Abstract
The enterococcal surface protein Esp, specifically linked to nosocomial Enterococcus faecium, is involved in biofilm formation. To assess the role of Esp in endocarditis, a biofilm-associated infection, an Esp-expressing E. faecium strain (E1162) or its Esp-deficient mutant (E1162Δesp) were inoculated through a catheter into the left ventricle of rats. After 24 hours, less E1162Δesp than E1162 were recovered from heart valve vegetations. In addition, anti-Esp antibodies were detected in Esp-positive E. faecium bacteremia and endocarditis patient sera. In conclusion, Esp contributes to colonization of E. faecium at the heart valves. Furthermore, systemic infection elicits an Esp-specific antibody response in humans.
Keywords: Enterococcus faecium, Enterococcal surface protein Esp, endocarditis, pathogenesis, immunity
1. Introduction
Enterococci have globally emerged as important multi-antibiotic resistant nosocomial pathogens [1]. They can cause a wide spectrum of diseases, including bacteremia, endocarditis, surgical wound, urinary tract, and device-related infections [2–5]. Although Enterococcus faecalis infections are more common, infections with Enterococcus faecium, which are more difficult to treat due to the accumulation of multiple antibiotic resistance traits, have increased in the last two decades. Molecular epidemiological studies have indicated that E. faecium clones that are responsible for the majority of nosocomial infections and hospital outbreaks are genetically distinct from indigenous intestinal isolates [1,6]. In addition to several antibiotic resistance genes, these hospital-acquired E. faecium clones have acquired potential virulence factors that may enhance their pathogenicity in hospitalized patients. Potential virulence genes specifically found in this subpopulation are esp (enterococcal surface protein) [7–9], sgrA (serine-glutamate repeat containing protein A), and ecbA (E. faecium collagen binding protein A) encoding surface-exposed proteins [10], hylefm encoding a potential hyaluronidase [11], and genes required for the biogenesis of pili [12,13]. Other genetic determinants, not specifically linked to this subpopulation, but also implicated in virulence of E. faecium are acm (adhesin of collagen of E. faecium) [14–16] and scm (second collagen adhesin of E. faecium) [17], both encoding surface-exposed proteins. Although acm is ubiquitously found among E. faecium, its expression is specifically linked to just mentioned subpopulation [15]. The exact role of these putative virulence factors is unknown, but some are known to be involved in adherence to extracellular matrix molecules, human cells, and biofilm formation. Adherence to (a)biotic surfaces and subsequent biofilm formation are key processes for colonization and initiation of infection [18]. Enterococci have been associated with biofilms in a variety of infections, including urinary tract infections, device-related infections, and endocarditis [18,19].
Endocarditis, an infection of the heart valves or inner lining, is assumed to be a biofilm-associated infection. It is difficult to treat, especially when caused by multi-resistant E. faecium and, as a result, is associated with high morbidity and mortality [5,20]. Bacteria may adhere to the heart valves at sites of injury and subsequently form biofilms leading to heart valve vegetations and valvular destruction [18]. Little is known about the pathogenesis of E. faecium endocarditis. Recent data indicate that the collagen adhesin Acm [14,15] is involved in the primary adherence of E. faecium to the collagen-rich heart valves [16]. In E. faecalis, the ebp operon, encoding endocarditis and biofilm associated pili, is involved in biofilm formation and contributes to the pathogenesis of endocarditis [21]. Although the role of the enterococcal surface protein (Esp) [22,23] has not been demonstrated in E. faecalis endocarditis, endocarditis isolates of E. faecalis more frequently expressed Esp than fecal isolates [23], and the presence of the esp gene in endocarditis isolates was associated with higher amounts of biofilm [19]. In E. faecium, Esp is specifically enriched in hospital-acquired E. faecium isolates [8,9]. By constructing an Esp-deficient mutant, we demonstrated that, in E. faecium, Esp is involved in initial adherence and biofilm formation [7] and contributes to the pathogenesis of urinary tract infection [24] and bacteremia in mice [25]. To assess the role of E. faecium Esp in infective endocarditis, we compared the Esp-deficient mutant and its Esp-expressing parent strain for their ability to colonize the heart valves in an experimental endocarditis model in rats. In addition, we investigated whether Esp is expressed in vivo and immunogenic by determining the presence of antibodies against Esp in sera of patients with E. faecium bacteremia and endocarditis.
