Abstract
Mesoporous Silica Nanoparticles (MSNs) have proven to be promising vehicles for drug delivery. However, despite the potential, there are few studies that extended the success of in vitro studies to animal settings. In this work, we report the efficacy of MSNs using two different human pancreatic cancer xenografts on different mouse species. Significant tumor-suppression effects were achieved with camptothecin-loaded MSNs. Dramatic improvement of the potency of tumor suppression was obtained by surface modifying MSNs with folic acid. Dose dependent tumor suppression was observed establishing 0.5 mg of CPT-loaded MSNs per mouse as a minimum dose sufficient for achieving complete tumor growth inhibition. Renal excretion of MSNs was also confirmed with TEM imaging. These findings highlight attractive features (biocompatibility, renal clearance and tumor-suppressing ability) of MSNs as a drug delivery system.
Keywords: Mesoporous silica nanoparticles, anticancer drug, drug delivery, tumor suppression, pancreatic cancer xenografts
Background
As low drug solubility in aqueous media of most anticancer drugs hampers the ability of drugs to be administered through the optimal route, the development of new delivery systems for these molecules without the use of organic solvents has received significant attention. Encapsulation of drugs within nanoparticles provides advantages when using hydrophobic, water-insoluble anticancer drugs by incorporating them into the matrix of nanoparticles.1-3 This loading also prevents premature release and degradation of drugs before the drugs reach the target lesion. Moreover, the passive targeting of nanoparticles to tumors by the enhanced permeability and retention (EPR) effect,4 as well as the positive targeting by conjugating nanoparticle surface with specific antibody or ligands,5-8 lead to significantly increased therapeutic efficacy and decreased side effect by reducing the undesirable total body release.
Among inorganic-based nanomaterials, Mesoporous Silica Nanoparticles (MSNs) have several attractive properties as a drug-delivery system, such as large surface areas, tailorable pore sizes, controllable particle sizes and shapes, dual-functional surfaces (exterior and interior), ease of large scale synthesis and stability.9 The size- and shape-controllable pores of MSNs can store pharmaceutical drugs and prevent their premature release and degradation before reaching their designated target.1, 10 Chemotherapeutic drugs can be loaded onto MSNs, replacing the need to use solvents that are often toxic for healthy tissues.1, 2
We and other groups have shown that MSNs could be used as drug-delivery vehicles, gene-transfection agents, cell markers, and carriers of molecules.5, 11-21 Conjugation with specific ligands or antibodies, such as folic acid, allows the targeting of nanoparticles to special cell types, such as cancer cells. In our previous studies, we have successfully incorporated a representative hydrophobic anticancer drug, camptothecin (CPT), into the pores of MSNs and delivered the drug to a variety of human cancer cells to induce cell death.1, 2, 10 These results suggest that the mesoporous silica nanoparticles can be used as a vehicle to overcome the insolubility problem of many anticancer drugs.1, 5, 10 Many studies also showed the biocompatibility of MSNs in vitro with culture cells as well as in vivo with animals.10, 22-25
The most important question regarding the use of mesoporous silica nanoparticles as a drug delivery vehicle for cancer therapy is how successful they are for delivering anticancer drugs to tumors and suppressing their growth in animal studies. Although there are many cell studies demonstrating that MSNs are promising materials for drug delivery in tissue culture cells, there are few studies that extended the success of in vitro studies to animal settings. The versatility of MSNs promises a new drug delivery vehicle with better blood circulation times, biocompatibility and clearance from the animal body,10, 26 which envisions promising nanomaterials for future clinical application. We were the first to demonstrate, in our previous study, that MSNs are effective for antitumor drug delivery and that the tumor suppression was significant with a subcutaneous human breast cancer xenograft in mice.10 In that study, the subcutaneous tumors in mice were virtually eliminated by treating with CPT-loaded MSNs or CPT-loaded folate-modified MSNs (FMSNs). However, much more work is needed. It is important to examine whether MSNs are capable of suppressing tumor types other than breast cancer. It is also important to employ different administration conditions as well as to optimize the dosages of MSN administration in animal. We also wanted to further examine the effects of targeting MSNs.
In this paper, we report an animal tumor study of MSN on pancreatic cancer xenografts. We show that CPT-loaded MSNs and folic acid-conjugated MSNs (FMSN) (folate receptor-targeting) exhibit significant tumor suppression effects. Different dosages were also examined to obtain dose dependent inhibition of tumor growth. In the present study, we also addressed our previous preliminary observation that MSNs are excreted. We show that MSNs excreted in the urine have similar features to those injected including their size.
Methods
Synthesis of mesoporous silica nanoparticles
MSNs were synthesized by first dissolving FITC (5.5 mg) in absolute ethanol (3 mL) before adding aminopropyltriethoxysilane (APTS; 12 μL). In another container, cetyltrimethylammonium bromide (CTAB; 0.5 g) was dissolved in a solution of distilled water (240 mL) and sodium hydroxide (2 M, 1.75 mL) that was heated to 80 °C and stirred vigorously. The FITC/APTS solution was stirred under an inert atmosphere for 2 h before adding tetraethylorthosilicate (TEOS; 2.5 mL). Once the temperature of the CTAB solution had stabilized, the ethanol solution containing TEOS and FITC/APTS was added. After 15 min, 0.63 mL 3-trihydroxysilylpropyl methylphosphonate was slowly added to the mixture. After 2 h, the solution was cooled to room temperature and the particles were filtered and washed with methanol using a fritted funnel. The particles were allowed to dry at room temperature overnight. To remove the surfactants from the pores of the particles, particles (850 mg) were dissolved in a solution of methanol (90 mL) and hydrochloric acid (12.1 M, 5 mL) and refluxed for 24 h. The particles were then filtered and washed thoroughly to remove the surfactants and unbound FITC.
