Abstract
One of the major limitations of studying cancer-bone metastasis has been the lack of an appropriate ex-vivo model which can be used under defined conditions that simulates closely the in vivo live bone microenvironment in response to cancer-bone interactions. We have developed and utilized a three-dimensional (3D) cancer-bone metastasis model using free floating live mouse calvarial bone organs in the presence of cancer cells in a roller-tube system. In such co-cultures under hypoxia and a specifically defined bone remodeling stage, viz., resorption system, cancer cells showed a remarkable affinity and specificity for the “endosteal side” of the bone where they colonize and proliferate. This was concurrent with differentiation of resident stem/progenitor cells to osteoclasts and bone resorption. In contrast, under bone formation conditions this model revealed different pathophysiology where the breast cancer cells continued to induce osteoclastic bone resorption whereas prostate cancer cells led to osteoblastic bone formation. The current 3D model was used to demonstrate its application to studies involving chemical and biochemical perturbations in the absence and presence of cancer cells and cellular responses. We describe proof-of-principle with examples of the broad versatility and multi-faceted application of this model that adds another dimension to the ongoing studies in the cancer-bone metastasis arena.
Keywords: Breast and Prostate Cancer Cells, Bone Resorption, Calvarial bone organ cultures, Indomethacin, Cancer-Bone Metastasis Model, Prostaglandin E2
1. INTRODUCTION
Bone metastases occur in a majority of patients with advanced breast and prostate cancers for reasons that are still not well understood. While bone is not the only organ where metastasis can occur, bone is one of the preferred sites [1]. Once in the bloodstream tumor cells reaching the bone marrow adhere to the “endosteal” surface and colonize bone. The site and formation of bone lesions is determined by multiple cellular and molecular interactions between cancer cells and the bone microenvironment [2-4]. These co-operative interactions rely on factors that are secreted by cancer cells that stimulate proliferation and differentiation of osteoblasts and osteoclasts with a net effect of either bone resorption or bone formation [5, 6]. Bone undergoes remodeling continuously throughout life with the coupling of formation and resorption involving osteoblasts and osteoclasts, respectively. The physico-chemical properties of the bone microenvironment and its cellular components play a significant role in pathogenesis. To date a clear understanding of the cellular and molecular mechanisms involved in cancer-bone interactions and other factors that may play a critical role are limited. This is partly due to a lack of an ideal experimental model(s) that could recapitulate these complex multicellular physiological events under defined conditions to effectively study them at physical, cellular and molecular levels.
A variety of two-dimensional (2D) cell cultures and in vivo animal models have been developed and used to investigate the mechanistic complexities of cancer-bone metastasis [7-9]. It is well known that cell-cell and cell matrix interactions play a major role in tumor morphogenesis and cancer metastasis. However, thus far there is no ideal model that incorporates all of these in vivo physiological events. In a 2D cell culture system there is no three-dimensional (3D) microenvironment consisting of an extracellular matrix (ECM) comparable to native tissue. This lack of cell-matrix interaction has an adverse impact by deviating from the in vivo condition which results in changes in cancer cell phenotype and gene expressions [10, 11]. Such inconsistencies may provide less reliable data with limitations for translating to clinical applications. While in vivo animal models have more relevance and lack the disadvantages of the 2D cell culture systems, they too have limitations. There is the ethical and cost issues which can restrict the number of experiments to obtain statistically reliable data. The pathology and disease progression may not be accurately represented between humans and animal models. In response to demands for new and improved cancer-bone metastasis models, more recent studies have attempted to overcome these limitations by the development of three-dimensional (3D) in vitro models. These include the use of cancer cells embedded in collagen type I gel and co-cultured with mouse calvarial bone directly in contact with the gel or separated by a filter in a normal culture dish and conditions [12]. A variation of such co-cultures was the development of cancer cells grown on the bottom of the culture dish with calvarial bone placed on an elevated metal grid under normal culture conditions [13]. Other studies used engineered bio-organic polymer scaffolds to create 3D systems which have yielded important new information regarding cell signaling, phenotypic changes, angiogenesis, chemo-resistance of cancer cells and interaction of cancer cells with osteoblastic matrices [14-19]. Despite these advances, however, these latter bioengineered 3D-constructs do not reflect the in vivo conditions of live bone. This is because there is no well organized multicellular cell population consisting of stem/progenitor cells such as those related to mesenchymal, hematopoeitic and stromal origin that can respond to the presence of cancer cells and in turn have dual-feedback communications. Furthermore, there is no true bone matrix which contains embedded osteocyte cells and ECM that has well organized structural components such as collagen and many other proteins that generate architectural integrity. These ECM components together with the multicellular “periosteal and endosteal” layers of real bone play critical roles in assisting cancer cell attachment, proliferation, cell signaling processes and bi-directional communications between cancer and bone cells.
We have developed and utilized a roller-tube model system which simulates closely the in vivo tissue under defined conditions and specific bone remodeling stages, i.e., resorption or formation, including hypoxic conditions which both solid tumors and bone experience naturally. This current report describes the development and multi-faceted application of this model for studies of cancer-bone metastasis/interactions.
2. MATERIALS AND METHODS
2.1. Roller-tube system for calvarial bone organ cultures and cancer cell studies under hypoxic conditions and in the absence of immuno-reaction
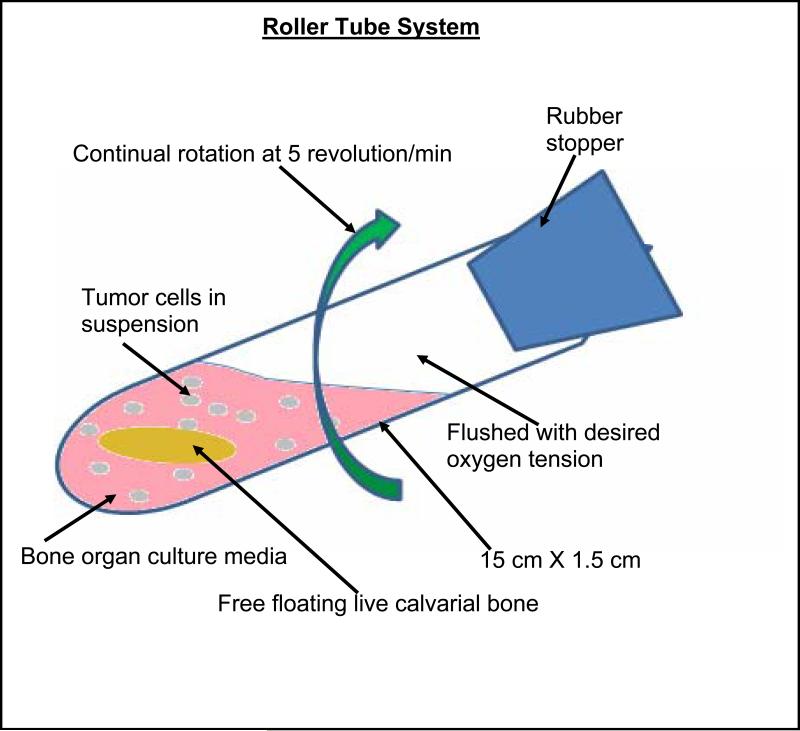
Calvaria from 5-7 day old neonatal CD-1 mice (Charles River Laboratories, MA) were dissected under sterile conditions. Calvaria were cut in the occipital lobe, and partially in the frontal lobe, to produce a trapezoid structure and rinsed in culture medium. The live bone organ culture medium consisted of DMEM supplemented with bovine serum albumin (BSA), fraction V (5 mg/ml, Sigma Co.), 100 U/ml penicillin and 100 μg/ml streptomycin and 250 ng/ml amphotericin B (Gibco, Grand Island, NY) with “no” fetal calf serum (FCS) or ascorbate. The approach permits studies to be performed with remodeling events dissociated whereby when stimulated by inclusion of parathyroid hormone (PTH) [20] or PGE2 or conditioned media from cancer cells, calvarial bone undergoes predominantly osteoclastic bone resorption. For the bone formation model in addition to the above conditions, 150 μg/ml of sodium ascorbate was included in the bone culture media. In this system for co-cultures, a single calvarium was placed in each roller-tube (15 cm × 16 cm, Bellco, Inc. N.J.) containing 2 ml of bone culture media with respective cancer cells (5 × 105 per tube) in suspension. (a) For the bone resorption model the culture media contained no FCS or ascorbate. (b) For the bone formation model the culture media contained no FCS but sodium ascorbate was included. The media and the tubes were flushed with a gas mixture of 5% O2, 5% CO2, and 90% N2, for 60 seconds, the roller tubes were sealed with a stopper, and placed horizontally in a roller tube apparatus (Bellco Biotechnology, Inc. Vineland, N.J.) that makes 5 revolutions per min. in a 37°C incubator, see Scheme 1. For individual cultures of bone alone or cancer cells alone the appropriate media and a single respective specimen was used and tubes processed as above.
Scheme 1.
The roller tube system was comprised of pyrex tubes of 15 cm × 1.6 cm with 2 ml of culture media, one free floating calvarial bone and cancer cells (5×105) flushed with a mixture of 5% O2, 5% CO2 and 90% N2 (using a gas cylinder ordered to specification, Medical Gas Supplier, Inc.) for hypoxic conditions. The tubes with sterile stoppers were placed horizontally in a roller tube apparatus with a setting of 5 revolutions per min in a 37°C incubator.
