Abstract
The hematopoietic actin regulatory protein HS1 is required for cell spreading and signaling in lymphocytes, but the scope of HS1 function in antigen presentation has not been addressed. We show that dendritic cells (DCs) from HS1−/− mice differentiate normally and display normal LPS-induced upregulation of surface markers and cytokines. Consistent with their normal expression of MHC and costimulatory molecules, HS1−/− DCs present OVA peptide efficiently to CD4+ T cells. However, presentation of ovalbumin protein is defective. Similarly, MHC Class I-dependent presentation of VSV8 peptide to CD8+ T cells occurs normally, but cross-presentation of GRP94/VSV8 complexes is defective. Analysis of antigen uptake pathways shows that HS1 is required for receptor-mediated endocytosis, but not for phagocytosis or macropinocytosis. HS1 interacts with dynamin 2, a protein involved in scission of endocytic vesicles. However, HS1−/− DCs showed decreased numbers of endocytic invaginations, whereas dynamin-inhibited cells showed accumulation of these endocytic intermediates. Taken together, these studies show that HS1 promotes an early step in the endocytic pathway that is required for efficient antigen presentation of exogenous antigen by DCs.
Keywords: dendritic cells, HS1, antigen presentation, receptor-mediated endocytosis, actin, cortactin
Introduction
Dendritic cells (DCs) are highly specialized for presentation of antigens to naïve T cells. DCs survey peripheral tissues, ingesting large volumes of material by receptor-mediated endocytosis, phagocytosis and macropinocytosis (1–5). In the presence of inflammatory signals, DCs undergo a maturation process that results in diminished endocytosis of antigen, enhanced acidification of antigen processing compartments, redistribution of MHC molecules to the cell surface, upregulation of costimulatory molecules, and increased cell motility. As they mature, DCs migrate to lymphoid organs, where they present peptides derived from non-self antigens to T cells, initiating an adaptive immune response.
Many of these aspects of DC function rely on actin and its regulatory proteins. During endocytosis, actin polymerization produces forces that promote internalization of plasma membrane vesicles. This is particularly obvious for phagocytosis and macropinocytosis, which involve large actin-rich cell surface protrusions (6–8). However, receptor-mediated endocytosis is also dependent on actin filaments, which work together with clathrin and other proteins such as dynamin 2 to drive the internalization of plasma membrane vesicles (9, 10). After vesicle internalization, the actin cytoskeleton serves as a highway to transport vesicles to compartments where antigen is processed, loaded onto MHC molecules, and transported back to the cell surface for recognition by T cells (11, 1, 2).
Macropinocytosis and phagocytosis depend on the Rho family GTPases CDC42 and Rac (12–14), and diminished uptake through these pathways in mature DCs has been linked to downregulation of CDC42 function (13). Interestingly, however, receptor-mediated endocytosis is not dependent on Rho GTPases, nor is it downregulated in mature DCs (13). In keeping with this finding, recent analysis has shown that mature DCs take up antigens efficiently via receptor-mediated endocytosis (15), a process that may be very important for presentation of antigens by lymphoid resident DCs (16). Receptor-mediated antigen uptake by both immature and mature DCs is likely to be particularly important at low antigen dose. In addition to playing an important role in normal immune responses, the ability of DCs to take up material by receptor-mediated endocytosis has been widely exploited to target these cells for therapeutic purposes (e.g. (17–21)).
Defects in actin regulatory proteins have far-reaching effects on DC function. Mutations in Wiskott-Aldrich Syndrome protein (WASp), and its binding partner WASp interacting protein (WIP) exhibit defects in antigen uptake, migration and immunological synapse formation (22–27). We have recently found that WASp and WIP interact in DCs with a third protein, hematopoietic lineage cell-specific protein 1 (HS1, also called HCLS1, LckBP1) (28). HS1 is the hematopoietic lineage-specific homologue of the more widely expressed protein cortactin (29), and we have shown that HS1 is the only cortactin family member expressed in murine BMDCs (28). Like WASp, HS1 can activate the Arp2/3 complex to drive the formation of branched actin filaments (30, 31). However HS1 also binds to F-actin, and stabilizes the branched actin network generated by WASp and other proteins (32). HS1 is a modular protein, with an N-terminal domain that binds to the Arp2/3 complex followed by a repeat region that binds to actin filaments (33, 34, 30, 31). The C-terminal half of the protein functions as a signaling adaptor, and consists of an extended proline-rich region and a C-terminal SH3 domain. The proline-rich region contains sites for immunoreceptor-induced tyrosine phosphorylation (35–37), and provides docking sites for several SH3 and SH2 domain containing proteins including Lck, Vav1 PLCγ1 and Itk (38–40). The HS1 SH3 domain mediates binding to the WIP/WASp heterodimer (28). The SH3 domain of cortactin also binds to dynamin 2 (41), though this interaction has not been shown for HS1.
