Abstract
Growing evidence suggests that adult-born granule cells integrate into hippocampal networks and are required for proper cognitive function. Although neuroinflammation is involved in many disorders associated with cognitive impairment, it remains unknown whether it impacts the recruitment of adult-born neurons into behaviorally relevant hippocampal networks. Under similar behavioral conditions, exploration-induced expression of the immediate-early gene Arc in hippocampal cells has been linked to cellular activity observed by electrophysiological recording. By detecting exploration-induced Arc protein expression, we investigated whether neuroinflammation alters the recruitment of adult-born neurons into behaviorally relevant hippocampal networks. Neuroinflammation was induced in rats by intra-cerebroventricular infusion of lipopolysaccharide for 28 days. Animals received bromodeoxyuridine injections starting on day 29 (five days) and were euthanized two months later. Persistent lipopolysaccharide-induced neuroinflammation was reliably detected by microglial activation in the hippocampus. Neuroinflammation did not impact the number of adult-born neurons but did alter their migration pattern through the granule cell layer. There was a positive correlation between the density of activated microglia and alterations in the fraction of existing granule neurons expressing Arc, suggesting that neuroinflammation induced a long-term disruption of hippocampal network activity. The proportion of adult-born neurons expressing behaviorally induced Arc was significantly lower in lipopolysaccharide-treated rats than in controls. This observation supports the fact that neuroinflammation significantly impacts adult-born neurons recruitment into hippocampal networks encoding spatial information.
Keywords: adult neurogenesis, immediate early gene, Arc, hippocampus, immediate-early gene, inflammation, synaptic plasticity
1. Introduction
The subgranular zone of the dentate gyrus (DG) is one of the brain regions where robust neurogenesis continues throughout adulthood (Altman and Das, 1965). New neurons migrate into the granule cell layer, develop dendritic arborization and display passive membrane properties, action potentials and functional synaptic inputs similar to those found in mature dentate granule cells (Toni et al., 2008; van Praag et al., 2002). Growing evidence supports the findings that adult-born neurons integrate into hippocampal networks (Kee et al., 2007; Ramirez-Amaya et al., 2006) and are necessary for hippocampal functions, including spatial learning (Goodman et al., 2010; Jessberger et al., 2009).
Numerous evidences suggest that neuroinflammation plays a crucial role in the development and progression of neurodegenerative, neuropsychiatric and viral illnesses including Alzheimer's disease, Parkinson's disease, depression and human immunodeficiency associated dementia (Cagnin et al., 2001; Garden, 2002; McGeer et al., 1988; Miller et al., 2009; Rao et al., 2010). Microglia are the resident immune cells of the brain parenchyma and play a central role in neuroinflammation (Barger and Basile, 2001). Depending on the specific conditions, neuroinflammation can either enhance or suppress neurogenesis (Butovsky et al., 2006). Although previous observations have revealed that neuroinflammation influences the electrophysiological properties of adult-born neurons (Jakubs et al., 2006; Jakubs et al., 2008), it remains unknown whether neuroinflammation affects their functional recruitment into behaviorally relevant hippocampal networks.
The plasticity-related immediate-early gene Arc (activity-regulated cytoskeleton-associated protein) and its protein product are induced in hippocampal neurons following spatial exploration in percentages similar to those recorded electrophysiologically (Guzowski et al., 1999; Ramirez-Amaya et al., 2005; Rosi et al., 2009), Arc is required for engaging durable plasticity processes that underlie memory consolidation (Guzowski et al., 2000; Lyford et al., 1995). The expression of behaviorally-induced Arc can be used to study the recruitment of adult-born mature neurons into functional neural networks (Ramirez-Amaya et al., 2006).
The present study was designed to examine the impact of chronic neuroinflammation on the functional integration of adult-born neurons into hippocampal networks that mediate spatial and contextual information processing.
