Abstract
Ceramides (Cer) comprise the major constituent of sphingolipids in the epidermis and are known to play diverse roles in the outermost layers of the skin including water retention and provision of a physical barrier. In addition, they can be hydrolyzed into free sphingoid bases such as C18 sphingosine (SO) and C18 sphinganine (SA) or can be further metabolized to C18 So-1-phosphate (S1P) and C18 Sa-1-phosphate (Sa1P) in keratinocytes. The significance of ceramide metabolites emerged from studies reporting altered levels of SO and SA in skin disorders and the role of S1P and Sa1P as signaling lipids. However, the overall metabolism of sphingoid bases and their phosphates during keratinocyte differentiation remains not fully understood. Therefore, in this study, we analyzed these Cer metabolites in the process of keratinocyte differentiation. Three distinct keratinocyte differentiation stages were prepared using 0.07 mM calcium (Ca2+) (proliferation stage), 1.2 mM Ca2+ (early differentiation stage) in serum-free medium, or serum-containing medium with vitamin C (50 µL/mL) (late differentiation stage). Serum-containing medium was also used to determine whether vitamin C increases the concentrations of sphingoid bases and their phosphates. The production of sphingoid bases and their phosphates after hydrolysis by alkaline phosphatase was determined using high-performance liquid chromatography. Compared to cells treated with 0.07 mM Ca2+, levels of SO, SA, S1P, and SA1P were not altered after treatment with 1.2 mM Ca2+. However, in keratinocytes cultured in serum-containing medium with vitamin C, levels of SO, SA, S1P, and SA1P were dramatically higher than those in 0.07- and 1.2-mM Ca2+-treated cells; however, compared to serum-containing medium alone, vitamin C did not significantly enhance their production. Taken together, we demonstrate that late differentiation induced by vitamin C and serum was accompanied by dramatic increases in the concentration of sphingoid bases and their phosphates, although vitamin C alone had no effect on their production.
Keywords: Keratinocyte differentiation, calcium, vitamin C, sphingoid bases, sphingoid base 1 phosphates
Introduction
The epidermis is composed of a single layer of basal cells, called the basal layer, which, after formation, divide and move upward through the stratum spinosum and stratum granulosum (SG) to the stratum corneum (SC). This process, known as keratinocyte differentiation, is accompanied by sphingolipid synthesis. In the SG, synthesized lipids are packaged into cytosolic organelles called lamella bodies and are excreted into the interface between the SG and the SC. The lipids are then incorporated into the SC as ceramides (Cer) [1-3]. Several nutrients have been reported to modulate the course of keratinocyte differentiation, particularly calcium (Ca2+) and vitamin C [4-6]. A Ca2+ gradient exists in the epidermis, with the highest levels of extracellular Ca2+ present in the SG, resulting in differentiation of this layer [4,7]. In addition, vitamin C is known to enhance the late differentiation of keratinocytes by increasing the production of certain Cer [5,6].
In the epidermis, Cer have heterogeneity and play diverse roles including water retention and the provision of a physical barrier between the internal organs and the environment [8,9]. Cer also act as precursors for metabolites such as free sphingoid bases (sphingosine [SO], sphinganine [SA], phytophingosine, or 6-hydroxy-SO) [1,10]. Free sphingoid bases have been reported to account for 0.5% of the total lipids in the SC; C18 chains are known to dominant other chain lengths [11-13]. Specifically, C18 SO and C18 SA have been proposed to be associated with abnormal skin conditions [13]. C18 SO exerts natural antiinflammatory activity by inhibiting protein kinase C and exerting its antimicrobial activity in atopic dermatitis [14-16]. Changes in the C18 SA levels in skin diseases such as psoriasis have also been reported [13]. In addition, these free sphingoid bases can be phosphorylated through the action of SO kinase. Many studies have focused on SO 1-phosphate (S1P) rather than SA 1-phosphate (SA1P) because S1P is a bioactive lipid that is implicated in keratinocyte growth arrest and differentiation [17]. Moreover, it also plays different roles in the epidermis such as re-epithelialization of wounds and antimicrobial activity on the surfaces of acne lesions [17,18].
Although many studies have focused on the roles of sphingoid bases and their phosphates in the epidermis, their metabolism during in vitro keratinocyte differentiation remains unclear and their levels have not been studied in response to vitamin C, which has been shown to stimulate the production of other sphingolipids [6]. Therefore, we examined the metabolisms of C18 SO and C18 SA and their phosphates at different stages of cell differentiation using cultured human keratinocytes (CHKs) grown in high calcium (early differentiation stage) and serum-containing medium with vitamin C (late differentiation stage) [19]. We also investigated whether vitamin C alone enhances the production of these ceramide metabolites.
Materials and Methods
Materials
Human neonatal epidermal keratinocytes (HEKn) and their culture medium and growth supplements were purchased from Cascade Biologics (Portland, OR, USA). Dulbecco's modified Eagle's medium (DMEM) and Ham's F-12 were obtained from GIBCO (San Diego, CA, USA). Insulin and hydrocortisone were obtained from Sigma-Aldrich (St. Louis, MO, USA). All sphingolipids such as D-erthyro-SO and D-erythro- as external stands and D-erythro-C17-SO and D-erythro-C17-SO1-phosphate were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL, USA). The solvents used for high-performance liquid chromatography (HPLC) analysis were purchased from Merck (Dermstadt, Germany).
