Abstract
Drug discovery and development efforts critically rely on cell-based assays for high-throughput screening. These assay systems mostly utilize immortalized cell lines, such as human embryonic kidney cells, and can provide information on cytotoxicity and cell viability, permeability and uptake of compounds as well as receptor pharmacology. While this approach has proven extremely useful for single-target pharmacology, there is an urgent need for neuropharmacological studies to screen novel drug candidates in a cellular environment resembles neurons in vivo more closely, in order to gain insight into the involvement of multiple signaling pathways. Primary cultured neuronal cells, such as cortical neurons, have long been used for basic research and low-throughput screening and assay development, and may thus be suitable candidates for the development of neuropharmacological high-throughput screening approaches. We here developed and optimized protocols for the use of primary cortical neuronal cells in high-throughput assays for neuropharmacology and neuroprotection, including calcium mobilization, cytotoxicity and viability as well as ion channel pharmacology. Our data show low inter-experimental variability and similar reproducibility as conventional cell line assays. We conclude that primary neuronal cultures provide a viable alternative to cell lines in high-throughput assay systems by providing a cellular environment more closely resembling physiological conditions in the central nervous system.
Keywords: Primary neuronal culture, Cytotoxicity, Calcium mobilization, Drug screening, Neuroprotection, High-throughput screening
1. Introduction
Drug discovery and development efforts for a variety of targets, including G-protein coupled receptors and ion channels, rely significantly on the use of cell-based assays for high-throughput screening (HTS) (Johnston, 2002; Horrocks et al., 2003; Pereira and Williams, 2007; Eglen et al., 2008). Cell-based assays provide for a more physiologically-predictive outcome over other cell-free assay systems, and can provide information on cytotoxicity and viability, permeability, uptake as well as receptor pharmacology (Johnston, 2002; Horrocks et al., 2003; Pereira and Williams, 2007; Eglen et al., 2008).
Drug screening approaches, especially HTS, typically utilize immortalized cell lines as vehicles for the transient or stable expression of proteins of interest (Pereira and Williams, 2007). This is achieved by the introduction of plasmid vectors under the control of viral promoters that can exploit the cells protein synthesis and translation machinery. Perhaps the most prominent example for immortalized cell lines is human embryonic kidney (HEK) cells, which were permanently transformed by exposure to human adenovirus type 5 (Thomas and Smart, 2005; Eberle et al., 2010); others include CHO (Chinese hamster ovary) (Eberle et al., 2010; Omasa et al., 2010) and Cos (CV-1 Origin SV-40) (Eberle et al., 2010) cells. While similarly well suited for HTS, the choice of cell line is most critically influenced by the presence of the appropriate intracellular signaling pathways, which is largely determined by the species and tissue of origin.
Neuropharmacological HTS studies utilizing immortalized cell lines are faced with cellular properties being very different from those of neurons (Eglen et al., 2008). Immortalized cells typically lack regenerative currents making them non- or only moderately excitable (Thomas and Smart, 2005; Eglen et al., 2008), even when of neuronal origin. For instance, the resting potential of HEK cells is approximately −40 mV, compared to around −70 mV for neurons (Thomas and Smart, 2005), rendering them unsuitable for studies involving most voltage-activated ion channels. Recently, this particular challenge could be overcome by co-expression of inward-rectifier channels (Ashen et al., 1995; Thomas and Smart, 2005; Dai et al., 2008). However, this approach is only useful for single-target pharmacological studies using over-expression of target proteins.
Furthermore, there is a need to screen novel drug candidates in cells that resemble neurons in vivo more closely, in order to gain insight into the involvement of different signaling pathways, compensatory effects, etc., requiring an alternative to protein overexpressing cell lines (Eglen et al., 2008; Kaja et al., 2011).
Primary cultured neuronal cells, such as cortical neurons, have long been used for basic research and low-throughput screening and assay development (Richards et al., 2006; Melli and Hoke, 2009). A neuronal environment more close resembling physiological conditions together with well-established protocols for dissociation and in vitro maintenance make primary neuronal cultures suitable candidates for the development of HTS screening approaches.
Insufficient signal-to-noise ratios, prohibitive cost and, most importantly, experimental variability have often been named as reasons for the continued use of cell lines (Johnston, 2002; Eglen et al., 2008). Yet, there is no report methodically assessing the suitability of primary neuronal cells for HTS assays.
