Abstract
Renal involvement in systemic lupus erythematosus (SLE) remains a major cause of morbidity and mortality. Although immune parameters that instigate renal damage have been characterized, their link to local processes, which execute tissue damage, is poorly understood. Using genetic deletion and pharmalogical inhibition approaches we demonstrate that calcium/calmodulin-dependent protein kinase type IV (CaMKIV) which contributes to altered cytokine production in SLE patients controls spontaneous and platelet derived growth factor (PDGF)-stimulated mesangial cell proliferation and promotes IL-6 production through AP-1. Our studies identify CaMKIV as a valuable treatment target for lupus nephritis and point out the importance of local kidney factors in the expression of tissue damage which if properly targeted should enhance clinical benefit and limit toxicity.
Keywords: autoimmunity, lupus nephritis, systemic lupus erythematosus, mesangial cells, CaMKIV
INTRODUCTION
Lupus nephritis is still the major cause of morbidity and mortality in patients with systemic lupus erythematosus (SLE) (1). IL-6 can be produced by mesangial cells (MCs) and has been reported to orchestrate the cytokine network of glomerular inflammation. There is clear evidence that IL-6 is involved in mesangial proliferation and pathogenesis of lupus nephritis (2–5). Various transcription factors such as AP-1, cAMP response element (CRE)-binding protein (CREB) and nuclear factor for IL-6 expression (NF-IL-6) have been identified to control IL6 promoter activity (6–8). Amongst them, AP-1 has been implicated in transcriptional regulation of a wide range of genes participating in cell survival, proliferation, and apoptosis (9–11). The multifunctional calcium/calmodulin dependent protein kinase type IV (CaMKIV) belongs to a family of serine/threonine protein kinases that regulate autoimmunity and cell proliferation (12–14). A small molecule inhibitor of CaMKIV, KN-93 mitigates disease development in lupus-prone mice by suppressing cytokine production and co-stimulatory molecule expression in lymphocytes (15). In this report, we provide evidence that pharmacologic inhibition or genetic depletion of CaMKIV in lupus-prone MRL/lpr mice results in decreased mesangial IL-6 production, reduced MC proliferation and less kidney damage. Our data suggest a prominent role for CaMKIV not only in expression of systemic autoimmunity, but also that of local renal damage.
MATERIALS AND METHODS
Mice
Female MRL/lpr, Camk4tm1Tch/J and MRL/MPJ mice were purchased from Jackson Laboratory. MRL/lpr.Camkiv−/− mice were generated on a MRL/lpr background. Experiments were approved by the Institutional Animal Care Committee of Beth Israel Deaconess Medical Center. Measurement of anti-dsDNA antibody levels were performed as described previously (15). Proteinuria was measured in a semiquantitative manner as described before (15). Briefly, mice in each group (n=4) were placed together overnight in a Nalgene metabolic cage to collect urine. This procedure was repeated in 2 independent experiments, so that the presented data display the average from a total of 8 mice/group. Kidneys from 16-week old mice were formalin-fixed, paraffin sections were PAS-stained and renal lesions were evaluated according to previously described criteria (16, 17). Scoring was performed blindly by a nephropathologist.
Primary culture of mesangial cells (MCs)
Primary MCs were isolated according to Allam et al. (20) and purity of isolated MCs was assessed by morphologic characteristics, positivity for smooth muscle actin (>99%) and negativity for cytokeratin 18 (>99%). Cultured MCs were used for experiments between passages 3 and 7. MCs were plated in 12- or 6-well plates and serum-starved for 24 h before experiments were performed. Cells were treated with 20 ng/ml of PDGF-BB (Peprotech) for 24 h. As indicated, cells were pre-treated with KN-93 (20 µM) for 48 h prior to addition of PDGF-BB. For RNA and protein analyses, EMSAs and luciferase experiments mesangial cells were pooled from 5 mice/group and each experiment was performed in 2–3 independent replicates.
