Abstract
PURPOSE
To evaluate the relationship between “Look-Locker” (LL) and modified Look-Locker Inversion recovery (MOLLI) approaches for T1 mapping of the myocardium.
MATERIALS AND METHODS
168 myocardial T1 maps using MOLLI and 165 maps using LL were obtained in human subjects at 1.5 Tesla. The T1 values of the myocardium were calculated before and at five time points after gadolinium administration. All time and heart rate normalizations were done. The T1 values obtained were compared to determine the absolute and bias agreement.
RESULTS
The pre-contrast global T1 values were similar when measured by the LL and by MOLLI technique (mean 1004.9 ms +/- 120.3 vs. 1034.1 ms +/- 53.1, respectively, p = 0.26). Post-contrast myocardial T1 time from LL was significantly longer than MOLLI from 5 to 25 minutes (mean difference, LL - MOLLI was +61.8 +/- 46.4 ms, p < 0.001). No significant differences in T1 values were noted between long and short axis measurements for either MOLLI or LL.
CONCLUSION
Post-contrast LL and MOLLI showed very good agreement, although LL vaules are higher than MOLLI. Pre-contrast T1 values showed good agreement, however LL has greater limits of agreement. Short and long axis planes can reliably assess T1 values.
Keywords: Magnetic resonance imaging, diffuse myocardium fibrosis, T1 mapping
INTRODUCTION
Myocardial T1 determination using cardiac magnetic resonance imaging (CMR) is an emerging technique that may improve diagnostic accuracy and quantification of myocardial fibrosis. The quantitative assessment of myocardial T1 values (“T1 mapping”) has been used recently to identify patients with acute and chronic myocardial infarction(1,2), myocardial fibrosis in chronic aortic regurgitation(3), myocardial fibrosis in heart failure(4), diffuse fibrosis in dilated cardiomyopathy(5), and myocardial damage in hypertrophic cardiomyopathy(6). Thus far, most studies have been observational and no standardized approach is agreed upon for T1 determination in the myocardium after gadolinium administration. Standardization of T1 data would be helpful to determine any potential cut-off values to predict normal versus diseased myocardium. Understanding the limitations of various T1 mapping techniques as applied in cardiac imaging will help to ascertain true differences between patient groups resulting from disease.
The most common approaches to measure left ventricular (LV) T1 values with CMR include the Look-Locker (LL) sequence(6,7) (also known as “TI scout”). The Look-Locker sequence approach has been widely implemented by magnetic resonance scanner manufacturers over many years. As a consequence, an abundance of retrospective data is available for the LL sequence. A new sequence, termed modified Look-Locker (MOLLI) is currently investigational (8,9). The MOLLI sequence has the advantage of T1 determination at the same phase of the cardiac cycle, with potential for more accurate T1 measurement, and less heart rate dependence. Using either technique, average T1 values obtained from a single slice have been assumed to reflect diffuse myocardial fibrosis. However, differences in T1 values obtained either from different pulse sequences or from different regions of the myocardium have not been well investigated. Thus, the purpose of this study was to compare the T1 values obtained from different pulse sequences for myocardial T1 determination, and to determine the impact of slice position/ orientation on average T1 values.
MATERIALS AND METHODS
Subjects
The inclusion criteria for study subjects were: no history of cardiovascular or systemic disease, no history of infection within 4 weeks before the CMR exam, and no contraindication to magnetic resonance imaging (MRI) such as claustrophobia or presence of metallic implants. Subjects were excluded if they could not follow instructions for breath-holding. Height, weight, creatinine, glomerular filtration rate (GFR), heart rate, and contrast dose were recorded. The study was approved by the local ethics committee, and all subjects gave written informed consent.
CMR protocol
All the subjects were scanned on a 1.5 Tesla Avanto scanner (Siemens, Erlangen, Germany) with a phase array cardiac coil. A three-lead vector cardiogram was used for ECG gating. The procedure was performed with the patient in supine position, during expiratory apnea, and without chest or abdomen movements during the image acquisitions. All patients underwent the complete examination without complications.
