Abstract
In eyes wearing negative lenses, the D2 dopamine antagonist spiperone was only partly effective in preventing the ameliorative effects of brief periods of vision (Nickla et al., 2010), in contrast to reports from studies using form deprivation. The present study was done to directly compare the effects of spiperone, and the D1 antagonist SCH-23390, on the two different myopiagenic paradigms. 12-day old chickens wore monocular diffusers (form deprivation) or − 10 D lenses attached to the feathers with matching rings of Velcro. Each day for 4 days, 10 µl intravitreal injections of the dopamine D2/D4 antagonist spiperone (5 nmoles) or the D1 antagonist SCH-23390, were given under isoflurane anesthesia, and the diffusers (n=16; n=5, respectively) or lenses (n=20; n=6) were removed for 2 hours immediately after. Saline injections prior to vision were done as controls (form deprivation: n=11; lenses: n=10). Two other saline-injected groups wore the lenses (n=12) or diffusers (n=4) continuously. Axial dimensions were measured by high frequency A-scan ultrasonography at the start, and on the last day immediately prior to, and 3 hours after the injection. Refractive errors were measured at the end of the experiment using a Hartinger’s refractometer. In form-deprived eyes, spiperone, but not SCH-23390, prevented the ocular growth inhibition normally effected by the brief periods of vision (change in vitreous chamber depth, spiperone vs saline: 322 vs 211 µm; p=0.01). By contrast, neither had any effect on negative lens-wearing eyes given similar unrestricted vision (210 and 234 µm respectively, vs 264 µm). The increased elongation in the spiperone-injected form deprived eyes did not, however, result in a myopic shift, probably due to the inhibitory effect of the drug on anterior chamber growth (drug vs saline: 96 vs 160 µm; p<0.01). Finally, spiperone inhibited the vision-induced transient choroidal thickening in form deprived eyes, while SCH-23390 did not. These results indicate that the dopaminergic mechanisms mediating the protective effects of brief periods of unrestricted vision differ for form deprivation versus negative lens-wear, which may imply different growth control mechanisms between the two.
Keywords: dopamine, spiperone, form deprivation, myopia, emmetropization
1. Introduction
The retinal neuromodulator dopamine has been implicated in the process of the visual regulation of eye growth in chicks (Stone et al., 1989; Rohrer et al., 1993; Schaeffel et al., 1995; Schmid and Wildsoet, 2004) and monkeys (Iuvone et al., 1991): the non-specific dopamine agonist apomorphine inhibits the myopia and excessive ocular elongation caused by form deprivation (Stone et al., 1989; Iuvone et al., 1991; Schmid and Wildsoet, 2004) or wearing negative lenses (Schmid and Wildsoet, 2004; Nickla et al., 2010). Because this inhibitory effect is also found for the D2 specific agonist quinpirole, but not for the D1 agonist SKF-38393 for both form deprivation (McCarthy et al., 2007) and negative lenses (Nickla et al., 2010), the effect appears to be mediated by a D2 receptor. The fact that the D2 agonist “mimics” the effects of brief daily vision during form deprivation, largely preventing the development of myopia, and that spiperone can block the protective effect of vision, argues that dopamine D2 receptors mediate the growth inhibition effected by the vision (McCarthy et al., 2007). However, results using negative lens-induced hyperopic defocus suggests a more complex scenario: When either spiperone (D2) or SCH-23390 (D1) was combined with 2 daily hours of unrestricted vision, eyes developed about −3.0 D of myopia, which was midway between the continuous lens-wear controls (−6 D) and saline-injected vision controls (0.6 D). This evinces a partial effect of both drugs in blocking the protective effects of vision on the development of myopia (Nickla et al., 2010), in contrast to the reports that spiperone was fully effective at blocking the effects of brief vision (McCarthy et al., 2007) or bright lights (Ashby and Schaeffel, 2010) in form-deprived eyes. One possible explanation for this discrepancy is that D2 mechanisms predominate in the absence of visual feedback as occurs in form deprivation, but not in the responses to hyperopic defocus caused by negative lenses (Nickla et al., 2010). To further resolve this issue, we tested both antagonists on the two visual paradigms. Our results imply that the dopaminergic mechanisms mediating ocular growth do indeed differ in form deprivation and negative lens-wear.
