Abstract
Efficient clearance of apoptotic cells by phagocytes (efferocytosis) is critical for normal tissue homeostasis and regulation of immune system. Apoptotic cells are recognized by a vast repertoire of receptors on macrophage that lead to transient formation of phosphatidylinositol-3,4,5-trisphosphate (PtdIns(3,4,5)P3), and subsequent cytoskeletal reorganization necessary for engulfment. Certain PI3K isoforms are required for engulfment of apoptotic cells, but relatively little is known about the role of lipid phosphatases in this process. Here we report that the activity of the phosphatidylinositol 3-phosphatase, PTEN (phosphatase and tensin homolog deleted on chromosome 10) is elevated upon efferocytosis. Depletion of PTEN in macrophage results in elevated PtdIns(3,4,5)P3 production and enhanced phagocytic ability both in vivo and in vitro, while overexpression of wild type PTEN abrogates this process. Loss of PTEN in macrophage leads to activation of the PH domain containing guanine-nucleotide exchange factor Vav1 and subsequent activation of Rac1 GTPase, resulting in increased amounts of F-actin upon engulfment of apoptotic cells. PTEN disruption also leads to increased production of anti-inflammatory cytokine IL-10, and decreased production of proinflammatory IL-6 and TNFα, upon engulfment of apoptotic cells. These data suggests that PTEN exerts control over efferocytosis potentially by regulating PtdIns(3,4,5)P3 levels that modulate Rac GTPase and F-actin reorganization through Vav1 exchange factor and enhancing apoptotic cell-induced anti-inflammatory response.
Keywords: Macrophage, phagocytosis of apoptotic cell, signal transduction
Introduction
Cells undergo apoptosis, or programmed cell death, during diverse physiological processes. The efficient clearance of apoptotic cells is critical for the maintenance of tissue homeostasis and for the prevention of inflammation and autoimmunity. The process of engulfment of apoptotic cells by both professional (e.g., macrophages and dendritic cells) and non-professional phagocytes has been termed efferocytosis (1). The initial event in efferocytosis is the sensing of secreted “find me” signals such as nucleotides (2) and lysophosphatidylcholine (LPC) (3) released by apoptotic cells. Next, “eat me” signals, the most well-studied of which is phosphatidylserine (PS (4)), that are exposed on the apoptotic cell surface are recognized by a broad set of receptors on the phagocyte. Apoptotic cells can bind directly to macrophages via PS-receptors such as TIM4, BAI1 and stabilin-2 (5-8), or indirectly through soluble bridging molecules such as milk-fat globule E8 (MFG-E8) (9-11), growth-arrest-specific 6 (Gas6) and β2-glycoprotein-I (β2-GPI) that link apoptotic cells to macrophages via vitronectin receptor (αvβ3-integrin), receptor tyrosine kinase Mer (12) and β2-GPI receptor on the macrophages, respectively. It is generally believed that different signaling cascades converge to activate the small GTPase Rac1, resulting in actin reorganization and engulfment of the apoptotic cells (13). The recognition and clearance of apoptotic cells is anti-inflammatory, mediated by the release of the anti-inflammatory cytokines transforming growth factor (TGF-β1), IL-10, platelet activating factor (PAF) and prostaglandin E2 with concurrent inhibition of the proinflammatory cytokines TNFα, IL-6, IL-12 and IL-1β (14).
Apoptotic cells are generated during various physiological processes, including inflammation and development. In host defense, neutrophils are recruited to the site of infection to combat invading pathogens. Neutrophils have a very short life span (~ 6-24 hrs). They are loaded with granules containing cytotoxic enzymes, such as neutrophil elastase, peroxidases, proteinases and collagenase; thus, neutrophils need to be cleared properly to avoid aggravated tissue damage and autoimmunity (15). Likewise, cell death is also one of the default fates of thymocytes; more than 95% of thymocytes die without reaching maturity through the process of thymic selection.
Recognition of an apoptotic cell by a macrophage results in the activation of several signaling cascades, including PtdIns(3,4,5)P3 mediated signaling, leading to actin reorganization at the phagocytic cup and engulfment of the apoptotic cell. PtdIns(3,4,5)P3 can activate Rac via activation of Rac-guanine exchange factors such as Vav, P-rex and DOCK2. The ELMO-DOCK180 complex is the most well characterized exchange factor that activates Rac during the process of efferocytosis (7, 16-18). Although phosphoinositide 3-kinase (PI3K), the enzyme that produces PtdIns(3,4,5)P3, is required for efferocytosis, it is not the only enzyme that regulates the levels of PtdIns(3,4,5)P3 in the cell. PTEN (Phosphatase and tensin homolog deleted on chromosome ten), a phosphatidylinositol 3-phosphatase, and SHIP (SH2 domain containing inositol phosphatase), a phosphatidylinositol 5-phosphatase, convert PtdIns(3,4,5)P3 to PtdIns(4,5)P2 and PtdIns(3,4)P2, respectively. Relatively little is known about the role of these lipid phosphatases in the regulation of apoptotic cell-mediated signaling. The roles of PtdIns(3,4,5)P3 and PTEN in infection and inflammation have been extensively examined. Blocking the kinase activity of PI3K leads to the impaired recruitment of neutrophils to inflammatory sites in vivo (19-26). On the other hand, increasing PtdIns(3,4,5)P3 signaling by depleting PTEN enhances cell mobility (27-31) and improves neutrophil recruitment to the inflamed peritoneal cavity (32). The disruption of PTEN also enhances neutrophil function in a bacterial pneumonia model, leading to increased engulfment of bacteria (33, 34). In the current study, we have investigated whether the loss of PTEN can result in macrophage activation, leading to the enhanced clearance of apoptotic cells and faster resolution of inflammation.
Herein, we demonstrate that PTEN negatively regulates efferocytosis. Overexpression of PTEN reduces the engulfment of apoptotic cells, and disruption of PTEN increases the engulfment of apoptotic cells both in vitro and in vivo. We also show that PTEN activity is up-regulated during the engulfment of apoptotic cells, although PTEN does not localize to the phagocytic cup like PtdIns(3,4,5)P3. The loss of PTEN in macrophages results in the enhanced formation of PtdIns(3,4,5)P3, leading to activation of the PH-domain containing exchange factor Vav1, which induces the activation of Rac GTPase and the subsequent polymerization of F-actin and enhances the engulfment of apoptotic cells. Consistent with augmented engulfment, the loss of PTEN increases the production of anti-inflammatory cytokine (IL-10) and decreases the production of proinflammatory (IL-6 and TNFα) cytokines upon efferocytosis.
Materials and Methods
Mice
The conditional PTEN knockout mouse (PTENloxP/loxP) and the myeloid-specific Cre mouse were purchased from The Jackson Laboratories (Bar Harbor, ME). The experimental myeloid-specific PTEN knockout mice were generated as previously described (32). In all the experiments performed using knockout mice (PTENloxP/loxP; Cre+/+ or PTENloxP/loxP; Cre+/-), we included corresponding littermates (PTENwt/wt; Cre+/+ or PTENwt/wt; Cre+/–) as wild-type controls. Rac1loxP/loxP (35), Rac2-/- (36), Rac3-/- (37) mice have been described elsewhere. All procedures involving mice were approved and monitored by the Children's Hospital Animal Care and Use Committee.
Cells, Antibodies, Plasmids and Reagents
RAW264.7 (ATCC) cells were maintained in Dulbecco's Modified Eagle Medium (DMEM) containing 10% FCS and transfected with plasmids encoding PTEN-Citrine (38), myristoylated-Akt-enhanced green fluorescent protein (myr-Akt-EGFP) or Akt-PH-EGFP using the Amaxa nucleofection kit according to the manufacturer's protocol. Mouse peritoneal macrophages were prepared by injecting 1 ml of 3% thioglycollate (Sigma) intraperitoneally. The peritoneal lavage fluid was collected after 3 days, and the collected cells were cultured in RPMI with 10% fetal calf serum (FCS). Mouse thymocytes were isolated by surgically removing the thymus glands. The glands were rinsed, and the thymocytes were resuspended in 10 ml RPMI containing 10% FCS by smashing the glands with the flat plastic head of a 10 ml syringe plunger. Murine bone marrow neutrophils were isolated using the neutrophil enrichment kit from Stem Cell Technologies Inc. according to the manufacturer's protocol. Antibodies to PTEN (Ser-380/Thr-382/Thr-383), total-PTEN, phospho-Akt, phospho-Erk1/2, phospho-Pyk2 and actin were obtained from Cell Signaling Technologies. The antibodies to Vav1 and Rac1 were obtained from Santa Cruz Biotech, and the anti-phosphoTyr antibody was from Millipore. TGX221 and AS252424 were obtained from Cayman Chemicals. Compound 15e was from Santa Cruz Biotech. The Akti-VIII was from EMD Biosciences, and the wortmannin was from Tocris Biosciences.
In vitro phagocytosis of apoptotic cells
RAW264.7 macrophages or thioglycollate-elicited peritoneal macrophages were incubated with apoptotic neutrophils or thymocytes for 90 minutes. The unengulfed cells were removed by washing with PBS, and the phagocytosis of apoptotic cells by macrophages was detected by staining with HEMA3 (Fisher) following the manufacturer's guidelines. The percentage of macrophages containing at least one apoptotic body was quantified to determine the percent efferocytosis, and the number of apoptotic bodies per macrophage was determined to calculate the efferocytic index. Apoptotic neutrophils were obtained by culturing neutrophils in RPMI for 1 day, which typically resulted in 30-40% apoptotic cells. Thymocyte apoptosis was induced by incubation in 1 μM dexamethasone overnight, which generated over 85% apoptotic cells.