2. Materials and methods
2.1. Bacterial strains and growth conditions
E. faecium strain E1162 is a clinical blood isolate and positive for Esp expression [7]. The isogenic Esp-deficient mutant, E1162Δesp, was previously constructed by introduction of a chloramphenicol resistance cassette resulting in allelic replacement of the esp gene [7]. E. faecium strains were grown in brain heart infusion (BHI) broth or on BHI Agar (Difco Laboratories). No difference in growth rates was observed between E1162 and E1162Δesp [7].
2.2. Rats
Male Sprague-Dawley rats (~250 g) were purchased from Harlan Sprague-Dawley (Indianapolis, IN). The Animal Welfare Committee of the University of Texas Health Science Center at Houston approved all experiments.
2.3. Induction of endocarditis
Plate-grown bacteria were inoculated into BHI and grown at 37°C for 24 hrs, while shaking. Cells were washed and resuspended in saline and OD660 adjusted prior to inoculation. Surgical procedures were carried out under general anesthesia. Vegetations were produced in rats by inserting a polyethylene catheter (Intramedic PE10; Braintree Scientific) via the right carotid artery, across the aortic valve into the left ventricle. After twenty minutes, E1162 or E1162Δesp660 (OD adjusted with the aim to infect with 1×107 CFU) were injected intraventricularly through the catheter in 16 and 11 rats, respectively. Subsequently, the catheter was heat sealed, imbedded in subcutaneous tissue, and the skin was closed. A single inoculum per strain was prepared for rats infected at the same day. After the injection this inoculum was plated to determine viable counts. Actual CFU of E1162 was 0.99×107 (10 rats) and 1.87×107 (6 rats). Actual CFU of E1162Δesp was 1.59×107 (2 rats), 2.16×107 (6 rats), and 1.21×107 (3 rats). Rats were sacrificed 24 hrs after inoculation. Aortic vegetations were excised, weighed, and homogenized in 1 ml saline. Serial dilutions were made and 50 μl of each dilution was plated onto BHI plates and incubated at 37°C for 24 hrs. Colonies were counted and corrected for the dilution factor to calculate CFU per gram of vegetation. In a second experiment, a different inoculum was used (OD660 adjusted with the aim to infect with 5×106 CFU). Twenty rats were inoculated with E1162 and 20 rats with E1162Δesp. Actual CFU of E1162 was 5.6×106 (8 rats), 3.0×106 (3 rats), 3.55×106 (7 rats), and 2.35×106 (2 rats). Actual CFU of E1162Δesp was 3.0×106 (4 rats), 4.45×106 (6 rats), 6.2×106 (3 rats), 9.5×106 (4 rats), and 7.5×106 (3 rats). In a third experiment, rats were sacrificed 3 hrs after inoculation (OD660 adjusted with the aim to infect with 1×107 CFU). Six rats were inoculated with 2.4×107 CFU of E1162 and 6 rats with 4.2×107 CFU of E1162Δesp.
2.4. PFGE
A total of 15 colonies obtained from vegetations were randomly picked and pulsed-field gel electrophoresis (PFGE) was performed to confirm strain identity. PFGE analysis was performed with agarose plugs containing genomic DNA, which was digested with SmaI and separated by electrophoresis using a clamped homogeneous electric field (CHEF-DRII, Bio-Rad Laboratories), with ramped pulse times beginning with 2 s and ending with 50 s, at 200 V for 23 hrs.
2.5. Bacteremia and endocarditis sera
Serum was obtained from three patients with endocarditis and from five patients with bacteremia caused by esp-positive E. faecium strains. In addition, serum was obtained from one patient with endocarditis caused by an esp-negative E. faecium isolate and from healthy volunteers. Six sera from volunteers were pooled before testing. Whole blood was allowed to clot at room temperature, centrifuged at 4°C, and sera were stored in aliquots at 70°C. The sera were investigated for the presence of IgM and IgG antibodies against Esp.
