Abstract
α-Lipoic acid and L-carnosine are powerful antioxidants and are often used as a health supplement and as an ergogenic aid. The objective of this study was to investigate the effects of α-lipoic acid and/or L-carnosine supplementation on antioxidant activity in serum, skin, and liver of rats and blood lipid profiles for 6 weeks. Four treatment groups received diets containing regular rat chow diet (control, CON), 0.5% α-lipoic acid (ALA), 0.25% α-lipoic acid + 0.25% L-carnosine (ALA + LC), or 0.5% L-carnosine (LC). Superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) and lipid peroxidation products, malondialdehyde (MDA) concentrations, were analyzed in serum, skin, and liver. Blood lipid profiles were measured, including triglycerides (TG), total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), and low density lipoprotein cholesterol (LDL-C). Skin and liver SOD activities of the ALA and LC groups were higher than those of the CON group (P < 0.05), but serum SOD activity was higher only in the LC group compared to that in the CON group (P < 0.05). Additionally, only liver GSH-Px activity in the LC group was higher than that of the CON and the other groups. Serum and skin MDA levels in the ALA and LC groups were lower than those in the CON group (P < 0.05). Serum TG and TC in the ALA and ALA + LC groups were lower than those in the CON and LC groups (P < 0.05). The HDL-C level in the LC group was higher than that in any other group (P < 0.05). LDL-C level was lower in the ALA + LC and LC groups than that in the CON group (P < 0.05). Thus, α-lipoic acid and L-carnosine supplementation increased antioxidant activity, decreased lipid peroxidation in the serum, liver, and skin of rats and positively modified blood lipid profiles.
Keywords: α-Lipoic Acid, L-carnosine, antioxidant activity, glutathione peroxidase, superoxide dism
Introduction
α-Lipoic acid (ALA) is a naturally occurring short chain fatty acid with sulfhydryl groups and a powerful micronutrient with various biological functions [1,2]. ALA functions as an E2 subunit cofactor of the pyruvate dehydrogenase complex and α-ketoglutarate dehydrogenase and is a potent scavenger of hydroxyl radicals, superoxide radicals, peroxyl radicals, singlet oxygen, and nitric oxide [3,4]. ALA also plays a role regenerating endogenous antioxidants such as coenzyme Q10, vitamin C, vitamin E, and glutathione and chelates metal ions and repairs oxidized proteins [1,5]. ALA is reduced by the cytosolic enzymes GSH-reductase and thioredoxin reductase, and also the E3 mitochondrial enzyme dihydrolipoyl dehydrogenase. The mitochondrial E3 enzyme reduces ALA to DHLA at the expense of NAD(P)H [6]. ALA is also a substrate for the NADPH-dependent enzyme GSH-reductase [7].
Observations of ALA as an antioxidant in vitro and in vivo were previously based on hyperglycemic ambience [8-10]. However, no experimental design has addressed whether ALA could work against atherosclerosis in vivo; thus, preventing proliferation and propagation of this degenerative disease. Lipid peroxidation, the oxidative deterioration of polyunsaturated fatty acids, leads to the formation of hydroperoxides, short-chain aldehydes, ketones, and other oxygenated compounds. This process is responsible for the development of various diseases such as atherosclerosis [8], diabetes [11], cancer [12], and may be one of the main contributing factors in aging [13].
Free radical-mediated lipid peroxidation has been proposed to be critically involved in disease states including brain dysfunction, cardiovascular disease, and cancer as well as in the degenerative processes associated with aging. Enzymatic antioxidants such as superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) protect cell membranes from lipid peroxidation. ALA administered to aged rats replenishes glutathione, ascorbic acid, and alpha-tocopherol due to its capacity to enhance the levels of these antioxidants by reducing their radicals to repair oxidative damage. In contrast, the combination of two different antioxidants can be beneficial for limiting pathological processes in which excess production of free radicals may be involved and progress to damage (e.g., diabetic neuropathy, Alzheimer's disease, and cardiac ischemia). Some studies have suggested a possible synergistic effect of different non-enzymatic antioxidant compounds [14,15].
Human skin is constantly exposed to ultraviolet irradiation, which increases reactive oxygen species (ROS) production and leads to a number of pathological changes in the skin. A recent photoprotection strategy is to support the endogenous antioxidant system of the skin with natural substances through the diet or dermatological preparations. ALA easily penetrates the skin due to its lipid soluble characteristics in combination with a low molecular weight (206.3) [16]. Freisleben et al. [17] demonstrated that topical ALA cream applied to hairless mice penetrates the epidermis and is distributed in the dermis and the subcutaneous tissue after 4 h. However, no studies have investigated the effects of dietary administration of ALA on changes in antioxidant activity in human skin, blood, and tissues [18].
L-carnosine (β-alanyl-L-histidine) (LC) is an endogenously synthesized peptide found in muscles, brain, and other tissues [19]. LC is a potent antioxidant [20] and has an important role as a pH buffer to protect against damage caused by oxidation under anaerobic respiration conditions. During exercise, carnosine suppresses acidification of intracellular environments and maintains muscle activity. It also decreases malonaldehyde (MDA)-induced lipid peroxidation by the combined actions of free radical scavenging and metal chelation. The protective effects of carnosine have been demonstrated by brain ischemia in two animal models.