Cell culture
Human epidermal keratinocytes were cultured in keratinocyte growth medium with bovine pituitary extract (0.2%, v/v), insulin (5 µg/mL), hydrocortisone (0.18 µg/mL), bovine transferrin (5 µg/mL), human epidermal growth factor (0.2 ng/mL), and 0.07 mM calcium chloride (Cascade Biologics, Portland, OR, USA). Cells at 80-90% confluence were either harvested for use as keratinocytes in the proliferative stage or were grown further in differentiation-inducing medium in two consecutive steps as described below [19]. First, early-differentiation keratinocytes were generated via culturing cells in medium supplemented with 1.2 mM calcium and then harvesting after three days. Second, late differentiation keratinocytes were obtained by replacing the medium with DMEM and Ham's F-12 (2:1, v/v) containing 1.2 mM calcium, 10% fetal bovine serum, 10 µg/mL insulin, and 0.4 µg/mL hydrocortisone, either without or with 50 µg/mL vitamin C. Cells were maintained for 11 days, underwent media changes three times per week, and were then harvested for the experiments. All cultures were maintained at 37℃ in an incubator with 5% CO2 in air.
DNA assay
Following brief sonication, the cell lysates were used for DNA determination after treatment with the fluorescent dye bisbenzimidazole (Bisbenzimide H 33258; Sigma-Aldrich), which binds to A-T base pairs in double-stranded DNA. Cell lysates were mixed with 200 µL of 0.1 µg/mL bisbenzimide in 1 × fluorescent assay buffer [20] (100 mM Tris-HCl [pH 7.4] with 10 mM EDTA and 2 M NaCl), and fluorescence was immediately determined using a fluorescent plate reader (TECAN, Switzerland) at wavelengths of 360 nm for excitation and 460 nm for emission. The fluorescence intensity was compared with those of DNA standards sodium salt from calf thymus DNA (Sigma-Aldrich) with concentrations in the range of 0.1-10 µg/mL.
Lipid extraction for sphingoid analysis
Cultured cells were rinsed twice with ice-cold phosphate-buffered saline (PBS) and harvested via scraping into PBS. After centrifugation at 240 × g for 5 min, the pellet was homogenized in 500 µL of PBS and ultrasonicated for 10 sec. Cell suspension aliquots (50 µL of supernatant) were collected for DNA and protein determination. Extraction of sphingoid bases and sphingoid base 1-phosphates was performed by addition of 350 µL of MeOH, 150 µL of 1M NaCl, and 300 µL of CHCl3 to the cell homogenates. The pH was then adjusted to 10-11 through the addition of 35 µg 3N NaOH to separate the sphingoid bases and sphingoid base 1-phosphates to the lower and upper phases, respectively. The non-naturally occurring species C17 SO (100 pmol) and C17 S1P (100 pmol) were added as internal standards, and the mixture was vortexed vigorously for 1 h [21,22]. After centrifugation at 240 × g for 3 min, the upper phase containing the S1P was transferred to a new tube for dephosphorylation, while the lower phase was used for free sphingoid base extraction as described below. The organic phase was first removed using an evaporator and then dissolved in 500 µg of 0.15 M methanolic KOH by vortexing for 20 min at 37℃. To this, 500 µg of CHCl3, 100 µg of 2N NH4OH, and 400 µg of alkaline water were added, and the solution was vortexed for 1 h before centrifugal separation. After the organic phase containing SO and SA with 800 µg of alkaline water was washed twice [21], the CHCl3 was evaporated using a Speed Vac concentrator (Hanil, Seoul, Korea).
Dephosphorylation of sphingoid base 1-phosphates
S1P extracted from the aqueous fraction was dephosphorylated through the addition of ten units of alkaline phosphatase (New England Biolabs, Ipswich, MA, USA) in 100 µL of reaction buffer (200 mM Tris-HCl [pH 7.4], 7.5 mM MgCl2 in 2M glycine buffer, pH 9.0-9.2) and gentle mixing [22]. The enzyme efficiently converted sphingoid base 1-phosphates into their corresponding sphingoid bases at 37℃ for 2 h. The dephosphorylated sphingoid bases were extracted by addition of 600 µL of CHCl3 and vortexed for 30 min. After centrifugation at 240 g for 3 min, the CHCl3 phase was transferred to a new tube, washed once with 800 µL of alkaline water, and then dried in a Speed Vac concentrator.
O-Phtalaldehyde (OPA) derivatization and HPLC analysis
Levels of sphingoid bases and their phosphates were determined using HPLC analysis as described below. The extracted lipid fraction was resolved in 120 µL of MeOH, followed by the addition 20 µL of OPA derivatization (Sigma-Aldrich), 50 mg of O-phthaldialdehyde, 1 mL of ethanol, 100 µL of 2-mercaptoethanol, 50 mL of 3% (w/v) boric acid solution, and a 30-min incubation at room temperature [21-23]. OPA is known to react with the primary amine groups of sphingoid bases, and it becomes highly fluorescent at excitation and emission wavelengths of 340 nm and 455 nm, respectively. The derivatives were analyzed via HPLC equipped with a Jasco PU-980 pump (Tokyo, Japan), a Jasco AS-1559 autosampler, and a Jasco FP-920 fluorescence detector. The isocratic eluent composition of methanol (deionized distilled water (92:8 v/v) with 0.1% triethylamine) was used at a flow rate of 1 mL/min, while 70 µL of the derivatives were injected and separated on a C18 column (Waters, Sunfire 4.6 × 150 mm ID) at room temperature. Fluorescence of sphingoid derivatives was measured at emission and excitation wavelengths of 455 nm and 340 nm, respectively [22], to measure the concentrations of C17 SO (8.5 min), C18 SO (10.5 min), and C18 SA (14.2 min).