Immunoblotting
Briefly, MCs were homogenized in RIPA buffer at 4°C for 30 min. After centrifugation (14,000 rpm; 30 min; 4°C) supernatants were collected. The following polyclonal rabbit antibodies were used for immunoblotting: anti-CDK2, anti-Cyclin D1, anti-CaMKIV (all from Cell signaling), anti-c-Jun (Santa Cruz), anti-Histone-H3 (Abcam) and anti-actin (Sigma).
RNA extraction and PCR
Primary MCs were homogenized, total RNA was extracted using the RNeasy Mini Kit (Qiagen) and cDNA was generated using the Reverse Transcription kit (Promega). PCR primers were as follows: IL-6: 5’-CCGGAGAGGAGACTTCACAG-3’ (forward) and 5’- CCAGTTTGGTAGCATCCATC -3’ (reverse); CaMKIV: 5’-TCACATGGACACTGCTCAGA-3’ (forward) and 5’-TGCATCTTTCTCCACCTCCT-3’ (reverse). 18S rRNA primers were reported previously (15).
IL-6 ELISA
400,000 primary MCs were plated on 6-well plates and serum-starved for 24 hrs. Then, cells were pretreated with KN-93 (20 µM) for 48 h before the addition of PDGF-BB (20 ng/ml; 24 h). IL-6 concentrations were detected with a commercial ELISA kit (R&D Systems).
Cell-cycle analyses
MCs were trypsinized, washed twice with PBS, fixed in cold 95% ethanol and stored at 4°C until use. Before flow cytometric analysis, cell pellets were washed and resuspended in a solution of RNAse (0.5 mg/ml) in PBS and incubated at 37°C for 20 min. Then, propidium iodide (40 µg/ml) was added for 30 min. Stained cells were analyzed on FACS Scan (BD Biosciences). Data were acquired using CellQuest software (BD Biosciences); at least 10,000 events were collected for each histogram. Data analysis was performed with FlowJo version 7.6.1 (Tree Star).
Luciferase assays
Mouse IL6 promoter luciferase plasmid (in pGL3-Basic vector, Invitrogen) was kindly provided by Dr. David L. Allen (University of Colorado). Transient transfections were performed in primary MCs (seeded at 1 × 105 cells/well) by using 1 µg of reporter DNA, 10 ng PRTK plasmid per transfection and 2 µl of Lipofectamine 2000 (Invivogen). 24 h after transfection, cells were either incubated with or without PDGF-BB (20 ng/ml) for another 24 h. Luciferase activities in the cell lysates was measured using the Dual-Luciferase Reporter Assay System (Promega). Experiments were repeated at least four times. Values in the bar diagrams are given as mean ± S.D.
EMSAs
500,000 MCs were used for preparation of nuclear protein extracts as described before (21). A double-stranded DNA probe harboring the AP-1 site (−327) of the murine IL6 promoter (5'- AGTGCTGAGTCACTTTTAAAG -3') was [γ-32P]-ATP-radiolabeled using a T4-polynucleotide kinase. EMSA was performed as described before using 5 µg of nuclear protein and 1 µg of poly(dG)·poly(dC) per reaction. (21). Unlabeled AP-1 probe was used for competition assays in 50- and 100-fold molar excess as indicated.
Densitometries and statistical analyses
Densitometries of western blot and PCR images were perfomed using Image J software (NIH). Kruskal-Wallis test was used to determine statistical significance (*P ≤ 0.05; **P ≤ 0.01; NS = not significant).