LC dietary supplementation leads to increased concentrations in animal tissues, and high concentrations of LC in tissues delays diabetic deterioration in a mouse model by protecting low density lipoprotein (LDL) against methyl glycosylation and antioxidative activity [21]. LC is involved in blood glucose regulation [22] and has cardiovascular functions [8]. The combined effects of LC and alpha-tocopherol enhance antioxidative properties [23-25]. However, few reports are available on the interactions of antioxidants in an in vivo model. The synergistic or additive effect of other antioxidants with LC has not been investigated so far.
Thus, whether oral administration of ALA and/or LC is able to exert antioxidative effects in rats by reducing free radical formation, and the effects of combined dietary supplementation of different naturally occurring antioxidants have not been investigated. Therefore, the objectives of this study were to investigate the effects of ALA or LC supplementation and to examine the combined effects of ALA and LC on antioxidant activities and blood lipid profiles in high-fat fed rats.
Materials and Methods
Animals and diets
Forty male Sprague-Dawley rats were supplied by Hanlim Experimental Animal Laboratory (Seoul, Korea), (average body weight, 231.5 ± 8.0 g) and were randomly assigned to four groups of 10 rats each. The rats were kept individually in stainless-steel cages in a room controlled for temperature (22 ± 1℃), relative humidity (50-60%), and light (12-hour light/dark cycle). All experimental protocols followed established guidelines for the care and handling of laboratory animals and were approved by the Institutional Animal Ethics Committee of Chung-Ang University, Korea. After a 1 week adaptation period, the four groups were fed four different diets for 6 weeks. The control group (CON) was fed the AIN-93G diet [18]. The energy composition of AIN-93G diet is 64.0% from carbohydrate, 19.3% from protein, and 16.7% from fat. The ALA supplementation level was determined based on previous studies. It was reported that a 0.5% ALA diet reduces plasma MDA and blood lipids in Otsuka Long-Evans Tokushima Fatty rats [11], and that an intraperitoneal injection of ALA (35 mg/kg body weight) decreases lipid peroxidation [12]. We investigated carnosine supplementation (0.01, 0.1, and 1.0%) on muscle carnosine concentrations and hematological indices of rats prior to the study. The 0.1% and 1.0% carnosine supplementation led to drop in blood LDL-cholesterol but an increase in HDL-cholesterol levels [26]. Groups receiving ALA or LC in this study were fed one of the following diets containing 0.5% ALA (ALA group), 0.25% ALA and 0.25% LC (ALA + LC group), or 0.5% LC (LC group). Table 1 summarizes the experimental design.
Table 1.
Experimental design
CON, Control; ALA, α-lipoic acid added group; ALA + LC, α-lipoic acid and L-carnosine added group; LC, L-carnosine added group
The rats were allowed free access to the experimental diets and to deionized water. No significant differences in body weight were observed among the groups at the start of the experiment. Feed consumption was checked every other day, and body weight was measured weekly. After 6 weeks of ad libitum feeding, the animals were fasted overnight, anesthetized with a combination of ketamine (50 mg/kg) and xylazine (0.1 mg/kg), and sacrificed. The liver, heart, kidneys, and spleen were removed and weighed. Blood was collected immediately from the abdominal aorta, and the serum was obtained by centrifugation (3,000 rpm, 10 min, 4℃). All samples were stored at -70℃ until analysis.
Skin homogenates were prepared for the antioxidant activity measurements using the method of Kim and Lee [27]. The animals were shaved of hair on the back after dissection, and then the skin tissue was removed. Subcutaneous fat was removed from the skin tissue using a buffer (10 mM phosphate buffer, 1.15% KCl, 5 mM EDTA, pH 7.4) at 4℃ and then the skin tissue was cut into 0.5 g pieces. Ten volumes of 0.1 M phosphate buffer were added to each skin sample (0.5 g), which was then homogenized for 1 min at 4℃ and centrifuged at 5,000 rpm and 4℃ for 30 min. Only the supernatant was used for the assay. Each supernatant sample was stored at -70℃ until analysis. To obtain the liver homogenates, 0.5 g of liver was combined with 10 volumes of 0.1 M phosphate buffer, homogenized, and centrifuged at 6,000 rpm and 4℃ for 30 min. The supernatant was used for the lipid peroxidation assay. For SOD activity measurements, 1 ml of the supernatant was combined with 0.4 ml of mixed ethanol and chloroform (5:3, v/v), and then centrifuged at 10,000 rpm and 4℃ for 30 min. The supernatant was used for SOD activity. To measure GSH-Px activity, the first supernatant from the homogenate after centrifugation at 6,000 rpm for 30 min was re-centrifuged at 15,000 rpm and 4℃ for 50 min; this supernatant was used for the GSH-Px assay. All supernatant samples were stored at -70℃ until antioxidant activity analyses.
Measurement of SOD activity
SOD activity was estimated using the method of Marklund and Marklund [28]. In a cap tube containing 0.2 ml of sample, 2.6 ml of Tris EDTA HCl buffer (50 mM Tris, 10 mM EDTA, pH 8.5) was added and then mixed with 0.2 ml of 7.2 mM pyrogallol solution. After 10 min at 25℃, 0.1 ml of 1 N HCl was added to the tube and vortexed for several seconds. The absorbance of the solution was measured at 520 nm. One unit was the amount of enzyme required to cause 50% inhibition of pyrogallol oxidation, and SOD activity was expressed as unit/mg protein.