Statistical analysis
Data are expressed as means ± SEM (n = 3). Statistical comparisons were conducted using one-way analysis of variance followed Duncan's post-hoc multiple comparison test using SPSS 18.0 for Windows (SPSS Inc., Chicago, IL, USA) and the differences among the groups were determined by Duncan's multiple comparison test. Differences of P < 0.05 were considered significant.
Results
Morphological characteristics during keratinocyte differentiation
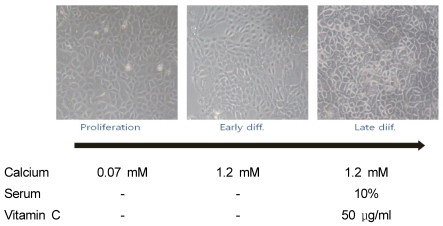
Keratinocytes showed distinct morphological features in the differentiation process (Fig. 1). At the proliferative stage induced by 0.07 mM Ca2+, cells grew to confluency as a monolayer of non-stratified cells with no detectable intercellular spaces. However, when cells at this stage came into contact with their neighbors, they did not form well. Cells at the early differentiation stage induced by 1.2 mM Ca2+ also grew as a monolayer but became more compact and grew away from their neighbors. Conversely, keratinocytes cultured in serum-containing medium with vitamin C grew as multilayered sheets with thicker cell layers. Furthermore, after 11 days of cell culture in serum-containing medium with vitamin C, the cells secreted a number of cytosolic lamella bodies (data not shown). According to morphologic criteria, cells cultured in serum-containing medium with vitamin C showed a greater differentiation than did those grown in high Ca2+-containing medium without serum or vitamin C.
Fig. 1.
Morphological characteristics of keratinocytes in the proliferative and early and late different differentiation stages. Cultured human keratinocytes grown in three different types of medium were observed under the microscope at a magnification 100×. Cells in the proliferation stage were grown in serum-free medium with 0.07 mM Ca2+ for six days and either harvested or induced to the early differentiation stage with 1.2 mM Ca2+. Late differentiation was induced by culturing cells in serum-containing medium with 1.2 mM Ca2+ and vitamin C (50 µL/mL). Late differentiated cells were maintained for 11 days, underwent media changes three times per week, and were then harvested.
DNA and protein levels during keratinocyte differentiation
Table 1 shows the changes in total DNA and protein content of the keratinocytes in the proliferative and early and late differentiation stages. Total protein content increased as cells became more differentiated [24]. In particular, increases in protein content were significant (P < 0.05) in the late differentiation stage because of a large increase in structural proteins such as cornified envelope proteins and keratins, which are known to characterize late keratinocyte differentiation [24]. In contrast, increases in DNA content were less profound and stayed relatively constant during the differentiation process.
Table 1.
Altered levels of DNA and protein during keratinocyte differentiation
Keratinocytes were grown in culture medium with 0.07 mM Ca2+ (proliferation), 1.2 mM Ca2+ (early diff.), and control medium containing vitamin C (late diff.) as described in Materials and Methods. Values are presented as means ± SEM (n = 3). Means with different letters are significantly different (P < 0.05) according to Duncan's multiple range test.
Analysis of sphingoid bases
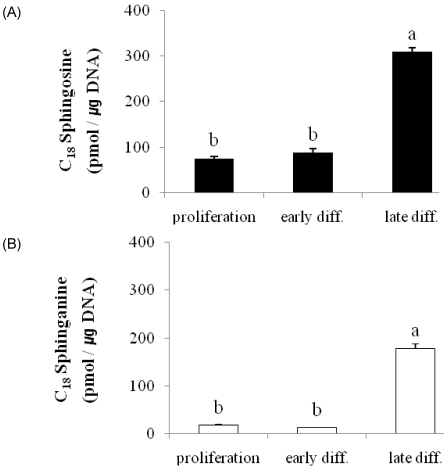
We first investigated the levels of C18 SO and C18 SA in the process of keratinocyte differentiation by generating three distinct keratinocyte differentiation stages (Fig. 2). Cells designated as being in the proliferation and early differentiation stage were prepared with 0.07 mM Ca2+ and 1.2 mM Ca2+, respectively, in serum-free culture medium. Late stage differentiated keratinocytes were cultured in serum-containing medium with vitamin C (50 µL/mL) and 1.2 mM Ca2+. Because of the large increase in the number of structural proteins (data not shown) known to characterize keratinocyte differentiation [24], the results were normalized with respect to DNA rather than protein content. Cellular levels of C18 SO and C18 Sa in keratinocytes increased dramatically when keratinocytes were induced into late differentiation; a four-fold increase in So and a ten-fold increase in Sa were observed compared with the levels in the proliferation stage (P < 0.001) (Fig. 2). However, high Ca2+ treatment alone did not increase the levels of SO or SA, suggesting that free sphingoid bases were required for late differentiation of keratinocytes.
Fig. 2.