2.6. Cloning N-terminal Esp
A 2545-bp-long DNA fragment of the N-terminal domain of esp was amplified from genomic DNA of TX2465 by using a Platinum® Pfx DNA polymerase (Invitrogen) with the primers EspExpF2 (5′-CGCAGATCTCAGGTCGATCCAAAGAAAGGAATTG) and EspExpR1 (5′-CGGGGTACCTTAAGTTACTGCTAAATCGGTCGTGC) (introduced restriction sites are underlined). The PCR product, digested with BglII and KpnI, was cloned into the pQE30 expression vector (Invitrogen), digested with BamHI and KpnI, followed by electroporation into E. coli DH5α cells (Invitrogen). Electroporation was carried out using a Bio-Rad gene pulser. Plasmid DNA was purified by using the QIAprep Spin Miniprep Kit (QIAGEN) and electroporated into E. coli host M15(pREP4) cells (QIAGEN). One of the colonies was verified by sequencing. Recombinant N-terminal Esp (rN-Esp) was overexpressed and purified as described elsewhere [14].
2.7. Western blotting
For western blotting, 100 ng rN-Esp in electrophoresis sample buffer (100 mM Tris-HCl, 5% dithiothreitol, 2% SDS, 0.004% bromophenol blue, and 20% glycerol) was boiled for 5 min and electrophoresed through a 10% SDS-polyacrylamide gel and transferred to nitrocellulose using a Bio-Rad Trans-Blot Cell tank transfer unit at 12 V overnight in 20 mM Tris, 0.15 M glycine, and 20% methanol at pH 8.3. Non-specific sites in the blot were blocked by incubation for 1 h at room temperature with 5% skim milk powder in PBS with 0.05% Tween 20. Binding of antibodies to rN-Esp was assayed by overnight incubation at 4°C with bacteremia or endocarditis patient sera (1:500 dilution) as primary antibody, followed by incubation for 1 h at room temperature with horseradish peroxidase-conjugated goat anti-human IgG or goat anti-human IgM (both 1:5000 dilution; Santa Cruz Biotechnology) as secondary antibody. Both primary and secondary antibodies were diluted in PBS with 1% skim milk powder and 0.05% Tween 20. rN-Esp was detected by using light-emitting ECL™ Western Blotting Detection Reagents (GE Healthcare).
2.8. Statistical analysis
The geometric means of CFU of E1162 and E1162Δesp per ml inoculum or per gram vegetation were calculated. Differences between groups were analyzed by Mann-Whitney U test using GraphPad Prism version 4.0, GraphPad Software (San Diego, CA). A P-value < 0.05 was considered statistically significant.
3. Results
3.1. Experimental endocarditis
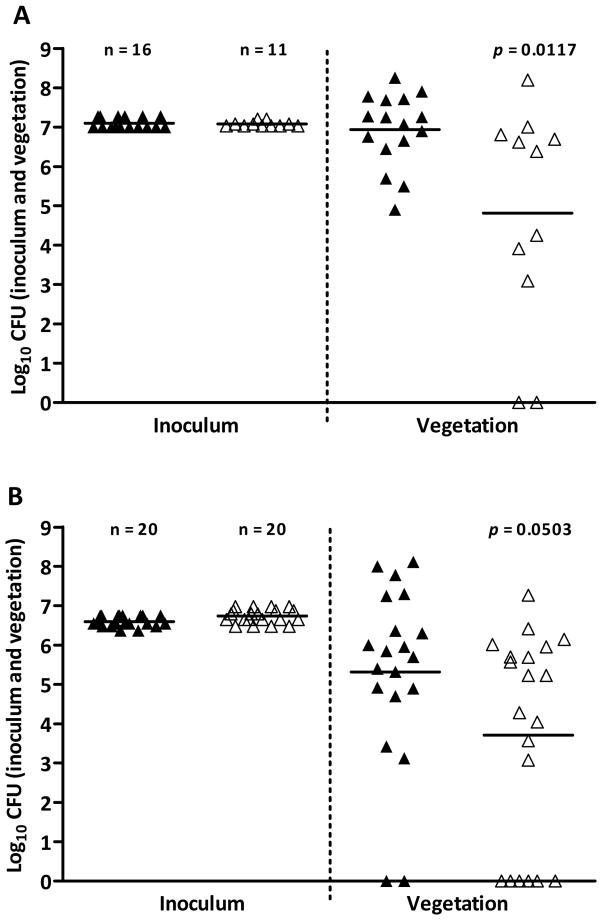
To investigate the role of E. faecium Esp in infective endocarditis, the Esp-expressing strain (E1162) and its isogenic Esp-deficient mutant (E1162Δesp) were compared for their ability to colonize the heart valves in an experimental rat endocarditis model. Our previous data demonstrate that Esp increased biofilm formation on polystyrene at 24 hours [7], influenced the initial course (at 24 hours) of urinary tract infection, and increased