Measurement of GSH-Px activity
GSH-Px activities in the serum, skin, and liver were determined using the method described by Flohé and Günzler [29]. In a tube, 0.1 ml of sample was combined with 0.5 ml of 0.1 M sodium phosphate buffer (pH 7.0) containing 0.1 mM EDTA, 0.1 ml glutathione reductase, and 0.1 ml 10 mM GSH. After 10 min at 37℃, 0.1 ml 1.5 mM NADPH was added. Then, 0.1 ml 12 mM cumene hydroperoxide was added to the reaction solution after 3 min. The decrease in absorbance of the solution was measured every min for 5 min at 340 nm. Values are expressed as unit/mg protein/min.
Measurement of lipid peroxidation
The amount of MDA reacting with thiobarbituric acid (TBA) was measured by the method of Ohkawa et al. [30] in the serum, skin, and liver. Sample (0.2 ml), 0.2 ml of 8.1% sodium dodecyl sulfate, and 3 ml of 20% acetic acid containing 0.8% TBA were combined in a capped tube. Distilled water was added until the total volume was 4 ml. After reacting at 95℃ for 60 min, the tube was cooled in a cold water bath and 1 ml of distilled water and 5 ml of n-butanol: pyridine (15:1, v/v) were added. The vortexed tube was centrifuged at 4,000 rpm for 20 min. The absorbance of the supernatant was measured at 532 nm. MDA content are expressed as nmol/mg protein.
Determination of serum lipid profiles and liver function
Serum lipid profiles (TG, total cholesterol, HDL-C, and LDL-C) were determined using an autoanalyzer (SPOTCEM™, Arkray, Tokyo, Japan). Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured using enzymatic kits (Asan Pharmaceutical, Seoul, Korea) based on the Reitman-Frankel method [31].
Statistical analysis
All data are expressed as means ± standard deviations using SPSS for Windows version 12.0 (SPSS, Inc., Chicago, IL, USA). The statistical analyses included a one-way analysis of variance, and mean differences were analyzed by Duncan's multiple range test. P-values < 0.05 were considered significant.
Results
Body weight, feed intake, feed efficiency ratio, and organ weights
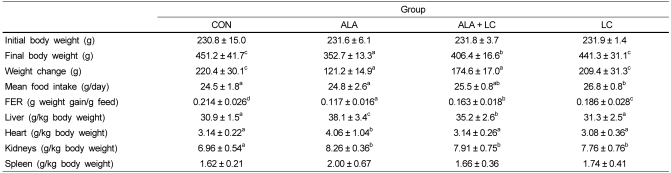
Table 2 shows the body weight, feed intake, feed efficiency ratio (FER), and organ weights of rats fed the four different diets for 6 weeks. No significant differences in initial body weight were observed among the groups; however, final body weights were lowest in the ALA group after 6 weeks (P < 0.05). The CON group had the highest final body weight (P > 0.05), which was similar to the final body weight of the LC group. The mean feed intake in the LC group was significantly higher than that in the CON and ALA groups (P < 0.05); the CON and ALA groups did not significantly differ from each other. The FER of the CON group was highest (P < 0.05) and that of the ALA group was lowest among the groups (P < 0.05). The feed intake amounts and FER of the groups supplemented with ALA tended to be lower than those of the other groups. Organ weights (per kg body weight) were highest in the ALA group, but liver, heart, and spleen weights did not differ significantly between the LC group and the CON group.
Table 2.
Body weight, food intake, feed efficiency, and organ weights
Values are expressed as means ± SD.
Means with different superscripts in the same row are significantly different by Duncan's multiple range test (P < 0.05).
CON, Control; ALA, α-lipoic acid added group; ALA + LC, α-lipoic acid and L-carnosine added group; LC, L-carnosine added group; FER, food efficiency rate.
SOD activity
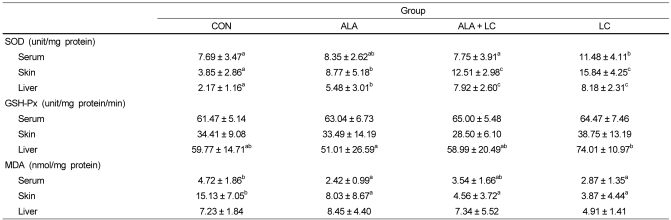
The highest SOD activities in the serum, skin, and liver were found in the LC group compared to those in the other groups (Table 3). The skin and liver SOD activities in the CON group were significantly lower than those of the groups fed the ALA or LC supplemented diets (P < 0.05). Although SOD activities in the skin and liver of the ALA group were significantly higher than those in the CON group, these activities were significantly lower in the ALA group than those in the LC supplemented groups, ALA + LC and LC (P < 0.05).
Table 3.
SOD and GSH-Px activities and MDA concentrations in serum, skin, and liver of rats
Values are expressed as means ± SD.
