Abstract
Background
Recent studies have hypothesized that endothelial and microvascular dysfunction may play a role in the development of obesity. Previous studies have shown that retinal microvascular changes are associated with diabetes, hypertension, and cardiovascular disease. In contrast, few prospective studies have examined the association between retinal microvascular changes and the risk of developing obesity.
Methods
We examined n=2,089 nonobese subjects from a population-based cohort in Beaver Dam, Wisconsin (aged 44-85 years, 49% women). Retinal arteriolar and venular diameters were measured from baseline retinal photographs. The main outcome-of-interest was 15-year incidence of obesity.
Results
Retinal venular widening was positively associated with incident obesity over a 15-year follow-up period. This association was independent of age, gender, smoking, alcohol intake, education, physical activity, body mass index, serum cholesterol, and C-reactive protein levels. Compared to subjects with retinal venular diameter in the lowest tertile (referent), the multivariable RR (95% CI) of obesity among subjects in the highest tertile was 1.68 (1.24-2.28); p-trend=0.0005. In contrast, narrow retinal arterioles were not associated with obesity.
Conclusions
In a population-based cohort, we found that wider retinal venules are positively associated with risk of developing obesity, suggesting a role for microvascular dysfunction in its etiology.
Keywords: Retinal vessel diameters, venular widening, obesity, cohort study, BMI
Obesity is a major public health problem in the US with its prevalence exceeding 30% in most age and sex groups1. Obesity is also a strong risk factor for diabetes mellitus, hypertension and cardiovascular disease (CVD)2. Recent studies have hypothesized that endothelial and microvascular dysfunction may play a role in the development of obesity3;4. The retinal vasculature offers a unique opportunity to investigate the systemic effects of microvascular dysfunction as it can be viewed non-invasively and also it has been shown to be structurally and functionally similar to microvasculature elsewhere in the body5. Recent advances in retinal imaging have allowed quantitative measurement of retinal microvascular caliber in large epidemiological studies5. These previous studies have shown that narrower retinal arterioles are associated with diabetes6, hypertension7;8, and CVD9 whereas wider retinal venules with inflammation10;11, metabolic abnormalities12, higher glycosylated hemoglobin levels13 and impaired fasting glucose14. In contrast, few prospective studies have examined the association between retinal microvascular changes and the risk of developing obesity15. Therefore, we examined the association between retinal vessel diameter and the risk of developing obesity in a large population based study of adults from Wisconsin, after controlling for the main confounders.
METHODS
Study population
The Beaver Dam Eye Study is a population-based cohort study primarily aimed at studying the distribution and determinants of age-related eye diseases among older Americans in Wisconsin. The methods used to identify and describe the population have appeared in previous reports16;17. In brief, a private census of the population of Beaver Dam, Wisconsin, was performed from September 1987 to May 1988 to identify all residents in the city or township of Beaver Dam who were 43-84 years of age. Of the 5,924 eligible individuals (98 percent Caucasians), 4,926 (83.1 percent) participated in the baseline examination between March 1, 1988 and September 14, 1990. The baseline examination was followed by follow-up cohort examinations every five years in 1993-1995 and 1998-2000 and 2005-2007. The main reason for non-participation at subsequent examinations was mortality. The follow-up rate for nonfatal events was 83 percent of the total potential person-years of follow-up. Comparisons between participants and nonparticipants at the time of the baseline and follow-up examinations have appeared elsewhere16;17. In short, those who did not participate in the baseline study were more likely to be older, had fewer years of education, lower income, and smoked more pack-years than those who participated in the study. Both the baseline and follow-up examinations followed a similar protocol. Written informed consent was obtained from each subject at each examination. The study was approved by the Human Subjects Committee of the University of Wisconsin School of Medicine and Public Health, Madison.
There were 3770 study participants who participated at the baseline examination and at least one of the follow-up examinations. We further excluded those with preexisting obesity (n=1,348), CVD (n=263), missing data on retinal vessel diameters (n=22), and other important covariates included in the multivariable model (n=18), including cigarette smoking, total or high density lipoprotein cholesterol levels. This resulted in 2,089 nonobese individuals at baseline who contributed person-time data to the current analysis. Among them 486 subjects developed obesity during the 15-year follow-up period.