2. Methods
2.1 Subjects
Subjects were White Leghorn chickens (Gallus gallus domesticus; Cornell University K-strain), hatched in an incubator and raised in temperature-controlled brooders. The light cycle was 12L/12D (8:00 am to 8:00 pm). Food and water were supplied ad libitum. In all experiments, the right eye was treated and the left eye served as the untreated control. The data presented here for the lens experiments are from chicks used for a previous publication (Nickla et al., 2010); these data (anterior chamber depth and vitreous chamber depth) were not presented in that publication. Care and use of the animals conformed to the ARVO Resolution for the Care and Use of Animals in Research.
2.2 Experimental design
At 12 days of age, diffusers or negative lenses (−10 D) mounted on Velcro rings were attached to the matching ring that was glued to the feathers around one eye. On noon of each day for 4 days, under isoflurane inhalation anesthesia, 10 µl intravitreal injections of spiperone (Tocris, D2/D4 antagonist; dose=5nmoles) were given and the diffusers (n=16) or lenses (n=20) were removed for 2 hours. Using the same paradigam, the D1 antagonist SCH-23390 (Sigma, dose=5nmoles) was also tested (diffusers: n=5; lenses: n=6). Saline (0.75%) injections were done as controls in a “saline + vision” group (diffusers, n=11; lenses, n=10). Two other saline-injected groups wore the lenses (n=12) or diffusers (n=4) continuously (“saline/no vision”).
Spiperone was dissolved in a 1mg/ml solution of ascorbic acid to yield a 500µM concentration, and heated to 30 degrees for 10 minutes while stirring (personal communication, Regan Ashby). The dose used was based on the results of McCarthy et al. (McCarthy et al., 2007). Injections used a 30G needle, going through the skin of the lids over the superior temporal sclera after removing the feathers and cleaning the skin with alcohol. Care was taken to use the same injection site for subsequent injections. The needle remained in place for 30 seconds before being slowly withdrawn while the skin around the site was held tightly together using a small forceps.
Axial dimensions were measured using high frequency A-scan ultrasonography (details in Nickla et al., 1998) at the start of lens wear, on day 4 immediately prior to the injections, and then again 3 hours later, in order to detect the transient choroidal thickening in response to the episode of normal vision. Refractive errors were measured using a Hartinger’s refractometer (details in (Wallman and Adams, 1987) at the end of the experiment. Statistical analyses between groups used ANOVAs and two-sample T-tests.
