Abstract
The norepinephrine transporter (NET) is an important target for a wide variety of antidepressants and psychostimulants. Despite its prominence as a drug target, there is only one radioligand in use for NET competitive binding assays, [3H]nisoxetine. However, traditional [3H]nisoxetine binding protocols often give an underestimation for the affinity of certain classes of NET ligands, particularly cocaine and other tropanes. Here, we explore the feasibility of using the phenyltropane [3H]CFT for labeling human NET (hNET) in heterologous cell-based binding studies. Assays were optimized for time and protein content and specific, one-site binding was observed. Potencies of tested NET ligands for inhibition of [3H]CFT binding to whole cells (at physiological [Na+] and 25°C) were similar to potencies observed in the [3H]NE uptake assay. Inhibition constants (Ki) for binding assays were highly correlated with uptake inhibition constants for all compounds tested (R2 = 0.99, p < 0.0001). Cell-free membrane preparations did not display the same pharmacological profile. Under conditions routinely used for measuring [3H]nisoxetine binding to membrane preparations (4°C for 3 h, [Na+] at 295 mM), the potency of nisoxetine and desipramine in inhibiting [3H]CFT binding became greater than that measured in a functional assay of [3H]NE uptake at physiological [Na+]. However, the opposite was true for CFT and cocaine. Interestingly, while investigating [3H]CFT as a potential NET radioligand, we uncovered evidence suggesting that CFT and nisoxetine are not mutually exclusive in binding to the NET. Dixon plots of the interaction between nisoxetine and CFT in inhibition of [3H]dopamine uptake by the NET indicate that the two compounds can simultaneously bind to the transporter.
Introduction
The norepinephrine transporter (NET)—a member of the neurotransporter/sodium symporter (NSS) protein family—plays a vital role in regulating adrenergic neurotransmission in the central and peripheral nervous systems (Bonisch and Bruss 2006). The NET is targeted by a variety of chemically heterogeneous antidepressant and psychostimulant drugs. For example, many clinically-used antidepressants act as NET inhibitors, including tricyclics (TCAs, such as desipramine and protriptyline), selective norepinephrine reuptake inhibitors (NRIs, such as reboxetine and atomoxetine), serotonin and norepinephrine reuptake inhibitors (SNRIs, such as duloxetine and milnacipran), and bupropion (a substituted cathinone with both adrenergic and dopaminergic activity). Despite the wide assortment of characterized ligands, there are a very limited number of radiolabeled compounds suitable for NET radioligand binding assays. The first radioligand employed in NET binding studies was [3H]desipramine (Hrdina et al., 1981; Lee et al., 1982), which was later supplanted by [3H]mazindol (Javitch et al., 1984) and [3H]nomifensine (Scatton et al., 1985) and eventually replaced by the contemporary industry-standard, [3H]nisoxetine (Bauer and Tejani-Butt, 1992; Tejani-Butt, 1992). As discovered by early researchers, [3H]desipramine binding assays are fraught with annoyances, such as the need for high KCl pretreatment and a low signal-to-noise ratio due to significant nonspecific binding of desipramine (Lee et al., 1982). This nonspecific binding is likely caused by desipramine's extremely high lipophilicity—a property that allows it to interact with cellular phospholipid membranes and makes it nearly impossible to remove from cell-culture-based binding assays, even after repeated washings and buffer replacements (Zhu et al., 2004). To our knowledge, [3H]nomifensine is no longer on the market; nomifensine itself was removed from the market after it was associated with anemia and liver failure. After initial promising results with [3H]mazindol as a NET radioligand (Javitch et al., 1984), this compound has, unfortunately, been largely ignored-more work is needed to validate its use for NET-related studies with animal tissue, likely hindering its adoption. Rather, [3H]nisoxetine has become the standard radioligand in modern high-throughput screening assays for monoamine transporter research and drug development. In NET membrane homogenate binding assays with [3H]nisoxetine, a high concentration of sodium ([Na+] is typically ∼295 mM) and long equilibration time in the cold is required, customarily 3 hours at 4°C (Tejani-Butt, 1992). Previous work (Kuhar et al., 1999), including our own prior investigation (Reith et al., 2005), demonstrated that under these conditions, radioligand competition assays with [3H]nisoxetine seriously underestimate the potency of phenyltropanes (like cocaine) and atypical ligands (such as vanoxerine (GBR12909) and similar compounds) at the NET. Clearly, studies with the NET could benefit from the development of additional radioligands.
The cocaine analogue β-CFT (2β-carbomethoxy-3β-(4-fluorophenyl)-tropane, also called WIN 35,428) is a relatively non-selective phenyltropane monoamine transporter inhibitor (Rothman et al., 1995; Kuhar et al., 1999; Eshleman et al., 1999), but [3H]CFT is routinely used as a selective dopamine transporter (DAT) label in striatal brain tissue where DAT predominates (Madras et al., 1989) and is the de facto radioligand for assays with cells heterologously expressing the DAT (Loland et al., 2004 and Kurian et al., 2009). [3H]CFT has not been previously reported as a radioligand for the serotonin transporter (SERT) and only few studies have made use of this radioligand for labeling the NET (Norregaard et al., 1998; Zhou et al., 2009). However, pharmacological characterization of [3H]CFT as a specific NET radiolabel—particularly its competitive binding profile, compared with [3H]nisoxetine—is lacking. The present work was undertaken to provide such characterization of the pharmacological profile of [3H]CFT labeling in hNET-expressing human embryonic kidney (HEK-293) cells. Both intact-cell and homogenized membrane preparations were used, and [3H]CFT binding was monitored under various conditions routinely used in monoamine transporter binding assays. The pharmacological profile of [3H]CFT competitive binding was assessed with structurally different compounds with varying affinity for NET: two distinct selective NET inhibitors (nisoxetine and desipramine), two non-selective phenyltropane monoamine uptake inhibitors (cocaine and β-CFT), as well as benztropine (which possesses a tropane moiety, but has an atypical binding profile and lacks addictive liability) and mazindol.
Methods
Cell lines
Human embryonic kidney 293 cells stably expressing hNET (HEK-hNET) were a gift from Dr. Randy Blakely (Vanderbilt University Medical Center, Nashville, TN). The HEK-hNET cells were cultured in DMEM, supplemented with 10% fetal bovine serum, 2 mM glutamine, and 200 μg/ml G418 at 5% CO2 and 37°C. After being cultured for 3 days, they were prepared for the experiments.
Materials
[3H]CFT (85.9 Ci/mmol), [3H]dopamine, Wallac filtermat A and B were purchased from Perkin Elmer (Boston, MA). Nonlabeled β-CFT was from the NIDA (Research Triangle Institute, Research Triangle Park, NC). Mazindol was from Sandoz Pharmaceuticals (E. Hanover, NJ). All other chemicals were commercially sourced, primarily from either Sigma Chemical (St. Louis, MO) or Fisher Scientific (Pittsburg, PA). Protease inhibitor cocktail (“100×”, EDTA-free) was from Thermo Scientific.
Intact HEK-hNET cells preparations
Intact HEK-hNET cell suspensions were prepared as described previously by our lab (Wang et al., 2000; Zhou et al., 2009). In brief, HEK-hNET cells were first harvested with 0.25% (w/v) trypsin-EDTA. The HEK-hNET cell pellet was washed once with PBS and gently suspended in assay buffer (containing 152 mM [Na+]) by repeated up and down movements through a 1-ml pipet tip, resulting in a homogeneous suspension in isotonic assay buffer C (see table 1). Tropolone (for final concentration of 0.1 mM) was added for inhibition of COMT before conducting the [3H]DA uptake assay or [3H]CFT binding assay.