RESULTS AND DISCUSSION
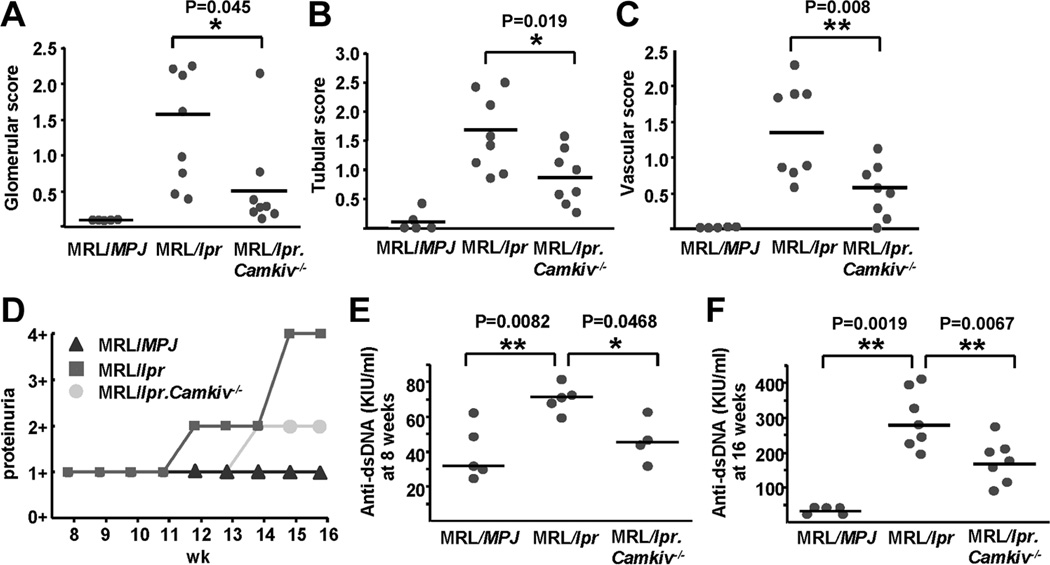
In our efforts to prove the importance of CaMKIV in the pathogenesis of SLE and lupus nephritis, we transferred the Camkiv null locus into the lupus-prone mouse strain MRL/lpr (18) (Suppl. figs. 1A/B). Total numbers of splenocytes, peripheral lymph node cells and major B and T cell compartments were not different between MRL/lpr and MRL/lpr.Camkiv−/− mice (data not shown). However, at 16 weeks of age kidney damage was largely diminished in MRL/lpr.Camkiv−/− when compared to age-matched MRL/lpr mice (Suppl. fig. 1C). MRL/lpr.Camkiv−/− mice developed significantly less glomerular, tubulointerstitial and perivascular lesions than MRL/lpr mice (Figs. 1A–C). As shown in Fig. 1D, proteinuria was also robustly decreased in MRL/lpr.Camkiv−/− mice. Furthermore, MRL/lpr.Camkiv−/− mice displayed significantly lower serum titers of anti-dsDNA antibodies at 8 and 16 weeks of age as compared to MRL/lpr mice (Figs. 1E and F).
Figure 1. CaMKIV deficiency improves lupus pathology.
(A–C) Mean scores of glomerular injury (A), tubular damage (B), and perivascular lymphocyte infiltration (C), from MRL/MPJ, MRL/lpr and MRL/lpr.Camkiv−/− mice are shown. (D) Proteinuria was quantified weekly starting from 8 weeks of age. (E) and (F) Anti-dsDNA IgG antibodies from 8-weeks old (E) and 16-weeks old (F) mice of each group were detected by ELISA (n=4–8 mice per group).
Mesangial proliferation is a hallmark of lupus nephritis. Incubation of MCs from MRL/MPJ and MRL/lpr mice with platelet derived growth factor (PDGF)-BB for 24 hours up-regulated protein levels of cell cycle regulators CDK-2 and cyclin D1, however, pre-treatment with CaMKIV inhibitor KN-93 suppressed these levels (Suppl. figs. 2A–C). In line with this, these proteins were down-regulated in MRL/lpr.Camkiv−/− MCs in the presence or absence of PDGF-BB stimulation (Suppl. figs. 2D–F).