Intravenous infusion of gadolinium contrast (gadopentetate dimeglumine; Bayer Healthcare Pharmaceuticals, New Jersey, USA) was performed (0.14-0.2 mmol/kg) at an injection rate of 2 ml/s, followed by a 20-30-ml saline flush. Standard four-chamber long-axis and midcavity short-axis section orientations were used for LL and MOLLI T1 mapping. The LL (6,7) and the MOLLI(1,8,9) pulse sequences were used as previously described. The LL single-slice T1 determinations were performed at six time points before and after gadolinium administration (pre contrast, 5, 10, 15, 20 and 25 minutes) using an inversion recovery True FISP LL sequence. The MOLLI single-slice T1 determinations were performed at six time points (pre contrast, 3.5, 8.5, 13.5, 18.5 and 23.5 minutes) using three inversion-recovery pulses obtained consecutively within one breath hold (17 heartbeats). All studies were completed by obtaining delayed enhancement images at the end of each study protocol to exclude positive cases of focal myocardial scar.
Scan parameters for the LL protocol were 260-400 mm FOV; 192×72 to 192×93 matrix size, 8 mm slice thickness; 2.2/1.1ms ≈ TR/TE; 23 to 25 ms phase interval; 50° flip angle; 8 turbofactor; 22 to 46 phases over two RR intervals (determined by heart rate). Scan parameters for the MOLLI protocol were 250 to 360 mm FOV; 192×122 to 192×183 matrix size; 6-8 mm slice thickness; 2.2/1.1ms ≈ TR/TE, 35° flip angle; GRAPPA factor = 2; 17 heart beats (which collects 3+3+5 samples) as previously described (9). The dependency of MOLLI T1 values on scan parameters have been discussed in more detail in reference(10).
CMR and Data Analysis
The analyses were done by two experienced observers (EBT and MSN) with 4 and 6 years of experience in CMR respectively. T1 measurements were performed twice by the same observer and the average value was used for analysis. The readers were blinded to each other's analysis.
LL image analysis
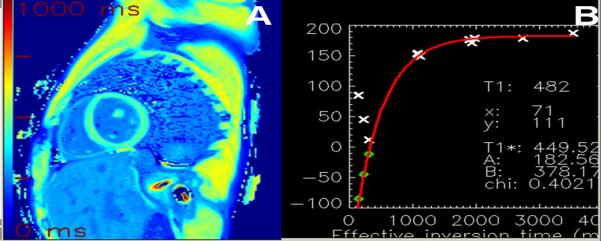
Images were processed off-line using MASS research software (MASS V2010-EXP, Leiden University Medical Center, Leiden, The Netherlands). Left ventricular endocardial and epicardial borders were traced on every fifth image and the contours were interpolated, semiautomatically, to all images for each sequence by a single reader. Pixel by pixel fit was performed to a three-parameter model (A–Bexp[–TI/T1*]) to obtain T1 (as T1=(B/A–1)T1*)(11). Only pixels where the χ2 test for goodness of fit was significant with level of significance α=0.05 were included in the final average T1 value. An example showing the myocardial wall contouring and mean T1 fit is shown in Figure 1.
Figure 1.
A) LL T1 fitting map after 10 minutes of contrast administration (average T1 time 479ms) and B) fitting curve using MASS software (MASS V2010-EXP, Leiden University Medical Center, Leiden, The Netherlands).
MOLLI image analysis
T1 maps from MOLLI image sets were calculated using MRmap(12), and motion correction was performed when necessary. The T1 maps were stored in the Digital Imaging and Communications in Medicine (DICOM) format. For extraction of myocardial mean T1 values, endocardial and epicardial contours were manually traced on all T1 maps using QMass (version 7.2; Medis, Leiden, the Netherlands). Care was taken to exclude epicardial structures and blood pool from the contours. An example showing the myocardial wall contouring and mean T1 fit is shown in Figure 2.
Figure 2.
A) MOLLI Color map after 10 minutes of contrast administration (T1 time 482 ms) using MRmap software and B) Fitting curve of the same study as on Figure 1.
Statistical analysis
The LL pre-contrast T1 values showed a linear dependence on the heart rate as previously described(13). Therefore, pre contrast LL T1 values were normalized to a heart rate of 60 bpm. Post-contrast LL values were not corrected. The MOLLI data were obtained 1.5 minutes before the LL data. In order to compare T1 values obtained by the two sequences, MOLLI data were adjusted to delays of 5, 10, 15, 20 and 25 minutes using a multi-compartmental model for which kinetic parameters were obtained from separately performed normal volunteer studies(11). For intrapatient comparisons, only a correction factor for delay time was applied. For interpatient comparison, correction factors for GFR, dose and delay time were applied. In the tables, and graphs below, only corrected T1 data is shown.
