoked by cortical stimulation were increased. At 7 and 12 months, after the development of the behavioral phenotype, glutamate release and AMPA synaptic currents were significantly reduced (Joshi et al., 2009). These effects were due to combined pre- and postsynaptic alterations.
These studies point to cell-cell interactions as important for striatal neuron dysfunction. If that is the case, removal of inputs to the striatum could retard disease progression. In fact, in our previous studies we found that removal of the cerebral cortex in corticostriatal slices markedly reduced the occurrence of large-amplitude events (Cepeda et al., 2003). Also, lesions of the cortex or elimination of DA neurons improves the behavioral and pathological HD phenotype in R6/2 mice, suggesting that synaptic stress more than trophic factors contribute to neurodegeneration (Stack et al., 2007). Further supporting this idea, studies have shown that selective removal of mutant htt from cortical pyramidal neurons can reduce striatal pathology (Gu et al., 2007; Gray et al., 2008a). While such cell-cell interactions are important, cell-autonomous alterations will also affect the progression of the phenotype (Brown et al., 2008; Kim et al., 2011; Thomas et al., 2011).
In marked contrast to reduced excitation of MSNs of symptomatic animals, the frequency of inhibitory GABAA receptor-mediated synaptic events is increased in MSNs in the R6/2 and other models of HD (Cepeda et al., 2004; Cummings et al., 2010) (Figure 1, Table 1). This increase in GABA-mediated synaptic input was associated with an increase in immunostaining for the GABAA α1 receptor subunit (Cepeda et al., 2004). New preliminary data indicate that the increased inhibitory input may be due to enhanced GABAergic activity of striatal interneuron populations, and not intrinsic connections from other MSNs (Rao et al., 2010). Increased inhibitory input onto MSNs predicts reduced output of MSNs to target structures along both the direct and indirect pathways. Increased GABA release also could dampen glutamate inputs either by shunting (increase in the membrane conductance) or by activation of GABAB receptors located on corticostriatal terminals.
6.3 Synaptic Transmission in the Cortex
Increased excitability in cortical networks occurs as the disease progresses in the R6/2 and several other mouse models (Cummings et al., 2009) (Figure 1). Examination of somatosensory cortical pyramidal neurons in layers II/III in slices from R6/2 mice revealed that spontaneous EPSCs occurred at a higher frequency in behaviorally phenotypic mice, whereas spontaneous inhibitory postsynaptic currents (IPSCs) were initially increased in frequency and subsequently decreased at 80–90 days when the disease phenotype was manifest (Cummings et al., 2009) (Figure 1, Table 1). Decreased inhibition in cortical pyramidal neurons also was observed in the BACHD model at 6 months when motor dysfunction occurs (Spampanato et al., 2008). Furthermore, compared with control animals, R6/2 mice demonstrate increased epileptiform activity in cortical slices and seizure susceptibility in vivo after blockade of GABAA receptors, providing more evidence that the cortex becomes hyperexcitable (Cummings et al., 2009).
The observation that excitatory inputs to pyramidal neurons are enhanced whereas inhibitory inputs are reduced is in the opposite direction to alterations observed in the striatum, once the behavioral phenotype is manifest (see above) (Cepeda et al., 2004) (Figure 1, Table 1). Because mutant htt is expressed ubiquitously throughout the body, one might predict that all regions of the brain would show similar electrophysiological dysfunctions. However, since opposite changes in these two brain regions occur, it appears that neuronal dysfunction is not only cell-autonomous, but that the cellular environment may determine the type of dysfunction. It is possible that the striatum is more affected in HD not only because MSNs are more inherently susceptible than cortical neurons per se, but also because the highly integrative nature of the striatum places it in the unique position of receiving a wide range of aberrant inputs from cortex and other regions. These findings suggest strongly that therapies may need to be regionally oriented as well as delivered early in the time course of disease progression.
7 Selective Vulnerability of MSNs
One of the enigmas of HD is the particular vulnerability of MSNs forming the indirect pathway as pointed out above. What makes these neurons more susceptible to degeneration? Previously, it was extremely difficult to isolate direct and indirect pathway MSNs for functional analyses. Recently, the use of the enhanced green fluorescent protein (EGFP) gene as a reporter to identify DA D1 (direct pathway) and D2 (indirect pathway) receptor-expressing striatal cells has made it possible to identify and separately study neurons originating each pathway.
7.1 Differential properties of indirect and direct pathway MSNs
In intact mice, anatomical evidence has demonstrated differential excitatory inputs onto direct and indirect pathway MSNs. Two types of pyramidal corticostriatal projections have been identified; one is ipsilateral and arises from collaterals of the pyramidal tract (PT-type), and the other is bilateral and projects only intratelencephalically to the cortex and striatum (IT-type). IT-type neuronal somata are medium-sized and located in layer III and upper layer V, whereas PT-type somata are larger and most commonly located in lower layer V. The corticostriatal terminals from IT-type neurons are approximately half the size of those from PT-type neurons (Reiner et al., 2003). Terminals making asymmetric axospinous contact with direct pathway MSNs are significantly smaller than those making contact with indirect pathway MSNs (Lei et al., 2004), suggesting that direct pathway neurons preferentially receive inputs from IT-type cortical neurons, whereas indirect pathway neurons receive greater inputs from PT-type cortical neurons. A possible functional consequence of this differential innervation is that indirect pathway cells would be subject to increased glutamate release from corticostriatal terminals (Reiner et al., 2003; however see Ballion et al., 2008), which could make them more susceptible in HD as the major glutamate input comes from the cortex. Interestingly, cortical pyramidal neurons innervating the indirect pathway receive more excitatory inputs due to enlarged dendritic trees in cortical layer I compared to pyramidal neurons innervating the direct pathway (Morishima and Kawaguchi, 2006).
Although it was generally believed that MSNs of the direct and indirect pathways were morphologically and electrophysiologically identical, this assumption has changed (Kreitzer and Malenka, 2007; Cepeda et al., 2008; Gertler et al., 2008; André et al., 2010a). Studies in vitro generally demonstrate that cell input resistance, capacitance, time constant and resting membrane potentials are similar in direct and indirect pathway MSNs (Kreitzer and Malenka, 2007; Cepeda et al., 2008). However, one study found that indirect pathway MSNs have higher input resistances and are slightly but significantly more depolarized than direct pathway MSNs (Gertler et al., 2008). Indirect pathway MSNs are more excitable than direct pathway MSNs as, at similar current intensities, these MSNs fire more action potentials (Kreitzer and Malenka, 2007), have a lower threshold for action potential generation (Cepeda et al., 2008), and exhibit a lower rheobase (Gertler et al., 2008). Indirect pathway MSNs also have significantly smaller dendritic surface areas, due to fewer primary dendrites, which makes them more compact and consequently more excitable (Gertler et al., 2008).
There also are differences in synaptic inputs. The frequency of spontaneous EPSCs is higher in indirect compared to direct pathway MSNs and large-amplitude events (>100 pA) only occur in indirect pathway MSNs (Cepeda et al., 2008). After addition of the sodium channel blocker tetrodotoxin, to isolate miniature (m)EPSCs, the difference in the frequency of mEPSCs between direct and indirect pathway MSNs is reduced but the cumulative inter-event interval distributions are still significantly different (Kreitzer and Malenka, 2007; Cepeda et al., 2008). Further, after addition of the GABAA receptor blockers bicuculline or picrotoxin, which induce epileptiform activity in cortical pyramidal neurons, indirect pathway MSNs display large membrane depolarizations rarely seen in direct pathway MSNs (Cepeda et al., 2008). The preferential propagation of epileptiform activity onto indirect pathway MSNs supports previous data demonstrating that enkephalin-positive neurons that also express D2 receptors are more selectively activated by cortical stimulation (Uhl et al., 1988; Berretta et al., 1997; Parthasarathy and Graybiel, 1997).
These results imply that indirect pathway MSNs reflect on-going cortical activity, particularly the activity generated by PT-type neurons, more faithfully than direct pathway cells. Inputs from PT-type neurons may provide indirect pathway MSNs with a copy of the cortical motor signal (Reiner et al., 2003). This signal could be crucial for motor coordination. Another implication of increased intrinsic excitability and tighter corticostriatal synaptic coupling of indirect pathway MSNs is that cells of the indirect pathway are more readily available for activation than cells of the direct pathway. In contrast to the tight synaptic coupling between cortical PT terminals and indirect pathway MSNs, the smaller size and diffuse nature of the projections of the IT-type terminals will produce less activation of direct pathway MSNs.
7.2 Direct and Indirect Pathway Alterations in HD
As described above, indirect pathway MSNs are believed to be affected earlier than direct pathway MSNs. If these neurons degenerate, a loss of input to the GPe would induce an imbalance in the basal ganglia circuitry, leading to inhibition of the subthalamic nucleus, which in turn would release inhibition to the thalamus, inducing overflow of glutamate activity in the cortex and hyperkinetic movements (Chevalier and Deniau, 1990). In support of this hypothesis, increased firing rates in GPe and decreased firing rates in GPi were found in an HD patient (Starr et al., 2008), which could explain why deep brain stimulation of the GPi can improve abnormal movements in some HD patients (Moro et al., 2004; Biolsi et al., 2008; Fasano et al., 2008).
It remains unclear, however, whether the early loss of enkephalin immunoreactivity in the GPe reflects a depletion of peptide content or an actual loss of axonal inputs to the GPe due to degenerative changes in indirect pathway MSNs. In addition, there is evidence of early alterations in MSNs originating the direct pathway, which also could lead to disinhibition of thalamocortical pathways (Hedreen and Folstein, 1995). Because activation of the direct and indirect pathways has differential effects on motor control, it is of paramount importance to understand the changes occurring in the two pathways. Taking advantage of the availability of mice expressing EGFP under the promoter of DA D1 and D2 receptors, glutamate and GABA synaptic function in direct and indirect pathway MSNs were examined in two models of HD, the YAC128 and the BACHD (André et al., 2011a). Electrophysiological recordings were performed at different stages of the disease phenotype in these mouse models, corresponding to the period of hyperkinesia (“early HD”, 1.5 to 2 months) and the period of hypokinesia (“late HD”, 12 months).
7.2.1 Direct Pathway MSNs
Early in HD, the first alterations in synaptic inputs to striatal neurons were apparent in the presynaptic activity onto direct pathway MSNs, and not indirect pathway MSNs (André et al., 2011a; André et al., 2011b). In the early HD stage, both glutamate and GABA transmission were increased onto direct pathway MSNs (Figure 1, Table 1). The change in glutamate transmission is likely to occur presynaptically because we observed changes in mEPSCs and in paired-pulse ratios suggesting increased probability of release. Furthermore, we showed that tonic activation of D1 receptors underlies the increase in excitatory neurotransmission in early HD, as the DA depleter tetrabenazine or blockade of D1 receptor function with a D1 antagonist reversed the increases in direct pathway MSNs from YAC128 and BACHD mice. The reversal was explained by a combination of pre- and postsynaptic mechanisms. Based on studies showing that endocannabinoid content is decreased by D1 receptor activation (Patel et al., 2003) and our own data (André et al., 2010a), we hypothesize that activation of postsynaptic D1 receptors increases presynaptic glutamate release through blockade of endocannabinoid release by MSNs, thereby releasing the negative control endocannabinoids normally exert onto neighboring terminals (André et al., 2010a). The early decrease in endocannabinoid receptors in the striatum in HD provides additional evidence that the interaction between DA and endocannabinoid receptors will play a prominent role in controlling both excitatory and inhibitory inputs to MSNs.
In late stage HD, direct pathway MSNs cells no longer displayed increased glutamate and GABA synaptic activity (Figure 1, Table 1). There was a clear decrease of EPSC frequency and increased paired-pulse ratios, indicative of decreased probability of glutamate release. Glutamate currents evoked by corpus callosum electrical stimulation displayed a marked decrease in amplitude in the direct pathway MSNs at 12 months (André et al., 2011a). Glutamate released by electrical stimulation activated mostly AMPA receptors, indicating that decreases in AMPA currents previously reported in 12 month old YAC128 mice occur primarily in D1 cells (Joshi et al., 2009).
7.2.2 Indirect Pathway MSNs
Changes in indirect pathway MSNs were also observed. In early HD, indirect pathway MSNs displayed larger evoked glutamate currents. As paired-pulse ratios and EPSC frequencies were not changed, this effect could be due to increased glutamate receptor expression at the surface of postsynaptic neurons. However, there were no significant changes in mEPSC amplitude, suggesting postsynaptic glutamate receptors were not increased. Currents evoked by electrical stimulation induce massive glutamate release and are likely to activate different sets of receptors than the smaller quantities of glutamate released spontaneously. One hypothesis is that electrical stimulation induces a spillover of glutamate, activating extrasynaptic receptors that are not activated during spontaneous activity (Millerwood et al. 2010). Although in our recording conditions AMPA currents are isolated, it is possible that NMDA currents also are increased early in these indirect pathway MSNs (see section 8.3). In contrast to direct pathway MSNs, neurotransmitter release onto indirect pathway MSNs displayed changes only at the later stage of the disease phenotype (Figure 1, Table 1). In 12 month-old YAC128 mice, indirect pathway MSNs exhibited a slightly lower frequency of EPSCs, although the paired-pulse ratios were not different, suggesting that changes in glutamate release are not as marked as in D1 cells at that age. This is surprising, as corticostriatal inputs onto indirect pathway MSNs are more prominent (Lei et al., 2004; Kreitzer and Malenka, 2007; Cepeda et al., 2008). These findings suggest that other factors, such as differential connectivity of striatal interneurons to MSNs of the direct and indirect pathways (Gittis et al., 2010) also might play a role in regulating glutamate and GABA inputs. In contrast to glutamate transmission, GABA transmission was increased onto indirect pathway MSNs. In late HD, a lack of DA or D2 receptors in indirect pathway MSNs could increase GABA release onto indirect pathway MSN via a lack of endocannabinoid release. This effect will not occur in direct pathway MSNs since they do not express D2 receptors.
Taken together the findings suggest that abnormal differential DA transmission contributes to anomalies in glutamate and GABA transmission in HD. Early in the progression of the disease, increased glutamate release onto direct pathway MSNs, which is correlated with increased stereotypies, is consistent with increased DA tone. In late stage HD, decreased glutamate release onto direct pathway MSNs, correlated with behavioral hypoactivity is consistent with decreased DA tone and/or loss of corticostriatal terminals. For treatment of hyperkinetic motor symptoms in early stage HD, these results suggest that, in addition to agents decreasing DA function (e.g., tetrabenazine) specific modulation of direct pathway neurons with D1 antagonists might prove a useful strategy. Even better would be development of agents that can reduce DA receptor activity when DA tone is too high, and increase it when DA tone is too low, a property of partial agonists. Although D1 agonists/antagonists are not currently used to treat symptoms of HD, early reports of treatment of HD patients with the partial D2-selective agonist aripiprazole indicate improvement of both voluntary and involuntary movement abnormalities (Lin and Chou, 2008; Brusa et al., 2009; Oulis et al., 2010). Clearly, more studies are necessary to understand the consequences of altered GABA and glutamate transmission onto MSNs in HD to help direct new therapies and understand the mechanisms underlying the phenotypic changes.
8 Role of NMDARs in HD
As discussed above, there are many extrinsic influences on MSNs that can contribute to their exquisite vulnerability in HD. However, it is clear that intrinsic factors are also important. The following sections of this review will summarize studies implicating a role for altered postsynaptic MSN glutamate transmission, especially mediated by NMDA-type glutamate receptors, in the earliest changes underlying selective neuronal vulnerability in HD.