Table 1.
Kd values of [3H]CFT binding at HEK-hNET under varing conditions. Kd values were determined as described in Methods and are expressed as mean ±SEM of three independent experiments.
| Condition | Buffer component (mM) | Total [Na+] (mM) | pH | Assay time, temperature | Sample preparation | Kd (nM) |
|---|---|---|---|---|---|---|
| A | Na2HPO4 (17.5)-NaH2PO4 (35), NaCl (260), KCl (5) | 295 | 7.4 | 3 hr, 4°C | Cell membranes | 168 + 39 |
| B | Na2HPO4 (17.5)-NaH2PO4 (35) | 35 | 7.4 | 15 min, 25°C | Cell membranes | 35.7±3.4 |
| C | Na2HPO4 (15)-NaH2PO4 (30), NaCl (122), KCl (5), MgSO4 (1.2), glucose (10), CaCl2 (1) | 152 | 7.4 | 15 min, 25°C | Intact cells | 22.4+ 3.6 |
Cell-free membranes preparations
After washing with cold PBS (without calcium and magnesium salts), the attached cells were lysed with 3 ml of cold lysis buffer (2 mM HEPES and 1 mM EDTA, adjusted to pH 7.6 with NaOH). Cell lysates were combined with another 3 ml of lysis buffer used to rinse the flask, transferred to a Beckman polycarbonate thick-wall centrifuge tube, and centrifuged at 31,000g for 20 min at 4°C. The pellets were homogenized with a Brinkmann Polytron (Brinkmann Instruments, Westbury, NY) at setting 6 for 15 sec in the assay buffer A or B, supplemented with 0.1mM tropolone (table 1).
[3H]CFT binding assays
Binding of [3H]CFT to hNET was measured by procedures as described by us for hDAT (Schmitt et al., 2008; Zhou et al., 2009). In brief, assays were conducted in a total volume of 0.2 ml in 96-well plates, with termination of the binding reaction by rapid filtration through a cold PEI-presoaked Wallac filtermat A (Perkinelmer, Boston, MA) with a semi-automatic Tomtec harvester (Tomtec, Hamden, CT). Intact cell preparations or cell-free membranes were incubated with [3H]CFT (∼3 nM final concentration) and varying concentrations of test compounds. To determine the dissociation constant (Kd) of [3H]CFT and maximal number of binding site (Bmax), cold saturation approach was applied that the final concentrations of nonradioactive CFT (0, 1, 3, 10, 30, 100 and 300 nM) were co-present with one fixed concentration of [3H]CFT (3nM as final). Assay temperature, incubation time, and buffer composition were varied as indicated (see table 1). Nonspecific binding was defined with 10 μM desipramine. Time course experiments were carried out at room temperature on cell membranes in 35 mM [Na+] buffer (buffer B, listed in table1) or intact cells 152 mM [Na+] buffer (buffer C, listed in table 1). Assay incubation time was extended to up to 240 minutes. To examine whether the decreased [3H]CFT binding was due to the protein degradation in the extended incubation time, a protease inhibitor cocktail (“100×” from Thermo Scientific) was added for a final concentration of 0.1× (i.e., a 1000-fold dilution of cocktail stock). [3H]CFT binding was determined at 30 min, 60 min, 120 min and 180 min in the presence of 0.1% ethanol (vehicle control) or 0.1× protease inhibitor cocktail. Results are expressed as percentage of specific [3H]CFT binding at 30 min.
[3H]DA uptake assays and Dixon plots
Intact cells were prepared as described above, in assay buffer C (table 1). Intact cells were first pre-incubated with various concentrations of nisoxetine or cocaine at 25°C for 5 minutes. The uptake process was then initiated by adding [3H]dopamine, a NET substrate that is taken up as well as—or perhaps better than—[3H]NE, at a final concentration of 10 nM (Coyle and Snyder, 1969; Pacholczyk et al., 1991; Loland et al., 2004). After 10 minutes of incubation, cell uptake of [3H]DA (which we found to be still in the linear phase at that point; data not shown) was stopped by rapid filtration and several washes through a cold PEI-presoaked Wallac filtermat B (Perkin Elmer, Boston, MA) with ice-cold saline with a Brandel 96-well harvester (Brandel, Gathersburg, MD). Nonspecific uptake was defined with 10 μM desipramine, as in the [3H]CFT binding assay. For Dixon plots, inhibition curves of nisoxetine and cocaine (varying concentrations) were measured with and without an additional fixed concentration of unlabeled CFT.
Data analysis and statistics
Protein level was determined with the Lowry method as described in (Zhen et al., 2005). Total [3H]DA and [3H]CFT retained on the filtermat was determined in Wallac Trilux 1450 β-scintillation counter (Perkin Elmer, Boston, MA). Compound IC50 values were converted to inhibition constants (Ki) by the Cheng-Prusoff equation (Cheng and Prusoff, 1973). IC50s of certain compounds to inhibit [3H]NE uptake were taken from previous studies (Eshleman et al., 1999; Reith et al., 2005) and converted to Inhibition constants(Ki) taking into account the [3H]NE used in the respective literatures. Results are expressed as mean ± S.E.M. Kinetic parameters (Kd, Bmax, and IC50) were calculated using the computer-fitting programs LIGAND (“cold” saturation module, Biosoft, Cambridge, UK) and ORIGIN (Microcal Software, Northampton, MA, USA). Data were analyzed by one-way analysis of variance (ANOVA) followed by Dunnett multiple comparisons test, one way repeated measures analysis of variance followed by Holm-Sidak multiple comparison or student t-test, as indicated in text. The accepted level of significance was p < 0.05. Graphical analysis consisted of least-squares linear regression and correlation analysis.
Results
Time course and effect of protease inhibitor cocktail on the binding of [3H]CFT to hNET
Total [3H]CFT binding to hNET on intact cells was measured at different protein levels, initially up to 262 μg protein per assay (data not shown). Subsequent experiments were carried out within this linear range and resulted in binding less than 10% of the total added radioactivity (generally ∼40-60 μg per well in membrane preparations and ∼80-100 μg in the whole cells assay). In most experiments with intact cells reported in this study, nonspecific binding was less than 30% of total binding, and approximately 10% with membrane preparations.