2.2. Effects of cancer cell conditioned media, added factors and drugs on calvarial bone cultures under hypoxic (5% O2) conditions
Five separate samples (a-e) for each breast tumor cell lines, MCF-7 and MDA-MB-231, and prostate tumor cell lines, LnCap Clone FGC and PC-3 (American Type Culture Collection), were grown in Dulbecco's Modified Eagle Medium (DMEM) in the presence of Fetal Calf Serum (FCS) for breast tumor cells or Roswell Park Memorial Institute media (RPMI) for prostate tumor cells. Tumor cells (1 × 106 per tube) were incubated at 37°C for 8 days, with each tumor cell type in five (a-e) separate sterile, rubber-stoppered roller-tubes at 5% oxygen tension (a total of 20 samples), see also Scheme 1. All tumor media was changed every 2-3 days, and freshly oxygenated with 5% oxygen in gas phase.
On day 6, media was changed for the second time, and all five (a-e) conditioned media for each of the four tumor types were collected for time point, T2, and stored at -20°C. On day 8 the conditioned media were also collected, time point T3, and the roller-tube experiment was terminated. T2 and T3 time point conditioned tumor media were combined for each of the individual a, b, c, d, and e-samples. These five different tumor conditioned media obtained for each of the different tumors were used to spike at 25% level the calvarial bone culture media. Conditioned media from four different human tumor cell lines grown under hypoxia were used to test their effects on live mouse calvarial bone cultures under conditions primed to undergo only bone resorption if presented with appropriate external stimuli. A single calvarium and 2.0 ml of bone culture media spiked with 25% of tumor media was added to each roller tube and incubated as described above. A total of 20 calvaria were used to set up one calvarium per conditioned cancer cell media from each of four cancer cell lines. A second set of such 20 calvaria with the same cancer cell media was set up but in the presence of 5 μM Indomethacin. A set of control calvarial bone cultures were carried out using: (a) five individual calvarial bone cultures with no cancer media addition, and (b) five calvarial bone cultured in the presence of added 20 nM PGE2 (Cayman Chemical Co., Ann Arbor, Michigan U.S.A.). These bone cultures were grown for 7 days with media changed every 2-3 days and the used media were stored at -20°C. At the end of 7 day culture the calvarial bones were fixed and stained with H & E for histological analysis and the media were analyzed for prostaglandin E2 (PGE2) levels using commercially available PGE2 ELISA Kit as described in the protocol (Cayman Chemical Co., Ann Arbor, Michigan U.S.A.).
2.3. Roller-tube system for three-dimensional co-cultures of free-floating live calvarial bones and cancer cells in suspension under hypoxic conditions
The roller tubes were set up as follows: (a) Ten tubes each containing one calvarium and 5 × 105 cells of a same cancer cell line, and this was repeated for each of the four cancer cell lines, (total of 40 co-cultures), (b) controls were 10 roller tubes each containing a single calvarium in the absence of any tumor cells. The media was first changed after 48 hrs, and again on day 5 followed by 2 additional days of incubation with a total co-culture time of 7 days. Each time the media was changed the tubes were flushed with a gas mixture of 5% O2, 5% CO2, and 90% N2. At the end of 7 days, the calvarial bones co-cultured with respective cancer cell lines were processed for histological observations, tartrate resistant acid phosphatase (TRAP) enzyme activity, neutral red (NR) and counter silver nitrate staining.
(a) Histological and TRAP enzyme activity analysis
For histological analysis 5 calvarial bones for each cancer cell line from co-cultures was split into two halves at the suture line. One half was fixed in 10% formalin and H & E staining and the other half used for TRAP enzyme activity measurements. Histological sections were obtained from 5 calvaria per cancer cell line co-cultures, with two different region serial sectioned for each calvaria. A total of 50 sections from 5 different cultured calvaria per group were used to obtain representative osteoclastic bone loss. Five control calvaria were also treated the same way. TRAP activity, a marker for osteoclast activity, for 5 calvaria per cell line was determined as described in detail [20].
(b) Neutral red staining to visualize osteoclasts
On day 7 the media of calvarial bones that were cultured with respective cancer cell lines were removed and replaced with bone media containing 70 μg/ml NR (Sigma, Co.), flushed with 5% O2 and incubated for 45 min at 37°C in roller tubes. In this live bone culture approach osteoclast cells take up NR rapidly and because of their large size and multinucleated nature provide a clear contrast between osteoclasts and other bone cells and matrix for microscopic observations.
(c) Quantitative analysis of bone resorption areas using total morphometric analysis
Following NR staining and microscopic examination, the same NR stained half calvarial bones were used for quantitative determination of bone resorption areas and mineral loss. NR stained calvaria were washed in PBS, fixed in 10% formalin overnight and counter stained with 2% silver nitrate for 30 min. The mineral loss and bone resorption areas were clearly apparent under the microscope where resorption regions were transparent to light and total resorption areas can be measured and quantified under low magnification, viz., 30x. The digital images captured using Dell computer with BioQuant software (BioQuant Image Analysis, Co., Nashville, TN, U.S.A.) were used to manually highlight by computer assisted tracing of the resorption areas and calculation of the unit area by the BioQuant software. The unit resorption areas were then used to calculate the % bone loss relative to controls and total calvarial bone surface area.
2.4. Evaluation of alteration in gene expression levels due to cancer-bone interactions under hypoxia by comprehensive human and mouse gene micrarrays
Calvaria were dissected as described above and co-cultured in roller tubes in the presence of 5 × 105 PC3 cells under hypoxic conditions. Total RNA was extracted from following sets of cultures: (i) for co-cultures of bone and PC3 cancer cells 15 roller tubes each containing one free floating calvarium and 5 × 105 PC3 cells in suspension; four sets of such cultures were carried out, two sets for RNA extraction to be used for human microarrays and two sets for mouse microarrays; (ii) for control calvaria, 15 roller tubes each containing one calvarium without cancer cells; two sets of such cultures were carried out for RNA extraction and for mouse microarrays; and (iii) for control PC3 cancer cells 15 roller tube each containing 5 × 105 PC3 cells without calvaria; two sets of such cultures were carried out for RNA extraction and human microarrays. The co-cultures and cultures with calvaria and PC3 cancer cells alone were maintained for 5 days with media changed after 48 hrs. Four sets, case (i) above, of 15 calvaria with PC3 cells growing on their surface were then collected for total RNA preparation. Similarly two sets of control free floating 15 clavaria, case (ii), and two sets of PC3 cells, case (iii), grown alone were processed for RNA extraction. The use of combined 15 roller tubes per set in (i)–(iii) was necessary to obtain sufficient RNA to perform microarrays.
2.5. RNA extraction
In brief, calvaria ± PC3 cancer cells and PC3 cancer cells alone were snap frozen and powdered in liquid nitrogen after washing with PBS and stored at -70°C. RNA was extracted with TRIzol reagent (Invitron, CA) according to instructions from the manufacturer, followed by isopropanol precipitation. Samples were washed with 75% ethanol and traces of contaminating DNA were removed by use of the DNA-free kit (Ambion, TX), according to manufacturer's instructions. After quantification of the RNA content at OD260, the RNA integrity was checked by 1.5% Tris-borate-EDTA agarose gel electrophoresis.
2.6. Comprehensive human and mouse microarray gene expression analysis
RNA samples prepared were submitted for comprehensive gene expression analysis to the gene analysis core facility at Childrens Hospital Boston, Department of Genetics, Boston, MA. The above model system developed using mouse calvarial bones and human cancer cell lines has the advantage of allowing for use of two different gene arrays to determine changes in the gene expression levels in human cancer cells and mouse calvarial bones as a result of cancer-bone interactions. Comprehensive human and mouse gene array analyses were performed in duplicates on the above sample sets utilizing the Affymetrix Human Genome U133A and Mouse Genome MOE430A Array, respectively. Data analysis was performed using the Microarray Suite software. Two comparisons were made: expression levels of PC3 cells alone vs. PC3 cells + bone (comparison 1, human genome), and bone cells alone vs. bone cells + PC3 cells (comparison 2, mouse genome)
2.7. Microscopic observations
All microscopic images were obtained using a Retiga 1300 digital camera (Qimaging, Canada) interfaced with a Leitz Dialux 20 EB microscope (Leitz, Germany). The digital images were captured using Dell computer with BioQuant software (BioQuant Image Analysis, Co., Nashville, TN, U.S.A.). This same software was used for determination of the quantitative bone resorption areas and % bone loss of the NR + silver nitrate stained histological sections.
2.8. Statistics
Data are presented as means ± S.D. The significance of differences between groups was determined with the Student's t-Test for pairs of samples using the Bonferroni option within InStat GraphPad, p < 0.05 was considered significant.
