Abstract
The airway epithelium possesses many mechanisms to prevent bacterial infection. Not only does it provide a physical barrier but it also acts as an extension of the immune system through the expression of innate immune receptors and corresponding effectors. One outcome of innate signaling by the epithelium is the production of type I interferons (IFN) , which have traditionally been associated with activation via viral and intracellular organisms. We discuss how three extracellular bacterial pathogens of the airway activate this intracellular signaling cascade through both surface components as well as via secretion systems and the differing effects of type I IFN signaling on host defense of the respiratory tract.
Bacterial activation of type I interferon signaling
Type I interferon (IFN) signaling has long been recognized as a critical component of innate immune defense to viral pathogens in the airway. Increasingly, its role in the response to bacterial infections is being appreciated. The participation of the type I IFN cascade in responses to intracellular bacteria within phagocytes has been well established. It is only with the recognition that airway epithelial cells respond to extracellular pathogens themselves that epithelial induction has become a focus of investigation. Typically bacteria activate type I IFN signaling through TLR-dependent mechanisms, through recognition of LPS in Gram negative organisms or via TLR-independent cytosolic receptors that respond to nucleic acids that are either endocytosed or secreted directly into the eukaryotic cells [1, 2]. The consequences of type I IFN signaling on host outcome can be either protective or damaging, depending on the organism [3, 4]. Below, we characterize how three major extracellular respiratory pathogens, Streptococcus pneumoniae, Pseudomonas aeruginosa and Staphylococcus aureus activate type I IFN signaling in the airway and how this impacts upon the early stages of host defense.
Induction of Type I Interferons
A large redundant array of receptors sense microorganisms through ligands known as pathogen-associated molecular patterns (PAMPs). One of the best studied groups of pattern recognition receptors (PRRs) is the toll-like receptor (TLR) family. TLRs sense PAMPs such as LPS, peptidoglycan and flagellin, which typically leads to proinflammatory signaling as well as the recruitment and activation of phagocytes, via the transcription factor NF-κB. However, upon activation, a subset of TLRs induce the type I IFNs (the main types produced during bacterial and viral infection are IFN-α (multiple genes) and IFN-β (a single gene); Table 1) [4]. The importance of type I IFN signaling in innate immune defense was most evident in studies of influenza infections of mice. Mice lacking the receptor to type I IFN succumbed to infection while wild-type mice survived in mouse models of influenza infection [5]. Susceptibility of mice to influenza infection requires the interferon-regulated Mx1 gene to be absent [6]. Activation of type I IFNs produces specific antiviral factors as well as various cellular responses such as T cell recruitment [7, 8].
Table 1.
Host receptors that lead to type I IFN signaling.
Bacteria are also able to activate type I IFNs through TLR4, 7/8 and 9 as well as additional non-TLR receptors (Table 1, Figure 1). TLR4 utilizes both the MyD88 and TRIF pathways of signal transduction. Type I IFN production occurs when TLR4 has been endocytosed after LPS binding [LPS interacts with TLR4 along with lipid binding protein (LBP), CD14 and MD2]. TLR4 is then brought to TRIF in early endosomes by TRAM before activation of TBK1 and IRF3 induce transcription of Ifnb [9, 10]. IFN-β then binds to the interferon alpha/beta receptor (IFNAR) that activates the JAK/STAT pathway. Janus kinase (JAK) members Jak1 and Tyk2 become phosphorylated leading to the recruitment and phosphorylation of STAT1 and STAT2 [11]. STAT1 and STAT2 then bind to IFN stimulated response elements (ISRE) that leads to the induction of >300 genes with both inflammatory and anti-inflammatory effects [8, 12, 13]. STAT1 mice are completely unresponsive to IFNs. STAT1 is also activated by a number of other cytokine pathways including IFN-γ and -λ and gp130 members IL-6, IL-23 and IL-27, although their levels of activation are typically more modest [11]. The role of IL-27 in bacterial infection is unknown while IFN-γ plays no role in P. aeruginosa infection [14], is important for S. pneumoniae clearance [15] and is potentially detrimental in S. aureus infections [16]. The role of IFN-λ has yet to be examined with these organisms, while its role is variable in viral infections [17]. Signaling through IFNAR also leads to MAPK activation [4]. A similar pathway is utilized by NOD2 (sensor of muramyl dipeptide, peptidoglycan) (Table 1, Figure 1). The endosomally located sensors TLR7/8 (TLR7 murine, TLR8 human) and TLR9 utilize MyD88 to signal type I IFN. Interaction with these receptors recruits IRAK-1 and IRAK-4 that activate IRF5 and IRF7. IRAK-1 also interacts with TRAF-6 that leads to NF-kB-dependent genes as well as type I IFNs [18-20]. NF-kB is also produced when these TLR are activated. This can also lead to type I IFN production [21].
