Abstract
This study investigated whether PRM–151 (Promedior, Inc., Malvern, PA), a recombinant form of human pentraxin-2 (PTX-2, also referred to as serum amyloid P, hSAP), that inhibits differentiation of circulating monocytes into fibrocytes and profibrotic macrophages, could modulate generation of myofibroblasts after opacity-producing corneal injury in rabbits, and, therefore, have potential to reduce or prevent haze after PRK. Nine diopter PRK for myopia was performed with the VISX S4 IR laser. Four groups of 6 animals were treated in masked fashion: Group 1: 30 μl of topical PRM–151 (20 mg/ml) 6 times a day for 5 days; Group 2: 30 μl topical vehicle 6 times a day for 5 days; Group 3: 200 μl subconjunctival PRM–151 (total injection of 4 mg) immediately after surgery and every other day until day 8; Group 4: 200 μl subconjunctival injections of vehicle according to the same schedule as group 3. At one month after PRK, the animals were euthanized and immunohistochemistry was performed for the myofibroblast marker α-smooth muscle actin (SMA). The density of SMA+ cells/400X field in the central stroma was determined in each cornea. Myofibroblast density at one month after surgery was significantly lower (p=0.006) after subconjunctival PRM-151 treatment (5.8±2.8 cells/400X stromal field) compared to subconjunctival vehicle treatment (15.3±2.9 cells/400X stromal field). There was no significant (p = 0.27) decrease in stromal myofibroblasts triggered by topical PRM-151 treatment (11.8±6.6 cells/400X stromal field) compared to the topical vehicle treatment (14.2.8±6.2 cells/400X stromal field). PRM-151 inhibits myofibroblast generation when administered by subconjunctival injection, but not when administered topically, after opacity-producing corneal injury. This study provides additional confirmation that bone marrow-derived cells contribute to corneal myofibroblast generation.
1. Introduction
Myofibroblast generation and persistence, along with extracellular matrix deposition by these cells, is associated with the development of stromal opacity (referred to clinically as haze) during corneal wound healing (Jester et al., 1999; Mohan et al., 2003; Funderburgh et al., 2003; Lee et al., 2007). Myofibroblasts are fibroblastic cells that can be derived from a variety of cell precursors, depending on the tissue and inciting event (Novo et al., 2009; Barbosa et al., 2010; Saika et al., 2010).
The identity of the progenitor cells for myofibroblasts in the corneal stroma remains a subject of active investigation. Corneal fibroblasts, bone marrow-derived cells, or even corneal epithelial cells, may give rise to corneal myofibroblasts, depending on the inciting injury, the genetic makeup of the individual, and other unknown factors (Direkze et al., 2003; Mohan et al., 2008; Barbosa et al., 2010). In the latter study in mice (Barbosa et al., 2010), more than nine times more myofibroblasts originated from bone marrow-derived cells than could have originated from keratocytes or other cells after opacity-producing corneal injury.
Human pentraxin-2 (PTX-2, also referred to as serum amyloid P, hSAP) is a highly conserved serum protein and a soluble pattern recognition receptor (PRR) of the innate immune system that regulates monocyte activation and differentiation (Castano, et al., 2009). Ligand-bound PTX-2 exerts anti-fibrotic effects via crosslinking of Fc gamma receptors, which has been shown to inhibit the differentiation of circulating monocytes into fibrocytes (Pilling, et al., 2003; Macdonald and Kilpatrick, 2006; Pilling and Gomer, 2007; Quan, Cowper, and Bucala, 2006; Pilling, et al., 2007) and profibrotic macrophages (Moreira, et al., 2010). In vivo studies showed that species-specific serum-derived PTX-2 inhibited fibrosis in bleomycin-induced lung fibrosis models in rats and mice (Pilling, et al., 2007) and in an ischemia reperfusion injury model in mouse hearts (Haudek, et al., 2006), and that human serum-derived PTX-2 also inhibited fibrosis in the bleomycin-induced lung model in mice (Murray, et al, 2010), in the TGF-β transgene-induced lung fibrosis model in mice (Murray, et al., 2011), and in both the unilateral ischemia-reperfusion injury kidney model and unilateral ureteral obstruction kidney model in mice (Castano, et al., 2009). Other later studies also demonstrated that PTX-2 is a potent regulator of monocyte and macrophage activation induced by exposure to damaged tissue in vitro and in vivo, and confirmed that this regulation is dependent upon specific interaction of PTX-2 with Fc gamma receptors (Castano, et al., 2009).
