Abstract
The content of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) (Et; EC 4.1.1.39) measured in different-aged leaves of sunflower (Helianthus annuus) and other plants grown under different light intensities, varied from 2 to 75 μmol active sites m−2. Mesophyll conductance (μ) was measured under 1.5% O2, as well as postillumination CO2 uptake (assimilatory charge, a gas-exchange measure of the ribulose-1,5-bisphosphate pool). The dependence of μ on Et saturated at Et = 30 μmol active sites m−2 and μ = 11 mm s−1 in high-light-grown leaves. In low-light-grown leaves the dependence tended toward saturation at similar Et but reached a μ of only 6 to 8 mm s−1. μ was proportional to the assimilatory charge, with the proportionality constant (specific carboxylation efficiency) between 0.04 and 0.075 μm−1 s−1. Our data show that the saturation of the relationship between Et and μ is caused by three limiting components: (a) the physical diffusion resistance (a minor limitation), (b) less than full activation of Rubisco (related to Rubisco activase and the slower diffusibility of Rubisco at high protein concentrations in the stroma), and (c) chloroplast metabolites, especially 3-phosphoglyceric acid and free inorganic phosphate, which control the reaction kinetics of ribulose-1,5-bisphosphate carboxylation by competitive binding to active sites.
Rubisco (EC 4.1.1.39) catalyzes the irreversible carboxylation of RuBP to form two PGA molecules (in this work the oxygenase reaction was not active since a low O2 concentration was used). RuBP carboxylation is the major rate-determining reaction in photosynthetic CO2 assimilation. All factors that influence the photosynthetic rate do so by influencing the activity of Rubisco and the concentration of its substrates, CO2 and RuBP. Et in leaves may be as high as 75 μmol m−2, and for the extracted enzyme Km(CO2) = 9.4 μm (Makino et al., 1985a) and Km(RuBP) = 30 to 40 μm (Yeoh et al., 1981). In leaves photosynthesizing under atmospheric conditions, the concentration of RuBP may increase to 10 to 15 mm (Badger et al., 1984; Sharkey et al., 1986), but the concentration of CO2 is usually about 4 to 8 μm in leaf intercellular spaces, depending on stomatal conductance. This CO2 concentration is well below the Km(CO2) of the enzyme, and it is the initial slope of the kinetic curve VM/Km(CO2), termed carboxylation conductance, that becomes important.
rc limits the CO2-fixation rate in series with the other resistances, rg and rmd. The carboxylation rates are usually expressed in relation to Ci or Cw. Cc is usually about 20% to 30% lower than Cw because of concentration decrease generated by the carboxylation flux on rmd. Considering the above, the carboxylation conductance in intact leaves in vivo may be found as the initial slope of the A versus Cc graph at low Cc values. If Cc cannot be calculated because rmd is unknown, the closest approximation is a plot of A versus Cw or A versus Ci. The true parameters of the carboxylase can be found only from experiments carried out in nonphotorespiratory conditions (1%–2% O2); otherwise the competing oxygenase reaction consumes a part of RuBP and partially inhibits carboxylase activity.
Because of technical problems with the measurement of A versus Cw relationships, in many studies only the net photosynthetic rate under atmospheric conditions (21% O2) was related to Rubisco activity or content. Nevertheless, good correlation has been found (Makino et al., 1983; Hudson et al., 1992; Jacob and Lawlor, 1992; Jiang and Rodermel, 1995; Nakano et al., 1997). These results indicated that the level of Rubisco protein could be a limiting factor in photosynthesis throughout the life span of the leaf under natural environmental conditions. On the other hand, when Rubisco levels in leaves exceeded 4 g m−2 (60 μmol m−2), the in vivo Rubisco activity (measured as photosynthesis under pCi = 20 to 30 Pa and 21% O2) became curvilinearly correlated with Et (Makino et al., 1994, 1997). When measurements were made over the whole life span of wheat leaves, the measured rates of photosynthesis were lower in young leaves, which had high protein content, than would have been expected from the amount and activity of Rubisco (Lawlor et al., 1989).
During senescence the decrease in Rubisco activity was initially greater than the decrease in net photosynthesis (Hall et al., 1978). In a willow canopy, Rubisco-specific activity was higher when the apparent Et (N content in leaves) was smaller (Vapaavuori and Vuorinen, 1989). A similar nonlinearity was found in our previous experiments (Eichelmann and Laisk, 1990), in which we obtained a saturating relationship when Et exceeded 30 μmol m−2. In the latter work the initial slope of the A versus Cw curves under nonphotorespiratory conditions (1.5% O2) was assumed to represent the Rubisco activity in vivo and was compared with the Et. We discovered that growth light had the strongest influence on the saturation of the relationship between μ and Et. In the present work we present insight into this relationship, using not only plants grown under different light intensities but also leaves adapted to different light intensities.
MATERIALS AND METHODS
Plant Material
Sunflower (Helianthus annuus L.), cotton (Gossypium hirsutum L.), bean (Phaseolus vulgaris L.), and English spinach (Rumex patientia L.) were grown in growth boxes in 3-L pots filled with a commercial fertilized peat-soil mixture in a 16-h/8-h 28°C/18°C day/night cycle at a PAD of 250 to 300 μmol m−2 s−1 (high light) or 40 to 60 μmol m−2 s−1 (low light). In increased-N experiments plants were watered every 3 d with 200 mL of a solution of 0.5 g carbamide L−1. For different phosphate treatments, plants were grown in a greenhouse under natural sunlight in June, and a nonfertilized peat-soil mixture was supplemented with fertilizers as described below. Leaves of different ages were used in experiments, as specified below.
Gas-Exchange Measurements
A rapid-response leaf gas-exchange measurement system (Fast-Est, Tartu, Estonia; Oja, 1983) was used (leaf chamber, 4.4 × 4.4 × 0.3 cm3; gas flow rate, 20 cm3 s−1). The system consisted of two similar, open gas channels, channels 1 and 2, which allowed independent gas preconditioning. The channels were equipped with laboratory-made psychrometers for water vapor measurements and IR CO2 analyzers (Infralyt 3, Junkalor, Jena, Germany [channel 1] and LI 6262, Li-Cor, Lincoln, NE [channel 2]). The leaf chamber could be rapidly switched from one channel to the other, which made it possible to produce rapid changes in CO2 concentration and to start gas-exchange recording 3 s after switching. The abaxial side of the leaf was sealed with starch paste to the chamber window, the temperature of which was controlled with a thermostat water at 22.3°C. This increased the heat-exchange coefficient between the leaf and the water to 30 mW cm−2 °C−1, which stabilized the leaf temperature within 1°C of the circulated water, even when the maximum PAD was applied. This procedure prevented gas exchange through the upper epidermis, but Cw was calculated for all measurements on the basis of leaf temperature, transpiration, CO2-exchange rate, and CO2 solubility (Laisk, 1977; Laisk and Oja, 1998). Since Cw is calculated on a micromolar basis (as dissolved in water), we also present Cw0 in micromolar units considering the concentration that would exist in water in equilibrium with the ambient gas.
AC
AC was measured in 1.5% O2 and Cw0 = 3.25 μm (Cw from 1.1 to 3.3 μm) independently of the previous conditions of steady-state photosynthesis. The low Cw0 was used to suppress additional postillumination PEP carboxylation, which increases considerably with CO2 concentration (Laisk, 1985). For the AC measurements, the light was switched off and the leaf chamber was simultaneously switched to channel 2, where CO2 and O2 concentrations were as specified above. Alternatively, in some experiments light was switched off but the leaf chamber was left in the same channel as previously. For the measurements of ACM, an additional 10- to 20-s exposure in CO2-free N2 with 1.5% O2 was added to the routine before the leaf chamber was darkened and switched to channel 2. We assumed that during this exposure most of the Calvin cycle metabolites were converted into RuBP, according to the thermodynamic free energy gradient. The degree of error of the CO2 concentration and exchange-rate measurements was less than 1%, the degree of error of the calculated Cw was about 5%, and the margin of error of the calculated μ was about 5% (Oja, 1983). Thus, the scattering of data was caused mainly by biological and temporal variation of leaf parameters during and between experiments rather than by measurement errors.