Synthesis of folic-acid-conjugated FMSNs and drug loading
To attach folic acid to MSNs, 20 mg of particles were washed with and resuspended in DMSO. In a flask, folic acid (0.1 mg, Sigma, 98%) and APTS (0.05 μL) were mixed in DMSO (1 mL). N-hydroxysuccinimide (0.03 mg, Aldrich, 98%) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (0.05 mg, Alfa Aesar, 98%) was then added into the mixture and stirred for 2 h. In a separate flask containing toluene (4 mL) and the MSNs–DMSO suspension, the folate/APTS solution was added and the mixture was stirred for 20 h at room temperature. The amount of folic acid left in the supernatant was negligible after the reaction. It is estimated that MSNs have approximately 0.5 wt% of folic acid grafted on the surface. The materials were recovered by centrifugation, washed twice with toluene, and dried under vacuum. To load camptothecin (CPT; Sigma, 95%), 10 mg of the particles were suspended in a solution of drugs (1 mg) and DMSO (0.25 mL) for 4 h. After centrifuging the drug-loaded FMSNs from the suspension and removing the supernatant completely, the materials were dried under vacuum. The drug-loaded FMSNs were then washed and sonicated with water before being resuspended in aqueous solution. In order to determine the amount of drugs inside the FMSNs, the aqueous drug-loaded FMSN suspension was incubated at 4 °C for 6 h before centrifugation to show that the drugs were not being slowly released from the mesopores. The resulting supernatant was mixed with the previous supernatant solution from the washing process and measured by UV/Vis absorption spectroscopy. The pellet of drug-loaded FMSNs was resuspended and sonicated in DMSO and collected by centrifugation. The process was repeated two more times (≈15 min, total time) to ensure that the drugs were completely removed from the pores. The DMSO (or methanol) supernatants were then measured using UV/Vis absorption.
Cells and culture
The human pancreatic cancer cell lines, PANC-1 and MiaPaca-2, obtained from American Type Culture Collection, were maintained in DMEM supplemented with fetal calf serum (10%, Sigma, MO), L-glutamine (2%), penicillin (1%), and streptomycin (1%). The medium was routinely changed every 3 d and the cells were separated by trypsinization before reaching confluency.
Animals
All animal experiments were performed following the protocols approved by the UCLA Animal Research Committee. Six-week-old female BALB/c regular or BALB/cAnNCrj-nu nude, or NOD/nCrCrl-Prkdc SCID (Severe combined immunodeficiency) mice (purchased from Charles River Laboratories) were maintained in disposable plastic cages with hardwood chips bedding in an air-conditioned room with a 12-h-light–12-h-dark cycle and given food (oriental CRF-1) irradiated with 30 Gy rays and filtered tap water ad libitum.
Human cancer xenograph establishment
For xenografts established from cultured cells, 1 × 107 human pancreatic cancer cells, MiaPaca-2, collected in ice cold DMEM (0.2 mL) containing 50% Matrigel (BioScience), were s.c. injected into the right lateral abdominal wall of the nude mice. Mice were then randomly divided into treatment or vehicle groups after tumors of ≈3 mm in diameter were palpable. For PANC-1 xenograft, the culture cell transplantation in nude mice did not produce reliable subcutaneous tumors in our first trial, therefore, 3 mice were firstly inoculated with 1 × 107 PANC-1 cells subcutaneously. 1 month later, all tumors were removed, washed with PBS, cut into small tumor chunks from the margins of tumors with similar size, and randomly transplanted subcutaneously into the right side of the backs of other immune compromised mice. Mice were then randomly divided into treatment or vehicle groups after tumors of ≈3 mm in diameter were palpable. For each experiment, thirty mice with the above-mentioned established xenografts were randomly divided into 5 groups. Animals in group 1 received intraperitoneal injection of saline solution (0.2 mL, 0.9%). Animals in group 2 received i.p. injection of CPT (0.2 mL, 7.8 μM, dissolved in DMSO, and then diluted in saline to achieve the final concentration; the final concentration of DMSO was <1.5%). Animals in group 3 received i.p. injection of MSNs (0.2 mL, 5 mg mL−1). Animals in group 4 received i.p. injection of MSNs loaded with CPT (0.2 mL, 5 mg mL−1, the final concentration of CPT was ≈7.8 μM). Animals in group 5 received i.p. injection of folic-acid-conjugated MSNs (FMSNs) loaded with CPT (0.2 mL, 5 mg mL−1). All injections were done twice per week from the right lower corner of the abdomen until the end of the experiment. The tumor volume and body weight were monitored every other day and the tumor volume was calculated using the following formula: tumor volume=π × (L × W2)/8 × 4/3 where L is the length and W is the width of the tumor. On the final day, all mice were sacrificed and subjected to autopsy.