We here tested primary cortical neuronal cells in high-throughput assays for neuropharmacology and neuroprotection, including calcium mobilization, cytotoxicity and viability as well as ion channel pharmacology.
2. Material and Methods
2.1. Primary neuronal culture
Sprague/Dawley rat E18 cortical neurons were obtained from Genlantis (San Diego, CA), dissociated and maintained essentially per the supplier’s recommendations, with the following modifications. Briefly, cortical tissue was centrifuged at 200 × g for one minute, the shipping media aspirated, and enzymatically dissociated with 2 ml NeuroPapain Enzyme Solution (2 mg/ml; Genlantis, San Diego, CA) for 30 min at 37 °C. Cells were centrifuged at 200 × g for 1 min, and re-suspended in 1 ml of the original shipping media. Cells were then gently triturated 10 times with a fire-polished glass Pasteur pipet and centrifuged at 200 × g for 1 min. Cells were then re-suspended in Neurobasal A medium supplemented with B27 serum-free supplement (all from Invitrogen, Carlsbad, CA; Brewer et al., 1993) by gently triturating 10 times with a fire-polished glass Pasteur pipet. Cells were counted in an automated cell counter (Nexcelom Auto T4; Nexcelom Bioscience LLC., Lawrence, MA) using 0.2% Trypan Blue as indicator for cell viability and seeded on poly-d-lysine/laminin-coated 96-well plates (BD PureCoat™, BD Biosciences, Bedford, MA) at a density of 25,000 cells per well (unless indicated otherwise) in a 100 µL volume. Primary neuronal cultures were maintained at 37°C, 5 %CO2/95% humidity for 5 days prior to experiments.
We found that using commercially available, poly-d-lysine/laminin-coated 96-well plates had significantly lower variability in the homogeneity of neuronal cultures, as assessed by microscopical analysis.
Cultures were pure neuronal cultures, essentially free of glia as assessed by fluorescent immunocytochemistry using glial fibrillary acidic protein and neuron-specific enolase as markers for neurons and glia, respectively (data not shown; Brewer et al., 1993; Brewer, 1995).
Cells were visually inspected using a Leica DM-IL microscope with fluorescence (North Central Instruments, Plymouth, MN), and images acquired with a QImaging color camera (QImaging, Surrey, BC).
2.2. Cell viability assays
Fluorimetric calcein-AM and colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) viability assays were conducted essentially as described by us previously (Kaja et al., 2011). Briefly, for the calcein-AM assay, media was aspirated and replaced with pre-warmed Hank’s balanced salt solution (HBSS, Lonza, Walkersville, MD) supplemented with 2 mM CaCl2 and calcein-AM dye (Axxora, San Diego, CA) at a final concentration of 4 µM for 20 min. Calcein fluorescence was then quantified using a fluorimetric plate reader (FlexStation3, Molecular Devices, Sunnyvale, CA).
The MTT assay was conducted as described previously (Kaja et al., 2011) and according to manufacturer’s instructions (Invitrogen/Molecular Probes, Eugene, OR) and was measured at an absorbance wavelength of 560 nm using a FlexStation3 plate reader (Molecular Devices, Sunnyvale, CA).
For oxidative stress and neuroprotection insults, cells were treated overnight (20 hr) with media supplemented with tert-butyl hydroperoxide (tBHP; 10–40 µM; see also Kaja et al., 2011) or 3-nitroproprionic acid (3-NP; 1 mM) or the appropriate vehicle (PBS and 1% ethanol, respectively). (±)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox™, Sigma Aldrich, St. Louis, MO) is a prototypic antioxidant and vitamin E derivative, and was applied 30 min prior to the 3-NP insult at a concentration of 1 mM.
2.3. Ca2+ mobilization assays
The FLIPR Calcium5 Assay kit (Molecular Devices, Sunnyvale, CA) for 96-well plates essentially was used according to the manufacturer’s recommendations. Briefly, media was aspirated and neurons were loaded with Calcium5 at 100 µl per well for 1 hr at 37°C, 5% CO2/95% humidity. Data was collected with a FlexStation3 plate reader (Molecular Devices, Sunnyvale, CA) at 485/525nm excitation/emission with 515nm emission cutoff, at 37°C. 100 mM KCl was used as stimulus. The following settings were optimized for neuronal cultures: Fluorescence bottom-read mode; PMT sensitivity: high; readings: 6; compound transfer: 25 µL at 32 µL/sec; pipette height: 80 µL. For calcium channel pharmacology, neurons were pre-treated for 30 min with 10 µM nifedipine. Data was background subtracted, normalized to baseline, and peak values used to determine the maximal response.