3. Results
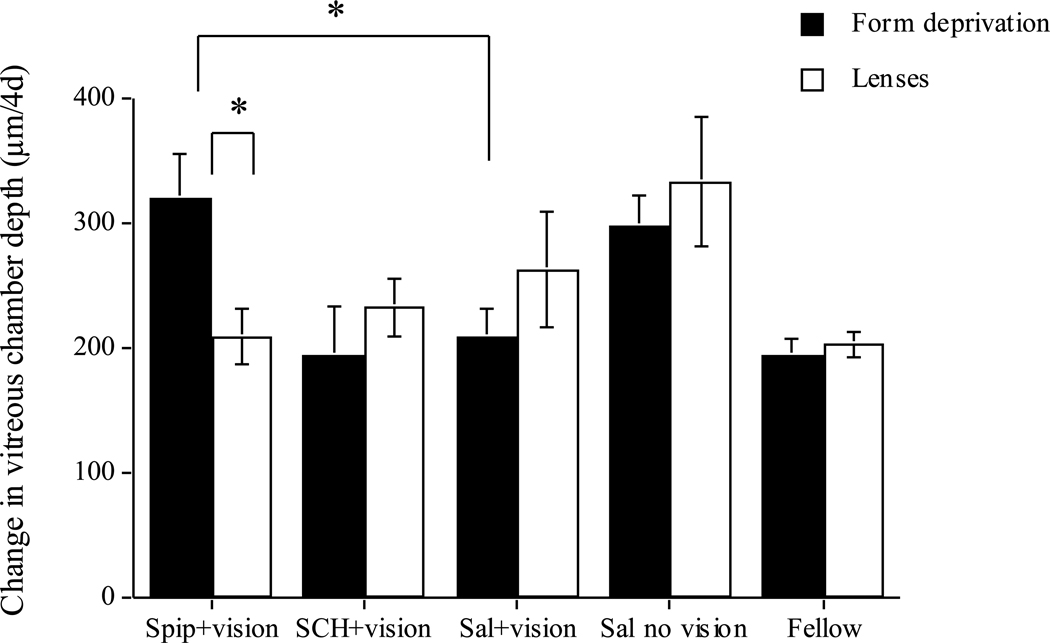
In agreement with McCarthy et al. (2007), injecting spiperone immediately prior to exposing form-deprived eyes to brief periods of daily vision prevented the inhibitory effect of the vision on vitreous chamber growth: drug-injected eyes grew significantly faster than saline+vision controls (Figure 1, black bars: 322 vs 211 µm/4d; p=0.01), and did not differ from saline-injected deprived eyes receiving no daily vision (black bars, spiperone vs saline/no vision: 322 vs 299 µm/4d; NS). By contrast, as previously reported (Nickla et al., 2010), spiperone did NOT prevent the effects of daily vision in eyes responding to negative lenses: vitreous chamber elongation in these eyes did not differ from that of saline+vision controls (Figure 1, white bars: 210 vs 264 µm) (nor was there any difference in axial elongation: 338 vs 273 µm; Nickla et al., 2010). Thus, spiperone was effective at blocking the effects of vision in eyes responding to form deprivation but not in eyes responding to lens-induced hyperopic defocus (spiperone; black vs white bars: 322 vs 210 µm; p=0.01).
Figure 1.
Change in vitreous chamber depth over the 4 days of the experiment for form-deprived (black bars) and lens-wearing (white bars) eyes. “Sal+vision” are saline-injected controls receiving the same daily exposures to unrestricted vision. “Sal no vision” are saline-injected eyes with continuous diffuser-wear (black bars) or lens-wear (white bars). “Fellow” are all fellow eyes combined. Spiperone prevents the vision-induced growth inhibition in form deprived eyes (black bars, compare “spip” to “sal+vision”), but not in lens-wearing eyes (white bars, same comparison). SCH-23390 has no effect. Bars are standard errors of the mean. * p<0.05.
The D1 antagonist SCH-23390 had no effect on the responses to periods of vision in either form-deprived eyes, or in lens-wearing eyes: in both, eyes grew at a similar rate as their saline+vision controls (Figure 1: compare SCH+ vision to saline+vision). There was also no effect on refractive error (0.7 vs −0.4 D; data not shown).