Measurement of MSN excretion in urine
To examine the excretion of MSN in urine and feces, special metabolic cages (Tecniplast) were used. Six regular mice were housed in 2 metabolic cages (3 for each). 0.5 mg of MSNs or FMSNs were i.p. injected into the mice for each cage. Urine and feces were collected into separate collection tubes before and after the injections at different time points. The collected urine were sent to the molecular instrumentation center at UCLA for ICP analysis of Si, and also subjected to TEM imaging examination.
Results
Synthesis and characterization of mesoporous silica nanoparticles (MSNs)
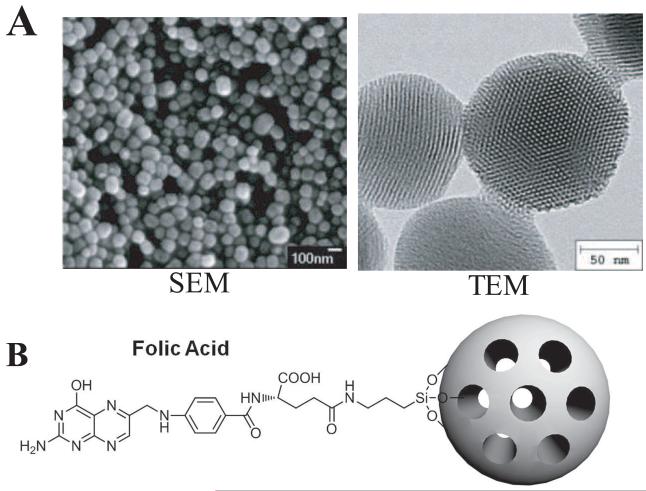
Mesoporous silica nanoparticles provide an attractive vehicle for delivering anticancer drugs. The nanoparticles were synthesized by the sol-gel method using surfactants as described previously.1 To increase dispersibility of the nanoparticles, phosphonates are included to surface modify the particles. Scanning electron microscopy and transmission electron microscopy analysis showed that the MSNs we used are roughly spherical in shape and approximately 100-130 nm in diameter, with hexagonal arrays of the pores (Figure 1A). An average pore diameter of approximately 2 nm was observed by using transmission electron microscopy. Based on UV/Vis absorption measurements, the amount of drug molecules that were stored in MSNs is approximately 29 nmol CPT in 1 mg MSNs. For this study, we used both untargeted MSNs as well as folate attached MSNs that target folate receptor. The folate-MSNs have approximately 0.5 wt% of folic acid covalently attached to the surface of the particles. Figure 1B shows the schematic illustration of MSNs with surface modifications of folic acid molecules.
Figure 1.
Characterization of MSN. A) Scanning electron microscope (left) and transmission electron microscope (right) images of FMSN. B) Schematic illustration of MSNs modified with folic acid targeting ligands on the surface.
Biocompatibility and excretion of MSNs in the urine
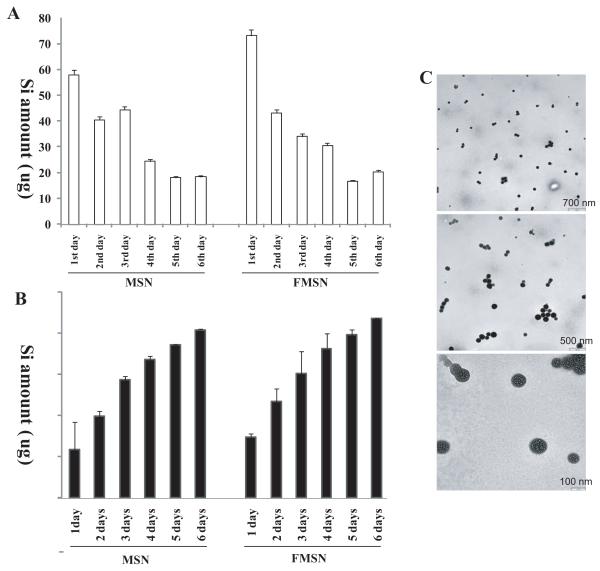
We have shown previously that MSNs prepared above are biocompatible and that they are excreted efficiently after administration into mice.10 In that study using nude mice, we found that a majority of MSNs injected into the mice bodies was excreted through urine within four days by measuring Si concentration in the urine with inductively coupled plasma optical emission spectrometry (ICP-OES).10 This feature of MSNs is important in designing tumor suppression experiments in mice, as their excretion can influence the schedule of MSN administration. Therefore, we addressed this excretion issue by examining the concentration of Si in the urine again, but with regular mice this time. A total of 1 mg of MSN or FMSN was injected into three mice for each kind of particle, which corresponds to ≈465 μg of Si. Urine and feces were collected into separate collection tubes before and after the injections at different time points as described in the methods for ICP analysis of Si concentrations. As shown in Figure 2A, within the first 24 h after injection, approximately 58 μg of Si was detected in urine of the MSN-injected mice, and 73 μg in the FMSN-injected mice. In the second day, 40 μg and 42 μg of Si was detected in the urine of MSN and FMSN injected mice, respectively. Figure 2B shows cumulative amount of Si detected. The renal excretion decreased in the following days. A part of the urine sample was examined with electron microscopy to confirm that the Si detected represents intact silica nanoparticles, not broken pieces. As shown in Figure 2C, MSNs were detected in the urine and the nanoparticles recovered had the same size as that of injected nanoparticles. Pores were clearly recognizable, confirming the urine excretion of intact nanoparticles. The total amount of Si excreted from mice within 6 days was approximately 360 μg and 350 μg from MSN and FMSN-injected mice respectively (Figure 2 and Supplementary Figure 1). Thus, it appears that MSNs can be excreted within a week after their administration. Taken together with our previous results, it appears that the majority of MSNs administered into mice can be excreted. This led us to conclude that twice per week administration of MSNs should provide effective means of delivering anticancer drugs.