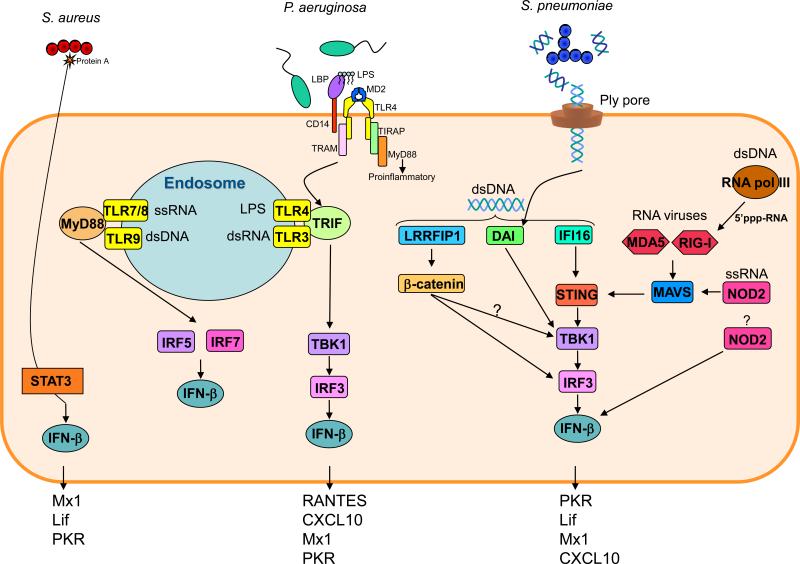
Figure 1.
Mechanisms involved in bacterial type I IFN signaling. A number of receptors are able to activate type I IFN signaling and the mechanisms for S. pneumoniae, P. aeruginosa and S. aureus are highlighted along with known type I-dependent gene products. The majority of type I IFN activating receptors are located inside cells, the exception being TLR4, which is internalized to signal with its adaptor TRIF. The MDA5 and RIG-I receptors that sense viral RNA can also sense bacterial DNA converted to RNA by RNA polymerase III. All signaling pathways lead to activation of an interferon regulatory factor (IRF) before producing IFN-β.
Recognition of nucleic acids to induce type I IFNs was first established in the context of viral infection. Viral RNA is sensed by the RIG-like receptors (RLR) receptors RIG-I retinoic acid inducible gene I (RIG-I) and melanoma differentiation-associated protein (MDA5), which converge to IPS-1 [also called mitochondrial antiviral signaling protein (MAVS)] before the signal goes to TANK binding kinase (TBK1) (Figure 1) [19]. Viral RNA is also sensed through TLR3 via TRIF that leads to TBK1 activation. Activation of TBK1 and IKK-related kinases lead to phosphorylation of interferon regulatory factor (IRF3) and IRF7 that results in production of IFN-β [22, 23]. A number of receptors have been recently identified that sense cytosolic nucleic acids from bacteria. RNA polymerase III is able to convert DNA to a 5’-triphosphate RNA that is sensed by the RIG helicases and along with DNA dependent activator of IFN-regulatory factors (DAI) and IFI16 through [24] stimulator of interferon genes (STING) all lead to activation of type I IFN signaling through TBK1 and IRF3 (Figure 1).
Type I Inteferon signaling in the airway
The connecting airways consist of polarized epithelial cells and clara cells that secrete mucus whereas the lower airways contain type I and type II cells [38]. The majority of studies utilize polarized cells (in the studies mentioned, ciliated epithelial), with significant data also available for alveolar cells types (i.e. A549). The epithelial cells lining the airway are the most numerous cell, initiating and coordinating the immune response to recruited immune cells, especially DCs and macrophages, but also T-cells. Airway epithelial cells themselves express the full complement of TLR and NOD genes, including those known to activate type I IFN signaling [39-42]. The epithelium is not just a physical barrier, it represents the first interaction of a pathogen with the immune system. Airway epithelial cells produce several antimicrobial peptides and secrete cytokine and chemokines that recruit and activate professional phagocytic cells [19]. The importance of type I IFN production (STAT1 production) by the airway epithelium in viral infection has been demonstrated. STAT1 null mice reconstituted with WT bone marrow were still susceptible to infection, indicating that epithelial cell gene products directly act or influence other immune cells to provide the protective response [43]. Downstream effects of STAT1 activation, such as CXCL8 production, leads to recruitment of neutrophils to the site of infection, while production of other cytokines that are recognized by immune cells such as dendritic cells (DCs) amplifies the response [19]. The components of the innate immune system associated with viral detection have been the most thoroughly studied in the airway, i.e. RIG, MDA5, TLR3 and TLR7, with TLR3 producing the strongest proinflammatory response [19, 44-46]. The newly identified cytosolic sensors of DNA have yet to be examined in the airway epithelium.
The production of type I IFN by epithelial cells is an important part of the mucosal immune response that includes several different types of lymphocytes. Macrophages and DCs are well known producers of type I IFNs, particularly the plasmacytoid DC (pDC). pDCs are especially responsive to nucleic acid (TLR 7 and 9) and produce significant quantities of type I IFN before maturing into mature DCs and triggering adaptive responses [47]. The roles of DCs and macrophages are important in pulmonary infections of S. aureus and S. pneumoniae [48, 49], macrophages play variable roles in P. aeruginosa infection [50, 51]. The respective roles of DC subsets in these organism is unknown. As seen with bone marrow chimera studies [43] type I IFN production by epithelial cells is important. Interferon-induced cytokines from epithelial cells could directly effect the lung or manipulate other cells types such as DCs. Epithelial cells produce an array of cytokines and chemokines (eg GM-CSF, IL-1β, thymic stromal lymphopoietin) that recruit DCs and change their activation and ability to uptake antigen [52, 53].