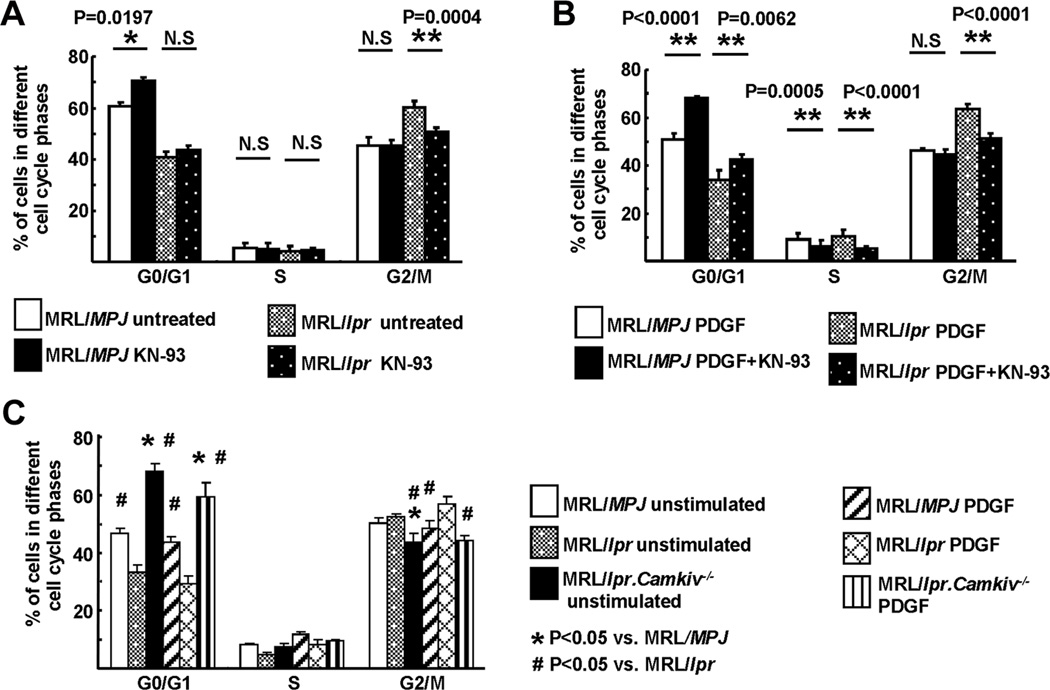
MCs from MRL/MPJ and MRL/lpr mice were G1-synchronized by serum starvation for 24 h, labeled with propidium iodide and DNA content was analyzed by flow cytometry. The percentage of G1-synchronized MCs entering the G2/M phase was higher in MRL/lpr mice compared to that of MRL/MPJ mice (Fig. 2A). This may indicate that MCs from MRL/lpr mice actively divide even in the absence of exogenous stimuli suggesting increased proliferation abilities of MRL/lpr MCs. Pre-treatment with KN-93 clearly diminished the percentage of MCs entering the G2/M phase with a concomitant increase in the percentage of MCs arrested in the G0/G1 phase in PDGF-BB-stimulated MCs from MRL/lpr mice (Fig. 2B). In addition, KN-93 pre-treatment reduced the frequency of cells entering the S phase (Fig. 2B). Next, we analyzed the cell cycle status of G1-synchronized MCs from the MRL/lpr.Camkiv−/− mice in the absence or presence of PDGF-BB. A large percentage of MCs from the MRL/lpr.Camkiv−/− mice was arrested at the G0/G1 phase compared to control MRL/lpr and MRL/MPJ mice (Fig. 2C). Interestingly, PDGF-BB was not able to overcome the G0/G1 block imposed by the genetic deletion of CaMKIV. These findings further support the hypothesis that MCs from MRL/lpr have an intrinsic ability to proliferate without exogenous stimulation.
Figure 2. CaMKIV suppresses cell cycle arrest of lupus MCs.