3. RESULTS
3.1. Evaluation of the biochemical and cellular characteristics of the live calvarial bone organ cultures in response to cancer cell media, as well as chemical and biochemical perturbations
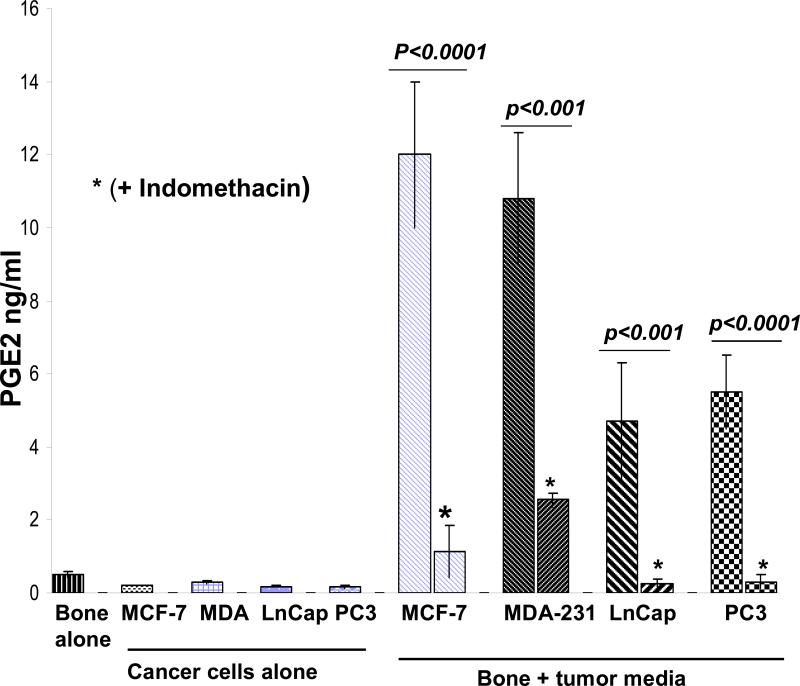
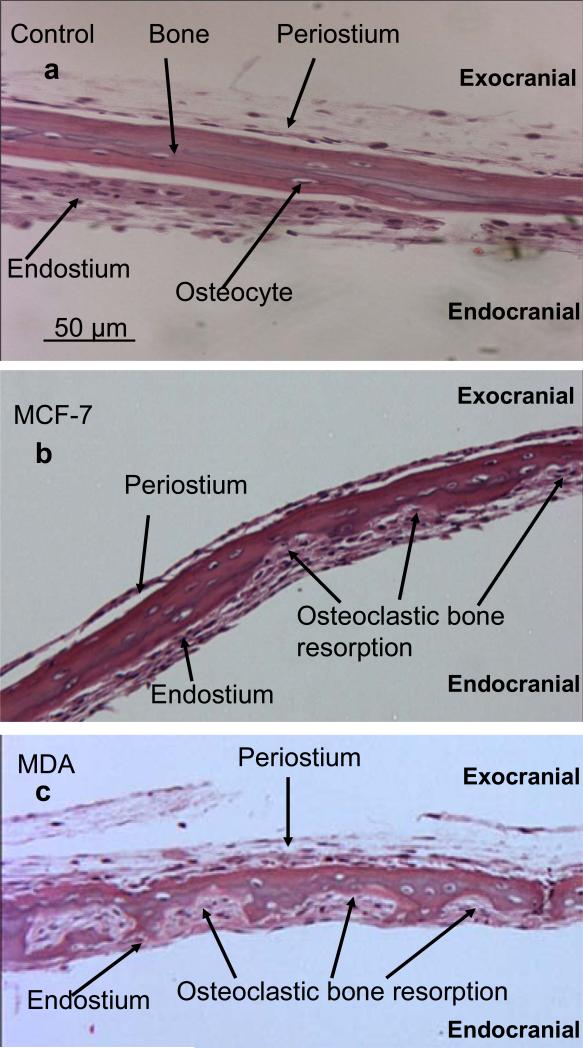
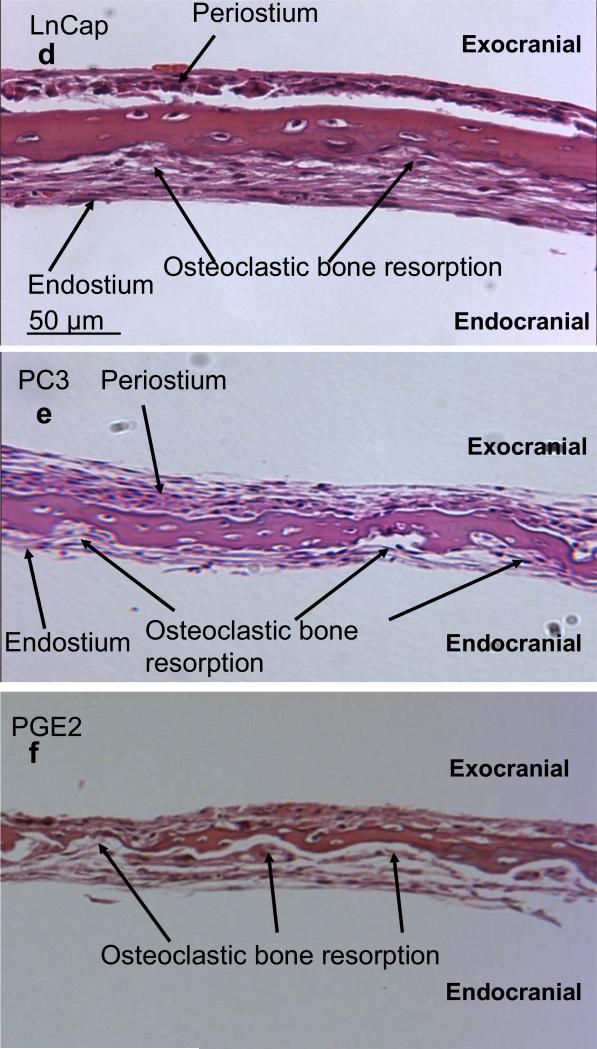
Our initial aim was to establish the effects of media from cancer cells grown under hypoxic conditions on mouse calvarial bone organ cultures using the “roller-tube model” under hypoxia in the absence and presence of indomethacin and prostaglanding E2 (PGE2). Figure 1 shows PGE2 levels in media of controls (bone alone and cancer cells alone) and calvarial bone cultures in the presence of different cancer cell media. The results clearly established that all of the tested cancer cell lines secreted factors into the media capable of stimulating live calvarial bones to synthesize substantial amounts of PGE2 and bone resorption through osteoclast differentiation. This was confirmed by inclusion of indomethacin, a known inhibitor of COX-2 enzyme and PGE2 synthesis, in the media of bone cultures which resulted in significant inhibition of PGE2 synthesis. There were, however, differences in the extent to which different cancer cell line media can stimulate bone to synthesize PGE2 and bone resorption. The highest level of PGE2 synthesis by bone was induced by media from MCF7 cells and MDA cells. The prostate, LnCap and PC3, cancer cell media induced much less PGE2 synthesis. These results were consistent with histological analysis of such cultures (Fig. 2). The number of osteoclasts and degree of bone resorption induced by the media derived from cancer cells were in the following order MDA-MB-231 > MCF7 > PC3 > LnCap as defined by the histological data. Importantly, in the live calvarial bone cultures using the roller-tube system under hypoxia, in all cases osteoclast differentiation and bone resorption was restricted to the “endocranial side” (endosteal cell layer) of these bones. This same biological response was also observed when such bone cultures were studied using a “Grid-System” under normoxia (21% O2) and stimulated by parathyroid hormone (PTH), as we have recently reported [20]. These inherent biological characteristics of calvarial bones will play a major role in the cancer-bone metastasis mechanism when co-cultures are used in roller-tubes as detailed below.
Figure 1.
PGE2 levels in media of calvarial bone cultures using hypoxic conditions and spiked with 25% media from cancer cells grown under hypoxic conditions in the absence and presence of indomethacin. The PGE2 levels in the media from calvarial bones grown alone and the media from cancer cells grown alone are as indicated. The two sample student's t test indicated that the difference in PGE2 synthesis in response to cancer media in the absence and presence of indometacin were statistically significant with p values as noted within the Figure. The PGE2 levels within tumor types, MCF-7 versus MDA-231, or LnCap versus PC3, were statistically not significant, p > 0.05. PGE2 levels in response to breast versus prostate cancer media were different and these were statistically significant, p < 0.001. The levels of PGE2 in tumor media alone for the four tumors were not different and statistically not significant, p >0.05, but PGE2 levels in bone alone versus tumor media alone were different and statistically significant p < 0.01.
Figure 2.
H & E stained sections of calvarial bones grown in the presence of cancer media under hypoxic conditions. (a) Control calvarial bone with no cancer media. (b)–(e) Calvarial bone cultured in the presence of media from cancer cell lines, MCF-7, MDA-231, LnCap, and PC3, respectively. (f) Calvarial bone cultured in the presence of added 20 nM PGE2 only. All sections are at 100x magnification.