μ
μ was taken as the closest measure of Rubisco activity in vivo. The reciprocal (1/μ) is rm. The parameter μ is close to the carboxylation conductance, except that there is an additional diffusion component in 1/μ caused by transport of the dissolved CO2. Experimentally, μ was calculated as the initial slope of an A versus Cw graph using two measurements, one carried out at a Cw0 of 0 and the other at a Cw0 of 3.25 μm. Nonlinearity of the response was corrected according to:
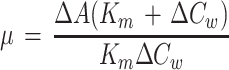 |
1 |
where Km is for CO2 and Δ indicates the difference. Km(CO2) = 11 μm, as previously estimated from gas-exchange measurements (Eichelmann and Laisk, 1990), in agreement with the Km(CO2) of 9 to 10 μm measured in vitro correcting the pK(HCO3) for the ionic strength in the chloroplast (Yokota and Kitaoka, 1985). If we consider that
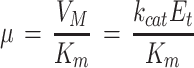 |
2 |
we see that
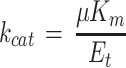 |
3 |
Equation 2 relates the measured μ to enzyme characteristics.
AM
AM was measured as the CO2 uptake rate at a Cw0 of 70 μm and a PAD of 1200 to 1400 μmol m−2s−1, after photosynthesis stabilized under the high CO2 concentration (about 15 min).
Et
A sample (6–8 cm2) was cut from an intact leaf, frozen in liquid N2, ground, and extracted in 3 mL of buffer (80 mm Tris-HCl, pH 6.8, 2% SDS, 100 mm DTT, and 850 mm glycerol). The extract was dissolved (1:2 or 1:4) for 10% SDS gel electrophoresis. The Et was determined on the basis of the large subunit band, stained with Coomassie blue, and measured photometrically (Eichelmann and Laisk, 1990). Purified Rubisco from sunflower was used as a standard (the concentration was determined gravimetrically) for calibration of the gels. The remaining part of the sampled leaf was left attached to the plant so that another sample could be taken later from the other half of the leaf symmetrically with respect to the midrib.
RESULTS
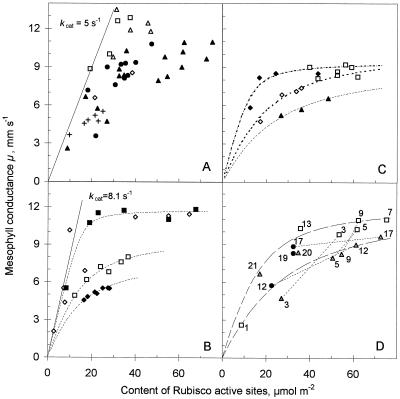
In leaves used in these experiments, Et varied between 2 and 75 μmol active sites m−2 (0.14–5 g Rubisco protein m−2). At high N nutrition (0.5 g carbamide L−1 in watering solution), Et varied from 10 to 75 μmol m−2 in leaves of different ages. In different soil phosphate treatments the maximum Et was 50 μmol m−2; in low-light-grown sunflower the range was from 10 to 30 μmol m−2 (Fig. 1; Table I). A linear relationship between Et and μ would be expected if all sites were equally active. However, the measured μ versus Et relationships were linear only at low Et, but they were saturated beyond an Et of approximately 30 μmol m−2 (2 g Rubisco protein m−2; Fig. 1, A and C). The kcat calculated from Equation 2 using the initial slope of the relationship in Figure 1A was 5 s−1, assuming a Mr of 550,000 and eight active sites per molecule. In low-light-grown sunflower the maximum μ was only about 5 mm s−1 compared with 9 to 13 mm s−1 in high-light-grown plants, but it still showed a tendency toward saturation. Such a saturating relationship between μ and Et is typical not only for sunflower but also for other C3-type plants such as cotton, bean, potato, and English spinach (Fig. 1C; Eichelmann and Laisk, 1990).
Figure 1.
Relationships between Et and μ in leaves. A, Influence of growth conditions. •, High-light-grown, commercially fertilized sunflower, different-aged leaves; ▴, high-light-grown sunflower, increased-N treatment, different-aged leaves; ⋄, 25 mg g−1 KH2PO4 kg−1 soil; ▵, 140 mg g−1 KH2PO4 kg−1 soil; □, 250 mg g−1 KH2PO4 kg−1 soil (plants grown in a greenhouse in May–June); ×, low-light-grown sunflower. Line corresponds to kcat of 5 s−1 per Rubisco active site. B, Adaptation to different light intensities. ♦, 2.5-week-old, low-light-grown plants; ⋄, low-light-grown plants adapted at high-light intensity for 2 weeks; ▪, 2-week-old, high-light-grown plants; □, high-light-grown plants adapted 2 weeks at low-light intensity. Line corresponds to kcat 8.1 s−1 per Rubisco active site. C, Different-aged leaves of different species. ♦, Cotton; ⋄, bean; □, potato; ▴, English spinach. D, Changes over the life span of single leaves. □, Different leaves of a 2-week-old plant; , 3.5-week-old plant; •, 5.5-week-old plant. Numbers at the points correspond to the plant leaf, numbered upward, not counting cotyledons and juvenile leaves. Dotted lines indicate changes in the same leaf. C and D, Data from Eichelmann and Laisk (1990).
Table I.
Et, μ, and AC in leaves
| Growth Condition | Et | μ | AC | SCE | |
|---|---|---|---|---|---|
| μmol m−2 | mm s−1 | μmol m−2 | μm−1 s−1 | ||
| 1 | LLa | 7.4 | 5.5 | 123 | 0.044 |
| 2 | LL | 17.3 | 5.7 | 143 | 0.040 |
| 3 | HLb | 66.0 | 10.0 | 255 | 0.040 |
| 4 | LL_HLc | 7.0 | 11.1 | 277 | 0.040 |
| 5 | LL | 17.9 | 4.9 | 123 | 0.039 |
| 6 | LL | 9.0 | 4.9 | 125 | 0.039 |
| 7 | LL_HL | 42.5 | 13.5 | 241 | 0.056 |
| 8 | LL_HL | 22.5 | 11.4 | 200 | 0.057 |
| 9 | LL | 22.5 | 8.9 | 118 | 0.076 |
| 10 | HL | 22.4 | 4.7 | 118 | 0.040 |
| 11 | LL | 7.4 | 5.5 | 124 | 0.044 |
| 12 | LL_HL | 7.0 | 11.1 | 277 | 0.040 |
| 13 | LL | 27.1 | 5.5 | 85 | 0.065 |
| 14 | LL_HL | 27.0 | 8.9 | 135 | 0.066 |
| 15 | HL | 66.0 | 10.0 | 255 | 0.040 |
| 16 | HL | 66.0 | 13.5 | 304 | 0.043 |
| 17 | HL | 25.0 | 6.6 | 145 | 0.046 |
| 18 | HL | 25.0 | 9.3 | 201 | 0.046 |
LL, Low-light-grown plant.
HL, High-light-grown plant.
LL_HL, Low-light-grown plant adapted to high light.
In the next experiment a low-light-grown plant was transferred to high light with the aim of inducing Rubisco adaptation (Fig. 1B). Low-light-grown plants are characterized by a low maximum μ of 4.5 to 5 mm s−1 and with a comparatively small range of Rubisco active sites in leaves of different ages (from 18 to 28 μmol m−2). Because of this narrow range, no data points were obtained on the initial slope of the relationship, and we can state only that the calculated kcat was ≥4.5 s−1. When the low-light-grown plants were transferred to high light, the Et in existing leaves decreased drastically during the 2-week adaptation time to the extent that the Et came to limit the μ (Fig. 1B). The calculated kcat was 8 s−1 for these leaves. New leaves grown under high light behaved like typical high-light-grown leaves, having a high μ and the ability to synthesize large amounts of Rubisco, up to 65 μmol m−2 (Fig. 1B).
In a reverse experiment, high-light-grown plants were readapted under low light. Leaves initially having high μ (11 mm s−1) and large Et (up to 68 μmol m−2 in fully expanded leaves) were converted into typical low-light-grown leaves, with a low maximum μ (6–7.5 mm s−1) and a limited range of Et (15–40 μmol active sites m−2).