In vivo efferocytosis
In the peritonitis model, wild-type and PTEN-/- mice were injected intraperitoneally with 3% thioglycollate to induce inflammation. At day three , when the number of peritoneal macrophages was maximal, 40×106 apoptotic thymocytes resuspended in PBS were injected into the peritoneum. After 90 minutes, the cells from the peritoneal lavage were analyzed for the percentage of macrophages containing engulfed apoptotic cells. The dexamethasone-induced thymic involution was performed as described previously with slight modifications (2). To induce thymic involution caused by thymocyte apoptosis, 250 μg dexamethasone was injected intraperitoneally into mice for 4 hrs or 8 hrs. Treatment with PBS in an equivalent amount of DMSO (solvent) for 8 hrs was used as the control. The mice were sacrificed, the thymuses were isolated and the total number of cells were counted. The total numbers of annexin V+ apoptotic cells and Mac1+ macrophages were determined by FACS.
PTEN activity assay
Thioglycollate-elicited peritoneal macrophages were treated with MFG-E8 (1μg/ml) or Gas6 (1 μg/ml) or incubated with apoptotic thymocytes for indicated time. The unengulfed apoptotic cells were washed with PBS three times. The macrophages were then lysed using RIPA buffer containing 150 mM NaCl, 1.0% IPEGAL, 0.5% sodium deoxycholate, 0.1% SDS and 50 mM Tris, pH 8.0 (Sigma). The cell lysates were incubated overnight at 4°C with protein-A sepharose beads coated with anti-PTEN (Ser-380/Thr-382/Thr-383) antibody (Cell Signaling Technologies). The beads were washed three times with the PTEN reaction buffer containing 25 mM Tris-HCl, pH 7.4, 140 mM NaCl, 2.7 mM KCl and 10 mM DTT. The PTEN pulled down was incubated with 3000 pmol of water-soluble PtdIns(3,4,5)P3 substrate (Echelon Biosciences) for 30-60 minutes at 37°C. The free phosphate generated through PTEN activity was then quantified using the malachite green phosphatase assay kit, according to the manufacturer's protocol (Echelon Biosciences). The percent of PtdIns(3,4,5)P3 conversion was determined at each time point as [(free phosphate in test reaction, pmol) -(free phosphate in background, pmol)] X 100% / 3000 pmol. The free phosphate in the background was the amount of phosphate in the “substrate only [PtdIns(3,4,5)P3]” controls. The cell lysates were also analyzed for the levels of total PTEN.
ELISA
Peritoneal macrophages (0.5×106) from WT or PTEN-/- mice were plated in a 24-well plate and cultured for 12-16 hrs in RPMI containing 10% FCS at 37°C. The cells were then treated with LPS in the presence or absence of apoptotic cells (5 ×106 per well) for 16 hrs. The levels of the proinflammatory cytokines IL-6 and TNFα and the anti-inflammatory cytokine IL-10 in the culture supernatants were determine by ELISA (eBiosciences), according to the manufacturer's guidelines.
Rac and Vav1 activation assays
RAW264.7 or peritoneal macrophages were treated with apoptotic cells for the indicated time periods, and the unengulfed cells were washed off as previously described. For the Vav1 activation assay, cells were lysed in lysis buffer containing 20 mM Tris-HCl, pH 8, 150 mM NaCl, 10% glycerol, 1% Triton X-100 and 2 mM EDTA and supplemented with protease inhibitor cocktail (Roche). The cell lysates were incubated with Protein A/G Plus sepharose beads coated with Vav1 antibody (Santa Cruz Biotech) for 16 hrs at 4°C. The immunoprecipitates were washed with lysis buffer, resolved by SDS-PAGE and analyzed for levels of Vav1 and phospho-Tyr (clone 4G10, Millipore). For the Rac activation assay, the cells were lysed in lysis buffer containing 50 mM Tris pH 7.5, 10 mM MgCl2, 300 mM NaCl and 1% Triton X-100 supplemented with protease inhibitors (Roche). Portions of the cell lysates were saved for input controls, and 500 μg of total protein from each sample was then incubated with glutathione sepharose beads coated with GST-PAK-PBD to pull down active Rac. The pull-down eluates were washed in wash buffer containing 25 mM Tris pH 7.5, 30 mM MgCl2 and 40 mM NaCl, resolved by SDS PAGE and immunoblotted with Rac1 antibody (Cytoskeleton Inc). The immunoblotted proteins were detected using an enhanced chemiluminescence-based detection system (GE Healthcare).
Quantification of F-actin levels
Peritoneal macrophages from WT or PTEN-/- mice were isolated, and 2 × 106 cells were plated in 35 mm dishes. The macrophages were incubated with or without apoptotic thymocytes for 30 minutes, and the unengulfed cells were washed off with four PBS washes. The cells were lysed in a cytoskeletal fraction buffer (CB) containing 50 mM Tris HCl, pH 7.5, 100 mM NaCl, 10 mM NaF, 1 mM EDTA, 1 mM EGTA, 10% glycerol, 1 mM DTT and 1% NP-40 and were kept on ice for 10 minutes. The total cell lysates were taken at this step. The cell lysates were then kept at room temperature for 10 minutes and spun at 10,000 × g for 5 minutes. The cytoskeletal pellet was washed once with CB and resuspended in Laemmeli buffer. The total cell lysates and cytoskeletal fractions were resolved by SDS-PAGE and stained with Coomassie brilliant blue dye. The amounts of actin in the two fractions were quantified by measuring the intensities of the protein band using ImageJ software (NIH).
Image acquisition
For the visualization of phagocytosis after HEMA3 staining, an Olympus BX51 microscope was used. For the visualization of fluorescently labeled cells, images were acquired using an Olympus IX71 microscope and processed using IPlab 3.5.6 software and Adobe Photoshop CS2.
Statistical analysis
Analyses of statistical significance for the indicated data sets were performed using the Student's t test capability of Microsoft Excel (Redman, WA).
Results
Efferocytosis is PtdIns(3,4,5)P3-dependent but Akt-independent
PI3K catalyzes the conversion of PtdIns(4,5)P2 to PtdIns(3,4,5)P3, a critical second messenger in signal transduction processes. Among the classes of PI3Ks, class I PI3Ks can be divided into class IA and IB based on the receptors they are activated by. Signals from receptor tyrosine kinases and G protein coupled receptors activate class IA and IB PI3Ks, respectively. PI3Ks are heterodimers consisting of the catalytic subunit p110 (p110α, p110β, p110δ or p110γ) and p85 regulatory subunits. During phagocytosis, PI3Ks are activated in response to several receptors and have been shown to be required for Fcγ receptor-mediated phagocytosis and apoptotic cell engulfment (39). To address the role of each individual PI3K in efferocytosis, we treated thioglycollate-elicited peritoneal macrophages with pharmacological isoform-specific PI3K inhibitors and co-incubated the cells with dexamethasone-induced apoptotic thymocytes for 90 min. The percentage of macrophages containing one or more apoptotic cells was quantified after staining with HEMA3. Treatment with wortmannin, a pan-specific PI3K inhibitor, significantly reduced efferocytosis, indicating a requirement for PI3K and its PtdIns(3,4,5)P3 products in the process. Compound 15e, which targets p110α and TGX221, which targets p110β, also showed significant inhibitory effects, indicating that class IA PI3Ks are important for efferocytosis. On the other hand, AS252424, which targets p110γ, did not have any effect on efferocytosis, indicating that the p110γ (the major GPCR-activated PI3K isoform) mediated formation of PtdIns(3,4,5)P3 is not involved in the process (Figure 1A). As a control for the efficacy of AS252424 in inhibiting p110γ mediated PI3K-Akt signaling, we treated macrophages with fMLP (a GPCR ligand) in the presence or absence of 50 nM AS252424 for 30 minutes. The cell lysates were analyzed for Akt phosphorylation. The addition of AS252424 completely inhibited Akt activation in macrophages upon stimulation with fMLP, indicating the inhibition of p110γ by AS252424 (Supplementary Figure 1A).
Figure 1. PI3K but not Akt is required for engulfment of apoptotic cell by macrophage.
(A) Effect of isoform specific PI3K inhibitors on engulfment of apoptotic cells by macrophages. Macrophages pretreated with 50 nM Wortmannin (pan-), 50 nM Compound 15e (p110α-specific), 50 nM TGX-221 (p110β-specific), or 50 nM AS252424 (p110γ-specific) were incubated with apoptotic thymocytes for 90 min and the engulfment of apoptotic cells was analyzed by HEMA3 staining. Percentage of macrophage containing one or more apoptotic body is counted. (B) Transient generation of PtdIns(3,4,5)P3 during efferocytosis. RAW264.7 macrophage-like cells were transfected with a PH-Akt-EGFP construct and fed with SNARF1 labeled apoptotic thymocytes for 60 min. Cells were washed, fixed and analyzed by fluorescence microscopy. Top panel represents early phase of apoptotic cell recognition by macrophage and the bottom panel represents a macrophage with an apoptotic cell after completion of engulfment. Bar represents 10 μm. (C) Pharmacological inhibition of Akt by Akti-VIII (3 μM) in peritoneal macrophages did not affect engulfment of apoptotic cells. (D) Overexpression of a constitutively activated Akt (myr-Akt) in RAW264.7 cells did not affect engulfment of apoptotic cells. Inset, Akt activation (Ser473-phosphorylation) upon expression of myr-Akt. Results shown are means ± SD (n>600). *p<0.005.
Next, we examined the localization of PtdIns(3,4,5)P3 during efferocytosis. We transfected RAW264.7 macrophage-like cells with an Akt-PH-EGFP construct. One day after the transfection, RAW264.7 cells were co-incubated with CMTMR-labeled apoptotic thymocytes for 1 hr. Akt-PH-EGFP was enriched at the phagocytic cup during efferocytosis, indicating that PtdIns(3,4,5)P3 was formed at this location (Figure 1Btop panel). The enrichment of PtdIns(3,4,5)P3 at the phagocytic cup was transient. It returned to basal level upon the completion of engulfment, suggesting that PtdIns(3,4,5)P3 may play a role in the engulfment of apoptotic cells by macrophages (Figure 1Bbottom panel).