Early work suggested a role for glutamate-mediated excitotoxicity, especially neuronal dysfunction and death mediated by overactivation of NMDA-type glutamate receptors, in the pathogenesis of HD. Excitotoxicity refers to neuronal death resulting from prolonged exposure to the neurotransmitter glutamate (Lucas and Newhouse, 1957), leading to overactivation of its receptors with associated sustained neuronal membrane depolarization, Ca2+ overload and mitochondrial energy failure (Coyle and Puttfarcken, 1993). Although glutamate binds a variety of metabotropic and ionotropic receptors, NMDARs play a larger role than others in mediating neuronal toxicity due to high Ca2+ permeability and slow deactivation and desensitization (Choi et al., 1987; DiFiglia, 1990).
NMDARs are tetrameric complexes of two GluN1 (formerly NR1) with two GluN2 (formerly NR2) and/or GluN3 (NR3) subunits. GluN2 subunits are encoded by four genes, GluN2A, GluN2B, GluN2C and GluN2D. These subunits determine differences in receptor channel properties, pharmacology, and temporal and spatial brain distribution (Dingledine et al., 1999; Cull-Candy and Leszkiewicz, 2004). NMDARs are activated by the binding of glutamate and the allosteric modulator glycine to gate a cation-selective channel with significant Ca2+ permeability. Because this channel exhibits voltage-dependent Mg2+ block (Mayer and Westbrook, 1987), receptor activation requires presynaptic glutamate release coupled with postsynaptic depolarization to allow ion flux through the channel. Therefore, NMDARs serve as coincidence detectors for inducing activity-dependent synaptic plasticity (Bliss and Collingridge, 1993). On the other hand, overstimulation of NMDARs results in activation of proteases, lipases, and DNAases, mitochondrial energy failure, and/or signaling to a variety of apoptotic proteins, leading to neuronal death (Lipton and Rosenberg, 1994; Arundine and Tymianski, 2003). Thus, NMDARs are ideal candidates for contributing to early neuronal dysfunction and learning deficits, as well as later neuronal degeneration in HD.
A role for NMDAR-mediated excitotoxicity was suggested almost 30 years ago. Glutamate was shown to be the transmitter of the corticostriatal projection providing the major excitatory input to the striatum (McGeer et al., 1977; Fonnum et al., 1981). At about the same time, specific glutamate analogs were shown to produce selective, axon sparing lesions in the brain (Coyle et al., 1978). As striatal cell loss is the primary neuropathologic landmark in HD, one of the first rodent models used the excitotoxin kainic acid to selectively destroy MSNs (Coyle and Schwarcz, 1976; McGeer and McGeer, 1976; Schwarcz and Coyle, 1977). Later, quinolinic acid (a selective NMDAR agonist) was used as it better replicated the neurochemical, neuropathological, and behavioral manifestations of HD in rodents and non-human primates (Coyle, 1979; Schwarcz et al., 1983; Schwarcz et al., 1984; Beal et al., 1986; Sanberg et al., 1989; Hantraye et al., 1990; Beal et al., 1991; Ferrante et al., 1993). NMDAR agonists were not only more effective than other glutamate receptor agonists in producing striatal lesions, but also were more selective, in that they triggered loss of MSNs while interneurons were spared. In contrast, excitotoxic lesions induced by injection of other glutamate receptor agonists, such as kainic acid, resulted in more widespread neuronal loss (Coyle and Schwarcz, 1976; McGeer and McGeer, 1976), a finding that was reproduced in acute corticostriatal brain slices (Cepeda et al., 2001b).
Furthermore, systemic exposure to chemicals such as 3-nitropropionic acid, which compromise mitochondrial function and reduce energy production, also caused striatal lesions similar to those found in HD (Beal et al., 1993; Damiano et al., 2009; Brouillet et al., 1999), and this effect was rescued by NMDAR antagonists (Greene et al., 1993; Bogdanov et al., 1998; Lee et al., 2006). Interestingly, human autopsy brain tissue from presymptomatic and early stage HD showed reduced NMDAR radioligand binding in the striatum that was disproportionate to neuronal loss, suggesting that neurons expressing high levels of these receptors were most susceptible to degeneration (Young et al., 1988; Albin et al., 1990b).
Together, these early studies with chemically-lesioned animal models of HD, along with findings in human brain tissue, pointed toward a role for NMDAR excitotoxicity in selective striatal neuronal degeneration. Identification of the HD gene and development of genetic mouse models of HD allowed the hypothesis to be further tested.
8.1 Altered NMDAR Activity Occurs Early in Mouse Models of HD
Studies showing that MSNs are more vulnerable to NMDAR-mediated toxicity compared to other striatal neuronal types led to the hypothesis that enhanced NMDAR activation could contribute to selective striatal degeneration in HD. In contrast to that prediction, R6/2 and R6/1 transgenic mice developed resistance to striatal quinolinate toxicity with emergence of the HD phenotype, and showed similar sensitivity as WT littermates during presymptomatic stages (Hansson et al., 2001). In contrast, YAC72 and YAC128 mice showed increased striatal lesion size after quinolinate injection compared to WT controls during presymptomatic and early disease stages, while also developing resistance as the phenotype progressed (Zeron et al., 2002; Graham et al., 2009). Notably, R6/1 and R6/2 mice exhibit widespread brain atrophy, without selective striatal neuronal degeneration, whereas YAC transgenic HD mice show mild but significant striatal neuronal loss with lesser losses of cortical neurons and no significant atrophy in other brain regions (Slow et al., 2003). These studies suggest that protein context is important for accurately modeling selective neuronal degeneration, and that the YAC and other full-length mutant htt models may be better suited for study of the earliest molecular mechanisms underlying striatal neuronal vulnerability.
Enhanced sensitivity to striatal quinolinate toxicity in younger YAC HD mice suggested that mutant htt expression altered striatal NMDAR signaling. Consistent with this idea, NMDAR EPSC amplitudes were found to be larger, upon strong stimulation of cortical and/or thalamic glutamatergic afferents, in recordings from striatal neurons in acute corticostriatal brain slices from 1–2 month old YAC72 and YAC128 mice (Li et al., 2003a; Li et al., 2004; Milnerwood and Raymond, 2007; Graham et al., 2009; Milnerwood and Raymond, 2010). Notably, evoked NMDAR EPSCs were dramatically reduced with progression of the behavioral phenotype, consistent with development of resistance to striatal quinolinate toxicity (Graham et al., 2009). Interestingly, NMDAR currents also were shown to be increased in a subpopulation of striatal neurons in brain slices and dissociated neurons from R6/2 mice (Cepeda et al., 2001a; Starling et al., 2005) at ages at which the mice were resistant to NMDAR excitotoxicity. One possible explanation for such differences comes from studies indicating that signaling downstream of NMDARs is dramatically altered with disease onset. In mouse models of HD and/or human HD brains, Ca2+ handling is more efficient (Hansson et al., 2001), scaffolding and signaling proteins in the NMDAR signaling complex show altered expression (Luthi-Carter et al., 2003; Jarabek et al., 2004), and some downstream death signaling proteins, such as calcineurin, are downregulated (Goto et al., 1989; Hernandez-Espinosa and Morton, 2006; Xifro et al., 2009). These changes could all contribute to resistance to excitotoxicity, but would also be expected to reduce capacity for learning and promote deficits in motor performance.
Results in YAC HD mice suggest increased NMDAR activation is an early mechanism underlying striatal neuronal vulnerability. Data indicate increased NMDAR current amplitude and/or toxicity in primary embryonic or early postnatal cultured striatal neurons, as well as acutely dissociated striatal neurons, from YAC46, YAC72 and YAC128 mice, demonstrating that NMDAR function itself is altered from birth in a polyQ length-dependent and cell autonomous fashion (Cepeda et al., 2001a; Zeron et al., 2002; Zeron et al., 2004; Shehadeh et al., 2006; Fan et al., 2007; Zhang et al., 2008). Consistent with this idea, conditional transgenic BACHD mice in which mutant htt is expressed selectively in the striatum still show enhanced striatal NMDAR current (Gu et al., 2005). Importantly, increased striatal neuronal vulnerability to NMDA toxicity correlates well with augmented NMDAR current and Ca2+ influx in YAC HD MSNs (Shehadeh et al., 2006).
8.2 GluN2B-type NMDARs Contribute to Selective Striatal Neuron Vulnerability
Since both NMDAR and htt expression are widespread throughout the brain, differential distribution of these proteins cannot explain selective striatal MSN degeneration on the basis of sensitivity to NMDAR toxicity. On the other hand, NMDARs containing the GluN2B subunit show high expression levels in mature striatal MSNs compared to other NMDAR subtypes and brain regions (Landwehrmeyer et al., 1995; Ghasemzadeh et al., 1996; Rigby et al., 1996; Standaert et al., 1999; Christie et al., 2000; Li et al., 2003c). Recent studies suggest GluN2B-type NMDARs are preferentially linked to cell death signaling pathways compared to GluN2A-containing NMDARs (Kim et al., 2005; Liu et al., 2007; Tu et al., 2010). Moreover, a study in transfected non-neuronal cells demonstrated increased GluN2B- but not GluN2A-type NMDAR current when co-expressed with mutant htt (Chen et al., 1999). Consistent with this, enhanced striatal MSN NMDAR current and toxicity in cultured neurons and acute brain slices are abolished by treatment with the GluN2B-selective antagonist ifenprodil (Zeron et al., 2002; Tang et al., 2005; Fan et al., 2007; Milnerwood et al., 2010). In addition, a recent study demonstrated that GluN2B-containing NMDARs contribute more to total NMDA-evoked current in D2 MSNs than in D1 cells (Jocoy et al., 2011), consistent with the early vulnerability of D2 MSNs in HD. Strong support for the hypothesis that mutant htt enhances cell death signaling by modulating GluN2B-type NMDARs comes from a recent study showing a dramatic increase in striatal neuronal loss in HD knock-in mice crossed with GluN2B-overexpressing mice (Heng et al., 2009).
8.3 Role of Extrasynaptic (Ex-) NMDARs in Striatal Neuronal Dysfunction and Degeneration
In addition to subunit composition, the subcellular localization of NMDARs plays a critical role in determining whether the resulting signal transduction cascades promote neuronal plasticity and survival or stimulate cell death. Specifically, activation of synaptic NMDARs triggers survival and plasticity pathways associated with promoting neuronal health and learning, respectively. Among others, a major consequence of synaptic NMDAR transmission is increased phosphorylation and activity of the master transcriptional regulator Cyclic-AMP Responsive Element Binding protein (CREB) (Hardingham et al., 2001; Papadia et al., 2005; Zhang et al., 2009). In this way synaptic transmission promotes expression of a variety of anti-apoptotic, anti-oxidant and pro-survival proteins, including BDNF (Favaron et al., 1993; Hansen et al., 2004; Jiang et al., 2005; Zuccato and Cattaneo, 2007; Papadia et al., 2008). In contrast, activation of Ex-NMDARs leads to CREB shut-off, promotion of pro-death gene expression, mitochondrial calcium overload and release of apoptotic factors, increased neuronal nitric oxide synthase activity with free radical formation, and activation of cell death signaling p38 and c-Jun N-terminal Kinase Mitogen-Activated Protein Kinases (JNK MAPKs) (Hardingham et al., 2002; Cao et al., 2004; Ivanov et al., 2006; Leveille et al., 2008; Soriano et al., 2008; Wahl et al., 2009; Xu et al., 2009; Hardingham and Bading, 2010). Notably, a variety of studies suggest that GluN2B-containing NMDARs predominate at extrasynaptic sites (Kew et al., 1998; Tovar and Westbrook, 1999; Barria and Malinow, 2002), although this point has been disputed (Groc et al., 2006; Thomas et al., 2006; Harris and Pettit, 2007; Petralia et al., 2010).
Given the weight of evidence indicating a role for Ex-NMDARs in stimulating pro-apoptotic signaling, as well as opposing CREB activation and pathways required for learning and memory, it is striking that recent studies demonstrate increased expression and signaling of Ex-NMDARs in the YAC HD mouse model (Okamoto et al., 2009; Milnerwood et al., 2010) (Figure 2). This effect is mediated predominantly by GluN2B-type NMDARs. Moreover, data indicate that this process contributes to enhanced vulnerability to NMDAR-mediated toxicity in striatal neurons, and deficits in skilled motor learning, of young YAC128 mice. Treatment of these mice beginning at 2 months of age with low doses of memantine, which preferentially silences Ex-NMDARs (Lipton, 2006; Xia et al., 2010), results in restoration of striatal phosphorylated CREB (pCREB) and rotarod learning performance to WT levels at 4 months of age (Milnerwood et al., 2010). The same treatment also rescues striatal atrophy at 12 months of age (Okamoto et al., 2009). However, memantine also can affect glutamate release by reducing Ca2+ conductances (Lu et al., 2010).
Figure 2.
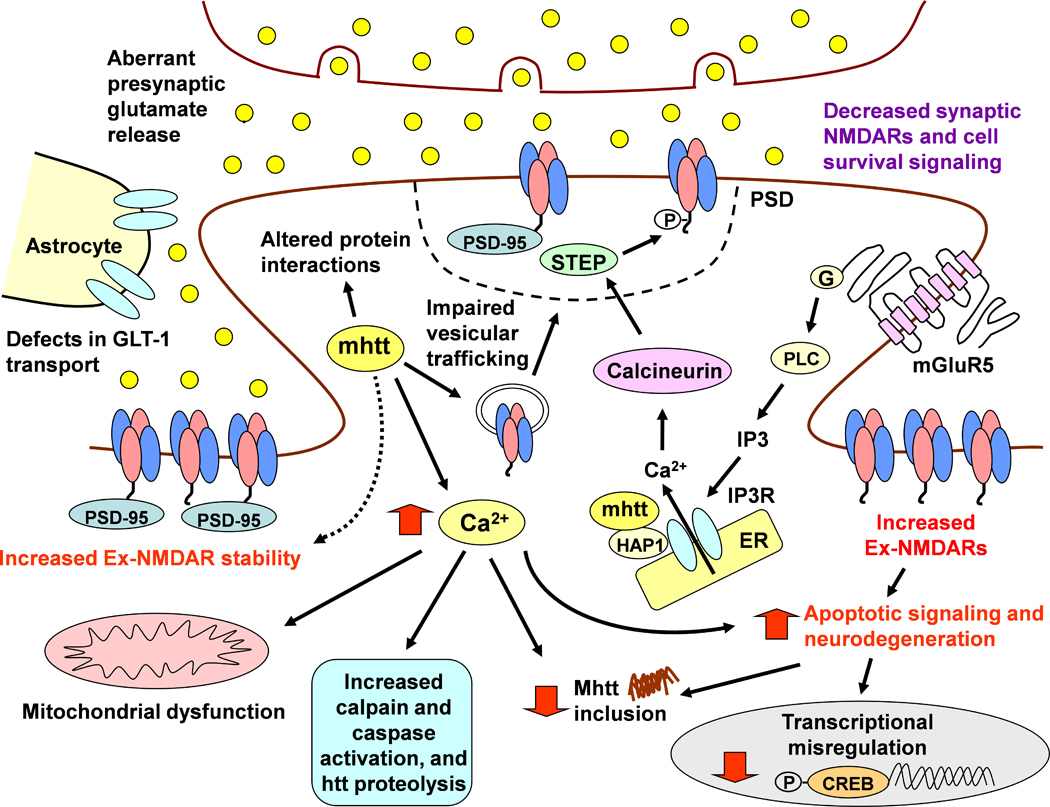
Model of mutant htt induced synaptic dysfunction in HD. Aberrant glutamate release from cortical and thalamic afferents stimulates postsynaptic and Ex-NMDARs on MSNs. Overactivation of Ex-NMDARs also may be facilitated by deficits in glutamate uptake by the transporter GLT-1 on astrocytes. The mutant htt protein affects multiple cellular processes such as Ca2+ homeostasis, mitochondria function, transcriptional regulation, protein-protein interactions and vesicular transport of proteins including neurotransmitter receptors. Mutant htt also affects group 1 metabotropic receptor signalling such that ER IP3 receptor activation and release of intracellular Ca2+ is increased. HD is also associated with decreased synaptic NMDAR stability and increased expression, function and signalling of Ex-NMDARs. Posttranslational modifications and altered protein interactions may facilitate synaptic NMDAR instability. For example, increased Ca2+ levels activating calcineurin may lead to increased activation of STEP in the PSD, which dephosphorylates the GluN2B Y1472 residue and mediates lateral diffusion to extrasynaptic sites. It is postulated that enhanced Ex-NMDAR stability may be mediated by increased binding to scaffolding proteins such as PSD-95. Ex-NMDAR stimulation leads to downregulation of pro-survival signalling such as CREB-mediated gene transcription, decreased mutant htt inclusion formation, and increased activation of pro-apoptotic signalling that facilitates further neuronal dysfunction and neurodegeneration.