2.4. Data analysis and statistics
For every experimental condition, four replicate wells were measured, and the mean value was used for statistical analyses. At least three separate experiments (n=3) obtained from different cultures of primary cultured neurons were performed. Data was analyzed and plotted using Prism 5.0 software (GraphPad Software Inc., La Jolla, CA). Statistical significance was assessed using a Student’s t-test.
3. Results
3.1. Primary neuronal cultures maintained in 96-well plates are amenable to cell viability and neuroprotection assays
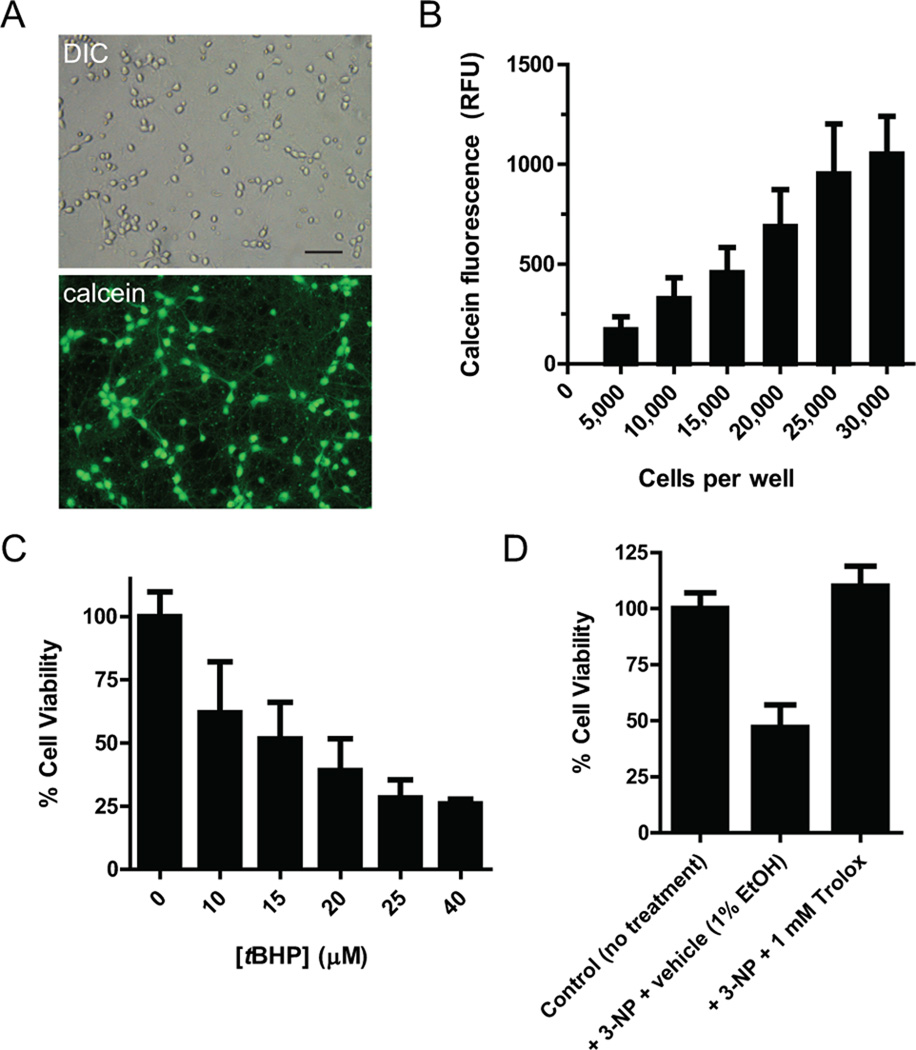
Using differential interference contrast (DIC) and fluorescence microscopy, we investigated primary cortical neurons grown on poly-d-lysine pre-coated 96-well plates. Neurons differentiated and formed synaptic connections and dendrites (Fig. 1A). Calcein fluorescence as measured in an automated multimode plate-reader correlated linearly with the number of cells seeded per well up to 25,000 cells per well and when tested at 5 days in vitro (Fig. 1B). Fluorescence, measured in relative fluorescence units (RFU) and corrected for background, ranged from 200 to 1,000 for seeding densities between 5,000 and 30,000 cells per well. We determined the optimal seeding density for primary cultured cortical neurons at 25,000 cells per well. This density yielded the maximal signal, providing a larger experimental window, while higher densities favored the formation of neurospheres, as assessed by microscopical analysis, and resulting in larger experimental variability.