The serine/threonine kinase Akt is one of the most well-studied PtdIns(3,4,5)P3 effector molecules. The binding of Akt to PtdIns(3,4,5)P3 through its PH domain facilitates its membrane localization and activation, which subsequently regulates diverse cellular processes like cell proliferation, cell cycle and cell migration. To test whether Akt influences the process of efferocytosis, we first used a pharmacological inhibitor of Akt, Akti-VIII. Treatment of macrophages with Akti-VIII for 30 minutes completely blocked Akt phosphorylation and thus its activation stimulation with fMLP (Supplementary Figure 1B). Peritoneal macrophages were either pretreated with 50 nM wortmannin or 7.6 μM Akti or left untreated, then fed with apoptotic thymocytes for 90 minutes. Treatment with wortmannin severely reduced efferocytosis, but treatment with Akti had no significant effect on efferocytosis (Figure 1C). To further investigate the role of Akt in efferocytosis, we transfected RAW264.7 cells with a constitutively activated form of Akt, myr-Akt, in which Akt is myristoylated and is always in the plasma membrane. As expected, transfection with myr-Akt resulted in dramatic Akt phosphorylation (activation). However, no detectable difference in efferocytosis was noted between the cells transfected with myr-Akt and control cells (Figure 1D). These results further indicate that the process of efferocytosis does not require Akt activity.
PTEN activity increases during efferocytosis
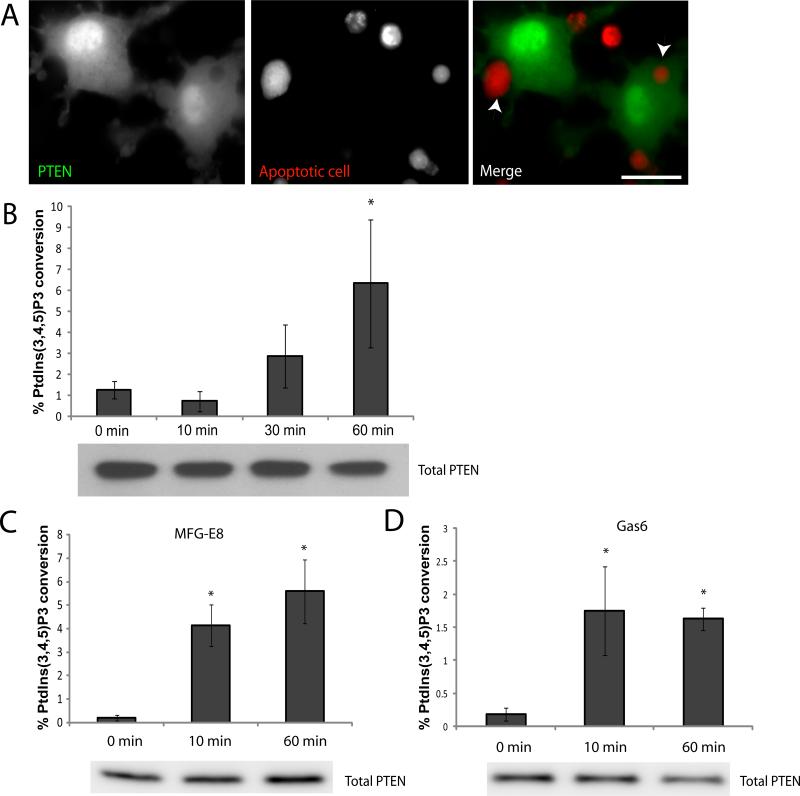
Cellular PtdIns(3,4,5)P3 levels are not only regulated by PI3K but also by PTEN, which converts PtdIns(3,4,5)P3 back into PtdIns(4,5)P2. To determine the role of PTEN, we analyzed its localization during the process of efferocytosis. To this end, we transfected RAW246.7 macrophage-like cells with an mPTEN-Citrine (enhanced YFP) expression construct and co-incubated the transfected cells with CMTMR-labeled apoptotic cells for 1 hr. We observed that PTEN was present throughout the cytosol and in the nucleus and was not enriched in the phagocytic cup during efferocytosis (Figure 2A).
Figure 2. PTEN activity is elevated during efferocytosis.
(A) Subcellular localization of PTEN during efferocytosis. RAW264.7 cells transfected with a PTEN-citrine construct were incubated with SNARF1 labeled apoptotic thymocytes for 60 min. Cells were washed, fixed and analyzed by fluorescence microscopy. Bar represents 10 μm. (B) PTEN activity during engulfment of apoptotic cells. Peritoneal macrophages were incubated with apoptotic thymocytes for indicated time and washed to remove unadhered thymocytes. Cells were then lysed and PTEN was immunoprecipitated. PTEN activity was assessed by its ability to convert PtdIns(3,4,5)P3 to PtdIns(4,5)P2 and free phosphate. Free phosphates generated were measured by a colorimetric assay using malachite green. Data is a representative of 3 independent experiments done in triplicates. Results shown are means ± SD. * p<0.05. (C) PTEN activity in macrophages stimulated with MFG-E8. Wild type and PTEN-/- macrophages were stimulated with 1μg/ml MFG-E8 for indicated time points and PTEN activity was assessed as described above. Results shown are means ± SD. * p<0.05. (D) PTEN activity in macrophages stimulated with Gas6. Wild type and PTEN-/- macrophages were stimulated with 1μg/ml Gas6 for indicated time points and PTEN activity was assessed as described above. Results shown are means ± SD. * p<0.05.
PTEN possesses a C-terminal non-catalytic domain with three phosphorylation sites (Ser-380/Thr-382/Thr-383) that regulate protein activity and stability (40, 41). To examine whether PTEN activity is altered during the process of apoptotic cell engulfment, we used a PTEN (Ser-380/Thr-382/Thr-383) antibody to immunoprecipitate PTEN from macrophages incubated with apoptotic cells for different lengths of time and measured PTEN activity. The levels of PTEN protein were undetectable in apoptotic cells (Supplementary Figure 2), indicating that the PTEN measured would be entirely derived from the macrophages. PTEN converts PtdIns(3,4,5)P3 to PtdIns(4,5,)P2 and free phosphate. The amount of free phosphate generated reflects PTEN activity and can be measured using the malachite green phosphatase assay kit (Echelon Biosciences). Upon incubation of apoptotic cells with macrophages, PTEN activity was significantly elevated (Figure 2B), indicating a role for PTEN in this process. As increasing numbers of macrophages engulfed apoptotic cells, PTEN was also activated. The activity reached the peak about 60 minutes after the initiation of efferocytosis, which is consistent with the maximum engulfment detected at the same time point. From these results, we can infer that PTEN may act as a global regulator of PtdIns(3,4,5)P3 formation during efferocytosis and may be responsible for the transient formation of PtdIns(3,4,5)P3 at the phagocytic cup. Interestingly, the lipid phosphatase activity of PTEN could also be elevated directly by either MFG-E8 or Gas6 which are bridging molecules that bind to phosphatidylserine on apoptotic cells and αvβ3/5 integrin or Mer tyrosine kinase on macrophages. This suggests that the efferocytosis-associated PTEN activation can be elicited by multiple receptor signaling (Figure 2C and D).
Disruption of PTEN enhances engulfment of apoptotic cells by macrophages
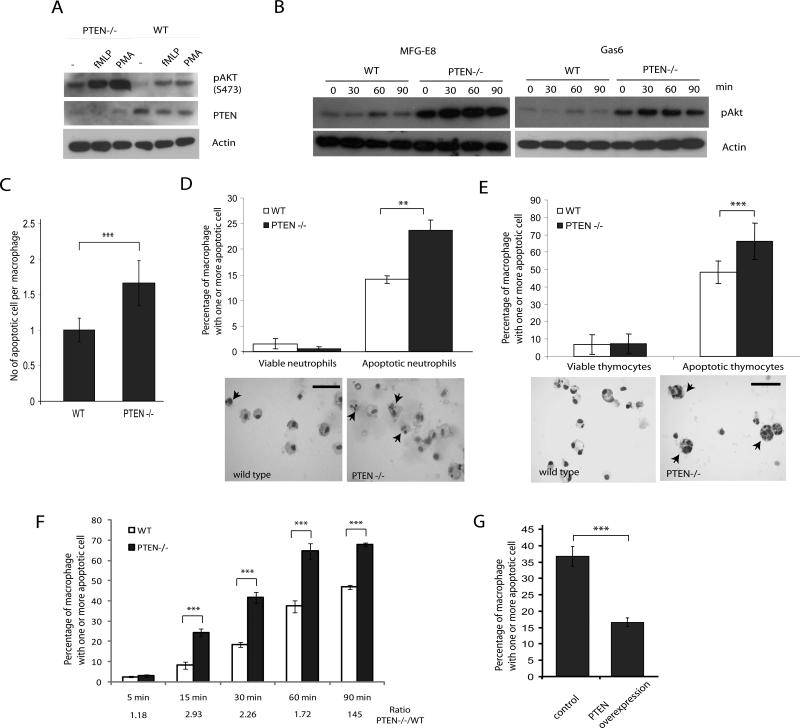
The complete loss of PTEN in mice leads to embryonic lethality. To examine the role of PTEN and PtdIns(3,4,5)P3 signaling in efferocytosis, we used a conditional myeloid-specific PTEN knockout mouse generated by crossing a PTEN-floxed mouse with a line expressing myeloid-specific Cre. In this Cre line, the expression of the Cre recombinase gene is under the control of the lysozyme promoter, which is expressed only in cells of the myeloid lineage, including monocytes, mature macrophages and neutrophils. Western blot analysis indicated that the expression of PTEN protein was completely abolished in thioglycollate-elicited peritoneal macrophages isolated from either PTENloxP/loxP; Cre+/– or PTENloxP/loxP; Cre+/+mice (PTEN-/- macrophages) (Figure 3A). The loss of PTEN resulted in elevated basal PtdIns(3,4,5)P3 signaling in the cell, as evidenced by the increased levels of phosphorylated Akt (phospho-Akt), which serves as a marker for PtdIns(3,4,5)P3 formation. In addition, PTEN disruption resulted in increased levels of activated Akt in macrophages stimulated with fMLP or PMA (Figure 3A). In addition, Akt phosphorylation induced by efferocytosis bridging proteins MFG-E8 and Gas6 which elicit downstream signaling through αvβ3-integrin and receptor tyrosine kinase Mer, respectively, was also elevated in PTEN-/- macrophages (Figure 3B). To determine whether PTEN plays a role in efferocytosis, we co-incubated PTEN-null peritoneal macrophages with either dexamethasone-induced apoptotic thymocytes or apoptotic