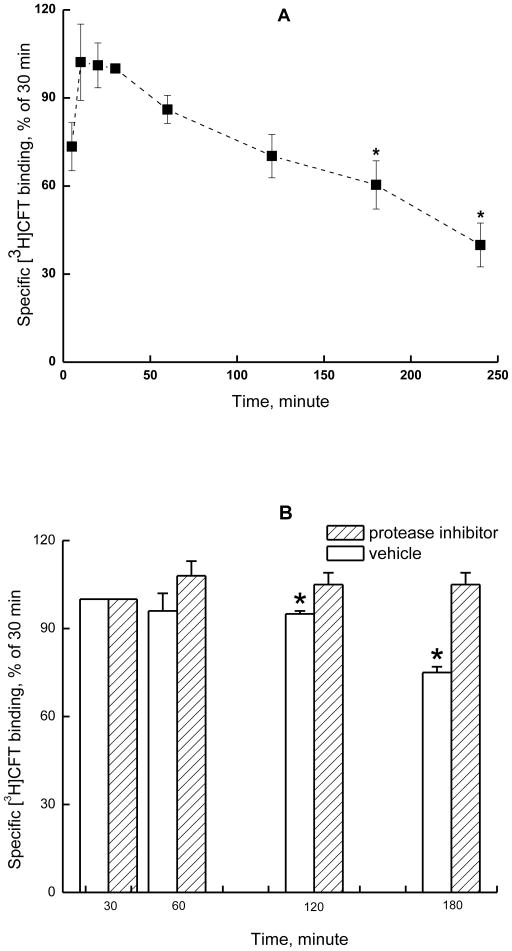
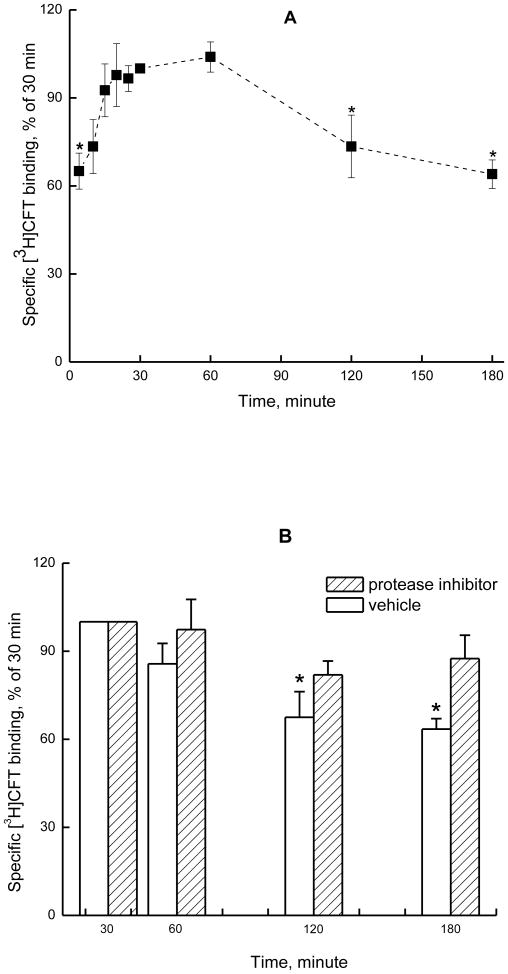
Maximal [3H]CFT binding to hNET on cell-free membranes was reached in 15 min (Fig.1A) and intact cells (Fig.2A) and remained at a plateau for at least 30 min at 25°C. At 2 h, an apparent reduction in binding was seen; and a significant drop occurred at the next time point measured, 180 min (one way repeated measures analysis of variance followed by Holm-Sidak post-hoc test, compared with the value at 30 min, Fig 1A, B and 2A, B). The loss of [3H]CFT binding upon prolonged incubation (3 h) of cell-free membranes is also shown in Table 2 (panel 1), with a significant reduction in the apparent binding density (Bmax) without a change in the dissociation constant (Kd). It is possible that extended incubation allows degradative processes to impact the NET, or, alternatively, conformational changes occur, such that a portion of transporters cannot bind CFT anymore. In the presence of protease inhibitor cocktail (1000-fold dilution of stock), [3H]CFT bound to cell-free membrane preparations (Fig. 1B) or intact cells (Fig.2B) remained at plateau for at least 3 h. Therefore, the loss of [3H]CFT binding to hNET after incubation at prolonged times is likely due to degradation of the NET protein.
Fig. 1.
A, Time course for binding of [3H]CFT to HEK-hNET cell-free membranes at 25oC in 35 mM sodium buffer. Total [3H]CFT binding and nonspecific binding (defined with 10 μM desipramine) were determined at the indicated time to yield the specific binding values, which were normalized by the value at 30 min and expressed as the mean ± S.E.M for three independent experiments performed in triplicate. B, Protease inhibitor cocktail (0.×, see Methods) can prevent, over the tested incubation period of 180 minutes, the decrease in [3H]CFT binding to HEK-hNET. The specific [3H]CFT binding values were normalized with the value at 30 min (three independent experiments, mean + S.E.M. - vertical bar). In both panels, data were analyzed with one way repeated measures analysis of variance followed by Holm-Sidak multiple comparison versus binding at 30 minutes ( *: p < 0.001)
Fig. 2.
A, Time course for binding of [3H]CFT to HEK293-hNET intact cells in 153 mM sodium buffer at 25°C. B, Protease inhibitor cocktail (0.×, see Methods) can prevent, over the tested incubation period of 180 minutes, the decrease in [3H]CFT binding to HEK-hNET. All other details are as in Fig. 1.
Table 2.
Dissociate constant (Kd) and maximal binding sites (Bmax) of [3H]CFT to hNET determined under different conditions as described in the text.
| Binding assay conditions | Kd (nM) | Bmax (pmol/mg protein) |
|---|---|---|
| I: membranes, 35 mM [Na+] | ||
| Vehicle, 15 min1 | 35.7±3.4 | 1.38±0.21 |
| Vehicle, 3 hrs | 22.1±2.0 | 0.53±0.09* |
| II: membranes, 35 mM [Na+] | ||
| Vehicle, 15 min1 | 34.5±4.1 | 2.53±0.31 |
| 100 nM nisoxetine, 15 min | 36.0±1.9 | 1.61±0.14* |
| III: cells, 152 mM [Na+] | ||
| Vehicle, 15 min2 | 25.8.±4.5 | 1.84±0.22 |
| 5 nM nisoxetine, 15 min | 38.3±7.6 | 1.15±0.14* |
Values are the mean ± S.E.M. of three to eight independent experiments.
condition B in table 1
condition C in table 1
P<0.05 (student t test. compared with t vehicle group)
Binding properties for [3H]CFT under various conditions
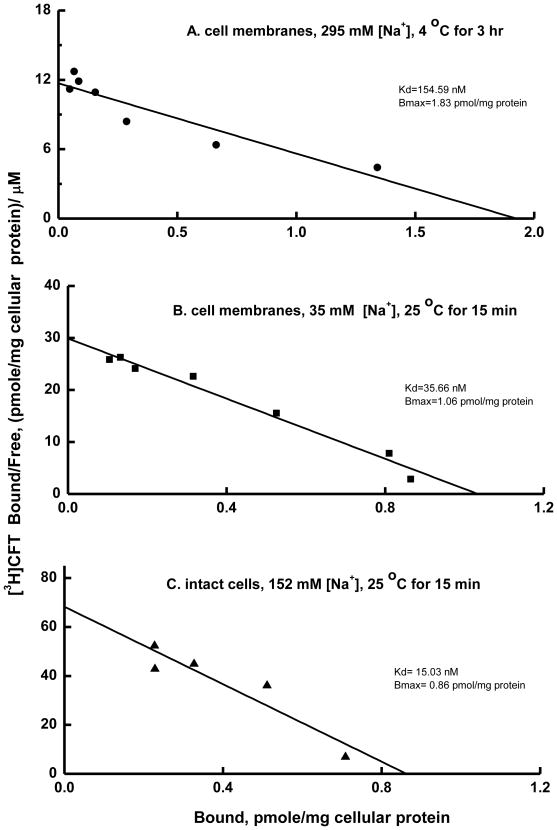
In the following experiments, saturation analysis of [3H]CFT binding was performed with an incubation time sufficient for maximal binding and at a protein concentration sustaining binding in the linear range. Because we previously found that differing buffer systems and assay temperature dramatically impacted the inhibition of [3H]nisoxetine binding to hNET by certain classes of compounds—particularly cocaine analogues (Reith et al., 2005)—we examined [3H]CFT binding to hNET under varying conditions. Three different assay conditions were applied (see Table 1): conditions routinely used for studying [3H]nisoxetine binding to NET in homogenate assays (condition A), conditions commonly used for [3H]CFT binding to DAT (condition B), and intact-cell binding conditions similar to those used in monoamine uptake assays (condition C). Under all conditions, the binding isotherms indicated one-site binding (Fig. 3). The Kd of CFT binding (168 nM) in membrane preparations at high [Na+] (condition A, Table 1, Fig. 3A) was substantially higher than previously obtained Ki values for inhibition of [3H]NE uptake (Ki = 26.0 nM, calculated from the IC50=33.3 nM of Eshleman et al., 1999). The Kd values for CFT binding observed for membranes at low [Na+] (Kd = 35.7 nM) or for intact cells at physiological [Na+] (Kd = 22.4 nM) (conditions B and C in Table 1, Figs. 3B, C and D) are in alignment with prior Ki values for β-CFT in the hNET functional assay(Eshleman et al., 1999).