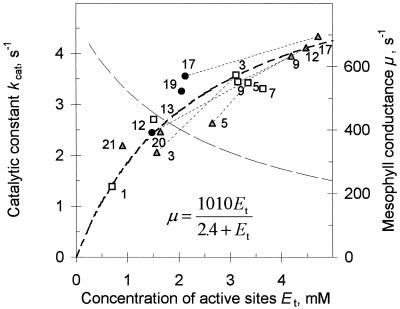
Figure 1D demonstrates changes in μ and Et during the life span of one plant grown under a light intensity of 180 to 200 μmol m−2 s−1. To eliminate the effect of the leaf mesostructure (cell and chloroplast anatomy) on the data, Et from this experiment was transformed into the molar concentration of active sites (Fig. 2). Chloroplast volume per meter squared was assumed to be proportional to chlorophyll concentration using the conversion factor 50 μL stroma mg−1 chlorophyll (Eichelmann and Laisk, 1990). Three measurement series were carried out at plant ages of 2, 3.5, and 5.5 weeks. The leaves were numbered upward and did not include cotyledons and juvenile leaves. Data points connected with dashed lines correspond to the same leaf at different plant ages. In the molar presentation of Figure 2 the scattering of data is smaller than in the area presentation of Figure 1D, but the curvilinear relationship clearly remains. The data show that when a leaf is young its Et is low (0.8–1.5 mm active sites, leaf no. 13 in the 2-week-old plant and leaf nos. 20 and 21 in the 3.5-week-old plant).
Figure 2.
μ and kcat presented as functions of Et (data from Fig. 1D). Thick, dashed line was calculated from the equation; the thin, dashed line corresponds to kcat calculated from the thick line.
In fully expanded leaves Et reached a maximum (3–4 mm in leaf nos. 3–9 in the 2-week-old plants or 4–5 mm in leaf nos. 9–17 in the 3.5-week-old plant). During the senescing of leaves Et and μ both decreased and followed the same relationship as during leaf expansion (senesced leaf no. 1 in the 2-week-old plant, leaf nos. 3 and 5 in the 3.5-week-old plant, and leaf no. 12 in the 5.5-week-old plant). For example, in leaf nos. 12 and 17, during aging from 3.5 to 5.5 weeks, Et decreased 3-fold, whereas μ decreased only 30%. In the 5.5-week-old plant the relatively young leaf no. 19 did not accumulate as much Rubisco as leaf nos. 3 to 9 from the 2-week-old plant or leaf nos. 9 to 17 in the 3.5-week-old plants, probably because of whole-plant senescence, limitations in mineral nutrition under the pot-bound conditions, and outflow of N to regenerative organs. The saturating relationship between Et and μ is characterized with the value of kcat, which decreased from 4.5 to 1.5 s−1 in the range of Et from 0.5 to 5 mm (Fig. 2).
μ and AC
μ of a leaf depends on Et and also on the pool of RuBP. The latter can be estimated from AC in intact leaves. AC was measured in different leaves by darkening the leaf during steady-state photosynthesis at Cw0 of 3.25 μm and 1.2% O2. μ was calculated from the CO2-fixation rate just before the darkening. In different leaves chosen at different ages from different growth treatments, Et differed considerably, whereas μ values differed very little in the same leaves (Table I). On the other hand, it was also possible to find pairs with very close Et values but different μ values (nos. 9–18). Usually, close AC values corresponded to close μ values. The last column in Table I presents the SCE, which is the slope of the μ versus AC curve (Laisk et al., 1984). This parameter is close to 0.045 mm s−1 μmol−1 m2 (μm−1 s−1 expressed as a second-order rate constant), being similar in leaves of different ages and from different growth treatments. However, this parameter does not seem to be a basic constant of Rubisco, because in some leaves (mostly in low-light-grown leaves with a small AC) the SCE was significantly higher (0.066–0.076 μm−1 s−1).
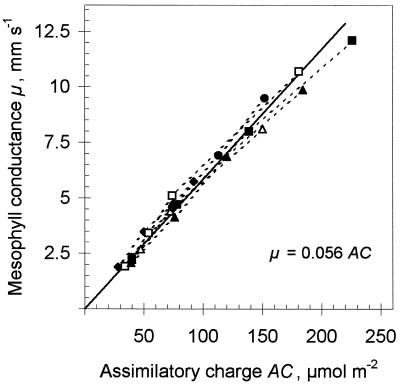
As long as SCE remains constant, μ proportionally depends on AC. This dependence was checked in the next experiment as AC changed under varying light intensity. In a leaf of a high-light-grown plant, AC and μ were measured at a Cw0 of 3.25 μm and O2 of 1.5% by darkening the leaf during steady-state photosynthesis. After the postillumination CO2 uptake ceased, the light was turned on again and the steady-state photosynthetic rate was recovered. Then PAD was decreased and after 15 s the light was switched off to measure AC from the low-light state. Fifteen seconds under decreased PAD was sufficient to establish a lower equilibrium RuBP and a higher PGA concentration but too short to change Rubisco activity. Different AC values were obtained by jumping from the maximum PAD to different, lower PADs. From Equation 1 the corresponding μ was calculated for every AC, whether light saturated or light limited, and the calculated μ was plotted against AC (Fig. 3). Data for five different-aged leaves of the same plant all present a proportional dependence, with a SCE of 0.056 mm s−1 μmol−1 m2.
Figure 3.
Relationship between μ and AC for five different-aged leaves of one plant. Different assimilatory charges were generated by decreasing light intensity from 1300 μmol m−2 s−1 to different levels for 10 s, and then μ and AC were measured at a Cw0 of 3.25 μm. SCE was 0.056 mm s−1 μmol−1 m2 (the slope of the solid line).
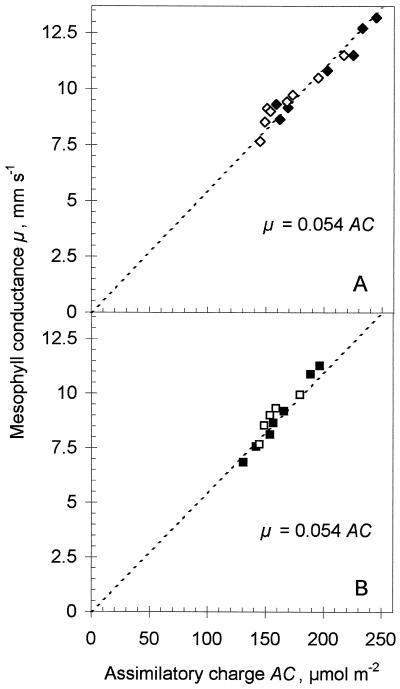
In another experiment, Pi or Man was fed to excised leaves through the petiole. Increasing the Pi concentration in the cytosol is expected to drain triose phosphates out of the chloroplast stroma as a result of the activity of the Pi translocator. Man binds Pi in the cytosol and thus decreases the drainage of triose phosphates from chloroplasts, increasing the concentration of Pi esters in the stroma. Both of these treatments result in decreasing AC, either because the pools of the carbon reduction cycle generally decrease (Pi feeding) or, more likely, because they accumulate in hexose phosphates because of active starch synthesis in Man-fed leaves (Eichelmann and Laisk, 1994). Most remarkably, μ varied proportionally with AC, with an SCE of 0.054 mm s−1 μmol−1 m2 (Fig. 4). After Pi or Man was removed, the μ and AC values tended to recover, maintaining the SCE value constant. Although in the above-described experiments we could only decrease AC, a temperature treatment resulted in an increase in AC and μ. After a leaf was exposed at 27°C for 10 min, both AC and μ, measured after a 30-min stabilization following transfer to 23°C, increased by 40%, but μ remained proportional to AC and SCE remained constant (Table I, nos. 17 and 18).
Figure 4.
Relationships between μ and AC obtained by varying the Pi concentration in the cytosol by feeding Pi or Man through the petiole. A, Feeding 10 mm Man for 110 min (♦) and reversing the experiment in distilled water for 125 min (⋄). B, Feeding 33 mm Pi for 190 min (▪) and reversing the experiment in distilled water for 120 min (□). The slope of the dashed lines corresponds to a SCE of 0.054 mm s−1 μmol−1 m2.