adherence to epithelial cells at one hour [24]. Based on these results, we chose to assess the role of Esp in a 24-hour and 3-hour endocarditis model. The E1162 or E1162Δesp were injected intraventiculary through a catheter in 16 and 11 rats, respectively. Vegetations recovered at autopsy 24 hours after inoculation (0.99–1.87×107 CFU of E1162 and 1.08–1.59×107 CFU of E1162Δesp) contained fewer E1162 Δesp CFU (8×104 CFU/g) than E1162 (9×106 CFU/g) (P=0.0117) (Fig. 1A). This was confirmed in a second experiment with inoculations of 2.35–5.6×106 CFU of E1162 in 20 rats and 3–9.5×106 CFU of E1162Δesp in 20 rats, in which vegetations again contained less E1162 Δesp (7×103 CFU/g) than E1162 (2×105 CFU/g) (P=0.0503) (Fig. 1B), demonstrating that Esp contributes to infective endocarditis in rats. Subsequently, the role of Esp in a 3-hour endocarditis model was tested. An additional 12 rats were inoculated with 2.4×107 CFU of E1162 (6 rats) or 4.2×107 CFU of E1162Δesp and CFU counts of viable bacteria were determined three hours after infection. Vegetations from six rats, three inoculated with E1162 and three inoculated with E1162Δesp, contained comparable amounts of bacteria (5×102, 1.56×103, or 2.67×104 CFU/g of E1162 and 8.88×102, 1.5×103, or 1.77×103 CFU/g of E1162Δesp). From six rats, three inoculated with E1162 and three with E1162Δesp, no bacteria were recovered from vegetations. PFGE analysis confirmed correct strain identity. Previously, we described a strain in which we complemented the esp mutation [7]. Unfortunately, the complementation strain could not be used in the endocarditis model because the plasmid appeared to be highly unstable.
Fig. 1.
Recovery of E1162 wild-type and its Esp-deficient mutant from heart valve vegetations in experimental endocarditis in rats. Panel A and B depict the number of wild-type E1162 (closed triangles) and Esp-deficient mutant E1162Δesp (open triangles) recovered from inocula (CFU/ml) and from vegetations (CFU/gram) 24 hours after infection. Horizontal lines represent geometric means.
3.2. Determination of antibodies against Esp in human patient sera
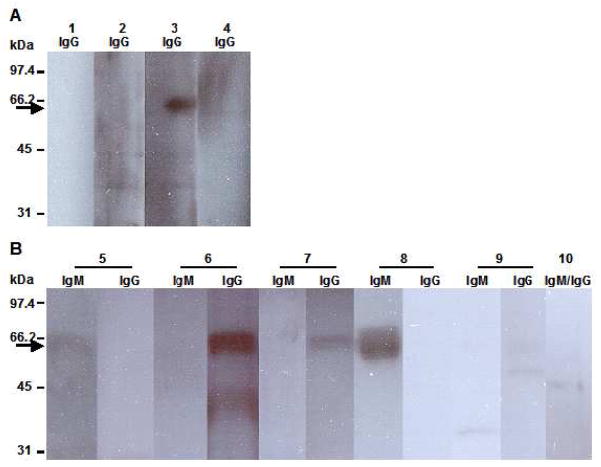
To investigate whether Esp is expressed in vivo and is immunogenic, the presence of antibodies against Esp was assessed in endocarditis and bacteremia sera from patients infected with esp-positive E. faecium isolates by Western blotting. Of four endocarditis sera, strong reactivity (lane 3) was observed in one (Fig. 2A). There was no reactivity in the endocarditis serum from a patient infected with esp-negative E. faecium isolate (lane 1). Of five bacteremia sera, strong reactivity was observed in two sera (lane 6 and 8), moderate and weak reactivity were observed in one sera (lane 7 and 5, respectively) (Fig. 2B). No reactivity could be demonstrated in pooled sera from healthy volunteers (lane 10).