Altered levels of sphingoid bases associated with keratinocyte differentiation. Panel A: Sphingosine; Panel B: Sphinganine. Sphingoid bases were extracted from keratinocytes grown in culture medium with 0.07 mM Ca2+ (proliferation), 1.2 mM Ca2+ (early diff.), and control medium containing vitamin C (late diff.) and analyzed using high-performance liquid chromatography as described in Materials and Methods. Values are presented as means ± SEM (n = 3) Means with different letters are significantly different (P < 0.001) according to Duncan's multiple range test.
Analysis of sphingoid base 1-phosphates
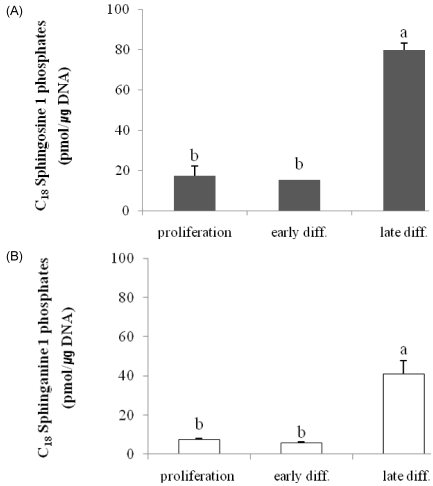
Cellular levels of C18 S1P and SA1P per microgram of DNA were increased in keratinocytes induced into late differentiation by vitamin C in serum-containing medium, similar to the levels of their sphingoid bases. Compared with cells in the proliferation stage, S1P and SA1P levels decreased in early differentiation and then increased significantly when the cells progressed to late differentiation (P < 0.001) (Fig. 3).
Fig. 3.
Altered levels of sphingoid base phosphates associated with keratinocyte differentiation. Panel A: Sphingosine-1-phosphate; Panel B: Sphinganine-1-phosphate. Sphingoid base phosphates were extracted and dephosphorylated using an alkaline phosphatase assay from keratinocytes grown in culture medium with 0.07 mM Ca2+ (proliferation), 1.2 mM Ca2+ (early diff.), and control medium containing vitamin C (late diff.) and then analyzed using high-performance liquid chromatography as described in Materials and Methods. Values are presented as means ± SEM (n = 3) Means with different letters are significantly different (P < 0.001) according to Duncan's multiple range test.
Effect of vitamin C on ceramide metabolites
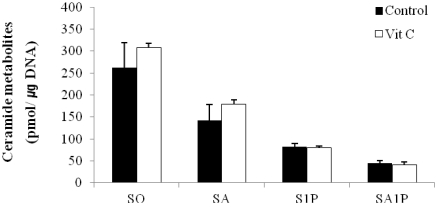
To demonstrate whether addition of vitamin C, which has been previously shown to increase the concentrations of other sphingolipids [6] and related enzymes, had an effect on the production of free sphingoid bases and their phosphates, keratinocytes were cultured in serum-containing medium with either vehicle or vitamin C (50 µL/mL). Although treatment with vitamin C moderately increased the productions of both C18 SO and C18 SA, these increases were not statistically significant. In addition, productions of S1P and SA1P that were formed through the phosphorylation of C18 SO and C18 SA, respectively, by SO kinase also did not change in response to vitamin C (Fig. 4).
Fig. 4.
Effect of vitamin C on production of ceramides metabolites. Sphingoid bases and their phosphates were extracted from keratinocytes grown in serum-containing culture medium with 50 µg/mL of vitamin C (Vit C) or without (control) and were then analyzed by high-performance liquid chromatography. Values are presented as means ± SEM (n = 3).
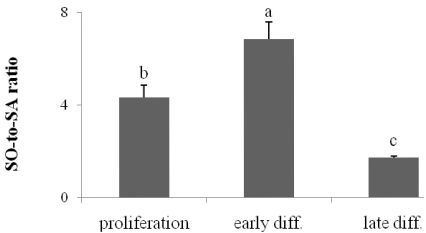
The ratio of SO to SA during keratinocyte differentiation
Changes in sphingoid bases have recently been implicated in membrane permeability. In this study, we observed a marked change in the ratio of SO to SA during keratinocyte differentiation. In basal cells and early differentiated cells, the ratios were 4.45 and 6.85, respectively. As the CHKs progressed into the late differentiation stage, the ratio decreased to 1.73 (Fig. 5).
Fig. 5.
The ratio of sphingosine (SO) to sphinganine (SA) during keratinocyte differentiation. C18 sphingoid bases were extracted from keratinocytes grown in culture medium with 0.07 mM Ca2+ (proliferation), 1.2 mM Ca2+(early diff.), and control medium containing vitamin C (late diff.) and were then analyzed using high-performance liquid chromatography. The ratios of SO to SA were compared during differentiation. Values are presented as means ± SEM (n = 3). Means with different letters are significantly different (P < 0.05) according to Duncan's multiple range test.
Discussion
CHKs grown in culture medium with low Ca2+ (0.02 to 0.1 mM) continue to proliferate and maintain the morphologies of basal epidermal cells. When the extracellular Ca2+ level is increased (from 1 to 2 mM), the cells stop differentiating and develop into an early differentiation stage that is accompanied by increased synthesis of sphingolipids [4,7,24]. Furthermore, when grown in serum-containing culture medium with vitamin C and a high concentration of Ca2+, CHKs undergo late differentiation associated with the distribution of epidermal-like sphingolipids [6,19]. In the present study, we employed the same model for culturing differentiated keratinocytes as described in previous studies and expanded upon those studies to gain a better understanding of the relationship between sphingolipid metabolism and epidermal differentiation. Levels of C18 SO and C18 SA, S1P, and SA1P were analyzed during keratinocyte differentiation.
