Abstract
Subcutaneous IgG treatment for primary immunodeficiencies (PI) is particularly well suited for children because it does not require venous access and is mostly free of systemic adverse events (AEs). In a prospective, open-label, multicenter, single-arm, Phase III study, 18 children and five adolescents with PI were switched from previous intravenous (IVIG) or subcutaneous (SCIG) IgG treatment to receive dose-equivalent, weekly subcutaneous infusions of Hizentra® for 40 weeks. Mean IgG trough levels were maintained in patients previously on SCIG, or increased in those previously on IVIG, regardless of age. No serious bacterial infections were reported during the efficacy period of the study. The rates of non-serious infections were 4.77 (children) and 5.18 (adolescents) infections per patient per year. Related AEs were observed in seven children (38.9%) and two adolescents (40%). Three serious AEs and two AEs leading to discontinuation (all unrelated) were reported in children. Hizentra® is an effective and well-tolerated treatment for pediatric patients.
Keywords: Subcutaneous immunoglobulin (SCIG), primary immunodeficiency, local tolerability, serum IgG levels, pediatric patients
Introduction
Primary immunodeficiency (PI) disorders, such as common variable immunodeficiency (CVID) and X-linked agammaglobulinemia (XLA), are often diagnosed in childhood and require life-long replacement therapy with intravenous (IVIG) or subcutaneous (SCIG) IgG [1–5]. Patients with PI are predisposed to recurrent respiratory, skin, or gastrointestinal infections that may lead to chronic damage. IgG replacement is an effective therapy, usually administered intravenously [6–12]. Subcutaneous IgG administration, which is an increasingly widely used alternative, is particularly suitable for children, as it does not require venous access and is mostly free of systemic adverse events (AEs) [13–17]. Moreover, compared to adults, children require lower volumes for the infusion of prescribed doses, which makes them well suited for SCIG therapy.
Therapy with the newly developed, highly concentrated SCIG products can be easily integrated into daily activities, thus offering patients more flexibility. Hizentra® (IgPro20, approved in the USA in March 2010) is a new 20% liquid formulation with stability at 25°C for at least 24 months, which has high purity and proven pathogen safety [18–20]. Hizentra® can be administered at relatively high infusion rates, which, together with low infusion volumes, contributes to potentially short infusion times.
In this prospective, open-label, multicenter, single-arm, Phase III study (NCT00542997), the efficacy and tolerability of Hizentra® were evaluated in patients switched from previous IVIG or SCIG therapy at equivalent doses [21]. The rationale for using SCIG doses equivalent to previous IVIG or SCIG dosing was based on the European experience showing that SCIG administered at doses equivalent to IVIG is sufficient to achieve comparable IgG trough levels in patients with PI. This approach is reflected in the European Medicines Agency requirement to show that equivalent serum IgG levels can be achieved with the same doses [22]. Here we report efficacy and safety results in pediatric patients in comparison with adults.
Patients and Methods
Patients
Eighteen children (2–11 years) and five adolescents (12–15 years) with CVID, XLA, or autosomal recessive agammaglobulinemia were enrolled in the study. Major inclusion criteria were stable IVIG therapy at regular 3- or 4-week intervals or weekly SCIG therapy for at least 6 months before study start, at a monthly dose of 0.2 to 0.8 g/kg body weight (bw), and at least three documented serum IgG trough levels of ≥5 g/L. Patients who had never been treated with immunoglobulin replacement therapy were excluded from the study. Patients were also excluded if they had any condition that was likely to interfere with evaluation of the study medication or satisfactory conduct of the study. Detailed description of inclusion and exclusion criteria is given elsewhere [21].
This study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice guidelines and the Declaration of Helsinki. The study protocol and all other study-related documents were reviewed and approved by the appropriate local Independent Ethics Committees. Written informed consent was obtained from all patients or patients’ parents or legal guardians.
Study Design
This was a prospective, open-label, multicenter, single-arm, Phase III study with a 12-week wash-in/wash-out period followed by a 28-week efficacy period [21]. A pharmacokinetic (PK) substudy was conducted in week 28 ± 1, in which nine children and three adolescents participated.
The primary objective of this study was to show that sustained serum IgG levels can be achieved with IgG doses comparable to previous IgG treatment. Individual IgG doses were equivalent to previous IVIG or SCIG doses, with weekly administrations. Dose adjustments were made in the wash-in/wash-out period in case IgG trough levels were <5 g/L.
Premedication prior to infusion for alleviating AEs was not allowed. Oral and parenteral steroids were allowed if the average daily dose was <0.15 mg of prednisone equivalent per kilogram per day. Local anesthetics could be used before infusion to reduce the pain associated with needle insertion if required.
Hizentra® was administered subcutaneously using infusion pumps in sites on the abdomen, thighs, and/or lateral hip [21]. Infusion techniques varied according to local practice and patients already on SCIG treatment continued with their established infusion technique. The maximal volume per injection site was limited to 25 mL. The infusion rate per site was not to exceed 35 mL/h during the efficacy period, depending on tolerability. Several infusions were performed under supervision at the study site, the rest were administered at home by the patient or patient’s parent/guardian.
Efficacy and Safety Assessments
The primary efficacy objective was achievement of IgG trough levels at six consecutive weeks at steady state (Infusions 12 to 17) similar to three pre-study IgG trough levels (during the last 3 to 6 months prior to the study). Trough serum IgG levels were measured before Infusions 1, 4, 8, 12–17, 20, and every fourth infusion thereafter.
Secondary efficacy endpoints included rate of clinically documented serious bacterial infections (SBIs), number of infection episodes, number of days missed from school/day care or unable to perform normal activities due to infections (abbreviated further as number of days missed from school/day care), number of days of hospitalization due to infections, and use of antibiotics for infection prophylaxis or treatment. SBIs (bacterial pneumonia, bacteremia/septicemia, osteomyelitis/septic arthritis, bacterial meningitis, and visceral abscess) were diagnosed using pre-defined criteria adjusted to both adult and pediatric patients [23]. Non-serious infections were diagnosed at the discretion of the Investigators according to the national standard of medical practice.