Fig. 2.
Determination of antibodies against Esp in human patient sera. Panel A and B depict Western blots of recombinant Esp protein (molecular mass of 65 kDa) probed with four endocarditis (lane 1–4) and five bacteremia patient sera (lane 5–9). Lanes 1 and 10 represent the negative controls, endocarditis sera from a patient infected with an esp-negative E. faecium isolate and pooled sera of healthy human volunteers, respectively. Numbers depicted on the left indicate molecular masses in kDa; arrow indicates the predicted position of the recombinant Esp protein; 100 ng of Esp was used for each lane.
4. Discussion
Infections caused by multi-resistant E. faecium have increased since the late 1980s. In most E. faecium infections, like urinary tract infections, device-related infections, and endocarditis, adherence to (a)biotic surfaces and subsequently biofilm formation is thought to be the first step in pathogenesis. Previous studies have demonstrated that Esp, specifically associated with hospital-acquired multi-resistant E. faecium [8,9], is involved in initial adherence and biofilm formation to abiotic material [7]. To investigate the contribution of Esp in the pathogenesis of endocarditis, thought to be biofilm-associated infection, we studied an Esp-deficient mutant and its Esp-expressing parent strain in a rat endocarditis model. When rats were challenged with either the Esp-deficient mutant or the wild-type strain, vegetations recovered after 24 hours contained significantly fewer Esp-deficient mutants than wild-types indicating that Esp is important in this endovascular infection. Although the Esp-deficient mutant was significantly attenuated, Esp-deficient mutants were nonetheless recovered from vegetations demonstrating that the mutant strain was able to colonize the heart valves of these rats. This indicates that, besides Esp, other determinants are involved in adherence to and biofilm development on heart valves. For instance, Acm also contributes to the pathogenesis of experimental endocarditis caused by E. faecium. An Acm-deficient mutant was highly attenuated compared to the wild-type strain in both established vegetations (72 hours) and colonization of the heart valves (one and three hours) [16]. Other factors that could be implicated in this process are a set of potential adhesins described recently including novel surface proteins or pili [10,12,13,17]. It is interesting in this respect that PilA of E. faecium [12] exhibits high similarity with the EbpC pilin protein of E. faecalis, which is involved in biofilm formation and endocarditis [21].
Subsequent analysis of vegetations obtained at three hours post-inoculation with Esp-deficient mutants or wild-types demonstrated low level colonization or no (detectable) colonization of the heart valves.
In order to assess whether Esp is expressed in humans during a natural infection, we screened three endocarditis and five bacteremia sera from patients infected with esp-positive E. faecium for a serological response against Esp by Western blotting. In five of eight patient sera, we were able to detect anti-Esp antibodies indicating that Esp is expressed and recognized by the immune system yielding an anti-Esp response, at least in some patients. Explanations for absence of an anti-Esp response in three sera may be lack of Exp-expression, sampling of sera before an immune response was elicited, or that some individuals do not recognize certain epitopes and some do. Anti-Esp antibodies could not be detected in sera from a patient with endocarditis caused by an esp-negative E. faecium strain nor in pooled sera from healthy volunteers. These results indicate that reactivity is specific and probably not caused by cross-reacting antibodies. In conclusion, the identification of Esp as an immunogenic surface protein and its association with biofilm formation and endocarditis confirms the importance of Esp in the pathogenesis of infections caused by hospital-acquired E. faecium strains.
Further elucidating the role of Esp has improved our understanding how this emerging nosocomial pathogen initiates infections and interacts with its environment. Improved understanding may contribute to the development of novel intervention strategies, possibly targeting Esp, to prevent infections with and the spread of multi-resistant E. faecium in hospitals.