neutrophils for 90 min. The efficiency of efferocytosis (% of macrophage containing one or more apoptotic body) and the phagocytic index (number of apoptotic bodies per macrophage) were analyzed. The PTEN-/- macrophages consistently engulfed increased quantities of apoptotic neutrophils or thymocytes. WT macrophages had a phagocytic index of about 1, and PTEN-/- macrophages had a phagocytic index of 1.6 (Figure 3C). About 15% of the wild-type macrophages engulfed apoptotic neutrophils, while 23% of PTEN-/- macrophages engulfed apoptotic neutrophils. Likewise, about 50% of wild-type macrophages engulfed apoptotic thymocytes while more than 65% of PTEN-/- macrophages engulfed apoptotic thymocytes. Viable cells were not engulfed by either wild-type or PTEN-/- macrophages (Figure 3D, E), and wild-type and PTEN-/-macrophages exhibited similar binding to apoptotic cells (Supplementary Figure 3). Next, we compared the kinetics of apoptotic cell engulfment in wild-type and PTEN-/- macrophages. Wild-type and PTEN-/- macrophages were fed with apoptotic cells for 0, 15, 30, 60 or 90 minutes, and the percentages of macrophages that engulfed apoptotic cells were assessed. At all of the time points, we observed that PTEN-/- macrophages engulfed more apoptotic cells, but the difference in engulfment of apoptotic cells was larger at the relatively earlier time points such as 15 and 30 minutes (Figure 3F). Interestingly, the kinetics of apoptotic cell engulfment in wild-type was similar to the kinetics of PTEN up-regulation observed during efferocytosis. It is possible that PTEN activity was up-regulated during efferocytosis and remained elevated for a prolonged time to maintain the low PtdIns(3,4,5)P3 in the cells that have finished engulfment. We next tested if the overexpression of PTEN, which reduces the cellular levels of PtdIns(3,4,5)P3, could affect efferocytosis. RAW264.7 macrophages were transfected with PTEN-Citrine or p-Max-GFP (as control). The transfected cells were then co-incubated with apoptotic cells. Supporting the idea that PTEN is a negative regulator of efferocytosis, overexpression of PTEN significantly reduced the ability of macrophages to engulf apoptotic cells (Figure 3G). We also used wortmannin to inhibit PtdIns(3,4,5)P3 formation in PTEN-/- macrophages and found that inhibiting PtdIns(3,4,5)P3 formation resulted in a similar reduction in efferocytosis as in wild-type macrophages (Supplementary Figure 4). Taken together, these results demonstrate that increasing PtdIns(3,4,5)P3 signaling by disrupting PTEN can result in the enhanced efficiency of efferocytosis.
Figure 3. Loss of PTEN leads to enhanced efferocytosis in vitro.
(A) Loss of PTEN in macrophages led to increased Akt phosphorylation. Thioglycollate-elicited peritoneal macrophages from wild type or PTEN-/- mice were stimulated with 1 μM fMLP or 100 nM PMA for 30 min. Cell lysates were analyzed by western blotting with phosphor-Akt (Ser473), PTEN and Actin antibodies. (B) Wild type and PTEN-/- macrophages were stimulated with 1μg/ml MFG-E8 or 1μg/ml Gas6 for indicated time points and cell lysates were analyzed for phospho-Akt. Actin is used as a loading control. (C) Wild type or PTEN-/- peritoneal macrophages were incubated with apoptotic cells at a density of (1:10) for 90 minutes at 37°C. Cells were washed and efferocytosis was analyzed by HEMA3 staining. Number of apoptotic cells per macrophage was counted and represented as efferocytic index, n>600. (D-E) Wild type or PTEN-/- peritoneal macrophages were incubated with fresh or one-day cultured bone marrow neutrophils (D), or viable or dexamethasone-induced apoptotic thymocytes (E) at a density of (1:10) for 90 minutes at 37°C. Cells were washed and efferocytosis was analyzed by HEMA3 staining. Number of macrophages containing one or more apoptotic cell was scored as % efferocytosis. Arrowheads indicate macrophage containing engulfed apoptotic cells. Bar represents 50μm. (F) Wild type and PTEN-/- macrophages were incubated with apoptotic thymocytes for indicated time points and engulfment of apoptotic cells was analyzed by HEMA3 staining. Ratio of percentage of macrophages with one or more apoptotic cells between PTEN-/- and wild type macrophages is depicted. (G) RAW264.7 cells were transfected with a PTEN-citrine construct or pMax-GFP (control) and incubated with apoptotic cells. Cells that expressed fluorescent proteins were then scored and their ability to engulf apoptotic cells was measured as described above. Results shown are mean ± SD (n>600). **p<0.01, ***p<0.005.
Disruption of PTEN enhances clearance of apoptotic cells in vivo
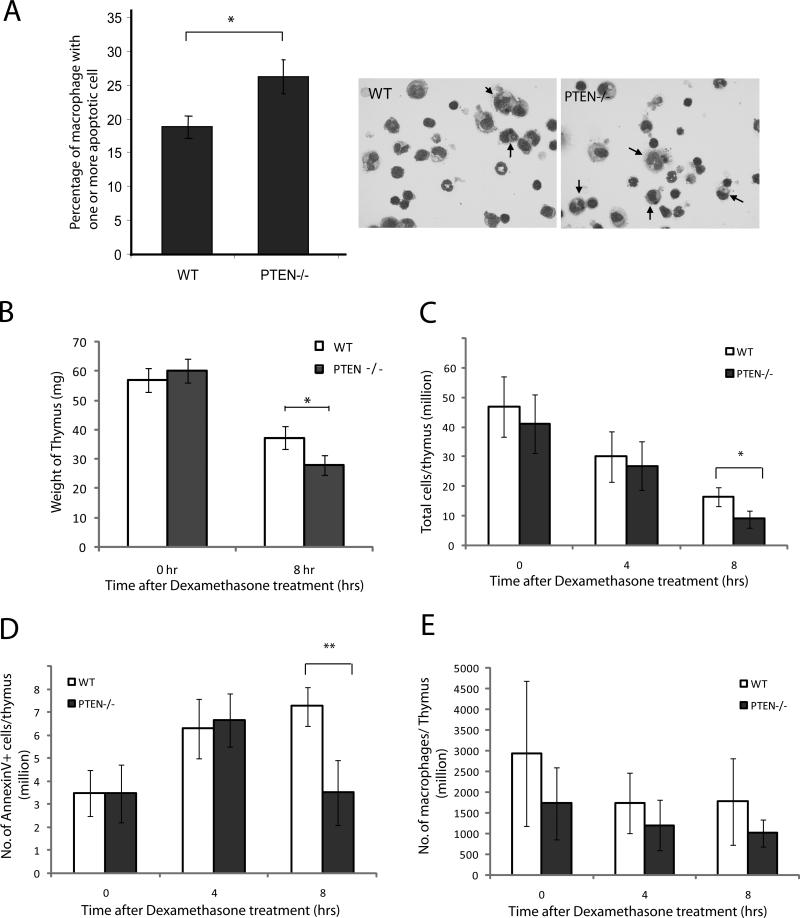
To determine whether the loss of PTEN in macrophages had any effect on the clearance of apoptotic cells in vivo, we used the following two in vivo models: the clearance of apoptotic cells in thioglycollate-elicited peritonitis and the clearance of dexamethasone-induced apoptotic thymocytes in the thymus. Thioglycollate is a polysaccharide mixture that has been widely used to induce mild inflammation, thus providing information about events that occur during inflammation. In the peritonitis model, wild-type and PTEN-/- mice were treated intraperitoneally with 3% thioglycollate to induce inflammation. After three days, macrophages represented the major cell type in the peritoneal cavity. Apoptotic thymocytes (40 × 106) were injected into the peritoneum and incubated for 90 minutes. The peritoneal cells were flushed out and analyzed by HEMA3 staining. We observed engulfment of apoptotic thymocytes by peritoneal macrophages in both the wild-type and PTEN-/- mice, but significantly more macrophages from the PTEN-/- mice had engulfed apoptotic cells (~26%) than had the wild-type macrophages (~18%) (Figure 4A).
Figure 4. Disruption of PTEN enhances engulfment of apoptotic cells in vivo.
(A) WT and PTEN-/- mice were injected intraperitoneally with 3% thioglycollate (TG) to induce recruitment of macrophages. Three days after the TG injection, 40 million apoptotic thymocytes were injected into the peritoneum and peritoneal lavage was prepared after 90 minutes. Efferocytosis was analyzed by HEMA3 staining and percentage of macrophage containing one or more apoptotic cell was counted. Data is a representative of three independent experiments. (B-E) Dexamethasone-induced thymic involution. Dexamethasone (250 μg) was dissolved in 1 ml PBS and then injected into wild type or PTEN-/- mice, DMSO alone was used as a control. At each indicated time points, thymus was extracted from the mice and analyzed. (B) Weight of thymus upon dexamethasone treatment for 8 hrs. (C) Total number of cells isolated from thymus at different time points. (D) Total number of annexin-positive apoptotic cells from thymus at different time points. (E) Total number of Mac1-positive cells (macrophages) from the thymus. Results shown are means ± SD (n=5), * p<0.05.