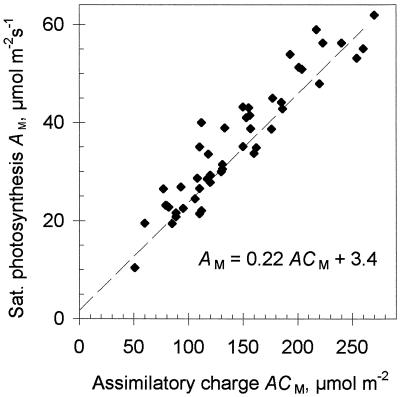
Although AM is not determined by Rubisco, but in most cases by the end-product synthesis rate and/or the capacity of the RuBP-regeneration chain, a good correlation was still found between AM and ACM (maximum AC measured after an exposure to CO2-free gas) in different-aged leaves from differently treated plants (Fig. 5). To the extent that ACM reflects the carbon reduction cycle pool of Pi esters, AM is proportional to that pool. In experiments including high- and low-light-grown and readapted plants, the correlation between AM and ACM was high and the slope of the dependence was the same.
Figure 5.
Relationship between AM and ACM. Different data points were obtained from different leaves grown at high or low light, re-adapted to different PADs or by feeding Man and Pi. Sat., Saturated.
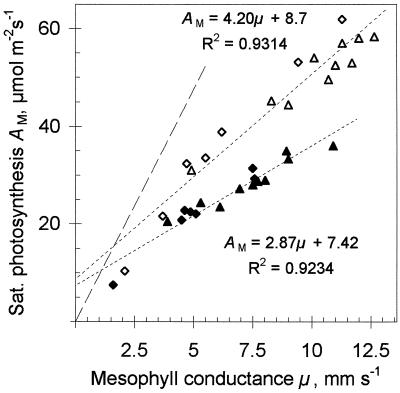
Since proportionality exists in the AC versus μ and the AC versus AM relationships, proportionality between μ and AM is also expected. The slope of this regression depends on light conditions during leaf growth (Fig. 6). It is remarkable that adapting plants from one light intensity to another moves the data points precisely from one regression line to another. Feeding Pi concentration changers (Man, Pi, and vanadate) to a leaf through the petiole decreases μ and AM simultaneously, moving data points along the same lines of proportionality.
Figure 6.
AM as a function of μ in sunflower leaves adapted to different light intensities. ♦, 2.5-week-old low-light-grown plant; ⋄, low-light-grown plant adapted 2 weeks at high light intensity; ▵, 2-week-old high-light-grown plant; ▴, high-light-grown plants adapted for 2 weeks at low light intensity. Dotted lines are regression lines; dashed line corresponds to VM of Rubisco calculated from the μ assuming the Michaelis-Menten kinetics.
DISCUSSION
By varying growth conditions and leaf age we obtained leaves that had a wide range of Et (0.14–5 g protein m−2, 2–75 μmol active sites m−2, or 0.5–5 mm active sites in the chloroplast stroma) to find relationships between Et and photosynthetic parameters of the leaf. In contrast to most previous researchers, we did not compare the Et with the photosynthetic rate at atmospheric CO2 and O2 concentrations but, instead, with μ under nonphotorespiratory conditions, which is the closest parameter to the carboxylation conductance measurable from leaf gas exchange. μ underestimates the carboxylation conductance by the proportion of rmd (since the total rm = rmd + rc). rmd usually makes up 20% to 30% of the total rm (Laisk and Loreto, 1996), and the rest of the resistance is due to the limited speed of carboxylation.
Carboxylation conductance is proportional to the Et or VM provided that Km(CO2) is constant. We found that μ was linearly related to Et at low Et values but was saturated at higher Et values. This saturation phenomenon caused a 2- to 3-fold decrease in the apparent kcat calculated from μ and Et (Fig. 2). This saturation is at variance with what was found in other studies, in which the photosynthetic rate at atmospheric levels of CO2 and O2 was linearly correlated with Et (Makino et al., 1983; Jacob and Lawlor, 1992; Jiang and Rodermel, 1995). A linear relationship between Et and VM of Rubisco, calculated from measured μ, was also obtained by von Caemmerer et al. (1994). However, in these investigations the Et did not exceed 35 μmol m−2, whereas the calculated kcat was 3.5 s−1. With this average kcat, the data in Figure 1 would also approximately satisfy a linear regression below 35 μmol active sites m−2. Saturating relationships between Et and photosynthesis have been found in other studies. For example, when Et exceeded 4 g m−2 in rice (Makino et al., 1994, 1997), wheat (Lawlor et al., 1989), or leaves of a willow canopy (Vapaavuori and Vuorinen, 1989), the dependence between the rate of CO2 assimilation and Et declined from linearity. In Chlorella pyrenoidosa cultures grown under high CO2, Et was higher but specific activity was lower than in cultures grown under low CO2 (Yokota and Canvin, 1986). These results agree with those in Figure 1, which shows a decrease of kcat with increasing Et. In our experiments the linearity between μ and Et broke down above 2 g protein m−2 or 25 μmol active sites m−2.
The saturating dependence between the Et and μ may be caused by physical and/or chemical properties. Physically, as rcdecreases, the rmd eventually begins to determine μ. Calculations based on leaf anatomy have shown that an rmd of 0.01 s mm−1 corresponds to a diffusion distance of 1 μm in chloroplasts (Laisk et al., 1970). rmd for CO2 has been estimated to be sufficient to cause depletion of CO2 at the carboxylation sites and to introduce curvilinearity into the relationship between photosynthesis and Et (Evans, 1983). Other calculations have shown that a decrease in Ci cannot be large enough to cause curvilinearity in the relationship (Makino et al., 1985a; Lawlor et al., 1989). Calculations from fluorescence-based measurements of the electron-transport rate and the net CO2-exchange rate (Laisk and Loreto, 1996) showed that rmd was usually between 0.02 and 0.04 s mm−1 in herbaceous plants and did not exceed 20% to 30% of the total rm. Measurements of sunflower in our laboratory have given similar results, estimating rmd from 0.02 to 0.06 s mm−1 for full-grown leaves (Laisk and Sumberg, 1994; V. Oja and E. Eichelmann, unpublished results). In this work the minimum values of rm were about 0.1 s mm−1, which means that diffusionally limited μ is about 2 to 5 times greater than the actual maximum μ. This estimate shows that μ could still be carboxylase limited, even at the maximum Et. This was clear in low-light-grown plants.
rc is determined by the content and activity of Rubisco on one hand and by inhibiting stroma metabolites, e.g. PGA and free Pi, on the other (Badger and Lorimer, 1981; Foyer et al., 1987). Because our measurements of μ were carried out at limiting CO2 and low O2 concentrations, it is likely that Pi and PGA accumulated at very low levels and RuBP was at a maximum (Badger et al., 1984; Dietz and Heber, 1986; Seemann and Sharkey, 1986). Therefore, we initially ignore the metabolite effects and discuss the μ as a function of the content and activity of Rubisco. The data in Figure 1B most clearly show that, in high-light-grown leaves at low Et, μ increases proportionally with Et. The calculated kcat was 8.1 s−1, slightly higher than the highest values obtained in Rubisco assays in vitro (5–6 s−1; Evans and Seemann, 1984; Flachmann et al., 1997), whereas the modeled kcat values in mature leaves are 3 to 3.6 s−1 (von Caemmerer et al., 1994; Mate et al., 1996).
In leaves that had the highest kcat values we assume that all of the Rubisco was completely active. When the Et exceeded 20 μmol active sites m−2 and μ approached 11 mm s−1, the relationship was saturated, although Et further increased to 65 μmol m−2. The abrupt saturation of the relationship (Fig. 1, B and C) supports the case of chemical limitation, because the limitation by rd would have resulted in a slowly saturating rectangular hyperbola. However, the abrupt saturation might be induced by changes in leaf mesostructure that took place in parallel with the changes in Et, because the relationship shown in Figure 2 is close to a rectangular hyperbola. Proceeding from the model of chemical limitation, we see that there was a ceiling that limited the amount of active Rubisco to about 20 μmol reaction sites m−2 in high-light-grown leaves. In low-light-grown leaves the relationship tended toward saturation at slightly higher Et than in the high-light-grown leaves, but the maximum μ was considerably lower.