2. Methods
2.1 Animal procedures
This research was conducted under the supervision and with the approval of the University of California Institutional Animal Care and Use Committee. Fifteen 3-month-old male F344 rats (Charles River Labs) living in an inverted 12 hour light/dark cycle (lights off at 8 a.m. and on at 8 p.m.) were individually caged with food and water freely available. Throughout the duration of the study, all animals were handled and given forage mix (dried fruit, seeds and pellets) three times a week. Surgeries were performed as previously described in details (Rosi et al., 2005); each rat was anesthetized with isoflurane gas and placed in a stereotaxic instrument with the incisor bar set 3.0 mm below the ear bars. The scalp was incised and retracted and a hole was made at the appropriate location in the skull with a dental drill. A chronic indwelling cannula was inserted into the 4th ventricle. Coordinates for the 4th ventricle infusions were as follows: 2.5 mm posterior to lambda, on the mid-line, and 7.0 mm ventral to the dura. An osmotic minipump (Alzet, Palo Alto, CA, model 2004, to deliver 0.25 ul/h; 28-day delivery) was attached via a catheter to a chronic indwelling cannula that had been positioned stereotaxically so that the tip extended to the coordinates given above. Each minipump was prepared to inject either the vehicle artificial cerebrospinal fluid (aCSF, n=5) or lipopolysaccharide (LPS, n=10; Sigma-Aldrich, E.coli, serotype 055:B5, TCA extraction, 1.0 μg/μl dissolved in aCSF). The rats were closely monitored during recovery and kept in an incubator at temperatures ranging from 30–33°C. Body weights were determined daily and general behavior was monitored for seizures. Starting on day 29, all rats received five daily intraperitoneal injections of BrdU (50 mg/kg/day; Sigma-Aldrich). Two months after the last BrdU injection, rats were allowed to explore a novel environment (a 60 × 60 cm open square box with 35 cm–high walls) for five minutes and were euthanized 30 minutes later; the brains were quickly extracted, frozen in isopentane at -70 °C (Ramirez-Amaya et al., 2006; Rosi et al., 2005).
2.2 Histological procedures
Brains were cryosectioned in the coronal plane (20μm) and collected on microscopic slides (Rosi et al., 2005). Sections were selected from the medial portion of the dorsal hippocampus (approximately from 3.2 to 4.00 mm posterior to bregma) and stained for activated microglia (MHC Class II RT1B mouse monoclonal antibody clone OX-6; BD Pharmingen) (Rosi et al., 2005) or mature neurons (NeuN monoclonal antibody clone A60; Chemicon), BrdU (BrdU mouse monoclonal antibody clone BMC9318; Roche Applied Science) and Arc protein (antibody kindly provided by Paul F. Worley laboratory) (Ramirez-Amaya et al., 2006; Rosi et al., 2005).
2.3 Image acquisition and analysis
Unbiased stereology was used to determine the total number of neurons and BrdU-labeled neurons (Gundersen and Jensen, 1987; West, 1990), One out of 6 serial coronal sections stained for NeuN and BrdU was sampled, with a total of 8 sections per brain. Eight z-stack images (1μm optical thickness per plane) from the DG were imaged using a Zeiss Apotome microscope. The area of the DG enclosed blade was measured in each sampled section by tracing its contour with Zeiss AxioVision software. Based on the Cavalieri method (Gundersen and Jensen, 1987; West, 1990), we estimated the reference volume (Vref) of the granule cell layer by multiplying the sum of the areas in the series of sections by the distance between sections (120μm). Neuronal density in the DG enclosed blade was estimated by using the optical dissector method (Gundersen et al., 1988). The counting frame was 60 × 60μm2, with a 8μm depth; nuclei intercepting the uppermost focal plane or intercepting the exclusion lines of the count frames were not counted. The number of neurons was evaluated as the product of Vref and the neuronal density in the sample. The density of OX-6 immunoreactive microglia and the percentage of existing and BrdU-labeled neurons expressing Arc were analyzed from six to ten sections per animal for each endpoint as described previously (Ramirez-Amaya et al., 2006; Rosi et al., 2005; Rosi et al., 2010). The migration of adult-born neurons throughout the enclosed blade of the dentate granule cell layer was analyzed as follows: for each BrdU-labeled neuron, the distance between the center of the nucleus and the subgranular zone (distance of migration = m) and the width of the granule cell layer (width = w) were measured. The index of migration was calculated as im = m / w × 100 (Fig. 1C).
Fig. 1.