In the second model, dexamethasone was used to induce thymic involution. Dexamethasone is taken up rapidly by T-cells and induces apoptosis, leading to shrinkage in the size of the thymus and a reduction in cell number. The apoptotic cells are cleared by macrophages present in the thymus. In our study, dexamethasone was injected into wild-type or PTEN-/- mice, and efferocytosis was assessed 4 and 8 hrs after the injection. The thymuses were extracted from the mice, and the weights and numbers of thymocytes were analyzed. Upon dexamethasone treatment, the thymuses from both wild-type and PTEN-/- mice exhibited reduced size and weight and manifested decreased total thymocyte numbers. However, weight hours after dexamethasone treatment, the thymuses isolated from PTEN-/- mice weighed less (Figure 4B) and contained fewer thymocytes than those from wild-type mice (Figure 4C). When the number of apoptotic thymocytes was analyzed, we found that in wild-type mice, the number of apoptotic cells per thymus gradually increased in the first 8 hrs. On the other hand, in PTEN-/- mice, there was an increase in the number of apoptotic cells upon treatment with dexamethasone for the first 4 hrs, followed by a reduction in apoptotic cells in the thymus by 8 hrs (Figure 4D). Because the numbers of macrophages in the thymus were similar in the wild-type and PTEN-/- mice (Figure 4E), the reduced levels of apoptotic thymocytes in the PTEN-/- mice was likely due to enhanced clearance by macrophages in these mice.
Alteration of cytokine production by PTEN-/- macrophages in response to engulfment of apoptotic cells
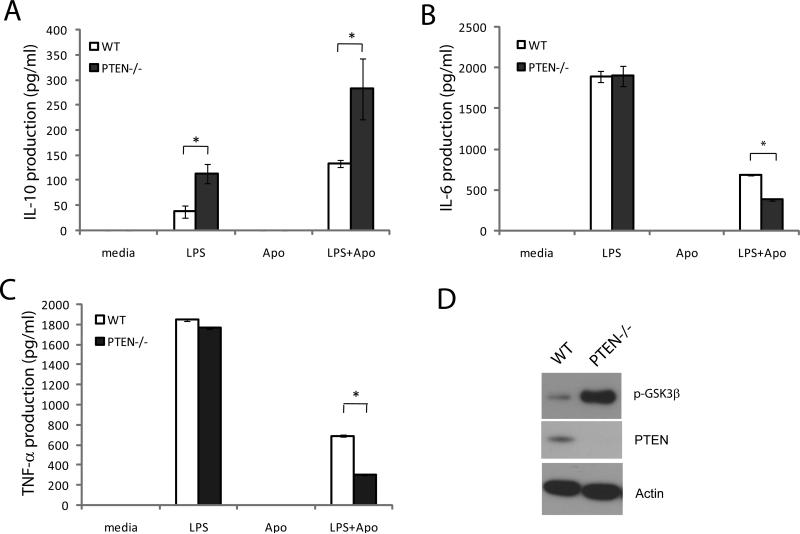
It has been established that the phagocytosis of apoptotic cells suppresses the autoimmune response by releasing immunosuppressive cytokines, such as TGFβ and IL-10, and inhibiting the production of proinflammatory cytokines, such as IL-6, TNFα, IL-12 and IL-1β. As the deletion of PTEN led to more efficient clearance of apoptotic cells through the upregulation of PtdIns(3,4,5)P3 signaling, we wondered whether the loss of PTEN would also affect cytokine production upon efferocytosis. We stimulated thioglycollate–elicited wild-type and PTEN-/- macrophages with lipopolysaccharide (LPS) in the presence or absence of apoptotic cells. The cytokines secreted in response to LPS and efferocytosis were analyzed by ELISA. As expected, incubation with apoptotic cells significantly augmented LPS-induced secretion of the anti-inflammatory cytokine IL-10 (Figure 5A) and reduced the secretion of the inflammatory cytokines IL-6 (Figure 5B) and TNFα (Figure 5C) in both wild-type and PTEN-/- macrophages. However, in PTEN-/- macrophages, the levels of the proinflammatory cytokines IL-6 and TNFα were much lower, and the production of the anti-inflammatory cytokine IL-10 was significantly enhanced in the presence of apoptotic cells.
Figure 5. Abnormal cytokine production upon efferocytosis by PTEN-/- macrophages.
Thioglycollate-elicited peritoneal macrophages from wild type and PTEN-/- mice were stimulated with or without lipopolysaccharide (LPS) in the presence or absence of apoptotic cells for 16 hrs. Culture supernatants were analyzed for anti-inflammatory cytokines IL-10 (A), proinflammatory cytokine IL-6 (B), and TNFα (C) by ELISA. Data is a representative of 4 independent experiments. Results shown are means ± SD. * p<0.0005. (D) Constitutive phosphorylation of GSK3β (Ser9) in resting macrophages from wild type and PTEN-/- mice. Actin was used as loading control.
Interestingly, upon treatment with LPS alone, the levels of IL-10 secreted by PTEN-/- macrophages were also significantly higher than those secreted by wild-type macrophages, while the levels of the proinflammatory cytokines IL-6 and TNFα were similar in wild-type and PTEN-/- macrophages. The PtdIns(3,4,5)P3-Akt signaling pathway has been implicated in the regulation of both proinflammatory and anti-inflammatory cytokines. It has been demonstrated that TLR-induced PI3K/Akt activation and the subsequent phosphorylation and inactivation of GSK-3β inhibits the NFκB -driven proinflammatory response (42-47) and enhances the expression of the immunosuppressive cytokine IL-10 (48). Accordingly, we analyzed the levels of phosphorylated GSK3β (Ser 9) in wild-type and PTEN-/- macrophages, and found that the levels of phospho-GSK3β (inactive form) in PTEN-/- macrophages were drastically elevated compared to in wild-type macrophages (Figure 5D). Thus, the augmentation of LPS-induced IL-10 production could simply be a results of enhanced GSK-3β phosphorylation and inactivation induced by PTEN disruption. However, it appeared that PTEN disruption-induced elevation of PtdIns(3,4,5)P3 signaling was not sufficient to alter the production of inflammatory cytokines (IL-6 and TNF-α) in our experimental system (Figure 5B-C).
Enhanced Rac activation in PTEN-/- macrophages
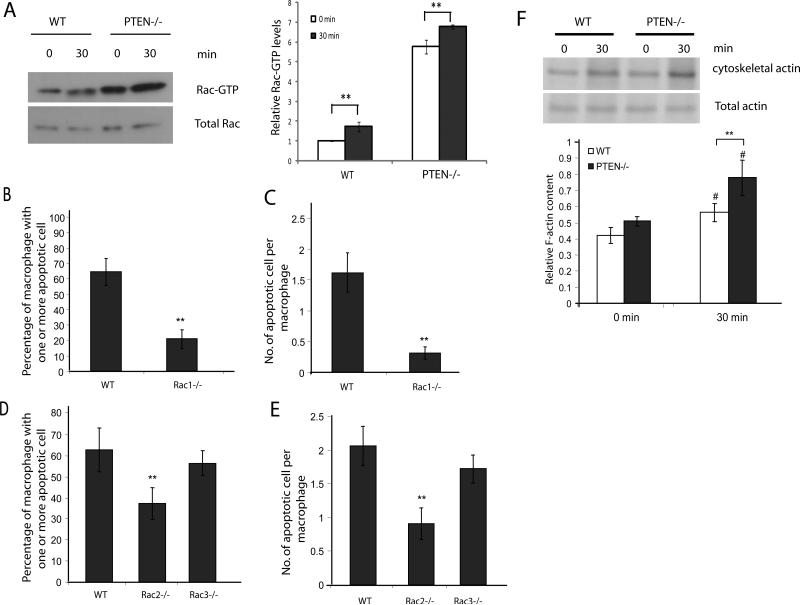
Rac subfamily GTPases have been implicated in the process of phagocytosis and have been shown to be key regulators of apoptotic cell engulfment. We next examined the activation of Rac GTPase in wild-type and PTEN-/- macrophages using GST-PAK-PBD to pull down activated Rac. Upon engulfment of apoptotic cells, an increase in Rac activation was observed. Both under basal conditions and when stimulated with apoptotic cells for 30 minutes, PTEN-/- macrophages had much higher levels of Rac1-GTP (Figure 6A). We next examined the roles of different Rac isoforms in efferocytosis. The mouse genome encodes 3 Rac isoforms: Rac 1, 2 and 3. Rac1 and Rac2 comprise 99% of the Rac in macrophages, and Rac3 is a minor isoforms (49, 50). We used mice deficient in the three Rac isoforms to test their roles in efferocytosis. As expected, loss of Rac1 and Rac2 resulted in a significant reduction in efferocytic ability, while the loss of Rac3 had no significant effect (Figure 6B-E).
Figure 6. Increased Rac activation and F-actin polymerization in PTEN-/- macrophages during efferocytosis.
(A) Wild type or PTEN-/- peritoneal macrophages were incubated with or without apoptotic cells for 30 minutes. Unadhered cells were washed and cell lysates were analyzed for levels of activated Rac-GTPase (Rac-GTP) by GST-pulldown using GST-PAK-PBD coated glutathione beads. Cell lysates and pulldown eluates were probed for Rac1. Relative level of active GTP-bound Rac1 to total Rac1 was quantified using ImageJ and depicted below. Wild type, Rac1-/- (B-C), Rac2-/-or Rac3-/- (D-E) peritoneal macrophages were incubated with apoptotic cells at a density of (1:10) for 90 minutes at 37°C. Cells were washed and efferocytosis was analyzed by HEMA3 staining. Number of macrophages containing one or more apoptotic cell was scored as % efferocytosis, n>600 (B and D). Number of apoptotic cells engulfed by one macrophage was counted and represented as efferocytic index, n>600 (C and E). (F) Wildtype and PTEN-/- macrophages were incubated with or without apoptotic cells for 30 minutes. Unadhered cells were washed and cells were lysed in CB. Cell lysates were centrifuged and the pellets were collected, washed, and analyzed by SDS-PAGE. Graph (below) indicates mean of four independent experiments. Results shown are means ± SD. #p<0.05; **p<0.005.