Promedior, Inc. (Malvern, PA) recently developed PRM-151, a recombinant form of human pentraxin-2 (hPTX-2) (Duffield and Lupher, 2010). The purpose of this study was to determine whether PRM-151 would modulate myofibroblast generation after haze-producing injury to the rabbit cornea rabbits, and, therefore, have potential to reduce or prevent haze after PRK.
2. Materials and methods
2.1. PRM-151
PRM-151 is formulated in P5SP vehicle (10 mM sodium phosphate, 5% (w/v) sorbitol, 0.01% (w/v) polysorbate 20, pH 7.5) at a concentration of approximately 20 mg/ml. The drug was shipped frozen and stored at −20 °C until initial use. Frozen drug was gently thawed and vigorous agitation was avoided.
2.2. Animals, surgery, and drug application groups
All animals were treated in accordance with the tenets of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the Animal Control Committee at the Cleveland Clinic approved these studies.
Twenty-four 12 to 15 week old female New Zealand white rabbits weighing 2.5–3.0 kg each were included in this study. Haze-generating injury was performed in one cornea of each rabbit by applying a -9 diopter photorefractive keratectomy (PRK) with a VISX S4 IR excimer laser (AMO, Irvine, CA), as previously described (Mohan, et al., 2003).
The rate of closure of the corneal epithelium was monitored in each eye with slit lamp photos performed at 12 cm with topical fluorescein drops under ketamine/xylaxine IM anesthesia at one-day intervals for 5 days after surgery. The area of the epithelial defect on the cornea was quantitated for each animal for each day using the photographs and ImageJ software (http://rsb.info.nih.gov/ij/).
One eye of each rabbit selected at random was treated with masked aliquots of test solutions that had been labeled prior to shipment from Promedior. The key to masking was held by a neutral party and was not disclosed to the investigators until data was finalized and tabulated for all of the rabbits. The four groups with six rabbits each were as follows:
-
Group 1
Received 30 μl (given with sterile Pipetman) of 20 mg/ml PRM–151 six times a day until epithelium closed (3 to 5 days) beginning immediately after PRK surgery.
-
Group 2
Received 30 μl of control P5SP vehicle solution six times a day until epithelium closed (3 to 5 days) beginning immediately after PRK surgery.
-
Group 3
Received a 200 μl subconjunctival injection of 4 mg of PRM–151 immediately following the PRK surgery. The injected drug was distributed over the entire subconjunctival space and posterior into the orbital area and in this way provided a depot effect that lasted for 12 to 24 hours. The subconjunctival injection was repeated at 2, 4, 6, and 8 days after the surgery after anesthesia with 30mg/kg ketamine and 5mg/kg xylazine IM.
-
Group 4
Received a 200 μl subconjunctival injection of P5SP vehicle immediately following the PRK surgery and repeated at 2, 4, 6, and 8 days after the surgery after anesthesia with 30mg/kg ketamine and 5mg/kg xylazine IM.
All rabbits were provided with Tylenol in drinking water at a concentration of 6 mg/ml post-procedure until the epithelium healed at 3 to 5 days after surgery.
Rabbits were euthanized at one month after PRK with a pentobarbitol overdose and the corneoscleral rim collected without manipulation of the cornea using 0.12 forceps and sharp Westcott scissors. The corneoscleral rims were embedded in liquid OCT compound (Sakura Finetek, Torrance, CA, USA) within a 24 mm × 24 mm × 5 mm mould (Fisher, Pittsburgh, PA). Specimens were centered within the mould so that the block could be bisected and transverse sections cut from the center of the cornea. Frozen tissue blocks were stored at −80° C until sectioning was performed. Central corneal sections (7 μm thick) were cut with a cryostat (HM 505M, Micron GmbH, Walldorf, Germany). Sections were placed on 25 mm × 75 mm × 1 mm microscope slides (Superfrost Plus, Fisher) and maintained frozen at −80° C until staining was performed.