Figure 1. Cell viability and neuroprotection assays using primary cultured cortical neurons in 96 well-plate assays.
(A) Representative images of primary cortical culture at 5 days in vitro. Cells differentiation was obvious by significant connections and dendrite formation. Calcein fluorescence indicates cell viability and activity of endogenous esterases. Scale bar: 10 µM. (B) Calcein fluorescence correlates with plating density of cells per well, at 5 days in vitro. Data is shown as mean ± s.e.m. (n=3). (C) tBHP treatment reduces cell viability, which was confirmed by MTT assay. Data is shown as mean ± s.e.m. (n=3). (D) Representative neuroprotection experiment utilizing 3-NP as insult and 1 mM Trolox™ as neuroprotectant. Trolox™ pre-treatment prevents 3-NP-induced loss in cell viability. Data is shown as mean ± s.d.
In addition, we measured calcein-AM uptake and fluorescence in HEK-293 cells. Calcein fluorescence increased linearly with seeding density, and signal intensity and experimental variability were similar in HEK-293 cells compared to primary cultured neurons when taking into account cell doubling time (Supplemental Figure 1).
We next tested, whether MTT assays, a standard assay of cell viability for cell lines, can reliably be employed for primary neuronal cultures in a 96 well-plate format. We chose tBHP as insult to induce oxidative stress-induced toxicity and cell death, as recently used by us for a neuronal cell line (Kaja et al., 2011). 20 hr incubation with tBHP resulted in a dose-dependent reduction of MTT absorbance indicative of diminishing levels of cell viability (Fig. 1C). Cell loss was confirmed by visual analysis using DIC microscopy (data not shown). Similarly, tBHP treatment reduced viability of HEK-293 cells in a dose-dependent manner (Supplemental Figure 2). Using 3-NP as insult, we also tested, whether the MTT assay could be used for neuroprotection studies in primary cortical culture in a high-throughput approach. Treatment with 1 mM 3-NP resulted in a reduction of cell viability of 45% as assessed by MTT assay (Fig. 1D). This loss in cell viability could be completely prevented by co-application of 1 mM Trolox™, a prototypic anti-oxidant and Vitamin E-derivative, 1 hr prior to the insult (Fig. 1D).
3.2. Primary neuronal cultures are well-suited for HTS approaches and ion channel pharmacology
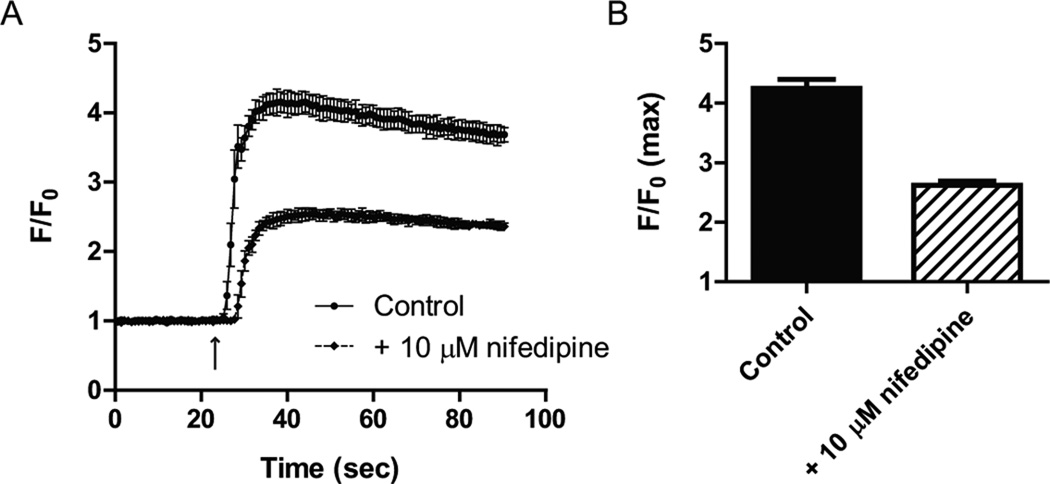
In order to assess the feasibility of cultured primary cortical neurons for HTS approaches, we tested the Ca2+ response to chemical depolarization, using the FLIPR Calcium5 kit. Maximum response as expressed normalized to baseline (F/F0) was 4.45 ± 0.39 (n=3; Fig. 2A), with a combined coefficient of variance of 0.046 for all three separate experiments (data not shown). The fluorescence response in our FlexStation3 plate reader averaged 33 ± 3, compared with 145 ± 17 for the maximal KCl-induced response (n=3; P<0.01; Fig. 2B).