The augmented pool of Ex-NMDARs in YAC128 striatal neurons could be stimulated under physiological conditions of repetitive cortical glutamatergic afferent firing at frequencies as low as 25 Hz (Harris and Pettit, 2008). Ex-NMDAR stimulation in striatal neurons would be exacerbated by mutant htt-mediated alterations discussed above (see section 6.2), such as augmentation of presynaptic glutamate release (Joshi et al., 2009) and deficits in glutamate uptake by the transporter, GLT-1 (Lievens et al., 2001; Shin et al., 2005; Miller et al., 2008a; Faideau et al., 2010; Huang et al., 2010) (see Figure 2). Alterations in microglial activation, leading to enhanced release of the endogenous NMDAR agonist quinolinate (Guidetti et al., 2004; Pavese et al., 2006; Tai et al., 2007), could also contribute to stimulation of Ex-NMDARs. Mutant htt also induces alterations in Group 1 metabotropic glutamate receptor signaling, by increasing sensitivity of inositol trisphosphate 3 (IP-3) receptors and release of intracellular Ca2+ stores (Tang et al., 2003; Tang et al., 2005). This alteration, coupled with mhtt-induced mitochondrial dysfunction and consequent reduced Ca2+ and free radical buffering capacity (Panov et al., 2002; Panov et al., 2003; Milakovic et al., 2006; Oliveira et al., 2006), also would act synergistically with Ex-NMDAR signaling to augment vulnerability to cell death (Figure 2).
8.4 Mechanisms of Enhanced Ex-NMDAR Signaling
The mechanisms underlying mis-trafficking of NMDARs in MSNs expressing mutant htt are a topic of current investigation. There is evidence for an increased surface to internal ratio of GluN2B-containing receptors in primary cultures of striatal neurons from YAC72 mice, correlating well with the enhanced ifenprodil-sensitive NMDAR current (Fan et al., 2007). Data indicate that the increase in steady-state surface expression is linked to accelerated forward trafficking to the surface rather than enhanced stability of surface receptors (Fan et al., 2007). Although the precise molecular mechanism(s) responsible for enhanced forward trafficking have not yet been elucidated, htt interacts with a variety of proteins associated with vesicular trafficking at the level of the ER, Golgi and plasma membranes, suggesting a number of candidates (Kaltenbach et al., 2007). Aside from enhanced forward trafficking, the mechanisms underlying accumulation of NMDARs at extrasynaptic sites in striatal neurons in vivo remain to be explored. Altered posttranslational modification of GluN2B-containing receptors is one potential mechanism, since GluN2B retention in synapses is regulated by C-terminal protein-protein interaction motifs and phosphorylation (Mori et al., 1998; Roche et al., 2001; Lavezzari et al., 2004; Lin et al., 2004; Prybylowski et al., 2005; Chen and Roche, 2007; Goebel-Goody et al., 2009; Salter et al., 2009; Sanz-Clemente et al., 2010) (reviewed by Chen and Roche, 2007; Salter et al., 2009; Gladding and Raymond, 2011). Synaptic and Ex-NMDAR distribution may also be regulated by other posttranslational modifications such as ubiquitination (Ehlers, 2003; Kato et al., 2005) and palmitoylation (El-Husseini Ael et al., 2002; Hayashi et al., 2009). In addition, altered interactions between mutant htt and the palmitoyl acyl-transferase (PAT) htt-interacting protein 14 (HIP14, also known as DHHC17), may lead to defects in palmitoylation and trafficking of key synaptic proteins (Singaraja et al., 2002; Huang et al., 2004). In this regard, NMDAR synaptic stability may be reduced in HD as a result of alterations in palmitoylation of GluN2B and/or the scaffolding protein postsynaptic density protein 95 (PSD-95), since palmitoylation has been shown previously to regulate subcellular distribution of these proteins (Craven et al., 1999; El-Husseini et al., 2000a; El-Husseini et al., 2000b; Christopherson et al., 2003; Hayashi et al., 2009). PSD-95-GluN2B interactions are increased in YAC128 mice (Fan et al., 2009), and it is postulated that this contributes to increased NMDAR stability at extrasynaptic sites (Fan et al., 2011) (Figure 2).
Activation of Ca2+-dependent proteases such as calpain may also modulate plasma membrane NMDAR localization in HD (Guttmann et al., 2001; Simpkins et al., 2003; Wu et al., 2007). Calpain activation is elevated in HD mice (Gafni and Ellerby, 2002; Gafni et al., 2004; Cowan et al., 2008), which facilitates enhanced activation of the serine protein phosphatase, calcineurin (Kim et al., 2002; Wu et al., 2007). Once dephosphorylated and activated by calcineurin (Braithwaite et al., 2006), the STriatal Enriched tyrosine Phosphatase (STEP) dephosphorylates the GluN2B tyrosine 1472 residue, leading to reduced synaptic NMDAR expression (Pelkey et al., 2002; Prybylowski et al., 2005; Snyder et al., 2005; Braithwaite et al., 2006). It is hypothesized that enhanced synaptic STEP activity may mediate increased lateral diffusion of GluN2B-containing receptors to extrasynaptic sites in HD (Gladding et al., 2011) (Figure 2). An additional mechanism that may contribute to NMDAR redistribution away from synapses is aberrant cortical glutamatergic input (see sections 6.2 and 6.3); previous studies suggest that high levels of synaptic activity reduce synaptic GluN2B (Ehlers, 2003) (Figure 2).
9 Conclusions and Implications
The evolving alterations that occur with disease stage in the corticostriatal circuit of HD mouse models suggest that therapeutic strategies in human HD must be targeted to different molecular mechanisms in prodromal, early and late HD. The prominent cognitive and mood changes that occur in the prodromal and early stages of HD may largely reflect neuronal dysfunction, suggesting a critical role for altered firing and glutamate release from cortical afferents onto striatal MSNs, as well as altered trafficking of MSN neurotransmitter receptors and changes in DA modulation. The late stages of HD are characterized by combinations of neuronal dysfunction and degenerative changes in cortex and striatum. In future studies, it will be important to determine more precisely which components of the altered circuit contribute to deficits in learning, memory and mood in the early stages, because development of more specific therapies for these symptoms would significantly improve quality of life in affected individuals. Moreover, it will be critical to identify the earliest molecular mechanisms that lead to neuronal dysfunction and death in order to develop therapies that can delay onset of overt HD.
Highlights.
Understanding mechanisms of HD pathology using genetic animal models
Timeline of electrophysiological alterations in cerebral cortex and striatum are different
Role of extrasynaptic NMDA receptors in early pathogenesis
Alterations in intracellular signaling systems differ for synaptic and extrasynaptic receptors in prodromal and early stages of Huntington’s disease
Acknowledgements
L.A.R. is supported by the Canadian Institutes of Health Research (CIHR, MOP 12699) and the Cure Huntington Disease Initiative (CHDI). C.M.G. holds a joint CIHR-Huntington Society of Canada postdoctoral fellowship. Supported by USPHS NS41574 and contracts from CHDI awarded to M.S.L. The authors wish to thank Donna Crandall for help with the illustrations.
Abbreviations
- AMPA
α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate
- BAC
Bacterial Artificial Chromosome
- BDNF
Brain Derived Neurotrophic Factor
- CAG
Cytosine Adenine Guanine
- CREB
Cyclic-AMP Responsive Element Binding protein
- DA
Dopamine
- EEG
Electroencephalogram
- EGFP
Enhanced Green Fluorescent Protein
- EPSCs
Excitatory Post-synaptic Currents
- Ex-NMDAR
Extrasynaptic N-methyl-D-aspartate Receptor
- GABA
γ-aminobutyric acid
- GLT-1
Glutamate Transporter-1
- GluN1
Glutamate NMDA receptor subunit 1
- GluN2A-D
Glutamate NMDA receptor subunit 2A–D
- GP
Globus Pallidus
- GPi
Internal Globus Pallidus
- GPe
External Globus Pallidus
- HD
Huntington’s Disease
- HIP14
htt-interacting protein 14
- Htt
huntingtin
- IP-3
Inositol trisphosphate-3
- IPSCs
Inhibitory Post-synaptic Currents
- IT15
Interesting Transcript 15
- IT-type
Intratelencephalic-type
- JNK-MAPKs
c-Jun-N-terminal Kinase - Mitogen-Activated Protein Kinases
- MRI
Magnetic Resonance Imaging
- MSNs
Medium Sized-Spiny Neurons
- NADPH
Nicotinamide Adenine Dinucleotide Phosphate
- NMDA(R)
N-methyl-D-aspartate (Receptor)
- PAT
Palmitoyl Acyl-Transferase
- PSD-95
Post-Synaptic Density-95
- PT-type
Pyramidal Tract-type
- REM
Rapid Eye Movements
- SN
Substantia Nigra
- SNc
Substantia Nigra pars compacta
- SNr
Substantia Nigra pars reticulata
- SP
Substance P
- STEP
STriatal Enriched tyrosine Phosphatase
- WT
Wild Type
- YAC
Yeast Artificial Chromosome
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albin RL, Reiner A, Anderson KD, Dure LSt, Handelin B, Balfour R, Whetsell WO, Jr, Penney JB, Young AB. Preferential loss of striato-external pallidal projection neurons in presymptomatic Huntington's disease. Ann Neurol. 1992;31:425–430. doi: 10.1002/ana.410310412. [DOI] [PubMed] [Google Scholar]
- Albin RL, Reiner A, Anderson KD, Penney JB, Young AB. Striatal and nigral neuron subpopulations in rigid Huntington's disease: implications for the functional anatomy of chorea and rigidity-akinesia. Ann Neurol. 1990a;27:357–365. doi: 10.1002/ana.410270403. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB, Handelin B, Balfour R, Anderson KD, Markel DS, Tourtellotte WW, Reiner A. Abnormalities of striatal projection neurons and N-methyl-D-aspartate receptors in presymptomatic Huntington's disease. N Engl J Med. 1990b;322:1293–1298. doi: 10.1056/NEJM199005033221807. [DOI] [PubMed] [Google Scholar]
- André VM, Cepeda C, Cummings DM, Jocoy EL, Fisher YE, William Yang X, Levine MS. Dopamine modulation of excitatory currents in the striatum is dictated by the expression of D1 or D2 receptors and modified by endocannabinoids. Eur J Neurosci. 2010a;31:14–28. doi: 10.1111/j.1460-9568.2009.07047.x. [DOI] [PubMed] [Google Scholar]
- André VM, Cepeda C, Fisher YE, Huynh M, Bardakjian N, Singh S, Yang XW, Levine MS. Differential electrophysiological changes in striatal output neurons in Huntington's disease. J Neurosci. 2011a;31:1170–1182. doi: 10.1523/JNEUROSCI.3539-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André VM, Cepeda C, Levine MS. Dopamine and glutamate in Huntington's disease: A balancing act. CNS Neurosci Ther. 2010b;16:163–178. doi: 10.1111/j.1755-5949.2010.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André VM, Cepeda C, Venegas A, Gomez Y, Levine MS. Altered cortical glutamate receptor function in the R6/2 model of Huntington's disease. J Neurophysiol. 2006;95:2108–2119. doi: 10.1152/jn.01118.2005. [DOI] [PubMed] [Google Scholar]
- André VM, Fisher YE, Levine MS. Altered balance of activity in the striatal direct and indirect pathways in mouse models of Huntington's disease. Front Syst Neurosci. 2011b;5:46.. doi: 10.3389/fnsys.2011.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews TC, Weeks RA, Turjanski N, Gunn RN, Watkins LH, Sahakian B, Hoddges JR. Huntington’s disease progression. PET and clinical observations. Brain. 1999;122(Pt 12):2353–2363. doi: 10.1093/brain/122.12.2353. [DOI] [PubMed] [Google Scholar]
- Ariano MA, Aronin N, Difiglia M, Tagle DA, Sibley DR, Leavitt BR, Hayden MR, Levine MS. Striatal neurochemical changes in transgenic models of Huntington's disease. J Neurosci Res. 2002;68:716–729. doi: 10.1002/jnr.10272. [DOI] [PubMed] [Google Scholar]
- Ariano MA, Cepeda C, Calvert CR, Flores-Hernandez J, Hernandez-Echeagaray E, Klapstein GJ, Chandler SH, Aronin N, DiFiglia M, Levine MS. Striatal potassium channel dysfunction in Huntington's disease transgenic mice. J Neurophysiol. 2005;93:2565–2574. doi: 10.1152/jn.00791.2004. [DOI] [PubMed] [Google Scholar]
- Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34:325–337. doi: 10.1016/s0143-4160(03)00141-6. [DOI] [PubMed] [Google Scholar]
- Backman L, Farde L. Dopamine and cognitive functioning: brain imaging findings in Huntington's disease and normal aging. Scand J Psychol. 2001;42:287–296. doi: 10.1111/1467-9450.00238. [DOI] [PubMed] [Google Scholar]
- Backman L, Robins-Wahlin TB, Lundin A, Ginovart N, Farde L. Cognitive deficits in Huntington’s disease are predicted by dopaminergic PET markers and brain volumes. Brain. 1997;120(Pt 12):2207–2217. doi: 10.1093/brain/120.12.2207. [DOI] [PubMed] [Google Scholar]
- Ballion B, Mallet N, Bézard E, Lanciego JL, Ganon F. Intratelencephalic corticostriatal neurons equally excite striatonigral and striatopallidal neurons and their discharge activity is selectively reduced in experimental parkinsonism. Eur J Neurosci. 2008;27:2313–2321. doi: 10.1111/j.1460-9568.2008.06192.x. [DOI] [PubMed] [Google Scholar]
- Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35:345–353. doi: 10.1016/s0896-6273(02)00776-6. [DOI] [PubMed] [Google Scholar]
- Beal MF, Brouillet E, Jenkins BG, Ferrante RJ, Kowall NW, Miller JM, Storey E, Srivastava R, Rosen BR, Hyman BT. Neurochemical and histologic characterization of striatal excitotoxic lesions produced by the mitochondrial toxin 3-nitropropionic acid. J Neurosci. 1993;13:4181–4192. doi: 10.1523/JNEUROSCI.13-10-04181.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal MF, Ferrante RJ, Swartz KJ, Kowall NW. Chronic quinolinic acid lesions in rats closely resemble Huntington's disease. J Neurosci. 1991;11:1649–1659. doi: 10.1523/JNEUROSCI.11-06-01649.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal MF, Kowall NW, Ellison DW, Mazurek MF, Swartz KJ, Martin JB. Replication of the neurochemical characteristics of Huntington's disease by quinolinic acid. Nature. 1986;321:168–171. doi: 10.1038/321168a0. [DOI] [PubMed] [Google Scholar]