3.2. Three-dimensional cancer-bone metastasis model using co-cultures of calvarial bone organs and cancer cells in suspension using the roller-tube system under hypoxia
(a) Bone resorption model
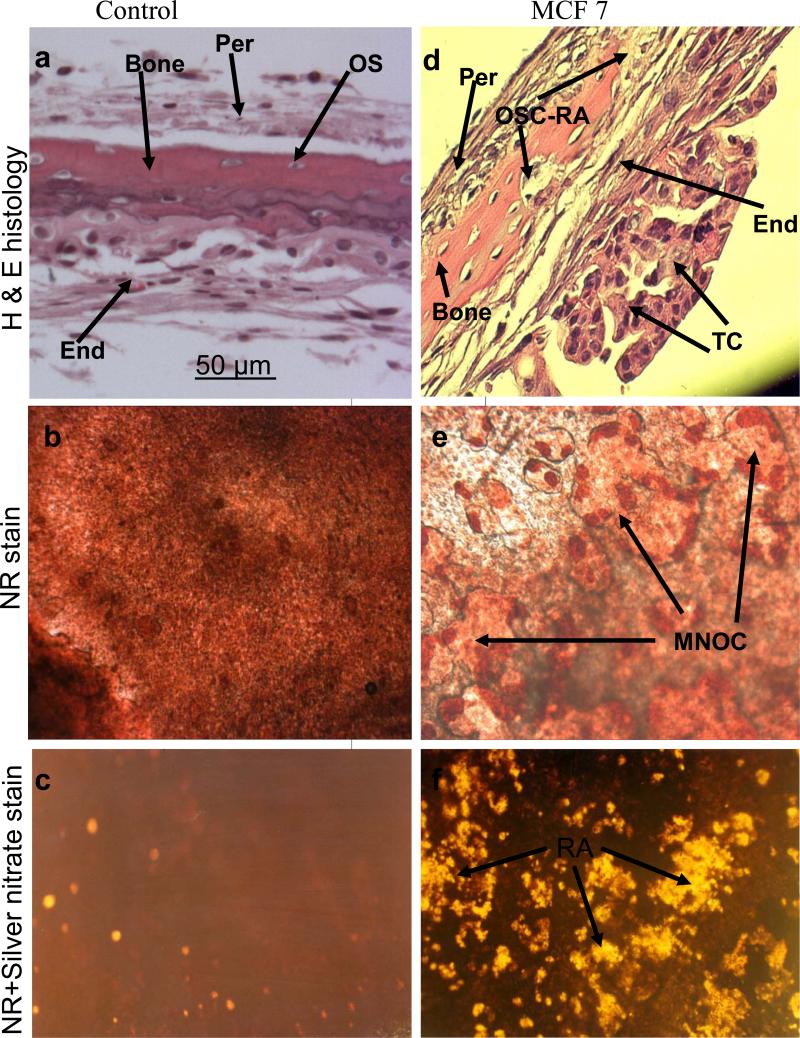
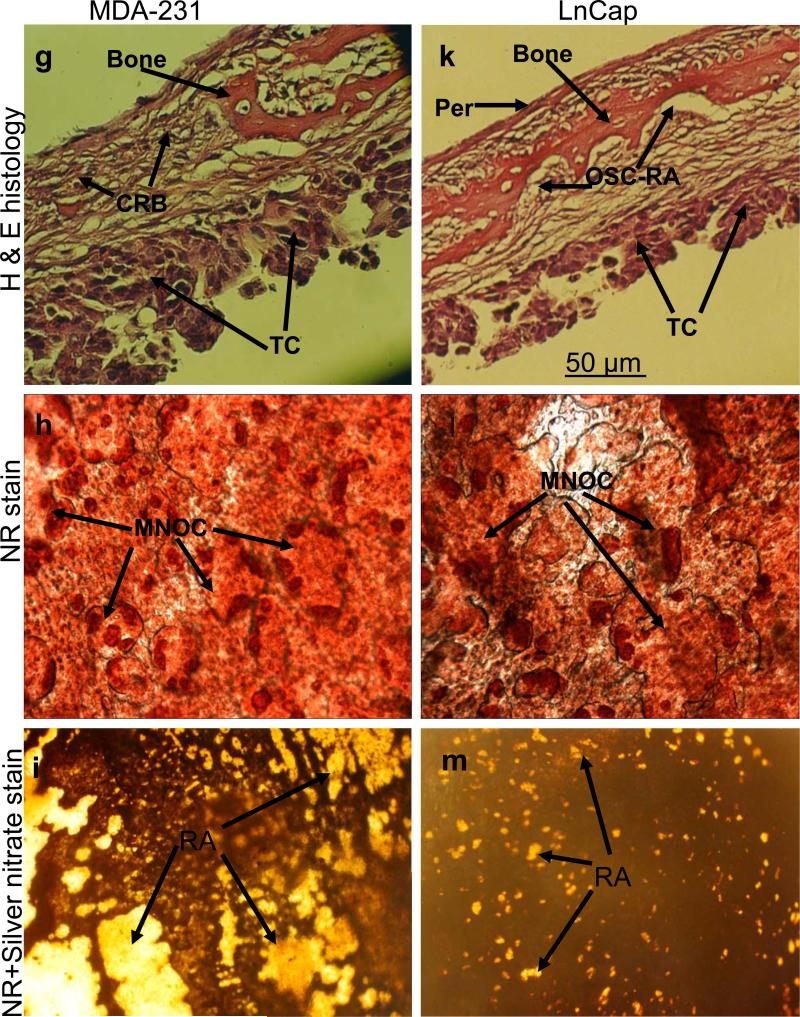
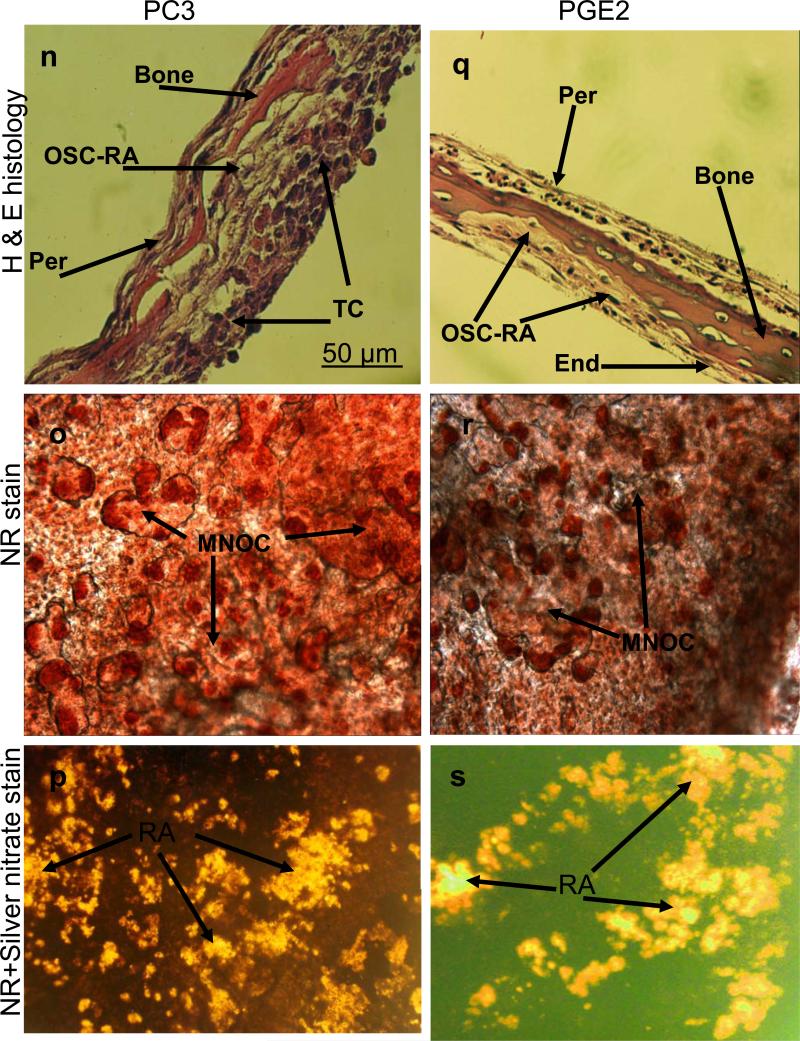
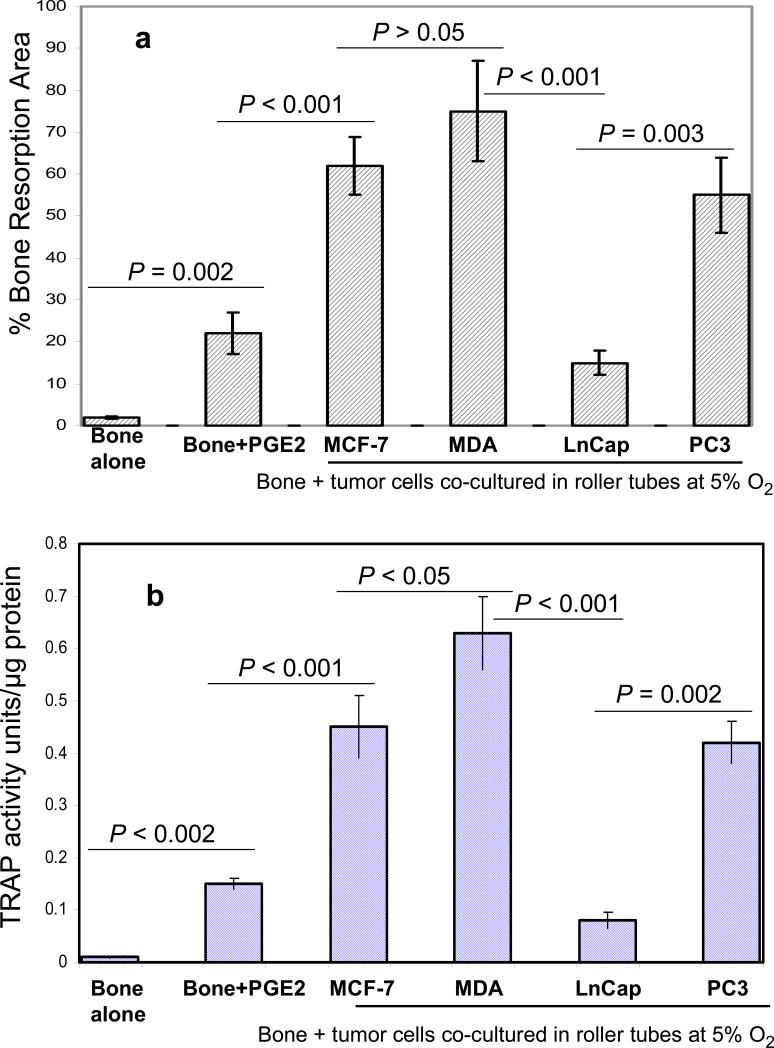
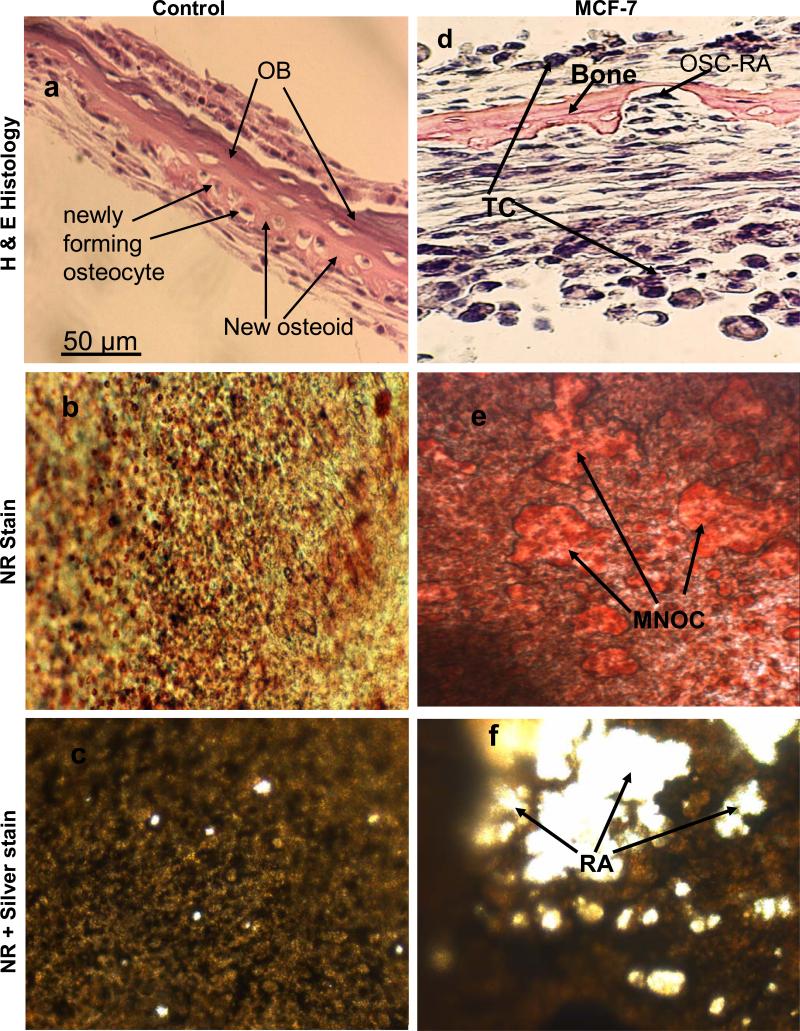
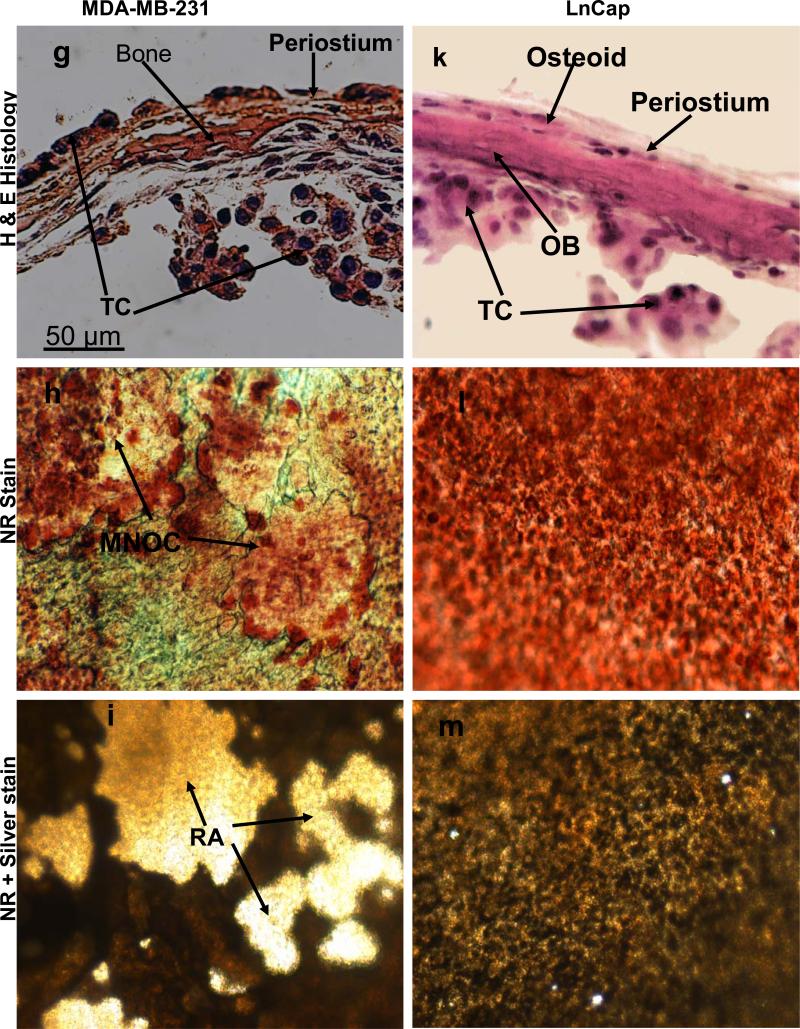
The results obtained above revealed important clues related to the possible significance of the specific cellular components and microenvironment of the endosteal cell layer of calvarial bones that may play a critical role during cancer-bone metastasis. To investigate this we used a model of 3D free floating live calvarial bones co-cultured with cancer cells in suspension in the roller-tube system under hypoxia and defined culture conditions where predominantly bone resorption can occur (Scheme 1). Figure 3 shows live calvarial bones co-cultured with each of the four cancer cell lines evaluated by histological analysis for the effects of cancer-bone interactions on osteoclast formation and bone resorption using live neutral red (NR) staining for multi-nucleated osteoclasts, and counter stained with silver nitrate. Figures 3a-c show control calvaria alone with no cancer cells and very little to no osteoclastic activity in the mid-parietal region of calvaria. By contrast, calvarial bones co-cultured with cancer cells showed a substantial increase of differentiated multinucleated active osteoclasts and bone resorption throughout the parietal bone (Figs.3d-s). In all of these co-cultures it was clearly apparent that the tumor cells preferentially attached to the fibro-stroma endosteal cell layer, and colonize and grow on the endocranial side of the calvarial bone, despite the fact that these clavaria were free floating and both periosteal and endosteal sides of the calvarial bone were equally exposed to the cancer cells in suspension (Figs. 3d, g, k and n). The impact of cancer cells on bone and the differentiation of numerous multinucleated resorptive osteoclasts were clearly visible in the NR stained live calvaria (Figs. 3e, h, l, and o). It should be noted that NR and silver staining were used at the terminal stage and no further culturing of such bones were carried out. Remarkably, in most instances the bone at many areas was either completely dissolved or significantly resorbed as evidenced from the histology (Figs.3 d, g, and n), and observation of silver nitrate stained bones, showing light transparency where the mineral and matrix has been removed (Figs. 3c, f, i, m, p, and s). Quantitative morphological measurements of the degree of bone resorption were determined for all these co-cultures (Fig. 4a). It is noteworthy that the effects seen on bone biology by media derived from the cancer cells (data presented above) is quite distinct with much less extent of bone resorption over the same time period as compared to the bone surface colonized by cancer cells. However, the relative impact of each cancer type is intrinsically consistent and in the order of MDA > MCF-7 > PC3 > LnCap for degree of bone loss. The direct quantitative TRAP activity, a measure of differentiated osteoclasts, of the bone cultures were consistent with the histological analysis (Fig. 4b). These were consistent with the microscopic observations H & E, NR staining and silver nitrate counter stained samples.