Figure 2.
ICP-OES analysis of the Si concentration in urine of mice collected after injection of MSNs. A total of 1 mg of MSNs or FMSNs was injected into three mice for each kind of particle, which corresponds to ≈465 μg of Si. Urines were collected before and after the injections at different time points for ICP analysis of Si concentrations. The amount of Si in urines in each single day is shown in (B) and the total cumulative amount is shown in (A). TEM examination of the nanoparticles in urine smear confirmed the shape and size of MSNs or FMSNs (C).
Tumor suppressing effect of CPT-loaded MSNs on PANC-1 xenograft in nude mice
To examine the ability of MSNs to deliver anticancer drugs and to suppress tumor growth in mice, we used camptothecin (CPT)-loaded MSN as well as CPT-loaded folate modified MSN (FMSN) on human pancreatic cancer xenografts (PANC-1) established on nude mice. The amount of CPT-loaded MSN was determined based on our previous experiments.10 In that study, we examined acute and long term toxicity profiling of MSN and FMSN in mice, and determined that 1 mg per mouse per day is the highest dosage that can be injected into mice safely without inducing any toxicity to the animals. This concentration of both CPT-loaded MSN and FMSN was proved to be able to completely eliminate the subcutaneous breast tumors in that study. In addition, in our previous in vitro studies, we observed similar cell-killing effects of CPT-loaded MSN and FMSN on human breast cancer and pancreatic cancer cell lines. Therefore, we decided to test the tumor suppressing effect of CPT-loaded MSN and FMSN with the concentration of 1 mg per mouse.
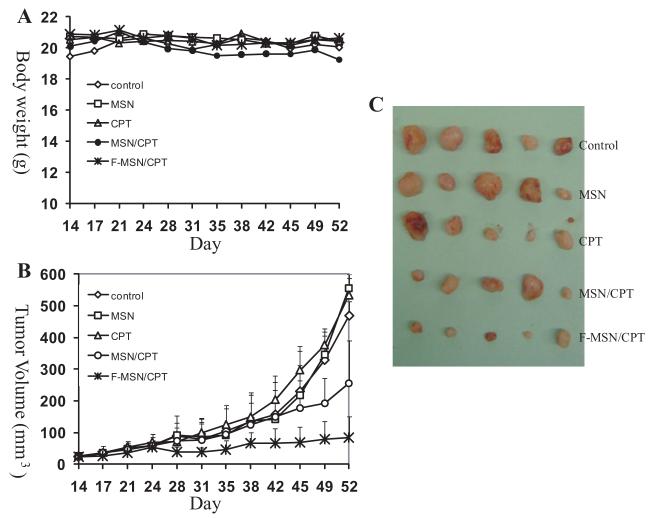
25 nude mice with established xenografts (described in Methods) of human pancreatic cancer cells PANC-1 were randomly divided into 5 groups (n = 5) and intraperitoneal injections were started when the palpable subcutaneous tumors were detected (14th day after inoculation). Animals in the first group received saline solution as a control. Group 2 was treated with empty MSNs without loading. Group 3 was treated with camptothecin dissolved in dimethyl sulfoxide (DMSO) and diluted in saline. Group 4 was treated with MSNs loaded with CPT (the final concentration of CPT is the same as group 3). Group 5 was treated with folate-modified MSNs (FMSNs) loaded with CPT. Injections were performed twice per week until the end of the experiment (the 52nd day). Each mouse was injected 12 times in total. The average body weights are shown in Figure 3A. No obvious body weight loss was observed in all the treated mice compared with the control mice group. Until the end of injections, no mouse showed severe toxicity, defined by shivering, inactivity, severe necrosis of the tail, ataxic gait, or sudden drop of body weight.
Figure 3.
Antitumor effects of CPT-loaded MSNs and FMSNs on human pancreatic cancer PANC-1 xenograft on nude mice. 25 nude mice with established xenografts of human pancreatic cancer cells PANC-1 were randomly divided into 5 groups (n = 5) and intraperitoneal injections were started when palpable subcutaneous tumors were detected (14th day after inoculation). Animals in the first group received saline solution as a control (control). Group 2 was treated with empty MSNs without loading (MSN).Group 3 was treated with camptothecin dissolved in dimethyl sulfoxide (DMSO) and diluted in saline (CPT). Group 4 was treated with MSNs loaded with CPT (the final concentration of CPT is the same as group 3) (MSN/CPT). Group 5 was treated with folate-modified MSNs (FMSNs) loaded with CPT (F-MSN/CPT). Injections were performed twice per week until the end of the experiment (the 52nd day). Each mouse was injected 12 times in total. A) Average body weights are shown. B) The average tumor volumes are shown as means ± SD. C) Images of subcutaneous tumors collected at the end of experiment.