Means with different superscripts in the same row are significantly different by Duncan's multiple range test (P < 0.05). CON, Control; ALA, α-lipoic acid supplemented group; ALA + LC, α-lipoic acid and L-carnosine supplemented group; LC, L-carnosine supplemented group; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; MDA, malondialdehyde
GSH-Px activity
GSH-Px activities in the serum, skin, and liver are shown in Table 3. GSH-Px activities in the serum and skin were not significantly different among the groups. Only liver GSH-Px activity was highest in the LC group (P < 0.05), but no significant differences were observed in the CON, ALA, and or ALA + LC groups.
Lipid peroxidation concentrations
The highest levels of MDA in the serum and skin were found in the CON group (Table 3). Serum MDA level in the CON group was significantly higher than that in the ALA and LC groups. Furthermore, the skin MDA level in the CON group was significantly higher than that of the other groups (P < 0.05). Although the liver MDA level in the LC group was the lowest, no significant differences were found in liver MDA levels among the groups.
Serum lipid profiles and liver function
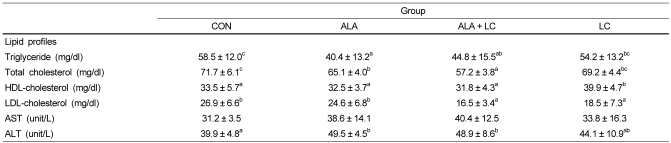
The lipid profiles and AST and ALT levels in the serum of rats fed ALA or LC supplemented diets are presented in Table 4. Serum TG and TC levels were significantly lower in the ALA group compared to those in the CON group (P < 0.05), but no difference was observed between the CON and LC groups. No significant differences among the ALA, ALA + LC, and CON groups were observed for HDL-C, whereas HDL-C level was highest in the LC group (P < 0.05). LDL-C level was significantly lower in the ALA + LC and LC groups than that in the CON and ALA groups (P < 0.05). No significant difference in serum AST levels was observed among the groups, whereas serum ALT levels were significantly higher in the ALA and ALA + LC supplemented groups than those in the CON group.
Table 4.
Lipid profiles and AST and ALT concentrations in serum
Values are expressed as means ± SD.
Means with different superscripts in the same row are significantly different by Duncan's multiple range test (P < 0.05). AST, aspartate aminotransferase; ALT, alanine aminotransferase; CON, Control; ALA, α-lipoic acid added group; ALA + LC, α-lipoic acid and L-carnosine added group; LC, L-carnosine added group.
Discussion
A significant weight reduction was observed in ALA fed rats. Similar to our data, Prieto-Hontoria et al. [23] showed a reduction in body weight in Wistar rats treated with a lipoic acid supplemented high-fat diet for 56 days. They suggested that lipoic acid reduces feed intake and feed efficiency by inhibiting intestinal sugar transport and preventing body weight gain in lean and obese models. In contrast, Dicter et al. [24] showed contradictory data for glycogen synthesis in rat soleus muscle supplemented with LA. LA causes mitochondrial uncoupling and induces the production of reactive oxygen species. LA regulates glucose metabolism as a oxidant to inhibit glycogen synthesis in muscle. Glucose metabolism by LA is regulated differently in muscle. Because of these contradictory data, our data confirm the anti-obese effect of LA supplementation in rats.
This study was performed to investigate the effects of ALA and/or LC supplementation on antioxidant activities and blood lipid profiles in rats. The rats received diets containing 0.5% ALA, 0.5% LC, 0.25% ALA + 0.25% LC, and regular rat chow diet. The results showed that the groups receiving 0.5% ALA dietary supplementation had significantly reduced final body weight and FER compared to rats fed the control diet (P < 0.05). Shen et al. [32] reported that 0.5% and 1.0% ALA supplementation for 3 weeks resulted in a decrease in final mouse body weight and carcass weights as the levels of dietary ALA increased. They concluded that ALA supplementation caused the significant decrease in carcass fat, which, in turn, decreased carcass weight. Song et al. [33] suggested that the anti-obesity effect of a 0.5% ALA diet was possibly due to an increase in energy expenditure induced by the increased gene expression of uncoupling protein-1 (UCP1) mRNA in brown adipose tissue, which generates body heat by non-shivering thermogenesis. In the current study, our results agreed with other reports pertaining to the effect of weight loss from dietary ALA supplementation, although we did not measure UCP 1 expression. Final body weight in the LC group did not differ from that in the CON group but was greater than that in the ALA group (P > 0.05). Dietary supplementation with a 5% LC diet seems to inhibit the growth of rats; however, a 0.9% and 1.8% LC diet has no effects on growth [34]. Body weight in the ALA + LC group was in between that of the ALA and LC groups, indicating that 0.25% ALA was still sufficient to affect body weight loss and to cancel out the LC effect on body weight.
The organ weights relative to body weight in the antioxidant supplemented groups were different (P < 0.05), except for spleen weight. The mean weights per kg body weight of the liver, heart, and kidneys of rats fed the 0.5% ALA diet were significantly higher than those in the CON group. However, LC group organ weights were not significantly different with those of the CON group except for kidney weight. Such differences in organ weights might simply be due to lower body weights of the ALA group rather than due to any adverse effects of the treatments, as no growth failures or signs occurred during the experimental period.