(A) and (B) MCs were stained by propidium iodide (PI) and cell cycle analysis was performed by flow cytometry. 400,000 MCs derived from MRL/MPJ and MRL/lpr mice were treated with or without KN-93 for 48 hrs and stimulated with or without PDGF-BB for 24 hrs. Cumulative data of flow cytometry (percentage of cells in each cell cycle phases) are shown from (A) unstimulated and (B) PDGF-BB-stimulated MCs. Values are the mean ± SD of n=4–5 mice/group and performed in three independently replicates. (C) 400,000 MCs derived from MRL/MPJ, MRL/lpr and MRL/lpr.Camkiv−/− mice were stimulated with or without PDGF-BB for 24 hrs. Cumulative data of flow cytometry (percentage of cells in each cell cycle phases) are shown. Values indicate mean ± SD of n=4–5 mice/group performed in three independently replicates.
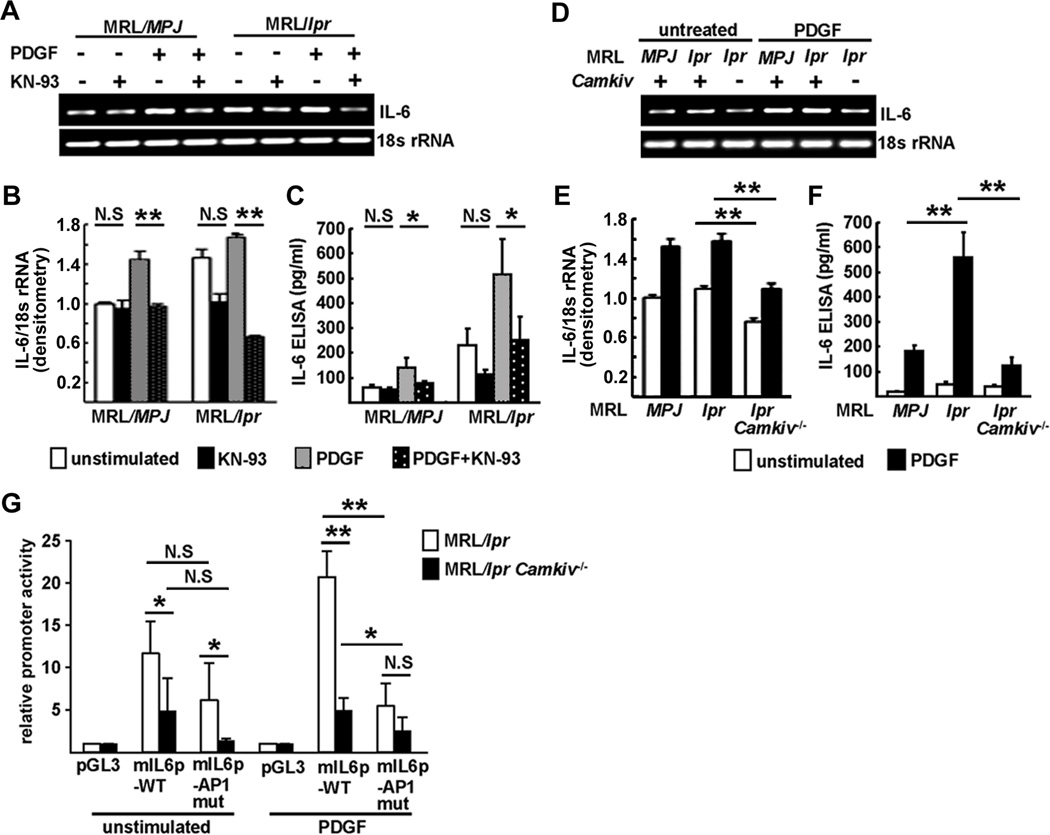
Mesangial IL-6 production was induced by PDGF-BB stimulation and effectively suppressed by KN-93 treatment in MRL/lpr MCs at mRNA and protein levels (Figs. 3A–C). Similarly, MRL/lpr.Camkiv−/− MCs showed decreased IL-6 expression as compared to MRL/MPJ and MRL/lpr MCs upon stimulation with PDGF-BB (Figs. 3D–F). Additionally, IL-6 mRNA expression in activated splenocytes was significantly decreased in MRL/lpr.Camkiv−/− mice (Suppl. fig. 3A).