Rac activation leads to F-actin polymerization, so the observation that PTEN-/- macrophages exhibit increased Rac activation prompted us to measure actin levels in preparations of macrophage cytoskeletal ghosts under basal conditions and after stimulation with apoptotic cells. Actin present in this fraction represents the detergent-insoluble filamentous F-actin. Wild-type and PTEN-/- macrophages both exhibited an increase in F-actin content upon stimulation with apoptotic cells. However, PTEN-/- macrophages contained much higher levels of F-actin when stimulated with apoptotic cells compared to wild-type macrophages (Figure 6F). These results indicate that loss of PTEN in macrophages leads to an enhancement in efferocytosis-associated F-actin polymerization. However, it is intriguing that although PTEN-/- macrophages have elevated levels of activated Rac1 under basal conditions, the levels of F-actin are similar to the levels in wild-type macrophages. It is possible that PTEN-/- macrophages have evolved compensatory mechanisms to limit basal F-actin level despite high levels of Rac1 activation. Such a phenomenon of F-actin polymerization is also observed in PTEN--/- neutrophils stimulated with fMLP (32).
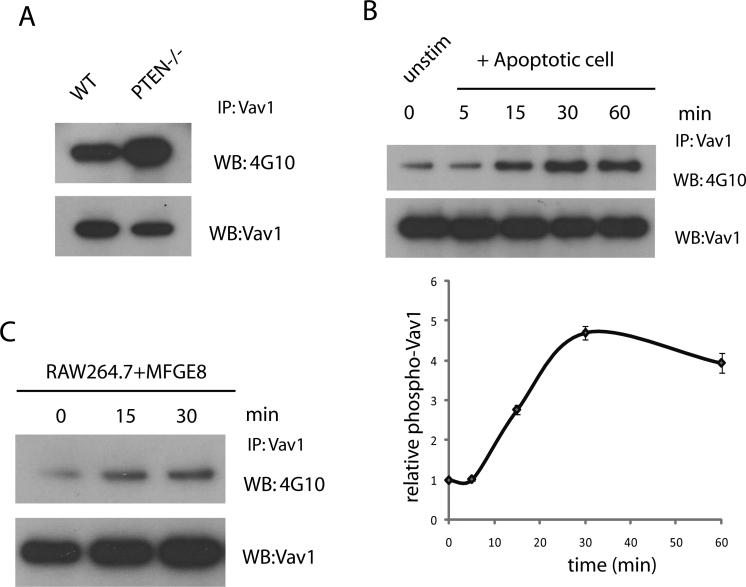
Enhanced Vav1 activation in PTEN-/- macrophages
To investigate the mechanism by which Rac activity is elevated in PTEN-/- macrophages, we investigated the involvement of other PH domain-containing Rac guanine nucleotide exchange factors (GEFs) in the regulation of efferocytosis. Vav-family guanine exchange factors are PH domain-containing proteins that are recruited to the membrane upon PtdIns(3,4,5)P3 formation and activated by tyrosine phosphorylation by protein tyrosine kinases. Vav-family GTPases act as exchange factors for Rac and cdc42 GTPases, which regulate actin polymerization. The engulfment of apoptotic cells requires the activation of Rac GTPase and subsequent actin polymerization to form the phagocytic cup. Immunoprecipitation analysis revealed that PTEN-/- macrophages had much higher levels of phosphorylated (activated) Vav1 compared to wild-type macrophages (Figure 7A), indicating that in PTEN-/- macrophages, the increased Vav1 activation may result in the enhanced Rac1 activation and augmented actin reorganization required for more efficient engulfment of apoptotic cells. We next examined whether Vav1 is activated by phosphorylation upon efferocytosis. Immunoprecipitation analysis showed that treatment with apoptotic cells resulted in gradually increased levels of Vav1 phosphorylation, which reached a maximum at 30 minutes (Figure 7B). These results indicate that Vav1 is activated in macrophages upon the engulfment of apoptotic cells. Since the levels of Vav1 protein were undetectable or extremely low in apoptotic cells (Supplementary Figure 2), the detected Vav1 was derived largely from macrophages.
Figure 7. Increased Vav1 activation in PTEN-/- macrophages during efferocytosis.
(A) Wild type and PTEN-/- peritoneal macrophages were lysed and Vav1 was immunoprecipitated. Pulldown eluates were analyzed by immunoblotting using anti-phospho-Tyr (4G10) and anti-Vav1 antibodies. (B) Peritoneal macrophages were incubated with or without apoptotic cells at indicated time points. Unadhered cells were washed and cell lysates were immunoprecipitated for Vav1 and analyzed by immunoblotting using anti-phospho-Tyr and anti-Vav1 antibodies. Relative level of phosphorylated Vav1 to total Vav1 was quantified using ImageJ and depicted. (C) Peritoneal macrophages were treated with 1μg/ml MFG-E8 for indicated time points and cell lysates were immunoprecipitated using Vav1 antibody. Levels of phosphorylated and total Vav1 were analyzed by immunoblotting using anti-phospho-Tyr and anti-Vav1 antibodies as described above.
The intracellular signaling elicited by efferocytosis is mediated by receptors or bridge proteins that recognize apoptotic cells. It was previously reported that Vav1 can be phosphorylated by the bridge protein Gas6, that cross-bridges apoptotic cells to macrophages through a receptor tyrosine kinase, Mer (51, 52). MFG-E8 is another protein that cross-bridges apoptotic cells to phagocytic macrophages. It can bind to phosphatidylserine on apoptotic cells and associate with αvβ3/5 integrin on the macrophages through an RGD motif. It was reported that Vav1 can be selectively phosphorylated at Y160 and activated after αvβ3-mediated adhesion on vitronectin and by the engagement of collagen, fibronectin and fibrinogen (53, 54). Thus, we tested whether MFG-E8, a ligand for αvβ3, could also induce Vav1 phosphorylation. Indeed, peritoneal macrophages treated with mouse recombinant MFG-E8 (1 μg/ml) manifested a gradual increase in Vav1 phosphorylation, suggesting that Vav1 can be directly activated by MFG-E8 (Figure 7C).
Discussion
The present study demonstrates that increased PtdIns(3,4,5)P3 signaling, caused by PTEN depletion, can enhance the ability of macrophages to clear apoptotic cells by elevating Rac GTPase activity. Using pharmacological inhibitors, we determined the roles of different PI3K isoforms in the process of efferocytosis. We found that the inhibition of p110α and p110β suppressed the engulfment of apoptotic cells, but the inhibition of p110γ did not cause any significant reduction in engulfment, indicating that signals from the G-protein coupled receptor (GPCR) signaling pathway that lead to p110γ mediated PtdIns(3,4,5)P3 production are not involved in the engulfment of apoptotic cells (Figure 1A). Because the Ser/Thr kinase Akt is an important downstream target of PtdIns(3,4,5)P3, we also investigated its role in efferocytosis. To our surprise, we found that neither pharmacological inhibition of Akt nor overexpression of a constitutively activated form of Akt caused any change in the efficiency of efferocytosis, indicating that Akt may not play a key role in the engulfment of apoptotic cells.
The cellular levels of PtdIns(3,4,5)P3 can be regulated by lipid phosphatases; thus we reasoned that the dephosphorylation of PtdIns(3,4,5)P3 by PTEN may also regulate efferocytosis. To address this question, we studied the role of PTEN in efferocytosis using a myeloid-specific PTEN knockout mouse. We observed an increase in PTEN activity in macrophages during the engulfment of apoptotic cells. The mechanisms that regulate PTEN activation are not completely understood. The lipid phosphatase activity of PTEN could also be elevated directly by either MFG-E8 or Gas6, suggesting that the efferocytosis-associated PTEN activation can be elicited by multiple receptor signaling. PTEN activity can be regulated by various mechanisms. Phosphorylation of the 50-amino acid C-terminal tail domain has been proposed to be critical for protein stability and phosphorylation. PTEN can be phosphorylated by CK2 at three residues (S380, T382 and T383) in the C-terminal tail domain, which increases its activity but concomitantly reduces its stability (40, 41). It is possible that conformational changes induced by protein-protein interactions cause the phosphorylation of PTEN by CK2. It has also been shown that the inactivated form of the PI3K p110δ isoform could activate PTEN in a mechanism requiring RhoA activity (55, 56). It is possible that the downregulation of PI3K after its initial activation by the binding and engulfment of apoptotic cells may activate PTEN through a similar mechanism that requires RhoA and ROCK (57, 58). The exact nature of the association between Rho activation and PTEN activity during efferocytosis is a matter for further investigation.
The loss of PTEN in macrophages led to enhanced basal levels of PtdIns(3,4,5)P3, as inferred from the phosphorylation of Akt. PTEN-/- peritoneal macrophages engulfed both apoptotic neutrophils and thymocytes with higher efficiency than wild-type macrophages. Conversely, the forced expression of wild-type PTEN dramatically reduced the engulfment of apoptotic cells. These results imply that PTEN negatively regulates the engulfment of apoptotic cells. In vivo animal models also led to similar conclusions. In a dexamethasone-induced thymic involution model, the loss of PTEN resulted in the faster clearance of apoptotic thymocytes. In a peritonitis model involving the introduction of apoptotic cells into the peritoneum, PTEN-/- macrophages also engulfed apoptotic cells more efficiently (Figure 4A).
The generation of apoptotic cells and efferocytosis are continuous processes. Clearance of apoptotic cells is a non-inflammatory event. The roles of PtdIns(3,4,5)P3/Akt and PTEN signaling in the process of inflammation have been studied previously and remain controversial, with reports suggesting both pro- and anti-inflammatory activities (44, 59). Recent studies have demonstrated that PtdIns(3,4,5)P3/Akt signaling exerts an anti-inflammatory effect in response to sepsis (60), bacterial pneumonia (33) and viral infections (61), which is likely mediated by the downregulation of NFκβ transcriptional activity through deactivation of GSK-3β upon Akt phosphorylation (42). Activation of PI3K/Akt also leads to a blockade of NFκβ activation in dendritic cells exposed to apoptotic cells (62). Consistent with these early reports, we also observed that the loss of PTEN led to increased GSK3β phosphorylation, which may result in the modulation of NFκβ and increased production of anti-inflammatory cytokine IL-10. It appeared that PTEN disruption-induced elevation of PtdIns(3,4,5)P3 signaling was not sufficient to alter LPS-induced production of proinflammatory cytokines (IL-6 and TNF-α) in our experimental system. Nevertheless, PTEN disruption could both increase the production of IL-10 and reduce the production of IL-6 and TNFα during efferocytosis. These results further imply that due to the enhanced anti-inflammatory response and the augmented efferocytic properties, PTEN-/- macrophages can clear apoptotic cells more efficiently under physiological conditions and may potentially lead to the faster resolution of inflammation. Thus, PTEN disruption can be a potential strategy for elevating efferocytosis in certain infectious and inflammatory diseases.