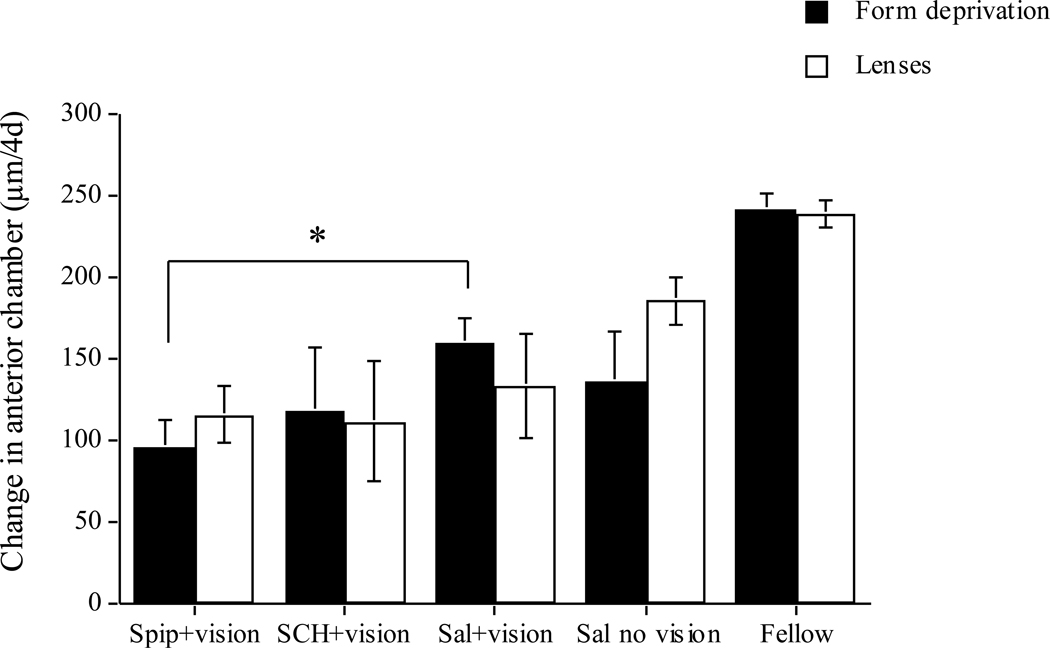
Despite the increase in vitreous chamber growth rate in the spiperone-injected eyes, the refractive errors were not significantly different from those of saline-injected controls (−1.1 vs −0.9 D; data not shown). This discrepancy is probably the result of the inhibitory effect of spiperone on anterior chamber growth in these form-deprived eyes (Figure 2, black bars: drug vs saline: 96 vs 160 µm; p<0.01), which may have changed corneal power. As a result of shallower anterior chambers in the experimental eyes, there was no significant effect on axial elongation between the two experimental groups (data not shown). While SCH-23390 also appears to have inhibited anterior chamber growth as well (118 vs 160 µm), the variability due to the small number of animals precludes statistical significance, so this must be interpreted with caution.
Figure 2.
Change in anterior chamber depth over the 4 days of the experiment for form-deprived (black bars) and lens-wearing (white bars) eyes. The groups are defined above. Spiperone inhibits anterior chamber growth in form-deprived eyes but not in lens-wearing eyes. Bars are standard errors of the mean. * p<0.05.
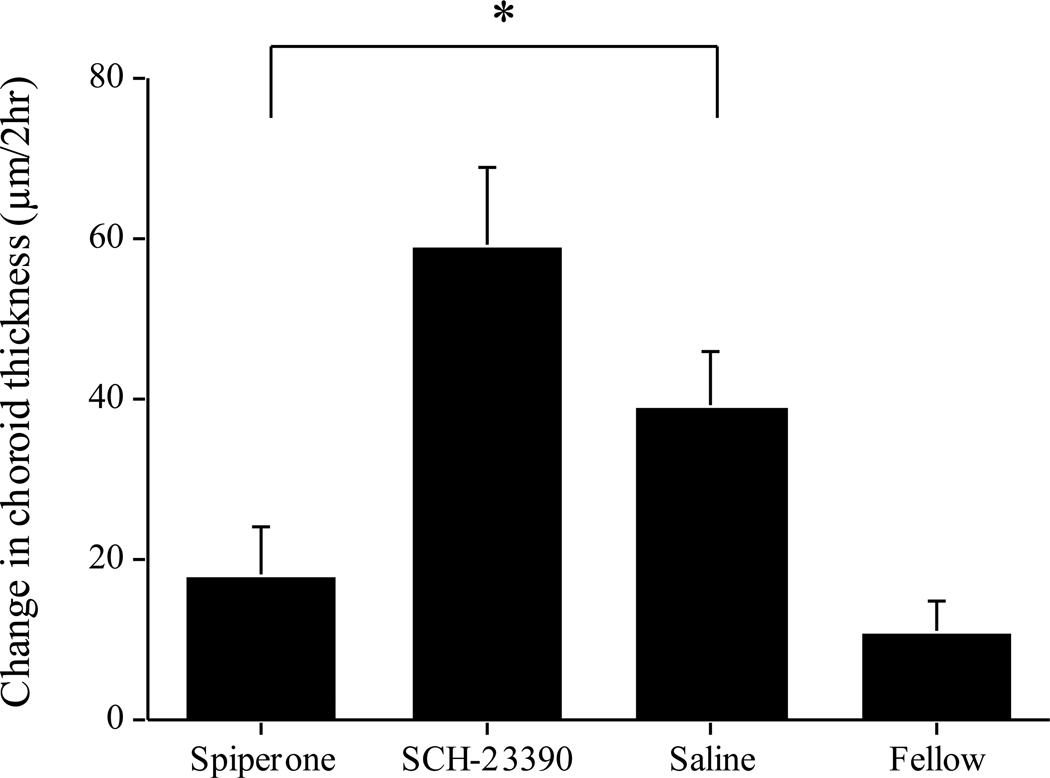
Finally, spiperone significantly inhibited the choroidal thickening in response to brief vision in form deprived eyes compared to saline controls (Figure 3: 18 vs 38 µm; p<0.05), in contrast to the lack of effect previously found in lens-wearing eyes (Nickla et al., 2010). SCH-23390 had no such inhibitory effect: choroids showed a similar response as that of saline-injected controls (Figure 3: 59 vs 39 µm), similar to what was found in lens-wearing eyes (Nickla et al., 2010).
Figure 3.
Change in choroidal thickness over the two hours of daily unrestricted vision in form-deprived eyes. “Saline” are the saline-injected eyes receiving daily vision. Spiperone inhibits the choroidal expansion in response to vision, while SCH-23390 has no effect. “Fellow” are all fellow eyes combined. Bars are standard errors of the mean. * p<0.05.