Maximal serum IgG concentration (Cmax), time to reaching Cmax (Tmax), and area under the concentration/time curve (AUC) until the last measured concentration were evaluated. Blood samples for IgG determination were taken before Infusion 28, 10 ± 5 min prior to end of infusion, 2 ± 1 h after infusion, and after 1, 2, 3, 4, and 7 days.
Information about AEs was collected continuously. Patients or their guardians documented any findings in patient diaries and Investigators evaluated them at visits. For the analysis of local reactions, 25 preferred terms of the category “General disorders and administration site conditions” were combined in the category of local reactions. Local tolerability was assessed by the patients within 24 to 72 h after infusion and reviewed by the investigator during visits. Standard hematology, serum chemistry, and urinalysis laboratory parameters were determined at screening, before Infusions 1, 4, 28, and at the completion visit.
Statistical Methodology
Efficacy analyses were carried out on the intent-to-treat (ITT) population (17 children, five adolescents, and 24 adults) defined as all patients who were treated with Hizentra® during the efficacy period (starting from week 13). Safety analyses were carried out on the all-treated population (18 children and five adolescents) defined as all patients who were treated with Hizentra® during any study period. PK analyses were carried out on the per-protocol pharmacokinetic population (nine children and three adolescents) comprising all patients who had received uniformly repeated weekly doses of Hizentra® at least 12 weeks before week 28 ± 1, with not more than three missing samples or outliers in the AUC sampling period.
For primary efficacy analyses, six consecutive steady-state IgG trough values were aggregated to the patient’s median value. The same procedure was used for the three most recent IgG trough values ≥5 g/L from the previous treatment [21]. A post hoc analysis with point estimates and a two-sided 95% confidence interval (CI) for the change from pre-study serum IgG levels was performed using the Hodges–Lehmann nonparametric analysis.
The rate of SBIs per patient per year was estimated with a 99% upper confidence limit (CL), and the number of infection episodes was estimated with a two-sided 95% CI. Other secondary endpoints were summarized with descriptive statistics. AUC was obtained by the trapezoidal rule between the first and last IgG measurement, ignoring outliers.
Adverse events were coded using the Medical Dictionary for Regulatory Activities (MedDRA), version 12.0. Temporally associated AEs were defined as occurring between the start of infusion and 72 h after the end of infusion.
Results
Patients
All 23 pediatric patients screened were enrolled in the study. Two of these discontinued due to unrelated adverse events (after week 2 and after week 36). The remaining 21 pediatric patients completed the study. Of 28 adults enrolled, 22 completed the study.
The proportion of females among children was three times lower than that of males (Table I), due to the high proportion of patients with XLA (all males).
Table I.
Demographic and baseline characteristics (AT population)
| Characteristic | Children | Adolescents | Adults |
|---|---|---|---|
| 2–11 years | 12–15 years | 16–64 years | |
| N = 18 | N = 5 | N = 28 | |
| Gender, n (%) | |||
| Female | 5 (27.8) | 0 (0) | 11 (39.3) |
| Male | 13 (72.2) | 5 (100) | 17 (60.7) |
| Age (years) | |||
| Mean (SD) | 7.2 (2.5) | 14.0 (1.0) | 34.1 (12.7) |
| Median (Range) | 7.5 (3–11) | 14.0 (13–15) | 32.5 (16–60) |
| Weight (kg) | |||
| Mean (SD) | 25.9 (11.06) | 57.1 (15.84) | 69.2 (13.34) |
| Median (Range) | 22.0 (13–56) | 53.3 (36–77) | 70.2 (41–96) |
| BMI (kg/m2) | |||
| Mean (SD) | 16.4 (2.88) | 20.6 (2.29) | 23.3 (3.96) |
| Median (Range) | 15.3 (12–24) | 20.1 (18–23) | 23.0 (16–32) |
| Primary disease | |||
| CVID, n (%) | 7 (38.9) | 0 (0) | 23 (82.1) |
| XLA, n (%) | 10 (55.6) | 5 (100) | 5 (17.9) |
| ARAG, n (%) | 1 (5.6) | 0 (0) | 0 (0) |
AT all treated (23 pediatric patients), N number of patients in age group, SD standard deviation, CVID common variable immunodeficiency, XLA X-linked agammaglobulinemia, ARAG autosomal recessive agammaglobulinemia
Study Drug Administration
All patients in the ITT population received the intended 12 infusions during the wash-in/wash-out period. Most patients received the planned 40 infusions during the study: 16 children (94%), five adolescents (80%), and 17 (71%) adults. Five adults (21%) received 41 infusions.
The mean (SD) of individual median Hizentra® doses per week for the entire study period was 129.9 (46.2) mg/kg bw in children (range, 76–262 mg/kg bw) and 113.7 (28.0) mg/kg bw in adolescents (range, 72–150 mg/kg bw), both very similar to the dose of 114.3 (27.6) mg/kg bw administered in adults (range, 59–189 mg/kg bw).
Dose increases of >10% of the planned dose were made in four children and one adolescent during the wash-in/wash-out period. One child and one adolescent had dose decreases of >10%. Dose adjustments in adults were not necessary.
The mean of the individual median infusion rates was lower in children (19.0 mL/h) compared to adolescents and adults (31.2 and 28.5 mL/h, respectively). The median duration of infusion per week was 0.78 h (range, 0.3–2.5 h) in children and 1.0 h (range, 0.5–2.5 h) in adolescents, which was lower than that in adults (1.42 h; range, 0.7–3.3 h) because of the higher total dose administered in adults.