All participants had 30 degree color retinal photographs taken of both eyes at baseline. Details of retinal measurement from these photographs have been published before18. After converting the field 1 photographs to digitized images, retinal measurements were carried out by trained graders masked to participant characteristics using a computer-assisted software. All arterioles and venules coursing through a specified zone of 0.5 to 1 disc diameter surrounding the optic disc margin were measured and summarized as central retinal arteriolar equivalent (CRAE) or central retinal venular equivalent (CRVE) using a modification of the Parr-Hubbard formula19 as described by Knudtson et al20. These equivalents are the projected diameters of the central retinal vessels, measured away from the optic disc. Reproducibility of these retinal measurements has been previously reported with intragrader and intergrader intraclass correlation coefficients ranging from 0.78 to 0.9921. For the current analysis, average of the right and left eye measurements were taken as the CRAE and CRVE value for each participant.
The baseline and follow-up examinations included measuring weight, height, systolic and diastolic blood pressure by a trained observer, administering standardized questionnaire that collected information regarding participants’ demographic characteristics, details regarding cigarette smoking, alcohol intake, physical activity, medical histories and medications taken, including diagnosis of diabetes or hypertension by a physician. Non-fasting blood specimens were obtained for measurement of total cholesterol and high sensitivity C-reactive protein (CRP). Blood pressure was measured in seated position with a random-zero sphygmomanometer. The average of last two out of three total measurements was used as the blood pressure value for this analysis.
Age was defined as the participants’ age at the time of baseline examination. Education was categorized as below high school, high school, or beyond high school. Moderate physical activity frequency was defined as the frequency of exercise severe enough to work up sweat per week. Body mass index (BMI) was defined as participants’ weight in kilograms divided by the height in meter squared. Obesity was defined as a BMI of >30 kg/m1. The main study outcome was incident obesity, defined as the new development of obesity at the 5-, 10- or 15-year follow-up examination among subjects free of obesity at baseline. As secondary outcomes, we also examined longitudinal change in body weight (lbs), and incident severe body mass gain, defined as a change in BMI of >20% over the 15 year follow-up period, since this is the body mass gain required to change from a BMI of 25 kg/m2 to 30 kg/m2.
Statistical methods
Retinal vessel diameters were categorized into tertiles for the analysis. As previous studies have shown that retinal arteriolar narrowing and venular widening are possibly separate pathophysiological processes10, we used the widest quartile of retinal arteriolar diameter (quartile 4) as the reference category in the retinal arteriolar narrowing analysis, and the narrowest quartile of retinal venular diameter (quartile 1) as the reference category in the retinal venular widening analysis. We used chi-square test and analysis of variance to compare the relationship of selected baseline characteristics to increasing retinal venular tertiles. First, we used Cox proportional hazards models with discrete handling of ties to determine the relative risk (RR) and 95 percent confidence interval (CI) of incident obesity, controlling simultaneously for potential confounders. Second, smoking may be a strong confounder of the association between retinal vessel diameters and obesity as smoking has been shown to be associated with both retinal vessel diameters22 and obesity,23. Therefore, we performed subgroup analysis stratified by current smoking status to examine if the observed association between retinal vessel diameters and obesity is consistently present among smokers and nonsmokers. Third, we examined the association between baseline retinal arteriolar and venular diameters and estimated annual change in weight (in lbs) over the 15-year follow-up period using linear regression using PROC MIXED in SAS to account for the correlation within subjects due to repeated measures. Fourth, we used Cox proportional hazards models to determine the RR and 95% CI of incident severe weight gain, controlling for potential confounders. For all these analyses, we used the most parsimonius model adjusting for age (years), sex (male, female), education categories (<high school, high school, >high school), smoking (never, former, current), alcohol intake (never, former, current), moderate physical activity (times/week), mean arterial blood pressure (mm Hg), serum total cholesterol (mg/dL), high sensitivity CRP (mg/dL), and the fellow vessel diameter (μm, retinal arteriolar diameter in models of venular diameter and vice-versa following the recommendation by Liew et al)24.
We also performed the following supplementary analyses. First, we additionally adjusted for baseline BMI in the multivariable model to examine if the observed findings are confounded by variations in baseline BMI. Second, we examined the association between retinal vessel diameters and incident obesity employing time varying covariates in proportional hazards models. Third, when we examined the association between retinal vessel diameters and incident obesity after excluding all subjects with diagnosed cancer or cardiovascular disease at baseline as well as follow-up. SAS version 9.2 was used for all analyses.
Role of funding source
This study was supported by grants from the National Institute of Health, National Institute of Diabetes and Digestive and Kidney Diseases and American Heart Association. The funding bodies were not involved in data analysis, interpretation of findings or writing of the manuscript.
RESULTS
Table 1 presents the baseline characteristics of the cohort by increasing categories of retinal venular diameter. Subjects with wider venular diameters were more likely to be younger, current smoker, more likely to have lower serum HDL cholesterol levels; and less likely to be women.