For all continuous parameters, the means and their SD were calculated. We used Bland-Altman to compare T1 values from the two methods. A p value ≤ 0.05 was considered statistically significant. We used exponential regression analysis to compare the gadolinium washout of the two protocols. Statistical analysis was performed using STATA®, version 9.0 (StataCorp LP College Station, Texas, USA) and Excel plug-in (XL Toolbox© 2008-2010 Daniel Kraus, Boston, Massachusetts, USA).
RESULTS
Demographic characteristics of the study population are presented in Table 1. The mean age was 38±10.9 years; 57% of study participants were men. All volunteers had normal ejection fraction on cine images and had no myocardial scar on delayed enhancement images. Three hundred thirty three myocardial T1 maps that included 168 from MOLLI (84 in long axis and 84 in short axis) and 165 from LL (82 in long axis and 83 in short axes) were available for analysis. Three LL sequences inadvertently were not acquired.
Table 1.
Participant characteristics
| Subject | Age | Sex | Height | Weight | Creatinine | GFR | HR | CD |
|---|---|---|---|---|---|---|---|---|
| 1 | 55 | F | 163 | 63 | 0.87 | 65 | 63 | 0.19 |
| 2 | 39 | M | 193 | 109 | 0.82 | 108 | 59 | 0.15 |
| 3 | 28 | M | 180 | 73 | 1.17 | 72 | 53 | 0.19 |
| 4 | 18 | M | 173 | 73 | 0.92 | 110 | 70 | 0.19 |
| 5 | 37 | F | 173 | 80 | 1.14 | 68 | 60 | 0.20 |
| 6 | 47 | F | 163 | 64 | 0.69 | 90 | 68 | 0.15 |
| 7 | 24 | M | 188 | 92 | 1.02 | 82 | 58 | 0.20 |
| 8 | 45 | F | 173 | 115 | 0.76 | 78 | 50 | 0.20 |
| 9 | 52 | M | 178 | 99 | 0.76 | 102 | 78 | 0.20 |
| 10 | 45 | M | 165 | 104 | 0.57 | 146 | 54 | 0.14 |
| 11 | 39 | F | 160 | 61 | 0.36 | 178 | 61 | 0.17 |
| 12 | 34 | M | 168 | 70 | 0.97 | 86 | 60 | 0.16 |
| 13 | 43 | M | 168 | 91 | 0.81 | 106 | 88 | 0.17 |
| 14 |
26 |
F |
163 |
60 |
0.63 |
121 |
52 |
0.17 |
| Total | 38.0±10.9 | - | 172±09.8 | 82.4±18.9 | 0.82±0.22 | 100.8±31.5 | 62.4±10.5 | 0.170±0.02 |
Note – Age in years, Height in cm, Weight in Kg, Creatinie in mg/dl, GFR (glomerular filtration rate) in mL/min/1.73 m2, HR = heart rate, CD = contrast dose in mmol/kg. Values are reported by mean±1SD.
Comparison of raw data and normalized data
Raw and normalized pre-contrast myocardial T1 values were similar (mean 998.7 ms +/- 196.3 vs. 1000.4 ms +/- 126.0, respectively, p = 0.64). Normalized post-contrast myocardial T1 times were greater than LL values (mean 523.3 ms +/- 72.8 vs. 486.7 ms +/- 69.6, respectively, p < 0.001). , The average change in T1 values was less than 1% of the mean value.
For MOLLI, similar comparisons between raw and normalized data were present. Pre-contrast T1 times were similar (mean 1034.0 ms +/- 56.0 vs. 1029.4 ms +/- 56.8 for raw and normalized data respectively, p = 0.75). Post-contrast T1 times from normalized MOLLI data were greater than raw data (mean 462.4 ms +/- 62.2 vs. 418.1 ms +/- 70.2, respectively, p < 0.001). The average change in T1 values was less than 1% of the mean difference.