Fig. 3.
Scatchard plots for saturation binding of [3H]CFT to HEK-hNET cell membranes (A and B) and intact cells (C). Data are from representative experiments under different conditions: 295 mM, 35 mM, and 152 mM Na+ (see Table 1).
Pharmacological properties of [3H]CFT binding under various conditions
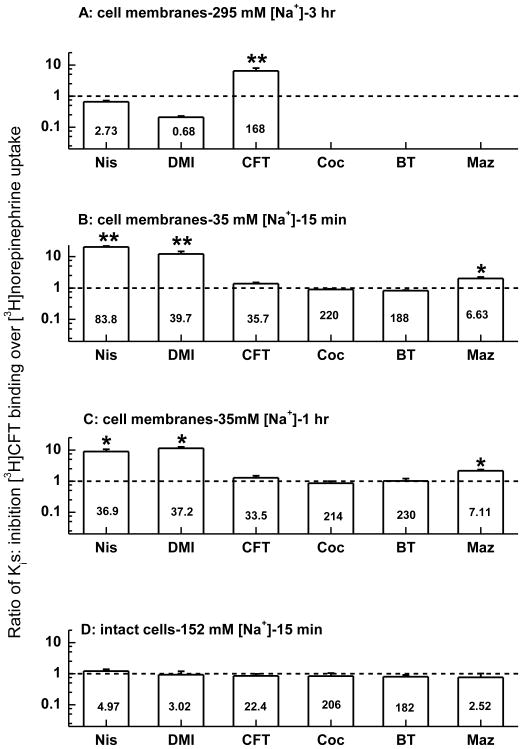
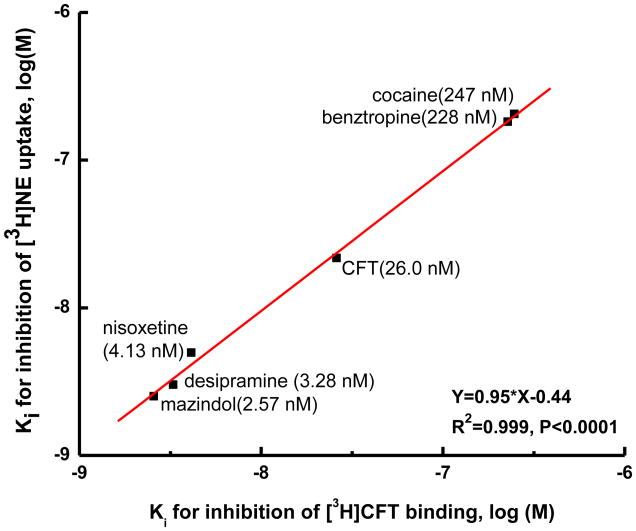
We previously found that different assay conditions (buffer, temperature) significantly impacted the potency of compounds, especially cocaine analogues, in inhibiting [3H]nisoxetine binding to hNET by (Reith et al., 2005). We therefore examined the impact of varying conditions (see Table 1) on [3H]CFT binding to hNET in the present work. In high Na+ buffer at 4°C with membrane preparations (condition A), nisoxetine and desipramine tended to be more potent in inhibiting [3H]CFT binding than [3H]NE uptake (see Ki ratio in Fig. 4A, bars compared with horizontal dotted line for equal binding and uptake Ki). In contrast, the potency of CFT was significantly lower in binding than uptake assays (p < 0.005, Fig. 4A). Compounds were grouped according to their different chemical structures and requirement of Na+. Sodium differentially modified the potencies of various classes of compounds in [3H]CFT binding inhibition. Thus, in 35 mM [Na+] buffer with membrane preparations (condition B, Fig. 4B), the affinity of nisoxetine and desipramine became weaker relative to the uptake affinity (higher Ki, p < 0.005), whereas CFT and the other tropanes, cocaine and benztropine, were equipotent at binding and uptake, and mazindol was weaker in the binding assay (p < 0.05, Fig. 4B). The same membrane conditions with prolonged 1-hr incubation gave almost identical results (Fig. 4C), indicating that the relative weak potencies of nisoxetine, desipramine, and mazindol were not due to insufficient equilibration time for these drugs. Strikingly, when [3H]CFT binding was measured in intact cells at normal Na+ (condition C), the potencies for all of the tested compounds were similar to those observed in [3H]NE uptake assays (Fig. 4D). Indeed, a plot of the Ki values for inhibition of [3H]CFT binding to intact cell expressing hNET against Ki values in an [3H]NE uptake assay (Eshleman et al., 1999; Reith et al., 2005) shows a high correlation (R2 = 0.99, p < 0.0001) with an almost 1 : 1 relationship between the results of the two assays (Fig. 5).
Fig. 4.
Potencies of compounds in inhibiting [3H]CFT binding to HEK-hNET membranes (A, B, C) or whole cells (D) under various conditions. Fig. 4A, 4B and 4D correspond to condition A, B, and C as described in the table 1, respectively. Fig. 4C corresponds to condition B with the incubation time extended to 1 h. Results are expressed as affinity (Ki) relative to that in inhibiting [3H]NE uptake (for source and computation of uptake Ki values see legend to Fig. 5). The dotted line indicates a ratio of unity (i.e. Ki-binding equals Ki-uptake). The figures inside each bar denote the averaged Ki of each compound from three to eight independent experiments for the given condition in the [3H]CFT binding assay. For all compounds, Ki values measured under various conditions were analyzed by one-way ANOVA, followed by Dunnet multiple comparison to the uptake Ki. *p < 0.05; **p < 0.005.
Fig. 5.
Correlation of inhibition of [3H]CFT binding and [3H]NE uptake in HEK-hNET cells. [3H]CFT binding was measured in intact cells at 25°C for 15 minutes. Uptake inhibition constants (Ki) were converted from the uptake IC50s of mazindol, desipramine, nisoxetine, CFT, benztropine (Eshleman et al.,1999), and cocaine (Reith et al., 2005). The values of the uptake inhibition constant are listed in parentheses for each compound. The line represents the linear regression for all drugs (R2 = 0.99, p < 0.0001).