Many inhaled pathogens are entrapped in mucus and if not efficiently cleared may replicate. Even if pathogens do not gain access to the epithelial surface, they can lyse and shed surface components, PAMPs, which can activate type I IFN signaling. The nature of the PAMP depends upon the characteristics of the infecting organism. For example, pneumococci often colonize the upper respiratory tract but can be aspirated and initiate pneumonia. During periods of rapid proliferation pneumococci undergo autolysis releasing intracellular components such as peptidoglycan [54, 55] . Gram negative organisms, such as P. aeruginosa, spontaneously shed the major PAMP LPS during growth. S. aureus, a cause of severe pneumonia particularly following influenza, produce large amounts of surface proteins, such as protein A during active growth, which gets released into the airways and functions as an immunostimulant [56, 57]. Thus, depending upon the nature of the infection, common bacterial pathogens release PAMPS that can activate both NF-κB mediated signaling, but also can stimulate the type I IFN cascade-with important consequences for the host.
Streptococcus pneumoniae
S. pneumoniae the most common cause of acute bacterial pneumonia activates type I IFN signaling at multiple sites in the respiratory tract [58, 59]. In nasal associated lymphoid tissue Ifnb along with transcripts encoding STAT1 and STAT2 are induced by pneumococcus [58], while in the lung Ifnb along with phosphorylation of STAT1 and 3 as well as type I dependent genes such as Mx1 and PKR are induced [59].
Type I IFN signaling appears to play a protective role against S. pneumoniae. One of the earliest studies investigating the role of IFN-β in bacterial infections [60] observed that giving mice anti IFN-β antibody increased death as a result of S. pneumoniae infection. In a colonization model Ifnar-/- mice displayed increased carriage of S. pneumoniae [59]. This is an important observation given that the nasopharynx is the first site for colonization by these organisms. S. pneumoniae is also a significant cause of bacterial pneumonia post influenza infection. After a five day infection (intratracheal inoculation) of S. pneumoniae of wild-type and Ifnar-/- mice equivalent concentrations of S. pneumoniae were observed in their lungs. However, previously influenza infected mice had significantly higher bacterial counts in the lung that was potentiated by type I IFNs [61]. The increased bacterial loads in co-infected mice was apparently associated with diminished neutrophil responses. These studies highlight the differing roles played by type I IFN signaling in various body sites.
As type I IFN signaling appears to be protective in murine models of respiratory infection, the bacterial components responsible for activating this response were investigated. The cholesterol dependent cytolysin of S. pneumoniae, pneumolysin (Ply), a highly conserved virulence factor critical for virulence, was found to be required for activation of type I IFN signaling. In nasal epithelial cells a time dependent induction of Ifnb is observed in response to S. pneumoniae, but not with a pneumolysin mutant [59]. A similar requirement for Ply is observed in vivo in the lungs of mice with significantly reduced Ifnb activation and downstream genes. A different study [58] did not observe an absolute requirement, but also commented on the role of Ply in type I IFN induction in the nasopharynx. It is the pore of Ply that is required for activation and not the protein itself. S. pneumoniae strains expressing a defective pore do not complement the phenotype as well and purified, endotoxin free Ply does not act as a ligand for Ifnb activation. Indicative of a bacterial product utilizing the Ply pore to activate signaling, Ply was observed on the surface of epithelial cells and the activation of Ifnb was not dependent on bacterial uptake [59].
While the Ply pore is necessary for type I IFN induction, pneumococcal DNA activates type I IFN signaling via cytosolic DNA receptors. Several lines of evidence support the hypothesis that DNA is a ligand: a mutant in the autolysin gene lytA that does not lyse and release cellular components does not activate type I IFN signaling. Treatment of cell lysates with DNase reduced Ifnb induction and 16S rRNA levels were decreased inside cells that were stimulated with the ply strain. Although the typical receptors and adaptors involved in type I IFN activation were not involved in the response to pneumococcus in the lung, the DNA sensor DAI was observed to be upregulated in lung tissue. Pneumococcal DNA was sensed by DAI and STING, TBK1 and IRF3 [59]. Incubation of S. pneumoniae with DAI and STING null cells induced significantly less Ifnb production than wild-type cells. S. pneumoniae is thus an example of how an extracellular organism can activate an intracellular pathway via a pore-forming toxin that potentially allows ligands to enter and activate signaling.
Pseudomonas aeruginosa
P. aeruginosa is a common Gram negative opportunist, a major cause of hospital associated pneumonia and infection in cystic fibrosis (CF) patients. The ability of P. aeruginosa to activate type I IFN signaling has been demonstrated both in vivo as well as in vitro [62]. As the P. aeruginosa cell wall contains LPS, which is actively shed during bacterial growth, it is not surprising that the TLR4 signaling complex (TLR4, TRIF, MD2, CD14), along with the necessary downstream components (TBK1, IRF3) [62], are all required to initiate type I IFN signaling. P. aeruginosa is an example of how the immune system has mechanisms in place to sense components of bacterial cell walls.