Efficacy
Primary Efficacy Endpoint
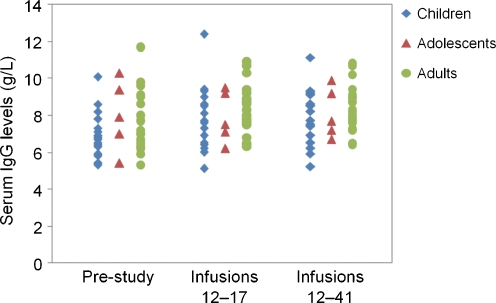
The study objective was met: Hizentra® treatment resulted in serum IgG trough levels comparable to or higher than those achieved with previous therapy. The mean of the individual pre-study median IgG trough level in children was 6.94 g/L, and in adolescents, 7.99 g/L compared with 7.81 g/L in adults (Table III). The mean of the individual median IgG trough levels measured before Infusions 12 to 17 were 7.86 g/L in children, and 7.91 g/L in adolescents compared with 8.31 g/L in adults, suggesting that IgG levels were maintained in all age groups.
Table III.
Efficacy endpoints (ITT population)
| Efficacy endpoint | Children | Adolescents | Adults |
|---|---|---|---|
| 2–11 years | 12–15 years | 16–64 years | |
| N = 17 | N = 5 | N = 24 | |
| IgG trough levels | |||
| Pre-study | |||
| Mean ± SD | 6.94 ± 1.223 | 7.99 ± 1.946 | 7.81 ± 1.666 |
| Median (range) | 6.77 (5.3–10.1) | 7.88 (5.4–10.3) | 7.49 (5.3–11.7) |
| Infusions 12–17a, e | |||
| Mean ± SD | 7.86 ± 1.720 | 7.91 ± 1.432 | 8.31 ± 1.250 |
| Median (range) | 7.66 (5.1–12.4) | 7.54 (6.2–9.5) | 8.15 (6.3–10.9) |
| Infusions 12–41f | |||
| Mean ± SD | 7.78 ± 1.510 | 8.14 ± 1.390 | 8.32 ± 1.211 |
| Median (range) | 7.67 (5.2–11.2) | 7.71 (6.7–9.9) | 8.25 (6.4–10.8) |
| SBIs | |||
| Number (%) of patientsb | 0 (0) | 0 (0) | 0 (0) |
| Number of events (annual rate per patient) | 0 (0) | 0 (0) | 0 (0) |
| [upper 99% CL] | [0.511] | [1.705] | [0.376] |
| [0.543]d | NA | NA | |
| All infections | |||
| Number (%) of patientsb | 15 (88.2) | 3 (60.0) | 18 (75.0) |
| Number of events (annual rate per patient) | 43 (4.77) | 14 (5.18) | 67 (5.47) |
| 40 (4.714)d | NA | NA | |
| [95% CI] | [3.452; 6.426] | [2.833; 8.695] | [4.241; 6.949] |
| [3.368, 6.419]d | NA | NA | |
| Days missed from school/day care | |||
| Number (%) of patientsc | 8 (47.1) | 4 (80.0) | 8 (33.3) |
| Number of days (annual rate per patient) | 139 (14.90) | 5 (1.79) | 54 (4.28) |
| 68 (7.74)d | NA | NA | |
| Days hospitalized due to infection | |||
| Number (%) of patientsc | 3 (17.7) | 0 (0) | 1 (4.2) |
| Number of days (annual rate per patient) | 78 (8.36) | 0 (0) | 8 (0.63) |
| 15 (1.71)d | NA | NA | |
| Days with antibiotics for infection prophylaxis or treatment | |||
| Number (%) of patientsb | 11 (64.7) | 1 (20.0) | 17 (70.8) |
| Number of days (annual rate per patient) | 442 (49.04) | 1 (0.37) | 727 (59.38) |
| 260 (30.64)d | NA | NA | |
With the exception of IgG trough levels, data are for the efficacy period
ITT intent-to-treat, N number of patients in age group, SBI serious bacterial infections, CL confidence limit, CI confidence interval, NA not applicable
aPrimary efficacy endpoint
bThe total number of days in the study was 3,290 (children), 986 (adolescents), and 4,469 (adults)
cThe total number of days from patient diaries was 3,406 (children), 1,020 (adolescents), and 4,607 (adults)
dData excluding the patient who suffered from recurrent pneumonias
eData from a post hoc nonparametric analysis of the change in IgG levels from baseline to the efficacy period (Infusions 12–17): children (mean change from baseline, 0.920; Hodges–Lehmann point estimate, 0.680; two-sided 95% CI, 0.030, 1.500); adolescents (mean change from baseline, −0.089; Hodges–Lehmann point estimate, −0.152; two-sided 95% CI, −0.770, 0.810); adults (mean change from baseline, 0.366; Hodges–Lehmann point estimate, 0.377; two-sided 95% CI, −0.058, 0.838)
fData from a post hoc nonparametric analysis of the change in IgG levels from baseline to the entire study period (Infusions 12–41): children (mean change from baseline, 0.846; Hodges–Lehmann point estimate, 0.683; two-sided 95% CI, 0.055, 1.445); adolescents (mean change from baseline, 0.145; Hodges–Lehmann point estimate, −0.020; two-sided 95% CI, −0.370, 1.300); adults (mean change from baseline, 0.386; Hodges–Lehmann point estimate, 0.355; two-sided 95% CI, −0.090, 0.820)
Children achieved the largest increase in mean of the individual median IgG trough levels from baseline to study end (13.3%), while the change in adolescents and adults was small (Fig. 1). Most likely, this observation was due to the fact that two thirds of the children (n = 11) had been treated with IVIG prior to the study and had a pre-study IgG trough level of 6.47 g/L (mean of individual medians). The IgG trough level measured before Infusions 12 to 17 in these children was 7.92 g/L (mean of individual medians), which corresponded to an increase of 22.4%. In comparison, the pre-study IgG trough level in children previously treated with SCIG (n = 6, mean of individual medians 7.80 g/L) was similar to the level achieved with Hizentra®. The four adolescents who had previously been on SCIG had a pre-study IgG trough level of 8.65 g/L (mean of individual medians). Adults previously treated with IVIG had a larger increase in IgG levels (n = 13, from 7.15 to 8.14 g/L) compared with those previously on SCIG (n = 9, from 8.76 to 8.56 g/L), similarly to children.