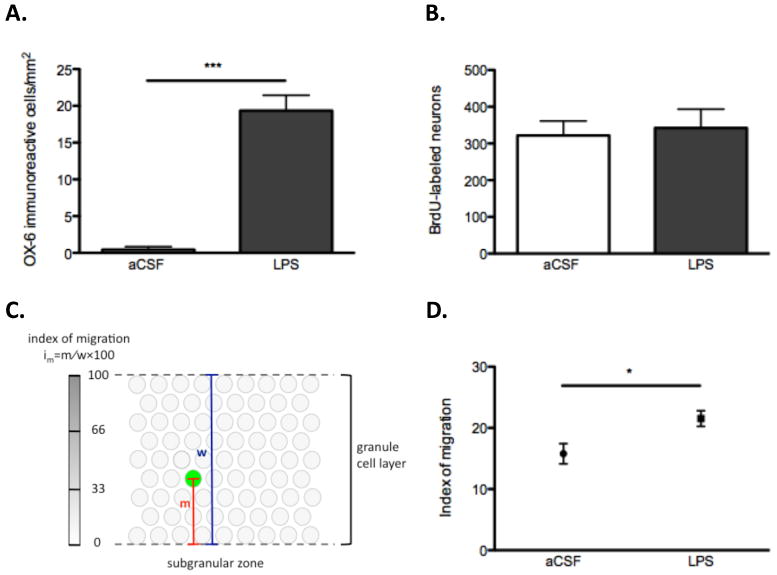
Impact of chronic neuroinflammation on neurogenesis and the migration of adult-born neurons two months after LPS infusion terminated. (A) Numbers of activated microglia (OX-6 immunoreactive) within the DG of aCSF- and LPS-treated rats (***p=0.0001 vs. aCSF). (B) The number of BrdU-labeled neurons in the DG enclosed blade did not significantly differ between aCSF-treated and LPS-treated animals (p=0.7577). (C) Index of migration calculated as im = m / w × 100, “m” is the distance between the center of the nucleus and the subgranular zone; “w” is the width of the granule cell layer. (D) The index of migration was higher in LPS-treated rats than in aCSF-treated rats (*p=0.0192).
2.4 Statistical analysis
Statistical analyses were performed with GraphPad Prism software version 5.0 using a Student's t-test or one-way ANOVA followed by a post-hoc Newman-Keuls multiple comparison test. The correlation between the density of activated microglia and the percentage of Arc immunoreactive neurons in the DG granule cell layer was calculated using Pearson's analysis as previously reported (Rosi et al., 2005). The results are expressed as the mean ± SEM, and p<0.05 was considered significant for all tests.
3. Results
3.1 LPS-infusion induced persistent activation of microglia
Two months after the LPS infusion ended immunofluorescence staining for OX-6 (MHC class II) revealed numerous highly activated microglia distributed primarily within the DG and the CA3 areas of the hippocampus, similar to what had been previously described following 28 days of intraventricular LPS infusion (Rosi et al., 2005; Rosi et al., 2009). Quantitative MHC Class II positive cell count analysis revealed a statistically significant difference in the number of activated microglia per square millimeter in LPS-treated versus aCSF-treated rats in both the DG (19.34 ± 2.12 vs. 0.42 ± 0.42 cells/mm2; p=0.0001; Fig. 1A) and CA3 (21.75 ± 2.04 vs. 0.87 ± 0.50 cells/mm2; p<0.0001; data not shown) areas of the hippocampus.
3.2 Chronic neuroinflammation did not alter the number of adult-born neurons but did affect their migration
Based on previous studies showing that behaviorally-induced Arc expression occurs primarily in the DG enclosed blade (Ramirez-Amaya et al., 2006; Rosi et al., 2005), we exclusively analyzed this subregion. The number of neurons (NeuN+) in the DG enclosed blade did not differ between a-CSF (142905 ± 4540) and LPS animals (158874 ± 18874; p=0.4422). There was no statistical difference in the number of BrdU-labeled neurons (BrdU+ / NeuN+) between aCSF- (321.8 ± 39.78) and LPS-treated animals (342.3 ± 51.27; p=0.7577; Fig. 1B). Migration analysis of adult-born neurons revealed that the average index of migration of BrdU-labeled neurons was higher in LPS-treated rats (21.53 ± 1.28) than in aCSF-treated controls (15.78 ± 1.66; p=0.0192; Fig. 1C, D). DG enclosed blade width was comparable between LPS-treated and aCSF-treated (77.17 ± 1.41μm vs. 73.47 ± 1.73μm; p=0.1661).