It appears that in low-light-grown leaves the same or only a slightly greater maximum number of reaction sites could be activated as in high-light leaves, but each site apparently had a slower kcat. Proceeding from this, a corresponding model would involve two processes that determine the Rubisco activity in vivo: one that determines the specific activity (turnover rate) of each active site and another that limits the maximum number of active sites. The first process is sensitively regulated by growth light, whereas the second is relatively insensitive to this parameter. For example, there may be a dynamic equilibrium between activation by the Rubisco activase system and nonenzymatic deactivation of Rubisco by a large molar excess of RuBP and other metabolites (Portis et al., 1986; Zhu and Jensen, 1991).
In leaf extracts containing a constant concentration and specific activity of Rubisco activase (about 0.45 μmol min−1 mg−1), the specific activity of Rubisco declined as the amount of Rubisco increased (Portis et al., 1986). This result is similar to what we found in intact leaves: an increase in the Et resulted in a decrease of kcat (Fig. 1). In experiments by Portis et al. (1986) the optimum concentration was 100 μg mL−1 Rubisco and 300 μg mL−1 Rubisco activase. In intact leaves this ratio seems to be shifted toward a higher Et. In transgenic leaves with limited expression of Rubisco activase, photosynthesis was not influenced before activase content decreased more than 10 times (Mate et al., 1996). This result seemingly contradicts our above-described model, which emphasizes the limiting role of activase; however, several cofactors are needed to activate the activase itself.
ATP is an important cofactor of Rubisco activase in isolated chloroplasts (Robinson and Portis, 1988) but not in intact leaves (Brooks et al., 1988). In leaves light-induced electron transport through the PSI region of the electron-transport chain and the establishment of a transthylakoid pH gradient are obligatory for full activation of Rubisco (Campbell and Ogren, 1990, 1992). A good correlation between the dark-light pH difference in chloroplast stroma and μ has been observed (Eichelmann and Laisk, 1990). These results suggest that its binding sites at the PSI region of thylakoids may govern the activation process of Rubisco, not the content and activity of the Rubisco activase. Another important factor limiting the activation process may be the limited diffusibility of activase and Rubisco proteins, which could limit the protein-to-protein interaction required for activation. In our experiments Et increased to 350 mg mL−1 in the chloroplast stroma, or to about 700 mg mL−1 of total soluble protein. At such a concentration the state of the protein is close to crystalline, strongly limiting the movement of the activase and the carboxylation substrates. In crystalline Rubisco, the water content is 200 mg g−1 protein (Paulsen and Lane, 1966). In protein-bound water, diffusion of small molecules is from 2 to 5 times slower than in free water (Lawlor et al., 1989), and the Michaelis-Menten kinetics of Rubisco catalysis may no longer apply because of limited diffusibility of RuBP and PGA.
Whatever the mechanistic reasons, our results confirm the notion that a part of Rubisco is not available for carboxylation and plays the role of storage protein. This idea was suggested as early as the 1960s, when Rubisco was discovered and found to make up as much as 60% of soluble protein in the chloroplast (Paulsen and Lane, 1966). Alteration of the source-to-sink ratio by removing the fruit in soybean resulted in the formation of an insoluble form of Rubisco in leaf extracts, with a specific activity 5 times less than that of the soluble form (Crafts-Brandner et al., 1991).
In transgenic rice leaves it was necessary to reduce Rubisco levels by about 55% to achieve 100% Rubisco activation (Makino et al., 1997). Using radioactive label Mae et al. (1983) demonstrated that Rubisco is a major N depot to support the growth of young tissues. This is in good accordance with our measurements of Et during the life span of sunflower leaves (Figs. 1D and 2). During leaf expansion Et increased, and during senescence it decreased again (e.g. leaf nos. 3 and 9 in Fig. 2). In a 2-week-old plant, the two leaves had similar Et (3.2 mm) and μ (570 s−1) values. Later (1.5 weeks), leaf no. 9 was fully expanded and Et increased to 4.2 mm, whereas μ (620 s−1) increased only 8%. Leaf no. 3 had begun to senesce and the Et (1.6 mm) had decreased by 50%, whereas μ (330 s−1) had decreased by 40%. The process of remobilizing the N depot from old leaves to support the growth of new leaves explains why, during the adaptation of high-light-grown leaves to low light, the data points of the μ versus Et graph are above the data points for normal low-light-grown leaves (Fig. 1B): the readapted leaf still has a larger N content than the low-light-grown leaf.
As defined above, the second component of carboxylation resistance is related to inhibiting stroma metabolites. μ correlated surprisingly well with assimilatory charge (Figs. 3 and 4), which is a gas-exchange measure of the RuBP pool (Laisk et al., 1984, 1987; Eichelmann and Laisk, 1990; Ruuska et al., 1998). It is not easy to explain why the μ versus AC relationships were so linear up to RuBP concentrations of 10 to 20 mm, considering that Km(RuBP) for Rubisco is about 40 μm in vitro (Servaites et al., 1991). Farquhar (1979) and von Caemmerer and Farquhar (1985) attempted to resolve this problem by assuming that not all RuBP is available for carboxylation, only its nonchelated part, whereas the RuBP-Mg2+ complex cannot be a substrate for carboxylation. In the presence of ample Mg2+ only a minor fraction of RuBP remains free to be the carboxylation substrate. When the RuBP concentration is less than the concentration of active sites (2 mm in the activated state in our experiment) the reaction kinetics remain proportional to the RuBP concentration (Farquhar, 1979). This is a plausible explanation, except that it does not explain why the concentration of substrate RuBP, a small residual of the budget, is so constant and well reproducible from experiment to experiment, as is the measured maximum μ.
According to Michaelis-Menten kinetics, the reaction rate is expected to have little dependence on the RuBP concentration until it decreases to the concentration of active sites. The measured proportionality between the RuBP pool (AC) and reaction rate (μ) suggests that Rubisco must become progressively inhibited while the RuBP concentration decreases. In experiments with extracted Rubisco having a concentration up to 1.6 mm active sites, Paech (1986) found that an initial, fast PGA formation soon faded, indicating decreasing Rubisco activity in time. Eichelmann (1985) measured kinetic curves of RuBP utilization in extracts from sunflower leaves and in solutions of partially purified enzyme with Et values from 5 to 30 mg mL−1 protein (0.07–0.4 mm active sites). No RuBP saturation plateau was observed, and the kinetic curves of RuBP utilization were very similar to postillumination uptake curves in leaves (Laisk et al., 1987).
The continuous decrease in Rubisco activity during the RuBP utilization reaction was explained by competitive inhibition of the enzyme by the product, PGA. The KI(PGA) calculated from the in vitro curves was 0.54 mm. However, when external PGA was added or when RuBP was repeatedly injected, leaving previously generated PGA in the reaction mixture, its KI(PGA) was much higher (3.7 mm). It was concluded that enzyme-bound, freshly generated PGA has a higher efficiency for inhibition than the free PGA. A similar result was reported later (Fong and Butcher, 1988), confirming that enzyme-bound PGA molecules formed by RuBP carboxylation are not equivalent to free PGA. The explanation is that PGA formed from C-3, C-4, and C-5 of the six-carbon intermediate (2-carboxy-3-keto-arabinitol-1,5-bisphosphate) is rapidly released, but PGA formed from C-1, C-2, and C-2′ is tightly bound to the Mg2+ of the enzyme and occupies the active site for a relatively long time.