The full scope of HS1 function within leukocyte lineages is not known. Mutations and polymorphisms in HS1 are associated with autoimmune disease (42, 43), though the mechanistic basis for this association has not been determined. Aberrant HS1 expression and/or phosphorylation in B cells are associated with chronic lymphocytic leukemia (44–46). Deficiency for HS1 is associated with disruption of actin responses and Ca++ signaling events leading to IL-2 promoter activation in T cells (39, 40), with defects in activation induced cell death in B cells (47, 48), and with defects in chemotaxis and cytolysis in NK cells (49).
In comparison with lymphocytes and NK cells, much less is known about HS1 function in professional antigen presenting cells. As we recently showed, WT murine BMDCs do not express cortactin, and DCs from HS1−/− mice lack both cortactin family members (28). These cells exhibit defects in podosome organization and lamellipodial protrusion, resulting in a diminished directional persistence during chemotaxis. Since several studies have demonstrated a role for cortactin in endocytosis (50–56), we asked if HS1 expression is required for antigen uptake and presentation by DCs. Our results demonstrate that HS1 expression is essential for efficient antigen processing and presentation to T cells, and that this is due, at least in part, to a requirement for HS1 in receptor-mediated endocytosis of protein antigens.
Materials and Methods
Mice
HS1−/− mice (48) were fully backcrossed onto C57BL/6J or Balb/c backgrounds. DO-11.10 mice, which express a TCR specific for chicken OVA(323–339)/I-Ad, and C57BL/6J mice were obtained from Jackson Laboratories. OTII mice, which express a TCR specific for chicken OVA(323–339)/I-Ab were obtained from Taconic. Mice were housed under pathogen-free conditions in the Children’s Hospital of Philadelphia animal facility. All studies involving animals were reviewed and approved by the Children’s Hospital of Philadelphia Institutional Animal Care and Use Committee.
Cell Culture
Primary cultures of bone marrow-derived dendritic cells (BMDCs) were prepared as previously described (57). Briefly, bone marrow progenitor cells were flushed from femurs and tibia of 2–4 month old mice, and cells were cultured in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 10% FBS, recombinant mouse granulocyte/macrophage-colony stimulating factor (GM-CSF), glutamine, β-mercaptoethanol and penicillin/streptomycin. Medium was replaced every 2 days, and cells were used between 6–8 days of culture, when >70% of the cells are CD11c+. Maturation of BMDCs was induced by culturing for 16–24h in the presence of 100 ng/ml LPS (E. coli 055:B5, Sigma) or, where indicated, 500 nM CpG (ODN 1668) or control oligonucleotides (InvivoGen).
Primary CD4+ T cells from DO-11.10 or OT II mice were prepared as described previously (40). Briefly, CD4+ T cells were enriched from spleens and lymph nodes by negative selection with anti-CD8 and anti-MHC Class II using anti-rat IgG magnetic beads (Qiagen). The T cell hybridoma N15, which expresses a TCR specific for VSV8/H2-Kb (58), was obtained from Dr. E. Reinherz (Dana-Farber Cancer Institute, Boston, MA) and cultured in RPMI, 10% FCS, 0.1 mg/ml G418 and 0.2 mg/ml hygromycin. N15 cells were incubated overnight with PMA (10 ng/ml) prior to use in antigen presentation assays, a treatment necessary for optimal activation (58). Maximal activation of N15 cells was measured by cross-linking with plate-bound anti-CD3.
Analysis of BMDC maturation
For flow cytometry, BMDCs were incubated with rat anti-mouse CD16/CD32 (clone 2.4G2) to block FcγR, followed by staining with the following mAbs (all from BD Biosciences): allophycocyanin-conjugated hamster anti-mouse CD11c, FITC-conjugated hamster anti-mouse CD40, PE-conjugated rat anti-mouse mAbs to CD80, CD86, and MHC class-I and -II. Non-specific staining was defined using isotype-matched antibodies. Cells were analyzed on a FACSCalibur (BD Biosciences) using FLOWJO software (Treestar). To measure TNFα production, BMDC culture supernatants were harvested at the indicated times and assayed by ELISA (R&D Systems).