Fig. 1.
Individual median serum IgG trough levels before and during the study. Median values were calculated for each patient for pre-study IgG levels and IgG levels measured before Infusions 12 to 17 (efficacy period; primary efficacy endpoint) and before Infusions 12 to 41 (entire study period)
A post hoc statistical analysis of the change from baseline in serum IgG trough levels confirmed that IgG levels were maintained in adolescents and adults, and increased in children. For the efficacy period of the study (Infusions 12–17), the mean change from baseline in IgG levels was most pronounced in children (0.920 g/L), with a Hodges–Lehmann point estimate for the change from baseline of 0.680 g/L. The two-sided 95% CI for this change (0.030, 1.500) suggested a significant difference in IgG levels from pre-study level to the level achieved during the efficacy period. The corresponding change from baseline in adolescents and adults indicated stable IgG levels and no significant difference from pre-study to study IgG levels (Table III). Similar results were obtained for the entire study period (Infusions 12–41). A significant increase in IgG levels from baseline was observed in children, while the IgG levels in the other age groups remained stable (Table III).
In a robustness analysis of IgG trough values excluding the seven pediatric patients who had dose adjustments, no relevant difference was observed compared to the analysis for the ITT population.
Pharmacokinetic Parameters
Nine children and three adolescents participated in the PK substudy. There were no clinically relevant differences between children, adolescents, and adults (n = 11) in the PK parameters for serum IgG.
In children, slightly lower mean values compared to adults were observed for Cmax (8.09 vs. 8.31 g/L) and AUC (52.30 vs. 54.52 day × g/L), which was in agreement with the slightly lower mean IgG trough concentrations in children. Corresponding values in adolescents were similar to those in adults (Table II).
Table II.
PK parameters by age group (PPK population)
| Parameter | Children | Adolescents | Adults |
|---|---|---|---|
| 2–11 years | 12–15 years | 16–64 years | |
| N = 9 | N = 3 | N = 11 | |
| Cmax (g/L), mean (SD) | 8.09 (1.492) | 8.60 (1.443) | 8.31 (1.096) |
| Tmax (day), median (range) | 2.06 (0.94–6.92) | 1.98 (1.93–2.94) | 2.07 (0.95–3.98) |
| AUC (day × g/L), mean (SD) | 52.30 (9.987) | 54.91 (11.548) | 54.52 (8.672) |
PPK per-protocol pharmacokinetic, N number of patients in age group, Cmax maximum concentration, Tmax time point of maximum concentration, AUC area under the concentration–time curve until last measured concentration, SD standard deviation
Secondary Efficacy Endpoints
Hizentra® was effective in maintaining the rate of infections at a very low level. No SBIs were reported during the efficacy period of the study (Table III). A child with a history of recurrent severe pneumonia experienced an SBI of pneumonia during the wash-in/wash-out period.
Although the annual rate of all infections (including SBIs) in children was slightly lower than in adults (4.77 vs. 5.47 infections per patient per year; Table III), no age-related trends in the incidence of specific types of infection were observed. The most frequent type of infection was cough (seven events in children and six events in adolescents), followed by upper respiratory tract infection (eight events in children and two events in adolescents) and bronchitis (four events in children only).
Eight children missed 139 days from school/day care and three children spent a total of 78 days in hospital (Table III). Of these, the child with recurrent pneumonia missed 71 days from school and spent 63 days in hospital. This child’s experience differed substantially from that of the other patients in the study, regardless of age. Therefore, a post hoc analysis excluding this child was performed to evaluate the efficacy in patients with less severe disease (Table III). In comparison, four adolescents missed only 5 days and none was hospitalized, while eight adults missed 54 days and one adult spent 8 days in hospital. Treatment with antibiotics for prophylaxis or against infections was prescribed to 11 children (442 days) and one adolescent (1 day). The child with recurrent pneumonia was treated for 182 days. Excluding this child, the rate of days on antibiotics in children was twofold lower than the one in adults (Table III).
Safety and Tolerability
Local Reactions
Local tolerability results showed that patients tolerated Hizentra® administration well. Children and adolescents experienced 27 and 7 local reactions, respectively (0.040 and 0.035 events per infusion). In children, the most frequent local reactions were infusion site mass (five events), infusion site pruritus (five events), and infusion site pain (four events), followed by infusion site reaction (three events). Adolescents experienced infusion site pruritus (four events), infusion site swelling (two events), and infusion site extravasation (one event). Most of the local reactions were mild in intensity (26 events in children and three in adolescents; Table IV). A total of four moderate local reactions were reported, and only one was severe. Adults reported more local reactions in general, most being mild in intensity (67 of 76 events), yet the rates of moderate local reactions were similar to those in pediatric patients.
Table IV.