Table 1.
Baseline characteristics of study participants by tertiles of retinal venular diameter
| Retinal venular diameter (μm) |
||||
|---|---|---|---|---|
| Tertile 1 (n=699) |
Tertile 2 (n=701) |
Tertile 3 (n=689) |
P value* | |
| Age, mean (SD), y | 60.9 (10.8) | 58.9 (10.3) | 58.6 (10.0) | <0.0001 |
| Female, % | 68.7 | 58.8 | 52.1 | <0.0001 |
| Less than high school education, % | 18.3 | 22.5 | 21.0 | 0.1 |
| Current smoking, % | 10.3 | 21.8 | 38.6 | <0.0001 |
| Heavy drinker, % | 6.6 | 6.3 | 8.7 | 0.2 |
| Exercise frequency per week, mean (SD) | 1.6 (2.3) | 1.5 (2.2) | 1.2 (2.0) | 0.007 |
| Systolic blood pressure, mean (SD), mm Hg | 129.0 (20.4) | 127.0 (19.0) | 126.9 (18.1) | 0.07 |
| Diastolic blood pressure, mean (SD), mm Hg | 76.4 (9.9) | 76.7 (9.9) | 76.5 (10.4) | 0.9 |
| Mean arterial blood pressure, mean (SD), mm Hg | 93.8 (11.5) | 93.3 (11.5) | 93.1 (11.3) | 0.5 |
| Serum total cholesterol, mean (SD), mg/dL | 232.2 (42.7) | 230.6 (42.4) | 230.4 (41.0) | 0.7 |
| Serum HDL cholesterol, mean (SD), mg/dL | 58.4 (18.8) | 56.3 (18.6) | 54.0 (17.7) | <0.0001 |
| C-reactive protein mg/dL | 2.8 (6.9) | 3.3 (11.0) | 3.8 (7.8) | 0.09 |
Abbreviations: HDL, High density lipoprotein; SD, standard deviation;
P value represents difference in characteristic by retinal venular diameter tertiles, using analysis of variance or chi square test.
Table 2 presents the multivariable association between retinal vessel diameters and the risk of developing obesity over the 15 year follow-up period. Retinal arteriolar narrowing was not found to be associated with obesity. However, retinal venular widening was positively associated with increased risk of incident obesity even after adjusting for high sensitivity CRP levels; models evaluating trend in this association were also statistically significant.
Table 2.
Association between retinal vessel diameters and incident obesity
| No. at risk | obesity cases | Multivariable-adjusted relative risk (95% confidence interval)* |
|
|---|---|---|---|
| Retinal arteriolar diameter, μm | |||
| Tertile 3 (156.0-199.7) | 700 | 156 | 1 (referent) |
| Tertile 2 (144.6-155.9) | 697 | 172 | 1.32 (0.97-1.78) |
| Tertile 1 (89.8-144.5) | 692 | 158 | 1.27 (0.99-1.64) |
| p-trend | 0.07 | ||
| Retinal venular diameter, μm | |||
| Tertile 1 (165.9- 220.0) | 699 | 131 | 1 (referent) |
| Tertile 2 (220.1-237.3) | 701 | 174 | 1.44 (1.10-1.87) |
| Tertile 3 (237.3-339.7) | 689 | 181 | 1.68 (1.24-2.28) |
| p-trend | 0.0005 |
Adjusted for age (years), sex (men, women), education categories (<high school, high school, >high school), smoking (never, former, current), alcohol intake (never, former, current), exercise frequency (number of times per week), mean arterial blood pressure (mm Hg), serum total cholesterol (mg/dL), C-reactive protein (mg/dL), fellow vessel diameter (μm, central retinal arteriolar in models of central retinal venular diameter and vice-versa)
Table 3 presents the multivariable association between retinal vessel diameters and the risk of developing obesity by current smoking status. We found that the association between retinal venular widening and incident obesity was consistently present among both current smokers and nonsmokers (p-interaction between current smoking and CRVE tertile =0.9). Retinal arteriolar narrowing was found to be borderline positively associated with obesity only among nonsmokers (p-trend=0.06, p-interaction between current smoking and CRAE tertile=0.4).
Table 3.