The effects of an imbalance between free radical generation and the defense system have been implicated in the pathogenesis of several disease states, such as cancer, atherosclerosis, diabetes mellitus, and Parkinson's disease. Thus, antioxidant enzymes are influenced by oxidation status, and changes in their activity can serve as a biomarker for oxidative stress [35]. In the present study, SOD activities in the serum, skin, and liver of the LC group were significantly higher than those of rats in the CON and ALA groups. LC supplementation seemed to be more effective for increasing SOD activity than that of ALA supplementation, although SOD activities in the skin and liver of the ALA group were still significantly higher than those in the CON group. Significantly higher GSH-Px activity was only shown in the liver of the LC group compared to that in the other groups. A study conducted by Liu et al. [36] indicated that LC supplementation increases liver GSH-Px activity in ethanol-induced liver injured rats. ALA supplementation (100 mg/kg/day) in rats increases antioxidant enzyme activities, including SOD and GSH-Px in blood and tissue [37-39]. However, in the current study, no significant differences in GSH-Px activity in the serum, skin, or liver were observed following ALA supplementation when compared to the CON group, which might be due to the lower ALA and LC supplementation levels (12-14 mg/day) compared to the levels in previous studies [37-39]. Combined ALA and LC supplementation (ALA + LC group) showed synergistic effects for increasing skin and liver SOD and GSH-Px activities.
Lipid peroxidation occurs when hydroxyl radicals attack fatty acid side chains of membrane phospholipids. Certain chromosomal aberrations and carcinogenesis may develop as a result of lipid peroxidation [40]. MDA is a product of lipid peroxidation, and TBARS, as indicated by the MDA concentration, serve as an oxidative damage index [41]. Zulkhairi et al. [42] reported that TBARS levels were significantly lower in an ALA treated group compared to those in an untreated group, which agrees with our results. In our study, ALA and LC supplementation significantly lowered serum and skin lipid peroxide levels as measured by MDA (P < 0.05), but not in the liver. These effects of LC supplementation might contribute to inhibiting atherosclerosis in blood vessels. Dobrota et al. [43] reported that LC exhibits a significant protective effect in brain tissue damaged by ischemic-reperfusion injury when it was administered to animals before an ischemic episode, and LC prevents higher lipid peroxidation in the brain membrane of patients with a stroke and increases the resistance of neuronal membranes to in vitro induced oxidation. In another study, LC treatment resulted in significant decreases in lipid peroxide levels in thioacetamide-induced oxidative stress and hepatotoxicity [44]. ALA supplementation also appeared to be effective for reducing oxidative stress in the rats in our study. ALA supplementation markedly lowered oxidant production and the attendant increase in oxidative damage associated with aging by ameliorating mitochondrial decay with age [42]. Furthermore, ALA supplementation with carnitine improves antioxidant status and reduces TBARS levels [45]. In our study, the serum and skin MDA levels in the ALA + LC group were not significantly different compared to those of the ALA and LC groups. Thus, any synergistic effects by combined ALA and LC supplementation were not indicated by MDA levels, although the MDA values were in between these two groups. Determining SOD and GSH-Px activities and MDA concentrations reflects the level of oxygen free radical metabolism and the extent of oxidative stress, as SOD and GSH-Px are the major antioxidant enzymes that eliminate free radicals and possess antioxidative stress functions. MDA is a lipid peroxidation product formed after free radical attack cell membranes. Supplementation with ALA and ALA + LC in this study improved SOD activities with reduced MDA levels in skin. Both SOD and GSH-Px activities increased significantly in the liver, and MDA concentrations decreased with LC supplementation. Therefore, ALA and/or LC contributed to antioxidant defense by improving SOD and GSH-Px activities and by reducing lipid peroxidation in the skin and liver of rats. The MDA levels tended to decrease with increased SOD and GSH-Px activity.
ALA supplementation decreased serum TG and TC levels significantly compared to those in the CON group, whereas ALA + LC and LC supplementation decreased serum LDL-C levels. Furthermore, LC supplementation lead to significantly increased HDL-C levels compared to those in other groups. Ford et al. [46] reported that ALA supplementation induces a decrease in plasma TG levels in diabetic rats, but that TC and HDL-C levels do not differ. Although the mechanism of how ALA and LC reduce TG, TC, and LDL-C levels is unknown [47], it is likely via lipoprotein lipase activity [48] or through cholesterol metabolism by the liver [49]. Butler et al. [50] reported that ALA supplementation in diabetic rats offsets rises in blood and liver triglycerides by inhibiting liver lipogenic gene expression, lowering hepatic triglyceride secretion, and stimulating clearance of triglyceride-rich lipoproteins. Rashid et al. [51] reported that ALA inhibits LDL glycation in vitro, which promotes foam cell formation. Foam cells are hallmarks of atherosclerosis and play a critical role in the development of the disease [38,52]. Therefore, the evident decrease in blood lipids following ALA and LC supplementation could improve endothelial function, which could have beneficial effects for preventing cardiovascular diseases.
Serum ALT concentrations were significantly higher in rats fed the ALA-supplemented diets than those of the CON (P < 0.05), and serum AST concentrations in the ALA and ALA + LC groups tended to be higher than those in the CON and LC groups. However, the serum ALT and AST concentrations were within the normal range for rats [53]. Cremer et al. [54] reported that administering 31.6 or 61.9 mg ALA/kg body weight/day for 4 weeks to rats does not result in any adverse effects, whereas a high-dose of 121 mg ALA/kg body weight is associated with slight alterations in liver enzymes and histopathological effects on the liver. Our results are in good agreement with other studies.