In this study, the overall amount of SO was greater than that of Sa, and both showed the same pattern during differentiation, even though SO (with a 4,5-trans double bond) has been considered a more active analog than SA (without a 4,5-trans double bond). SO and SA levels were dramatically increased in late differentiated cells grown in serum-containing medium with vitamin C and were present in low concentrations in the proliferative and early differentiation stages. In the basal layer of the epidermis, SO and S1P derived from ceramide hydrolysis have been shown to protect keratinocytes against cellular Cer accumulation, which is known to mediate cell apoptosis through oxidative stress. Other studies have also suggested that SO plays a role in cell proliferation through signaling pathways and, in turn, can be phosphorylated by SO kinase to form S1P, which is widely known for its anti-apoptotic effects in keratinocytes [25-27]. The activities of neutral and phytoalkaline ceramidases account for this finding [19]. However, in the early differentiation stage, induced by increasing the concentration of Ca2+ (1.2 mM), the cells contained almost the same amounts of SO and SA as were present in the basal layer. When keratinocytes reach the SG containing high Ca2+ concentrations, sphingoid bases are produced via a de novo biosynthetic pathway in which SA acts as a pathway intermediate, and SO is associated with sphingolipid and Cer degradation [24,28]. Prior studies revealed that treatment with a high concentration of extracellular Ca2+ (1.8 mM) activates acid and alkaline ceramidases, both of which more efficiently hydrolyze SO-containing Cer than they do SA-containing Cer (dihydroceramides). In comparison, inhibition of alkaline ceramidase activity by a negative regulator such as epidermal growth factor (EGF) has also been proposed [29]. The fact that early differentiated cells were grown in medium containing both Ca2+ and EGF supports this finding. Similar to our results, another study showed that mRNA levels for both acid and alkaline ceramidases were not altered in response to changes in Ca2+ concentration [19]. On the other hand, sphingoid bases were much more abundant (P < 0.001) in late differentiated CHKs compared with proliferative and early differentiated cells. Increased levels of Cer in late differentiated cells account are shown in Fig. 3. The concentrations of Cer, the major components of the SC permeability barrier, were almost ten-fold higher than those of sphingoid bases [11]. Unrestricted Cer hydrolysis could threaten the lamellar structures of the SC, and the acid and alkaline ceramidases that require optimum pH conditions are reported to mediate this conversion [28,30,31]. Another recent study reported that acid and alkaline ceramidases have activities of 127 ± 63 pmol/mg of protein/h and 42 ± 16 pmol/mg of protein/h, respectively [19]. Moreover, the level of alkaline ceramidase mRNA was increased more than 100-fold in late differentiated CHKs compared with those in high Ca2+-treated cells. Releases of SO and SA from Cer have also been shown to induce growth arrest or differentiation in keratinocytes [11].
Studies have suggested that vitamin C itself increases sphingolipid production by not only promoting the hydroxylation of sphingoid bases and free fatty acids of Cer but also enhancing the production of non-hydroxylated Cer and specific glucosylceramides [5,6,31]. Therefore, we next investigated whether the vitamin C or the medium-containing serum enhanced the generation of ceramide metabolites. In a previous study, CHKs maintained in serum-containing medium with 1.2 mM Ca2+ were more fully differentiated and had greater glucosylceramide production [6,32]. Likewise, in this study, we demonstrated higher levels of SO (3.8-fold increase) and SA (8.17-fold increase) in CHKs cultured in serum-containing medium than in basal cells. Vitamin C did not increase the production of SO or SA compared with vitamin C-untreated late differentiation of CHKs (control) (Fig. 4). However, vitamin C has been previously reported to enhance the preferential formation of Cer 2 and 5, which contain SO or SA as their backbone bases. Further research investigating the heterogeneity of ceramidases is needed to determine the consequences of sphingoid base production in response to vitamin C. Interestingly, the formation of acylCer is enhanced by vitamin C, leading to the release of free sphingoid bases and the ω-OH-FFA that is associated with the cornified envelope of SC corneocytes [6,33,34]. Generation of ω-OH-FFA has been observed in vitamin C-treated cells but not in controls. Additional studies investigating the role of dihydroceramide delta 4-5 desaturase will be helpful to fully elucidate the effects of vitamin C on the production of C18 SO and C18 SA since this enzyme is a key regulator in SO production [35]. The ratio of SO to SA is also worth noting. as shown in Fig. 5, in basal cells and in the early differentiation stage, the SO:SA ratio was 4.45:6.85, respectively. As the cells progressed to late differentiated CHKs, the ratio decreased to 1.73. A significant increase in SA level in late differentiation might have resulted from its pro-differentiation activity in CHKs. Interestingly, recent research has reported that the ratio of SO to SA is a critical determinant of membrane stability in atopic dermatitis. The epidermis of mice with skin disease presented a markedly increased ratio of 5.5-15.2, suggesting that the higher ratio might contribute to barrier defects. In our study, the ratio was notably decreased in late differentiation, which is associated with a more rigid cell layer compared to all of the other epidermal cell layers [8,19]. On the other hand, another similar study showed that a lack of dihydroceramide delta 4-5 desaturase, which catalyzes the conversion of SA to SO in a de novo ceramide synthesis pathway, leads to scaly skin, suggesting that sphingoid bases are not only elements of Cer but are also essential for barrier function in the epidermis [9,36]. In the SC of the epidermis, C18 SO is known to exert powerful antimicrobial and anti-inflammatory effects by inhibiting protein kinase C activity [14,16,37]. In addition, SA also has also been reported to inhibit microbial adherence.