Local reactions (AT population)
| Local reaction | Children | Adolescents | Adults |
|---|---|---|---|
| 2–11 years | 12–15 years | 16–64 years | |
| N = 18 | N = 5 | N = 28 | |
| Ni = 678 | Ni = 199 | Ni = 954 | |
| Total, no. of AEs (rate per infusion) | |||
| 27 (0.040) | 7 (0.035) | 76 (0.080) | |
| Mild local reactions, no. of AEs (rate per infusion) | |||
| Total | 26 (0.038) | 3 (0.015) | 67 (0.070) |
| Moderate local reactions, no. of AEs (rate per infusion) | |||
| Total | 1 (0.001) | 3 (0.015) | 9 (0.009) |
| Infusion site nodule | 1 (0.001) | 0 | 0 |
| Infusion site pruritus | 0 | 2 (0.010) | 0 |
| Infusion site swelling | 0 | 1 (0.005) | 1 (0.001) |
| Infusion site inflammation | 0 | 0 | 1 (0.001) |
| Infusion site pain | 0 | 0 | 2 (0.002) |
| Infusion site reaction | 0 | 0 | 3 (0.003) |
| Infusion-related reaction | 0 | 0 | 2 (0.002) |
| Severe local reactions, no. of AEs (rate per infusion) | |||
| Total | 0 | 1 (0.005) | 0 |
| Infusion site pruritus | 0 | 1 (0.005) | 0 |
AT all treated, N number of patients in age group, Ni number of infusions, AEs adverse events
Patients or their guardians evaluated the local tolerability as “very good” or “good” for 98.5% of the infusions in children; a rating of “fair” or “poor” was given only for five (0.7%) and two (0.3%) infusions, respectively. In adolescents, local tolerability was rated as “very good” or “good” for 95.5% of the infusions and “fair” for nine (4.5%) infusions. Local tolerability assessments in adults were similar to those in pediatric patients: 95.5% of the infusions were rated as “very good” or “good,” 4.0% as “fair,” and 0.4% as “poor.”
Overall Adverse Events
A summary of all AEs, including local reactions, is provided in Table V.
Table V.
Summary of adverse events including local reactions (AT population)
| AE category | Children | Adolescents | Adults |
|---|---|---|---|
| 2–11 years | 12–15 years | 16–64 years | |
| N = 18 | N = 5 | N = 28 | |
| Ni = 678 | Ni = 199 | Ni = 954 | |
| Patients with AEs, n (%) | 18 (100) | 5 (100) | 27 (96.4) |
| Patients with related AEs, n (%) | 7 (38.9) | 2 (40.0) | 22 (78.6) |
| Patients with temporally associated AEs (72 h), n (%) | 17 (94.4) | 5 (100) | 26 (92.9) |
| Patients with related, temporally associated AEs (72 h), n (%) | 7 (38.9) | 2 (40.0) | 20 (71.4) |
| Patients with serious AEs, n (%) | 3 (16.7) | 0 | 2 (7.1) |
| Patients discontinued due to AEs, n (%) | 2 (11.1) | 0 | 4 (14.3) |
| Patients discontinued due to related AEs, n (%) | 0 | 0 | 3 (10.7) |
AT all treated, N number of patients in age group, Ni number of infusions, AEs adverse events
The rates of AEs, excluding local reactions, were similar in all age groups: 107 AEs occurred during 678 infusions in children (rate 0.158 AEs per infusion) and 41 AEs occurred during 199 infusions in adolescents (rate 0.206 AEs per infusion), compared with 269 AEs during 954 infusions in adults (0.282 AEs per infusion). Excluding local reactions, the most common AEs in children were cough and upper respiratory tract infection (12 and 10 events, respectively; rates 0.018 and 0.015 events per infusion); in adolescents, cough was the most common AE (eight events, rate 0.040).
The rates of temporally associated AEs other than local reactions were somewhat lower in pediatric patients than in adults: 0.090 AEs per infusion in children and 0.085 AEs per infusion in adolescents, compared with 0.148 AEs per infusion in adults. The most common temporally associated AEs were respiratory infections, such as cough, upper respiratory tract infection, and bronchitis.
The frequency of related AEs was lower in children and adolescents than in adults. Excluding local reactions, 20 related AEs were reported in children and four in adolescents (rates 0.029 and 0.020 AEs per infusion, respectively). Excluding local reactions and infections, the most common related AEs in children were pruritus (six events, rate 0.009 events per infusion), erythema (three events, rate 0.004 events per infusion), and psychomotor hyperactivity (three events, rate 0.004 events per infusion). Two related events of headache were reported in children (rate 0.003 events per infusion). In adolescents, the only related AE reported more than once was pruritus (three events, rate 0.015 events per infusion). Adults experienced more related AEs, which were neither local reactions nor infections, the most frequent being headache (11 events, rate 0.012 events per infusion), fatigue (five events, rate 0.005 events per infusion), and pruritus (five events, rate 0.005 events per infusion).
Almost all AEs, excluding local reactions, were mild or moderate in intensity: 95.3% in children and 97.6% in adolescents. Five patients experienced six severe AEs (chest pain, C-reactive protein increased, appendicitis, bronchitis, pneumonia, and cough). In adults, no severe AEs were reported.
Serious Adverse Events and Adverse Events Leading to Discontinuation
No deaths occurred during the study. Serious AEs and AEs leading to discontinuation (all unrelated) were reported only in children.
Three children (16.7%) experienced five SAEs, none of them related to study medication. A 5-year-old girl with a diagnosis of CVID experienced three SAEs: one event of pyrexia and two events of X-ray proven pneumonia. The first event of pneumonia, occurring in the wash-in/wash-out period, was moderate in intensity and was considered an SBI according to the pre-specified FDA criteria because chest X-ray/CT scan showed distinctive nodular and new infiltrative changes in both lungs, despite negative sputum and blood cultures. Approximately a month later, when the patient was hospitalized for diagnostic purposes, bronchoalveolar lavage was positive for atypical mycobacteria in polymerase chain reaction test. Tests for Mycobacterium tuberculosis were negative. With appropriate antibiotic treatment, this SBI resolved after 34 days without sequelae. Four days after Infusion 14, during the efficacy period, the patient experienced pyrexia that was reported as an SAE of moderate intensity; after treatment with antibiotics and ibuprofen, the event resolved after 2 days without sequelae. Four days after Infusion 22, the patient experienced pneumonia that was reported as an SAE of severe intensity. She had fever and cough, and her laboratory tests were indicative of an ongoing inflammatory process (white blood cells 25.69 × 109; C-reactive protein 31.8 mg/L; hemoglobin 9.7 g/dL; ALT 13.1 U/L). Because of the underlying lung disease and medical history of recurrent pneumonia, this event was an acute exacerbation of the existing pneumonia, and thus not considered an additional SBI. The patient completed the study as planned. Two other SAEs—diarrhea (mild) experienced by a 7-year-old girl and appendicitis (severe) experienced by a 10-year-old girl—resolved without sequelae after 2 and 5 days, respectively.