- Beglinger LJ, O'Rourke JJ, Wang C, Langbehn DR, Duff K, Paulsen JS. Earliest functional declines in Huntington disease. Psychiatry Res. 2010;178:414–418. doi: 10.1016/j.psychres.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benn CL, Slow EJ, Farrell LA, Graham R, Deng Y, Hayden MR, Cha JH. Glutamate receptor abnormalities in the YAC128 transgenic mouse model of Huntington’s disease. Neurosci. 2007;147:354–372. doi: 10.1016/j.neuroscience.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta S, Parthasarathy HB, Graybiel AM. Local release of GABAergic inhibition in the motor cortex induces immediate-early gene expression in indirect pathway neurons of the striatum. J Neurosci. 1997;17:4752–4763. doi: 10.1523/JNEUROSCI.17-12-04752.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrios GE, Wagle AC, Markova IS, Wagle SA, Ho LW, Rubinsztein DC, Whittaker J, Ffrench-Constant C, Kershaw A, Rosser A, Bak T, Hodges JR. Psychiatric symptoms and CAG repeats in neurologically asymptomatic Huntington's disease gene carriers. Psychiatry Res. 2001;102:217–225. doi: 10.1016/s0165-1781(01)00257-8. [DOI] [PubMed] [Google Scholar]
- Bibb JA, Yan Z, Svenningsson P, Snyder GL, Pieribone VA, Horiuchi A, Nairn AC, Messer A, Greengard P. Severe deficiences in dopamine signaling in presymptomatic Huntington's disease mice. Proc Natl Acad Sci USA. 2000;97:6809–6814. doi: 10.1073/pnas.120166397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biolsi B, Cif L, Fertit HE, Robles SG, Coubes P. Long-term follow-up of Huntington disease treated by bilateral deep brain stimulation of the internal globus pallidus. J Neurosurg. 2008;109:130–132. doi: 10.3171/JNS/2008/109/7/0130. [DOI] [PubMed] [Google Scholar]
- Bird ED. Chemical pathology of Huntington’s disease. Annu Rev Pharmacol Toxicol. 1980;20:533–551. doi: 10.1146/annurev.pa.20.040180.002533. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Boecker H, Ceballos-Baumann A, Bartenstein P, Weindl A, Siebner HR, Fassbender T, Munz F, Schwaiger M, Conrad B. Sensory processing in Parkinson's and Huntington's disease: investigations with 3D H(2)(15)O-PET. Brain. 1999;122(Pt 9):1651–1665. doi: 10.1093/brain/122.9.1651. [DOI] [PubMed] [Google Scholar]
- Bogdanov MB, Ferrante RJ, Kuemmerle S, Klivenyi P, Beal MF. Increased vulnerability to 3-nitropropionic acid in an animal model of Huntington's disease. J Neurochem. 1998;71:2642–2644. doi: 10.1046/j.1471-4159.1998.71062642.x. [DOI] [PubMed] [Google Scholar]
- Bohanna I, Georgiou-Karistianis N, Hannan AJ, Egan GF. Magnetic resonance imaging as an approach towards identifying neuropathological biomarkers for Huntington's disease. Brain Res Rev. 2008;58:209–225. doi: 10.1016/j.brainresrev.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Hanley JJ, Booth PA, Bevan MD. Synaptic organisation of the basal ganglia. J Anat. 2000;196(Pt 4):527–542. doi: 10.1046/j.1469-7580.2000.19640527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam JP, Smith Y. The GABA and substance P input to dopaminergic neurones in the substantia nigra of the rat. Brain Res. 1990;529:57–78. doi: 10.1016/0006-8993(90)90811-o. [DOI] [PubMed] [Google Scholar]
- Braithwaite SP, Paul S, Nairn AC, Lombroso PJ. Synaptic plasticity: one STEP at a time. Trends Neurosci. 2006;29:452–458. doi: 10.1016/j.tins.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouillet E, Conde F, Beal MF, Hantraye P. Replicating Huntington's disease phenotype in experimental animals. Prog Neurobiol. 1999;59:427–468. doi: 10.1016/s0301-0082(99)00005-2. [DOI] [PubMed] [Google Scholar]
- Brown TB, Bogush AI, Ehrlich ME. Neocortical expression of mutant huntingtin is not required for alterations in striatal gene expression or motor dysfunction in a transgenic mouse. Hum Mol Genet. 2008;17:3095–3104. doi: 10.1093/hmg/ddn206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusa L, Orlacchio A, Moschella V, Iani C, Bernardi G, Mercuri NB. Treatment of the symptoms of Huntington’s disease: preliminary results comparing aripiprazole and tetrabenazine. Mov Disord. 2009;24:126–129. doi: 10.1002/mds.22376. [DOI] [PubMed] [Google Scholar]
- Cao J, Semenova MM, Solovyan VT, Han J, Coffey ET, Courtney MJ. Distinct requirements for p38alpha and c-Jun N-terminal kinase stress-activated protein kinases in different forms of apoptotic neuronal death. J Biol Chem. 2004;279:35903–35913. doi: 10.1074/jbc.M402353200. [DOI] [PubMed] [Google Scholar]
- Carter RJ, Lione LA, Humby T, Mangiarini L, Mahal A, Bates GP, Dunnett SB, Morton AJ. Characterization of progressive motor deficits in mice transgenic for the human Huntington's disease mutation. J Neurosci. 1999;19:3248–3257. doi: 10.1523/JNEUROSCI.19-08-03248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, André VM, Yamazaki I, Wu N, Kleiman-Weiner M, Levine MS. Differential electrophysiological properties of dopamine D1 and D2 receptor-containing striatal medium-sized spiny neurons. Eur J Neurosci. 2008;27:671–682. doi: 10.1111/j.1460-9568.2008.06038.x. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Ariano MA, Calvert CR, Flores-Hernandez J, Chandler SH, Leavitt BR, Hayden MR, Levine MS. NMDA receptor function in mouse models of Huntington disease. J Neurosci Res. 2001a;66:525–539. doi: 10.1002/jnr.1244. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Cummings DM, André VM, Holley SM, Levine MS. Genetic mouse models of Huntington's disease: focus on electrophysiological mechanisms. ASN Neuro. 2010;2 doi: 10.1042/AN20090058. e00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Hurst RS, Calvert CR, Hernandez-Echeagaray E, Nguyen OK, Jocoy E, Christian LJ, Ariano MA, Levine MS. Transient and progressive electrophysiological alterations in the corticostriatal pathway in a mouse model of Huntington's disease. J Neurosci. 2003;23:961–969. doi: 10.1523/JNEUROSCI.23-03-00961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Itri JN, Flores-Hernandez J, Hurst RS, Calvert CR, Levine MS. Differential sensitivity of medium- and large-sized striatal neurons to NMDA but not kainate receptor activation in the rat. Eur J Neurosci. 2001b;14:1577–1589. doi: 10.1046/j.0953-816x.2001.01783.x. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Starling AJ, Wu N, Nguyen OK, Uzgil B, Soda T, André VM, Ariano MA, Levine MS. Increased GABAergic function in mouse models of Huntington's disease: reversal by BDNF. J Neurosci Res. 2004;78:855–867. doi: 10.1002/jnr.20344. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Wu N, André VM, Cummings DM, Levine MS. The corticostriatal pathway in Huntington's disease. Prog Neurobiol. 2007;81:253–271. doi: 10.1016/j.pneurobio.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha JH, Kosinski CM, Kerner JA, Alsdorf SA, Mangiarini L, Davies SW, Penney JB, Bates GP, Young AB. Altered brain neurotransmitter receptors in transgenic mice expressing a portion of an abnormal human huntington disease gene. Proc Natl Acad Sci U S A. 1998;95:6480–6485. doi: 10.1073/pnas.95.11.6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BS, Roche KW. Regulation of NMDA receptors by phosphorylation. Neuropharmacology. 2007;53:362–368. doi: 10.1016/j.neuropharm.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Luo T, Wellington C, Metzler M, McCutcheon K, Hayden MR, Raymond LA. Subtype-specific enhancement of NMDA receptor currents by mutant huntingtin. J Neurochem. 1999;72:1890–1898. doi: 10.1046/j.1471-4159.1999.0721890.x. [DOI] [PubMed] [Google Scholar]
- Cherry SD. PhD Thesis. The University of Tennessee Health Science Center; 2009. Calcium channel dysfunction in Huntington's disease; p. 128. [Google Scholar]
- Chevalier G, Deniau JM. Disinhibition as a basic process in the expression of striatal functions. Trends Neurosci. 1990;13:277–280. doi: 10.1016/0166-2236(90)90109-n. [DOI] [PubMed] [Google Scholar]
- Choi DW, Maulucci-Gedde M, Kriegstein AR. Glutamate neurotoxicity in cortical cell culture. J Neurosci. 1987;7:357–368. doi: 10.1523/JNEUROSCI.07-02-00357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM, Jane DE, Monaghan DT. Native N-methyl-D-aspartate receptors containing NR2A and NR2B subunits have pharmacologically distinct competitive antagonist binding sites. J Pharmacol Exp Ther. 2000;292:1169–1174. [PubMed] [Google Scholar]
- Christopherson KS, Sweeney NT, Craven SE, Kang R, El-Husseini Ael D, Bredt DS. Lipid- and protein-mediated multimerization of PSD-95: implications for receptor clustering and assembly of synaptic protein networks. J Cell Sci. 2003;116:3213–3219. doi: 10.1242/jcs.00617. [DOI] [PubMed] [Google Scholar]
- Cicchetti F, Prensa L, Wu Y, Parent A. Chemical anatomy of striatal interneurons in normal individuals and in patients with Huntington's disease. Brain Res Brain Res Rev. 2000;34:80–101. doi: 10.1016/s0165-0173(00)00039-4. [DOI] [PubMed] [Google Scholar]
- Cowan CM, Fan MM, Fan J, Shehadeh J, Zhang LY, Graham RK, Hayden MR, Raymond LA. Polyglutamine-modulated striatal calpain activity in YAC transgenic huntington disease mouse model: impact on NMDA receptor function and toxicity. J Neurosci. 2008;28:12725–12735. doi: 10.1523/JNEUROSCI.4619-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT. An animal model for Huntington's disease. Biol Psychiatry. 1979;14:251–276. [PubMed] [Google Scholar]
- Coyle JT, Molliver ME, Kuhar MJ. In situ injection of kainic acid: a new method for selectively lesioning neural cell bodies while sparing axons of passage. J Comp Neurol. 1978;180:301–323. doi: 10.1002/cne.901800208. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Schwarcz R. Lesion of striatal neurones with kainic acid provides a model for Huntington's chorea. Nature. 1976;263:244–246. doi: 10.1038/263244a0. [DOI] [PubMed] [Google Scholar]
- Craven SE, El-Husseini AE, Bredt DS. Synaptic targeting of the postsynaptic density protein PSD-95 mediated by lipid and protein motifs. Neuron. 1999;22:497–509. doi: 10.1016/s0896-6273(00)80705-9. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004;2004:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- Cummings DM, André VM, Uzgil BO, Gee SM, Fisher YE, Cepeda C, Levine MS. Alterations in cortical excitation and inhibition in genetic mouse models of Huntington's disease. J Neurosci. 2009;29:10371–10386. doi: 10.1523/JNEUROSCI.1592-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings DM, Cepeda C, Levine MS. Alterations in striatal synaptic transmission are consistent across genetic mouse models of Huntington's disease. ASN Neuro. 2010;2 doi: 10.1042/AN20100007. e00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings DM, Milnerwood AJ, Dallérac GM, Vatsavayai SC, Hirst MC, Murphy KPSJ. Abnormal Cortical Synaptic Plasticity in Mice Transgenic for Exon 1 of the Human Huntington's Disease Mutation. Brain Res Bul. 2007;72:103–107. doi: 10.1016/j.brainresbull.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Cummings DM, Milnerwood AJ, Dallerac GM, Waights V, Brown JY, Vatsavayai SC, Hirst MC, Murphy KP. Aberrant cortical synaptic plasticity and dopaminergic dysfunction in a mouse model of huntington's disease. Hum Mol Genet. 2006;15:2856–2868. doi: 10.1093/hmg/ddl224. [DOI] [PubMed] [Google Scholar]
- Damiano M, Galvan L, Deglon N, Brouillet E. Mitochondria in Huntington's disease. Biochim Biophys Acta. 2009;1802:52–61. doi: 10.1016/j.bbadis.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Davies SW, Turmaine M, Cozens BA, DiFiglia M, Sharp AH, Ross CA, Scherzinger E, Wanker EE, Mangiarini L, Bates GP. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell. 1997;90:537–548. doi: 10.1016/s0092-8674(00)80513-9. [DOI] [PubMed] [Google Scholar]
- Deng YP, Albin RL, Penney JB, Young AB, Anderson KD, Reiner A. Differential loss of striatal projection systems in Huntington's disease: a quantitative immunohistochemical study. J Chem Neuroanat. 2004;27:143–164. doi: 10.1016/j.jchemneu.2004.02.005. [DOI] [PubMed] [Google Scholar]
- DiFiglia M. Excitotoxic injury of the neostriatum: a model for Huntington's disease. Trends Neurosci. 1990;13:286–289. doi: 10.1016/0166-2236(90)90111-m. [DOI] [PubMed] [Google Scholar]
- DiFiglia M, Sapp E, Chase KO, Davies SW, Bates GP, Vonsattel JP, Aronin N. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277:1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Ehlers MD. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci. 2003;6:231–242. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000a;290:1364–1368. [PubMed] [Google Scholar]
- El-Husseini AE, Topinka JR, Lehrer-Graiwer JE, Firestein BL, Craven SE, Aoki C, Bredt DS. Ion channel clustering by membrane-associated guanylate kinases. Differential regulation by N-terminal lipid and metal binding motifs. J Biol Chem. 2000b;275:23904–23910. doi: 10.1074/jbc.M909919199. [DOI] [PubMed] [Google Scholar]
- El-Husseini Ael D, Schnell E, Dakoji S, Sweeney N, Zhou Q, Prange O, Gauthier-Campbell C, Aguilera-Moreno A, Nicoll RA, Bredt DS. Synaptic strength regulated by palmitate cycling on PSD-95. Cell. 2002;108:849–863. doi: 10.1016/s0092-8674(02)00683-9. [DOI] [PubMed] [Google Scholar]
- Faideau M, Kim J, Cormier K, Gilmore R, Welch M, Auregan G, Dufour N, Guillermier M, Brouillet E, Hantraye P, Deglon N, Ferrante RJ, Bonvento G. In vivo expression of polyglutamine-expanded huntingtin by mouse striatal astrocytes impairs glutamate transport: a correlation with Huntington's disease subjects. Hum Mol Genet. 2010;19:3053–3067. doi: 10.1093/hmg/ddq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Cowan CM, Zhang LY, Hayden MR, Raymond LA. Interaction of postsynaptic density protein-95 with NMDA receptors influences excitotoxicity in the yeast artificial chromosome mouse model of Huntington's disease. J Neurosci. 2009;29:10928–10938. doi: 10.1523/JNEUROSCI.2491-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Gladding CM, Wang L, Zhang LYJ, Raymond LA. P38 MAPK is involved in enhanced NMDA receptor-dependent excitotoxicity in YAC transgenic mouse model of Huntington disease. Soc for Neurosci Abstracts, 41st Annual Meeting; 2011. [DOI] [PubMed] [Google Scholar]
- Fan MM, Fernandes HB, Zhang LY, Hayden MR, Raymond LA. Altered NMDA receptor trafficking in a yeast artificial chromosome transgenic mouse model of Huntington's disease. J Neurosci. 2007;27:3768–3779. doi: 10.1523/JNEUROSCI.4356-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan MM, Raymond LA. N-methyl-D-aspartate (NMDA) receptor function and excitotoxicity in Huntington's disease. Prog Neurobiol. 2007;81:272–293. doi: 10.1016/j.pneurobio.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Fasano A, Mazzone P, Piano C, Quaranta D, Soleti F, Bentivoglio AR. GPi-DBS in Huntington's disease: results on motor function and cognition in a 72-year-old case. Mov Disord. 2008;23:1289–1292. doi: 10.1002/mds.22116. [DOI] [PubMed] [Google Scholar]