In this study, we showed similar results for C18 S1P and C18 SA1P to those of their sphingoid base precursors in the course of keratinocyte differentiation. In the proliferative and early differentiation stages, levels of these phosphates remained low and then increased in the suprabasal layer. In serum-containing media with vitamin C and 1.2 mM Ca2+, S1P and Sa1P levels increased by 4.56-fold and 5.60 fold, respectively, over those in serum-free medium with low Ca2+. In addition, neither low nor high Ca2+ treatment influenced the production of S1P or Sa1P. This finding is supported by previous studies demonstrating that exogenous Ca2+ has no effect on SO kinase, which converts sphingoid bases into their respective phosphates [38]. Interestingly, this enzyme is highly associated with Ca2+ and has binding sites for calmodulin, leading to Ca2+ mobilization in many different cell types [39,40]. Moreover, its activity increases the productions of differentiation markers such as involucrin, loricrin, filaggrin, and keratin 5, resulting in keratinocyte differentiation [26,41]. Consistent with this, we observed dramatic upregulations of C18 S1P and SA1P levels in late differentiated CHKs, which are thought to be derived from the activity of SO kinase and their precursors, C18 SO and C18 SA, respectively. S1P has been considered a significant factor involved in motility and morphological change in human cells such as arterial smooth muscle cells, endothelial cells, and keratinocytes [17,42-45]. S1P may regulate the morphological changes associated with differentiation since morphological changes in keratinocytes are concomitant with keratinocyte differentiation. On the other hand, treatment with vitamin C did not increase the production of these sphingoid base phosphates, suggesting that SO kinase was inactive despite the presence of vitamin C. However, we cannot confirm that the SO kinase was inactive since its activity was not investigated in this study.
In conclusion, we demonstrated that Cer metabolites, specifically C18 SO, C18 SA, C18 S1P, and C18 SA1P, were significantly increased in late but not early stage differentiated CHKs, suggesting that not only Cer but also high levels of their metabolites are required for late differentiation of keratinocytes. However, vitamin C, which is known to increase Cer production [6], did not stimulate the generation of C18 SO, C18 SA, C18 S1P, or C18 SA1P.
Footnotes
This work was supported by a grant from the Kyung Hee University in 2010 (KHU-20100156).
References
- 1.Houben E, De Paepe K, Rogiers V. A keratinocyte's course of life. Skin Pharmacol Physiol. 2007;20:122–132. doi: 10.1159/000098163. [DOI] [PubMed] [Google Scholar]
- 2.Downing DT, Stewart ME, Wertz PW, Colton SW, Abraham W, Strauss JS. Skin lipids: an update. J Invest Dermatol. 1987;88:2s–6s. doi: 10.1111/1523-1747.ep12468850. [DOI] [PubMed] [Google Scholar]
- 3.Elias PM, Menon GK. Structural and lipid biochemical correlates of the epidermal permeability barrier. Adv Lipid Res. 1991;24:1–26. doi: 10.1016/b978-0-12-024924-4.50005-5. [DOI] [PubMed] [Google Scholar]
- 4.Hennings H, Michael D, Cheng C, Steinert P, Holbrook K, Yuspa SH. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980;19:245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- 5.Ponec M, Weerheim A, Kempenaar J, Mulder A, Gooris GS, Bouwstra J, Mommaas AM. The formation of competent barrier lipids in reconstructed human epidermis requires the presence of vitamin C. J Invest Dermatol. 1997;109:348–355. doi: 10.1111/1523-1747.ep12336024. [DOI] [PubMed] [Google Scholar]
- 6.Uchida Y, Behne M, Quiec D, Elias PM, Holleran WM. Vitamin C stimulates sphingolipid production and markers of barrier formation in submerged human keratinocyte cultures. J Invest Dermatol. 2001;117:1307–1313. doi: 10.1046/j.0022-202x.2001.01555.x. [DOI] [PubMed] [Google Scholar]
- 7.Menon GK, Grayson S, Elias PM. Ionic calcium reservoirs in mammalian epidermis: ultrastructural localization by ion-capture cytochemistry. J Invest Dermatol. 1985;84:508–512. doi: 10.1111/1523-1747.ep12273485. [DOI] [PubMed] [Google Scholar]
- 8.Holleran WM, Takagi Y, Uchida Y. Epidermal sphingolipids: metabolism, function, and roles in skin disorders. FEBS Lett. 2006;580:5456–5466. doi: 10.1016/j.febslet.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 9.Mizutani Y, Mitsutake S, Tsuji K, Kihara A, Igarashi Y. Ceramide biosynthesis in keratinocyte and its role in skin function. Biochimie. 2009;91:784–790. doi: 10.1016/j.biochi.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Geilen CC, Barz S, Bektas M. Sphingolipid signaling in epidermal homeostasis. Current knowledge and new therapeutic approaches in dermatology. Skin Pharmacol Appl Skin Physiol. 2001;14:261–271. doi: 10.1159/000056356. [DOI] [PubMed] [Google Scholar]