Look-Locker sequence: intrasequence reproducibility
Long and short axis T1 values for LL were compared for intrasequence reproducibility. Examination of the data showed differences for pre-gadolinium measurements (long T1 values) and post-gadolinium data (shorter T1 values), so the two groups were analyzed separately (figure 3). Before gadolinium administration, the mean difference in T1 time between the long and short axis was -26.5 +/- 165.7 ms (p=0.6). The 95% limits of agreement between the two imaging planes were large [-358.0 to +304.8 ms]. A small bias towards longer T1 values in the short axis plane was detected (bias, 26.5 ms).
Figure 3.
Bland-Altman analysis to express the intrasequence agreement of the myocardial T1 values using Look-Locker. The mean difference (bias) is represented by the black line and the 95% limits of agreement limit are represented by the gray lines. (A) Pre-contrast. (B) Post-contrast.
After gadolinium administration, the mean difference (paired measurements) between the long and short axis planes was +10.0 +/- 37.8 ms (p=0.03). The 95% limits of agreement between the two cardiac planes were -65.5 to +85.7 ms. A slight trend towards longer T1 time measurement in the long axis plane was detected (bias, 10 ms longer in the long axis plane).
MOLLI sequence: intrasequence reproducibility
MOLLI pre- and post-gadolinium sequences were evaluated separately to be consistent with LL analysis (figure 4). Before gadolinium administration, the mean difference in T1 time between the long and short axis was +40.6 +/- 37.0 ms (p<0.01). The 95% limits of agreement between the two imaging planes were smaller than for pre-contrast LL [-33.4 to +114.7 ms]. A small bias towards longer T1 values in the long axis plane was detected (bias, 40.6 ms longer than short axis).
Figure 4.
Bland-Altman analysis to express the intrasequence agreement of the myocardial T1 values using MOLLI. The mean difference (bias) is represented by the black line and the 95% limits of agreement limit are represented by the gray lines. (A) Pre-contrast. (B) Post-contrast.
After gadolinium administration, the mean difference (paired measurements) between the long and short axis planes was comparable to Look-Locker (-14.9 +/- 26.1 ms, p<0.0001). The 95% limits of agreement between the two cardiac planes (-66.6 to +37.7 ms) were also comparable to LL. A slight trend towards longer T1 time measurement in the short axis plane was detected (bias, 14.9 ms longer in the short axis plane compared to the long axis).
Comparison of Look-Locker and MOLLI sequences
As described above, inspection of the pre- and post-gadolinium data showed different trends for T1 data so that each group was separately compared. According to the above analysis, there was no statistically significant difference between cardiac planes so long and short axes were pooled (figure 5).
Figure 5.
Bland-Altman analysis to express the intersequence agreement of the myocardial T1 values using LL compared to MOLLI. The mean difference (bias) is represented by the black line and the 95% limits of agreement limit are represented by the gray lines. (A) Pre-contrast. (B) Post-contrast.
For pre-gadolinium measurements, myocardial T1 values were slightly greater when measured using MOLLI (mean 1034.0 ± 56.0 ms) compared to LL (mean 1004.8 ± 126.0 ms). The difference in T1 time between LL and MOLLI was -29.1 +/- 125.7 ms (p = 0.26, t-test for paired comparison). The 95% limits of agreement between the 2 cardiac sequences were -280.7 to +222.3 ms.
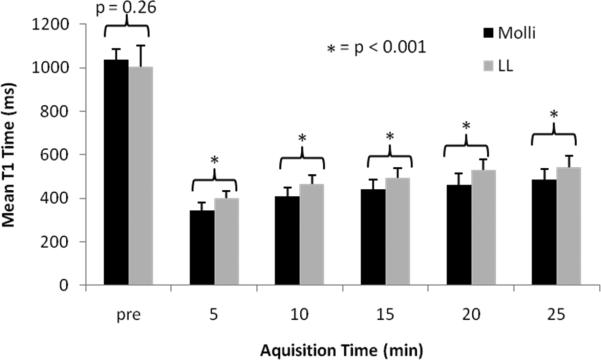
After gadolinium administration, there was a significant difference in mean T1 time between LL and MOLLI of +61.8 +/- 46.4 ms (p < 0.001, t-test for paired comparison). The 95% limits of agreement between the 2 cardiac sequences were -31.1 to +154.8 ms. Mean differences between each measured time point showed consistently greater T1 time for LL compared to MOLLI (Figure 6).
Figure 6.
Mean myocardial T1 relaxation times for each acquisition before and after gadolinium administration using MOLLI (black) and LL (gray). (Pre = pre-contrast acquisition time).