- Favaron M, Manev RM, Rimland JM, Candeo P, Beccaro M, Manev H. NMDA-stimulated expression of BDNF mRNA in cultured cerebellar granule neurones. Neuroreport. 1993;4:1171–1174. [PubMed] [Google Scholar]
- Ferrante RJ, Beal MF, Kowall NW, Richardson EP, Jr, Martin JB. Sparing of acetylcholinesterase-containing striatal neurons in Huntington's disease. Brain Res. 1987;411:162–166. doi: 10.1016/0006-8993(87)90694-9. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Kowall NW, Beal MF, Richardson EP, Jr, Bird ED, Martin JB. Selective sparing of a class of striatal neurons in Huntington's disease. Science. 1985;230:561–563. doi: 10.1126/science.2931802. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Kowall NW, Cipolloni PB, Storey E, Beal MF. Excitotoxin lesions in primates as a model for Huntington's disease: histopathologic and neurochemical characterization. Exp Neurol. 1993;119:46–71. doi: 10.1006/exnr.1993.1006. [DOI] [PubMed] [Google Scholar]
- Fonnum F, Storm-Mathisen J, Divac I. Biochemical evidence for glutamate as neurotransmitter in corticostriatal and corticothalamic fibres in rat brain. Neuroscience. 1981;6:863–873. doi: 10.1016/0306-4522(81)90168-8. [DOI] [PubMed] [Google Scholar]
- Gafni J, Ellerby LM. Calpain activation in Huntington's disease. J Neurosci. 2002;22:4842–4849. doi: 10.1523/JNEUROSCI.22-12-04842.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafni J, Hermel E, Young JE, Wellington CL, Hayden MR, Ellerby LM. Inhibition of calpain cleavage of huntingtin reduces toxicity: accumulation of calpain/caspase fragments in the nucleus. J Biol Chem. 2004;279:20211–20220. doi: 10.1074/jbc.M401267200. [DOI] [PubMed] [Google Scholar]
- Garrett MC, Soares-da-Silva P. Increased cerebrospinal fluid dopamine and 3,4-dihydroxyphenylacetic acid levels in Huntington's disease: evidence for an overactive dopaminergic brain transmission. J Neurochem. 1992;58:101–106. doi: 10.1111/j.1471-4159.1992.tb09283.x. [DOI] [PubMed] [Google Scholar]
- Gencik M, Hammans C, Strehl H, Wagner N, Epplen JT. Chorea Huntington: a rare case with childhood onset. Neuropediatrics. 2002;33:90–92. doi: 10.1055/s-2002-32367. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Herkenham M, Thibault J. The neostriatal mosaic: II. Patch- and matrix-directed mesostriatal dopaminergic and non-dopaminergic systems. J Neurosci. 1987;7:3915–3934. doi: 10.1523/JNEUROSCI.07-12-03915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization. Trends Neurosci. 1992a;15:133–139. doi: 10.1016/0166-2236(92)90355-c. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci. 1992b;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gertler TS, Chan CS, Surmeier DJ. Dichotomous anatomical properties of adult striatal medium spiny neurons. J Neurosci. 2008;28:10814–10824. doi: 10.1523/JNEUROSCI.2660-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Sharma S, Surmeier DJ, Eberwine JH, Chesselet MF. Multiplicity of glutamate receptor subunits in single striatal neurons: an RNA amplification study. Mol Pharmacol. 1996;49:852–859. [PubMed] [Google Scholar]
- Ginovart N, Lundin A, Farde L, Halldin C, Backman L, Swahn CG, Pauli S. PET study of the pre- and post-synaptic dopaminergic markers for the neurodegenerative process in Huntington's disease. Brain. 1997;120(Pt 3):503–514. doi: 10.1093/brain/120.3.503. [DOI] [PubMed] [Google Scholar]
- Gittis AH, Nelson AB, Thwin MT, Palop JJ, Kreitzer AC. Distinct roles of GABAergic interneurons in the regulation of striatal output pathways. J Neurosci. 2010;30:2223–2234. doi: 10.1523/JNEUROSCI.4870-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladding CM, Milnerwood AJ, Zhang LYJ, Xu J, Lombroso PJ, Raymond LA. Investigating the role of calpain and striatal-enriched tyrosine phosphatase (STEP) in mediating increased extrasynaptic NMDA receptor localization in a Huntington Disease mouse model. Soc for Neurosci Abstracts, 41st Annual Meeting.2011. [Google Scholar]
- Gladding CM, Raymond LA. Mechanisms underlying NMDA receptor synaptic/extrasynaptic distribution and function. Mol Cell Neurosci. 2011 doi: 10.1016/j.mcn.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Goebel-Goody SM, Davies KD, Alvestad Linger RM, Freund RK, Browning MD. Phospho-regulation of synaptic and extrasynaptic N-methyl-d-aspartate receptors in adult hippocampal slices. Neuroscience. 2009;158:1446–1459. doi: 10.1016/j.neuroscience.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Goto S, Hirano A, Rojas-Corona RR. An immunohistochemical investigation of the human neostriatum in Huntington's disease. Ann Neurol. 1989;25:298–304. doi: 10.1002/ana.410250315. [DOI] [PubMed] [Google Scholar]
- Graham RK, Pouladi MA, Joshi P, Lu G, Deng Y, Wu NP, Figueroa BE, Metzler M, André VM, Slow EJ, Raymond L, Friedlander R, Levine MS, Leavitt BR, Hayden MR. Differential Susceptibility to Excitotoxic Stress in YAC128 Mouse Models of Huntington Disease between Initiation and Progression of Disease. J Neurosci. 2009;29:2193–2204. doi: 10.1523/JNEUROSCI.5473-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M, Gu X, Shiraski D, Cepeda C, Yamazaki I, Levine MS, Yang X. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2008a. Cortical control of striatal pathogenesis in the Cre/LoxP conditional BAC transgenic mouse model of Huntington's Disease (BACHD) Program No. 114.6. [Google Scholar]
- Gray M, Shirasaki DI, Cepeda C, André VM, Wilburn B, Lu XH, Tao J, Yamazaki I, Li SH, Sun YE, Li XJ, Levine MS, Yang XW. Full-length human mutant huntingtin with a stable polyglutamine repeat can elicit progressive and selective neuropathogenesis in BACHD mice. J Neurosci. 2008b;28:6182–6195. doi: 10.1523/JNEUROSCI.0857-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990;13:244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- Greene JG, Porter RH, Eller RV, Greenamyre JT. Inhibition of succinate dehydrogenase by malonic acid produces an "excitotoxic" lesion in rat striatum. J Neurochem. 1993;61:1151–1154. doi: 10.1111/j.1471-4159.1993.tb03634.x. [DOI] [PubMed] [Google Scholar]
- Groc L, Heine M, Cousins SL, Stephenson FA, Lounis B, Cognet L, Choquet D. NMDA receptor surface mobility depends on NR2A-2B subunits. Proc Natl Acad Sci U S A. 2006;103:18769–18774. doi: 10.1073/pnas.0605238103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, André VM, Cepeda C, Li SH, Li XJ, Levine MS, Yang XW. Pathological cell-cell interactions are necessary for striatal pathogenesis in a conditional mouse model of Huntington's disease. Mol Neurodegener. 2007;2:8. doi: 10.1186/1750-1326-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Li C, Wei W, Lo V, Gong S, Li SH, Iwasato T, Itohara S, Li XJ, Mody I, Heintz N, Yang XW. Pathological cell-cell interactions elicited by a neuropathogenic form of mutant Huntingtin contribute to cortical pathogenesis in HD mice. Neuron. 2005;46:433–444. doi: 10.1016/j.neuron.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Guidetti P, Luthi-Carter RE, Augood SJ, Schwarcz R. Neostriatal and cortical quinolinate levels are increased in early grade Huntington's disease. Neurobiol Dis. 2004;17:455–461. doi: 10.1016/j.nbd.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Guttmann RP, Baker DL, Seifert KM, Cohen AS, Coulter DA, Lynch DR. Specific proteolysis of the NR2 subunit at multiple sites by calpain. J Neurochem. 2001;78:1083–1093. doi: 10.1046/j.1471-4159.2001.00493.x. [DOI] [PubMed] [Google Scholar]
- Haber SN, Nauta WJ. Ramifications of the globus pallidus in the rat as indicated by patterns of immunohistochemistry. Neuroscience. 1983;9:245–260. doi: 10.1016/0306-4522(83)90291-9. [DOI] [PubMed] [Google Scholar]
- Hansen HH, Briem T, Dzietko M, Sifringer M, Voss A, Rzeski W, Zdzisinska B, Thor F, Heumann R, Stepulak A, Bittigau P, Ikonomidou C. Mechanisms leading to disseminated apoptosis following NMDA receptor blockade in the developing rat brain. Neurobiol Dis. 2004;16:440–453. doi: 10.1016/j.nbd.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Hansson O, Guatteo E, Mercuri NB, Bernardi G, Li XJ, Castilho RF, Brundin P. Resistance to NMDA toxicity correlates with appearance of nuclear inclusions, behavioural deficits and changes in calcium homeostasis in mice transgenic for exon 1 of the huntington gene. Eur J Neurosci. 2001;14:1492–1504. doi: 10.1046/j.0953-816x.2001.01767.x. [DOI] [PubMed] [Google Scholar]
- Hantraye P, Riche D, Maziere M, Isacson O. A primate model of Huntington's disease: behavioral and anatomical studies of unilateral excitotoxic lesions of the caudate-putamen in the baboon. Exp Neurol. 1990;108:91–104. doi: 10.1016/0014-4886(90)90014-j. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Arnold FJ, Bading H. A calcium microdomain near NMDA receptors: on switch for ERK-dependent synapse-to-nucleus communication. Nat Neurosci. 2001;4:565–566. doi: 10.1038/88380. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Harper PS, Jones L. Huntington's disease: Genetic and molecular studies. In: Bates GP, et al., editors. Huntington's Disease. Oxford: Oxford University Press; 2002. pp. 113–158. [Google Scholar]
- Harris AZ, Pettit DL. Extrasynaptic and synaptic NMDA receptors form stable and uniform pools in rat hippocampal slices. J Physiol. 2007;584:509–519. doi: 10.1113/jphysiol.2007.137679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AZ, Pettit DL. Recruiting extrasynaptic NMDA receptors augments synaptic signaling. J Neurophysiol. 2008;99:524–533. doi: 10.1152/jn.01169.2007. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Thomas GM, Huganir RL. Dual palmitoylation of NR2 subunits regulates NMDA receptor trafficking. Neuron. 2009;64:213–226. doi: 10.1016/j.neuron.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedreen JC, Folstein SE. Early loss of neostriatal striosome neurons in Huntington's disease. J Neuropathol Exp Neurol. 1995;54:105–120. doi: 10.1097/00005072-199501000-00013. [DOI] [PubMed] [Google Scholar]
- Heinsen H, Rub U, Gangnus D, Jungkunz G, Bauer M, Ulmar G, Bethke B, Schuler M, Bocker F, Eisenmenger W, Gotz M, Strik M. Nerve cell loss in the thalamic centromedian-parafascicular complex in patients with Huntington's disease. Acta Neuropathol. 1996;91:161–168. doi: 10.1007/s004010050408. [DOI] [PubMed] [Google Scholar]
- Heng MY, Detloff PJ, Wang PL, Tsien JZ, Albin RL. In vivo evidence for NMDA receptor-mediated excitotoxicity in a murine genetic model of Huntington disease. J Neurosci. 2009;29:3200–3205. doi: 10.1523/JNEUROSCI.5599-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng MY, Tallaksen-Greene SJ, Detloff PJ, Albin RL. Longitudinal evaluation of the Hdh(CAG)150 knock-in murine model of Huntington's disease. J Neurosci. 2007;27:8989–8998. doi: 10.1523/JNEUROSCI.1830-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Espinosa D, Morton AJ. Calcineurin inhibitors cause an acceleration of the neurological phenotype in a mouse transgenic for the human Huntington's disease mutation. Brain Res Bull. 2006;69:669–679. doi: 10.1016/j.brainresbull.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Hickey MA, Kosmalska A, Enayati J, Cohen R, Zeitlin S, Levine MS, Chesselet MF. Extensive early motor and non-motor behavioral deficits are followed by striatal neuronal loss in knock-in Huntington's disease mice. Neuroscience. 2008;157:280–295. doi: 10.1016/j.neuroscience.2008.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey MA, Reynolds GP, Morton AJ. The role of dopamine in motor symptoms in the R6/2 transgenic mouse model of Huntington's disease. J Neurochem. 2002;81:46–59. doi: 10.1046/j.1471-4159.2002.00804.x. [DOI] [PubMed] [Google Scholar]
- Hodgson JG, Agopyan N, Gutekunst CA, Leavitt BR, LePiane F, Singaraja R, Smith DJ, Bissada N, McCutcheon K, Nasir J, Jamot L, Li XJ, Stevens ME, Rosemond E, Roder JC, Phillips AG, Rubin EM, Hersch SM, Hayden MR. A YAC mouse model for Huntington's disease with full-length mutant huntingtin, cytoplasmic toxicity, and selective striatal neurodegeneration. Neuron. 1999;23:181–192. doi: 10.1016/s0896-6273(00)80764-3. [DOI] [PubMed] [Google Scholar]
- Huang K, Kang MH, Askew C, Kang R, Sanders SS, Wan J, Davis NG, Hayden MR. Palmitoylation and function of glial glutamate transporter-1 is reduced in the YAC128 mouse model of Huntington disease. Neurobiol Dis. 2010;40:207–215. doi: 10.1016/j.nbd.2010.05.027. [DOI] [PubMed] [Google Scholar]
- Huang K, Yanai A, Kang R, Arstikaitis P, Singaraja RR, Metzler M, Mullard A, Haigh B, Gauthier-Campbell C, Gutekunst CA, Hayden MR, El-Husseini A. Huntingtin-interacting protein HIP14 is a palmitoyl transferase involved in palmitoylation and trafficking of multiple neuronal proteins. Neuron. 2004;44:977–986. doi: 10.1016/j.neuron.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Imarisio S, Carmichael J, Korolchuk V, Chen CW, Saiki S, Rose C, Krishna G, Davies JE, Ttofi E, Underwood BR, Rubinsztein DC. Huntington's disease: from pathology and genetics to potential therapies. Biochem J. 2008;412:191–209. doi: 10.1042/BJ20071619. [DOI] [PubMed] [Google Scholar]
- Ivanov A, Pellegrino C, Rama S, Dumalska I, Salyha Y, Ben-Ari Y, Medina I. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. J Physiol. 2006;572:789–798. doi: 10.1113/jphysiol.2006.105510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi A, Vlamings R, Kaya AH, Lim LW, Janssen ML, Tan S, Visser-Vandewalle V, Steinbusch HW, Temel Y. Hyperdopaminergic status in experimental Huntington disease. J Neuropathol Exp Neurol. 2010;69:910–917. doi: 10.1097/NEN.0b013e3181ee005d. [DOI] [PubMed] [Google Scholar]
- Jarabek BR, Yasuda RP, Wolfe BB. Regulation of proteins affecting NMDA receptor-induced excitotoxicity in a Huntington's mouse model. Brain. 2004;127:505–516. doi: 10.1093/brain/awh058. [DOI] [PubMed] [Google Scholar]
- Jiang X, Tian F, Mearow K, Okagaki P, Lipsky RH, Marini AM. The excitoprotective effect of N-methyl-D-aspartate receptors is mediated by a brain-derived neurotrophic factor autocrine loop in cultured hippocampal neurons. J Neurochem. 2005;94:713–722. doi: 10.1111/j.1471-4159.2005.03200.x. [DOI] [PubMed] [Google Scholar]