- 11.Wertz PW, Downing DT. Free sphingosine in human epidermis. J Invest Dermatol. 1990;94:159–161. doi: 10.1111/1523-1747.ep12874122. [DOI] [PubMed] [Google Scholar]
- 12.Wertz PW, Downing DT. Free sphingosines in porcine epidermis. Biochim Biophys Acta. 1989;1002:213–217. doi: 10.1016/0005-2760(89)90289-0. [DOI] [PubMed] [Google Scholar]
- 13.Wakita H, Nishimura K, Takigawa M. Composition of free long-chain (sphingoid) bases in stratum corneum of normal and pathologic human skin conditions. J Invest Dermatol. 1992;99:617–622. doi: 10.1111/1523-1747.ep12668019. [DOI] [PubMed] [Google Scholar]
- 14.Bibel DJ, Aly R, Shinefield HR. Antimicrobial activity of sphingosines. J Invest Dermatol. 1992;98:269–273. doi: 10.1111/1523-1747.ep12497842. [DOI] [PubMed] [Google Scholar]
- 15.Arikawa J, Ishibashi M, Kawashima M, Takagi Y, Ichikawa Y, Imokawa G. Decreased levels of sphingosine, a natural antimicrobial agent, may be associated with vulnerability of the stratum corneum from patients with atopic dermatitis to colonization by Staphylococcus aureus. J Invest Dermatol. 2002;119:433–439. doi: 10.1046/j.1523-1747.2002.01846.x. [DOI] [PubMed] [Google Scholar]
- 16.Gupta AK, Fisher GJ, Elder JT, Nickoloff BJ, Voorhees JJ. Sphingosine inhibits phorbol ester-induced inflammation, ornithine decarboxylase activity, and activation of protein kinase C in mouse skin. J Invest Dermatol. 1988;91:486–491. doi: 10.1111/1523-1747.ep12476635. [DOI] [PubMed] [Google Scholar]
- 17.Kawanabe T, Kawakami T, Yatomi Y, Shimada S, Soma Y. Sphingosine 1-phosphate accelerates wound healing in diabetic mice. J Dermatol Sci. 2007;48:53–60. doi: 10.1016/j.jdermsci.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Vogler R, Sauer B, Kim DS, Schäfer-Korting M, Kleuser B. Sphingosine-1-phosphate and its potentially paradoxical effects on critical parameters of cutaneous wound healing. J Invest Dermatol. 2003;120:693–700. doi: 10.1046/j.1523-1747.2003.12096.x. [DOI] [PubMed] [Google Scholar]
- 19.Houben E, Holleran WM, Yaginuma T, Mao C, Obeid LM, Rogiers V, Takagi Y, Elias PM, Uchida Y. Differentiation-associated expression of ceramidase isoforms in cultured keratinocytes and epidermis. J Lipid Res. 2006;47:1063–1070. doi: 10.1194/jlr.M600001-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980;102:344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- 21.Ruwisch L, Schäfer-Korting M, Kleuser B. An improved high-performance liquid chromatographic method for the determination of sphingosine-1-phosphate in complex biological materials. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:358–363. doi: 10.1007/s002100000365. [DOI] [PubMed] [Google Scholar]
- 22.Min JK, Yoo HS, Lee EY, Lee WJ, Lee YM. Simultaneous quantitative analysis of sphingoid base 1-phosphates in biological samples by o-phthalaldehyde precolumn derivatization after dephosphorylation with alkaline phosphatase. Anal Biochem. 2002;303:167–175. doi: 10.1006/abio.2002.5579. [DOI] [PubMed] [Google Scholar]
- 23.Yoon HT, Yoo HS, Shin BK, Lee WJ, Kim HM, Hong SP, Moon DC, Lee YM. Improved fluorescent determination method of cellular sphingoid bases in high-performance liquid chromatography. Arch Pharm Res. 1999;22:294–299. doi: 10.1007/BF02976365. [DOI] [PubMed] [Google Scholar]
- 24.Pillai S, Bikle DD, Hincenbergs M, Elias PM. Biochemical and morphological characterization of growth and differentiation of normal human neonatal keratinocytes in a serum-free medium. J Cell Physiol. 1988;134:229–237. doi: 10.1002/jcp.1041340208. [DOI] [PubMed] [Google Scholar]
- 25.Herzinger T, Kleuser B, Schäfer-Korting M, Korting HC. Sphingosine-1-phosphate signaling and the skin. Am J Clin Dermatol. 2007;8:329–336. doi: 10.2165/00128071-200708060-00002. [DOI] [PubMed] [Google Scholar]
- 26.Youm JK, Jo H, Hong JH, Shin DM, Kwon MJ, Jeong SK, Park BD, Choi EH, Lee SH. K6PC-5, a sphingosine kinase activator, induces anti-aging effects in intrinsically aged skin through intracellular Ca2+ signaling. J Dermatol Sci. 2008;51:89–102. doi: 10.1016/j.jdermsci.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Manggau M, Kim DS, Ruwisch L, Vogler R, Korting HC, Schäfer-Korting M, Kleuser B. 1Alpha,25-dihydroxyvitamin D3 protects human keratinocytes from apoptosis by the formation of sphingosine-1-phosphate. J Invest Dermatol. 2001;117:1241–1249. doi: 10.1046/j.0022-202x.2001.01496.x. [DOI] [PubMed] [Google Scholar]