Association between retinal vessel diameters and incident obesity, by current smoking
| Current smoker, absent | Current smoker, present | |||
|---|---|---|---|---|
| No. at risk (cases) |
Multivariable-adjusted relative risk (95% confidence interval)* |
No. at risk (cases) |
Multivariable-adjusted relative risk (95% confidence interval)* |
|
| Retinal arteriolar diameter, μm | ||||
| Tertile 3 (156.0-199.7) | 456 (107) | 1 (referent) | 97 (17) | 1 (referent) |
| Tertile 2 (144.6-155.9) | 547 (128) | 1.17 (0.87-1.58) | 150 (44) | 1.65 (1.00-2.72) |
| Tertile 1 (89.8-144.5) | 595 (141) | 1.39 (0.99-1.96) | 244 (49) | 0.92 (0.45-1.88) |
| p-trend | 0.06 | 491 (110) | 0.76 | |
| Retinal venular diameter, μm | ||||
| Tertile 1 (165.9- 220.0) | 627 (122) | 1 (referent) | 72 (9) | 1 (referent) |
| Tertile 2 (220.1-237.3) | 548 (136) | 1.38 (1.04-1.84) | 153 (38) | 2.46 (1.10-5.52) |
| Tertile 3 (237.3-339.7) | 423 (118) | 1.70 (1.21-2.38) | 266 (63) | 2.31 (1.01-5.32) |
| p-trend | 0.002 | 491 (110) | 0.14 | |
Adjusted for age (years), sex (men, women), education categories (<high school, high school, >high school), alcohol intake (never, former, current), exercise frequency (number of times per week), mean arterial blood pressure (mm Hg), serum total cholesterol (mg/dL), C-reactive protein (mg/dL), fellow vessel diameter (μm, central retinal arteriolar in models of central retinal venular diameter and vice-versa)
When we examined the multivariable association between retinal vessel diameters and longitudinal change in body weight over the 15 year follow-up period, neither retinal arteriolar narrowing nor venular widening was found to be associated with weight change as a linear variable; for example the estimated annual change in weight in kg (95% CI) was −0.06 (−0.18-0.05) in tertile 1 of CRAE compared to tertile 3 with a p-trend of 0.18 and 0.06 (−0.09-0.15) in tertile 3 of CRVE compared to tertile 1 with a p-trend of 0.74 (data not shown).
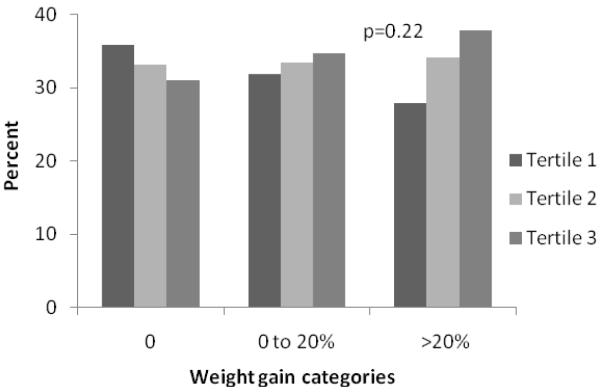
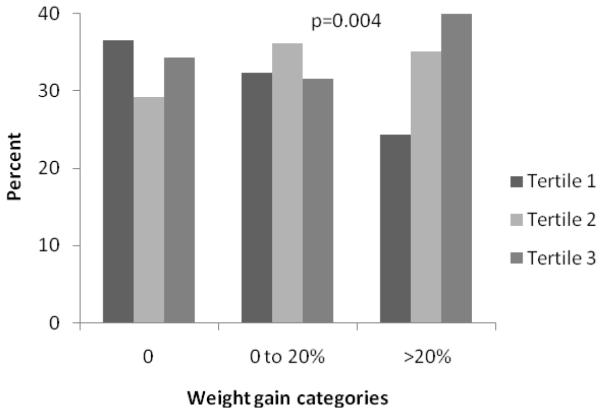
To further examine the association between retinal vessel diameters and longitudinal weight change, we categorized the study population into 3 weight gain categories classified as 0, 0 to 20% and >20% gain in BMI over the 15-year follow-up period to reflect none, modest and severe weight gain. Subsequently, in Figure 1, we examined the distribution of these three weight gain categories by tertiles of baseline retinal vessel diameter. We found that percentages of none or modest weight gain categories were distributed more or less similarly by retinal vessel diameters, but severe weight gain was associated with both narrow retinal arteriolar tertiles and wider venular tertiles; however results were statistically significant only for venular diameter.
Fig 1.
A. Relationship between retinal arteriolar diameter and 15-year body mass gain
B. Relationship between retinal venular diameter and 15-year body mass gain
We categorized the study population into 3 weight gain categories classified as 0, 0 to 20% and >20% gain in BMI over the 15-year follow-up period to reflect none, modest and severe weight gain. P-value for the difference in weight gain categories by retinal vessel diameter tertiles were calculated by chi square test.