Figure 2. Ca2+ mobilization assays in 96-well plate format using primary cultured cortical neurons.
(A) Depolarization of primary cultured neurons with 100 mM KCl (stimulus indicated by arrow) increases the intracellular Ca2+ concentration 4.45-fold. Data from three separate neuronal cultures is shown as mean ± s.e.m. and as ratio (F/F0) of the relative fluorescence (F) and normalized background fluorescence (F0). (B) Absolute RFU values obtained for the experiment shown in (A) were 33 ± 3 for background and 145 ± 17 for the maximal response (n=3, P<0.01). Data is shown as mean ± s.e.m. ** P<0.01. (C) Representative experiment showing the effect of the Ca2+ channel blocker nifedipine on the intracellular Ca2+ concentration, to highlight experimental variability. Data is shown as mean ± s.d. The arrow indicated KCl stimulus. (D) Nifedipine reduces the maximal response (F/F0 max) by 38%. Data is shown as mean ± s.d.
Pharmacology of (voltage-gated) ion-channel is a frequently tested parameter in HTS drug discovery and development. Using nifedipine, we here tested the contribution of dihydropyridine receptor (CaV1, L-type voltage-gated Ca2+ channels) to depolarization-induced Ca2+ mobilization. Pre-incubation with 10 µM nifedipine reduced Ca2+ response by 38% (Fig. 2C). The maximum response (F/F0 max) was 2.6 ± 0.1 in the presence of nifedipine, compared with 4.2 ± 0.1 for vehicle control (Fig. 2D).
4. Discussion
Drug discovery and development HTS approaches and neuroprotection assays rely largely on the use of cell lines, serving as vehicles for overexpressing proteins of interest for single-target pharmacology. Despite an urgent need for assay systems more closely resembling a neuronal environment, the suitability of primary neuronal cultures for HTS and plate-based assays has not been assessed. We here show feasibility, reproducibility and low experimental variability of primary neuronal cultures in cell viability/toxicity assays, neuroprotection assays, Ca2+ mobilization and ion channel pharmacology using plate-based, high throughput assay systems.
We modified existing standard protocols for cell lines to make them amenable to the use with primary cultured neurons. In contrast to cell line-based assays, primary neurons do not propagate or divide in culture, thereby eliminating one level of variability. However, we found that seeding density of primary cortical neurons is critical, as low seeding densities invariably result in lower signal-to-noise ratios that increase experimental variability, whereas too high seeding densities favor the formation of neurospheres after several days in culture. By utilizing commercially available, poly-d-lysine pre-coated 96-well plates, we could reduce inter-experimental variability even further (data not shown).
We used two experimental approaches for investigating the feasibility of primary neuronal cultures for high-throughput, 96-well plate-based cell viability and toxicity studies: calcein-AM is a as fluorescent indicator dye, uptake of which relies on endogenous esterases that cleave the acetoxymethyl ester group. We found a linear relationship between seeding density and calcein fluorescence. Furthermore, our data show low variability and high reproducibility between experiments and cultures. The presence of endogenous esterase activity is also an important marker for neuronal health, indicating that primary cultured cortical neurons develop and differentiate well when seeded on 96-well plates. MTT assays are frequently employed for neuroprotection studies (e.g. (Kaja et al., 2011). We here chose tBHP to induce oxidative stress-mediated loss is in cell viability and observed a dose-dependent decline in cell viability. Sensitivity of primary cortical neurons to tBHP was more pronounced than that for a cell line of neuronal origin (Kaja et al., 2011), with 10 – 25 µM tBHP providing a suitable window for neuroprotection studies. Our proof-of-concept data utilizing 3-NP as insult (Almeida et al., 2006) shows complete protection by the prototypic antioxidant Trolox™, and provides evidence that cultured primary cortical neurons are amenable to neuroprotection studies using insults and paradigms originally established for cell lines.