Apparent noncompetitive inhibition by nisoxetine of [3H]CFT binding to NET
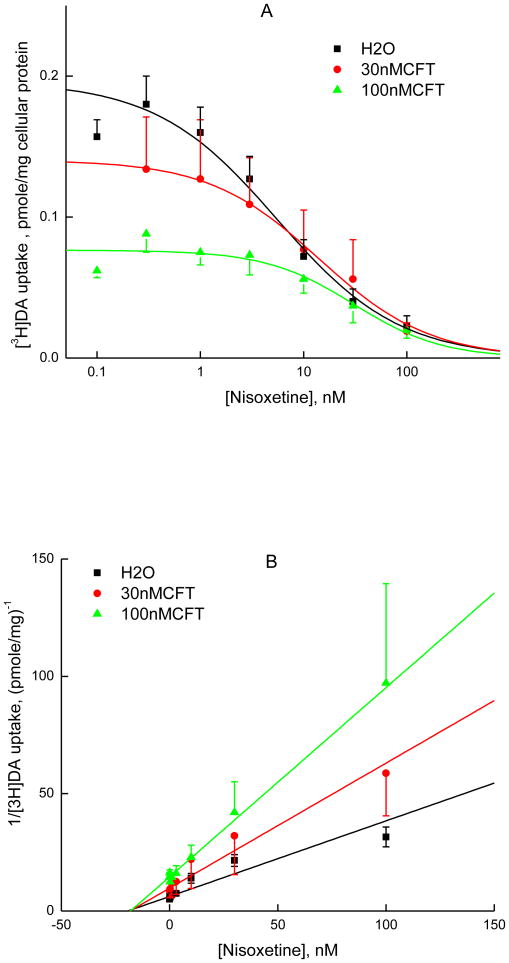
The tremendous increase of the Ki value for nisoxetine in [3H]CFT binding to HEK-hNET cell membranes compared with the Ki for inhibiting [3H]NE uptake in the functional assay prompted the question as to whether nisoxetine and CFT bind in a mutually exclusive manner. First, we determined the Kd and Bmax of [3H]CFT binding in the presence or absence of nisoxetine. In experiments with fractionated cell membranes at low [Na+] (condition B, Table 1), where nisoxetine is relatively weak, 100 nM nisoxetine reduced the Bmax without changing the Kd (Table 2, panel II). Along the same lines, in intact HEK-hNET cells at physiological [Na+], where nisoxetine is more potent (condition C), the Kd was not statistically significantly altered by 5 nM nisoxetine but the Bmax was reduced almost 2-fold (Table 2, panel III). This behavior can be described as noncompetitive (involving two separate sites where nisoxetine can bind simultaneously with CFT); but only in descriptive, not mechanistic terms (see e.g. Tomlinson, 1988). That is, if the transporter can bind nisoxetine and [3H]CFT both simultaneously and entirely independently, nisoxetine would not inhibit [3H]CFT binding at all. This incomplete binding relationship—not mutually exclusive, but also not fully independent—could indicate an interaction between binding sites.
Simultaneous binding of nisoxetine and CFT to NET as determined by Dixon plots of [3H]DA uptake
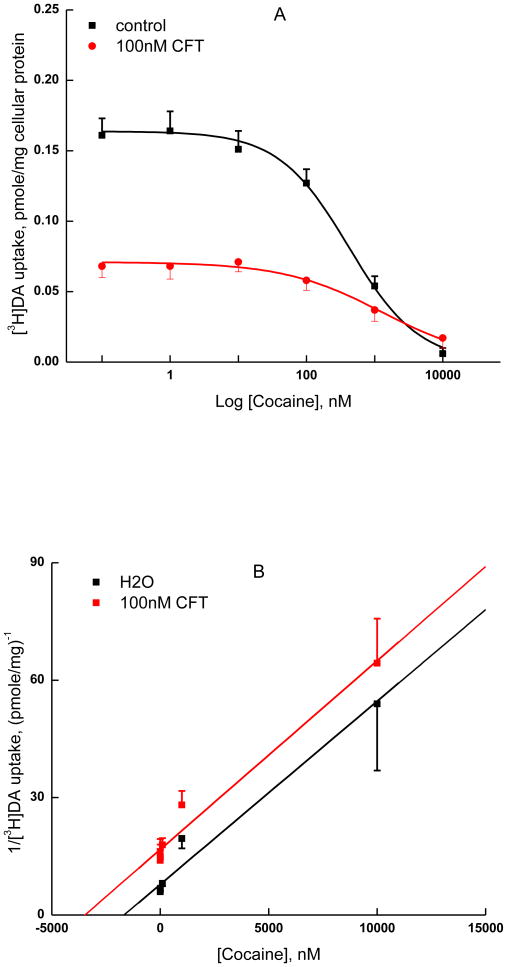
As the above analysis only points to a mechanism of inhibition of [3H]CFT binding by nisoxetine that deviates from competitive inhibition, without providing additional information regarding their binding interaction, we took a different approach to probe the binding mechanism of these two compounds. We studied the interaction between nisoxetine and CFT as inhibitors of the uptake of [3H]DA by NET (Coyle and Snyder, 1969; Pacholczyk et al., 1991; Loland et al., 2004). Dixon plots of such experiments provide a diagnostics as to whether both inhibitors can bind simultaneously, on the one hand, or in a mutually exclusive manner, on the other hand (Segel et al., 1975; Sarker et al., 2010). Nisoxetine was able to reduce [3H]DA uptake by hNET activity to zero (Fig. 6A, filled square); its Hill number of 0.93 ± 0.1 (n = 7), i.e. close to unity, is consonant with nisoxetine being a competitive inhibitor of [3H]DA uptake. The same is true for cocaine, closely related to CFT (Fig. 7A, filled square, Hill number of 0.84 ± 0.09 (n = 4). Plots of 1/[3H]dopamine uptake versus the concentration of nisoxetine at a fixed concentration of CFT (30 nM or 100 nM) are shown in Fig. 6B. Cocaine and CFT are structural analogues and are thus likely bind to the same site. Unlike the cocaine experiment, in which the Dixon plots showed two parallel lines with overlapping 95% confidence intervals for the absence and presence of fixed cold [CFT] (Fig. 7B) —indicating mutually exclusive binding of CFT and cocaine—the nisoxetine experiment gave Dixon plots in which the lines for nisoxetine inhibition in the presence of 30 nM or 100 nM CFT were not parallel to the line for vehicle, but rather intersected (Fig. 6B, note the non-overlapping 95% confidence intervals for 0 and 100 nM CFT). Therefore, nisoxetine and CFT can bind simultaneously to NET. The intercept on the x-axis (concentration of nisoxetine) equals -αKi (Segel et al., 1975). Here, Ki is the inhibition constant of nisoxetine in the HEK-hNET functional assay ([3H]DA uptake). Consonant with some degree of interaction between nisoxetine and CFT observed in the [3H]CFT binding experiments, the constant α, indicated by the intersection point, is more than 1. Thus, the results indicate that nisoxetine and CFT bind to separate sites; however, these sites do interact with each other, with each of them probably being mutually exclusive with the recognition site for DA (or NE).
Fig. 6.
Mutually non-exclusive binding of nisoxetine to NET in the presence of CFT. A, Inhibition of [3H]dopamine uptake into NET cells by varying concentrations of nisoxetine was measured in the presence of vehicle (■), or non-labeled CFT at 30 nM (●) or 100 nM (▲). B, Data shown in panel A were transformed into a Dixon plot by expressing the reciprocal of [3H]dopamine uptake (pmole/mg)-1 as a function of nisoxetine (nM). Linear regression was applied to the three plots with R2 s equal to 0.89, 0.96, and 1.00, respectively for the presence of vehicle, 30 nM CFT and 100 nM CFT. The slopes of the lines (coefficient with 95% confidence intervals in parentheses) were 0.26 (0.21-0.32) mg/mL (■ H2O), 0.49 (0.26-0.73) mg/mL(● 30 nM CFT), and 0.84 (0.54-1.14) mg/mL(▲100 nM CFT). Data are shown as mean ± S.E.M. of three to nine independent experiments in triplicate.