Table 4 presents the multivariable association between retinal vessel diameters and the risk of severe weight gain, defined as >20% gain in BMI, over the 15 year follow-up period. Similar to table 2, retinal arteriolar narrowing was not found to be associated with >20% weight gain. Whereas, retinal venular widening was positively associated with increased risk of >20% weight gain.
Table 4.
Relationship of retinal vessel diameters with >20% gain in body mass over the 15 year follow-up period
| No. at risk (cases) |
Multivariable-adjusted relative risk (95% confidence interval)* |
|
|---|---|---|
| Retinal arteriolar diameter, μm | ||
| Tertile 3 (156.0-199.7) | 692 (31) | 1 (Referent) |
| Tertile 2 (144.6-155.9) | 697 (38) | 1.00 (0.37-2.73) |
| Tertile 1 (89.8-144.5) | 700 (42) | 1.10 (0.58-2.06) |
| p-trend | 0.93 | |
| Retinal venular diameter, μm | ||
| Tertile 1 (165.9- 220.0) | 699 (27) | 1 (Referent) |
| Tertile 2 (220.1-237.3) | 701 (39) | 1.50 (0.88-2.58) |
| Tertile 3 (237.3-339.7) | 698 (45) | 1.85 (1.01-3.38) |
| p-trend | 0.04 |
Adjusted for age (years), sex (men, women), education categories (<high school, high school, >high school), smoking (never, former, current), alcohol intake (never, former, current), exercise frequency (number of times per week), mean arterial blood pressure (mm Hg), serum total cholesterol (mg/dL), C-reactive protein (mg/dL), height (inches) and fellow vessel diameter (μm , central retinal arteriolar in models of central retinal venular diameter and vice-versa)
We also performed several supplementary analyses. First, when we additionally adjusted for baseline BMI in the multivariable model, the results were attenuated but still statistically significant. Compared to tertile 1 of retinal venular diameter (referent), the RR (95% CI) of incident obesity was 1.27 (0.95 to 1.70) in tertile 2 and 1.55 (1.11 to 2.18) in tertile 3; p-trend=0.0006. Second, when we examined the association between retinal vessel diameters and incident obesity employing time varying covariates in proportional hazards models, the results were slightly attenuated, but still significantly present. Compared to tertile 1 of retinal venular diameter (referent), the RR (95% CI) of incident obesity was 1.22 (0.90 to 1.66) in tertile 2, and 1.39 (1.02 to 1.88) in tertile 3; p-trend=0.005. Third, when we examined the association between retinal vessel diameters and incident obesity after excluding all subjects with diagnosed cancer or cardiovascular disease at baseline as well as all follow-up examinations, the total sample size was reduced to 1476. However, we found that the association between retinal venular dilatation and incident obesity was stronger. Compared to tertile 1 of retinal venular diameter (referent), the RR (95% CI) of incident obesity was 1.55 (1.14 to 2.11) in tertile 2, and 1.72 (1.21 to 2.45) in tertile 3; p-trend=0.003.
DISCUSSION
In a population-based study of adults from Wisconsin, we found that retinal venular widening was positively associated with increased risk of becoming obese over a 15-year follow-up period. This association was found to be independent of age, gender, smoking, alcohol intake, education, physical activity, BMI, serum cholesterol, high sensitivity CRP levels, and the fellow vessel diameter. In a parallel analysis, we also found that retinal venular widening was associated with increased risk of developing severe body mass gain, defined as >20% gain in BMI from the baseline, over the 15-year follow-up period. In contrast, retinal arteriolar narrowing was not found to be associated with incident obesity or severe weight gain.
Three previous cross-sectional studies in adults10;12;15 and one cross-sectional study in 7 to 9 years old school children in Singapore26 have reported that obesity is associated with larger retinal venular diameters. To date, the only prospective study that examined the association between retinal vessel diameters and incident obesity was in the Blue Mountains Eye Study of elderly Australians where Wang et al.15 showed that retinal venular widening was associated with 5-year risk of developing obesity. Our results extend the previous findings by Wang et al. by providing longer-term follow-up data on incident obesity (15 years vs. 5 years) and adjusting for additional confounders such as high sensitivity CRP levels. Since markers of inflammation have been shown to be positively associated with retinal venular widening10;11 and also with obesity27;28, potential confounding by changes in inflammatory marker levels unrelated to microvascular disease is a concern. In the current study, we have demonstrated that retinal venular widening is associated with incident obesity even after additionally adjusting for high sensitivity CRP levels, suggesting an independent role for microvascular component in the pathogenesis of obesity and severe body mass gain. Similarly, smoking may be a confounder in the association between retinal vessel diameters and obesity as smoking has been shown to be inversely related to BMI29 and also to retinal vessel changes30. In a subgroup analysis, we found that retinal venular widening was positively associated with incident obesity among both current smokers and nonsmokers. Finally, when we analyzed the association between retinal vessel diameters and incident obesity after excluding subjects with a diagnosis of cancer or cardiovascular disease, the findings were found to be consistent with the main results, suggesting that our findings are not biased by weight changes related to these diseases.