- Jocoy EL, André VM, Cummings DM, Rao SP, Wu N, Ramsey AJ, Caron M, Cepeda C, Levine MS. Dissecting the contribution of individual receptor subunits to the enhancement of N-methyl-D-aspartate currents by dopamine D1 receptor activation in striatum. Front Syst Neurosci. 2011;5:28. doi: 10.3389/fnsys.2011.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MA, Rajan V, Miller CE, Wightman RM. Dopamine release is severely compromised in the R6/2 mouse model of Huntington’s disease. J Neurochem. 2006;97:737–746. doi: 10.1111/j.1471-4159.2006.03762.x. [DOI] [PubMed] [Google Scholar]
- Joshi PR, Wu NP, André VM, Cummings DM, Cepeda C, Joyce JA, Carroll JB, Leavitt BR, Hayden MR, Levine MS, Bamford NS. Age-dependent alterations of corticostriatal activity in the YAC128 mouse model of Huntington disease. J Neurosci. 2009;29:2414–2427. doi: 10.1523/JNEUROSCI.5687-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach LS, Romero E, Becklin RR, Chettier R, Bell R, Phansalkar A, Strand A, Torcassi C, Savage J, Hurlburt A, Cha GH, Ukani L, Chepanoske CL, Zhen Y, Sahasrabudhe S, Olson J, Kurschner C, Ellerby LM, Peltier JM, Botas J, Hughes RE. Huntingtin interacting proteins are genetic modifiers of neurodegeneration. PLoS Genet. 2007;3:e82. doi: 10.1371/journal.pgen.0030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassubek J, Bernhard Landwehrmeyer G, Ecker D, Juengling FD, Muche R, Schuller S, Weindl A, Peinemann A. Global cerebral atrophy in early stages of Huntington's disease: quantitative MRI study. Neuroreport. 2004;15:363–365. doi: 10.1097/00001756-200402090-00030. [DOI] [PubMed] [Google Scholar]
- Kato A, Rouach N, Nicoll RA, Bredt DS. Activity-dependent NMDA receptor degradation mediated by retrotranslocation and ubiquitination. Proc Natl Acad Sci U S A. 2005;102:5600–5605. doi: 10.1073/pnas.0501769102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Emson PC. Projection subtypes of rat neostriatal matrix cells revealed by intracellular injection of biocytin. J Neurosci. 1990;10:3421–3438. doi: 10.1523/JNEUROSCI.10-10-03421.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp JM. The site of termination of afferent fibres on the neurones of the caudate nucleus. J Physiol. 1970;210:17P–18P. [PubMed] [Google Scholar]
- Kew JN, Richards JG, Mutel V, Kemp JA. Developmental changes in NMDA receptor glycine affinity and ifenprodil sensitivity reveal three distinct populations of NMDA receptors in individual rat cortical neurons. J Neurosci. 1998;18:1935–1943. doi: 10.1523/JNEUROSCI.18-06-01935.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron. 2005;46:745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Jo DG, Hong GS, Kim BJ, Lai M, Cho DH, Kim KW, Bandyopadhyay A, Hong YM, Kim DH, Cho C, Liu JO, Snyder SH, Jung YK. Calpain-dependent cleavage of cain/cabin1 activates calcineurin to mediate calcium-triggered cell death. Proc Natl Acad Sci U S A. 2002;99:9870–9875. doi: 10.1073/pnas.152336999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Thomas CA, André VM, Cummings DM, Cepeda C, Levine MS, Ehrlich ME. Forebrain striatal-specific expression of mutant huntingtin protein in vivo induces cell-autonomous age-dependent alterations in sensitivity to excitotoxicity and mitochondrial function. ASN Neuro. 2011 doi: 10.1042/AN20110009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Kitai ST. Glutamate decarboxylase immunoreactive neurons in rat neostriatum: their morphological types and populations. Brain Res. 1988;447:346–352. doi: 10.1016/0006-8993(88)91138-9. [DOI] [PubMed] [Google Scholar]
- Klapstein GJ, Fisher RS, Zanjani H, Cepeda C, Jokel ES, Chesselet MF, Levine MS. Electrophysiological and Morphological Changes in Striatal Spiny Neurons in R6/2 Huntington's Disease Transgenic Mice. J Neurophysiol. 2001;86:2667–2677. doi: 10.1152/jn.2001.86.6.2667. [DOI] [PubMed] [Google Scholar]
- Kraft JC, Osterhaus GL, Ortiz AN, Garris PA, Johnson MA. In vivo dopamine release and uptake impairments in rats treated with 3-nitropropionic acid. Neurosci. 2009;161:940–949. doi: 10.1016/j.neuroscience.2009.03.083. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- Kremer HP, Roos RA, Dingjan G, Marani E, Bots GT. Atrophy of the hypothalamic lateral tuberal nucleus in Huntington's disease. J Neuropathol Exp Neurol. 1990;49:371–382. doi: 10.1097/00005072-199007000-00002. [DOI] [PubMed] [Google Scholar]
- Kung VW, Hassam R, Morton AJ, Jones S. Dopamine-dependent long term potentiation in the dorsal striatum is reduced in the R6/2 mouse model of Huntington’s disease. Neurosci. 2007;146:1571–1580. doi: 10.1016/j.neuroscience.2007.03.036. [DOI] [PubMed] [Google Scholar]
- Laforet GA, Sapp E, Chase K, McIntyre C, Boyce FM, Campbell M, Cadigan BA, Warzecki L, Tagle DA, Reddy PH, Cepeda C, Calvert CR, Jokel ES, Klapstein GJ, Ariano MA, Levine MS, DiFiglia M, Aronin N. Changes in cortical and striatal neurons predict behavioral and electrophysiological abnormalities in a transgenic murine model of Huntington's disease. J Neurosci. 2001;21:9112–9123. doi: 10.1523/JNEUROSCI.21-23-09112.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landwehrmeyer GB, Standaert DG, Testa CM, Penney JB, Jr, Young AB. NMDA receptor subunit mRNA expression by projection neurons and interneurons in rat striatum. J Neurosci. 1995;15:5297–5307. doi: 10.1523/JNEUROSCI.15-07-05297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzari G, McCallum J, Dewey CM, Roche KW. Subunit-specific regulation of NMDA receptor endocytosis. J Neurosci. 2004;24:6383–6391. doi: 10.1523/JNEUROSCI.1890-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AD, Hodges JR, Rosser AE, Kershaw A, ffrench-Constant C, Rubinsztein DC, Robbins TW, Sahakian BJ. Evidence for specific cognitive deficits in preclinical Huntington's disease. Brain. 1998;121(Pt 7):1329–1341. doi: 10.1093/brain/121.7.1329. [DOI] [PubMed] [Google Scholar]
- Lee ST, Chu K, Park JE, Kang L, Ko SY, Jung KH, Kim M. Memantine reduces striatal cell death with decreasing calpain level in 3-nitropropionic model of Huntington's disease. Brain Res. 2006;1118:199–207. doi: 10.1016/j.brainres.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Lei W, Jiao Y, Del Mar N, Reiner A. Evidence for differential cortical input to direct pathway versus indirect pathway striatal projection neurons in rats. J Neurosci. 2004;24:8289–8299. doi: 10.1523/JNEUROSCI.1990-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveille F, El Gaamouch F, Gouix E, Lecocq M, Lobner D, Nicole O, Buisson A. Neuronal viability is controlled by a functional relation between synaptic and extrasynaptic NMDA receptors. FASEB J. 2008;22:4258–4271. doi: 10.1096/fj.08-107268. [DOI] [PubMed] [Google Scholar]
- Levine MS, Cepeda C, Hickey MA, Fleming SM, Chesselet MF. Genetic mouse models of Huntington's and Parkinson's diseases: illuminating but imperfect. Trends Neurosci. 2004;27:691–697. doi: 10.1016/j.tins.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Levine MS, Klapstein GJ, Koppel A, Gruen E, Cepeda C, Vargas ME, Jokel ES, Carpenter EM, Zanjani H, Hurst RS, Efstratiadis A, Zeitlin S, Chesselet MF. Enhanced sensitivity to N-methyl-D-aspartate receptor activation in transgenic and knockin mouse models of Huntington's disease. J Neurosci Res. 1999;58:515–532. [PubMed] [Google Scholar]
- Li H, Wyman T, Yu ZX, Li SH, Li XJ. Abnormal association of mutant huntingtin with synaptic vesicles inhibits glutamate release. Hum Mol Genet. 2003a;12:2021–2030. doi: 10.1093/hmg/ddg218. [DOI] [PubMed] [Google Scholar]
- Li JY, Plomann M, Brundin P. Huntington's disease: a synaptopathy? Trends Mol Med. 2003b;9:414–420. doi: 10.1016/j.molmed.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Li L, Fan M, Icton CD, Chen N, Leavitt BR, Hayden MR, Murphy TH, Raymond LA. Role of NR2B-type NMDA receptors in selective neurodegeneration in Huntington disease. Neurobiol Aging. 2003c;24:1113–1121. doi: 10.1016/j.neurobiolaging.2003.04.003. [DOI] [PubMed] [Google Scholar]
- Li L, Murphy TH, Hayden MR, Raymond LA. Enhanced striatal NR2B-containing N-methyl-D-aspartate receptor-mediated synaptic currents in a mouse model of Huntington disease. J Neurophysiol. 2004;92:2738–2746. doi: 10.1152/jn.00308.2004. [DOI] [PubMed] [Google Scholar]
- Lievens JC, Woodman B, Mahal A, Spasic-Boscovic O, Samuel D, Kerkerian-Le Goff L, Bates GP. Impaired glutamate uptake in the R6 Huntington's disease transgenic mice. Neurobiol Dis. 2001;8:807–821. doi: 10.1006/nbdi.2001.0430. [DOI] [PubMed] [Google Scholar]
- Lin CH, Tallaksen-Greene S, Chien WM, Cearley JA, Jackson WS, Crouse AB, Ren S, Li XJ, Albin RL, Detloff PJ. Neurological abnormalities in a knock-in mouse model of Huntington's disease. Hum Mol Genet. 2001;10:137–144. doi: 10.1093/hmg/10.2.137. [DOI] [PubMed] [Google Scholar]
- Lin WC, Chou YH. Aripiprazole effects on psychosis and chorea in a patient with Huntington’s disease. Am J Psychiatry. 2008;165:1207–1208. doi: 10.1176/appi.ajp.2008.08040503. [DOI] [PubMed] [Google Scholar]
- Lin Y, Skeberdis VA, Francesconi A, Bennett MV, Zukin RS. Postsynaptic density protein-95 regulates NMDA channel gating and surface expression. J Neurosci. 2004;24:10138–10148. doi: 10.1523/JNEUROSCI.3159-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lione LA, Carter RJ, Hunt MJ, Bates GP, Morton AJ, Dunnett SB. Selective discrimination learning impairments in mice expressing the human Huntington's disease mutation. J Neurosci. 1999;19:10428–10437. doi: 10.1523/JNEUROSCI.19-23-10428.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat Rev Drug Discov. 2006;5:160–170. doi: 10.1038/nrd1958. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, Wu DC, Lu J, Tymianski M, Craig AM, Wang YT. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci. 2007;27:2846–2857. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C-W, Lin T-Y, Wang S-J. Memantine depresses glutamate release through inhibition of voltage-dependent Ca2+ entry and a protein C kinase in rat cerebral cortex nerve terminals: An NMDA receptor-dependent mechanism. Neurochem Int. 2010;57:168–176. doi: 10.1016/j.neuint.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Lucas DR, Newhouse JP. The toxic effect of sodium L-glutamate on the inner layers of the retina. AMA. 1957;58:193–201. doi: 10.1001/archopht.1957.00940010205006. [DOI] [PubMed] [Google Scholar]
- Luthi-Carter R, Apostol BL, Dunah AW, DeJohn MM, Farrell LA, Bates GP, Young AB, Standaert DG, Thompson LM, Cha JH. Complex alteration of NMDA receptors in transgenic Huntington's disease mouse brain: analysis of mRNA and protein expression, plasma membrane association, interacting proteins, and phosphorylation. Neurobiol Dis. 2003;14:624–636. doi: 10.1016/j.nbd.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Luthi-Carter R, Strand A, Peters NL, Solano SM, Hollingsworth ZR, Menon AS, Frey AS, Spektor BS, Penney EB, Schilling G, Ross CA, Borchelt DR, Tapscott SJ, Young AB, Cha JH, Olson JM. Decreased expression of striatal signaling genes in a mouse model of Huntington's disease. Hum Mol Genet. 2000;9:1259–1271. doi: 10.1093/hmg/9.9.1259. [DOI] [PubMed] [Google Scholar]
- Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies SW, Bates GP. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J Physiol. 1987;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL. Duplication of biochemical changes of Huntington's chorea by intrastriatal injections of glutamic and kainic acids. Nature. 1976;263:517–519. doi: 10.1038/263517a0. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG, Scherer U, Singh K. A glutamatergic corticostriatal path? Brain Res. 1977;128:369–373. doi: 10.1016/0006-8993(77)91003-4. [DOI] [PubMed] [Google Scholar]
- Menalled L, Zanjani H, MacKenzie L, Koppel A, Carpenter E, Zeitlin S, Chesselet MF. Decrease in striatal enkephalin mRNA in mouse models of Huntington's disease. Exp Neurol. 2000;162:328–342. doi: 10.1006/exnr.1999.7327. [DOI] [PubMed] [Google Scholar]
- Menalled LB, Sison JD, Dragatsis I, Zeitlin S, Chesselet MF. Time course of early motor and neuropathological anomalies in a knock-in mouse model of Huntington's disease with 140 CAG repeats. J Comp Neurol. 2003;465:11–26. doi: 10.1002/cne.10776. [DOI] [PubMed] [Google Scholar]
- Menalled LB, Sison JD, Wu Y, Olivieri M, Li XJ, Li H, Zeitlin S, Chesselet MF. Early motor dysfunction and striosomal distribution of huntingtin microaggregates in Huntington's disease knock-in mice. J Neurosci. 2002;22:8266–8276. doi: 10.1523/JNEUROSCI.22-18-08266.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milakovic T, Quintanilla RA, Johnson GV. Mutant huntingtin expression induces mitochondrial calcium handling defects in clonal striatal cells: functional consequences. J Biol Chem. 2006;281:34785–34795. doi: 10.1074/jbc.M603845200. [DOI] [PubMed] [Google Scholar]
- Miller BR, Dorner JL, Shou M, Sari Y, Barton SJ, Sengelaub DR, Kennedy RT, Rebec GV. Up-regulation of GLT1 expression increases glutamate uptake and attenuates the Huntington's disease phenotype in the R6/2 mouse. Neuroscience. 2008a;153:329–337. doi: 10.1016/j.neuroscience.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BR, Walker AG, Shah AS, Barton SJ, Rebec GV. Dysregulated information processing by medium spiny neurons in striatum of freely behaving mouse models of Huntington's disease. J Neurophysiol. 2008b;100:2205–2216. doi: 10.1152/jn.90606.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milnerwood AJ, Cummings DM, Dallerac GM, Brown JY, Vatsavayai SC, Hirst MC, Rezaie P, Murphy KP. Early development of aberrant synaptic plasticity in a mouse model of Huntington's disease. Hum Mol Genet. 2006;15:1690–1703. doi: 10.1093/hmg/ddl092. [DOI] [PubMed] [Google Scholar]
- Milnerwood AJ, Gladding CM, Pouladi MA, Kaufman AM, Hines RM, Boyd JD, Ko RW, Vasuta OC, Graham RK, Hayden MR, Murphy TH, Raymond LA. Early increase in extrasynaptic NMDA receptor signaling and expression contributes to phenotype onset in Huntington's disease mice. Neuron. 2010;65:178–190. doi: 10.1016/j.neuron.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Milnerwood AJ, Raymond LA. Corticostriatal synaptic function in mouse models of Huntington's disease: early effects of huntingtin repeat length and protein load. J Physiol. 2007;585:817–831. doi: 10.1113/jphysiol.2007.142448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milnerwood AJ, Raymond LA. Early synaptic pathophysiology in neurodegeneration: insights from Huntington's disease. Trends Neurosci. 2010;33:513–523. doi: 10.1016/j.tins.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Mori H, Manabe T, Watanabe M, Satoh Y, Suzuki N, Toki S, Nakamura K, Yagi T, Kushiya E, Takahashi T, Inoue Y, Sakimura K, Mishina M. Role of the carboxy-terminal region of the GluR epsilon2 subunit in synaptic localization of the NMDA receptor channel. Neuron. 1998;21:571–580. doi: 10.1016/s0896-6273(00)80567-x. [DOI] [PubMed] [Google Scholar]