3.3 Activated microglia correlated with altered behaviorally-induced Arc expression
By assessing the expression of the plasticity-related immediate early gene Arc in response to spatial exploration, we were able to study the effect of long-lasting chronic neuroinflammation on the activity pattern of granule neurons engaged in spatial information processing. We found a significant correlation between the density of activated microglia and the increase in the percentage of Arc-expressing neurons in the DG of LPS-treated rats, above that of aCSF-treated rats (r=0.7063; p=0.0334; Fig. 2A, B). These data, which were obtained two months after intraventricular LPS-infusion was ended, expand previous reports in which we demonstrated that, after 28 days of LPS-infusion, the number of activated microglia directly correlated with increased numbers of neurons overexpressing Arc in the hippocampus (Rosi et al., 2005).
Fig. 2.
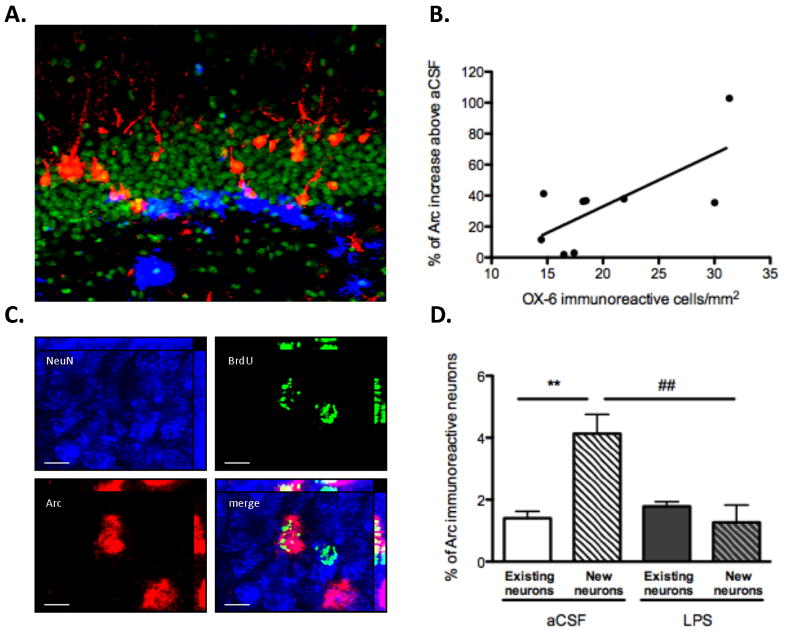
Impact of chronic neuroinflammation on the expression of behaviorally-induced Arc in existing and adult-born neurons measured two months after LPS infusion terminated. (A) Representative image of double immunofluorescence staining for activated microglia (OX-6 positive) (blue) and Arc protein (red) in the DG of an LPS-treated animal. Nuclei were counterstained with SYTOX Green. (B) There was a significant correlation between the density of activated microglia and the percentage increase of exploration-induced Arc in the DG of LPS-treated rats, above that of aCSF-treated rats (r=0.7063; p=0.0334). (C) Triple immunofluorescence staining for NeuN (Blue), BrdU (green) and Arc (red). The white arrow points to a triple-labeled cell (scale = 10 μm). (D) In aCSF-treated rats, the percentage of BrdU-labeled neurons expressing Arc was higher than the percentage of neurons that expressed Arc in the already existing population of granule cells (**p<0.01). In LPS-treated animals, the percentage of BrdU-labeled neurons expressing Arc was not significantly different than that of the already existing population of neurons. The proportion of BrdU-labeled neurons expressing Arc was significantly lower in LPS-treated animals than in aCSF-treated animals (##p<0.01).
3.4 Chronic neuroinflammation altered behaviorally-induced Arc expression in adult-born neurons
To analyze the percentage of adult-born neurons expressing Arc in response to spatial exploration, we performed triple staining for NeuN, Arc and BrdU (Fig. 2C). In aCSF-treated rats, the proportion of BrdU-labeled adult-born neurons expressing Arc (4.13 ± 0.62%) was higher than the proportion of neurons expressing Arc in the already-existing population of granule neurons (1.405 ± 0.22%; p<0.01; Fig. 2D). In LPS-treated rats, the proportion of BrdU-labeled neurons expressing Arc (1.27 ± 0.56%) was not significantly different than that of the already-existing population of neurons (1.781 ± 0.15%; Fig. 2D). Importantly, the proportion of BrdU-labeled neurons that expressed Arc in response to spatial exploration was significantly lower in LPS-treated animals than in aCSF-treated controls (p<0.01; Fig. 2D).