Fig.7.
Mutually exclusive binding of cocaine to NET in the presence of CFT. A, Inhibition of [3H]dopamine uptake into NET cells by varying concentrations of cocaine was measured in the presence of presence of vehicle (■) or non-labeled CFT at 100 nM (●). B, Data shown in panel A were transformed into a Dixon plot by expressing the reciprocal of [3H]dopamine uptake (pmole/mg)-1 as a function of cocaine (nM). Linear regression was applied to the two plots with R2 s equal to 0.97 and 0.97, respectively for the presence of vehicle or 100 nM CFT. The slopes of the lines (coefficient with 95% confidence intervals in parentheses) were 0.047 (0.037-0.056) mg/mL (■ H2O) and 0.048 (0.038-0.059) mg/mL (● 100nM CFT). Data are shown as mean ± S.E.M of four independent experiments in triplicate.
Discussion
In the present work, [3H]CFT was found to label an apparent single binding site on NET, as has been found for other NET radioligands. Thus, [3H]nisoxetine labels one site in homogenate binding assays with membrane preparations of rat cerebral cortex (Bauer and Tejani-Butt, 1992), as does [3H]nomifensine for the rabbit cerebral cortex (Dubocovich and Zahniser, 1985). Although [3H]desipramine displays high and low-affinity binding sites (Hrdina, 1981; Lee et al., 1982), the latter low-affinity binding occurs to sites other than NET (Backstrom et al., 1989). Among the above four different radioligands, only two are currently on the market: [3H]CFT and [3H]nisoxetine. We therefore examined the following questions: (i) how do different assay conditions (preparation, buffer, temperature) affect [3H]CFTs binding properties?; (ii) do CFT and nisoxetine label the same site?; and (iii) which NET radiolabeling assay is preferable and under what reaction conditions?
Based on our previous study (Reith et al., 2005) of assay conditions affecting the pharmacological profile of [3H]nisoxetine binding, together with the current results on [3H]CFT binding, compounds interacting with the NET can be divided in two primary groups: nisoxetine, desipramine, and mazindol on one hand, and cocaine, β-CFT, β-CIT (2β-carbomethoxy-3β-(4-iodophenyl)tropane), benztropine, and GBR 12909 on the other. The weak potency for CFT in inhibiting [3H]CFT binding in the high [Na+] buffer at 4°C (Fig. 4A) was also observed in our previous work with NET cells labeled with [3H]CIT (Table 1, column 6 in (Reith et al., 2005)). Most likely, the very high [Na+] is the determining factor, inhibiting the binding of cocaine-like compounds to NET, as observed for DAT (Li and Reith, 1999; Li and Reith, 2000), but potentiating the binding of nisoxetine and related compounds. At high [Na+] and 4°C (conditions commonly used for [3H]nisoxetine assays), nisoxetine and desipramine became more potent in inhibiting [3H]CFT binding to membranes prepared from NET cells relative to their uptake inhibitory potency (Fig. 4A). At low [Na+] and 25°C (conditions commonly used for DAT studies) these compounds became weaker, as did mazindol (Fig. 4B and C). Therefore, contrasting these two conditions, cocaine-like compounds went from displaying weak binding inhibition (relative to their uptake inhibitory activity) to a near-“normal” (closer to the uptake value) Ki in the binding assay (Fig. 4A, B and C). In the window of 0 to 300 mM [Na+], [3H]CFT binding to DAT cell-free membrane is initially stimulated (with [Na+], going from 0 to 60 mM), then stays at a plateau and is ultimately suppressed (with [Na+], going from 60 to 300 mM) (Li and Reith, 1999; Bonnet, 2003); in contrast, throughout this [Na+] window the specific binding of [3H]nisoxetine to NET cell-free membranes increases proportionally to [Na+] (Tejani-Butt 1992). Thus, at physiological [Na+] (152 mM), the potency of nisoxetine in inhibiting [3H]CFT binding to NET cell-free membranes is expected to be intermediate between the value observed at low [Na+] (Ki=83.8 ± 10.3 nM, Fig. 4B) and at high [Na+] (Ki=2.73 ± 0.25 nM, Fig. 4A). Indeed, under the same assay conditions as used for monitoring [3H]CFT binding to intact cells, i.e. at room temperature for 15 minutes and 152 mM [Na+] (condition C), we found the Ki of nisoxetine to be 51.7 ± 5.19 nM for cell membranes (mean ± S.E.M., n=3); this potency is also considerably weaker than the whole cell value (Ki=4.97 ± 0.80 nM, Fig.4D). In contrast, no difference was detected in the Kd of CFT between membranes (18.0 ± 5.0 nM, mean ± S.E.M., n=3) and cells (22.4 ± 3.6 nM, Fig. 4C) assayed at 152 mM [Na+].
Such differences in binding properties between compounds in the cocaine group and those in the nisoxetine group—revealed in membrane preparations subjected to different ion concentrations and temperatures—suggest an important difference between binding sites on the NET for cocaine-like and nisoxetine-like drugs.
Consonant with the non-identical nature of CFT and nisoxetine binding sites, the present [3H]CFT saturation binding experiments with and without nisoxetine revealed a nisoxetine-induced reduction in Bmax without a change in Kd. Theoretically, pure noncompetitive inhibition would not be observed in binding assays, because inhibitor and radioligand can bind simultaneously and independently; however, functional assays will detect the resulting inactivated complex (as in enzyme kinetic, or transport, experiments) (Tomlinson, 1988). Be that as it may, the present binding results do indicate an interaction between nisoxetine and CFT binding sites, i.e. the two ligands do not bind independently from each other. Dixon analysis of [3H]DA uptake by NET was applied to further examine the relationship between CFT/cocaine and nisoxetine binding sites. In the absence and presence of CFT, cocaine Dixon plots were parallel, consistent with mutually exclusive binding sites for cocaine and CFT. This is to be expected, with these closely structurally related analogues competing for one and the same binding pocket. In contrast, Dixon plots of nisoxetine's interaction effect did not show parallel lines but rather lines that intersected at a point close to the x-axis. These findings suggest that binding of nisoxetine and CFT at the NET is not mutually exclusive. Thus, nisoxetine and CFT can bind at the same time, implying different, distal binding sites.
In this context, it is of interest that mutation of either Thr381 or Asp473 in the NET (T381K and D473A, respectively) severely impacts nisoxetine's affinity (Paczkowski et al., 2007). In silico homology modeling of the NET—based upon the crystal structure of LeuT, a bacterial NSS protein—has suggested that the two residues are part of a secondary substrate site (S2), located in the extracellular vestibule, ∼13 Å above the primary substrate site (Paczkowski et al., 2007; Ravna et al., 2009; Beuming et al., 2008). A recent molecular simulation study indicated that the antidepressant imipramine—traditionally considered to be a competitive NET inhibitor—can occupy the S2 site in the outer vestibule, while also interacting with residues of the primary substrate binding site (S1) in the SERT (Sarker et al., 2010). In the DAT, there is evidence for CFT binding to the S1 site (Beuming et al., 2008). Though hDAT and hNET have 70% sequence identity, the cocaine-bound state of the NET may be different from the cocaine-bound state of the DAT (Loland et al., 2004). Other evidence suggests binding sites for antidepressants, cocaine, and substrates are overlapping but not identical in NET (Bryan-Lluka et al., 2003; Sharpe et al., 2003; Sucic and Bryan-Lluka, 2005). We interpret the available data as indicating that nisoxetine and CFT both competitively inhibit substrate recognition—nisoxetine likely by interacting with the S2 site, but also with some S1 residues and CFT by binding squarely in the S1 site. Importantly, the binding pockets for nisoxetine and CFT are separate, as shown by the Dixon plots: residues sharing interactions between nisoxetine and substrate (DA or NE) are not involved in CFT binding. However, although nisoxetine and CFT can bind simultaneously to NET, this does not mean their binding is completely independent. Allosteric interactions between two binding pockets can result in mutual effects, with ligand binding at one site affecting the ligand binding properties of the other. In our case, interaction between the two binding domains is also supported by the location of the intersection point of the Dixon plot lines (Fig. 6B) and the apparent noncompetitive inhibition of [3H]CFT binding by nisoxetine (Table 2).