As shown in Figure 3B, the tumors in the control group (group 1) and in the empty MSN-treated group (group 2) kept growing, showing a fast growing pattern, ending up with big tumors (average tumor volumes are approximately 500-550 mm3 at the 52nd day). No significant difference in average tumor volumes was observed between these two groups, confirming again that MSNs themselves do not affect the tumor growth in mice. On the other hand, the administration of CPT-loaded MSN (group 4) resulted in a significantly slower tumor growth, ending up with much smaller tumors (approximately 250 mm3 in average at the 52nd day). Noticeably, the tumors in the group 5 (CPT-loaded FMSN) stopped growing from the 24th day, remaining relatively smaller tumors within the entire treatment period, ending up with very small tumors at the end of experiment (90 mm3 in average). 4 out of 5 tumors in this group are almost completely eliminated at the end (Figure 3C). In the group 3 (CPT treated mice), the average tumor volumes are not significantly different from the control group, only showing a slightly smaller average tumor volumes at the end of experiment, as shown in Figure 3B. This was due to a variation of tumor volumes in this group; although 4 out of 5 tumors in this group showed smaller volume, there is one unusually big tumor in one mouse.
Tumor suppressing effect of CPT-loaded MSNs/FMSNs on MiaPaca-2 xenograft in nude mice
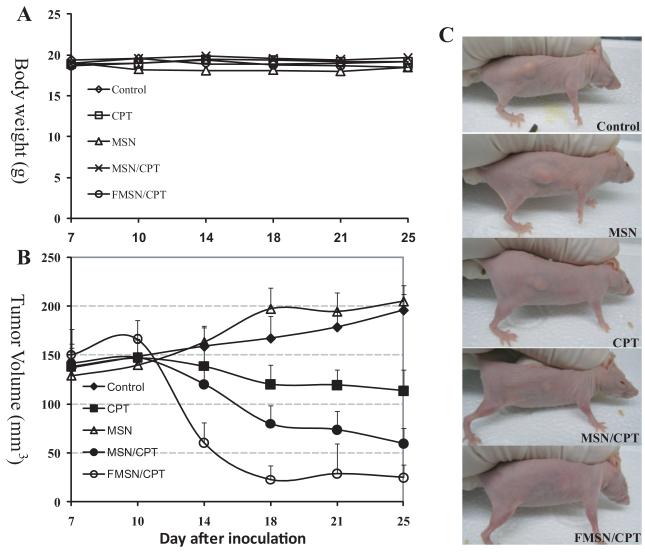
In the next experiment, we used a different pancreatic cancer cell line. The tumors are established with cultured MiaPaca-2 cells on nude mice as described in Methods. 7 days after the inoculation, palpable subcutaneous tumors were detected; therefore the treatments were started in a manner similar to that described above for PANC-1 xenografts. The body weight of all mice showed similar increase and no obvious body weight loss was observed as shown in Figure 4A, and all mice were active during the entire period.
Figure 4.
A human pancreatic cancer cell line, MiaPaca-2, was used for xenograft establishment on nude mice. 7 days after the inoculation when palpable subcutaneous tumors were detected, the treatments were started in a similar manner to that with PANC-1 xenografts. A) Average body weights are shown. B) The average tumor volumes are shown as means ± SD. C) Images of representative nude mice with subcutaneous tumors at the last day of experiment are shown.
As shown in Figure 4B, the tumors in the control and MSN groups showed a slow but stable growth. CPT treatment inhibited the tumor growth slightly in this xenograft model, reducing the tumor volume to approximately 60% of the control. Dramatically, the CPT-loaded MSNs showed a swift inhibition of the tumor growth. After only one week of the treatment, the tumors in this group had shrunk significantly. By the end of experiment (the 25th day), the tumors were barely palpable, having an average tumor volume of approximately 60 mm3, compared to 200 mm3 in the control group. Even more significantly, the tumors in CPT-loaded FMSN treated group disappeared after 11 days of injection (4 times of injections). 4 out of 5 mice in this group did not bear visible tumors any longer at the end of experiment, and the 5th mouse showed a very tiny subcutaneous tumor only (approximately 1 mm in diameter). Figure 4C shows representative pictures of mice in each group after treatments.
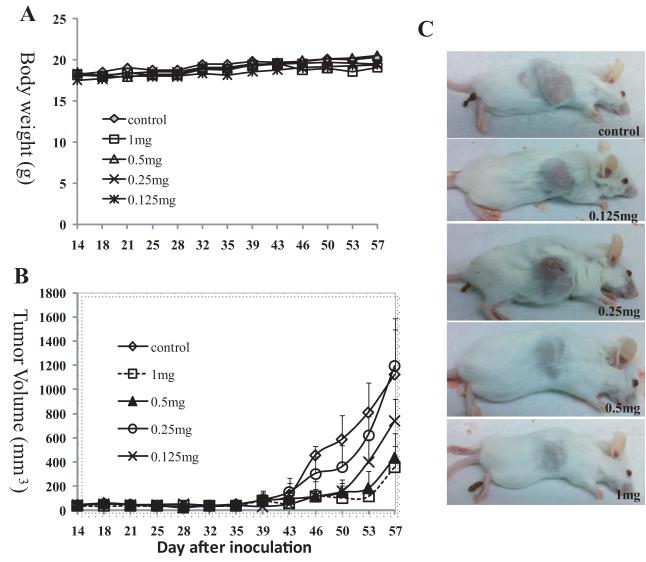
Dose-dependent tumor-suppressing effects of CPT-loaded FMSNs with SCID mice
The above two experiments together with our previous study showed that MSNs loaded with CPT can suppress growth of xenografts tumors from 3 different human cancer cell lines. Among them, the MiaPaca-2 xenograft showed most drastic tumor shrinking effect. For these three experiments, we applied the highest dosages of MSNs that are possible and safe for the animals. It is therefore logical to determine the optimal dosage that will be similarly effective, hence less stress and burden to the animals. And also to vary the experiment, we tested SCID mice for this experiment instead of nude mice. 25 shaved SCID mice were subcutaneously transplanted with 1×107 MiaPaca-2 culture cells. 14 days after the inoculation, palpable tumors on all mice reached 2-3 mm in diameter, and then the mice were divided into 5 groups and the treatments were started. Mice in group 1 (control) received injection of saline solution as described above. Group 2 mice received 1 mg CPT-loaded FMSN per mouse per injection (0.2 mL, 5 mg mL−1, the final concentration of CPT was ≈7.8 μM). Group 3 mice were injected with 0.5 mg CPT-loaded FMSN per mouse per injection (0.2 mL, 2.5 mg mL−1, the final concentration of CPT was ≈3.9 μM). Group 4 mice were injected with 0.25 mg CPT-loaded FMSN per mouse per injection (0.2 mL, 1.25 mg mL−1, the final concentration of CPT was ≈1.9 μM). Group 5 mice were injected with 0.125 mg CPT-loaded FMSN per mouse per injection (0.2 mL, 0.6 mg mL−1, the final concentration of CPT was ≈0.9 μM). All injections were through intraperitoneal and twice per week. As shown in Figure 5A, the dosages used did not alter the body weight gain of all mice.