Calcium mobilization assays on automated platforms, such as the FLIPR™ system, are important components of today’s HTS approaches for drug discovery, but also provide critical information about cell viability and cell signaling pathways. Utilizing the FLIPR Calcium5 kit, we here show low experimental variability and high reproducibility of depolarization-induced responses in cultured primary cortical neurons, when utilizing our optimized settings outlined in the Material and Methods section. The Ca2+ response obtained from our HTS approach were similar to those obtained from manual, low-throughput experiments employing Ca2+ imaging (Martin-Montanez et al., 2010). Similarly, the contribution of CaV1 channels as measured by the effect of the dihydropyridine nifedipine was similar to those observed in Ca2+ imaging experiments (Martin-Montanez et al., 2010). Our studies using primary cortical neurons showed experimental variability that is comparable with that of cell lines. Specifically, inter-experimental variability was similar to that of HEK-cells in cell viability assays (Supplemental Figure 1) and that of a neuronal cell line (HT-22 cells), which is widely used for neuroprotection studies (Aoun et al., 2003; Duncan et al., 2009; Kaja et al., 2011). Primary neuronal cultures obtained from embryonic tissue (harvested at E18) are significantly more homogeneous than cultures obtained from tissue harvested postnatally and express a highly similar complement of signaling receptors as a result of their in vitro differentiation under controlled conditions; this makes E18 primary cortical neurons the preferred model system for in vitro analyses and explains the low experimental variability obtained in Ca2+ mobilization assays (Dichter, 1978; Brewer, 1995; Chen and Herrup, 2008).
Taken together, we here show the feasibility of primary neuronal cultures for both high-throughput neuroprotection and cell viability studies and HTS approaches utilizing calcium mobilization. Primary cortical neuronal cultures show low inter-experimental variability and similar reproducibility as conventional cell line assays. While cell line assays are likely to remain the primary screen for most drug discovery assays, we here show for the first time that primary neuronal culture HTS assays provide a viable alternative to cell-lines based systems when a cellular environment more closely resembling physiological conditions in the central nervous system is required.
Highlights.
-
⇨
Primary neuronal cultures are suitable for high-throughput screening (HTS) approaches.
-
⇨
We developed protocols for primary neuronal cultures in HTS assays for neuroprotection.
-
⇨
We optimized protocols for primary neuronal cultures in HTS Ca2+ mobilization assays.
-
⇨
Neuronal cultures yield low inter-experimental variability and high reproducibility.
Supplementary Material
Acknowledgements
This study was supported in part by the National Headache Foundation (S.K.), and grants EY014227 from NIH/NEI, RR022570, RR027093 from NIH/NCRR and AG010485, AG022550 and AG027956 from NIH/NIA, the Felix and Carmen Sabates Missouri Endowed Chair in Vision Research (P.K.), and the Vision Research Foundation of Kansas City. We thank Margaret, Richard and Sara Koulen for generous support and encouragement.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almeida S, Brett AC, Gois IN, Oliveira CR, Rego AC. Caspase-dependent and - independent cell death induced by 3-nitropropionic acid in rat cortical neurons. J Cell Biochem. 2006;98:93–101. doi: 10.1002/jcb.20748. [DOI] [PubMed] [Google Scholar]
- Aoun P, Watson DG, Simpkins JW. Neuroprotective effects of PPARgamma agonists against oxidative insults in HT-22 cells. Eur J Pharmacol. 2003;472:65–71. doi: 10.1016/s0014-2999(03)01867-3. [DOI] [PubMed] [Google Scholar]
- Ashen MD, O'Rourke B, Kluge KA, Johns DC, Tomaselli GF. Inward rectifier K+ channel from human heart and brain: cloning and stable expression in a human cell line. Am J Physiol. 1995;268:H506–H511. doi: 10.1152/ajpheart.1995.268.1.H506. [DOI] [PubMed] [Google Scholar]