Figure 3.
Co-cultures of free floating calvarial bones with cancer cells in suspension using bone resorption model and the roller tube system under hypoxic conditions. (a)–(c) Control calvaria: (a) H & E sections of the parietal bone region with no osteoclastic bone resorption. (b) NR stained calvaria showing no multinucleated osteoclasts. (c) NR + silver nitrate counter stained calvaria showing very little light transparency reflecting lack of bone resorption. (d)–(f) Co-cultures of MCF-7 cells and calvarial bone: (d) H & E sections of the parietal bone region showing MCF-7 cells colonizing the endosteal side of the bone in direct contact with endosteal cells. (e) NR stained calvaria showing numerous active multinucleated osteoclasts. (f) NR + silver nitrate counter stained calvaria showing significant areas with light transparency reflecting bone resorption. (g)-(i) Co-cultures of MDA-231 cells and calvarial bone: (g) H & E sections of the parietal bone region showing MDA-231 cells colonizing the endosteal side of the bone. CBR, complete bone resorption. (h) NR stained calvaria showing numerous active multinucleated osteoclasts. (i) NR stained calvaria counter stained with silver nitrate showing substantial areas with light transparency reflecting bone resorption. (k)-(m) Co-cultures of LnCap cancer cells and calvarial bone: (k) H & E sections of the parietal bone region showing LnCap cells colonizing the endosteal side of the bone. (l) NR stained calvaria showing active multinucleated osteoclasts. (m) NR + silver nitrate counter stained calvaria showing much less light transparency and hence reduced bone resorption compared to other cancer cell lines. (n)-(p) Co-cultures of PC3 cancer cells and calvarial bone: (n) H & E sections of the parietal bone region showing PC3 cells colonizing the endosteal side. (o) NR stained calvaria showing numerous active multinucleated osteoclasts. (p) NR + silver nitrate counter stained calvaria showing large areas of light transparency reflecting osteoclastic bone resorption. (q)-(s) Calvarial bones cultured in the presence of added pure PGE2 only: (q) H & E sections of the parietal bone showing osteoclastic bone resorption on the endocranial side. (r) NR stained calvaria showing numerous active multinucleated osteoclasts. (s) NR + silver nitrate counter stained calvaria showing areas of light transparency and osteoclastic bone resorption. Per, periostium; End, endostium, OSC-RA, osteoclastic resorption areas; TC, tumor cells; MNOC, multinucleated osteoclasts; RA, resorption areas. All magnifications were: H & E 100x, NR 200x and silver nitrate counter stain 30x.