Figure 5.
Dose-dependent tumor-suppressing effects of CPT-loaded FMSNs. 25 shaved SCID mice were subcutaneously transplanted with 1×107 MiaPaca-2 culture cells. 14 days after the inoculation when palpable subcutaneous tumors were detected, the mice were divided into 5 groups and the treatments were started. Mice in group 1 (control) received injection of saline solution. Group 2 mice received 1 mg CPT-loaded FMSN per mouse per injection (0.2 mL, 5 mg mL–1, the final concentration of CPT was ≈7.8 μM) (1 mg as shown in figure). Group 3 mice were injected with 0.5 mg CPT-loaded FMSN per mouse per injection (0.2 mL, 2.5 mg mL–1, the final concentration of CPT was ≈3.9 μM) (0.5 mg as shown in figure). Group 4 mice were injected with 0.25 mg CPT-loaded FMSN per mouse per injection (0.2 mL, 1.25 mg mL–1, the final concentration of CPT was ≈1.9 μM) (0.25 mg shown in figure). Group 5 mice were injected with 0.125 mg CPT-loaded FMSN per mouse per injection (0.2 mL, 0.6 mg mL–1, the final concentration of CPT was ≈0.9 μM) (0.125 mg shown in figure). All injections were through intraperitoneal and twice per week. A) Average body weights are shown. B) The average tumor volumes are shown as means ± SD. C) Images of representative SCID mice with subcutaneous tumors at the last day of experiment are shown.
The MiaPaca-2 xenografts in SCID grew much slower than those in nude mice and also responded to CPT-loaded MSNs slower (Figure 5B). The tumor-suppressing effects appeared only after the 39th day (treated for 25 days, 8 injections). As expected, 1 mg of CPT-FMSNs inhibited the tumor growth dramatically at the end of the experiment (the 57th day). The same suppression effect was achieved in the group treated with 0.5 mg/mouse. Further reducing dosage to 0.125 mg resulted in weaker suppression of tumor growth, final average tumor volume in this group being the middle of the control and 0.5 mg group, suggesting a dose-dependent response. Figure 5C shows representative pictures of mice in each group after treatments. A large deviation of tumor volumes in the group treated with 0.25 mg was observed; 2 out of 5 mice in this group bearing unusual large tumors. Overall, these results show that FMSNs loaded with CPT are effective in blocking growth of pancreatic tumor in SCID mice.
Discussion
In the present study, we have shown that CPT-loaded MSNs are effective in suppressing tumor growth of two different human pancreatic cancer xenografts, PANC-1 and MiaPaCa-2. In addition, we used two different animal species, nude mice and SCID mice. The growth of PANC-1 tumors in the nude mice treated with CPT-loaded MSNs was significantly suppressed, ending up with very small tumors or almost complete elimination at the end of the treatment. With MiaPaCa-2 xenografts in nude mice, we observed regression of the tumor only one week after the treatment and the tumor was completely eliminated by the end of the treatment. These results extend our previous study with breast cancer xenograft and demonstrate remarkable ability of MSNs to deliver anticancer drugs and suppress tumor growth in mice.
The efficacy of CPT-loaded MSNs to suppress tumor growth was further examined with MiaPaca-2 xenograft in SCID mice and by using reduced dosages of drug-loaded FMSNs. Responses of MiaPaca-2 tumors in SCID mice showed dose-dependency. We also found that 0.5 mg/mouse (25 mg/kg) with 2 injections every week is enough to completely suppress tumor growth, achieving similar results observed with 1 mg dosage. These results suggest that 25 mg/kg with 2 injections per week would be an appropriate dosage for further animal experiments. Whether fewer injections work as well is currently being investigated in our laboratory. Interestingly, with nude mice, we observed complete elimination of the tumor when treated with drug-loaded FMSNs, while with SCID mice the tumors regained the growth after the 53rd day. The reason for this different response is unclear at this point and requires further experiments to determine. It is possibly due to a different speed of tumor growth in different mouse species. With nude mice, the average tumor size reached 130 mm3 one week after the transplantation, while in SCID mice it took 40 days to reach a similar size. Faster growing tumors are expected to possess more extensive angiogenesis and hence more hypervasculature, therefore more defective vascular architecture, more increased production of permeability mediators, facilitating the enhanced permeability and retention (EPR) effect for macromolecular agents such as MSNs to accumulate in the tumors.