In conclusion, our results suggest that ALA and/or LC contribute to antioxidant defense by increasing SOD and GSH-Px activities and by decreasing lipid peroxidation in the serum, skin, and liver of rats. LC supplementation resulted in better antioxidant activities compared to that of ALA supplementation, and synergistic effects by the combined supplementation of ALA and LC were indicated by skin and liver SOD activity. ALA and LC supplementation improved serum lipid profiles in rats, which may contribute to preventing cardiovascular diseases.
References
- 1.Busby RW, Schelvis JP, Yu DS, Babcock GT, Marletta MA. Lipoic acid biosynthesis: LipA is an iron-sulfur protein. J Am Chem Soc. 1999;121:4706–4707. [Google Scholar]
- 2.Roy S, Packer L. Redox regulation of cell functions by alpha-lipoate: biochemical and molecular aspects. Biofactors. 1998;7:263–267. doi: 10.1002/biof.5520070324. [DOI] [PubMed] [Google Scholar]
- 3.Reed LJ. From lipoic acid to multi-enzyme complexes. Protein Sci. 1998;7:220–224. doi: 10.1002/pro.5560070125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J. The effects and mechanisms of mitochondrial nutrient alpha-lipoic acid on improving age-associated mitochondrial and cognitive dysfunction: an overview. Neurochem Res. 2008;33:194–203. doi: 10.1007/s11064-007-9403-0. [DOI] [PubMed] [Google Scholar]
- 5.Biewenga GP, Haenen GR, Bast A. The pharmacology of the antioxidant lipoic acid. Gen Pharmacol. 1997;29:315–331. doi: 10.1016/s0306-3623(96)00474-0. [DOI] [PubMed] [Google Scholar]
- 6.Cakatay U. Pro-oxidant actions of alpha-lipoic acid and dihydrolipoic acid. Med Hypotheses. 2006;66:110–117. doi: 10.1016/j.mehy.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Packer L, Roy S, Sen CK. Alpha-lipoic acid: a metabolic antioxidant and potential redox modulator of transcription. Adv Pharmacol. 1997;38:79–101. doi: 10.1016/s1054-3589(08)60980-1. [DOI] [PubMed] [Google Scholar]
- 8.Niijima A, Okui T, Matsumura Y, Yamano T, Tsuruoka N, Kiso Y, Nagai K. Effects of L-carnosine on renal sympathetic nerve activity and DOCA-salt hypertension in rats. Auton Neurosci. 2002;97:99–102. doi: 10.1016/s1566-0702(02)00048-6. [DOI] [PubMed] [Google Scholar]
- 9.Morganti P, Bruno C, Guarneri F, Cardillo A, Del Ciotto P, Valenzano F. Role of topical and nutritional supplement to modify the oxidative stress. Int J Cosmet Sci. 2002;24:331–339. doi: 10.1046/j.1467-2494.2002.00159.x. [DOI] [PubMed] [Google Scholar]
- 10.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 11.Song KH, Lee WJ, Koh JM, Kim HS, Youn JY, Park HS, Koh EH, Kim MS, Youn JH, Lee KU, Park JY. α-Lipoic acid prevents diabetes mellitus in diabetes-prone obese rats. Biochem Biophys Res Commun. 2005;326:197–202. doi: 10.1016/j.bbrc.2004.10.213. [DOI] [PubMed] [Google Scholar]
- 12.Thirunavukkarasu V, Nandhini AT, Anuradha CV. Lipoic acid prevents collagen abnormalities in tail tendon of high-fructose-fed rats. Diabetes Obes Metab. 2005;7:294–297. doi: 10.1111/j.1463-1326.2004.00418.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee JC, Kim KH, Ahn SM, Lee HW, Lee BG. Effect of lipoic acid on antioxidant enzyme system in aged hairless mouse skin in vivo. Korean J Dermatol. 2003;41(Suppl 2):161. [Google Scholar]
- 14.Böhm F, Edge R, Lange L, Truscott TG. Enhanced protection of human cells against ultraviolet light by antioxidant combinations involving dietary carotenoids. J Photochem Photobiol B. 1998;44:211–215. doi: 10.1016/s1011-1344(98)00146-8. [DOI] [PubMed] [Google Scholar]
- 15.Böhm F, Edge R, McGarvey DJ, Truscott TG. Beta-carotene with vitamins E and C offers synergistic cell protection against NOx. FEBS Lett. 1998;436:387–389. doi: 10.1016/s0014-5793(98)01173-9. [DOI] [PubMed] [Google Scholar]
- 16.Beitner H. Randomized, placebo-controlled, double blind study on the clinical efficacy of a cream containing 5% α-lipoic acid related to photoageing of facial skin. Br J Dermatol. 2003;149:841–849. doi: 10.1046/j.1365-2133.2003.05597.x. [DOI] [PubMed] [Google Scholar]
- 17.Freisleben HJ, Groth N, Fuchs J, Rudolph P, Zimmer G, Herrling T. Penetration of spin-labeled dihydrolipoate into the skin of hairless mice. Modification of epidermal and dermal polarity. Arzneimittelforschung. 1994;44:1047–1050. [PubMed] [Google Scholar]