4. Discussion
To our knowledge, the present findings are the first to demonstrate that chronic neuroinflammation impacts the functional integration of adult-born neurons, based on their ability to express the plasticity-related immediate-early-gene Arc in response to spatial exploration.
Chronic neuroinflammation was induced by slow intraventricular infusion of LPS, a component of the outer membrane of Gram-negative bacteria known to selectively activate microglial cells through the stimulation of TLR4/CD14 receptors (Lehnardt et al., 2003). This treatment gave rise to long-lasting hippocampal microglial activation, as evidenced by the increased expression of MHC Class II antigen two months after the infusion was terminated.
Our data suggest that adult-born neurons are generated and survive differentiation during persistent chronic neuroinflammation induced by a slow and low-dosage intraventricular infusion of LPS (Fig. 1B). This observation differs from other reports that showed a detrimental effect of LPS-induced neuroinflammation on basal neurogenesis (Ekdahl et al., 2003; Fujioka and Akema, 2010; Monje et al., 2003). This apparent discrepancy could be attributable to experimental design differences in the dose of LPS delivered (5 μg/h vs. 0.25 μg/h) or its route of administration (intraperitoneal injection vs. intraventricular infusion). Changes in blood-brain barrier permeability induced by LPS treatment could also result in differences of BrdU brain bioavailability between studies (Banks and Erickson, 2010; Taupin, 2007).
In LPS-treated animals, two-month-old adult-born neurons migrated a longer average distance through the enclosed blade of the dentate granule cell layer compared to controls. This and similar observations made in the context of experimental trauma brain injury (Rosi et al., 2010) and experimental stroke (Kernie and Parent, 2010) suggest that the migration patterns of adult-born granule cells change under pathophysiological conditions such as neuroinflammation. The mechanisms underlying this change may involve the differential expression of factors that direct neuronal migration, such as matrix metalloproteases or chemokines released by neurons, activated glia and endothelial cells (Belmadani et al., 2006; Wang et al., 2006). Some insight on this aberrant migration may be gained from studies of the monocyte chemoattractant protein-1 (MCP-1) and its receptor CCR2. MCP-1 synthesis is increased by proinflammatory factors, and CCR2 is expressed by neural progenitors and regulates neuronal migration (Belmadani et al., 2006; Tran et al., 2007). Additionally, the chemokine fractalkine (FKN; CX3CL1) is a neuronally derived signal and the FKN receptor (CXCR3) is expressed exquisitely by microglia (Cardona et al., 2006). The FKN/CX3CR1 signaling pathway could drive an aberrant migration of adult born neurons.
The detection of the plasticity-related IEG Arc represents a reliable method for monitoring cellular activity in hippocampal neurons that is induced by behavior (Rosi et al., 2009; Rosi, 2011). Two month after LPS infusion was stopped, we show a positive correlation between the number of activated microglia and altered fractions of dentate granule cells expressing Arc following spatial exploration. This significant correlation suggests that neuroinflammation leads to a long-term disruption of hippocampal network activity. Based on the hypothesis that spatial information is sparsely encoded in the hippocampus (Jung and McNaughton, 1993), such disruption may significantly contribute to hippocampal-dependent and cognitive impairment-associated neuroinflammation, as previously discussed (Rosi et al., 2005; Rosi et al., 2009).
Several studies have reported that adult-born neurons can be activated by various stimuli, including high-frequency electrical perforant path stimulation (Bruel-Jungerman et al., 2006), Morris water-maze training (Jessberger and Kempermann, 2003; Kee et al., 2007) or spatial exploration (Ramirez-Amaya et al., 2006). Ramirez-Amaya and colleagues found that, in response to spatial exploration, the proportion of five-month-old neurons expressing Arc was higher than that of the already-existing granule neuron population (Ramirez-Amaya et al., 2006). In line with these findings, our study demonstrates that two-month-old adult-born neurons are preferentially activated in response to spatial exploration. These data support the finding that newly generated adult-born neurons are more likely than existing granule neurons to be recruited into hippocampal networks that process recent spatial and contextual information. This preferential recruitment is consistent with a decreased overall threshold for long-term potentiation and a greater excitability in new neurons (Schmidt-Hieber et al., 2004). Furthermore, this finding supports the hypothesis that newly generated adult-born neurons may play a special role in memory processing in the DG.