Figure 4.
Quantitative histomorphometric analysis for bone loss and TRAP enzyme activity for osteoclast quantification. (a) Quantitative morphometric measurements of the degree of bone loss showing significant loss of bone for those co-cultures of calvarial bone with MCF-7 (60%), MDA-231 (70%) and PC3 (50%) compared to bone grown alone. LnCap co-cultures showed much less bone loss (15%) and 20 nM PGE2 about 20%. The two sample student's t test indicated that the difference in bone resorption in response to cancer cells and added PGE2 were different and statistically significant with p values as noted within the Figure. (b) Quantitative TRAP enzyme activity analysis. The two sample student's t test indicated that the difference in TRAP activity in response to cancer cells and added PGE2 were different and statistically significant with p values as noted within the Figure.
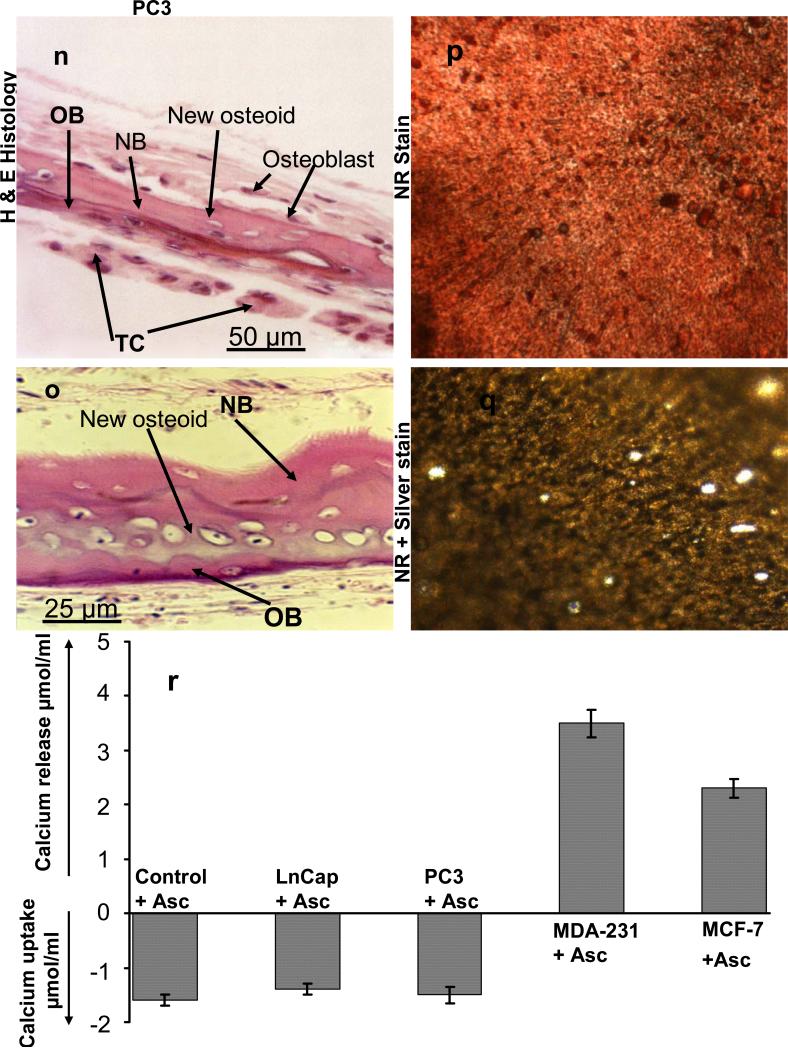
(b) Bone formation model
In order to further evaluate the cancer-bone interactions and effects on bone biology we developed bone culture condition that would represent the second bone remodeling stage, namely the bone formation. This was achieved by inclusion of ascorbate in the bone culture media. Under these conditions the breast and prostate cancer cells clearly behaved differently. Both the MCF-7 and MDA-231 cancer cells led still to significant induction of osteoclast formation and bone resorption with slight difference from the resorption model in that there was some colonization of the periostium in addition to the endostium cell layer, Fig. 5 d-f & g-i. The prostate cancer cells, however, behaved quite differently in that both LnCap and PC3 cancer cells permitted new bone formation, in particular the PC3 cells which stimulated osteoblastic activity, Fig. 5 k-m & n-q. Furthermore, prostate cancer cells had diminished level of bone attachment and colonization compared to those observed in the resorption model. The breast cancer cell co-cultures both MCF-7 and MDA-231 lead to significant calcium release consistent with the histological observations of bone resorption. The prostate cancer cell co-cultures both LnCap and PC3 lead to uptake of calcium from the media indicative of new bone formation, Fig. 5r.
Figure 5.
Co-cultures of free floating calvarial bones with cancer cells in suspension using bone formation model and the roller tube system under hypoxic conditions. (a)–(c) Control calvaria: (a) H & E sections of the parietal bone region. (b) NR stained calvaria showing no multinucleated osteoclasts. (c) NR + silver nitrate counter stained calvaria showing lack of bone resorption. (d)–(f) Co-cultures of MCF-7 cells and calvarial bone: (d) H & E sections of the parietal bone region. (e) NR stained calvaria showing numerous active multinucleated osteoclasts. (f) NR + silver nitrate counter stained calvaria with light transparency reflecting bone resorption. (g)-(i) Co-cultures of MDA-231 cells and calvarial bone: (g) H & E sections of the parietal bone region. (h) NR stained calvaria showing numerous active multinucleated osteoclasts. (i) NR stained calvaria counter stained with silver nitrate showing substantial bone resorption. (k)-(m) Co-cultures of LnCap cancer cells and calvarial bone: (k) H & E sections of the parietal bone region. (l) NR stained calvaria showing no multinucleated osteoclasts. (m) NR + silver nitrate counter stained calvaria. (n)-(q) Co-cultures of PC3 cancer cells and calvarial bone: (n) H & E sections of the parietal bone region showing PC3 cells colonizing the endosteal side with visible new osteoid and bone formation. (o) A higher magnification, 400 x, of a section of bone grown in the presence of PC3 cells showing clear new osteoid formation, active osteoblasts and new bone. (p) NR stained calvaria showing no multinucleated osteoclasts. (q) NR + silver nitrate counter stained calvaria. (r) Cummulative calcium release and/or uptake in control and those with different cancer cells. OB, old bone, NB, new bone, OSC-RA, osteoclastic resorption areas; TC, tumor cells; MNOC, multinucleated osteoclasts; RA, resorption areas. All magnifications unless otherwise stated were: H & E 100x, NR 200x and silver nitrate counter stain 30x.