- 18.Podda M, Rallis M, Traber MG, Packer L, Maibach HI. Kinetic study of cutaneous and subcutaneous distribution following topical application of [7,8-14C]rac-alpha-lipoic acid onto hairless mice. Biochem Pharmacol. 1996;52:627–633. doi: 10.1016/0006-2952(96)00337-1. [DOI] [PubMed] [Google Scholar]
- 19.Boldyrev AA, Severin SE. The histidine-containing dipeptides, carnosine and anserine: distribution, properties and biological significance. Adv Enzyme Regul. 1990;30:175–194. doi: 10.1016/0065-2571(90)90017-v. [DOI] [PubMed] [Google Scholar]
- 20.Decker EA, Livisay SA, Zhou S. A re-evaluation of the antioxidant activity of purified carnosine. Biochemistry (Mosc) 2000;65:766–770. [PubMed] [Google Scholar]
- 21.Lee YT, Hsu CC, Lin MH, Liu KS, Yin MC. Histidine and carnosine delay diabetic deterioration in mice and protect human low density lipoprotein against oxidation and glycation. Eur J Pharmacol. 2005;513:145–150. doi: 10.1016/j.ejphar.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Yamano T, Niijima A, Iimori S, Tsuruoka N, Kiso Y, Nagai K. Effect of L-carnosine on the hyperglycemia caused by intracranial injection of 2-deoxy-D-glucose in rats. Neurosci Lett. 2001;313:78–82. doi: 10.1016/s0304-3940(01)02231-5. [DOI] [PubMed] [Google Scholar]
- 23.Prieto-Hontoria PL, Pérez-Matute P, Fernández-Galilea M, Barber A, Martínez JA, Moreno-Aliaga MJ. Lipoic acid prevents body weight gain induced by a high fat diet in rats: effects on intestinal sugar transport. J Physiol Biochem. 2009;65:43–50. doi: 10.1007/BF03165968. [DOI] [PubMed] [Google Scholar]
- 24.Dicter N, Madar Z, Tirosh O. Alpha-lipoic acid inhibits glycogen synthesis in rat soleus muscle via its oxidative activity and the uncoupling of mitochondria. J Nutr. 2002;132:3001–3006. doi: 10.1093/jn/131.10.3001. [DOI] [PubMed] [Google Scholar]
- 25.Ramos LF, Kane J, McMonagle E, Le P, Wu P, Shintani A, Ikizler TA, Himmelfarb J. Effects of combination tocopherols and alpha lipoic acid therapy on oxidative stress and inflammatory biomarkers in chronic kidney disease. J Ren Nutr. 2011;21:211–218. doi: 10.1053/j.jrn.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yi HC. Supplementation to diet on muscle carnosine and blood chemical indices of rats. Seoul: Chung-Ang University; 2006. [master's thesis] [Google Scholar]
- 27.Kim YP, Lee SC. Superoxide dismutase activities in the human skin. In: Hayaishi O, Imamura S, Miyachi Y, editors. The Biological Role of Reactive Oxygen Species in Skin. Tokyo: University of Tokyo Press; 1987. pp. 225–320. [Google Scholar]
- 28.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 29.Flohé L, Günzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–121. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 30.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 31.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 32.Shen QW, Jones CS, Kalchayanand N, Zhu MJ, Du M. Effect of dietary alpha-lipoic acid on growth, body composition, muscle pH, and AMP-activated protein kinase phosphorylation in mice. J Anim Sci. 2005;83:2611–2617. doi: 10.2527/2005.83112611x. [DOI] [PubMed] [Google Scholar]
- 33.Song KH, Youn JY, Nam Koong C, Shin MJ, Ryu JW, Park HS, Kim MS, Park JY, Lee KU. Anti-obesity effects of α-lipoic acid in OLETF Rats. J Korean Diabetes Assoc. 2002;26:460–468. [Google Scholar]
- 34.Tamaki N, Funatsuka A, Fujimoto S, Hama T. The utilization of carnosine in rats fed on a histidine-free diet and its effect on the levels of tissue histidine and carnosine. J Nutr Sci Vitaminol (Tokyo) 1984;30:541–551. doi: 10.3177/jnsv.30.541. [DOI] [PubMed] [Google Scholar]
- 35.Voss P, Siems W. Clinical oxidation parameters of aging. Free Radic Res. 2006;40:1339–1349. doi: 10.1080/10715760600953859. [DOI] [PubMed] [Google Scholar]
- 36.Liu WH, Liu TC, Yin MC. Beneficial effects of histidine and carnosine on ethanol-induced chronic liver injury. Food Chem Toxicol. 2008;46:1503–1509. doi: 10.1016/j.fct.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 37.Arivazhagan P, Thilakavathy T, Panneerselvam C. Antioxidant lipoate and tissue antioxidants in aged rats. J Nutr Biochem. 2000;11:122–127. doi: 10.1016/s0955-2863(99)00079-0. [DOI] [PubMed] [Google Scholar]