- 28.Mao C, Obeid LM. Ceramidases: regulators of cellular responses mediated by ceramide, sphingosine, and sphingosine-1-phosphate. Biochim Biophys Acta. 2008;1781:424–434. doi: 10.1016/j.bbalip.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun W, Xu R, Hu W, Jin J, Crellin HA, Bielawski J, Szulc ZM, Thiers BH, Obeid LM, Mao C. Upregulation of the human alkaline ceramidase 1 and acid ceramidase mediates calcium-induced differentiation of epidermal keratinocytes. J Invest Dermatol. 2008;128:389–397. doi: 10.1038/sj.jid.5701025. [DOI] [PubMed] [Google Scholar]
- 30.Coderch L, López O, de la Maza A, Parra JL. Ceramides and skin function. Am J Clin Dermatol. 2003;4:107–129. doi: 10.2165/00128071-200304020-00004. [DOI] [PubMed] [Google Scholar]
- 31.Castiel-Higounenc I, Chopart M, Ferraris C. Stratum corneum lipids: specificity, role, deficiencies and modulation. Eur J Dermatol. 2004;11:401–406. [Google Scholar]
- 32.Sando GN, Howard EJ, Madison KC. Induction of ceramide glucosyltransferase activity in cultured human keratinocytes. Correlation with culture differentiation. J Biol Chem. 1996;271:22044–22051. doi: 10.1074/jbc.271.36.22044. [DOI] [PubMed] [Google Scholar]
- 33.Uchida Y, Holleran WM. Omega-O-acylceramide, a lipid essential for mammalian survival. J Dermatol Sci. 2008;51:77–87. doi: 10.1016/j.jdermsci.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Behne M, Uchida Y, Seki T, de Montellano PO, Elias PM, Holleran WM. Omega-hydroxyceramides are required for corneocyte lipid envelope (CLE) formation and normal epidermal permeability barrier function. J Invest Dermatol. 2000;114:185–192. doi: 10.1046/j.1523-1747.2000.00846.x. [DOI] [PubMed] [Google Scholar]
- 35.Ternes P, Franke S, Zähringer U, Sperling P, Heinz E. Identification and characterization of a sphingolipid delta 4-desaturase family. J Biol Chem. 2002;277:25512–25518. doi: 10.1074/jbc.M202947200. [DOI] [PubMed] [Google Scholar]
- 36.Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Motta S, Monti M, Sesana S, Caputo R, Carelli S, Ghidoni R. Ceramide composition of the psoriatic scale. Biochim Biophys Acta. 1993;1182:147–151. doi: 10.1016/0925-4439(93)90135-n. [DOI] [PubMed] [Google Scholar]
- 38.Pitson SM, D'andrea RJ, Vandeleur L, Moretti PA, Xia P, Gamble JR, Vadas MA, Wattenberg BW. Human sphingosine kinase: purification, molecular cloning and characterization of the native and recombinant enzymes. Biochem J. 2000;350:429–441. [PMC free article] [PubMed] [Google Scholar]
- 39.Olivera A, Kohama T, Tu Z, Milstien S, Spiegel S. Purification and characterization of rat kidney sphingosine kinase. J Biol Chem. 1998;273:12576–12583. doi: 10.1074/jbc.273.20.12576. [DOI] [PubMed] [Google Scholar]
- 40.Meyer zu Heringdorf D, Lass H, Alemany R, Laser KT, Neumann E, Zhang C, Schmidt M, Rauen U, Jakobs KH, van Koppen CJ. Sphingosine kinase-mediated Ca2+ signalling by G-protein-coupled receptors. EMBO J. 1998;17:2830–2837. doi: 10.1093/emboj/17.10.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong JH, Youm JK, Kwon MJ, Park BD, Lee YM, Lee SI, Shin DM, Lee SH. K6PC-5, a direct activator of sphingosine kinase 1, promotes epidermal differentiation through intracellular Ca2+ signaling. J Invest Dermatol. 2008;128:2166–2178. doi: 10.1038/jid.2008.66. [DOI] [PubMed] [Google Scholar]
- 42.Xu SZ, Muraki K, Zeng F, Li J, Sukumar P, Shah S, Dedman AM, Flemming PK, McHugh D, Naylor J, Cheong A, Bateson AN, Munsch CM, Porter KE, Beech DJ. A sphingosine-1-phosphate-activated calcium channel controlling vascular smooth muscle cell motility. Circ Res. 2006;98:1381–1389. doi: 10.1161/01.RES.0000225284.36490.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bornfeldt KE, Graves LM, Raines EW, Igarashi Y, Wayman G, Yamamura S, Yatomi Y, Sidhu JS, Krebs EG, Hakomori S, Ross R. Sphingosine-1-phosphate inhibits PDGF-induced chemotaxis of human arterial smooth muscle cells: spatial and temporal modulation of PDGF chemotactic signal transduction. J Cell Biol. 1995;130:193–206. doi: 10.1083/jcb.130.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sauer B, Vogler R, von Wenckstern H, Fujii M, Anzano MB, Glick AB, Schäfer-Korting M, Roberts AB, Kleuser B. Involvement of Smad signaling in sphingosine 1-phosphate-mediated biological responses of keratinocytes. J Biol Chem. 2004;279:38471–38479. doi: 10.1074/jbc.M313557200. [DOI] [PubMed] [Google Scholar]
- 45.Kim J, Kim Y, Yun H, Park H, Kim SY, Lee KG, Han SM, Cho Y. Royal jelly enhances migration of human dermal fibroblasts and alters the levels of cholesterol and sphinganine in an in vitro wound healing model. Nutr Res Pract. 2010;4:362–368. doi: 10.4162/nrp.2010.4.5.362. [DOI] [PMC free article] [PubMed] [Google Scholar]