Reversible binding of external PGA to Mg2+ of the active site is less likely. PGA and free Pi are both known to be competitive inhibitors for Rubisco: KI(PGA) is 0.85 mm (Badger and Lorimer, 1981) and KI(Pi) is 0.65 mm (Jordan et al., 1983). It is a general rule that the RuBP pool is complementary to PGA and Pi pools (Badger et al., 1984), because the total Pi pool is a constant and other carbon reduction cycle pools (except hexose phosphates under some conditions) are smaller than those directly involved in RuBP carboxylation. In the initial state of our postillumination experiments, under saturating light and limiting CO2 concentrations, most of the Pi was probably bound in RuBP, leaving little of it in PGA or in a free state. Beginning from this state, decreases of AC were created by decreasing the light intensity for 10 s. This time was sufficient to convert a part of the RuBP into PGA, increasing the PGA-to-RuBP ratio. The relationship between μ and the RuBP pool (measured as AC) in the presence of a varying PGA-to-RuBP ratio was strictly linear, with the same proportionality constant for different leaves grown under similar conditions (Table I; Fig. 3) but also when Pi and Man were fed to influence the cytosolic and chloroplast Pi pools (Fig. 4). Linear kinetics have been modeled to describe the postillumination CO2-fixation process, during which the RuBP pool decreases and the PGA pool increases, using the above inhibition coefficient by PGA (Laisk et al., 1987), showing that product inhibition can explain the close-to-linear kinetics of Rubisco.
The proportionality constant relating μ to AC, SCE, was 0.04 to 0.065 mm s−1 μmol−1 m2. SCE varies when external conditions lead to variations in Rubisco activation state (e.g. waiting until Rubisco activity stabilizes at each limiting PAD; Laisk et al., 1984), but its maximum value is usually relatively constant in similarly grown leaves. It is a rule of thumb that SCE is higher in leaves that have lower maximum AC pools (Laisk et al., 1984, 1987) and decreases with senescence (Eichelmann and Laisk, 1990). This is consistent with the above product-inhibition model, which predicts that the true uninhibited μ can be measured only under conditions in which PGA and Pi pools are minimal, i.e. under saturating light and limiting CO2 concentrations and low O2 concentrations.
Under low light and in the presence of saturating CO2 or high O2 concentrations, the PGA pool is not minimized and it has an inhibitory effect on Rubisco. Evidently, this is one reason why μ decreases with increasing O2 concentration (Laisk and Loreto, 1996), despite the fact that RuBP pool may still be large enough to saturate the enzyme. During postillumination CO2 fixation, the initially maximum μ decreases continuously during the whole period of RuBP consumption, which lasts longer the greater the RuBP pool. However, the larger the chloroplast Pi pool, the larger the RuBP pool. Thus, SCE is steeper the lower the initial RuBP concentration, i.e. the lower the chloroplast Pi content. An important conclusion is that SCE is not a true characteristic of Rubisco but is influenced by the chloroplast Pi pool. To the extent the latter stays constant, relative changes in SCE may be interpreted to indicate changes in Rubisco-specific activity, but one should be cautious when comparing SCEs from different species or leaves. It is also important that only μ measured under conditions in which PGA and Pi are minimized can be safely interpreted as Rubisco activity and related to its content.
We also made some observations that are more complicated to explain by the competitive inhibition model. Incubation of a leaf for 10 min at only a 4°C higher temperature (27°C) caused a 40% increase in AC, whereas the μ versus AC relationship remained constant. Staying with the model, we conclude that a part of the Pi was converted from an inhibitory state (PGA, Pi) into the substrate state (RuBP). Thus, one must be careful in interpreting the maximum ACM pool and the corresponding μ obtained under the light-saturating and CO2-limiting conditions as a true maximum. Some Pi may still reside in an inhibitory form, e.g. as hexose phosphate.
Another way to change μ and AC while maintaining SCE constant was to feed compounds through the phloem (Fig. 4). Our treatments were expected to increase (feeding Pi) or decrease (feeding Man) Pi concentration in the cytosol and to influence the Pi concentration and Pi distribution in stroma sugar phosphates via the Pi translocator. Since these treatments were not expected to change the total Pi in the stroma but only its distribution between free and organic forms, the correlated changes between μ and AC were expected. Either increasing or decreasing cytosolic Pi resulted in a decrease in the RuBP pool. It is possible that the formation of hexose phosphates traps Pi and thereby decreases the amount available for RuBP formation, as suggested previously (Eichelmann and Laisk, 1994).
Aside from the proportionality between μ and AC, AM was also proportional to μ (Fig. 6). This proportionality was noted earlier when photosynthesis was influenced by salinity (Seemann and Sharkey, 1986), in ozonation of bean (Moldau et al., 1991; Moldau and Kull, 1993) and aspen (Kull et al., 1996), by varying growth light in sunflower (Eichelmann and Laisk, 1990), and in a Cyt b6/f-deficient antisense mutant of tobacco (H. Eichelmann, unpublished data). The clearest demonstration so far of the proportionality between μ and the rate of CO2 assimilation at 500 μbar CO2 in spinach leaves grown at different nitrate levels was given by Evans and Terashima (1988). The novelty of our present work is that the slope of μ versus AM was dependent on the growth light (Fig. 6).
The relationship between μ and AM deserves to be discussed because it was theoretically unexpected. AM is usually determined by end product (Suc and starch) synthesis rate and Pi turnover, which limits RuBP regeneration (Laisk and Laarin, 1983), whereas μ is determined by Rubisco and carbon reduction cycle pools. Such good correlative relationships between different photosynthetic parameters show that genes controlling different subsystems of leaf photosynthetic metabolism are proportionally expressed and controlled during development and adaptation.
It is possible that the total pool of Pi in chloroplasts is an important parameter controlling the development of the photosynthetic apparatus, or at least proportionally related to its amount. Presently we know very little about factors that determine the total Pi pool in chloroplasts. The good proportionality between different photosynthetic parameters may be a result of spatial compartmentation of the photosynthetic machinery into small units that can perform almost completely independently, or of association of enzymes into supercomplexes that channel intermediates (Gontero et al., 1988; Süss et al., 1995; Echeverria et al., 1997).
We suggest that saturation of the relationship between Et and μ is caused by three limiting components. Physical diffusion resistance in the liquid phase would limit the μ at about 2 to 5 times higher values than measured. An essential limitation of μ is caused by factors that allow only partial activation of Rubisco. We suggest that these are related to Rubisco activase, which itself is activated by thylakoid membrane-bound complexes such as PSI, ATPase, and the transthylakoid pH gradient. The slower diffusibility of Rubisco at high protein concentrations in stroma is a factor that may limit the activation process of Rubisco, breaking the balance between activation and deactivation processes. Finally, chloroplast metabolites, especially PGA and free Pi, control the reaction kinetics of RuBP carboxylation by competitively binding to active sites.
ACKNOWLEDGMENTS
We are grateful to two unknown reviewers and to Prof. G.E. Edwards for thorough analysis of the manuscript and comments.
Abbreviations:
- A
net CO2 uptake rate
- AM
light- and CO2-saturated CO2 uptake rate
- AC
assimilatory charge
- ACM
maximal assimilatory charge measured after exposure to CO2-free gas
- Cc
CO2 concentration at carboxylation sites
- Ci
CO2 concentration in the intercellular space
- Cw
dissolved cell wall CO2 concentration
- Cw0
external CO2 concentration
- Et
content of Rubisco sites
- kcat
catalytic constant
- KI
inhibition constant
- μ
mesophyll conductance (initial slope of the response curve of CO2 uptake versus dissolved cell wall CO2 concentration)
- PAD
absorbed photon flux density
- PGA
3-phosphoglyceric acid
- rc
carboxylation resistance
- rg
gas-phase resistance
- rm
mesophyll resistance
- rmd
liquid-phase diffusion resistance
- RuBP
ribulose-1,5-bisphosphate
- SCE
specific carboxylation efficiency
- VM
maximum rate of the enzyme
Footnotes
This work was supported by the Estonian Science Foundation (grant no. 1808).