Components of the type I IFN pathway in response to pulmonary infection with P. aeruginosa have been examined in vivo. Mice lacking TLR4, TRIF and IRF3 have all shown a reduction in the ability to clear pulmonary infection. Although two studies that have examined the role of TLR4 in clearance of acute infections showed no difference in bacterial clearance [63, 64], the cytokine response of Tlr4-/- mice to P. aeruginosa was significantly reduced for TNF and KC as well as the type I IFN regulated RANTES. Technical issues such as strain differences might explain the discrepancies between these studies and work [65] showing TLR4 null mice less capable of clearing infection.
Similar defects in P. aeruginosa clearance were also observed in TRIF and IRF3 null mice with decreased RANTES production [66, 67]. Analogous to the Trif-/- data, Irf3-/- mice had reduced IFN-β-dependent products, such as CXCL10 in response to P. aeruginosa, while TNF, KC and IL-1β that are not as dependent upon type I signaling were unaffected. A major cellular consequence of TRIF deficiency was reduced KC production leading to delayed neutrophil recruitment. This KC response of TRIF null mice to P. aeruginosa undoubtedly contributed to the impaired clearance (7-fold higher) of organism from the lung. The delayed clearance in Trif-/- mice was evident at 24 and 48 hours before all the organisms were cleared by the host, highlighting the importance of TRIF in acute infection. As TRIF can lead to NF-κB activation via RIP1 [68] the clearance effects seen in mice cannot be entirely contributed to type I IFN signaling, as deficiency of IRF3 also led to reduced clearance (3-fold higher) of P. aeruginosa from the lungs of IRF3 deficient mice [67]. This clearance defect was most apparent at 24 h. Analysis of lung tissue showed significant levels of Ifnb transcription and IFN-β production that were entirely dependent on the presence of IRF3. Although not observing a reduction in KC, the Irf3-/- mice, like their TRIF counterparts, also showed reduced neutrophil recruitment in response to infection, as well as stunted macrophage recruitment, indicating the clearance defect was attributable to the reduced neutrophil and/or macrophage presence in the lung. The data from TLR4, TRIF and IRF3 null mice are all consistent with a protective role for type I IFN signaling against P. aeruginosa infection.
The importance of type I IFN signaling in the host response to infection was corroborated in a study utilizing a gain of function model [62]. Mice previously instilled with poly(I:C) (which stimulates type I IFN through TLR3) had increased ability to clear P. aeruginosa from the lung and the spleen. Pre-treatment with poly(I:C) was found to increase the level of the macrophage DC activation marker CD86.
The role of epithelial type I IFN signaling has been examined in the context of CF. CF epithelial cell lines (IB3 and CFBE) compared to corrected wild-type lines (C38 and CFBE with WT CFTR) show a significant reduction in type I IFN signaling. Levels of Ifnb, phosphorylation of STAT1 as well as transcription and secretion of type I-dependent gene products such as CXCL10, RANTES and PKR are all significantly reduced. This is in contrast to NF-κB-dependent products such as IL-6 or CXCL8 that are comparable between CF and non-CF epithelial cells. This CF epithelial defect also appears to be specific to TLR4-induced production of type I IFNs. Stimulation of WT and CF cells with the TLR3 ligand poly(I:C) induced Ifnb and phosphorylation of IRF3 and STAT1 comparably, in contrast to the significant differences observed when stimulated with P. aeruginosa or LPS [62]. This type I IFN defect in CF epithelial cells is interesting in the context of natural infection. P. aeruginosa isolates from CF patients have been shown to have altered LPS structures (such as increased acylation) that show altered capacity to activate inflammatory products [69, 70]. Isolates obtained from the same patient were found to have diminished capacity to stimulate NF-kB dependent responses over time [70]. It remains to be determined what effect these LPS modifications have on the activation of type I IFN signaling and if this contributes to chronic infection.
Type I IFN signaling from epithelial cells influences phagocytic cell activation in the context of P. aeruginosa infection [62]. The increased induction of IFN-β from wild-type epithelial compared to CF cells was associated with increased activation of phagocytes, highlighting the importance of epithelial type I IFN production in influencing major immune cells types.
Staphylococcus aureus
S. aureus is a major cause of pneumonia both as a hospital-associated infection and in the community particularly after influenza infection, a condition associated with abundant type I IFN responses [71-73]. Unlike the previously described organisms, type I IFN signaling appears to play a negative role in S. aureus pneumonia. When Ifnar-/- mice were infected with S. aureus, significantly reduced mortality was observed as compared to wild-type mice [71]. This is likely a result of an over-exuberant host response. S. aureus infected Ifnar-/- mice at early time points showed significantly less TNF as well as decreased IL-6. It has also been shown that type I IFNs stimulate TNF, which contributes substantially to the pathophysiology of septic shock [74]. It is possible that this excessive inflammatory response is a result of DC-T-cell signaling. S. aureus infected Ifnar-/- mice had elevated DC counts [71], which were associated with potentially damaging chemokine production.
In S. aureus models of pneumonia, depletion of macrophages resulted in increased DC numbers and enhanced production of the CXCR3 chemokine IP-10/CXCL10 [48], a known T-cell chemoattractant. Blockade of CXCR3 decreased both CD4+ cells in bronchoalveolar lavage fluid and substantially reduced lung pathology in response to S. aureus [48, 75].