- Morishima M, Kawaguchi Y. Recurrent connection patterns of corticostriatal pyramidal cells in frontal cortex. J Neurosci. 2006;26:4394–4405. doi: 10.1523/JNEUROSCI.0252-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro E, Lang AE, Strafella AP, Poon YY, Arango PM, Dagher A, Hutchison WD, Lozano AM. Bilateral globus pallidus stimulation for Huntington's disease. Ann Neurol. 2004;56:290–294. doi: 10.1002/ana.20183. [DOI] [PubMed] [Google Scholar]
- Morton AJ, Lagan MA, Skepper JN, Dunnett SB. Progressive formation of inclusions in the striatum and hippocampus of mice transgenic for the human Huntington's disease mutation. J Neurocytol. 2000;29:679–702. doi: 10.1023/a:1010887421592. [DOI] [PubMed] [Google Scholar]
- Murphy KP, Carter RJ, Lione LA, Mangiarini L, Mahal A, Bates GP, Dunnett SB, Morton AJ. Abnormal synaptic plasticity and impaired spatial cognition in mice transgenic for exon 1 of the human Huntington's disease mutation. J Neurosci. 2000;20:5115–5123. doi: 10.1523/JNEUROSCI.20-13-05115.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto S, Pouladi MA, Talantova M, Yao D, Xia P, Ehrnhoefer DE, Zaidi R, Clemente A, Kaul M, Graham RK, Zhang D, Vincent Chen HS, Tong G, Hayden MR, Lipton SA. Balance between synaptic versus extrasynaptic NMDA receptor activity influences inclusions and neurotoxicity of mutant huntingtin. Nat Med. 2009;15:1407–1413. doi: 10.1038/nm.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira JM, Chen S, Almeida S, Riley R, Goncalves J, Oliveira CR, Hayden MR, Nicholls DG, Ellerby LM, Rego AC. Mitochondrial-dependent Ca2+ handling in Huntington's disease striatal cells: effect of histone deacetylase inhibitors. J Neurosci. 2006;26:11174–11186. doi: 10.1523/JNEUROSCI.3004-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulis P, Mourikis I, Konstantakopoulos G, Papageorgiou SG, Kouzoupis AV. Aripiprazole in the treatment of olanzapine-resistant psychotic and motor symptoms of Huntington’s disease. J Neuropsych Clin Neurosci. 2010;22:352c.e4–352.e5. doi: 10.1176/jnp.2010.22.3.352.e4. [DOI] [PubMed] [Google Scholar]
- Paladini CA, Tepper JM. GABAA and GABAB antagonists differentially affect the firing pattern of substantia nigra dopaminergic neurons in vivo. Synapse. 1999;32:165–176. doi: 10.1002/(SICI)1098-2396(19990601)32:3<165::AID-SYN3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Panov AV, Burke JR, Strittmatter WJ, Greenamyre JT. In vitro effects of polyglutamine tracts on Ca2+-dependent depolarization of rat and human mitochondria: relevance to Huntington's disease. Arch Biochem Biophys. 2003;410:1–6. doi: 10.1016/s0003-9861(02)00585-4. [DOI] [PubMed] [Google Scholar]
- Panov AV, Gutekunst CA, Leavitt BR, Hayden MR, Burke JR, Strittmatter WJ, Greenamyre JT. Early mitochondrial calcium defects in Huntington's disease are a direct effect of polyglutamines. Nat Neurosci. 2002;5:731–736. doi: 10.1038/nn884. [DOI] [PubMed] [Google Scholar]
- Papadia S, Soriano FX, Leveille F, Martel MA, Dakin KA, Hansen HH, Kaindl A, Sifringer M, Fowler J, Stefovska V, McKenzie G, Craigon M, Corriveau R, Ghazal P, Horsburgh K, Yankner BA, Wyllie DJ, Ikonomidou C, Hardingham GE. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci. 2008;11:476–487. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadia S, Stevenson P, Hardingham NR, Bading H, Hardingham GE. Nuclear Ca2+ and the cAMP response element-binding protein family mediate a late phase of activity-dependent neuroprotection. J Neurosci. 2005;25:4279–4287. doi: 10.1523/JNEUROSCI.5019-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy HB, Graybiel AM. Cortically driven immediate-early gene expression reflects modular influence of sensorimotor cortex on identified striatal neurons in the squirrel monkey. J Neurosci. 1997;17:2477–2491. doi: 10.1523/JNEUROSCI.17-07-02477.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Rademacher DJ, Hillard CJ. Differential regulation of the endocannabinoids anandamide and 2-arachidonylglycerol within the limbic forebrain by dopamine receptor activity. J Pharmacol Exp Ther. 2003;306:880–888. doi: 10.1124/jpet.103.054270. [DOI] [PubMed] [Google Scholar]
- Paulsen JS. Functional imaging in Huntington's disease. Exp Neurol. 2009;216:272–277. doi: 10.1016/j.expneurol.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen JS, Langbehn DR, Stout JC, Aylward E, Ross CA, Nance M, Guttman M, Johnson S, MacDonald M, Beglinger LJ, Duff K, Kayson E, Biglan K, Shoulson I, Oakes D, Hayden M. Detection of Huntington's disease decades before diagnosis: the Predict-HD study. J Neurol Neurosurg Psychiatry. 2008;79:874–880. doi: 10.1136/jnnp.2007.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavese N, Gerhard A, Tai YF, Ho AK, Turkheimer F, Barker RA, Brooks DJ, Piccini P. Microglial activation correlates with severity in Huntington disease: a clinical and PET study. Neurology. 2006;66:1638–1643. doi: 10.1212/01.wnl.0000222734.56412.17. [DOI] [PubMed] [Google Scholar]
- Pelkey KA, Askalan R, Paul S, Kalia LV, Nguyen TH, Pitcher GM, Salter MW, Lombroso PJ. Tyrosine phosphatase STEP is a tonic brake on induction of long-term potentiation. Neuron. 2002;34:127–138. doi: 10.1016/s0896-6273(02)00633-5. [DOI] [PubMed] [Google Scholar]
- Petersén A, Chase K, Puschban Z, DiFiglia M, Brundin P, Aronin N. Maintenance of susceptibility to neurodegeneration following intrastriatal injections of quinolinic acid in a new transgenic mouse model of Huntington's disease. Exp Neurol. 2002;175:297–300. doi: 10.1006/exnr.2002.7885. [DOI] [PubMed] [Google Scholar]
- Petersén A, Gil J, Maat-Schieman ML, Bjorkqvist M, Tanila H, Araujo IM, Smith R, Popovic N, Wierup N, Norlen P, Li JY, Roos RA, Sundler F, Mulder H, Brundin P. Orexin loss in Huntington's disease. Hum Mol Genet. 2005;14:39–47. doi: 10.1093/hmg/ddi004. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Hua F, Yi Z, Zhou A, Ge L, Stephenson FA, Wenthold RJ. Organization of NMDA receptors at extrasynaptic locations. Neuroscience. 2010;167:68–87. doi: 10.1016/j.neuroscience.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picconi B, Passino E, Sgobio C, Bonsi P, Barone I, Ghiglieri V, Pisani A, Bernardi G, Ammassari-Teule M, Calabresi P. Plastic and behavioral abnormalities in experimental Huntington's disease: a crucial role for cholinergic interneurons. Neurobiol Dis. 2006;22:143–152. doi: 10.1016/j.nbd.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Pisani A, Bernardi G, Ding J, Surmeier DJ. Re-emergence of striatal cholinergic interneurons in movement disorders. Trends Neurosci. 2007;30:545–553. doi: 10.1016/j.tins.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Pouladi MA, Graham RK, Karasinska JM, Xie Y, Santos RD, Petersén A, Hayden MR. Prevention of depressive behaviour in the YAC128 mouse model of Huntington disease by mutation at residue 586 of huntingtin. Brain. 2009;132:919–932. doi: 10.1093/brain/awp006. [DOI] [PubMed] [Google Scholar]
- Prybylowski K, Chang K, Sans N, Kan L, Vicini S, Wenthold RJ. The synaptic localization of NR2B-containing NMDA receptors is controlled by interactions with PDZ proteins and AP-2. Neuron. 2005;47:845–857. doi: 10.1016/j.neuron.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SP, André VM, Cepeda C, Levine MS. Neuroscience Meeting Planner. San Diego, CA: 2010. Altered inhibitory inputs onto striatal medium-sized spiny neurons in the R6/2 mouse model of Huntington’s disease. Program: 861.16. [Google Scholar]
- Rasmussen A, Macias R, Yescas P, Ochoa A, Davila G, Alonso E. Huntington disease in children: genotype-phenotype correlation. Neuropediatrics. 2000;31:190–194. doi: 10.1055/s-2000-7461. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Conroy SK, Barton SJ. Hyperactive striatal neurons in symptomatic Huntington R6/2 mice: variations with behavioral state and repeated ascorbate treatment. Neuroscience. 2006;137:327–336. doi: 10.1016/j.neuroscience.2005.08.062. [DOI] [PubMed] [Google Scholar]
- Reiner A, Albin RL, Anderson KD, D'Amato CJ, Penney JB, Young AB. Differential loss of striatal projection neurons in Huntington disease. Proc Natl Acad Sci U S A. 1988;85:5733–5737. doi: 10.1073/pnas.85.15.5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Jiao Y, Del Mar N, Laverghetta AV, Lei WL. Differential morphology of pyramidal tract-type and intratelencephalically projecting-type corticostriatal neurons and their intrastriatal terminals in rats. J Comp Neurol. 2003;457:420–440. doi: 10.1002/cne.10541. [DOI] [PubMed] [Google Scholar]
- Richfield EK, Maguire-Zeiss KA, Vonkeman HE, Voorn P. Preferential loss of preproenkephalin versus preprotachykinin neurons from the striatum of Huntington's disease patients. Ann Neurol. 1995;38:852–861. doi: 10.1002/ana.410380605. [DOI] [PubMed] [Google Scholar]
- Rigby M, Le Bourdelles B, Heavens RP, Kelly S, Smith D, Butler A, Hammans R, Hills R, Xuereb JH, Hill RG, Whiting PJ, Sirinathsinghji DJ. The messenger RNAs for the N-methyl-D-aspartate receptor subunits show region-specific expression of different subunit composition in the human brain. Neuroscience. 1996;73:429–447. doi: 10.1016/0306-4522(96)00089-9. [DOI] [PubMed] [Google Scholar]
- Roche KW, Standley S, McCallum J, Dune Ly C, Ehlers MD, Wenthold RJ. Molecular determinants of NMDA receptor internalization. Nat Neurosci. 2001;4:794–802. doi: 10.1038/90498. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Feigin AS, Hersch SM. Using advances in neuroimaging to detect, understand, and monitor disease progression in Huntington's disease. NeuroRx. 2004;1:263–272. doi: 10.1602/neurorx.1.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas HD, Hevelone ND, Zaleta AK, Greve DN, Salat DH, Fischl B. Regional cortical thinning in preclinical Huntington disease and its relationship to cognition. Neurology. 2005;65:745–747. doi: 10.1212/01.wnl.0000174432.87383.87. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Koroshetz WJ, Chen YI, Skeuse C, Vangel M, Cudkowicz ME, Caplan K, Marek K, Seidman LJ, Makris N, Jenkins BG, Goldstein JM. Evidence for more widespread cerebral pathology in early HD: an MRI-based morphometric analysis. Neurology. 2003;60:1615–1620. doi: 10.1212/01.wnl.0000065888.88988.6e. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Salat DH, Lee SY, Zaleta AK, Hevelone N, Hersch SM. Complexity and heterogeneity: what drives the ever-changing brain in Huntington's disease? Ann N Y Acad Sci. 2008a;1147:196–205. doi: 10.1196/annals.1427.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas HD, Salat DH, Lee SY, Zaleta AK, Pappu V, Fischl B, Greve D, Hevelone N, Hersch SM. Cerebral cortex and the clinical expression of Huntington's disease: complexity and heterogeneity. Brain. 2008b;131:1057–1068. doi: 10.1093/brain/awn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas HD, Tuch DS, Hevelone ND, Zaleta AK, Vangel M, Hersch SM, Salat DH. Diffusion tensor imaging in presymptomatic and early Huntington's disease: Selective white matter pathology and its relationship to clinical measures. Mov Disord. 2006;21:1317–1325. doi: 10.1002/mds.20979. [DOI] [PubMed] [Google Scholar]
- Salter MW, Dong Y, Kalia LV, Liu XJ, Pitcher G. Regulation of NMDA Receptors by Kinases and Phosphatases. In: VanDongen AM, editor. Biology of the NMDA Receptor. Chapter 7. Boca Raton, FL: CRC Press; 2009. [PubMed] [Google Scholar]
- Sanberg PR, Calderon SF, Giordano M, Tew JM, Norman AB. The quinolinic acid model of Huntington's disease: locomotor abnormalities. Exp Neurol. 1989;105:45–53. doi: 10.1016/0014-4886(89)90170-2. [DOI] [PubMed] [Google Scholar]
- Sanz-Clemente A, Matta JA, Isaac JT, Roche KW. Casein kinase 2 regulates the NR2 subunit composition of synaptic NMDA receptors. Neuron. 2010;67:984–996. doi: 10.1016/j.neuron.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapp E, Ge P, Aizawa H, Bird E, Penney J, Young AB, Vonsattel JP, DiFiglia M. Evidence for a preferential loss of enkephalin immunoreactivity in the external globus pallidus in low grade Huntington's disease using high resolution image analysis. Neuroscience. 1995;64:397–404. doi: 10.1016/0306-4522(94)00427-7. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Coyle JT. Striatal lesions with kainic acid: neurochemical characteristics. Brain Res. 1977;127:235–249. doi: 10.1016/0006-8993(77)90538-8. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Foster AC, French ED, Whetsell WO, Jr, Kohler C. Excitotoxic models for neurodegenerative disorders. Life Sci. 1984;35:19–32. doi: 10.1016/0024-3205(84)90148-6. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Whetsell WO, Jr, Mangano RM. Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science. 1983;219:316–318. doi: 10.1126/science.6849138. [DOI] [PubMed] [Google Scholar]
- Shehadeh J, Fernandes HB, Zeron Mullins MM, Graham RK, Leavitt BR, Hayden MR, Raymond LA. Striatal neuronal apoptosis is preferentially enhanced by NMDA receptor activation in YAC transgenic mouse model of Huntington disease. Neurobiol Dis. 2006;21:392–403. doi: 10.1016/j.nbd.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Shelbourne PF, Killeen N, Hevner RF, Johnston HM, Tecott L, Lewandoski M, Ennis M, Ramirez L, Li Z, Iannicola C, Littman DR, Myers RM. A Huntington's disease CAG expansion at the murine Hdh locus is unstable and associated with behavioural abnormalities in mice. Hum Mol Genet. 1999;8:763–774. doi: 10.1093/hmg/8.5.763. [DOI] [PubMed] [Google Scholar]