After Infusion 2, an 8-year-old boy with XLA discontinued because of myalgia, pyrexia, and nausea (moderate intensity), and chest pain and C-reactive protein increased (severe intensity). All these AEs were considered unrelated to study medication and resolved without sequelae. The patient was diagnosed with cystic lymphangioma, and thoracic surgery was performed. A 5-year-old boy with XLA discontinued because of anemia of moderate intensity occurring after Infusion 32. The patient had suffered from anemia for 1 year before entering the study and had low hemoglobin values throughout the study (≤10.1 g/dL), as well as abnormally low values for hematocrit, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, and mean corpuscular volume at all measurements. The AE was considered unrelated to study medication and was ongoing at final assessment.
Laboratory Tests
Laboratory tests were unremarkable. Median values and ranges of hematology and serum chemistry variables did not show any relevant changes over time. Individual hematology, blood chemistry, and urinalysis values lying outside the normal range were considered by the investigator as not clinically significant in all but two patients. A 10-year-old girl with a history of anemia had low values for hemoglobin, hematocrit, and mean corpuscular volume at most measurements during the study. She was diagnosed with mild iron deficiency anemia that was unrelated to study treatment and resolved without sequelae after 95 days. The 5-year-old boy who discontinued because of anemia had low values for hematocrit, hemoglobin, and several other hematology tests before Infusion 28 and at the completion visit, and a high platelet count at the completion visit.
Discussion
This is the first report of Hizentra® treatment in pediatric patients with PI at doses equivalent to those in previous therapy. Sustained IgG levels were achieved at equivalent SCIG doses compared with previous IVIG or SCIG therapy, and thus the primary objective of this study was met. A statistically significant increase in IgG levels from baseline was observed in children. Children and adolescents responded well to therapy and even achieved greater improvements in some endpoints compared with adults.
IgG trough levels achieved with equivalent doses were similar to or higher than those with previous therapy, suggesting that frequent subcutaneous administration contributed to stabilization of serum IgG levels. In particular, Hizentra® treatment resulted in an increase of 22.4% in IgG trough levels in children who had been previously treated with equivalent IVIG doses. The mean IgG trough levels in adolescents, most of whom had been treated with SCIG previously, were maintained at levels between 6.2 and 9.5 g/L, which are considered sufficient for protection against infections [24–26]. Similar IgG trough levels have been published previously in pediatric patients treated with IVIG or SCIG preparations. At monthly IgG doses of 200–741 mg/kg, serum IgG trough concentrations of 5.68–14.51 g/L in children and 8.2–13.9 g/L in adolescents were reported in a study of Privigen®, a 10% IVIG [9]. Mean IgG trough levels achieved with Vivaglobin®, a 16% SCIG, in children aged 2–11 years, were 9.2 (±2.4) g/L [15]. IgG trough levels obtained with other subcutaneous preparations were similar (5.2–9.6 g/L [13]) or were reported to be in the normal range [27, 28]. In a study of IVIG, 31 children with agammaglobulinemia achieved serum IgG levels between 5 and 11.4 g/L [29]. Thus, Hizentra® therapy normalized IgG levels similarly in all patients, regardless of their age.
The overall efficacy, assessed by the rate of infections and associated treatments, was similar in pediatric patients and adults. Therefore, SCIG therapy can be used for the treatment of PI from early childhood. The rates of all infections, including SBIs, were similar in all age groups and comparable to previously reported rates for IVIG or SCIG treatments [9, 12, 30]. The number of days missed from school, days spent in hospital, and days on antibiotics was influenced markedly by the experience of the child with recurrent pneumonia. The rates of these efficacy endpoints in the other patients were within the range of reported results [9, 12, 30–32].
An important observation in this study was that children tolerated SCIG administration well, with no difference to adolescents or adults. With respect to local reactions, pediatric patients even showed better tolerability compared to adults, with rates markedly lower than the rates observed in adults. In our experience beyond the scope of this study, the infusion of smaller volumes of the 20% SCIG yielded better local tolerability compared to the available 16% and 16.5% SCIG preparations.
As SCIG therapy could help minimize the impact of chronic disease on patients’ and families’ active lives without compromising therapy, it is particularly suitable for children. Only two children discontinued this study, with none withdrawing consent, suggesting that self-administration was accepted well. Because children need lower infusion volumes to administer the required IgG doses, Hizentra® infusions were relatively short (in general, less than an hour) and could be easily integrated in normal daily routine, without a negative impact on patients’ quality of life. This observation is in line with the results of previous studies showing quality of life improvement in PI children with SCIG therapy [33, 34]. Considering the problematic venous access in young children, it is much more practicable to use a subcutaneous way of administration in this age group. Therapy by the subcutaneous route obviates also the use of premedication, which is an additional benefit, as shown here and in previous studies [15]. In addition, subcutaneous infusions are administered with much smaller needles than intravenous infusions, and the small pumps used could be comfortably carried on the body if necessary. Frequent administration (two to seven times a week) of small volumes is another option for reducing the time for each infusion for more flexibility, and may be a further option for patients as was shown in a recent retrospective study [35]. Most importantly, SCIG therapy offers a broad variety of administration options, enabling each patient to find their preferred treatment regimen. Children at school age are more active than adults, and would feel better integrated in their environment if they did not have to miss activities due to their therapy.
In conclusion, Hizentra® is effective in maintaining or increasing IgG trough levels and in protecting pediatric patients from infections. No pediatric-specific dose requirement was necessary to achieve the desired serum IgG level. Hizentra® was well tolerated, with respect to both systemic and local reactions. The short infusion duration and the lack of severe local reactions favor self-administration, which appears to be widely accepted by patents and their families.