To analyze the molecular mechanisms driving enhanced efferocytosis, we focused on signaling events downstream of PtdIns(3,4,5)P3. Because efferocytosis was independent of Akt, we investigated the activation of other PH domain-containing proteins that can influence engulfment. PTEN has previously been shown to control Fc gamma receptor mediated phagocytosis by regulating Rac activation (63). We noted that the Vav1 exchange factor is activated upon efferocytosis and by MFG-E8 or Gas6. Apoptotic cells, via MFG-E8 or Gas6, activates Class IA PI3K, leading to formation of PtdIns(3,4,5)P3 in macrophages. PtdIns(3,4,5)P3 in turn results in Vav1 activation which peaks at 30 minute after apoptotic cell stimulation. Vav1 activates Rac1 and F-actin polymerization leading to efferocytosis. Binding to apoptotic cells also leads to activation of PTEN that peaks and plateaus between 30-60 minute to dephosphorylate PtdIns(3,4,5)P3, indicating that Vav1 and PTEN may function in the same pathway to regulate apoptotic cell engulfment. Conceivably, increased Vav1 activation in PTEN-/- macrophages enhances Rac activity and subsequent F-actin polymerization, leading to elevated engulfment of apoptotic cells.
In conclusion, our findings demonstrate that increased PtdIns(3,4,5)P3 signaling in PTEN-deficient mice results in enhanced clearance of apoptotic cells. This occurs through the regulation of Rac GTPase activation, which may be due to the augmented activity of Vav family guanine nucleotide exchange factor, Vav1. In addition to elevating engulfment, depletion of PTEN may also lead to enhanced phosphorylation and inactivation of GSK3β, which would lead to inactivation of NFκβ and subsequent suppression of the production of proinflammatory cytokines IL-6 and TNFα and concurrent increase in expression of anti-inflammatory cytokine IL-10.
Supplementary Material
Acknowledgements
We thank Catlyn Blanchard and Jia Zhong for help with mice lines used in the study. We thank Dr Joel Swanson for providing murine PTEN construct.
The work is supported by National Institute of Health grants HL085100, AI076471, HL092020, and GM076084 to HRL and by a post-doctoral fellowship from Deutscheforschungsgemeinshaft, Germany (DFG) to SM.
References
- 1.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7:964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- 2.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lauber K, Bohn E, Krober SM, Xiao YJ, Blumenthal SG, Lindemann RK, Marini P, Wiedig C, Zobywalski A, Baksh S, Xu Y, Autenrieth IB, Schulze-Osthoff K, Belka C, Stuhler G, Wesselborg S. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113:717–730. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- 4.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 5.Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi N, Karisola P, Pena-Cruz V, Dorfman DM, Jinushi M, Umetsu SE, Butte MJ, Nagumo H, Chernova I, Zhu B, Sharpe AH, Ito S, Dranoff G, Kaplan GG, Casasnovas JM, Umetsu DT, Dekruyff RH, Freeman GJ. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 2007;27:927–940. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park D, Tosello-Trampont AC, Elliott MR, Lu M, Haney LB, Ma Z, Klibanov AL, Mandell JW, Ravichandran KS. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450:430–434. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 8.Park SY, Jung MY, Kim HJ, Lee SJ, Kim SY, Lee BH, Kwon TH, Park RW, Kim IS. Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 2008;15:192–201. doi: 10.1038/sj.cdd.4402242. [DOI] [PubMed] [Google Scholar]
- 9.Akakura S, Singh S, Spataro M, Akakura R, Kim JI, Albert ML, Birge RB. The opsonin MFG-E8 is a ligand for the alphavbeta5 integrin and triggers DOCK180-dependent Rac1 activation for the phagocytosis of apoptotic cells. Exp Cell Res. 2004;292:403–416. doi: 10.1016/j.yexcr.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 11.Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 12.Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 13.Kinchen JM, Cabello J, Klingele D, Wong K, Feichtinger R, Schnabel H, Schnabel R, Hengartner MO. Two pathways converge at CED-10 to mediate actin rearrangement and corpse removal in C. elegans. Nature. 2005;434:93–99. doi: 10.1038/nature03263. [DOI] [PubMed] [Google Scholar]
- 14.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borregaard N. Neutrophils, from marrow to microbes. Immunity. 33:657–670. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Elliott MR, Zheng S, Park D, Woodson RI, Reardon MA, Juncadella IJ, Kinchen JM, Zhang J, Lysiak JJ, Ravichandran KS. Unexpected requirement for ELMO1 in clearance of apoptotic germ cells in vivo. Nature. 467:333–337. doi: 10.1038/nature09356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarzynka MJ, Hu B, Hui KM, Bar-Joseph I, Gu W, Hirose T, Haney LB, Ravichandran KS, Nishikawa R, Cheng SY. ELMO1 and Dock180, a bipartite Rac1 guanine nucleotide exchange factor, promote human glioma cell invasion. Cancer Res. 2007;67:7203–7211. doi: 10.1158/0008-5472.CAN-07-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gumienny TL, Brugnera E, Tosello-Trampont AC, Kinchen JM, Haney LB, Nishiwaki K, Walk SF, Nemergut ME, Macara IG, Francis R, Schedl T, Qin Y, Van Aelst L, Hengartner MO, Ravichandran KS. CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell. 2001;107:27–41. doi: 10.1016/s0092-8674(01)00520-7. [DOI] [PubMed] [Google Scholar]
- 19.Camps M, Ruckle T, Ji H, Ardissone V, Rintelen F, Shaw J, Ferrandi C, Chabert C, Gillieron C, Francon B, Martin T, Gretener D, Perrin D, Leroy D, Vitte PA, Hirsch E, Wymann MP, Cirillo R, Schwarz MK, Rommel C. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med. 2005;11:936–943. doi: 10.1038/nm1284. [DOI] [PubMed] [Google Scholar]
- 20.Smith DF, Deem TL, Bruce AC, Reutershan J, Wu D, Ley K. Leukocyte phosphoinositide-3 kinase {gamma} is required for chemokine-induced, sustained adhesion under flow in vivo. J Leukoc Biol. 2006;80:1491–1499. doi: 10.1189/jlb.0306227. [DOI] [PubMed] [Google Scholar]
- 21.Liu L, Puri KD, Penninger JM, Kubes P. Leukocyte PI3Kgamma and PI3Kdelta have temporally distinct roles for leukocyte recruitment in vivo. Blood. 2007;110:1191–1198. doi: 10.1182/blood-2006-11-060103. [DOI] [PubMed] [Google Scholar]
- 22.Puri KD, Doggett TA, Douangpanya J, Hou Y, Tino WT, Wilson T, Graf T, Clayton E, Turner M, Hayflick JS, Diacovo TG. Mechanisms and implications of phosphoinositide 3-kinase delta in promoting neutrophil trafficking into inflamed tissue. Blood. 2004;103:3448–3456. doi: 10.1182/blood-2003-05-1667. [DOI] [PubMed] [Google Scholar]
- 23.Puri KD, Doggett TA, Huang CY, Douangpanya J, Hayflick JS, Turner M, Penninger J, Diacovo TG. The role of endothelial PI3Kgamma activity in neutrophil trafficking. Blood. 2005;106:150–157. doi: 10.1182/blood-2005-01-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heit B, Liu L, Colarusso P, Puri KD, Kubes P. PI3K accelerates, but is not required for, neutrophil chemotaxis to fMLP. J Cell Sci. 2008;121:205–214. doi: 10.1242/jcs.020412. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, Wakeham A, Itie A, Bouchard D, Kozieradzki I, Joza N, Mak TW, Ohashi PS, Suzuki A, Penninger JM. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 26.Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287:1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 27.Liliental J, Moon SY, Lesche R, Mamillapalli R, Li D, Zheng Y, Sun H, Wu H. Genetic deletion of the Pten tumor suppressor gene promotes cell motility by activation of Rac1 and Cdc42 GTPases. Curr Biol. 2000;10:401–404. doi: 10.1016/s0960-9822(00)00417-6. [DOI] [PubMed] [Google Scholar]
- 28.Gao P, Wange RL, Zhang N, Oppenheim JJ, Howard OM. Negative regulation of CXCR4-mediated chemotaxis by the lipid phosphatase activity of tumor suppressor PTEN. Blood. 2005;106:2619–2626. doi: 10.1182/blood-2004-08-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwak YG, Song CH, Yi HK, Hwang PH, Kim JS, Lee KS, Lee YC. Involvement of PTEN in airway hyperresponsiveness and inflammation in bronchial asthma. J Clin Invest. 2003;111:1083–1092. doi: 10.1172/JCI16440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lacalle RA, Gomez-Mouton C, Barber DF, Jimenez-Baranda S, Mira E, Martinez AC, Carrera AC, Manes S. PTEN regulates motility but not directionality during leukocyte chemotaxis. J Cell Sci. 2004;117:6207–6215. doi: 10.1242/jcs.01545. [DOI] [PubMed] [Google Scholar]
- 31.Tamura M, Gu J, Matsumoto K, Aota S, Parsons R, Yamada KM. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science. 1998;280:1614–1617. doi: 10.1126/science.280.5369.1614. [DOI] [PubMed] [Google Scholar]