2.3. Immunohistochemistry
Immunofluorescence staining was performed on experimental and control tissue sections as previously described (Barbosa et al., 2010). Briefly, sections from the central cornea were stained for α-smooth muscle actin (α-SMA) using a monoclonal mouse anti-human smooth muscle actin clone1A4 (Cat. # M0851, Dako, Carpinteria, CA). The antibody was used on the sections at 1:50 dilution in 1% BSA and incubated at room temperature for 90 minutes. Sections were washed with PBS and then incubated at room temperature for 60 minutes in Alexa Fluor 568 (Cat. # A11031, Invitrogen, Carlsbad, CA) secondary antibody, goat anti-mouse IgG (H + L) (Red) diluted 1:100 in PBS before washing with PBS three times. Immunocytochemical controls were performed by both omitting primary antibody and substituting mouse non-specific IgG1 for the primary antibody. Coverslips were mounted with Vectashield containing DAPI (Vector Laboratories Inc., Burlingame, CA) to allow visualization of all nuclei in the tissue sections. The sections were viewed and photographed with a Leica DM5000 microscope equipped with Q-Imaging Retiga 4000RV (Surrey, BC, Canada) camera and ImagePro software. IHC was performed at least three times on sections from each cornea to insure the results were consistent.
2.4. Quantification of cells
SMA+ cells were counted real time at the microscope. In each case, counts of SMA+ cells were performed in masked fashion in the central cornea and counting the number of cells per 400X field tangent to the epithelial basement membrane in the central cornea and averaging the counts for three adjacent fields, as previously described (Barbosa et al., 2009).
2.5. Statistical analysis
Statistical comparisons between the groups were performed using analysis of variance (ANOVA) with Student-Newman-Keuls method test, where applicable (Sigma Stat software 3.5). A p values less than 0.05 was considered statistically significant.
3. Result
3.1. Effect of sub-conjunctival and topical PRM–151 on epithelial closure rates
There was no significant difference in the rate of epithelial healing between the drug or vehicle groups in the sub-conjuntival or topical arms of the study. There was also no significant difference in the rate of epithelial healing between the topical and sub-conjunctival groups for drug or vehicle treatments. In all eyes, the rate of epithelial closure was between four and five days after surgery.
3.2. Effect of sub-conjunctival PRM–151 on stromal myofibroblast density
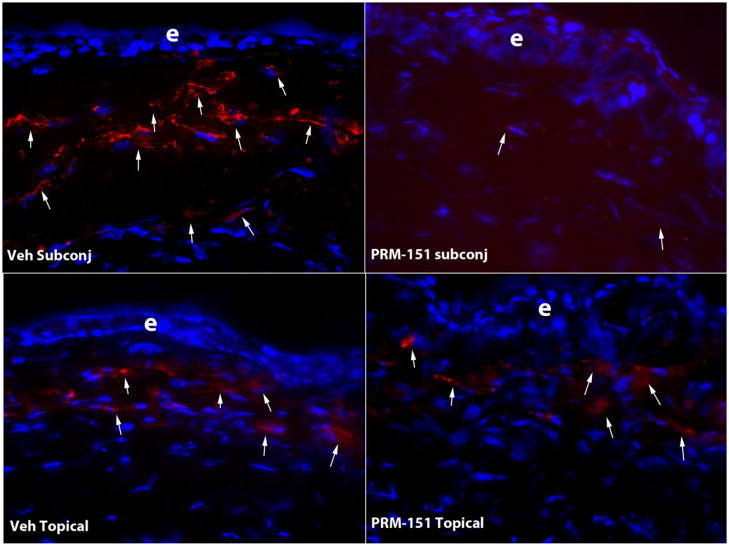
Myofibroblast density (Figure) at one month after surgery was significantly lower (p=0.006) after subconjunctival PRM-151 treatment (5.8±2.8 cells/400X stromal field) compared to subconjunctival vehicle treatment (15.3±2.9 cells/400X stromal field) at one month after PRK.
Fig. 1.
At one month after PRK, large numbers of SMA+ myofibroblasts (arrows) are noted in the anterior stroma of rabbit corneas treated with subconjunctival vehicle, topical vehicle or topical PRM-151. However, the density of SMA+ myofibroblasts (arrows) is much lower in a rabbit cornea treated with subconjunctival PRM-151. Cell nuclei are stained with DAPI. e indicates the epithelium in each panel. Magnification 400X.
3.3. Effect of topical PRM–151 on stromal myofibroblast density
There was no significant (p = 0.27) decrease in stromal myofibroblasts (Figure) triggered by topical PRM-151 treatment (11.8±6.6 cells/400X stromal field) compared to the topical vehicle treatment (14.2.8±6.2 cells/400X stromal field) at one month after PRK.