In the current study, we did not find a positive association between retinal microvascular changes and longitudinal weight gain analyzed as a linear variable. However, when we examined severe weight gain as the outcome, we found an independent association with retinal venular widening, consistent with the results for incident obesity. While this difference in findings between linear weight gain and severe weight gain is intriguing, one explanation is that retinal venular widening identifies generalized changes in the microvasculature among previously nonobese subjects who are likely to have weight change that is severe enough to develop obesity (for example, >20% body mass change, or a change in BMI from 25 to 30 kg /m2), but is not sensitive to non-pathological small changes/fluctuations in body mass (for example, a change in BMI from 25 to 25.6 kg/m2). A second explanation is that BMI, especially small changes/fluctuations in BMI, are probably not an accurate measure of true changes in fat mass31. There is a need to examine the relation of retinal vessel diameters and other, potentially more accurate measures of adiposity, such as visceral adiposity thickness measured from computed tomography32, or examine adipokines and hormones that are actively secreted by adipose tissue33. A final explanation is that our findings are due to chance.
In the current study, retinal arteriolar narrowing was not found to be associated with obesity, a finding consistent with the only previous longitudinal analysis on obesity from the Blue Mountains Eye Study15. Previous studies have shown that retinal arteriolar narrowing is associated with incident hypertension7;8 and diabetes6, conditions that are closely related to obesity. One emerging hypothesis is that retinal arteriolar narrowing and venular widening reflect different systemic pathophysiological processes10. For example, arteriolar changes appear to be strongly associated with elevated blood pressure7;10 while venular widening may reflect inflammation10;11, hyperperfusion resulting from hyperglycemia and lactic acidosis from retinal hypoxia, at least in people with diabetes34. Future analyses from other longitudinal cohorts such as the Atherosclerosis Risk in Communities (ARIC) study with retinal vessel measurements and longitudinal BMI data should be able to confirm or disprove this finding.
Major strengths of the current study include its large sample size, prospective design, long duration of follow-up, precise measurements of retinal vessel diameters and BMI, and information on potential confounders. Our study has some limitations. First, loss to follow-up might have resulted in selective mortality of those with retinal arteriolar narrowing and obesity. This might have masked the association between retinal arteriolar narrowing and obesity, and attenuated the association between venular widening and obesity. Second, it is possible that our results are biased by residual confounding from unmeasured variables including dietary factors (e.g. high intake of saturated fats, carbohydrates, high glycemic index diet) that play a role in obesity. However, since we have adjusted for serum cholesterol, blood pressure, diabetes, and C-reactive protein levels, which are known mediating factors by which diet may act, we believe that the magnitude of such residual confounding may be limited. Third, our adjustment for comorbid conditions including diabetes and hypertension in the multivariable model would likely lead to an overadjustment and may have under estimated the true association between retinal venular widening and obesity. Fourth, we excluded subjects with obesity at baseline and are examining the association between baseline retinal vessel diameters and the risk of developing obesity over a 10-year follow-up period among initially non-obese subjects. We acknowledge that some level of reverse causality may still be biasing our results. Therefore, at least some of the observed findings may be explained by the effect of obesity on retinal microcirculation.
In conclusion, in a large population-based study of adults from Wisconsin, retinal venular widening was found to be positively associated with increased risk of developing obesity as well as severe body mass gain, defined as >20% increase in BMI. This association was independent of age, gender, smoking, alcohol intake, education, physical activity, diabetes mellitus, hypertension, BMI, serum cholesterol, and high sensitivity CRP levels. In contrast, retinal arteriolar narrowing was not found to be associated with incident obesity or severe body mass gain. While the observed positive association between retinal venular widening and obesity warrants further replication in other populations, our findings suggest that microvascular disease may play a role in the development of obesity and interventions specifically targeting microcirculation may perhaps help reduce the burden of obesity.
ACKNOWLEDGEMENT
Details of funding: Supported by National Institutes of Health grant EYO6594 (RK, BEK), NIA grant AG11099 (KJC), NIDDK grant DK73217 (RK, AS), NIH Grant 5R03ES018888-02 and American Heart Association (AS).