- 38.Sahin M, Sağdiç G, Elmas O, Akpinar D, Derin N, Aslan M, Agar A, Alicigüzel Y, Yargiçoğlu P. Effect of chronic restraint stress and alpha-lipoic acid on lipid peroxidation and antioxidant enzyme activities in rat peripheral organs. Pharmacol Res. 2006;54:247–252. doi: 10.1016/j.phrs.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 39.Akpinar D, Yargiçoğlu P, Derin N, Alicigüzel Y, Ağar A. The effect of lipoic acid antioxidant status and lipid peroxidation in rats exposed to chronic restraint stress. Physiol Res. 2008;57:893–901. doi: 10.33549/physiolres.931284. [DOI] [PubMed] [Google Scholar]
- 40.Saygili EI, Konukoglu D, Papila C, Akcay T. Levels of plasma vitamin E, vitamin C, TBARS, and cholesterol in male patients with colorectal tumors. Biochemistry (Mosc) 2003;68:325–328. doi: 10.1023/a:1023010418230. [DOI] [PubMed] [Google Scholar]
- 41.Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 42.Zulkhairi A, Zaiton Z, Jamaluddin M, Sharida F, Mohd TH, Hasnah B, Nazmi HM, Khairul O, Zanariyah A. Alpha lipoic acid possess dual antioxidant and lipid lowering properties in atherosclerotic-induced New Zealand White rabbit. Biomed Pharmacother. 2008;62:716–722. doi: 10.1016/j.biopha.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Dobrota D, Fedorova T, Stvolinsky S, Babusikova E, Likavcanova K, Drgova A, Strapkova A, Boldyrev A. Carnosine protects the brain of rats and Mongolian gerbils against ischemic injury: after-stroke-effect. Neurochem Res. 2005;30:1283–1288. doi: 10.1007/s11064-005-8799-7. [DOI] [PubMed] [Google Scholar]
- 44.Mehmetçik G, Ozdemirler G, Koçak-Toker N, Cevikbaş U, Uysal M. Role of carnosine in preventing thioacetamide-induced liver injury in the rat. Peptides. 2008;29:425–429. doi: 10.1016/j.peptides.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Long J, Gao F, Tong L, Cotman CW, Ames BN, Liu J. Mitochondrial decay in the brains of old rats: ameliorating effect of alpha-lipoic acid and acetyl-L-carnitine. Neurochem Res. 2009;34:755–763. doi: 10.1007/s11064-008-9850-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ford I, Cotter MA, Cameron NE, Greaves M. The effects of treatment with α-lipoic acid or evening primrose oil on vascular hemostatic and lipid risk factors, blood flow, and peripheral nerve conduction in the streptozotocin-diabetic rat. Metabolism. 2001;50:868–875. doi: 10.1053/meta.2001.24914. [DOI] [PubMed] [Google Scholar]
- 47.Ziegler D, Nowak H, Kempler P, Vargha P, Low PA. Treatment of symptomatic diabetic polyneuropathy with the antioxidant α-lipoic acid: a meta-analysis. Diabet Med. 2004;21:114–121. doi: 10.1111/j.1464-5491.2004.01109.x. [DOI] [PubMed] [Google Scholar]
- 48.Ichikawa T, Liang J, Kitajima S, Koike T, Wang X, Sun H, Morimoto M, Shikama H, Watanabe T, Yamada N, Fan J. Macrophage-derived lipoprotein lipase increases aortic atherosclerosis in cholesterol-fed Tg rabbits. Atherosclerosis. 2005;179:87–95. doi: 10.1016/j.atherosclerosis.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 49.Marangon K, Devaraj S, Tirosh O, Packer L, Jialal I. Comparison of the effect of alpha-lipoic acid and alpha-tocopherol supplementation on measures of oxidative stress. Free Radic Biol Med. 1999;27:1114–1121. doi: 10.1016/s0891-5849(99)00155-0. [DOI] [PubMed] [Google Scholar]
- 50.Butler JA, Hagen TM, Moreau R. Lipoic acid improves hypertriglyceridemia by stimulating triacylglycerol clearance and downregulating liver triacylglycerol secretion. Arch Biochem Biophys. 2009;485:63–71. doi: 10.1016/j.abb.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rashid I, van Reyk DM, Davies MJ. Carnosine and its constituents inhibit glycation of low-density lipoproteins that promotes foam cell formation in vitro. FEBS Lett. 2007;581:1067–1070. doi: 10.1016/j.febslet.2007.01.082. [DOI] [PubMed] [Google Scholar]
- 52.Li AC, Glass CK. The macrophage foam cell as a target for therapeutic intervention. Nat Med. 2002;8:1235–1242. doi: 10.1038/nm1102-1235. [DOI] [PubMed] [Google Scholar]
- 53.Wolford ST, Schroer RA, Gohs FX, Gallo PP, Brodeck M, Falk HB, Ruhren R. Reference range data base for serum chemistry and hematology values in laboratory animals. J Toxicol Environ Health. 1986;18:161–188. doi: 10.1080/15287398609530859. [DOI] [PubMed] [Google Scholar]
- 54.Cremer DR, Rabeler R, Roberts A, Lynch B. Safety evaluation of alpha-lipoic acid (ALA) Regul Toxicol Pharmacol. 2006;46:29–41. doi: 10.1016/j.yrtph.2006.06.004. [DOI] [PubMed] [Google Scholar]