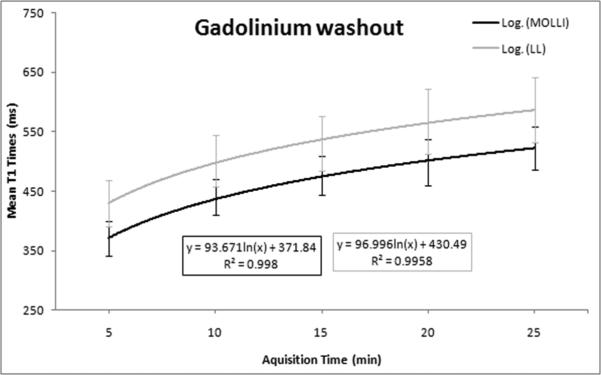
Gadolinium washout comparison using LL and MOLLI
An exponential regression curve was fit to LL and MOLLI data for the washout period from 5 to 25 minutes (Figure 7). This analysis emphasized consistency of measurements between the two sequences over this clinically relevant time period with similar model fit (MOLLI r2=0.998 and LL r2=0.995).
Figure 7.
Gadolinium washout curve from 5 to 25 minutes after the contrast injection for MOLLI and LL sequences show good consistency for both methods with similar curve fit to an exponential model (r2>0.99 for both methods).
DISCUSSION
This study examined limits of agreement of two rapid methods for quantifying myocardial T1: Look-Locker (LL) and MOLLI (modified Look-Locker). Over wide ranges in myocardial T1, both LL and MOLLI showed good agreement in intrasequence comparisons but MOLLI shows substantially tighter limits of agreement than LL. Short and long axis cardiac planes showed similar results for myocardial T1 values for both sequences.
T1 mapping via the LL method is one of the fastest approaches for T1 quantification(11). In addition, the LL sequence has been widely applied to delayed gadolinium CMR of the myocardium principally to determine the null point of the myocardium. In the LL technique, multiple phases are acquired after an inversion pulse. After a sufficient pause to allow magnetization to return to equilibrium, the experiment is repeated, until k-space is filled. There are some limitations in the LL method for quantifying myocardial T1. For post-contrast myocardial T1 time around 500 ms, two RR intervals enable almost full magnetization recovery and accurate T1 quantification. For non-contrast myocardium T1 time around 1100ms at 1.5T, two RR intervals are not sufficient for magnetization to return to equilibrium; thus, the estimated T1 will be in error and this error is increased with faster heart rate or at higher magnet field strength (when the T1 time is longer). Heart rate correction cannot entirely resolve this inaccuracy. In addition, LL data is prospective ECG triggered and acquired as a cine sequence. Thus, T1 mapping requires careful contouring of epicardial and endocardial boundaries on all cine phases, as described by Gai et al(11).
The MOLLI sequence is a variant of inversion recovery LL, which introduces two changes to the standard LL sequence: 1) selective data acquisition at a fixed delay time of the cardiac cycle over successive heartbeats, and 2) the possibility to combine multiple LL image sets into one data set to facilitate the final analysis. However, the accuracy of pixel-by-pixel T1 estimation is compromised by the myocardial motion between frames, mainly due to beat-to-beat variation and respiration. Xue et al(14), proposed a novel registration algorithm based on estimating motion-free synthetic images presenting similar contrast to original data. Robust motion correction is achieved by registering synthetic images to corresponding MOLLI frames(14). MOLLI allows a rapid and highly reproducible T1 map of the heart with high levels of intra and inter-observer agreement (8,13).
Overall, the MOLLI and LL sequences showed similar trends and consistency for post contrast images of the myocardium. However, the T1 values derived from the LL measurement are about 60 ms longer than those of LL. MOLLI images are easier to quantitatively analyze since artifacts from motion are more readily eliminated. This is likely to result in more reproducible data and thus smaller sample sizes for scientific studies. Nevertheless, it appears that either method, used consistently, may be applicable to post gadolinium MRI data acquisition.
In this study, the mean myocardial time was measured in both the long and short axis. No systematic difference was found between LL and MOLLI techniques. Since different segments of myocardial tissue are measured in the two imaging planes, these results suggest no global differences can be detected in myocardial T1 using either method. We did however observe greater standard deviation for LL short axis images compared to other acquisitions, including MOLLI. The reason for this is not clear, but could be related to small sample size or motion artifacts that are more apparent using LL sequence. A current limitation of both LL and MOLLI sequences are their two-dimensional nature. Ideally a 3D (15,16) acquisition of the entire myocardium with high resolution and accuracy will eventually be feasible, similar to applications in other areas of the body(17).