An important result of the present work is that the use of intact cells expressing NET, with traditional uptake buffer at 25°C and [3H]CFT as the radioligand, provides compound binding affinities that are close to those detected in a [3H]NE uptake assay. This is reminiscent of our previous finding with [3H]nisoxetine as radioligand for NET, which was also capable of detecting binding potencies close to uptake potencies— provided the assay is done with intact cells under physiological conditions with uptake buffer (Reith et al., 2005). Substitution of membranes for intact cells in such assays with [3H]CFT will not provide uptake potencies for all compounds as shown by the elevated Ki value for nisoxetine with membranes compared with cells in uptake buffer. The present results demonstrate there is no need to work with a separate radioligand for the NET if [3H]CFT is already at hand in laboratories studying both NET- and DAT-expressing cell lines. One should also consider the purpose of the assay: if the aim is to look for NET inhibitors that interfere with substrate uptake, [3H]CFT is a better choice than [3H]nisoxetine, as evidence suggests that the former binds more closely to the substrate recognition site than the latter. The present results indicate that [3H]nisoxetine must bind more distally because nisoxetine binding can be observed as being separate from that of CFT. Hence, if the purpose is to look for compounds that interfere with such more distal sites (perhaps the putative S2 site), then [3H]nisoxetine is the ligand of choice. An early study also indicates that [3H]mazindol can serve as a promising NET ligand (Javitch et al., 1984); but more work is needed to fully characterize the pharmacological profile of this ligand before accepting it as a tool in compound screening. Mazindol may bind, as nisoxetine, more distally from the substrate site, as mazindol's behavior under varying assay conditions resembles nisoxetine more than CFT (Reith et al., 2005). For experiments with brain tissue, use of [3H]CFT for labeling the NET requires caution as CFT is relatively nonselective among monoamine transporters (Kuhar et al., 1999; Rothman et al., 1995; Eshleman et al., 1999) and NET and SERT expression areas in the brain overlap. The commonly used high [Na+], [3H]nisoxetine binding assay with preparations of brain membranes, is not recommended. Such artificial conditions exaggerate the potency for NET of nisoxetine and related compounds, while underestimating the potency of drugs related to cocaine, GBR 12909, or methylphenidate (Reith et al., 2005). Even if an assay for [3H]nisoxetine binding to synaptosomal preparations with more physiologically-native conditions could be worked out (to our knowledge this has not been reported), the issue of measuring a more distal binding site (as compared to substrate) remains. As we suggested previously, for animal tissue experiments (i.e. synaptosomes from rat noradrenergic nerve terminals) with the NET, it is advisable to study inhibition of uptake of [3H]NE or [3H]DA (Reith et al., 2005).
Highlight.
>We examined that [3H]CFT is a valid radioligand for NET. >We concluded binding of CFT and nisoxetine to NET is not mutually exclusive. >Bindings of CFT and nisoxetine to HEK-NET are differently regulated by sodium concentration.
Acknowledgments
This work was supported by NIH grants R01 DA019676 (M.E.A.R.) and R01 MH084888 (A.K.D.). We thank Kyle C. Schmitt for his assistance in copy-editing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Backstrom IT, Ross SB, Marcusson JO. [3H]desipramine binding to rat brain tissue: binding to both noradrenaline uptake sites and sites not related to noradrenaline neurons. J Neurochem. 1989;52:1099–106. doi: 10.1111/j.1471-4159.1989.tb01853.x. [DOI] [PubMed] [Google Scholar]
- Bauer ME, Tejani-Butt SM. Effects of repeated administration of desipramine or electroconvulsive shock on norepinephrine uptake sites measured by [3H]nisoxetine autoradiography. Brain Res. 1992;582:208–14. doi: 10.1016/0006-8993(92)90134-u. [DOI] [PubMed] [Google Scholar]
- Beuming T, Kniazeff J, Bergmann ML, Shi L, Gracia L, Raniszewska K, Newman AH, Javitch JA, Weinstein H, Gether U, Loland CJ. The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nat Neurosci. 2008;11:780–9. doi: 10.1038/nn.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet J. Interactions of cations and anions with the binding of uptake blockers to the dopamine transporter. Eur J Pharm. 2003;479:199–212. doi: 10.1016/j.ejphar.2003.08.069. [DOI] [PubMed] [Google Scholar]
- Bönisch H, Brüss M. The norepinephrine transporter in physiology and disease. Handb Exp Pharmacol. 2006;175:485–524. doi: 10.1007/3-540-29784-7_20. [DOI] [PubMed] [Google Scholar]
- Bryan-Lluka LJ, Bonisch H, Lewis RJ. chi-Conopeptide MrIA partially overlaps desipramine and cocaine binding sites on the human norepinephrine transporter. J Biol Chem. 2003;278:40324–9. doi: 10.1074/jbc.M213101200. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Snyder SH. Catecholamine uptake by synaptosomes in homogenates of rat brain: stereospecificity in different areas. J Pharmacol ExpTher. 1969;170:221–31. [PubMed] [Google Scholar]
- Dubocovich ML, Zahniser NR. Binding characteristics of the dopamine uptake inhibitor [3H]nomifensine to striatal membranes. Biochem Pharmacol. 1985;34:1137–44. doi: 10.1016/0006-2952(85)90486-1. [DOI] [PubMed] [Google Scholar]
- Dutta AK, Ghosh B, Biswas S, Reith ME. D-161, a novel pyran-based triple monoamine transporter blocker: behavioral pharmacological evidence for antidepressant-like action. Eur J Pharmacol. 2008;589:73–9. doi: 10.1016/j.ejphar.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Eshleman AJ, Carmolli M, Cumbay M, Martens CR, Neve KA, Janowsky A. Characteristics of drug interactions with recombinant biogenic amine transporters expressed in the same cell type. J Pharmacol Exp Ther. 1999;289:877–85. [PubMed] [Google Scholar]
- Hrdina PD. Pharmacological characterization of [3H] desipramine binding in rat cerebral cortex. Prog Neuropsychopharmacol. 1981;5:553–7. doi: 10.1016/0364-7722(81)90045-x. [DOI] [PubMed] [Google Scholar]
- Javitch JA, Blaustein RO, Snyder SH. [3H]mazindol binding associated with neuronal dopamine and norepinephrine uptake sites. Mol Pharmacol. 1984;26:35–44. [PubMed] [Google Scholar]
- Kuhar MJ, McGirr KM, Hunter RG, Lambert PD, Garrett BE, Carroll FI. Studies of selected phenyltropanes at monoamine transporters. Drug Alcohol Depend. 1999;56:9–15. doi: 10.1016/s0376-8716(99)00005-8. [DOI] [PubMed] [Google Scholar]