Another important finding of this study is that folic acid-modified MSN (FMSN) dramatically increased tumor suppressing effects of CPT-loaded MSNs. With PANC-1 xenografts, the treatment with CPT-loaded FMSN resulted in very small tumors, while the tumor inhibition was less with CPT-loaded MSN. A dramatic difference was observed with MiaPaCa-2 xenografts; almost complete tumor regression was observed with CPT-loaded FMSN after 11 days, while with CPT-loaded MSN it took 25 days to achieve tumor regression approaching a similar level. Thus, while CPT loaded MSNs are effective in tumor growth inhibition, CPT-loaded FMSN is much more effective, pointing to the enhancement of tumor inhibition efficacy by adding a targeting moiety to MSNs. In our previous study10 with breast cancer xenograft, conjugation with folic acid on the surface showed a slight but not significant increase in the tumor-suppressing effect although increased cell killing in in vitro study and greater accumulation in tumors with FMSNs in animals were observed. We speculate that the high dosages and large number of injections might have suppressed the tumors quickly, thereby masking the enhanced effect of using FMSNs.
Our work also confirmed and extended our previous observation that MSNs are excreted. In our previous study, we observed that a majority of Si was excreted out of body through urine and feces, suggesting a quick clearance of nanoparticles from the animal.10 This phenomenon is confirmed in the present study. The clear TEM images of MSNs in the urine verify their excretion. The MSNs are excreted through urine without breakdown or degradation. He et al. reported that MSNs with sizes of ≈45 nm were safely excreted through the renal route.26 Recently, Ruggiero et al. reported a unique renal clearance of carbon nanotubes,27 large nanoparticles with high MW and high aspect ratio. Further work is needed to understand the mechanism of excretion through kidney. However, it can be pointed out that our observations suggesting that the MSNs can be cleared from the animal body reinforces the idea that MSNs can be used safely to suppress tumor growth.
Our findings, taken together with our previous animal studies, provide compelling evidence of FMSNs’ superior biocompatibility at concentrations adequate for pharmacological applications, capability of reducing adverse side effect of anticancer drugs, quick renal clearance, and excellent tumor-suppressing effect with anticancer loading. And lower concentration even showed similar encouraging results. These point to the features of MSNs as a promising drug delivery system for cancer therapy.
Supplementary Material
Acknowledgments
Sources of support for research: This work was supported by the NIH grant CA133697.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflict of interest was reported by the authors of this article.
References
- 1.Lu J, Liong M, Zink JI, Tamanoi F. Mesoporous silica nanoparticles as a delivery system for hydrophobic anticancer drugs. Small. 2007;3:1341–6. doi: 10.1002/smll.200700005. [DOI] [PubMed] [Google Scholar]
- 2.Lu J, Liong M, Sherman S, Xia T, Kovochich M, Nel AE, et al. Mesoporous Silica Nanoparticles for Cancer Therapy: Energy-Dependent Cellular Uptake and Delivery of Paclitaxel to Cancer Cells. Nanobiotechnology. 2007;3:89–95. doi: 10.1007/s12030-008-9003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenholm JM, Peuhu E, Eriksson JE, Sahlgren C, Linden M. Targeted intracellular delivery of hydrophobic agents using mesoporous hybrid silica nanoparticles as carrier systems. Nano Lett. 2009;9:3308–11. doi: 10.1021/nl901589y. [DOI] [PubMed] [Google Scholar]
- 4.Maeda H, Matsumura Y. EPR effect based drug design and clinical outlook for enhanced cancer chemotherapy. Adv Drug Deliv Rev. 2011;63:129–30. doi: 10.1016/j.addr.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE, et al. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano. 2008;2:889–96. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisichella M, Dabboue H, Bhattacharyya S, Lelong G, Saboungi ML, Warmont F, et al. Uptake of functionalized mesoporous silica nanoparticles by human cancer cells. J Nanosci Nanotechnol. 2010;10:2314–24. doi: 10.1166/jnn.2010.1917. [DOI] [PubMed] [Google Scholar]
- 7.Rosenholm JM, Peuhu E, Bate-Eya LT, Eriksson JE, Sahlgren C, Linden M. Cancer-cell-specific induction of apoptosis using mesoporous silica nanoparticles as drug-delivery vectors. Small. 2010;6:1234–41. doi: 10.1002/smll.200902355. [DOI] [PubMed] [Google Scholar]
- 8.Wang LS, Wu LC, Lu SY, Chang LL, Teng IT, Yang CM, et al. Biofunctionalized phospholipid-capped mesoporous silica nanoshuttles for targeted drug delivery: improved water suspensibility and decreased nonspecific protein binding. ACS Nano. 2010;4:4371–9. doi: 10.1021/nn901376h. [DOI] [PubMed] [Google Scholar]