Figure 3. PDGF-BB-mediated IL6 gene transcription is inhibited in MCs from MRL/lpr.Camkiv−/− mice.
(A)–(C) 400,000 MCs derived from MRL/MPJ and MRL/lpr mice were treated with or without KN-93 for 48 hrs and stimulated with or without PDGF-BB for 24 hrs. IL-6 mRNA expression was assessed by PCR (A) and quantified by densitometry of agarose gels (B). IL-6 levels in the supernatants were measured by ELISA (C). (D)–(F) 400,000 MCs derived from MRL/MPJ, MRL/lpr and MRL/lpr.Camkiv−/− mice were stimulated with or without PDGF-BB for 24 hrs. IL-6 mRNA expression was assessed by PCR (D) and quantified by densitometry of agarose gels (E). IL-6 levels in the supernatants were measured by ELISA (F). (G) MCs from MRL/lpr and MRL/lpr.Camkiv−/− mice were transfected with empty pGL3 luciferase vector, IL6 promoter construct (mIL6p-WT) or a mutated IL6 promoter construct with a disrupted AP-1 site (mIL6p-AP1 mut), incubated with or without PDGF-BB for 24 hrs and assayed for relative promoter activity (n=4–5 mice/group: performed in 3 independent experiments).
After we demonstrated that IL-6 expression was diminished in MRL/lpr.Camkiv−/− MCs, we sought to identify possible transcriptional mechanisms underlying IL-6 expression in response to PDGF-BB stimulation. IL6 promoter activity in MRL/lpr.Camkiv−/− MCs was significantly decreased compared to MRL/lpr MCs and this effect was even more pronounced following stimulation with PDGF-BB. AP-1 has been shown to be involved in IL6 gene transcription (19). Site-directed mutagenesis of an AP-1 site within the murine IL6 promoter (located 327 bp upstream of the first ATG) limited its promoter activity significantly (Fig. 3G). Whereas cytoplasmic protein levels of AP-1 member c-jun were increased in MRL/lpr.Camkiv−/− MCs compared to MRL/lpr MCs it was found to be decreased in the nuclei of these cells (Suppl. fig. 3B–D). DNA binding assays using nuclear MC extracts and a synthetic double-stranded oligonucleotide defining the AP-1 motif showed an increased binding of nucleoprotein from MRL/lpr MCs stimulated with PDGF-BB to this site which was diminished when nucleoprotein lysates from MRL/lpr.Camkiv−/− MCs were used (Suppl. fig. 2E). The binding was specific because it was competed out with the cold, unlabelled probe at a 10- and 50- fold molar excess (Suppl. fig. 2F).
Previously, CaMKIV was shown to contribute to decreased IL-2 production in SLE T cells and its inhibition in lupus-prone MRL/lpr mice mitigates disease pathology by interfering with immune parameters (15, 20). Our studies in the newly developed MRL/lpr.Camkiv−/− mice clearly demonstrate that CaMKIV is important in the expression of both, autoimmunity and kidney pathology. It reveals profound effects on MC proliferation and production of IL-6 which is known to be involved in the development of glomerulonephritis (5). It is undisputed that immune complexes, autoantibodies and autoreactive T cells are important in the instigation of lupus nephritis (1), yet little emphasis has been paid to the local kidney factors which eventually execute tissue damage. Previously, it was shown that the kallikrein genes contribute to lupus nephritis in mice and men and therefore, factors independent of the immune system are important in the expression of kidney pathology (21). Along these lines, a congenic mouse that was derived from the lupus-prone NZM2328 mouse develops severe glomerulonephritis without breaking tolerance to nuclear antigens (22). Here we show that MCs from MRL/lpr mice are able to proliferate in vitro in the absence of exogenous stimuli and that this proliferation along with the increased IL-6 production is significantly suppressed in the genetic absence or pharmacologic inhibition of CaMKIV. We have presented proof that CaMKIV, known to be important in the suppression of the production of IL-2, is important in MC proliferation and the expression of lupus nephritis. As such, CaMKIV represents a unique link between the immune system and the biology of MC and its targeting may prove of significant clinical value as it will mitigate systemic autoimmunity and suppress kidney inflammation.