LL methods demonstrate increased measurement variability in precontrast images compared with MOLLI methods. This poses a particular challenge for methods for interpreting T1 values that rely on both pre- and post-contrast T1 data – such as gadolinium volume of distribution (Vd) and extracellular volume calculations (ECV) (18-20). Our results suggest that MOLLI should be preferred for Vd and ECV calculations. When only LL is available, corrected post-contrast T1 measurements should be utilized(11).
While the use of MOLLI has advantages in prospectively acquired studies, there is a wealth of previously acquired CMR data in large population studies that included a Look-Locker sequence for optimization of delayed enhancement images (21,22). Our results provide validation for use of these previously acquired LL sequences to retrospectively measure myocardial T1 time in such study populations. While myocardial T1 measurement is a promising tool for the noninvasive assessment of fibrosis, there is as yet little data tying these measurements to clinical outcomes. Such retrospective analyses may help illuminate the most promising applications for this technique.
The study was limited to normal subjects, so that motion artifacts were probably less than for typical patient studies. Since we collected T1 values over a long period after contrast agent injection (3.5 to 25 minutes), a large range of T1 values (from about 350 to 1000 ms) was evaluated. This range likely covers the situation of low T1 values proposed to be associated with diffuse fibrosis in disease states. The overall T1 values we derived are in general agreement with previous studies (1,2,8,9,12,13). Only 1 MRI scanner manufacturer was included. In order to determine the applicability of T1 mapping, it will eventually be necessary to evaluate T1 values on different MRI scanners.
In conclusion, this study examined limits of agreement of two rapid methods for quantifying myocardial T1: Look-Locker (LL) and MOLLI (modified Look-Locker). Over wide ranges in myocardial T1, both LL and MOLLI showed good agreement in intrasequence comparisons but MOLLI shows substantially tighter limits of agreement than LL. Short and long axis cardiac planes showed similar results for myocardial T1 values for both sequences.
Acknowledgments
This research was supported by the NIH intramural research program.
Funding Sources
Funded by the National Institutes of Health (NIH) Intramural program.
Saman Nazarian is funded by grant K23-HL089333 from the NIH.
Rob J. van der Geest is consultant for Medis medical imaging systems.
Footnotes
Potential Conflict of Interest
The authors declare that they have no competing interests.
References
- 1.Messroghli DR, Walters K, Plein S, et al. Myocardial T1 mapping: application to patients with acute and chronic myocardial infarction. Magn Reson Med. 2007;58(1):34–40. doi: 10.1002/mrm.21272. [DOI] [PubMed] [Google Scholar]
- 2.Messroghli DR, Niendorf T, Schulz-Menger J, Dietz R, Friedrich MG. T1 mapping in patients with acute myocardial infarction. J Cardiovasc Magn Reson. 2003;5(2):353–359. doi: 10.1081/jcmr-120019418. [DOI] [PubMed] [Google Scholar]
- 3.Sparrow P, Messroghli DR, Reid S, Ridgway JP, Bainbridge G, Sivananthan MU. Myocardial T1 mapping for detection of left ventricular myocardial fibrosis in chronic aortic regurgitation: pilot study. AJR Am J Roentgenol. 2006;187(6):W630–635. doi: 10.2214/AJR.05.1264. [DOI] [PubMed] [Google Scholar]
- 4.Iles L, Pfluger H, Phrommintikul A, et al. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol. 2008;52(19):1574–1580. doi: 10.1016/j.jacc.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 5.Han Y, Peters DC, Dokhan B, Manning WJ. Shorter difference between myocardium and blood optimal inversion time suggests diffuse fibrosis in dilated cardiomyopathy. J Magn Reson Imaging. 2009;30(5):967–972. doi: 10.1002/jmri.21953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amano Y, Takayama M, Kumita S. Contrast-enhanced myocardial T1-weighted scout (Look-Locker) imaging for the detection of myocardial damages in hypertrophic cardiomyopathy. J Magn Reson Imaging. 2009;30(4):778–784. doi: 10.1002/jmri.21921. [DOI] [PubMed] [Google Scholar]