- 9.Coti KK, Belowich ME, Liong M, Ambrogio MW, Lau YA, Khatib HA, et al. Mechanised nanoparticles for drug delivery. Nanoscale. 2009;1:16–39. doi: 10.1039/b9nr00162j. [DOI] [PubMed] [Google Scholar]
- 10.Lu J, Liong M, Li Z, Zink JI, Tamanoi F. Biocompatibility, biodistribution, and drug-delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small. 2010;6:1794–805. doi: 10.1002/smll.201000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hom C, Lu J, Liong M, Luo H, Li Z, Zink JI, et al. Mesoporous silica nanoparticles facilitate delivery of siRNA to shutdown signaling pathways in mammalian cells. Small. 2010;6:1185–90. doi: 10.1002/smll.200901966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Y, Trewyn BG, Slowing II, Lin VS. Mesoporous silica nanoparticle-based double drug delivery system for glucose-responsive controlled release of insulin and cyclic AMP. J Am Chem Soc. 2009;131:8398–400. doi: 10.1021/ja901831u. [DOI] [PubMed] [Google Scholar]
- 13.Zhang R, Wu C, Tong L, Tang B, Xu QH. Multifunctional core-shell nanoparticles as highly efficient imaging and photosensitizing agents. Langmuir. 2009;25:10153–8. doi: 10.1021/la902235d. [DOI] [PubMed] [Google Scholar]
- 14.Vivero-Escoto JL, Slowing II, Wu CW, Lin VS. Photoinduced intracellular controlled release drug delivery in human cells by gold-capped mesoporous silica nanosphere. J Am Chem Soc. 2009;131:3462–3. doi: 10.1021/ja900025f. [DOI] [PubMed] [Google Scholar]
- 15.Stromme M, Brohede U, Atluri R, Garcia-Bennett AE. Mesoporous silica-based nanomaterials for drug delivery: evaluation of structural properties associated with release rate. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:140–8. doi: 10.1002/wnan.13. [DOI] [PubMed] [Google Scholar]
- 16.Qin F, Zhou Y, Shi J, Zhang Y. A DNA transporter based on mesoporous silica nanospheres mediated with polycation poly(allylamine hydrochloride) coating on mesopore surface. J Biomed Mater Res A. 2009;90:333–8. doi: 10.1002/jbm.a.31923. [DOI] [PubMed] [Google Scholar]
- 17.Hom C, Lu J, Tamanoi F. Silica nanoparticles as a delivery system for nucleic acid-based reagents. J Mater Chem. 2009;19:6308–16. doi: 10.1039/b904197d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen AM, Zhang M, Wei D, Stueber D, Taratula O, Minko T, et al. Co-delivery of doxorubicin and Bcl-2 siRNA by mesoporous silica nanoparticles enhances the efficacy of chemotherapy in multidrug-resistant cancer cells. Small. 2009;5:2673–7. doi: 10.1002/smll.200900621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slowing II, Trewyn BG, Lin VS. Mesoporous silica nanoparticles for intracellular delivery of membrane-impermeable proteins. J Am Chem Soc. 2007;129:8845–9. doi: 10.1021/ja0719780. [DOI] [PubMed] [Google Scholar]
- 20.Slowing II, Vivero-Escoto JL, Wu CW, Lin VS. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv Drug Deliv Rev. 2008;60:1278–88. doi: 10.1016/j.addr.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Torney F, Trewyn BG, Lin VS, Wang K. Mesoporous silica nanoparticles deliver DNA and chemicals into plants. Nat Nanotechnol. 2007;2:295–300. doi: 10.1038/nnano.2007.108. [DOI] [PubMed] [Google Scholar]
- 22.Liu HM, Wu SH, Lu CW, Yao M, Hsiao JK, Hung Y, et al. Mesoporous silica nanoparticles improve magnetic labeling efficiency in human stem cells. Small. 2008;4:619–26. doi: 10.1002/smll.200700493. [DOI] [PubMed] [Google Scholar]
- 23.Hsiao JK, Tsai CP, Chung TH, Hung Y, Yao M, Liu HM, et al. Mesoporous silica nanoparticles as a delivery system of gadolinium for effective human stem cell tracking. Small. 2008;4:1445–52. doi: 10.1002/smll.200701316. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Kim HS, Lee N, Kim T, Kim H, Yu T, et al. Multifunctional uniform nanoparticles composed of a magnetite nanocrystal core and a mesoporous silica shell for magnetic resonance and fluorescence imaging and for drug delivery. Angew Chem Int Ed Engl. 2008;47:8438–41. doi: 10.1002/anie.200802469. [DOI] [PubMed] [Google Scholar]
- 25.Taylor KM, Kim JS, Rieter WJ, An H, Lin W. Mesoporous silica nanospheres as highly efficient MRI contrast agents. J Am Chem Soc. 2008;130:2154–5. doi: 10.1021/ja710193c. [DOI] [PubMed] [Google Scholar]
- 26.He X, Nie H, Wang K, Tan W, Wu X, Zhang P. In Vivo Study of Biodistribution and Urinary Excretion of Surface-Modified Silica Nanoparticles. Anal Chem. 2008;80:9597–603. doi: 10.1021/ac801882g. [DOI] [PubMed] [Google Scholar]
- 27.Ruggiero A, Villa CH, Bander E, Rey DA, Bergkvist M, Batt CA, et al. Paradoxical glomerular filtration of carbon nanotubes. Proc Natl Acad Sci U S A. 2010;107:12369–74. doi: 10.1073/pnas.0913667107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.