Acknowledgments
This study was supported by CSL Behring AG, Bern, Switzerland. The opinions expressed in this paper are those of the authors. The assistance of Corinna Miede and Cordula Massion from Accovion GmbH for the statistical analyses is gratefully appreciated. The authors thank Dr. Andrea Sebald and Dr. Jeff Baggish for critical review of the manuscript. We also acknowledge the editorial assistance of Phocus Services Ltd supported by CSL Behring.
Disclosure of Conflicts of Interest Dr. Borte reports consultant fees from CSL Behring and Baxter, and honoraria for lectures from CSL Behring, Baxter, and Octapharma; Dr. Gonzalez-Quevedo, sponsorship for educational purposes and clinical trial support from CSL Behring; Dr. Grimbacher is a member of the IgPro20 Steering Committee and Advisory Boards, honoraria for presentations from Baxter and Grifols; Dr. Jolles, clinical trial support from CSL Behring, Baxter, and Octapharma, consultant fees from Baxter and Octapharma; and Dr. Zenker and J. Neufang-Hueber are employees of CSL Behring. No other potential conflicts of interest were reported.
References
- 1.Berger M. Principles of and advances in immunoglobulin replacement therapy for primary immunodeficiency. Immunol Allergy Clin North Am. 2008;28:413–37. doi: 10.1016/j.iac.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buckley RH. Primary immunodeficiency diseases due to defects in lymphocytes. N Engl J Med. 2000;343:1313–24. doi: 10.1056/NEJM200011023431806. [DOI] [PubMed] [Google Scholar]
- 3.Chapel H, Geha R, Rosen F. Primary immunodeficiency diseases: an update. Clin Exp Immunol. 2003;132:9–15. doi: 10.1046/j.1365-2249.2003.02110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ochs HD, Smith CIE, Puck JM. Primary immunodeficiency diseases: a molecular and genetic approach. 2. New York: Oxford University Press Inc, USA; 2007. [Google Scholar]
- 5.Park MA, Li JT, Hagan JB, Maddox DE, Abraham RS. Common variable immunodeficiency: a new look at an old disease. Lancet. 2008;372:489–502. doi: 10.1016/S0140-6736(08)61199-X. [DOI] [PubMed] [Google Scholar]
- 6.Busse PJ, Razvi S, Cunningham-Rundles C. Efficacy of intravenous immunoglobulin in the prevention of pneumonia in patients with common variable immunodeficiency. J Allergy Clin Immunol. 2002;109:1001–4. doi: 10.1067/mai.2002.124999. [DOI] [PubMed] [Google Scholar]
- 7.Bussel JB, Eldor A, Kelton JG, Varon D, Brenner B, Gillis S, et al. IGIV-C, a novel intravenous immunoglobulin: evaluation of safety, efficacy, mechanisms of action, and impact on quality of life. Thromb Haemost. 2004;91:771–8. doi: 10.1160/TH03-10-0650. [DOI] [PubMed] [Google Scholar]
- 8.Church JA, Leibl H, Stein MR, Melamed IR, Rubinstein A, Schneider LC, et al. Efficacy, safety and tolerability of a new 10% liquid intravenous immune globulin [IGIV 10%] in patients with primary immunodeficiency. J Clin Immunol. 2006;26:388–95. doi: 10.1007/s10875-006-9025-3. [DOI] [PubMed] [Google Scholar]
- 9.Church JA, Borte M, Taki H, Nelson RP, Sleasman JW, Knutsen AP, et al. Efficacy and safety of Privigen in children and adolescents with primary immunodeficiency. Pediatr Asthma Allergy Immunol. 2009;22:53–62. doi: 10.1089/pai.2009.0005. [DOI] [Google Scholar]
- 10.Gracia J, Vendrell M, Alvarez A, Pallisa E, Rodrigo MJ, Rosa D, et al. Immunoglobulin therapy to control lung damage in patients with common variable immunodeficiency. Int Immunopharmacol. 2004;4:745–53. doi: 10.1016/j.intimp.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Eijkhout HW, Meer JW, Kallenberg CG, Weening RS, Dissel JT, Sanders LA, et al. The effect of two different dosages of intravenous immunoglobulin on the incidence of recurrent infections in patients with primary hypogammaglobulinemia. A randomized, double-blind, multicenter crossover trial. Ann Intern Med. 2001;135:165–74. doi: 10.7326/0003-4819-135-3-200108070-00008. [DOI] [PubMed] [Google Scholar]
- 12.Stein MR, Nelson RP, Church JA, Wasserman RL, Borte M, Vermylen C, et al. Safety and efficacy of Privigen®, a novel 10% liquid immunoglobulin preparation for intravenous use, in patients with primary immunodeficiencies. J Clin Immunol. 2009;29:137–44. doi: 10.1007/s10875-008-9231-2. [DOI] [PubMed] [Google Scholar]
- 13.Abrahamsen TG, Sandersen H, Bustnes A. Home therapy with subcutaneous immunoglobulin infusions in children with congenital immunodeficiencies. Pediatrics. 1996;98:1127–31. [PubMed] [Google Scholar]
- 14.Chapel HM, Spickett GP, Ericson D, Engl W, Eibl MM, Bjorkander J. The comparison of the efficacy and safety of intravenous versus subcutaneous immunoglobulin replacement therapy. J Clin Immunol. 2000;20:94–100. doi: 10.1023/A:1006678312925. [DOI] [PubMed] [Google Scholar]
- 15.Gardulf A, Nicolay U, Asensio O, Bernatowska E, Bock A, Carvalho BC, et al. Rapid subcutaneous IgG replacement therapy is effective and safe in children and adults with primary immunodeficiencies—a prospective, multi-national study. J Clin Immunol. 2006;26:177–85. doi: 10.1007/s10875-006-9002-x. [DOI] [PubMed] [Google Scholar]