4. Discussion
PRM-151, a recombinant form of human pentraxin-2 (PTX-2, also referred to as serum amyloid P, hSAP), decreases stromal myofibroblast generation when administered by sub-conjunctival injection for eight days following stromal opacity-producing corneal injury. Topical PRM-151 administered six times a day until epithelial wound closure did not significantly decrease myofibroblast generation after PRK, although there appeared to be a trend towards efficacy. Subconjunctival administration of the drug may be effective because of the proximity of the drug to the limbal blood vessels from which bone marrow-derived cells enter the cornea after injury (Wilson, et al., 2004; Barbosa, et al., 2010) and, possibly, due to longer and more consistent exposure to PRM-151 from the subconjunctival depot. It is possible that improvements in formulation or more frequent or higher concentration topical administration of PRM-151 would lead to efficacy for this route of delivery. However, it is also possible that the topically delivered PRM-151 protein was unable to penetrate into the stroma at sufficient levels, regardless of the concentration, due to the size of the molecule. It is possible that other smaller drugs that inhibit monocyte differentiation would be more effective topical modulators of corneal myofibroblast generation.
hPTX-2 and recombinant PRM-151 have been shown to inhibit the differentiation of circulating monocytes into cells that contribute to fibrosis, such as fibrocytes and profibrotic macrophages, in several in vitro and in vivo models (Castano, et al., 2009; Pilling, et al., 2003; Macdonald and Kilpatrick, 2006; Pilling and Gomer, 2007; Quan, Cowper, and Bucala, 2006; Pilling, et al., 2007, Murray, et al. 2010; Moreira, et al. 2010; Murray, et al. 2011). The efficacy of sub-conjunctival PRM-151 in decreasing myofibroblast generation after opacity-producing PRK in rabbits supports the hypothesis that corneal myofibroblasts develop from bone marrow-derived cell in the rabbit, as has been conclusively demonstrated in mice (Barbosa, et al., 2010).
Highlights.
PRM—151, a recombinant form of human pentraxin-2, inhibits myofibroblast generation
PRM—151 acts as an inhibitor of circulating monocyte differentiation
Bone marrow-derived cells contribute to corneal myofibroblast generation.
Acknowledgments
Supported in part by US Public Health Service grants EY10056 and EY015638 from the National Eye Institute, National Institutes of Health, Bethesda, MD and Research to Prevent Blindness, New York, NY. Promedior, Inc., Malvern, PA provided PRM-151 and control solutions for this study.
Footnotes
Proprietary interest statement: SEW received research support and reagents from Promedior, Inc., Malvern, PA. None of the other authors have any proprietary or financial interests in the topics discussed in this manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barbosa FL, Chaurasia SS, Cutler A, Asosingh K, Kaur H, de Medeiros FW, Agrawal V, Wilson SE. Corneal myofibroblast generation from bone marrow-derived cells. Exp Eye Res. 2010;91:92–96. doi: 10.1016/j.exer.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castano A, Lin SL, Surowy T, Nowlin BT, Turlapati SA, Patel T, Singh A, Li S, Lupher ML, Jr, Duffield JS. Serum amyloid P inhibits fibrosis through FcgammaR-dependent monocyte/macrophage regulation in vivo. Science Translational Medicine. 2009;1:5ra13. doi: 10.1126/scitranslmed.3000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Direkze NC, Forbes SJ, Brittan M, Hunt T, Jeffery R, Preston SL, Poulsom R, Hodivala-Dilke K, Alison MR, Wright NA. Multiple organ engraftment by bone-marrow-derived myofibroblasts and fibroblasts in bone-marrow-transplanted mice. Stem Cells. 2003;21:514–520. doi: 10.1634/stemcells.21-5-514. [DOI] [PubMed] [Google Scholar]
- Duffield JS, Lupher ML., Jr PRM-151 (recombinant human serum amyloid P/pentraxin 2) for the treatment of fibrosis. Drug News Perspect. 2010;23:305–15. doi: 10.1358/dnp.2010.23.5.1444206. [DOI] [PubMed] [Google Scholar]