Supplementary Material
Acknowledgments
This work was supported by PHS NIH grant RO1 AI 49954 (to G.C.T.).
Footnotes
COMPETING FINANCIAL INTEREST
The authors declare no competing financial interest
REFERENCES
- 1.Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011 doi: 10.1056/NEJMra1100359. in press. [DOI] [PubMed] [Google Scholar]
- 2.Kiberd BA. Interleukin-6 receptor blockage ameliorates murine lupus nephritis. J Am Soc Nephrol. 1993;4:58–61. doi: 10.1681/ASN.V4158. [DOI] [PubMed] [Google Scholar]
- 3.Liang B, Gardner DB, Griswold DE, Bugelski PJ, Song XY. Anti-interleukin-6 monoclonal antibody inhibits autoimmune responses in a murine model of systemic lupus erythematosus. Immunology. 2006;119:296–305. doi: 10.1111/j.1365-2567.2006.02433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suematsu S, Matsusaka T, Matsuda T, Ohno S, Miyazaki J, Yamamura K, Hirano T, Kishimoto T. Generation of plasmacytomas with the chromosomal translocation t(12;15) in interleukin 6 transgenic mice. Proc Natl Acad Sci U S A. 1992;89:232–235. doi: 10.1073/pnas.89.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cash H, Relle M, Menke J, Brochhausen C, Jones SA, Topley N, Galle PR, Schwarting A. Interleukin 6 (IL-6) deficiency delays lupus nephritis in MRL-Faslpr mice: the IL-6 pathway as a new therapeutic target in treatment of autoimmune kidney disease in systemic lupus erythematosus. J Rheumatol. 2010;37:60–70. doi: 10.3899/jrheum.090194. [DOI] [PubMed] [Google Scholar]
- 6.Kishimoto T, Akira S, Taga T. Interleukin-6 and its receptor: a paradigm for cytokines. Science. 1992;258:593–597. doi: 10.1126/science.1411569. [DOI] [PubMed] [Google Scholar]
- 7.Tanabe O, Akira S, Kamiya T, Wong GG, Hirano T, Kishimoto T. Genomic structure of the murine IL-6 gene. High degree conservation of potential regulatory sequences between mouse and human. J Immunol. 1988;141:3875–3881. [PubMed] [Google Scholar]
- 8.Grassl C, Luckow B, Schlondorff D, Dendorfer U. Transcriptional regulation of the interleukin-6 gene in mesangial cells. J Am Soc Nephrol. 1999;10:1466–1477. doi: 10.1681/ASN.V1071466. [DOI] [PubMed] [Google Scholar]
- 9.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 10.Whitmarsh AJ, Davis RJ. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- 11.Leppa S, Bohmann D. Diverse functions of JNK signaling and c-Jun in stress response and apoptosis. Oncogene. 1999;18:6158–6162. doi: 10.1038/sj.onc.1203173. [DOI] [PubMed] [Google Scholar]
- 12.Shang S, Takai N, Nishida M, Miyazaki T, Nasu K, Miyakawa I. CaMKIV expression is associated with clinical stage and PCNA-labeling index in endometrial carcinoma. Int J Mol Med. 2003;11:181–186. [PubMed] [Google Scholar]
- 13.Takai N, Miyazaki T, Nishida M, Nasu K, Miyakawa I. Ca(2+)/calmodulin-dependent protein kinase IV expression in epithelial ovarian cancer. Cancer Lett. 2002;183:185–193. doi: 10.1016/s0304-3835(02)00107-6. [DOI] [PubMed] [Google Scholar]