- Brewer GJ. Serum-free B27/neurobasal medium supports differentiated growth of neurons from the striatum, substantia nigra, septum, cerebral cortex, cerebellum, and dentate gyrus. J Neurosci Res. 1995;42:674–683. doi: 10.1002/jnr.490420510. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- Chen J, Herrup K. Selective vulnerability of neurons in primary cultures and in neurodegenerative diseases. Rev Neurosci. 2008;19:317–326. doi: 10.1515/revneuro.2008.19.4-5.317. [DOI] [PubMed] [Google Scholar]
- Dai G, Haedo RJ, Warren VA, Ratliff KS, Bugianesi RM, Rush A, Williams ME, Herrington J, Smith MM, McManus OB, Swensen AM. A high-throughput assay for evaluating state dependence and subtype selectivity of Cav2 calcium channel inhibitors. Assay Drug Dev Technol. 2008;6:195–212. doi: 10.1089/adt.2008.136. [DOI] [PubMed] [Google Scholar]
- Dichter MA. Rat cortical neurons in cell culture: culture methods, cell morphology, electrophysiology, and synapse formation. Brain Res. 1978;149:279–293. doi: 10.1016/0006-8993(78)90476-6. [DOI] [PubMed] [Google Scholar]
- Duncan RS, Chapman KD, Koulen P. The neuroprotective properties of palmitoylethanolamine against oxidative stress in a neuronal cell line. Mol Neurodegener. 2009;4:50. doi: 10.1186/1750-1326-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle AN, Mild G, Zumsteg U. Cellular models for the study of the pharmacology and signaling of melanin-concentrating hormone receptors. J Recept Signal Transduct Res. 2010;30:385–402. doi: 10.3109/10799893.2010.524223. [DOI] [PubMed] [Google Scholar]
- Eglen RM, Gilchrist A, Reisine T. The use of immortalized cell lines in GPCR screening: the good, bad and ugly. Comb Chem High Throughput Screen. 2008;11:560–565. doi: 10.2174/138620708785204144. [DOI] [PubMed] [Google Scholar]
- Horrocks C, Halse R, Suzuki R, Shepherd PR. Human cell systems for drug discovery. Curr Opin Drug Discov Devel. 2003;6:570–575. [PubMed] [Google Scholar]
- Johnston P. Cellular assays in HTS. Methods Mol Biol. 2002;190:107–116. doi: 10.1385/1-59259-180-9:107. [DOI] [PubMed] [Google Scholar]
- Kaja S, Duncan RS, Longoria S, Hilgenberg JD, Payne AJ, Desai NM, Parikh RA, Burroughs SL, Gregg EV, Goad DL, Koulen P. Novel mechanism of increased Ca(2+) release following oxidative stress in neuronal cells involves type 2 inositol-1,4,5-trisphosphate receptors. Neuroscience. 2011;175:281–291. doi: 10.1016/j.neuroscience.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Montanez E, Acevedo MJ, Lopez-Tellez JF, Duncan RS, Mateos AG, Pavia J, Koulen P, Khan ZU. Regulator of G-protein signaling 14 protein modulates Ca(2)+ influx through Cav1 channels. Neuroreport. 2010;21:1034–1039. doi: 10.1097/WNR.0b013e32833f7b7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melli G, Hoke A. Dorsal Root Ganglia Sensory Neuronal Cultures: a tool for drug discovery for peripheral neuropathies. Expert Opin Drug Discov. 2009;4:1035–1045. doi: 10.1517/17460440903266829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omasa T, Onitsuka M, Kim WD. Cell engineering and cultivation of chinese hamster ovary (CHO) cells. Curr Pharm Biotechnol. 2010;11:233–240. doi: 10.2174/138920110791111960. [DOI] [PubMed] [Google Scholar]
- Pereira DA, Williams JA. Origin and evolution of high throughput screening. Br J Pharmacol. 2007;152:53–61. doi: 10.1038/sj.bjp.0707373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards GR, Jack AD, Platts A, Simpson PB. Measurement and analysis of calcium signaling in heterogeneous cell cultures. Methods Enzymol. 2006;414:335–347. doi: 10.1016/S0076-6879(06)14019-7. [DOI] [PubMed] [Google Scholar]
- Thomas P, Smart TG. HEK293 cell line: a vehicle for the expression of recombinant proteins. J.Pharmacol.Toxicol.Methods. 2005;51:187–200. doi: 10.1016/j.vascn.2004.08.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.