3.3. Alterations in gene expression levels in human PC3 cancer cells and mouse calvarial bone cells by comprehensive gene microarray analysis using the 3D cancer-bone metastasis model
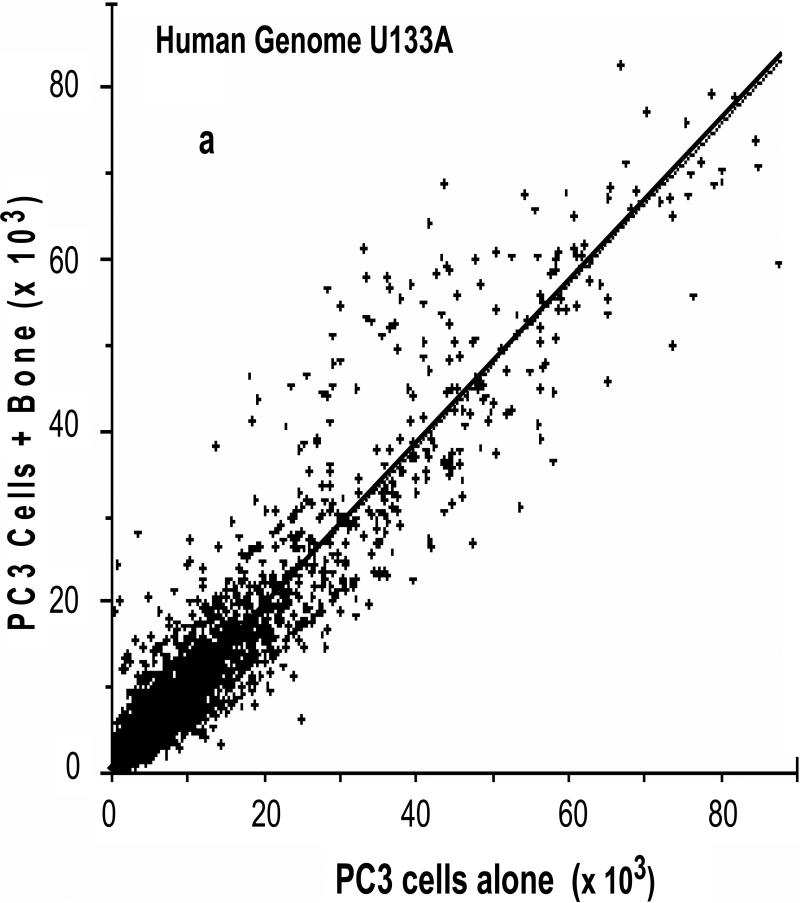
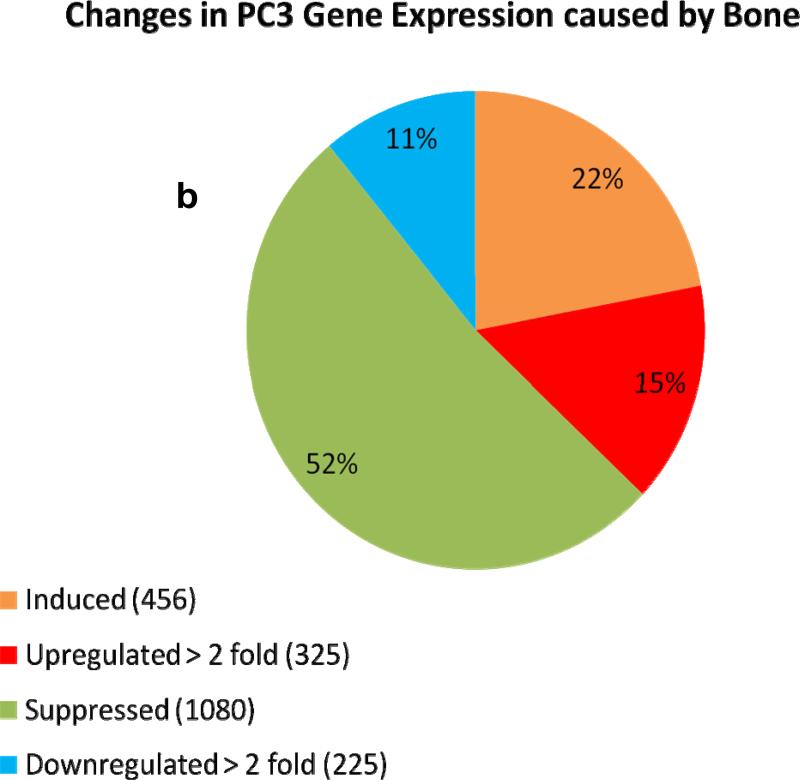
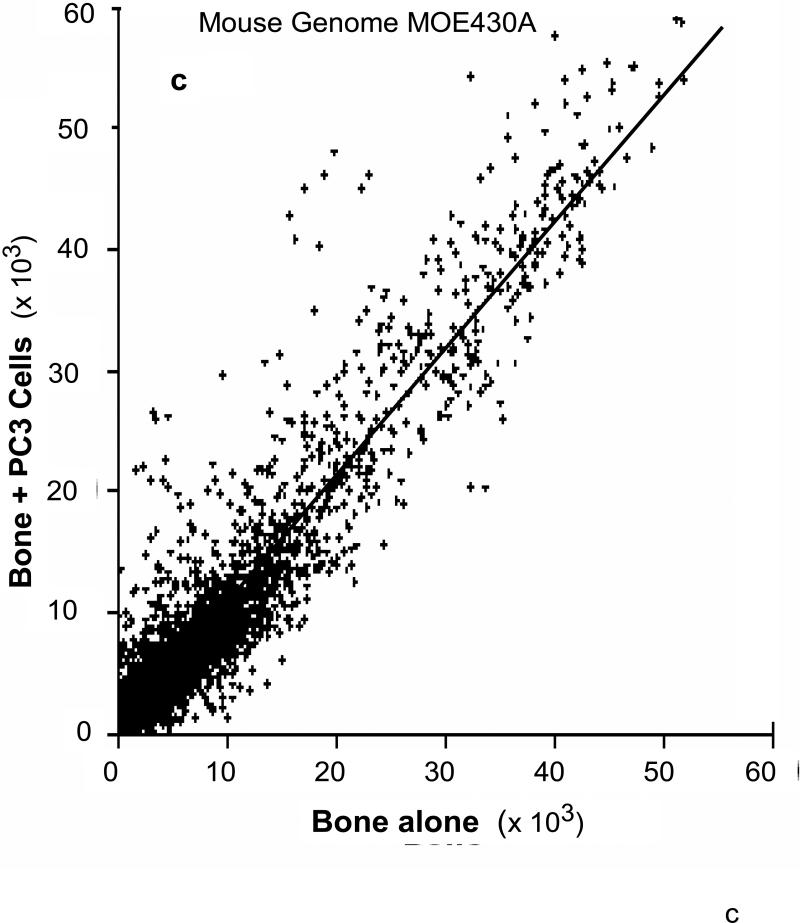
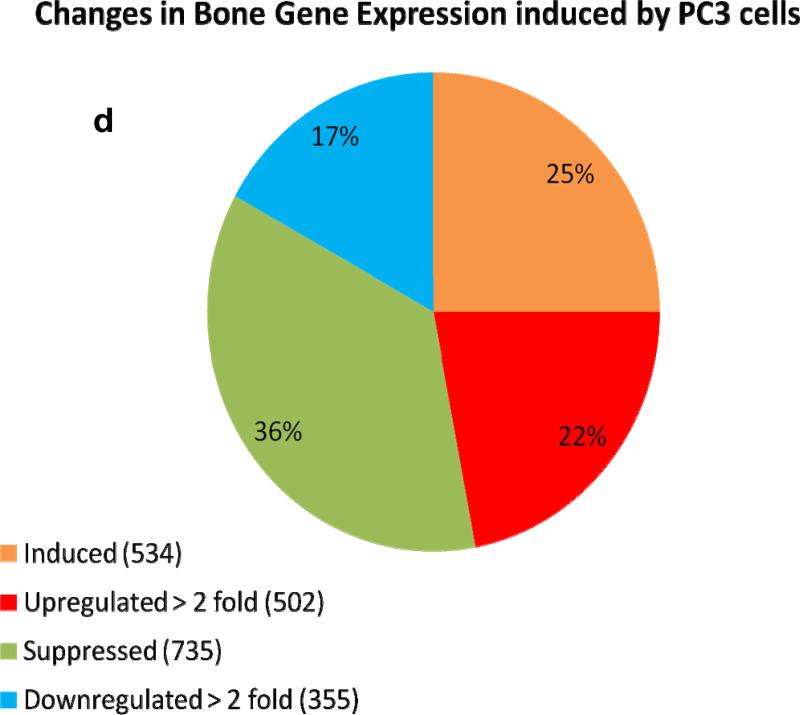
We used the roller-tube co-culture system to investigate alterations in gene expressions of cancer and bone cells as a result of cancer-bone interactions under hypoxic (5% O2) conditions. The interactions between bone and PC3 cells induced profound changes in gene expression levels in both bone and PC3 cells. Comprehensive human and mouse gene array analysis showed ~2000 genes (~10% of total genes ~20,000 analyzed) were affected in bone and cancer cells (Figs 6a-d). Comparable numbers but different genes were up-regulated > 2 fold, 325 genes, and down-regulated > 2 fold, 225 genes, in human PC3 cells (Fig.6a & b) and mouse calvarial bone up-regulated > 2 fold, 502 genes, and down-regulated >2 fold, 355 genes, (Fig. 6b & c) as a result of cancer-bone interactions. Among the highly expressed up-regulated PC3 genes were: cathepsin B, integrin receptor α2 subunit, IL-1-receptor antagonist; cytokeratin 17, and Il-6, all of which are at one level or another involved in invasiveness and metastasis phenotype. Among the highly expressed bone genes were classically well known components of bone ECM: decorin, an ECM proteoglycan; lysyl oxidase, matrix metalloproteinase-2 and matrix metalloproteinase-3, collagen type I; and tissue inhibitor of matrix metalloproteinase-1 each of which play a critical role in general bone biology.
Figure 6. Human and mouse comprehensive gene microarrays for cancer-bone interactions.
(a) Plot of identified human genes in PC3 cancer cells grown with calvarial bone versus PC3 cells grown alone. Line slope of 1.0 and the data point distribution of each gene relative to findings in PC3 cells grown in the presence of calvarial bone versus PC3 cells grown alone. The data represents an average of total of four analyses, i.e., duplicate gene array analysis of duplicate experimental samples. (b) Venn diagram of the total number of human genes impacted in PC3 cells, up- or down regulated. (c) Plot of identified mouse genes in calvarial bones grown with PC3 cancer cells versus calvarial bone grown alone. Line slope of 1.0 and the data point distribution of each gene relative to findings in calvaria grown in the presence of PC3 cells versus calvaria grown alone. The data represents average of total four analyses, i.e., duplicate gene array analyses of duplicate experimental samples. (d) Venn diagram of the total number of genes impacted in mouse calvarial bones, up- or down regulated.
4. DISCUSSION
We have developed an ex-vivo 3D cancer-bone metastatsis model by coupling free floating live mouse calvarial bone with cancer cells in suspension using a “roller-tube” system. This co-culture interactive system was found, under defined conditions, to undergo a specific bone remodeling stage, i.e., bone resorption or bone formation. Under resorption conditions the two bone remodeling stages are uncoupled and only osteoclast formation and bone resorption can occur if presented with appropriate external stimuli such as PTH, PGE2, cancer cell media or cancer cells. Under formation conditions bone has the capacity to form new bone but may undergo also resorption if presented with correct stimuli. This was based upon our concept that whether or not one observes osteolytic or osteoblastic bone metastasis depends on not only the cancer type/tissue origin but also influenced significantly by the degree of imbalance between the bone formation and bone resorption. The overriding force to develop this model was the lack of an appropriate ex-vivo model that can be used under a variety of defined conditions, and to formulate a system which simulate closely the in vivo bone microenvironment that contains constitutively responsive multicellular bone. In addition we coupled these studies under hypoxia in order to reflect the in vivo conditions where most solid tumors and the bone microenvironment are associated with hypoxia and such conditions are reflected in cancer cell malignant phenotype. Under intrinsic hypoxic conditions developing tumor cells produce prostaglandin E2 (PGE2) at increased levels [21, 22]. PGE2 is synthesized in bone mainly by osteoblasts to stimulate bone resorption and is regulated by cyclo-oxygenase (COX) enzymes. In cancer metastasis and bone resorption, studies have shown PGE2 binds to the EP4 receptor (a PGE receptor subtype) to induce RANKL expression and stimulation of bone destruction [23]. The effects of PGE2 on bone resorption has been highlighted using the mouse calvarial bone organ cultures originally developed by Goldhaber [24, 25]. In the present work we have established inherent characteristics of the calvarial bone cultures in response to chemical and biochemical perturbations as well as media from cancer cells. In response to cancer media the data clearly revealed induction of PGE2 synthesis by the calvarial bone cells and concurrent osteoclast differentiation and bone resorption. Surprisingly, in all cases the osteoclast differentiation in response to added pure PGE2, PTH or cancer media takes place strictly on the endocranial side (endosteal cell layer) of these calvarial bones. Collectively, these data highlight that the calvarial endostium which is rich in stem/progenitor cells essential for osteoclast differentiation provides an appropriate model to investigate details of the mechanism of osteolytic bone metastasis. Interestingly, the results are consistent with the well known process that occurs in patients with advanced breast cancers where the tumors at a distant location induce elevated bone resorption and hypercalcimia [26].