Acknowledgments
EH and RW were supported by the European Union Sixth Framework Programme “Approaches to Control multi-resistant Enterococci (ACE): Studies on molecular ecology, horizontal gene transfer, fitness and prevention” under contract LSHE-CT-2007-037410 and ZonMW “Vaccine-development to combat the emergence of vancomycin-resistant Enterococcus faecium” project number 0.6100.0008. BEM and KVS were supported in part by NIH grant R01 AI067861 to BEM from the Division of Microbiology and Infectious Diseases, NIAID.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leavis HL, Bonten MJ, Willems RJ. Identification of high-risk enterococcal clonal complexes: global dispersion and antibiotic resistance. Curr Opin Microbiol. 2006;9:454–460. doi: 10.1016/j.mib.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Hidron AL, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention. Infect Control Hosp Epidemiol. 2008;29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 3.Giannitsioti E, Skiadas I, Antoniadou A, Tsiodras S, Kanavos K, Triantafyllidi H, Giamarellou H. Nosocomial vs. community-acquired infective endocarditis in Greece: changing epidemiological profile and mortality risk. Clin Microbiol Infect. 2007;13:763–769. doi: 10.1111/j.1469-0691.2007.01746.x. [DOI] [PubMed] [Google Scholar]
- 4.McDonald JR, Olaison L, Anderson DJ, Hoen B, Miro JM, Eykyn S, brutyn E, Fowler VG, Habib G, Selton-Suty C, Pappas PA, Cabell CH, Corey GR, Marco F, Sexton DJ. Enterococcal endocarditis: 107 cases from the international collaboration on endocarditis merged database. Am J Med. 2005;118:759–766. doi: 10.1016/j.amjmed.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 5.Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001;345:1318–1330. doi: 10.1056/NEJMra010082. [DOI] [PubMed] [Google Scholar]
- 6.Willems RJ, Top J, van Santen-Verheuvel M, Robinson DA, Coque TM, Baquero F, Grundmann H, Bonten MJ. Global spread of vancomycin-resistant Enterococcus faecium from distinct nosocomial genetic complex. Emerg Infect Dis. 2005;11:821–828. doi: 10.3201/eid1106.041204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heikens E, Bonten MJ, Willems RJ. Enterococcal surface protein Esp is important for biofilm formation of Enterococcus faecium E1162. J Bacteriol. 2007;189:8233–8240. doi: 10.1128/JB.01205-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leavis H, Top J, Shankar N, Borgen K, Bonten M, van Embden J, Willems RJ. A novel putative enterococcal pathogenicity island linked to the esp virulence gene of Enterococcus faecium and associated with epidemicity. J Bacteriol. 2004;186:672–682. doi: 10.1128/JB.186.3.672-682.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willems RJ, Homan W, Top J, van Santen-Verheuvel M, Tribe D, Manzioros X, Gaillard C, Vandenbroucke-Grauls CM, Mascini EM, van Kregten E, van Embden JD, Bonten MJ. Variant esp gene as a marker of a distinct genetic lineage of vancomycin-resistant Enterococcus faecium spreading in hospitals. Lancet. 2001;357:853–855. doi: 10.1016/S0140-6736(00)04205-7. [DOI] [PubMed] [Google Scholar]
- 10.Hendrickx AP, van Luit-Asbroek M, Schapendonk CM, van Wamel WJ, Braat JC, Wijnands LM, Bonten MJ, Willems RJ. SgrA, a nidogen-binding LPXTG surface adhesin implicated in biofilm formation, and EcbA, a collagen binding MSCRAMM, are two novel adhesins of hospital-acquired Enterococcus faecium. Infect Immun. 2009;77:5097–5106. doi: 10.1128/IAI.00275-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rice LB, Carias L, Rudin S, Vael C, Goossens H, Konstabel CC, Klare I, Nallapareddy SR, Huang W, Murray BE. A potential virulence gene, hylEfm, predominates in Enterococcus faecium of clinical origin. J Infect Dis. 2003;187:508–512. doi: 10.1086/367711. [DOI] [PubMed] [Google Scholar]