In vivo models of secondary pneumonia post-influenza infection show increased lung damage and bacterial densities consistent with human disease [76]. Co-infection with both S. aureus and influenza generates increased concentrations of type I IFNs that can inhibit Th17 responses [77]. Consistent with the involvement of IFNAR in host damage [71] co-infected Ifnar-/- mice with S. aureus and influenza showed improved clearance of S. aureus from the lung [77].
The initiation of this damaging type I IFN response to S. aureus likely originates at the epithelium. Incubation of airway epithelial cells with S. aureus induces significant production of the type I IFN pathway [71]. Transcription of Ifnb is observed as early as 2 h in epithelial cells as well as phosphorylation of STAT transcription factors and type I dependent gene products such as Mx1 and LIF. In airway cells the staphylococcal surface virulence factor protein A appears to be involved. Lung sections of mice infected with a protein A null strain of S. aureus show reduced activation of STAT3, which can be induced via IL-6 [78]. Protein A is taken up by epithelial cells (inhibitable by cytochalasin D) as it is abundantly shed by organisms during growth and can activate signaling through TNFR1 and IRF1, which can be activated by TNF. The role of IRF1 in type I IFN signaling has gained prominence most recently after it was shown to be involved in TLR9 signaling in myeloid DCs [79]. The cell wall component, lipoteichoic acid has also been suggested to activate IRF1 and STAT1 to induce type I IFN signaling [80, 81]. Other virulence factors such as α-toxin may also be involved in activating immune cells via a mechanism analogous to that described for the pore-forming beta-hemolysin/cytolysin of group B Streptococcus [82]. As S. aureus is notorious for its multiple interactions of host immune effectors, it is likely that numerous staphylococcal PAMPs contribute to this deleterious type I IFN response.
The precise factors involved in orchestrating the protective and sensitizing effects of type I IFNs are still largely unknown. Type I IFNs could effect on processes that affect bacterial clearance including: cell migration, apoptosis, iNOS expression and secretion of chemokines [4]. One study that investigated the effect of IFN on gastrointestinal epithelial cells noted that interferon reduced the ability for organisms to invade cells [83]. Applicable to the sensitizing effect observed with S. aureus, in Listeria, interferon results in increased apoptosis of lymphocytes [84].
The three organisms discussed in this review are not the only extracellular respiratory pathogens to elicit type I IFNs. Type I IFN signaling plays an important role against Streptococcus pyogenes or group A streptococcus (GAS) in a model of cellulitis [85]. The mechanism of type I IFN activation by GAS has been examined in macrophages and dendritic cells. Macrophages sense GAS DNA and signal via STING, TBK1 and IRF3. In contrast, DCs sense GAS RNA and signal via MyD88 and IRF5 [85]. Group B streptococcus (GBS) (S. agalactiae) has also been investigated in multiple cells types. In macrophages, DNA from GBS appears to be a major ligand and requires TBK1 and IRF3 [82, 86]. The hemolysin of GBS, not unlike Ply from S. pneumoniae is a virulence factor and is also required for induction of type I IFN signaling. GBS ssRNA has also been reported to activate type I IFN through the MyD88-dependent pathway in macrophages [87]. In DCs GBS activates type I IFNs through TLR7, Myd88 and IRF1 [86]. Infection of Ifnar-/- mice (intraperitoneal and subcutaneous routes) with GBS results in significantly increased mortality [88]. Lastly, Haemophilus influenzae has been examined in the context of otitis media. H. influenzae is able to activate downstream genes of type I IFN signaling and organisms have increased persistence in a TRIF null animal [89]. However, what remains to be examined with this group of organisms is determining the role type I IFN signaling plays in their clearance from the lung and the contribution of epithelial cells in this process (Box 1).
Concluding remarks
With the recognition that the mucosal epithelium is an active participant in innate immune signaling, it should not be surprising that these cells contribute substantially to the type I IFN cascade. We would expect that type I IFN responses are likely induced by most of the organisms which cause pneumonia by inhalation and are recognized by epithelial cells. However, the type I IFN responses elicited by the organisms discussed in this review are very different: ranging from protective responses induced by S. pneumoniae that might be targets therapeutically to prevent infection versus the responses induced by S. aureus that caused excessive mortality, at least in a mouse model. While it has been useful to categorize innate immune responses to organisms that have primarily intracellular versus extracellular lifestyles, it is apparent that this distinction, at least in the airway epithelium, is blurred. Instead, these are highly conserved mechanisms to deal with the presence of PAMPs-regardless of the source of these stimuli. More important is likely the net effect of the multiple PAMPs expressed by a single pathogen and virulence components that facilitate their entry into the epithelium.
Box 1. Outstanding questions.
What are the host factors regulated by type I IFN production that induce protective and sensitizing phenotypes in response to bacterial infections?