Our experiment demonstrates that during chronic neuroinflammation, adult-born neurons maintained the ability to express the plasticity-related behaviorally induced protein Arc. However, in LPS-treated animals, the proportion of BrdU-labeled neurons expressing Arc in response to spatial exploration was lower than that of the existing population of granule neurons. In contrast with what was observed in control animals, during chronic neuroinflammation, adult-born neurons were not preferentially recruited into hippocampal networks that encode recent spatial information. Following spatial exploration, the proportion of activated adult-born neurons was significantly lower in LPS-treated versus control animals. Using whole-cell patch clamp recording, Jakubs and colleagues reported a high degree of synaptic plasticity and an increased inhibitory synaptic drive of new neurons that developed under conditions of neuroinflammation (Jakubs et al., 2008). Consistent with those findings our data show decreased induction of Arc expression in the adult-born neurons of LPS-treated animals following spatial exploration. Our study supports the hypothesis that neuroinflammation-induced changes in the functional synaptic plasticity of adult-born neurons impact the recruitment of those neurons into behaviorally relevant hippocampal networks. Given that adult-born neurons are preferentially activated in response to recent behavioral experiences (Kee et al., 2007; Ramirez-Amaya et al., 2006), this disruption may especially impact the encoding of recent spatial and contextual information. In the context of neuroinflammation otherwise marked by an increased hippocampal network activity (i.e., an increased fraction of hippocampal neurons expressing Arc) (Rosi et al., 2005; Rosi et al., 2009), it remains unclear whether this change acts to worsen or mitigate brain dysfunction.
Increasing lines of evidence support the importance of adult hippocampal neurogenesis in learning, memory and mood regulation (Goodman et al., 2010; Perera et al., 2011; Trouche et al., 2009). Chronic neuroinflammation is reliably detected in several neurodegenerative, neuropsychiatric and viral illnesses associated with cognitive impairments (Cagnin et al., 2001; Garden, 2002; McGeer et al., 1988; Miller et al., 2009; Rao et al., 2010). Our data are the first to demonstrate that chronic neuroinflammation impacts the recruitment of adult-born neurons into behaviorally relevant neuronal networks. Additional studies will be needed to elucidate the molecular mechanisms underlying this effect and to clarify its possible implication for cognitive impairments observed in pathological conditions associated with neuroinflammation.
Acknowledgments
Grant Sponsor: Alzheimer's Association, NIH
Grant Number: NIRG 08-90589, R01 CA133216
Footnotes
Neuroinflammation affects the ability of adult-born granule cells to functionally integrate into hippocampal networks necessary for spatial information processing
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Banks WA, Erickson MA. The blood-brain barrier and immune function and dysfunction. Neurobiol Dis. 2010;37:26–32. doi: 10.1016/j.nbd.2009.07.031. [DOI] [PubMed] [Google Scholar]
- Barger SW, Basile AS. Activation of microglia by secreted amyloid precursor protein evokes release of glutamate by cystine exchange and attenuates synaptic function. J Neurochem. 2001;76:846–854. doi: 10.1046/j.1471-4159.2001.00075.x. [DOI] [PubMed] [Google Scholar]
- Belmadani A, Tran PB, Ren D, Miller RJ. Chemokines regulate the migration of neural progenitors to sites of neuroinflammation. J Neurosci. 2006;26:3182–3191. doi: 10.1523/JNEUROSCI.0156-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Davis S, Rampon C, Laroche S. Long-term potentiation enhances neurogenesis in the adult dentate gyrus. J Neurosci. 2006;26:5888–5893. doi: 10.1523/JNEUROSCI.0782-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G, Schwartz M. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Cagnin A, Brooks DJ, Kennedy AM, Gunn RN, Myers R, Turkheimer FE, Jones T, Banati RB. In-vivo measurement of activated microglia in dementia. Lancet. 2001;358:461–467. doi: 10.1016/S0140-6736(01)05625-2. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka H, Akema T. Lipopolysaccharide acutely inhibits proliferation of neural precursor cells in the dentate gyrus in adult rats. Brain Res. 2010;1352:35–42. doi: 10.1016/j.brainres.2010.07.032. [DOI] [PubMed] [Google Scholar]
- Garden GA. Microglia in human immunodeficiency virus-associated neurodegeneration. Glia. 2002;40:240–251. doi: 10.1002/glia.10155. [DOI] [PubMed] [Google Scholar]