Footnotes
Conflict of interest: There are no conflicts of interest related to this manuscript.
Ethical Approval: This study followed the recommendations of Declaration of Helsinki and was approved by the Human Subjects Committee of the University of Wisconsin School of Medicine and Public Health, Madison, WI. Written, informed consent was obtained from all participants.
References
- (1).Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- (2).Malnick SD, Knobler H. The medical complications of obesity. QJM. 2006;99:565–579. doi: 10.1093/qjmed/hcl085. [DOI] [PubMed] [Google Scholar]
- (3).Skilton MR, Celermajer DS. Endothelial dysfunction and arterial abnormalities in childhood obesity. Int J Obes (Lond) 2006;30:1041–1049. doi: 10.1038/sj.ijo.0803397. [DOI] [PubMed] [Google Scholar]
- (4).de Jongh RT, Serne EH, IJzerman RG, de VG, Stehouwer CD. Impaired microvascular function in obesity: implications for obesity-associated microangiopathy, hypertension, and insulin resistance. Circulation. 2004;109:2529–2535. doi: 10.1161/01.CIR.0000129772.26647.6F. [DOI] [PubMed] [Google Scholar]
- (5).Liew G, Wang JJ, Mitchell P, Wong TY. Retinal vascular imaging: a new tool in microvascular disease research. Circ Cardiovasc Imaging. 2008;1:156–161. doi: 10.1161/CIRCIMAGING.108.784876. [DOI] [PubMed] [Google Scholar]
- (6).Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar narrowing and risk of diabetes mellitus in middle-aged persons. JAMA. 2002;287:2528–2533. doi: 10.1001/jama.287.19.2528. [DOI] [PubMed] [Google Scholar]
- (7).Sharrett AR, Hubbard LD, Cooper LS, et al. Retinal arteriolar diameters and elevated blood pressure: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1999;150:263–270. doi: 10.1093/oxfordjournals.aje.a009997. [DOI] [PubMed] [Google Scholar]
- (8).Wong TY, Shankar A, Klein R, Klein BE, Hubbard LD. Prospective cohort study of retinal vessel diameters and risk of hypertension. BMJ. 2004;329:79. doi: 10.1136/bmj.38124.682523.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. JAMA. 2002;287:1153–1159. doi: 10.1001/jama.287.9.1153. [DOI] [PubMed] [Google Scholar]
- (10).Ikram MK, de Jong FJ, Vingerling JR, et al. Are retinal arteriolar or venular diameters associated with markers for cardiovascular disorders? The Rotterdam Study. Invest Ophthalmol Vis Sci. 2004;45:2129–2134. doi: 10.1167/iovs.03-1390. [DOI] [PubMed] [Google Scholar]
- (11).Klein R, Klein BE, Knudtson MD, Wong TY, Tsai MY. Are inflammatory factors related to retinal vessel caliber? The Beaver Dam Eye Study. Arch Ophthalmol. 2006;124:87–94. doi: 10.1001/archopht.124.1.87. [DOI] [PubMed] [Google Scholar]
- (12).Wong TY, Duncan BB, Golden SH, et al. Associations between the metabolic syndrome and retinal microvascular signs: the Atherosclerosis Risk In Communities study. Invest Ophthalmol Vis Sci. 2004;45:2949–2954. doi: 10.1167/iovs.04-0069. [DOI] [PubMed] [Google Scholar]
- (13).Falck A, Laatikainen L. Retinal vasodilation and hyperglycaemia in diabetic children and adolescents. Acta Ophthalmol Scand. 1995;73:119–124. doi: 10.1111/j.1600-0420.1995.tb00650.x. [DOI] [PubMed] [Google Scholar]
- (14).Ikram MK, Janssen JA, Roos AM, et al. Retinal vessel diameters and risk of impaired fasting glucose or diabetes: the Rotterdam study. Diabetes. 2006;55:506–510. doi: 10.2337/diabetes.55.02.06.db05-0546. [DOI] [PubMed] [Google Scholar]
- (15).Wang JJ, Taylor B, Wong TY, et al. Retinal vessel diameters and obesity: a population-based study in older persons. Obesity (Silver Spring) 2006;14:206–214. doi: 10.1038/oby.2006.27. [DOI] [PubMed] [Google Scholar]
- (16).Klein R, Klein BE, Lee KE. Changes in visual acuity in a population. The Beaver Dam Eye Study. Ophthalmology. 1996;103:1169–1178. doi: 10.1016/s0161-6420(96)30526-5. [DOI] [PubMed] [Google Scholar]
- (17).Linton KL, Klein BE, Klein R. The validity of self-reported and surrogate-reported cataract and age-related macular degeneration in the Beaver Dam Eye Study. Am J Epidemiol. 1991;134:1438–1446. doi: 10.1093/oxfordjournals.aje.a116049. [DOI] [PubMed] [Google Scholar]