Identification of additional bacterial components that can activate type I IFN production
The role of type I IFN receptors in sensing bacteria in airway epithelial cells
The contribution of type I IFN signaling in the airway epithelium to overall host outcome
ACKNOWLEDGEMENTS
DP was supported by an NHMRC Biomedical Overseas Fellowship. The type I IFN studies in the laboratory was supported by NIH grant 1R21AI083491 to AP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Monroe KM, et al. Induction of type I interferons by bacteria. Cell Microbiol. 2010;12:881–890. doi: 10.1111/j.1462-5822.2010.01478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodward JJ, et al. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science. 2010;328:1703–1705. doi: 10.1126/science.1189801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trinchieri G. Type I interferon: friend or foe? J Exp Med. 2010;207:2053–2063. doi: 10.1084/jem.20101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Decker T, et al. The yin and yang of type I interferon activity in bacterial infection. Nat Rev Immunol. 2005;5:675–687. doi: 10.1038/nri1684. [DOI] [PubMed] [Google Scholar]
- 5.Muller U, et al. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 6.Staeheli P, et al. Influenza virus-susceptible mice carry Mx genes with a large deletion or a nonsense mutation. Mol Cell Biol. 1988;8:4518–4523. doi: 10.1128/mcb.8.10.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Debes GF, et al. Chemotactic responses of IL-4-, IL-10-, and IFN-gamma-producing CD4+ T cells depend on tissue origin and microbial stimulus. J Immunol. 2006;176:557–566. doi: 10.4049/jimmunol.176.1.557. [DOI] [PubMed] [Google Scholar]
- 8.Der SD, et al. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc Natl Acad Sci U S A. 1998;95:15623–15628. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kagan JC, et al. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hacker H, et al. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature. 2006;439:204–207. doi: 10.1038/nature04369. [DOI] [PubMed] [Google Scholar]
- 11.Schindler C, Plumlee C. Inteferons pen the JAK-STAT pathway. Semin Cell Dev Biol. 2008;19:311–318. doi: 10.1016/j.semcdb.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benveniste EN, Qin H. Type I interferons as anti-inflammatory mediators. Sci STKE. 2007;2007:e70. doi: 10.1126/stke.4162007pe70. [DOI] [PubMed] [Google Scholar]
- 13.Kawai T, et al. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J Immunol. 2001;167:5887–5894. doi: 10.4049/jimmunol.167.10.5887. [DOI] [PubMed] [Google Scholar]
- 14.Yu H, et al. Innate lung defenses and compromised Pseudomonas aeruginosa clearance in the malnourished mouse model of respiratory infections in cystic fibrosis. Infect Immun. 2000;68:2142–2147. doi: 10.1128/iai.68.4.2142-2147.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber SE, et al. CD8+ cells enhance resistance to pulmonary serotype 3 Streptococcus pneumoniae infection in mice. J Immunol. 2011;186:432–442. doi: 10.4049/jimmunol.1001963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satorres SE, et al. IFN-gamma plays a detrimental role in murine defense against nasal colonization of Staphylococcus aureus. Immunol Lett. 2009;123:185–188. doi: 10.1016/j.imlet.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Mordstein M, et al. Interferon-lambda contributes to innate immunity of mice against influenza A virus but not against hepatotropic viruses. PLoS Pathog. 2008;4:e1000151. doi: 10.1371/journal.ppat.1000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32:305–315. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Parker D, Prince A. Innate Immunity in the Respiratory Epithelium. Am J Respir Cell Mol Biol. 2011 doi: 10.1165/rcmb.2011-0011RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 21.Lenardo MJ, et al. The involvement of NF-kappa B in beta-interferon gene regulation reveals its role as widely inducible mediator of signal transduction. Cell. 1989;57:287–294. doi: 10.1016/0092-8674(89)90966-5. [DOI] [PubMed] [Google Scholar]
- 22.Sato M, et al. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity. 2000;13:539–548. doi: 10.1016/s1074-7613(00)00053-4. [DOI] [PubMed] [Google Scholar]
- 23.Fitzgerald KA, et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 24.Ishikawa H, et al. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexopoulou L, et al. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 26.Poltorak A, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 27.Diebold SS, et al. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 28.Heil F, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 29.Hemmi H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 30.Takaoka A, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 31.Unterholzner L, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ablasser A, et al. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat Immunol. 2009;10:1065–1072. doi: 10.1038/ni.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiu YH, et al. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell. 2009;138:576–591. doi: 10.1016/j.cell.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang P, et al. The cytosolic nucleic acid sensor LRRFIP1 mediates the production of type I interferon via a beta-catenin-dependent pathway. Nat Immunol. 2010;11:487–494. doi: 10.1038/ni.1876. [DOI] [PubMed] [Google Scholar]