- Goodman T, Trouche S, Massou I, Verret L, Zerwas M, Roullet P, Rampon C. Young hippocampal neurons are critical for recent and remote spatial memory in adult mice. Neuroscience. 2010;171:769–778. doi: 10.1016/j.neuroscience.2010.09.047. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubs K, Nanobashvili A, Bonde S, Ekdahl CT, Kokaia Z, Kokaia M, Lindvall O. Environment matters: synaptic properties of neurons born in the epileptic adult brain develop to reduce excitability. Neuron. 2006;52:1047–1059. doi: 10.1016/j.neuron.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Jakubs K, Bonde S, Iosif RE, Ekdahl CT, Kokaia Z, Kokaia M, Lindvall O. Inflammation regulates functional integration of neurons born in adult brain. J Neurosci. 2008;28:12477–12488. doi: 10.1523/JNEUROSCI.3240-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Kempermann G. Adult-born hippocampal neurons mature into activity-dependent responsiveness. Eur J Neurosci. 2003;18:2707–2712. doi: 10.1111/j.1460-9568.2003.02986.x. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr, Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung MW, McNaughton BL. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993;3:165–182. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Kernie SG, Parent JM. Forebrain neurogenesis after focal Ischemic and traumatic brain injury. Neurobiol Dis. 2010;37:267–274. doi: 10.1016/j.nbd.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Perera TD, Dwork AJ, Keegan KA, Thirumangalakudi L, Lipira CM, Joyce N, Lange C, Higley JD, Rosoklija G, Hen R, Sackeim HA, Coplan JD. Necessity of hippocampal neurogenesis for the therapeutic action of antidepressants in adult nonhuman primates. PLoS One. 2011;6:e17600. doi: 10.1371/journal.pone.0017600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Vazdarjanova A, Mikhael D, Rosi S, Worley PF, Barnes CA. Spatial exploration-induced Arc mRNA and protein expression: evidence for selective, network-specific reactivation. J Neurosci. 2005;25:1761–1768. doi: 10.1523/JNEUROSCI.4342-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA. Integration of new neurons into functional neural networks. J Neurosci. 2006;26:12237–12241. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao JS, Harry GJ, Rapoport SI, Kim HW. Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol Psychiatry. 2010;15:384–392. doi: 10.1038/mp.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S, Ramirez-Amaya V, Vazdarjanova A, Worley PF, Barnes CA, Wenk GL. Neuroinflammation alters the hippocampal pattern of behaviorally induced Arc expression. J Neurosci. 2005;25:723–731. doi: 10.1523/JNEUROSCI.4469-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S, Ramirez-Amaya V, Vazdarjanova A, Esparza EE, Larkin PB, Fike JR, Wenk GL, Barnes CA. Accuracy of hippocampal network activity is disrupted by neuroinflammation: rescue by memantine. Brain. 2009;132:2464–2477. doi: 10.1093/brain/awp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S, Belarbi K, Ferguson RA, Fishman K, Obenaus A, Raber J, Fike JR. Trauma-Induced Alterations in Cognition and Arc Expression are Reduced by Previous Exposure to 56Fe Irradiation. Hippocampus. 2010 doi: 10.1002/hipo.20920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S. Neuroinflammation and the plasticity-related immediate-early gene Arc. Brain Behav Immun. 2011 doi: 10.1016/j.bbi.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Rev. 2007;53:198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PB, Banisadr G, Ren D, Chenn A, Miller RJ. Chemokine receptor expression by neural progenitor cells in neurogenic regions of mouse brain. J Comp Neurol. 2007;500:1007–1033. doi: 10.1002/cne.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouche S, Bontempi B, Roullet P, Rampon C. Recruitment of adult-generated neurons into functional hippocampal networks contributes to updating and strengthening of spatial memory. Proc Natl Acad Sci U S A. 2009;106:5919–5924. doi: 10.1073/pnas.0811054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhang ZG, Zhang RL, Gregg SR, Hozeska-Solgot A, LeTourneau Y, Wang Y, Chopp M. Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration. J Neurosci. 2006;26:5996–6003. doi: 10.1523/JNEUROSCI.5380-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ. Stereological studies of the hippocampus: a comparison of the hippocampal subdivisions of diverse species including hedgehogs, laboratory rodents, wild mice and men. Prog Brain Res. 1990;83:13–36. doi: 10.1016/s0079-6123(08)61238-8. [DOI] [PubMed] [Google Scholar]