4. Discussion
Our previous results showed that neither the D2 antagonist spiperone, nor the D1 antagonist SCH-23390, prevented brief vision from inhibiting lens-induced ocular growth (Nickla et al., 2010). In contrast, we here show that spiperone does prevent the inhibitory effect of brief vision on vitreous chamber elongation in form-deprived eyes while SCH-23390 has no effect, as previously shown by McCarthy et al. (2007). Because the drugs and the daily episodes of unobstructed vision were given throughout the experiment and because the refractive status of the eyes did not differ substantially among the treatments, the visual experiences were identical. This leaves one with no obvious alternative to concluding that the dopaminergic mechanisms modulating the ocular elongation caused by the lenses and diffusers were different, and answers a crucial question left open in our previous publication (Nickla et al., 2010). The existence of this difference between paradigms was first suggested by the finding of Bartmann et al. (1994) that constant light suppressed form deprivation- but not negative lens-induced myopia. The same lab reported a similar finding for the neurotoxin 6-hydroxydopamine (Schaeffel et al., 1994) but this was later reversed (Diether and Schaeffel, 1997). Second, Schmid and Wildsoet (2004) reported that atropine was more effective at growth inhibition in form deprived eyes than was a combination of apomorphine and atropine, but both had similar effects on negative lens-wearing eyes. Finally, Kee et al. (2001) reported that the inhibitory effects of stroboscopic stimulation (which might influence retinal dopamine release) were more effective in form-deprived eyes than in negative lens-wearing ones.