- 35.Pandey AK, et al. NOD2, RIP2 and IRF5 play a critical role in the type I interferon response to Mycobacterium tuberculosis. PLoS Pathog. 2009;5:e1000500. doi: 10.1371/journal.ppat.1000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sabbah A, et al. Activation of innate immune antiviral responses by Nod2. Nat Immunol. 2009;10:1073–1080. doi: 10.1038/ni.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leber JH, et al. Distinct TLR- and NLR-mediated transcriptional responses to an intracellular pathogen. PLoS Pathog. 2008;4:e6. doi: 10.1371/journal.ppat.0040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franks TJ, et al. Resident cellular components of the human lung: current knowledge and goals for research on cell phenotyping and function. Proc Am Thorac Soc. 2008;5:763–766. doi: 10.1513/pats.200803-025HR. [DOI] [PubMed] [Google Scholar]
- 39.Mayer AK, et al. Differential recognition of TLR-dependent microbial ligands in human bronchial epithelial cells. J Immunol. 2007;178:3134–3142. doi: 10.4049/jimmunol.178.5.3134. [DOI] [PubMed] [Google Scholar]
- 40.Muir A, et al. Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol. 2004;30:777–783. doi: 10.1165/rcmb.2003-0329OC. [DOI] [PubMed] [Google Scholar]
- 41.Berube J, et al. Distinct intracellular signaling pathways control the synthesis of IL-8 and RANTES in TLR1/TLR2, TLR3 or NOD1 activated human airway epithelial cells. Cell Signal. 2009;21:448–456. doi: 10.1016/j.cellsig.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 42.Platz J, et al. Microbial DNA induces a host defense reaction of human respiratory epithelial cells. J Immunol. 2004;173:1219–1223. doi: 10.4049/jimmunol.173.2.1219. [DOI] [PubMed] [Google Scholar]
- 43.Shornick LP, et al. Airway epithelial versus immune cell Stat1 function for innate defense against respiratory viral infection. J Immunol. 2008;180:3319–3328. doi: 10.4049/jimmunol.180.5.3319. [DOI] [PubMed] [Google Scholar]
- 44.Xing Z, et al. Host immune and apoptotic responses to avian influenza virus H9N2 in human tracheobronchial epithelial cells. Am J Respir Cell Mol Biol. 2010;44:24–33. doi: 10.1165/rcmb.2009-0120OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Q, et al. Role of double-stranded RNA pattern recognition receptors in rhinovirus-induced airway epithelial cell responses. J Immunol. 2009;183:6989–6997. doi: 10.4049/jimmunol.0901386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ritter M, et al. Characterization of Toll-like receptors in primary lung epithelial cells: strong impact of the TLR3 ligand poly(I:C) on the regulation of Toll-like receptors, adaptor proteins and inflammatory response. J Inflamm (Lond) 2005;2:16. doi: 10.1186/1476-9255-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 48.Martin FJ, et al. Participation of CD11c+ leukocytes in the host response to MRSA infection of the lung. Infect Immun. 2011;79:1898–1904. doi: 10.1128/IAI.01299-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Z, et al. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest. 2009;119:1899–1909. doi: 10.1172/JCI36731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kooguchi K, et al. Role of alveolar macrophages in initiation and regulation of inflammation in Pseudomonas aeruginosa pneumonia. Infect Immun. 1998;66:3164–3169. doi: 10.1128/iai.66.7.3164-3169.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheung DO, et al. Role of pulmonary alveolar macrophages in defense of the lung against Pseudomonas aeruginosa. Infect Immun. 2000;68:4585–4592. doi: 10.1128/iai.68.8.4585-4592.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rate A, et al. Airway epithelial cells regulate the functional phenotype of locally differentiating dendritic cells: implications for the pathogenesis of infectious and allergic airway disease. J Immunol. 2009;182:72–83. doi: 10.4049/jimmunol.182.1.72. [DOI] [PubMed] [Google Scholar]
- 53.Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol. 2008;8:193–204. doi: 10.1038/nri2275. [DOI] [PubMed] [Google Scholar]
- 54.Kadioglu A, et al. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol. 2008;6:288–301. doi: 10.1038/nrmicro1871. [DOI] [PubMed] [Google Scholar]
- 55.Chetty C, Kreger A. Role of autolysin in generating the pneumococcal purpura-producing principle. Infect Immun. 1981;31:339–344. doi: 10.1128/iai.31.1.339-344.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Movitz J. Formation of extracellular protein A by Staphylococcus aureus. Eur J Biochem. 1976;68:291–299. doi: 10.1111/j.1432-1033.1976.tb10788.x. [DOI] [PubMed] [Google Scholar]
- 57.Gomez MI, et al. Staphylococcus aureus protein A induces airway epithelial inflammatory responses by activating TNFR1. Nat Med. 2004;10:842–848. doi: 10.1038/nm1079. [DOI] [PubMed] [Google Scholar]
- 58.Joyce EA, et al. Streptococcus pneumoniae nasopharyngeal colonization induces type I interferons and interferon-induced gene expression. BMC Genomics. 2009;10:404. doi: 10.1186/1471-2164-10-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parker D, et al. Streptococcus pneumoniae DNA Initiates Type I Interferon Signaling in the Respiratory Tract. MBio. 2011;2:e00016–00011. doi: 10.1128/mBio.00016-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weigent DA, et al. Role of interferon in streptococcal infection in the mouse. Microb Pathog. 1986;1:399–407. doi: 10.1016/0882-4010(86)90071-9. [DOI] [PubMed] [Google Scholar]
- 61.Shahangian A, et al. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J Clin Invest. 2009;119:1910–1920. doi: 10.1172/JCI35412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parker D, et al. Induction of Type I Interferon Signaling by Pseudomonas aeruginosa is Diminished in Cystic Fibrosis Epithelial Cells. Am J Respir Cell Mol Biol. 2011 doi: 10.1165/rcmb.2011-0080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Skerrett SJ, et al. Redundant Toll-like receptor signaling in the pulmonary host response to Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol. 2007;292:L312–322. doi: 10.1152/ajplung.00250.2006. [DOI] [PubMed] [Google Scholar]