LITERATURE CITED
- Badger MR, Lorimer GH. Interaction of sugar phosphates with the catalytic site of ribulose-1,5-bisphosphate carboxylase. Biochemistry. 1981;20:2219–2225. doi: 10.1021/bi00511a023. [DOI] [PubMed] [Google Scholar]
- Badger MR, Sharkey TD, von Caemmerer S. The relationship between steady-state gas exchange of bean leaves and the levels of carbon-reduction-cycle intermediates. Planta. 1984;160:305–313. doi: 10.1007/BF00393411. [DOI] [PubMed] [Google Scholar]
- Brooks A, Portis AR, Sharkey TD. Effect of irradiance and methyl viologen treatment on ATP, ADP and activation of ribulose bisphosphate carboxylase in spinach leaves. Plant Physiol. 1988;88:850–853. doi: 10.1104/pp.88.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WJ, Ogren WL. Electron transport through photosystem I stimulates light activation of ribulose bisphosphate carboxylase/oxygenase (Rubisco) by Rubisco activase. Plant Physiol. 1990;94:479–484. doi: 10.1104/pp.94.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WJ, Ogren WL. Light activation of Rubisco by Rubisco activase and thylakoid membranes. Plant Cell Physiol. 1992;33:751–756. [Google Scholar]
- Crafts-Brandner SJ, Salvucci ME, Egli DB. Fruit removal in soybean induces the formation of an insoluble form of ribulose-1,5-bisphosphate carboxylase/oxygenase in leaf extracts. Planta. 1991;183:300–306. doi: 10.1007/BF00197802. [DOI] [PubMed] [Google Scholar]
- Dietz KJ, Heber U. Light and CO2limitation of photosynthesis and states of the reactions regenerating ribulose 1,5-bisphosphate or reducing 3-phosphoglycerate. Biochim Biophys Acta. 1986;848:392–401. [Google Scholar]
- Echeverria E, Salvucci ME, Gonzales P, Paris G, Salerno G. Physical and kinetic evidence for an association between sucrose-phosphate synthase and sucrose-phosphate phosphatase. Plant Physiol. 1997;115:223–227. doi: 10.1104/pp.115.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson DL, Badger MR, Andrews TJ. Slow inactivation of ribulose bisphosphate carboxylase during catalysis is caused by accumulation of a slow, tight-binding inhibitor at the catalytic site. Plant Physiol. 1990;93:1390–1397. doi: 10.1104/pp.93.4.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichelmann H (1985) Gag exchange measurement of ribulosebisphosphate carboxylase activity in leaf extracts. In J Viil, G Grishina, A Laisk, eds, Kinetics of Photosynthetic Carbon Metabolism in C3 Plants. Valgus, Tallinn, Estonia, pp 90–95
- Eichelmann H, Laisk A. Ribulose-1,5-bisphosphate carboxylase content and kinetic characteristics of photosynthesis in leaves. Fiziol Rast. 1990;37:1053–1064. [Google Scholar]
- Eichelmann H, Laisk A. CO2 Uptake and electron transport rates in wild type and starchless mutant of Nicotiana sylvestris: the role and regulation of starch synthesis at saturating CO2concentrations. Plant Physiol. 1994;106:679–687. doi: 10.1104/pp.106.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JR. Nitrogen and photosynthesis in the flag leaf of wheat (Triticum aestivumL.) Plant Physiol. 1983;72:297–302. doi: 10.1104/pp.72.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JR, Seemann JR. Differences between wheat genotypes in specific activity of ribulose-1,5-bisphosphate carboxylase and relationship to photosynthesis. Plant Physiol. 1984;74:759–764. doi: 10.1104/pp.74.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JR, Terashima I. Photosynthetic characteristics of spinach leaves grown with different nitrogen treatments. Plant Cell Physiol. 1988;29:157–165. [Google Scholar]
- Farquhar GD. Models describing the kinetics of ribulose bisphosphate carboxylase-oxygenase. Arch Biochem Biophys. 1979;2:456–468. doi: 10.1016/0003-9861(79)90052-3. [DOI] [PubMed] [Google Scholar]
- Flachmann R, Zhu G, Jensen RG, Bohnert HJ. Mutations in the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase increase the formation of the misfire product xylulose-1,5-bisphosphate. Plant Physiol. 1997;114:131–136. doi: 10.1104/pp.114.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong FK, Butcher KA. Non-cyclic photoreductive carbon fixation in photosynthesis. Light and dark transient of the glycerate-3-P pair. Biochem Biophys Res Commun. 1988;150:399–404. doi: 10.1016/0006-291x(88)90534-7. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Furbank RT, Walker DA. Interaction between ribulose-1,5-bisphosphate carboxylase and stromal metabolites. I. Modulation of enzyme activity by Benson-Calvin cycle intermediates. Arch Biochem Biophys. 1987;894:157–164. [Google Scholar]
- Gontero B, Càrdenas ML, Ricard J. A functional five-enzyme complex of chloroplasts involved in Calvin cycle. Eur J Biochem. 1988;173:437–443. doi: 10.1111/j.1432-1033.1988.tb14018.x. [DOI] [PubMed] [Google Scholar]
- Hall NP, Keys AJ, Merrett MJ. Ribulose-1,5-diphosphate carboxylase protein during flag leaf senescence. J Exp Bot. 1978;29:31–37. [Google Scholar]
- Hudson GS, Evans JR, von Caemmerer S, Arvidsson YBC, Andrews TJ. Reduction of ribulose-1,5-bisphosphate carboxylase/oxygenase content by antisense RNA reduces photosynthesis in transgenic tobacco plants. Plant Physiol. 1992;98:294–302. doi: 10.1104/pp.98.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J, Lawlor DW. Dependence of photosynthesis of sunflower and maize leaves on phosphate supply, ribulose-1,5-bisphosphate carboxylase/oxygenase activity, and ribulose-1,5-bisphosphate pool size. Plant Physiol. 1992;98:801–807. doi: 10.1104/pp.98.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C-Z, Rodermel SR. Regulation of photosynthesis during leaf development in RbcSantisense DNA mutants of tobacco. Plant Physiol. 1995;107:215–224. doi: 10.1104/pp.107.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan DB, Chollet R, Ogren WL. Binding of phosphorylated effectors by active and inactive forms of ribulose-1,5-bisphosphate carboxylase. Biochemistry. 1983;22:3410–3418. [Google Scholar]
- Kull O, Sõber A, Coleman MD, Dickson RE, Isebrans JG, Gagnon Z, Karnosky DF. Photosynthetic responses of aspen clones to simultaneous exposures of ozone and CO2. Can J For Res. 1996;26:639–648. [Google Scholar]
- Laisk A (1977) Kinetics of Photosynthesis and Photorespiration in C3 Plants. Nauka, Moscow
- Laisk A (1985) Kinetics of photosynthetic CO2 uptake in C3 plants. In J Viil, G Grishina, A Laisk, eds, Kinetics of Photosynthetic Carbon Metabolism in C3-Plants. Valgus, Tallinn, Estonia, pp 21–34
- Laisk A, Kiirats O, Eichelmann H, Oja V. Gas exchange studies of carboxylation kinetics in intact leaves. In: Biggins J, editor. Progress in Photosynthesis Research. Dordrecht, The Netherlands: Martinus Nijhoff Publishers; 1987. pp. 245–252. [Google Scholar]
- Laisk A, Laarin P (1983) Feedback regulation of the potential rate of photosynthesis. In U Margna, ed, Regulation of Plant Growth and Metabolism. Valgus, Tallinn, Estonia, pp 135–150 (in Russian)
- Laisk A, Loreto F. Determining photosynthetic parameters from leaf CO2exchange and chlorophyll fluorescence: Rubisco specificity factor, dark respiration in the light, excitation distribution between photosystems, alternative electron transport, and mesophyll diffusion resistance. Plant Physiol. 1996;110:903–912. doi: 10.1104/pp.110.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laisk A, Oja V (1998) Dynamics of Leaf Photosynthesis. Rapid-Response Measurements and Their Interpretations. Commonwealth Scientific and Industrial Research Organization, Australia
- Laisk A, Oja V, Kiirats O. Assimilatory power (post-illumination CO2uptake) in leaves—measurement, environmental dependencies and kinetic properties. Plant Physiol. 1984;76:723–729. doi: 10.1104/pp.76.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laisk A, Oja V, Rahi M. Diffusion resistances as related to the leaf anatomy. Fiziol Rast. 1970;17:40–48. [Google Scholar]