- 14.Williams CL, Phelps SH, Porter RA. Expression of Ca2+/calmodulin-dependent protein kinase types II and IV, and reduced DNA synthesis due to the Ca2+/calmodulin-dependent protein kinase inhibitor KN-62 (1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-L-tyrosyl]-4-phenyl piperazine) in small cell lung carcinoma. Biochem Pharmacol. 1996;51:707–715. doi: 10.1016/s0006-2952(95)02393-3. [DOI] [PubMed] [Google Scholar]
- 15.Ichinose K, Juang YT, Crispin JC, Kis-Toth K, Tsokos GC. Suppression of autoimmunity and organ pathology in lupus-prone mice upon inhibition of calcium/calmodulin-dependent protein kinase type IV. Arthritis Rheum. 2011;63:523–529. doi: 10.1002/art.30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kikawada E, Lenda DM, Kelley VR. IL-12 deficiency in MRL-Fas(lpr) mice delays nephritis and intrarenal IFN-gamma expression, and diminishes systemic pathology. J Immunol. 2003;170:3915–3925. doi: 10.4049/jimmunol.170.7.3915. [DOI] [PubMed] [Google Scholar]
- 17.Sadanaga A, Nakashima H, Akahoshi M, Masutani K, Miyake K, Igawa T, Sugiyama N, Niiro H, Harada M. Protection against autoimmune nephritis in MyD88-deficient MRL/lpr mice. Arthritis Rheum. 2007;56:1618–1628. doi: 10.1002/art.22571. [DOI] [PubMed] [Google Scholar]
- 18.Ho N, Liauw JA, Blaeser F, Wei F, Hanissian S, Muglia LM, Wozniak DF, Nardi A, Arvin KL, Holtzman DM, Linden DJ, Zhuo M, Muglia LJ, Chatila TA. Impaired synaptic plasticity and cAMP response element-binding protein activation in Ca2+/calmodulin-dependent protein kinase type IV/Gr-deficient mice. J Neurosci. 2000;20:6459–6472. doi: 10.1523/JNEUROSCI.20-17-06459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen DL, Uyenishi JJ, Cleary AS, Mehan RS, Lindsay SF, Reed JM. Calcineurin activates interleukin-6 transcription in mouse skeletal muscle in vivo and in C2C12 myotubes in vitro. Am J Physiol Regul Integr Comp Physiol. 2010;298:R198–R210. doi: 10.1152/ajpregu.00325.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juang YT, Wang Y, Solomou EE, Li Y, Mawrin C, Tenbrock K, Kyttaris VC, Tsokos GC. Systemic lupus erythematosus serum IgG increases CREM binding to the IL-2 promoter and suppresses IL-2 production through CaMKIV. J Clin Invest. 2005;115:996–1005. doi: 10.1172/JCI200522854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu K, Li QZ, Delgado-Vega AM, Abelson AK, Sanchez E, Kelly JA, Li L, Liu Y, Zhou J, Yan M, Ye Q, Liu S, Xie C, Zhou XJ, Chung SA, Pons-Estel B, Witte T, de Ramon E, Bae SC, Barizzone N, Sebastiani GD, Merrill JT, Gregersen PK, Gilkeson GG, Kimberly RP, Vyse TJ, Kim I, D'Alfonso S, Martin J, Harley JB, Criswell LA, Wakeland EK, Alarcon-Riquelme ME, Mohan C. Kallikrein genes are associated with lupus and glomerular basement membrane-specific antibody-induced nephritis in mice and humans. J Clin Invest. 2009;119:911–923. doi: 10.1172/JCI36728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waters ST, McDuffie M, Bagavant H, Deshmukh US, Gaskin F, Jiang C, Tung KS, Fu SM. Breaking tolerance to double stranded DNA, nucleosome, and other nuclear antigens is not required for the pathogenesis of lupus glomerulonephritis. J Exp Med. 2004;199:255–264. doi: 10.1084/jem.20031519. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.