- 64.Ramphal R, et al. TLRs 2 and 4 are not involved in hypersusceptibility to acute Pseudomonas aeruginosa lung infections. J Immunol. 2005;175:3927–3934. doi: 10.4049/jimmunol.175.6.3927. [DOI] [PubMed] [Google Scholar]
- 65.Faure K, et al. TLR4 signaling is essential for survival in acute lung injury induced by virulent Pseudomonas aeruginosa secreting type III secretory toxins. Respir Res. 2004;5:1. doi: 10.1186/1465-9921-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Power MR, et al. A role of Toll-IL-1 receptor domain-containing adaptor-inducing IFN-beta in the host response to Pseudomonas aeruginosa lung infection in mice. J Immunol. 2007;178:3170–3176. doi: 10.4049/jimmunol.178.5.3170. [DOI] [PubMed] [Google Scholar]
- 67.Carrigan SO, et al. IFN regulatory factor 3 contributes to the host response during Pseudomonas aeruginosa lung infection in mice. J Immunol. 2010;185:3602–3609. doi: 10.4049/jimmunol.0903429. [DOI] [PubMed] [Google Scholar]
- 68.Meylan E, et al. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol. 2004;5:503–507. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]
- 69.Ernst RK, et al. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. Science. 1999;286:1561–1565. doi: 10.1126/science.286.5444.1561. [DOI] [PubMed] [Google Scholar]
- 70.Cigana C, et al. Pseudomonas aeruginosa exploits lipid A and muropeptides modification as a strategy to lower innate immunity during cystic fibrosis lung infection. PLoS One. 2009;4:e8439. doi: 10.1371/journal.pone.0008439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martin FJ, et al. Staphylococcus aureus activates type I IFN signaling in mice and humans through the Xr repeated sequences of protein A. J Clin Invest. 2009;119:1931–1939. doi: 10.1172/JCI35879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klevens RM, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 73.Hageman JC, et al. Severe community-acquired pneumonia due to Staphylococcus aureus, 2003-04 influenza season. Emerg Infect Dis. 2006;12:894–899. doi: 10.3201/eid1206.051141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huys L, et al. Type I interferon drives tumor necrosis factor-induced lethal shock. J Exp Med. 2009;206:1873–1882. doi: 10.1084/jem.20090213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Manicone AM, et al. CXCR3 ligands contribute to Th1-induced inflammation but not to homing of Th1 cells into the lung. Exp Lung Res. 2008;34:391–407. doi: 10.1080/01902140802221987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee MH, et al. A postinfluenza model of Staphylococcus aureus pneumonia. J Infect Dis. 2010;201:508–515. doi: 10.1086/650204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kudva A, et al. Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice. J Immunol. 2011;186:1666–1674. doi: 10.4049/jimmunol.1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hirano T, et al. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548–2556. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- 79.Schmitz F, et al. Interferon-regulatory-factor 1 controls Toll-like receptor 9-mediated IFN-beta production in myeloid dendritic cells. Eur J Immunol. 2007;37:315–327. doi: 10.1002/eji.200636767. [DOI] [PubMed] [Google Scholar]
- 80.Dietrich N, et al. Murine toll-like receptor 2 activation induces type I interferon responses from endolysosomal compartments. PLoS One. 2010;5:e10250. doi: 10.1371/journal.pone.0010250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liljeroos M, et al. Bacterial ligand of TLR2 signals Stat activation via induction of IRF1/2 and interferon-alpha production. Cell Signal. 2008;20:1873–1881. doi: 10.1016/j.cellsig.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 82.Charrel-Dennis M, et al. TLR-independent type I interferon induction in response to an extracellular bacterial pathogen via intracellular recognition of its DNA. Cell Host Microbe. 2008;4:543–554. doi: 10.1016/j.chom.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bukholm G, et al. Mouse fibroblast interferon modifies Salmonella typhimurium infection in infant mice. Infect Immun. 1984;45:62–66. doi: 10.1128/iai.45.1.62-66.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carrero JA, et al. Type I interferon sensitizes lymphocytes to apoptosis and reduces resistance to Listeria infection. J Exp Med. 2004;200:535–540. doi: 10.1084/jem.20040769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gratz N, et al. Type I Interferon Production Induced by Streptococcus pyogenes-Derived Nucleic Acids Is Required for Host Protection. PLoS Pathog. 2011;7:e1001345. doi: 10.1371/journal.ppat.1001345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mancuso G, et al. Bacterial recognition by TLR7 in the lysosomes of conventional dendritic cells. Nat Immunol. 2009;10:587–594. doi: 10.1038/ni.1733. [DOI] [PubMed] [Google Scholar]
- 87.Deshmukh SD, et al. Macrophages recognize streptococci through bacterial single-stranded RNA. EMBO Rep. 2011;12:71–76. doi: 10.1038/embor.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mancuso G, et al. Type I IFN signaling is crucial for host resistance against different species of pathogenic bacteria. J Immunol. 2007;178:3126–3133. doi: 10.4049/jimmunol.178.5.3126. [DOI] [PubMed] [Google Scholar]
- 89.Leichtle A, et al. The toll-Like receptor adaptor TRIF contributes to otitis media pathogenesis and recovery. BMC Immunol. 2009;10:45. doi: 10.1186/1471-2172-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]