- 12.Hendrickx AP, Bonten MJ, van Luit-Asbroek M, Schapendonk CM, Kragten AH, Willems RJ. Expression of two distinct types of pili by a hospital-acquired Enterococcus faecium isolate. Microbiology. 2008;154:3212–3223. doi: 10.1099/mic.0.2008/020891-0. [DOI] [PubMed] [Google Scholar]
- 13.Sillanpää J, Nallapareddy SR, Singh KV, Prakash VP, Fothergill T, Ton-That HH, Murray BE. Characterization of the ebpfm pilus-encoding operon of Enterococcus faecium and its role in biofilm formation and virulence in a murine model of urinary tract infection. Virulence. 2010;1:236–246. doi: 10.4161/viru.1.4.11966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nallapareddy SR, Weinstock GM, Murray BE. Clinical isolates of Enterococcus faecium exhibit strain-specific collagen binding mediated by Acm, a new member of the MSCRAMM family. Mol Microbiol. 2003;47:1733–1747. doi: 10.1046/j.1365-2958.2003.03417.x. [DOI] [PubMed] [Google Scholar]
- 15.Nallapareddy SR, Singh KV, Okhuysen PC, Murray BE. A functional collagen adhesin gene, acm, in clinical isolates of Enterococcus faecium correlates with the recent success of this emerging nosocomial pathogen. Infect Immun. 2008;76:4110–4119. doi: 10.1128/IAI.00375-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nallapareddy SR, Singh KV, Murray BE. Contribution of the collagen adhesin Acm to pathogenesis of Enterococcus faecium in experimental endocarditis. Infect Immun. 2008;76:4120–4128. doi: 10.1128/IAI.00376-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sillanpää J, Nallapareddy SR, Prakash VP, Qin XX, Hook M, Weinstock GM, Murray BE. Identification and phenotypic characterization of a second collagen adhesin, Scm, and genome-based identification and analysis of 13 other predicted MSCRAMMs, including four distinct pilus loci, in Enterococcus faecium. Microbiology. 2008;154:3199–3211. doi: 10.1099/mic.0.2008/017319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohamed JA, Huang W, Nallapareddy SR, Teng F, Murray BE. Influence of origin of isolates, especially endocarditis isolates, and various genes on biofilm formation by Enterococcus faecalis. Infect Immun. 2004;72:3658–3663. doi: 10.1128/IAI.72.6.3658-3663.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arias CA, Murray BE. Antibiotic-resistant bugs in the 21st century--a clinical super-challenge. N Engl J Med. 2009;360:439–443. doi: 10.1056/NEJMp0804651. [DOI] [PubMed] [Google Scholar]
- 21.Nallapareddy SR, Singh KV, Sillanpaa J, Garsin DA, Hook M, Erlandsen SL, Murray BE. Endocarditis and biofilm-associated pili of Enterococcus faecalis. J Clin Invest. 2006;116:2799–2807. doi: 10.1172/JCI29021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tendolkar PM, Baghdayan AS, Gilmore MS, Shankar N. Enterococcal surface protein, Esp, enhances biofilm formation by Enterococcus faecalis. Infect Immun. 2004;72:6032–6039. doi: 10.1128/IAI.72.10.6032-6039.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shankar V, Baghdayan AS, Huycke MM, Lindahl G, Gilmore MS. Infection-derived Enterococcus faecalis strains are enriched in esp, a gene encoding a novel surface protein. Infect Immun. 1999;67:193–200. doi: 10.1128/iai.67.1.193-200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leendertse M, Heikens E, Wijnands LM, van Luit-Asbroek M, Teske GJ, Roelofs JJ, Bonten MJ, van der Poll T, Willems RJ. Enterococcal surface protein transiently aggravates Enterococcus faecium-induced urinary tract infection in mice. J Infect Dis. 2009;200:1162–1165. doi: 10.1086/605609. [DOI] [PubMed] [Google Scholar]
- 25.Sava IG, Heikens E, Kropec AP, Theilacker C, Willems R, Huebner J. Enterococcal surface protein contributes to persistance in the host but is not a target of opsonic and protective antibodies in Enterococcus faecium infection. J Med Microbiol. 2010;59:1001–1004. doi: 10.1099/jmm.0.020578-0. [DOI] [PubMed] [Google Scholar]