A second result from our study was that spiperone had an inhibitory effect on the growth of the anterior chamber in form deprived eyes, the opposite of its (dis-inhibitory) effect on the vitreous chamber. This evidence for differing growth control mechanisms mediating anterior chamber versus vitreous chamber growth is in accord with similar reported effects of other molecules: the nitric oxide synthase inhibitor L-NAME (Nickla and Wildsoet, 2004), the retinal hormone melatonin (Summers Rada and Wiechmann, 2006), and kainic acid (Wildsoet and Pettigrew, 1988) all have dis-inhibitory effects on the back of the eye and inhibitory effects on the front, similar to our result with spiperone. By contrast, insulin (Feldkaemper et al., 2009) and quisqualic acid (Barrington et al., 1989) result in increases in anterior chamber growth but little or no change in growth of the back of the eye, again, supporting different mechanisms. Because both D1 and D2 receptors are found in the anterior segment of the eye, it is likely that dopamine plays a role in aqueous flow dynamics (Potter, 1995), which might be related to the effect reported here for spiperone.
Finally, we find that spiperone, but not SCH-23390, inhibits the choroidal thickening normally elicited by brief periods of vision in form deprived eyes. This result is in accord with the hypothesis that the transient choroidal thickening responses may be involved in ocular growth inhibition: choroids of eyes injected with spiperone did not thicken in response to brief periods of vision and ocular growth was not inhibited, while choroids of eyes injected with SCH-23390 did thicken in response to vision, and ocular growth was inhibited.
In conclusion, our results support the existence of two different underlying dopaminergic mechanisms in the protective effects of “normal vision” in form deprivation and lens-compensation myopia. Because dopamine has been shown to be involved in mediating the protective effects of high light intensities against form deprivation myopia in chicks (Ashby and Schaeffel 2010) and monkeys (Smith et al., 2011), and dopamine release stimulated by high light intensity has been speculated to be a factor in the protective effects of outdoor activities in children (Rose et al., 2008), it would be crucial to determine the relative roles of the different dopamine receptors before contemplating complementary pharmacological therapies based on the assumption that form-deprivation myopia is analogous to the myopia commonly found in children.
Acknowledgements
Supported by NIH-NEI-013636.
The authors give sincere thanks to Drs. Josh Wallman and Xiao Ying Zhu (City College of N.Y., CUNY) for their helpful comments on this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashby R, Schaeffel F. The effect of bright light on lens-compensation in chicks. Invest Ophthalmol Vis Sci. 2010;51:5247–5253. doi: 10.1167/iovs.09-4689. [DOI] [PubMed] [Google Scholar]
- Barrington M, Sattayasai J, Zappia J, Ehrlich D. Excitatory amino acids interfere with normal eye growth in posthatch chick. Curr. Eye Res. 1989;8:781–792. doi: 10.3109/02713688909000868. [DOI] [PubMed] [Google Scholar]
- Bartmann M, Schaeffel F, Hagel G, Zrenner E. Constant light affects retinal dopamine levels and blocks deprivation myopia but not lens-induced refractive errors in chickens. Visual Neuroscience. 1994;11:199–208. doi: 10.1017/s0952523800001565. [DOI] [PubMed] [Google Scholar]
- Diether S, Schaeffel F. Local changes in eye growth induced by imposed refractive error despite active accommodation. Vision Res. 1997;37:659–668. doi: 10.1016/s0042-6989(96)00224-6. [DOI] [PubMed] [Google Scholar]
- Feldkaemper MP, Neacsu I, Schaeffel F. Insulin acts as a powerful stimulator of axial myopia in chicks. Invest. Ophthalmol. Vis. Sci. 2009;50:13–23. doi: 10.1167/iovs.08-1702. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Tigges M, Stone RA, Lambert S, Laties AM. Effects of apomorphine, a dopamine receptor agonist, on ocular refraction and axial elongation in a primate model of myopia. Invest. Ophthalmol. Vis. Sci. 1991;32:1674–1677. [PubMed] [Google Scholar]