- 7.Karlsson M, Nordell B. Analysis of the Look-Locker T(1) mapping sequence in dynamic contrast uptake studies: simulation and in vivo validation. Magn Reson Imaging. 2000;18(8):947–954. doi: 10.1016/s0730-725x(00)00193-4. [DOI] [PubMed] [Google Scholar]
- 8.Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. 2004;52(1):141–146. doi: 10.1002/mrm.20110. [DOI] [PubMed] [Google Scholar]
- 9.Messroghli DR, Greiser A, Frohlich M, Dietz R, Schulz-Menger J. Optimization and validation of a fully-integrated pulse sequence for modified look-locker inversion-recovery (MOLLI) T1 mapping of the heart. J Magn Reson Imaging. 2007;26(4):1081–1086. doi: 10.1002/jmri.21119. [DOI] [PubMed] [Google Scholar]
- 10.Gai N, Stehning C, Nacif M, Bluemke D. Characterization of Modified Look Locker (MOLLI) using Bloch simulations and corroboration with scan measurements. Proc Intl Soc Mag Reson Med. 2011;19:4451. [Google Scholar]
- 11.Gai N, Turkbey EB, Nazarian S, et al. T(1) mapping of the gadolinium-enhanced myocardium: Adjustment for factors affecting interpatient comparison. Magn Reson Med. 2010 doi: 10.1002/mrm.22716. [Epub ahead of print]; DOI:10.1002/mrm.22716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Messroghli DR, Rudolph A, Abdel-Aty H, et al. An open-source software tool for the generation of relaxation time maps in magnetic resonance imaging. BMC Med Imaging. 2010;10:16. doi: 10.1186/1471-2342-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Messroghli DR, Plein S, Higgins DM, et al. Human myocardium: single-breath-hold MR T1 mapping with high spatial resolution--reproducibility study. Radiology. 2006;238(3):1004–1012. doi: 10.1148/radiol.2382041903. [DOI] [PubMed] [Google Scholar]
- 14.Xue H, Shah S, Greiser A, et al. Improved motion correction using image registration based on variational synthetic image estimation: application to inline t1 mapping of myocardium. J Cardiovasc Magn Reson. 2011;13(Suppl 1):21. [Google Scholar]
- 15.Warntjes MJ, Kihlberg J, Engvall J. Rapid T1 Quantification based on 3D Phase Sensitive Inversion Recovery. BMC Med Imaging. 2010;10(1):19. doi: 10.1186/1471-2342-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nkongchu K, Santyr G. An improved 3-D Look--Locker imaging method for T(1) parameter estimation. Magn Reson Imaging. 2005;23(7):801–807. doi: 10.1016/j.mri.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Siversson C, Tiderius CJ, Neuman P, Dahlberg L, Svensson J. Repeatability of T1-quantification in dGEMRIC for three different acquisition techniques: two-dimensional inversion recovery, three-dimensional look locker, and three-dimensional variable flip angle. J Magn Reson Imaging. 2010;31(5):1203–1209. doi: 10.1002/jmri.22159. [DOI] [PubMed] [Google Scholar]
- 18.Flett AS, Hayward MP, Ashworth MT, et al. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 122(2):138–144. doi: 10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]
- 19.Broberg CS, Chugh SS, Conklin C, Sahn DJ, Jerosch-Herold M. Quantification of diffuse myocardial fibrosis and its association with myocardial dysfunction in congenital heart disease. Circ Cardiovasc Imaging. 3(6):727–734. doi: 10.1161/CIRCIMAGING.108.842096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arheden H, Saeed M, Higgins CB, et al. Reperfused rat myocardium subjected to various durations of ischemia: estimation of the distribution volume of contrast material with echo-planar MR imaging. Radiology. 2000;215(2):520–528. doi: 10.1148/radiology.215.2.r00ma38520. [DOI] [PubMed] [Google Scholar]
- 21.Epidemiology of Diabetes Interventions and Complications (EDIC) Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22(1):99–111. doi: 10.2337/diacare.22.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turkbey EB, McClelland RL, Kronmal RA, et al. The impact of obesity on the left ventricle: the Multi-Ethnic Study of Atherosclerosis (MESA). JACC Cardiovasc Imaging. 3(3):266–274. doi: 10.1016/j.jcmg.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]