- 32.Subramanian KK, Jia Y, Zhu D, Simms BT, Jo H, Hattori H, You J, Mizgerd JP, Luo HR. Tumor suppressor PTEN is a physiologic suppressor of chemoattractant-mediated neutrophil functions. Blood. 2007;109:4028–4037. doi: 10.1182/blood-2006-10-055319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schabbauer G, Matt U, Gunzl P, Warszawska J, Furtner T, Hainzl E, Elbau I, Mesteri I, Doninger B, Binder BR, Knapp S. Myeloid PTEN promotes inflammation but impairs bactericidal activities during murine pneumococcal pneumonia. J Immunol. 185:468–476. doi: 10.4049/jimmunol.0902221. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Jia Y, Pichavant M, Loison F, Sarraj B, Kasorn A, You J, Robson BE, Umetsu DT, Mizgerd JP, Ye K, Luo HR. Targeted deletion of tumor suppressor PTEN augments neutrophil function and enhances host defense in neutropenia-associated pneumonia. Blood. 2009;113:4930–4941. doi: 10.1182/blood-2008-06-161414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gu Y, Filippi MD, Cancelas JA, Siefring JE, Williams EP, Jasti AC, Harris CE, Lee AW, Prabhakar R, Atkinson SJ, Kwiatkowski DJ, Williams DA. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science. 2003;302:445–449. doi: 10.1126/science.1088485. [DOI] [PubMed] [Google Scholar]
- 36.Roberts AW, Kim C, Zhen L, Lowe JB, Kapur R, Petryniak B, Spaetti A, Pollock JD, Borneo JB, Bradford GB, Atkinson SJ, Dinauer MC, Williams DA. Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity. 1999;10:183–196. doi: 10.1016/s1074-7613(00)80019-9. [DOI] [PubMed] [Google Scholar]
- 37.Corbetta S, Gualdoni S, Albertinazzi C, Paris S, Croci L, Consalez GG, de Curtis I. Generation and characterization of Rac3 knockout mice. Mol Cell Biol. 2005;25:5763–5776. doi: 10.1128/MCB.25.13.5763-5776.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamen LA, Levinsohn J, Swanson JA. Differential association of phosphatidylinositol 3-kinase, SHIP-1, and PTEN with forming phagosomes. Mol Biol Cell. 2007;18:2463–2472. doi: 10.1091/mbc.E07-01-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leverrier Y, Okkenhaug K, Sawyer C, Bilancio A, Vanhaesebroeck B, Ridley AJ. Class I phosphoinositide 3-kinase p110beta is required for apoptotic cell and Fcgamma receptor-mediated phagocytosis by macrophages. J Biol Chem. 2003;278:38437–38442. doi: 10.1074/jbc.M306649200. [DOI] [PubMed] [Google Scholar]
- 40.Torres J, Pulido R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J Biol Chem. 2001;276:993–998. doi: 10.1074/jbc.M009134200. [DOI] [PubMed] [Google Scholar]
- 41.Vazquez F, Ramaswamy S, Nakamura N, Sellers WR. Phosphorylation of the PTEN tail regulates protein stability and function. Mol Cell Biol. 2000;20:5010–5018. doi: 10.1128/mcb.20.14.5010-5018.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin M, Schifferle RE, Cuesta N, Vogel SN, Katz J, Michalek SM. Role of the phosphatidylinositol 3 kinase-Akt pathway in the regulation of IL-10 and IL-12 by Porphyromonas gingivalis lipopolysaccharide. J Immunol. 2003;171:717–725. doi: 10.4049/jimmunol.171.2.717. [DOI] [PubMed] [Google Scholar]
- 44.Luyendyk JP, Schabbauer GA, Tencati M, Holscher T, Pawlinski R, Mackman N. Genetic analysis of the role of the PI3K-Akt pathway in lipopolysaccharide-induced cytokine and tissue factor gene expression in monocytes/macrophages. J Immunol. 2008;180:4218–4226. doi: 10.4049/jimmunol.180.6.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H, Brown J, Gu Z, Garcia CA, Liang R, Alard P, Beurel E, Jope RS, Greenway T, Martin M. Convergence of the Mammalian Target of Rapamycin Complex 1- and Glycogen Synthase Kinase 3-{beta}-Signaling Pathways Regulates the Innate Inflammatory Response. J Immunol. 186:5217–5226. doi: 10.4049/jimmunol.1002513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang H, Brown J, Garcia CA, Tang Y, Benakanakere MR, Greenway T, Alard P, Kinane DF, Martin M. The role of glycogen synthase kinase 3 in regulating IFN-beta-mediated IL-10 production. J Immunol. 186:675–684. doi: 10.4049/jimmunol.1001473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang H, Brown J, Martin M. Glycogen synthase kinase 3: a point of convergence for the host inflammatory response. Cytokine. 53:130–140. doi: 10.1016/j.cyto.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pengal RA, Ganesan LP, Wei G, Fang H, Ostrowski MC, Tridandapani S. Lipopolysaccharide-induced production of interleukin-10 is promoted by the serine/threonine kinase Akt. Mol Immunol. 2006;43:1557–1564. doi: 10.1016/j.molimm.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 49.Wells CM, Walmsley M, Ooi S, Tybulewicz V, Ridley AJ. Rac1-deficient macrophages exhibit defects in cell spreading and membrane ruffling but not migration. J Cell Sci. 2004;117:1259–1268. doi: 10.1242/jcs.00997. [DOI] [PubMed] [Google Scholar]
- 50.Yamauchi A, Kim C, Li S, Marchal CC, Towe J, Atkinson SJ, Dinauer MC. Rac2-deficient murine macrophages have selective defects in superoxide production and phagocytosis of opsonized particles. J Immunol. 2004;173:5971–5979. doi: 10.4049/jimmunol.173.10.5971. [DOI] [PubMed] [Google Scholar]
- 51.Mahajan NP, Earp HS. An SH2 domain-dependent, phosphotyrosine-independent interaction between Vav1 and the Mer receptor tyrosine kinase: a mechanism for localizing guanine nucleotide-exchange factor action. J Biol Chem. 2003;278:42596–42603. doi: 10.1074/jbc.M305817200. [DOI] [PubMed] [Google Scholar]
- 52.Grommes C, Lee CY, Wilkinson BL, Jiang Q, Koenigsknecht-Talboo JL, Varnum B, Landreth GE. Regulation of microglial phagocytosis and inflammatory gene expression by Gas6 acting on the Axl/Mer family of tyrosine kinases. J Neuroimmune Pharmacol. 2008;3:130–140. doi: 10.1007/s11481-007-9090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao C, Schaefer E, Lakkis M, Blystone SD. Beta3 tyrosine phosphorylation and alphavbeta3-mediated adhesion are required for Vav1 association and Rho activation in leukocytes. J Biol Chem. 2005;280:15422–15429. doi: 10.1074/jbc.M414457200. [DOI] [PubMed] [Google Scholar]
- 54.Cichowski K, Brugge JS, Brass LF. Thrombin receptor activation and integrin engagement stimulate tyrosine phosphorylation of the protooncogene product, p95vav, in platelets. J Biol Chem. 1996;271:7544–7550. doi: 10.1074/jbc.271.13.7544. [DOI] [PubMed] [Google Scholar]
- 55.Papakonstanti EA, Ridley AJ, Vanhaesebroeck B. The p110delta isoform of PI 3-kinase negatively controls RhoA and PTEN. EMBO J. 2007;26:3050–3061. doi: 10.1038/sj.emboj.7601763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Z, Dong X, Wang Z, Liu W, Deng N, Ding Y, Tang L, Hla T, Zeng R, Li L, Wu D. Regulation of PTEN by Rho small GTPases. Nat Cell Biol. 2005;7:399–404. doi: 10.1038/ncb1236. [DOI] [PubMed] [Google Scholar]
- 57.Nakaya M, Tanaka M, Okabe Y, Hanayama R, Nagata S. Opposite effects of rho family GTPases on engulfment of apoptotic cells by macrophages. J Biol Chem. 2006;281:8836–8842. doi: 10.1074/jbc.M510972200. [DOI] [PubMed] [Google Scholar]
- 58.Tosello-Trampont AC, Nakada-Tsukui K, Ravichandran KS. Engulfment of apoptotic cells is negatively regulated by Rho-mediated signaling. J Biol Chem. 2003;278:49911–49919. doi: 10.1074/jbc.M306079200. [DOI] [PubMed] [Google Scholar]
- 59.Strassheim D, Kim JY, Park JS, Mitra S, Abraham E. Involvement of SHIP in TLR2-induced neutrophil activation and acute lung injury. J Immunol. 2005;174:8064–8071. doi: 10.4049/jimmunol.174.12.8064. [DOI] [PubMed] [Google Scholar]
- 60.Williams DL, Li C, Ha T, Ozment-Skelton T, Kalbfleisch JH, Preiszner J, Brooks L, Breuel K, Schweitzer JB. Modulation of the phosphoinositide 3-kinase pathway alters innate resistance to polymicrobial sepsis. J Immunol. 2004;172:449–456. doi: 10.4049/jimmunol.172.1.449. [DOI] [PubMed] [Google Scholar]
- 61.Schabbauer G, Luyendyk J, Crozat K, Jiang Z, Mackman N, Bahram S, Georgel P. TLR4/CD14-mediated PI3K activation is an essential component of interferon-dependent VSV resistance in macrophages. Mol Immunol. 2008;45:2790–2796. doi: 10.1016/j.molimm.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sen P, Wallet MA, Yi Z, Huang Y, Henderson M, Mathews CE, Earp HS, Matsushima G, Baldwin AS, Jr., Tisch RM. Apoptotic cells induce Mer tyrosine kinase-dependent blockade of NF-kappaB activation in dendritic cells. Blood. 2007;109:653–660. doi: 10.1182/blood-2006-04-017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim JS, Peng X, De PK, Geahlen RL, Durden DL. PTEN controls immunoreceptor (immunoreceptor tyrosine-based activation motif) signaling and the activation of Rac. Blood. 2002;99:694–697. doi: 10.1182/blood.v99.2.694. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.