- Kee C-S, Marzani D, Wallman J. Differences in time course and visual requirements of ocular responses to lenses and diffusers. Invest. Ophthalmol. Vis. Sci. 2001;42:575–583. [PubMed] [Google Scholar]
- McCarthy CS, Megaw P, Devadas M, Morgan I. Dopaminergic agents affect the ability of brief periods of normal vision to prevent form-deprivation myopia. Exp. Eye Res. 2007;84:100–107. doi: 10.1016/j.exer.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Nickla D, Wildsoet C. The effect of the nonspecific nitric oxide synthase inhibitor NG-nitro-L-arginine methyl ester on the choroidal compensatory response to myopic defocus in chickens. Optometry Vis. Sci. 2004;81:111–118. doi: 10.1097/00006324-200402000-00009. [DOI] [PubMed] [Google Scholar]
- Nickla DL, Totonelly K, Dhillon B. Dopaminergic agonists that result in ocular growth inhibition also elicit transient increases in choroidal thickness in chicks. Exp. Eye Res. 2010;91:715–720. doi: 10.1016/j.exer.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickla DL, Wildsoet C, Wallman J. Visual influences on diurnal rhythms in ocular length and choroidal thickness in chick eyes. Exp. Eye Res. 1998;66:163–181. doi: 10.1006/exer.1997.0420. [DOI] [PubMed] [Google Scholar]
- Potter DE. Do dopamine and dopamine receptors have roles in modulating function in the anterior segment?: The Evidence. Prog. Retin. Eye Res. 1995;15:103–111. [Google Scholar]
- Rohrer B, Spira AW, Stell WK. Apomorphine blocks form-deprivation myopia in chickens by a dopamine D2-receptor mechanism acting in retina or pigmented epithelium. Visual Neuroscience. 1993;10:447–453. doi: 10.1017/s0952523800004673. [DOI] [PubMed] [Google Scholar]
- Rose K, Morgan I, Ip J, Kifley A, Huynh S, Smith W, Mitchell P. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008;116:1229–1230. doi: 10.1016/j.ophtha.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Bartmann M, Hagel G, Zrenner E. Studies on the Role of the Retinal Dopamine/Melatonin System in Experimental Refractive Errors in Chickens. Vision Res. 1995;35:1247–1264. doi: 10.1016/0042-6989(94)00221-7. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Hagel G, Bartmann M, Kohler K. 6-hydroxydopamine does not affect lens-induced refractive errors but suppresses deprivation myopia. Vision Res. 1994;34:143–149. doi: 10.1016/0042-6989(94)90327-1. [DOI] [PubMed] [Google Scholar]
- Schmid KL, Wildsoet C. Inhibitory effects of apomorphine and atropine and their combination on myopia in chicks. Optometry Vis. Sci. 2004;81:137–147. doi: 10.1097/00006324-200402000-00012. [DOI] [PubMed] [Google Scholar]
- Smith EL, Hung L, Huang J. Effects of high ambient lighting on the development of form-deprivation myopia in infant rhesus monkeys. ARVO. 2011 doi: 10.1167/iovs.11-8652. E-Abstract #3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RA, Lin T, Laties AM, Iuvone PM. Retinal dopamine and form-deprivation myopia. Proc. Nat. Acad. Sci. 1989;86:704–706. doi: 10.1073/pnas.86.2.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers Rada J, Wiechmann A. Melatonin receptors in chick ocular tissues: Implications for a role of melatonin in ocular growth regulation. Invest Ophthalmol Vis Sci. 2006;47:25–33. doi: 10.1167/iovs.05-0195. [DOI] [PubMed] [Google Scholar]
- Wallman J, Adams JI. Developmental aspects of experimental myopia in chicks: susceptibility, recovery and relation to emmetropization. Vision Res. 1987;27:1139–1163. doi: 10.1016/0042-6989(87)90027-7. [DOI] [PubMed] [Google Scholar]
- Wildsoet CF, Pettigrew JD. Kainic acid-induced eye enlargement in chickens: differential effects on anterior and posterior segments. Invest. Ophthalmol. Vis. Sci. 1988;29:311–319. [PubMed] [Google Scholar]