- Funderburgh JL, Mann MM, Funderburgh ML. Keratocyte phenotype mediates proteoglycan structure: a role for fibroblasts in corneal fibrosis. J Biol Chem. 2003;278:45629–45637. doi: 10.1074/jbc.M303292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, Pilling D, Gomer RH, Trial J, Frangogiannis NG, Entman ML. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci U S A. 2006;103:18284–9. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Moller-Pedersen T, Huang J, Sax CM, Kays WT, Cavangh HD, Petroll WM, Piatigorsky J. The cellular basis of corneal transparency: evidence for corneal crystallins. J Cell Sci. 1999;112:613–622. doi: 10.1242/jcs.112.5.613. [DOI] [PubMed] [Google Scholar]
- Lee YC, Wang IJ, Hu FR, Kao WW. Immunohistochemical study of subepithelial haze after phototherapeutic keratectomy. J Refract Surg. 2001;17:334–341. doi: 10.3928/1081-597X-20010501-07. [DOI] [PubMed] [Google Scholar]
- Macdonald SL, Kilpatrick DC. Human serum amyloid P component binds to peripheral blood monocytes. Scand J Immunol. 2006;64:48–52. doi: 10.1111/j.1365-3083.2006.01774.x. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Hutcheon AEK, Choi R, Hong J, Lee J, Mohan RR, Ambrósio R, Zieske JD, Wilson SE. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp Eye Res. 2003;76:71–87. doi: 10.1016/s0014-4835(02)00251-8. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Stapleton WM, Sinha S, Netto MV, Wilson SE. A novel method for generating corneal haze in anterior stroma of the mouse eye with the excimer laser. Exp Eye Res. 2008;86:235–240. doi: 10.1016/j.exer.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira AP, Cavassani KA, Hullinger R, Rosada RS, Fong DJ, Murray L, Hesson DP, Hogaboam CM. Serum amyloid P attenuates M2 macrophage activation and protects against fungal spore-induced allergic airway disease. J Allergy Clin Immunol. 2010;126:712–721. doi: 10.1016/j.jaci.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Murray LA, Chen Q, Kramer MS, Hesson DP, Argentieri RL, Peng X, Gulati M, Homer RJ, Russell T, van Rooijen N, Elias JA, Hogaboam CM, Herzog EL. TGF-beta driven lung fibrosis is macrophage dependent and blocked by Serum amyloid P. Int J Biochem Cell Biol. 2011;43:154–62. doi: 10.1016/j.biocel.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Murray LA, Rosada R, Moreira AP, Joshi A, Kramer MS, Hesson DP, Argentieri RL, Mathai S, Gulati M, Herzog EL, Hogaboam CM. Serum amyloid P therapeutically attenuates murine bleomycin-induced pulmonary fibrosis via its effects on macrophages. PLoS One. 2010;5:e9683. doi: 10.1371/journal.pone.0009683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Sinha S, Sharma A, Dupps W, Wilson SE. Stromal haze, myofibroblasts, and surface irregularity after PRK. Exp Eye Res. 2006;82:788–97. doi: 10.1016/j.exer.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novo E, di Bonzo LV, Cannito S, Colombatto S, Parola M. Hepatic myofibroblasts: a heterogeneous population of multifunctional cells in liver fibrogenesis. Int J Biochem Cell Biol. 2009;41:2089–2093. doi: 10.1016/j.biocel.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Pilling D, Gomer R. Regulatory pathways for fibrocyte differentiation. In: Bucala R, editor. Fibrocytes: New insights into tissue repair and systemic fibroses. World Scientific; 2007. pp. 37–60. [Google Scholar]
- Pilling D, Buckley CD, Salmon M, Gomer RH. Inhibition of fibrocyte differentiation by serum amyloid P. J Immunol. 2993;171:5537–46. doi: 10.4049/jimmunol.171.10.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling D, Roife D, Wang M, Ronkainen SD, Crawford JR, Travis EL, Gomer RH. Reduction of bleomycin-induced pulmonary fibrosis by serum amyloid P. J Immunol. 2007;179:4035–44. doi: 10.4049/jimmunol.179.6.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan TE, Cowper SE, Bucala R. The role of circulating fibrocytes in fibrosis. Curr Rheumatol Rep. 2006;8:145–50. doi: 10.1007/s11926-006-0055-x. [DOI] [PubMed] [Google Scholar]
- Saika S, Yamanaka O, Sumioka T, Okada Y, Miyamoto T, Shirai K, Kitano A, Tanaka S. Transforming growth factor beta signal transduction: a potential target for maintenance/restoration of transparency of the cornea. Eye Contact Lens. 2010;36:286–289. doi: 10.1097/ICL.0b013e3181eef01c. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Mohan RR, Netto MV, Perez V, Possin D, Huang J, Kwon R, Alekseev A. RANK, RANKL, OPG, and M-CSF expression in stromal cells during corneal wound healing. Invest Ophthalmol Vis Sci. 2004;45:2201–2211. doi: 10.1167/iovs.03-1162. [DOI] [PubMed] [Google Scholar]