- Shin JY, Fang ZH, Yu ZX, Wang CE, Li SH, Li XJ. Expression of mutant huntingtin in glial cells contributes to neuronal excitotoxicity. J Cell Biol. 2005;171:1001–1012. doi: 10.1083/jcb.200508072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuen JA, Chen M, Gloss B, Calakos N. Drd1a-tdTomato BAC transgenic mice for simultaneous visualization of medium spiny neurons in the direct and indirect pathways of the basal ganglia. J Neurosci. 2008;28:2681–2685. doi: 10.1523/JNEUROSCI.5492-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins KL, Guttmann RP, Dong Y, Chen Z, Sokol S, Neumar RW, Lynch DR. Selective activation induced cleavage of the NR2B subunit by calpain. J Neurosci. 2003;23:11322–11331. doi: 10.1523/JNEUROSCI.23-36-11322.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singaraja RR, Hadano S, Metzler M, Givan S, Wellington CL, Warby S, Yanai A, Gutekunst CA, Leavitt BR, Yi H, Fichter K, Gan L, McCutcheon K, Chopra V, Michel J, Hersch SM, Ikeda JE, Hayden MR. HIP14, a novel ankyrin domain-containing protein, links huntingtin to intracellular trafficking and endocytosis. Hum Mol Genet. 2002;11:2815–2828. doi: 10.1093/hmg/11.23.2815. [DOI] [PubMed] [Google Scholar]
- Singaraja RR, Huang K, Sanders SS, Milnerwood A, Hines R, Lerch J, Franciosi S, Drisdel R, Vaid K, Young FB, Doty C, Wan J, Bissada N, Henkelman RM, Green WN, Davis NG, Raymond LA, Hayden MR. Altered palmitoylation and neuropathological deficits in mice lacking HIP14. Hum Mol Genet. Jul 20; doi: 10.1093/hmg/ddr308. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slow EJ, van Raamsdonk J, Rogers D, Coleman SH, Graham RK, Deng Y, Oh R, Bissada N, Hossain SM, Yang YZ, Li XJ, Simpson EM, Gutekunst CA, Leavitt BR, Hayden MR. Selective striatal neuronal loss in a YAC128 mouse model of Huntington disease. Hum Mol Genet. 2003;12:1555–1567. doi: 10.1093/hmg/ddg169. [DOI] [PubMed] [Google Scholar]
- Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 2004;27:520–527. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lmbroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Soriano FX, Martel MA, Papadia S, Vaslin A, Baxter P, Rickman C, Forder J, Tymianski M, Duncan R, Aarts M, Clarke P, Wyllie DJ, Hardingham GE. Specific targeting of pro-death NMDA receptor signals with differing reliance on the NR2B PDZ ligand. J Neurosci. 2008;28:10696–10710. doi: 10.1523/JNEUROSCI.1207-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampanato J, Gu X, Yang XW, Mody I. Progressive synaptic pathology of motor cortical neurons in a BAC transgenic mouse model of Huntington's disease. Neuroscience. 2008;157:606–620. doi: 10.1016/j.neuroscience.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spokes EGS. The neurochemistry of Huntington's chorea. TINS. 1981;4:115–118. [Google Scholar]
- Stack EC, Dedeoglu A, Smith KM, Cormier K, Kubilus JK, Bogdanov M, Matson WR, Yang L, Jenkins BG, Luthi-Carter R, Kowall NW, Hersch SM, Beal MF, Ferrante RJ. Neuroprotective effects of synaptic modulation in Huntington's disease R6/2 mice. J Neurosci. 2007;27:12908–12915. doi: 10.1523/JNEUROSCI.4318-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standaert DG, Friberg IK, Landwehrmeyer GB, Young AB, Penney JB., Jr Expression of NMDA glutamate receptor subunit mRNAs in neurochemically identified projection and interneurons in the striatum of the rat. Brain Res Mol Brain Res. 1999;64:11–23. doi: 10.1016/s0169-328x(98)00293-9. [DOI] [PubMed] [Google Scholar]
- Starling AJ, André VM, Cepeda C, de Lima M, Chandler SH, Levine MS. Alterations in N-methyl-D-aspartate receptor sensitivity and magnesium blockade occur early in development in the R6/2 mouse model of Huntington's disease. J Neurosci Res. 2005;82:377–386. doi: 10.1002/jnr.20651. [DOI] [PubMed] [Google Scholar]
- Starr PA, Kang GA, Heath S, Shimamoto S, Turner RS. Pallidal neuronal discharge in Huntington's disease: support for selective loss of striatal cells originating the indirect pathway. Exp Neurol. 2008;211:227–233. doi: 10.1016/j.expneurol.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Gerfen CR. Enkephalin regulates acute D2 dopamine receptor antagonist-induced immediate-early gene expression in striatal neurons. Neuroscience. 1999;88:795–810. doi: 10.1016/s0306-4522(98)00241-3. [DOI] [PubMed] [Google Scholar]
- Stern EA. Functional changes in neocortical activity in Huntington's disease model mice: An in vivo intracellular study. Front Syst Neurosci. 2011;5:47. doi: 10.3389/fnsys.2011.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong TV, Tagle DA, Valdes JM, Elmer LW, Boehm K, Swaroop M, Kaatz KW, Collins FS, Albin RL. Widespread expression of the human and rat Huntington's disease gene in brain and nonneural tissues. Nat Genet. 1993;5:259–265. doi: 10.1038/ng1193-259. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai YF, Pavese N, Gerhard A, Tabrizi SJ, Barker RA, Brooks DJ, Piccini P. Imaging microglial activation in Huntington's disease. Brain Res Bull. 2007;72:148–151. doi: 10.1016/j.brainresbull.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Tang TS, Slow E, Lupu V, Stavrovskaya IG, Sugimori M, Llinas R, Kristal BS, Hayden MR, Bezprozvanny I. Disturbed Ca2+ signaling and apoptosis of medium spiny neurons in Huntington's disease. Proc Natl Acad Sci U S A. 2005;102:2602–2607. doi: 10.1073/pnas.0409402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang TS, Tu H, Chan EY, Maximov A, Wang Z, Wellington CL, Hayden MR, Bezprozvanny I. Huntingtin and huntingtin-associated protein 1 influence neuronal calcium signaling mediated by inositol-(1,4,5) triphosphate receptor type 1. Neuron. 2003;39:227–239. doi: 10.1016/s0896-6273(03)00366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Koos T, Wilson CJ. GABAergic microcircuits in the neostriatum. Trends Neurosci. 2004;27:662–669. doi: 10.1016/j.tins.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Wilson CJ, Koos T. Feedforward and feedback inhibition in neostriatal GABAergic spiny neurons. Brain Res Rev. 2008;58:272–281. doi: 10.1016/j.brainresrev.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Huntington's Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Thomas CG, Miller AJ, Westbrook GL. Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J Neurophysiol. 2006;95:1727–1734. doi: 10.1152/jn.00771.2005. [DOI] [PubMed] [Google Scholar]
- Thomas EA, Coppola G, Tang B, Kuhn A, Kim S, Geschwind DH, Brown TB, Luthi-Carter R, Ehrlich ME. In vivo cell-autonomous transcriptional abnormalities revealed in mice expressing mutant huntingtin in striatal but not cortical neurons. Hum Mol Genet. 2011;20:1049–1060. doi: 10.1093/hmg/ddq548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thu DC, Oorschot DE, Tippett LJ, Nana AL, Hogg VM, Synek BJ, Luthi-Carter R, Waldvogel HJ, Faull RL. Cell loss in the motor and cingulate cortex correlates with symptomatology in Huntington's disease. Brain. 2010;133:1094–1110. doi: 10.1093/brain/awq047. [DOI] [PubMed] [Google Scholar]
- Tobin AJ, Signer ER. Huntington's disease: the challenge for cell biologists. Trends Cell Biol. 2000;10:531–536. doi: 10.1016/s0962-8924(00)01853-5. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu W, Xu X, Peng L, Zhong X, Zhang W, Soundarapandian MM, Balel C, Wang M, Jia N, Zhang W, Lew F, Chan SL, Chen Y, Lu Y. DAPK1 interaction with NMDA receptor NR2B subunits mediates brain damage in stroke. Cell. 2010;140:222–234. doi: 10.1016/j.cell.2009.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turmaine M, Raza A, Mahal A, Mangiarini L, Bates GP, Davies SW. Nonapoptotic neurodegeneration in a transgenic mouse model of Huntington's disease. Proc Natl Acad Sci U S A. 2000;97:8093–8097. doi: 10.1073/pnas.110078997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR, Navia B, Douglas J. Differential expression of preproenkephalin and preprodynorphin mRNAs in striatal neurons: high levels of preproenkephalin expression depend on cerebral cortical afferents. J Neurosci. 1988;8:4755–4764. doi: 10.1523/JNEUROSCI.08-12-04755.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oostrom JC, Dekker M, Willemsen AT, de Jong BM, Roos RA, Leenders KL. Changes in striatal dopamine D2 receptor binding in pre-clinical Huntington’s disease. Eur J Neurol. 2009;16:226–223. doi: 10.1111/j.1468-1331.2008.02390.x. [DOI] [PubMed] [Google Scholar]
- Van Raamsdonk JM, Murphy Z, Slow EJ, Leavitt BR, Hayden MR. Selective degeneration and nuclear localization of mutant huntingtin in the YAC128 mouse model of Huntington disease. Hum Mol Genet. 2005a;14:3823–3835. doi: 10.1093/hmg/ddi407. [DOI] [PubMed] [Google Scholar]
- Van Raamsdonk JM, Pearson J, Slow EJ, Hossain SM, Leavitt BR, Hayden MR. Cognitive dysfunction precedes neuropathology and motor abnormalities in the YAC128 mouse model of Huntington's disease. J Neurosci. 2005b;25:4169–4180. doi: 10.1523/JNEUROSCI.0590-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent S, Hokfelt T, Christensson I, Terenius L. Immunohistochemical evidence for a dynorphin immunoreactive striato-nigral pathway. Eur J Pharmacol. 1982;85:251–252. doi: 10.1016/0014-2999(82)90477-0. [DOI] [PubMed] [Google Scholar]
- von Hörsten S, Schmitt I, Nguyen HP, Holzmann C, Schmidt T, Walther T, Bader M, Pabst R, Kobbe P, Krotova J, Stiller D, Kask A, Vaarmann A, Rathke-Hartlieb S, Schulz JB, Grasshoff U, Bauer I, Vieira-Saecker AM, Paul M, Jones L, Lindenberg KS, Landwehrmeyer B, Bauer A, Li XJ, Riess O. Transgenic rat model of Huntington's disease. Hum Mol Genet. 2003;12:617–624. doi: 10.1093/hmg/ddg075. [DOI] [PubMed] [Google Scholar]
- Vonsattel JP, DiFiglia M. Huntington disease. J Neuropathol Exp Neurol. 1998;57:369–384. doi: 10.1097/00005072-199805000-00001. [DOI] [PubMed] [Google Scholar]
- Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP., Jr Neuropathological classification of Huntington's disease. J Neuropathol Exp Neurol. 1985;44:559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- Wahl AS, Buchthal B, Rode F, Bomholt SF, Freitag HE, Hardingham GE, Ronn LC, Bading H. Hypoxic/ischemic conditions induce expression of the putative pro-death gene Clca1 via activation of extrasynaptic N-methyl-D-aspartate receptors. Neuroscience. 2009;158:344–352. doi: 10.1016/j.neuroscience.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Wheeler VC, White JK, Gutekunst CA, Vrbanac V, Weaver M, Li XJ, Li SH, Yi H, Vonsattel JP, Gusella JF, Hersch S, Auerbach W, Joyner AL, MacDonald ME. Long glutamine tracts cause nuclear localization of a novel form of huntingtin in medium spiny striatal neurons in HdhQ92 and HdhQ111 knock-in mice. Hum Mol Genet. 2000;9:503–513. doi: 10.1093/hmg/9.4.503. [DOI] [PubMed] [Google Scholar]
- White JK, Auerbach W, Duyao MP, Vonsattel JP, Gusella JF, Joyner AL, MacDonald ME. Huntingtin is required for neurogenesis and is not impaired by the Huntington's disease CAG expansion. Nat Genet. 1997;17:404–410. doi: 10.1038/ng1297-404. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Chang HT, Kitai ST. Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J Neurosci. 1990;10:508–519. doi: 10.1523/JNEUROSCI.10-02-00508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JD, MacMillan JC, Harper PS, Lowenstein PR, Jones AL. Partial characterisation of murine huntingtin and apparent variations in the subcellular localisation of huntingtin in human, mouse and rat brain. Hum Mol Genet. 1996;5:481–487. doi: 10.1093/hmg/5.4.481. [DOI] [PubMed] [Google Scholar]
- Wu HY, Hsu FC, Gleichman AJ, Baconguis I, Coulter DA, Lynch DR. Fyn-mediated phosphorylation of NR2B Tyr-1336 controls calpain-mediated NR2B cleavage in neurons and heterologous systems. J Biol Chem. 2007;282:20075–20087. doi: 10.1074/jbc.M700624200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia P, Chen HS, Zhang D, Lipton SA. Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses. J Neurosci. 2010;30:11246–11250. doi: 10.1523/JNEUROSCI.2488-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xifro X, Giralt A, Saavedra A, Garcia-Martinez JM, Diaz-Hernandez M, Lucas JJ, Alberch J, Perez-Navarro E. Reduced calcineurin protein levels and activity in exon-1 mouse models of Huntington's disease: role in excitotoxicity. Neurobiol Dis. 2009;36:461–469. doi: 10.1016/j.nbd.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Xu J, Kurup P, Zhang Y, Goebel-Goody SM, Wu PH, Hawasli AH, Baum ML, Bibb JA, Lombroso PJ. Extrasynaptic NMDA receptors couple preferentially to excitotoxicity via calpain-mediated cleavage of STEP. J Neurosci. 2009;29:9330–9343. doi: 10.1523/JNEUROSCI.2212-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A, Lucas JJ, Hen R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington's disease. Cell. 2000;101:57–66. doi: 10.1016/S0092-8674(00)80623-6. [DOI] [PubMed] [Google Scholar]
- Young AB, Greenamyre JT, Hollingsworth Z, Albin R, D'Amato C, Shoulson I, Penney JB. NMDA receptor losses in putamen from patients with Huntington's disease. Science. 1988;241:981–983. doi: 10.1126/science.2841762. [DOI] [PubMed] [Google Scholar]
- Zeron MM, Fernandes HB, Krebs C, Shehadeh J, Wellington CL, Leavitt BR, Baimbridge KG, Hayden MR, Raymond LA. Potentiation of NMDA receptor-mediated excitotoxicity linked with intrinsic apoptotic pathway in YAC transgenic mouse model of Huntington's disease. Mol Cell Neurosci. 2004;25:469–479. doi: 10.1016/j.mcn.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Zeron MM, Hansson O, Chen N, Wellington CL, Leavitt BR, Brundin P, Hayden MR, Raymond LA. Increased sensitivity to N-methyl-D-aspartate receptor-mediated excitotoxicity in a mouse model of Huntington's disease. Neuron. 2002;33:849–860. doi: 10.1016/s0896-6273(02)00615-3. [DOI] [PubMed] [Google Scholar]
- Zhang H, Li Q, Graham RK, Slow E, Hayden MR, Bezprozvanny I. Full length mutant huntingtin is required for altered Ca2+ signaling and apoptosis of striatal neurons in the YAC mouse model of Huntington's disease. Neurobiol Dis. 2008;31:80–88. doi: 10.1016/j.nbd.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SJ, Zou M, Lu L, Lau D, Ditzel DA, Delucinge-Vivier C, Aso Y, Descombes P, Bading H. Nuclear calcium signaling controls expression of a large gene pool: identification of a gene program for acquired neuroprotection induced by synaptic activity. PLoS Genet. 2009;5 doi: 10.1371/journal.pgen.1000604. e1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Role of brain-derived neurotrophic factor in Huntington's disease. Prog Neurobiol. 2007;81:294–330. doi: 10.1016/j.pneurobio.2007.01.003. [DOI] [PubMed] [Google Scholar]