These observations led us to probe the possibility of whether the endostium and its cellular components were major players in the initial cancer cell homing and adherence to the bone surface. We developed therefore a “roller tube” co-culture system with free floating live calvarial bone organs and cancer cells in suspension under hypoxic conditions. The use of this unique model revealed remarkable specificity of cancer cell colonization and growth predominantly on the “endostium (endocranial)” side of the bone. This occurred despite the fact that the roller-tube model exposes equally both periostium and endostium of the free floating calvarial bone surfaces to the cancer cells in suspension. The cancer cells adhere specifically to the “fibro-stoma endosteal layer” which contains stem/progenitor cells for osteoclast differentiation. The bone resorption takes place strictly by the differentiated bone osteoclasts and even when the mineralized bone is completely dissolved the cancer cells remain on the surface of the endosteal cell layer with no migration beyond this cell layer. To the best of our knowledge this is the first time specific targeting by cancer cells has been observed with extraordinary discrimination between the two bone surfaces. This important and new information reflects the specific affinity and signals from the bone endosteal cell population that is critical in cancer cells homing to bone, attachment and colonization of specific bone microenvironments. The results also signify as to how tumors may utilize specific bone turnover states and cellular components during bone colonization with the potential for new avenues for mechanistic and molecular investigations. The model developed in the present work has direct relevance to in vivo bone metastasis since in clinical cases cancer cells colonize predominantly the bone marrow environment involving endosteal bone surfaces and trabecular bone, which are also in vivo localities for major bone turnover by osteoclasts. The extent of bone resorption observed in the current work was consistent with the presence of numerous differentiated osteoclasts stained through active uptake of NR by live osteoclasts. Analysis of the degree of bone resorption areas showed distinctions between different cancer cell types colonizing the live calvarial bone endostium. Quantitative morphological analysis indicated substantial bone loss (~70%) for breast cancer cells and ~50% for PC3 prostate cells with much less bone loss for LnCap. The data reflect closely the non-aggressive versus aggressive characteristics of these cancer cell lines and hence, the model has the capacity to correlate metastatic potential. Other investigators have used NR staining to visualize osteoclasts and quantitate their numbers and showed that the results were closely related to those of TRAP staining of osteoclasts [27] and NR stains and localizes the same osteoclasts as the TRAP staining [28]. In the present work we have complemented the NR-stained osteoclast density and quantitative bone loss with direct determination of TRAP enzyme activity.
Interestingly, most human prostate cancer bone metastasis manifest in osteoblastic bone formation. Contrary to this in vivo mouse models of prostate cancers are predominantly osteolytic [29] with the exception of WISH cell line [30] and direct implantation in vivo of normal canine prostate tissue on nude mouse calvaria [31]. Based up on our results above and general interest in the models using 3D osteoblastic matrices in bioreactors [14-19 and references therein] it was of importance to develop a formation model using calvarial bone organ cultures. In our mouse calvarial bone “formation model” the breast and prostate cancer cells impacted the bone biology very differently. Both of the breast cancer cells still induced significant osteoclastic bone resorption whereas prostate cancer cells did not. The less aggressive (non-metastatic) LnCap cancer cells showed no osteoclast formation and bone resorption but a limited bone formation. In contrast the metastatic PC3 cancer cells induced significant osteoblastic activity and new bone formation. The results from bone resorption and formation models revealed characteristics of cancer cells with significant phenotypic plasticity not observed previously. Furthermore, to best of our knowledge this is the first time osteoblastic bone formation phenomenon has been observed with the PC3 cancer cell line either in vivo or in vitro studies.
The unique nature of this model provided an opportunity also to define for the first time on a large scale the genetic alterations in human cancer and mouse bone cells as a consequence of cancer-bone interactions. Genomic alterations were impressive with nearly ~10% (~2000 genes) of the whole human PC3 cancer cell and mouse bone genomes affected. While the extended discussion on the analytical and biological implications of this data are beyond the scope of the current report, nevertheless the results show intriguing relevant molecular events in support of “the-proof-of-principle” and significance of the co-culture model developed. In brief, the results form a very clear picture that cancer-bone interactions lead to an array of genes being up regulated that participate in assisting cancer cells to establish cell-cell interactions, invasive and metastasis phenotype, survival and growth while simultaneously stimulate bone cells to produce a series of factors that lead to osteoclast differentiation and bone resorption. Alterations in PC3 cells were exemplified by increased levels of cathepsin B, a cysteine proteinase, capable of degrading ECM and known to promote progression, metastasis and survival of tumors [32]; integrin α2 subunit involved in a variety of cell-cell adhesion and interactions; cytokeratin 17 the increase of which correlate with poor prognosis for human clinical cancer patients [33] and promotes epithelial proliferation and tumor growth [34]; IL-6 induce increased tumor-associated macrophages, osteoclastogenesis, bone resorption and tumor size [35]. Impact on bone was revealed by increased levels of lysyl oxidase, an enzyme essential for collagen cross-linking and formation of mature bone collagen; MMP-3 which is associated with epithelial-mesenchymal transition [36] and induce genetic instability and malignant transformation [37] also capable of ECM degradation, and collagen type I which is the major ECM component of bone. It is interesting to note that although the microarray analyses were performed using combined RNA derived from both human and mouse cells, there was nevertheless discrimination of the specific genes. This was evident by the identification of specific genes only in mouse microarrays or human microarrays not found in the other. Furthermore, PC3 cells grown alone did not show the above genes found in mouse microarrays and calvaria grown alone did not show the above genes found in human microarrays. Overall, the model when utilized with human clinical primary cancer cells could be used to define the bone metastasis phenotype.
Interest in deciphering the molecular mechanisms of cancer-bone metastasis continue to evolve with new avenues that target specific molecules such as transforming growth factor-β signaling in breast cancer by an in vivo cancer-bone mouse model [38]. Other investigations, however, study aspects of cancer metastasis related to cancer progression and invasive characteristics which enable the cancer cells to depart from the original primary site. To understand these earlier events which precede cancer-bone interface, a 3D engineered organotropic tissue model has been developed which recapitulates natural features of tumor progression, including epithelial invasion through intact basement membrane [39]. This study which focused on the soft tissue organ model and the model presented here on the hard tissue organ model are complimentary for cancer-bone metastasis studies.
5. CONCLUSIONS
Our model overcomes many of the existing limitations and difficulties associated with 2D cell cultures, animal models, and 3D engineered bio-organic inert polymer scaffolds or 3D collagen matrices seeded with cells. First, it overcomes the “ethical” issues of live animal experimentation and avoids host immune complexity. Secondly, it is cost effective and hence a large number of experiments can be carried out to obtain extensive data sets for reliability, reproducibility and statistical analysis. Thirdly, it allows for studies to be carried out under specific and defined conditions and with the versatility of changing environmental and chemical compositions. Fourthly, this live 3D bone microenvironment with its multicellular components is biologically responsive to changes within its microenvironment through the presence of stem/progenitor cells that can differentiate to osteoclasts or osteoblasts. Therefore, it is remarkably conducive for studies of cancer-bone interactions under defined conditions and can identify a wide variety of primary tumor characteristics such as heterogeneity at both phenotypic and genetic levels. The results of such studies will open novel avenues for therapeutic drug testing and development aiming towards translational clinical application. Finally, the ability to dissociate the “two coupled” bone remodeling stages, i.e., bone formation and bone resorption provides some major advantages. The model can be used to study the various aspects of cancer-bone interactions at a physical, genetic, cellular and molecular level using uncoupled bone remodeling stages. This was clearly highlighted by the major differences found in the behavior of different cancer cells and their impact on bone biology when studies were carried out using resorption model versus formation model. Thus, the model provides opportunities to study cancer-bone metastasis/interactions with significant contribution to the understanding of the underlining molecular and cellular mechanisms.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant RO1 AG 17969 to E.S. and support from Harvard Medical School for undergraduate summer research project to H.Y. We thank J. Pudney and P. Trackman for presubmission review of the manuscript.
ABREVIATIONS
- ECM
Extracellular matrix
- PGE2
prostaglandin E2
- 2D
two-dimensional
- 3D
three- dimensional
- PTH
parathyroid hormone
- MMP-3
matrix metaloproteinase 3
- NR
neutral red
- Cox2
cyclo-oxygenase-2
- ELISA
enzyme-linked immunosobent assay
- DMEM
Dulbecco's Modified Eagle's Medium
- EDTA
ethylene diamine tetraacetic acid
- IL-6
interleukin-6
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None
CONFLICT OF INTEREST: NONE