- 16.Hansen S, Gustafson R, Smith CI, Gardulf A. Express subcutaneous IgG infusions: decreased time of delivery with maintained safety. Clin Immunol. 2002;104:237–41. doi: 10.1006/clim.2002.5215. [DOI] [PubMed] [Google Scholar]
- 17.Stiehm ER, Casillas AM, Finkelstein JZ, Gallagher KT, Groncy PM, Kobayashi RH, et al. Slow subcutaneous human intravenous immunoglobulin in the treatment of antibody immunodeficiency: use of an old method with a new product. J Allergy Clin Immunol. 1998;101:848–9. doi: 10.1016/S0091-6749(98)70314-8. [DOI] [PubMed] [Google Scholar]
- 18.Hagan JB, Fasano MB, Spector S, Wasserman RL, Melamed I, Rojavin MA, et al. Efficacy and safety of a new 20% immunoglobulin preparation for subcutaneous administration, IgPro20, in patients with primary immunodeficiency. J Clin Immunol. 2010;30:734–45. doi: 10.1007/s10875-010-9423-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maeder W, Lieby P, Sebald A, Spycher M, Pedrussio R, Bolli R. Local tolerance and stability up to 24 months of a new 20% proline-stabilized polyclonal immunoglobulin for subcutaneous administration. Biologicals. 2010;39:43–9. doi: 10.1016/j.biologicals.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Stucki M, Boschetti N, Schaefer W, Hostettler T, Kaesermann F, Nowak T, et al. Investigations of prion and virus safety of a new liquid IVIG product. Biologicals. 2008;36:239–47. doi: 10.1016/j.biologicals.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Jolles S, Bernatowska E, de Gracia J, Borte M, Cristea V, Peter HH, et al. Efficacy and safety of Hizentra® in patients with primary immunodeficiency after a dose-equivalent switch from intravenous or subcutaneous replacement therapy. Clin Immunol. 2011 (in press) [DOI] [PubMed]
- 22.European Agency for the Evaluation of Medicinal Products (EMEA), Committee for Proprietary Medicinal Products (CPMP). Note for guidance on the clinical investigation of human normal immunoglobulin for subcutaneous and intramuscular use. EMEA/CPMP/BPWG/282/00. 2002
- 23.FDA. Safety, efficacy, and pharmacokinetic studies to support marketing of immune globulin intravenous (human) as replacement therapy for primary humoral immunodeficiency. Guidance for industry. 2008.
- 24.Bonagura VR, Marchlewski R, Cox A, Rosenthal DW. Biologic IgG level in primary immunodeficiency disease: the IgG level that protects against recurrent infection. J Allergy Clin Immunol. 2008;122:210–2. doi: 10.1016/j.jaci.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 25.Orange JS, Hossny EM, Weiler CR, Ballow M, Berger M, Bonilla FA, et al. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2006;117:S525–53. doi: 10.1016/j.jaci.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Stiehm ER. Human intravenous immunoglobulin in primary and secondary antibody deficiencies. Pediatr Infect Dis J. 1997;16:696–707. doi: 10.1097/00006454-199707000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Gaspar J, Gerritsen B, Jones A. Immunoglobulin replacement treatment by rapid subcutaneous infusion. Arch Dis Child. 1998;79:48–51. doi: 10.1136/adc.79.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas MJ, Brennan VM, Chapel HH. Rapid subcutaneous immunoglobulin infusions in children. Lancet. 1993;342:1432–3. doi: 10.1016/0140-6736(93)92798-X. [DOI] [PubMed] [Google Scholar]
- 29.Quartier P, Debre M, Blic J, Sauverzac R, Sayegh N, Jabado N, et al. Early and prolonged intravenous immunoglobulin replacement therapy in childhood agammaglobulinemia: a retrospective survey of 31 patients. J Pediatr. 1999;134:589–96. doi: 10.1016/S0022-3476(99)70246-5. [DOI] [PubMed] [Google Scholar]
- 30.Ochs HD, Gupta S, Kiessling P, Nicolay U, Berger M. Safety and efficacy of self-administered subcutaneous immunoglobulin in patients with primary immunodeficiency diseases. J Clin Immunol. 2006;26:265–73. doi: 10.1007/s10875-006-9021-7. [DOI] [PubMed] [Google Scholar]
- 31.Berger M, Pinciaro PJ. Safety, efficacy, and pharmacokinetics of Flebogamma 5% [immune globulin intravenous (human)] for replacement therapy in primary immunodeficiency diseases. J Clin Immunol. 2004;24:389–96. doi: 10.1023/B:JOCI.0000029108.18995.61. [DOI] [PubMed] [Google Scholar]
- 32.Ochs HD, Pinciaro PJ. Octagam 5%, an intravenous IgG product, is efficacious and well tolerated in subjects with primary immunodeficiency diseases. J Clin Immunol. 2004;24:309–14. doi: 10.1023/B:JOCI.0000025453.23817.3f. [DOI] [PubMed] [Google Scholar]
- 33.Gardulf A, Nicolay U, Math D, Asensio O, Bernatowska E, Bock A, et al. Children and adults with primary antibody deficiencies gain quality of life by subcutaneous IgG self-infusions at home. J Allergy Clin Immunol. 2004;114:936–42. doi: 10.1016/j.jaci.2004.06.053. [DOI] [PubMed] [Google Scholar]
- 34.Gardulf A, Borte M, Ochs HD, Nicolay U. Prognostic factors for health-related quality of life in adults and children with primary antibody deficiencies receiving SCIG home therapy. Clin Immunol. 2008;126:81–8. doi: 10.1016/j.clim.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 35.Shapiro R. Subcutaneous immunoglobulin therapy by rapid push is preferred to infusion by pump: a retrospective analysis. J Clin Immunol. 2010;30:301–7. doi: 10.1007/s10875-009-9352-2. [DOI] [PubMed] [Google Scholar]