- Laisk A, Sumberg A. Partitioning of the leaf CO2 exchange into components using CO2exchange and fluorescence measurements. Plant Physiol. 1994;106:689–695. doi: 10.1104/pp.106.2.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DW, Kontturi M, Young AT. Photosynthesis by flag leaves of wheat in relation to protein, ribulose bisphosphate carboxylase activity and nitrogen supply. J Exp Bot. 1989;40:43–52. [Google Scholar]
- Mae T, Makino A, Ohira K. Changes in the amounts of ribulose bisphosphate carboxylase synthesized and degraded during the life span of rice leaf (Oryza salivaL.) Plant Cell Physiol. 1983;24:1079–1086. [Google Scholar]
- Makino A, Mae T, Ohira K. Photosynthesis and ribulose 1,5- bisphosphate carboxylase in rice leaves. Plant Physiol. 1983;73:1002–1007. doi: 10.1104/pp.73.4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino A, Mae T, Ohira K. Photosynthesis and ribulose 1,5-bisphosphate carboxylase/oxygenase in rice leaves from emergence through senescence. Quantitative analysis by carboxylation/oxygenation and regeneration of ribulose 1,5-bisphosphate. Planta. 1985a;166:414–420. doi: 10.1007/BF00401181. [DOI] [PubMed] [Google Scholar]
- Makino A, Mae T, Ohira K. Enzymic properties of ribulose-1,5-bisphosphate carboxylase/oxygenase purified from rice leaves. Plant Physiol. 1985b;79:57–61. doi: 10.1104/pp.79.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino A, Nakano H, Mae T. Responses of ribulose-1,5-bisphosphate carboxylase, cytochrome f, and sucrose synthesis enzymes in rice leaves to leaf nitrogen and their relationships to photosynthesis. Plant Physiol. 1994;105:173–179. doi: 10.1104/pp.105.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino A, Shimada T, Takumi S, Kaneko K, Matsuoka M, Shimamoto K, Nakano H, Miyao-Tokutomi M, Mae T, Yamamoto N. Does decrease in ribulose-1,5-bisphosphate carboxylase by antisense RbcS lead to a higher N-use efficiency of photosynthesis under conditions of saturating CO2 and light in rice plants? Plant Physiol. 1997;114:483–491. doi: 10.1104/pp.114.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mate JC, von Caemmerer S, Evans RJ, Hudson S, Graham, Andrews TJ. The relationship between CO2-assimilation rate, Rubisco carboxylation and Rubisco activase content in activase-deficient transgenic tobacco suggest a simple model of activase action. Planta. 1996;198:604–613. doi: 10.1007/BF00262648. [DOI] [PubMed] [Google Scholar]
- Moldau H, Kull O. Differential susceptibility of mesophyll CO2 exchange to ozone in soil- or sand-grown Phaseolus vulgarisL. plants. Photosynthetica. 1993;28:37–44. [Google Scholar]
- Moldau H, Kull O, Sõber J, Norby RJ. Differential response of CO2 uptake parameters of soil and sand-grown Phaseolus vulgaris(L.) plants to absorbed ozone flux. Environ Pollut. 1991;74:251–261. doi: 10.1016/0269-7491(91)90074-7. [DOI] [PubMed] [Google Scholar]
- Nakano H, Makino A, Mae T. The effect of elevated partial pressures of CO2on the relationship between photosynthetic capacity and N content in rice leaves. Plant Physiol. 1997;115:191–198. doi: 10.1104/pp.115.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oja VM. A rapid-response gas exchange measuring device for studying the kinetics of leaf photosynthesis. Fiziol Rast. 1983;30:1045–1052. [Google Scholar]
- Paech C. Kinetics of ribulosebisphosphate carboxylase at high protein concentration. Biochem Biophys Res Commun. 1986;134:506–511. doi: 10.1016/s0006-291x(86)80449-1. [DOI] [PubMed] [Google Scholar]
- Paulsen JM, Lane DM. Spinach ribulose diphosphate carboxylase. I. Purification of the enzyme. Biochemistry. 1966;5:2350–2357. doi: 10.1021/bi00871a025. [DOI] [PubMed] [Google Scholar]
- Portis AR, Salvucci ME, Ogren WL. Activation of ribulose bisphosphate carboxylase/oxygenase at physiological CO2and ribulose bisphosphate concentrations by Rubisco activase. Plant Physiol. 1986;82:967–971. doi: 10.1104/pp.82.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SP, Portis AR. Involvement of stromal ATP in the light activation of ribulose-1,5-bisphosphate carboxylase/oxygenase in intact isolated chloroplasts. Plant Physiol. 1988;86:293–298. doi: 10.1104/pp.86.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruuska S, Andrews TJ, Badger RM, Hudson SG, Laisk A, Osmond CB, Price GD, von Caemmerer S (1988) Assessing the RuBP-saturation of CO2-assimilation rates and leaf RuBP pools with rapid-response gas exchange measurement. Insight from transgenic tobacco with impaired photosynthesis. Aust J Plant Physiol (in press)
- Seemann JR, Sharkey TD. Salinity and nitrogen effects on photosynthesis, ribulose-1,5-bisphosphate carboxylase and metabolite pool sizes in Phaseolus vulgarisL. Plant Physiol. 1986;82:555–560. doi: 10.1104/pp.82.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites JC, Shieh W-J, Geiger DR. Regulation of photosynthetic carbon reduction cycle by ribulose bisphosphate and phosphoglyceric acid. Plant Physiol. 1991;97:1115–1121. doi: 10.1104/pp.97.3.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Seemann JR, Pearcy RW. Contribution of metabolites of photosynthesis to postillumination CO2assimilation in response to light flecks. Plant Physiol. 1986;82:1063–1068. doi: 10.1104/pp.82.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Süss K-H, Prokhorenko I, Adler K. In situ association of Calvin cycle enzymes, ribulose-1,5-bisphosphate carboxylase/oxygenase activase, ferredoxin-NADP+ reductase, and nitrite reductase with thylakoid and pyrenoid membranes of Chlamydomonas reinhardtiichloroplasts as revealed by immunoelectron microscopy. Plant Physiol. 1995;107:1387–1397. doi: 10.1104/pp.107.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vapaavuori EM, Vuorinen AH. Seasonal variation in the photosynthetic capacity of a willow (Salix cv. Aquatica gigantea) canopy. I. Changes in the activity and amount of ribulose 1,5-bisphosphate carboxylase-oxygenase and the content of nitrogen and chlorophyll at different levels in the canopy. Tree Physiol. 1989;5:423–444. doi: 10.1093/treephys/5.4.423. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Evans JR, Hudson GS, Andrews TJ. The kinetics of ribulose-1,5-bisphosphate carboxylase/oxygenase in vivo inferred from measurements of photosynthesis in leaves of transgenic tobacco. Planta. 1994;195:88–97. [Google Scholar]
- von Caemmerer S, Farquhar GD (1985) Kinetics and activation of Rubisco and some preliminary modelling of RuP2 pool sizes. In J Viil, G Grishina, A Laisk, eds, Kinetics of Photosynthetic Carbon Metabolism in C3 Plants. Valgus, Tallinn, Estonia, pp 46–58
- Yeoh HH, Badger MR, Watson L. Variation in kinetic properties of ribulose-1,5-bisphosphate carboxylase among plants. Plant Physiol. 1981;67:1151. doi: 10.1104/pp.67.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota A, Canvin DT. Changes of ribulose bisphosphate carboxylase/oxygenase content, ribulose bisphosphate concentration, and photosynthetic activity during adaptation of high-CO2 grown cells to low-CO2 conditions in Chlorella pyrenoidosa. Plant Physiol. 1986;80:341–345. doi: 10.1104/pp.80.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota A, Kitaoka S. Correct pK values for dissociation constant of carbonic acid lower the reported Kmvalues of ribulose bisphosphate carboxylase by half. Presentation of a monograph and an equation for determining the pK values. Biochem Biophys Res Commun. 1985;131:1075–1079. doi: 10.1016/0006-291x(85)90200-1. [DOI] [PubMed] [Google Scholar]
- Zhu G, Jensen RG. Fallover of ribulose 1,5-bisphosphate carboxylase/oxygenase activity. Plant Physiol. 1991;97:1354–1358. doi: 10.1104/pp.97.4.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]