- (18).Wong TY, Klein R, Klein BE, Meuer SM, Hubbard LD. Retinal vessel diameters and their associations with age and blood pressure. Invest Ophthalmol Vis Sci. 2003;44:4644–4650. doi: 10.1167/iovs.03-0079. [DOI] [PubMed] [Google Scholar]
- (19).Hubbard LD, Brothers RJ, King WN, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106:2269–2280. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- (20).Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BE. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27:143–149. doi: 10.1076/ceyr.27.3.143.16049. [DOI] [PubMed] [Google Scholar]
- (21).Wong TY, Klein R, Nieto FJ, et al. Retinal microvascular abnormalities and 10-year cardiovascular mortality: a population-based case-control study. Ophthalmology. 2003;110:933–940. doi: 10.1016/S0161-6420(03)00084-8. [DOI] [PubMed] [Google Scholar]
- (22).Kifley A, Liew G, Wang JJ, et al. Long-term effects of smoking on retinal microvascular caliber. Am J Epidemiol. 2007;166:1288–1297. doi: 10.1093/aje/kwm255. [DOI] [PubMed] [Google Scholar]
- (23).Chiolero A, Jacot-Sadowski I, Faeh D, Paccaud F, Cornuz J. Association of cigarettes smoked daily with obesity in a general adult population. Obesity (Silver Spring) 2007;15:1311–1318. doi: 10.1038/oby.2007.153. [DOI] [PubMed] [Google Scholar]
- (24).Liew G, Wong TY, Mitchell P, Wang JJ. Are narrower or wider retinal venules associated with incident hypertension? Hypertension. 2006;48:e10. doi: 10.1161/01.HYP.0000231652.97173.4c. [DOI] [PubMed] [Google Scholar]
- (25).Hanley JA. A heuristic approach to the formulas for population attributable fraction. J Epidemiol Community Health. 2001;55:508–514. doi: 10.1136/jech.55.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Cheung N, Saw SM, Islam FM, et al. BMI and retinal vascular caliber in children. Obesity (Silver Spring) 2007;15:209–215. doi: 10.1038/oby.2007.576. [DOI] [PubMed] [Google Scholar]
- (27).Barzilay JI, Forsberg C, Heckbert SR, Cushman M, Newman AB. The association of markers of inflammation with weight change in older adults: the Cardiovascular Health Study. Int J Obes (Lond) 2006;30:1362–1367. doi: 10.1038/sj.ijo.0803306. [DOI] [PubMed] [Google Scholar]
- (28).Barinas-Mitchell E, Cushman M, Meilahn EN, Tracy RP, Kuller LH. Serum levels of C-reactive protein are associated with obesity, weight gain, and hormone replacement therapy in healthy postmenopausal women. Am J Epidemiol. 2001;153:1094–1101. doi: 10.1093/aje/153.11.1094. [DOI] [PubMed] [Google Scholar]
- (29).Albanes D, Jones DY, Micozzi MS, Mattson ME. Associations between smoking and body weight in the US population: analysis of NHANES II. Am J Public Health. 1987;77:439–444. doi: 10.2105/ajph.77.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Liew G, Sharrett AR, Wang JJ, et al. Relative importance of systemic determinants of retinal arteriolar and venular caliber: the atherosclerosis risk in communities study. Arch Ophthalmol. 2008;126:1404–1410. doi: 10.1001/archopht.126.10.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Pischon T. Commentary: Use of the body mass index to assess the risk of health outcomes: time to say goodbye? Int J Epidemiol. 2010;39:528–529. doi: 10.1093/ije/dyp388. [DOI] [PubMed] [Google Scholar]
- (32).Gradmark AM, Rydh A, Renstrom F, et al. Computed tomography-based validation of abdominal adiposity measurements from ultrasonography, dual-energy X-ray absorptiometry and anthropometry. Br J Nutr. 2010:1–7. doi: 10.1017/S0007114510000796. [DOI] [PubMed] [Google Scholar]
- (33).Ahima RS, Osei SY. Adipokines in obesity. Front Horm Res. 2008;36:182–197. doi: 10.1159/000115365. [DOI] [PubMed] [Google Scholar]
- (34).Grunwald JE, DuPont J, Riva CE. Retinal haemodynamics in patients with early diabetes mellitus. Br J Ophthalmol. 1996;80:327–331. doi: 10.1136/bjo.80.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]