- Kurian MA, Zhen J, Cheng SY, Li Y, Mordekar SR, Jardine P, Morgan NV, Meyer E, Tee L, Pasha S, Wassmer E, Heales SJR, Gissen P, Reith MEA, Maher ER. Homozygous loss-of-function mutations in the dopamine transporter are associated with infantile parkinsonism-dystonia. J Clin Inv. 2009 doi: 10.1172/JCI39060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CM, Javitch JA, Snyder SH. Characterization of [3H]desipramine binding associated with neuronal norepinephrine uptake sites in rat brain membranes. J Neurosci. 1982;2:1515–25. doi: 10.1523/JNEUROSCI.02-10-01515.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LB, Reith ME. Modeling of the interaction of Na+ and K+ with the binding of dopamine and [3H]WIN 35,428 to the human dopamine transporter. J Neurochem. 1999;72:1095–109. doi: 10.1046/j.1471-4159.1999.0721095.x. [DOI] [PubMed] [Google Scholar]
- Li LB, Reith ME. Interaction of Na+, K+, and Cl- with the binding of amphetamine, octopamine, and tyramine to the human dopamine transporter. J Neurochem. 2000;74:1538–52. doi: 10.1046/j.1471-4159.2000.0741538.x. [DOI] [PubMed] [Google Scholar]
- Loland CJ, Granas C, Javitch JA, Gether U. Identification of intracellular residues in the dopamine transporter critical for regulation of transporter conformation and cocaine binding. J Biol Chem. 2004;279:3228–38. doi: 10.1074/jbc.M304755200. [DOI] [PubMed] [Google Scholar]
- Madras BK, Spealman RD, Fahey MA, Neumeyer JL, Saha JK, Milius RA. Cocaine receptors labeled by [3H]2 beta-carbomethoxy-3 beta-(4- fluorophenyl)tropane. Mol Pharmacol. 1989;36:518–24. [PubMed] [Google Scholar]
- Norregaard L, Frederiksen D, Nielsen EO, Gether U. Delineation of an endogenous zinc-binding site in the human dopamine transporter. EMBO J. 1998;17:4266–73. doi: 10.1093/emboj/17.15.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens MJ, Knight DL, Nemeroff CB. Second-generation SSRIs: human monoamine transporter binding profile of escitalopram and R-fluoxetine. Biol Psychiatry. 2001;50:345–50. doi: 10.1016/s0006-3223(01)01145-3. [DOI] [PubMed] [Google Scholar]
- Pacholczyk T, Blakely RD, Amara SG. Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature. 1991;350:350–4. doi: 10.1038/350350a0. [DOI] [PubMed] [Google Scholar]
- Paczkowski FA, Sharpe IA, Dutertre S, Lewis RJ. chi-Conotoxin and tricyclic antidepressant interactions at the norepinephrine transporter define a new transporter model. J Biol Chem. 2007;282:17837–44. doi: 10.1074/jbc.M610813200. [DOI] [PubMed] [Google Scholar]
- Ravna AW, Sylte I, Dahl SG. Structure and localisation of drug binding sites on neurotransmitter transporters. J Mol Model. 2009 doi: 10.1007/s00894-009-0478-1. [DOI] [PubMed] [Google Scholar]
- Reith MEA, Wang LC, Dutta AK. Pharmacological profile of radioligand binding to the norepinephrine transporter: Instances of poor indication of functional activity. J Neurosci Methods. 2005;143:87–94. doi: 10.1016/j.jneumeth.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Silverthorn ML, Baumann MH, Goodman CB, Cadet JL, Matecka D, Rice KC, Carroll FI, Wang JB, Uhl GR. Studies of the biogenic amine transporters. VI. Characterization of a novel cocaine binding site, identified with [125I]RTI-55, in membranes prepared from whole rat brain minus caudate. J Pharmacol Exp Ther. 1995;274:385–95. [PubMed] [Google Scholar]
- Sarker S, Weissensteiner R, Steiner I, Sitte HH, Ecker GF, Freissmuth M, Sucic S. The high-affinity binding site for tricyclic antidepressants resides in the outer vestibule of the serotonin transporter. Mol Pharmacol. 2010;78:1026–35. doi: 10.1124/mol.110.067538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scatton B, Dubois A, Dubocovich ML, Zahniser NR, Fage D. Quantitative autoradiography of 3H-nomifensine binding sites in rat brain. Life Sci. 1985;36:815–22. doi: 10.1016/0024-3205(85)90204-8. [DOI] [PubMed] [Google Scholar]
- Schmitt KC, Zhen J, Kharkar P, Mishra M, Chen N, Dutta AK, Reith ME. Interaction of cocaine-, benztropine-, and GBR12909-like compounds with wild-type and mutant human dopamine transporters: molecular features that differentially determine antagonist-binding properties. J Neurochem. 2008;107:928–40. doi: 10.1111/j.1471-4159.2008.05667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe IA, Palant E, Schroeder CI, Kaye DM, Adams DJ, Alewood PF, Lewis RJ. Inhibition of the norepinephrine transporter by the venom peptide chi-MrIA. Site of action, Na+ dependence, and structure-activity relationship. J Biol Chem. 2003;278:40317–23. doi: 10.1074/jbc.M213030200. [DOI] [PubMed] [Google Scholar]
- Sucic S, Bryan-Lluka LJ. Roles of transmembrane domain 2 and the first intracellular loop in human noradrenaline transporter function: pharmacological and SCAM analysis. J Neurochem. 2005;94:1620–30. doi: 10.1111/j.1471-4159.2005.03316.x. [DOI] [PubMed] [Google Scholar]
- Tejani-Butt SM. [3H]nisoxetine: a radioligand for quantitation of norepinephrine uptake sites by autoradiography or by homogenate binding. J Pharmacol Exp Ther. 1992;260:427–36. [PubMed] [Google Scholar]
- Tomlinson G. Potential misconceptions arising from the application of enzyme kinetic equations to ligand-receptor systems at equilibrium. Can J Physiol Pharmacol. 1988;66:342–9. doi: 10.1139/y88-059. [DOI] [PubMed] [Google Scholar]
- Wang S, Sakamuri S, Enyedy IJ, Kozikowski AP, Deschaux O, Bandyopadhyay BC, Tella SR, Zaman WA, Johnson KM. Discovery of a novel dopamine transporter inhibitor, 4-hydroxy-1-methyl-4-(4-methylphenyl)-3-piperidyl 4-methylphenyl ketone, as a potential cocaine antagonist through 3D-database pharmacophore searching. Molecular modeling, structure-activity relationships, and behavioral pharmacological studies. J Med Chem. 2000;43:351–60. doi: 10.1021/jm990516x. [DOI] [PubMed] [Google Scholar]
- Zhen J, Chen N, Reith MEA. Differences in interactions with the dopamine transporter as revealed by dimishment of Na+ gradient and membrane potential: dopamine versus other substrates. Neuropharmacol. 2005;49:769–79. doi: 10.1016/j.neuropharm.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Zhen J, Karpowich NK, Law CJ, Reith ME, Wang DN. Antidepressant specificity of serotonin transporter suggested by three LeuT-SSRI structures. Nat Struct Mol Biol. 2009;16:652–7. doi: 10.1038/nsmb.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MY, Kyle PB, Hume AS, Ordway GA. The persistent membrane retention of desipramine causes lasting inhibition of norepinephrine transporter function. Neurochem Res. 2004;29:419–27. doi: 10.1023/b:nere.0000013747.04964.46. [DOI] [PubMed] [Google Scholar]