Protein binding studies and western blotting
For GST-pulldown studies, recombinant GST-tagged SH3 domain of HS1 (aa 415–486) and a variant bearing an inactivating mutation (W456Y) were generated in bacteria as described (28). BMDCs were lysed in NP40 lysis buffer (50 mM Tris-HCl pH 8, 1% NP40, 100 mM NaCl, 5 mM EDTA, 0.5 mM CaCl2, protease inhibitors, 1 mM Na3VO4, and 5 mM NaF). Lysates were clarified by centrifugation and exposed to glutathione resin-bound GST fusion proteins for 2h. Beads were washed in NP-40 lysis buffer, eluted, and analyzed by western blotting. For co-immunoprecipitation studies, 293T cells were transiently transfected with FLAG-tagged HS1 (FLAG.HS1 FL), an N-terminal deletion mutant lacking amino acids 1–335, which contain the Arp2/3 complex and actin binding regions (FLAG.HS1ΔN) or a variant of FLAG.HS1 ΔN bearing the W456Y mutation (FLAG.HS1ΔNW->Y). In addition, cells were transfected with either eGFP-tagged dynamin 2 (GFP.Dyn2 FL), or a dynamin 2 mutant lacking the proline-rich domain (GFP.Dyn2 ΔPRD) (41), gifts from Drs. H. Cao and M. McNiven, Mayo Clinic, Rochester, MN). Cells were lysed in NP40 lysis buffer, and HS1 was immunoprecipitated using M2 anti-FLAG agarose (Sigma).
For western blotting, proteins were separated by SDS-PAGE, transferred to nitrocellulose and blocked in 3% BSA in PBS. Blots were probed with primary antibodies as indicated. Rabbit anti-mouse HS1 was described previously (40, 28). Goat anti-dynamin 2 (C-18) and rabbit anti-WIP (H-224) were from Santa Cruz, rabbit anti-WASp was from Upstate, rabbit anti-GFP was from Invitrogen, and mouse anti-GAPDH (6C5) was from EMD Chemicals. Primary antibodies were detected with secondary antibodies coupled to IR800 (Rockland) or AlexaFluor680 (Invitrogen) and visualized using an Odyssey Imager (Licor). Quantitation was performed within the linear range.
Macropinocytosis and phagocytosis assays
To measure macropinocytosis, BMDCs were washed and resuspended at 8 × 106/ml in IMDM containing 0.5% BSA. Cells were mixed with prewarmed FITC-Dextran (70,000 MW, Invitrogen) or Lucifer Yellow (Invitrogen) at 1mg/ml final concentration and incubated at 37°C for the indicated times. After washing 3 times with cold FACS buffer (5% FBS, 1 mM EDTA in PBS), internalized tracers were measured by flow cytometry. Time points were performed in duplicate and mean fluorescence intensity values were calculated.
To analyze phagocytosis, BMDCs were plated on coverslips in 6-well plates, and cultured overnight. Media was replaced with ice-cold media containing 25 µg/ml FITC-Zymosan (Invitrogen) and incubated for 1 hr at 4°C. After washing three times with cold media, prewarmed media was added to initiate uptake. Cells were incubated at 37°C for the indicated times, and then fixed in 3% paraformaldehyde/PBS. Cells were permeabilized with 0.3% Triton X-100, blocked with 0.05% saponin/1.25% fish skin gelatin in Tris buffered saline, and labeled with Alexa Fluor 594-phalloidin or with anti-LAMP1 (1D4B) followed by Alexa Fluor 647 anti-Rat Ig. The 1D4B monoclonal antibody developed by Dr. J.T. August was obtained from the Developmental Studies Hybridoma Bank, developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City,IA. Cells were imaged using a Leica SP2 confocal microscope. Zymosan particles were scored as internalized if they were surrounded by phalloidin or LAMP1 staining.
Antigen presentation assays
VSV8 peptide (RGYVYQGL) from the vesicular stomatitis virus (VSV) N protein and OVA323–329 peptide, (ISQAVHAAHAEINEAGR) from chicken ovalbumin were synthesized at the University of Chicago peptide facility, purified by HPLC, and verified by mass spectrometry. Ovalbumin protein was purchased from Worthington and further purified by size exclusion chromatography to ensure removal of contaminating peptides and LPS. For phagocytosis studies, latex beads (polystyrene 3 micron microspheres, Polysciences) were freshly coated by overnight incubation with the indicated ratios of ovalbumin and BSA, maintaining a concentration of 10 mg/ml total protein. Complexes of the VSV8 peptide and the chaperone glucose regulated protein of 94 KDa (GRP94) were generated as described in (57). The N-terminal portion of GRP94 (N1–355), is sufficient to mediate peptide binding, receptor-mediated uptake by DCs, and cross-presentation of peptide antigens to T cells (57). N1–355 was expressed in SF9 cells and purified by affinity and size exclusion chromatography under LPS-free conditions as detailed in Vogen et al. (59). Peptide-GRP94 complex was prepared as previously described (59). Briefly, N1–355 and VSV8 were mixed in buffer A (20 mM HEPES, pH 7.2, 150 mM NaCl, 10 mM KCl, 1 mM MgCl2 and 0.1 mM CaCl2), heat shocked for 10 min at 50°C and then incubated at room temperature for 30 min. The complex was separated from unbound peptide by size exclusion chromatography.
To measure antigen presentation on MHC Class II molecules, BMDCs were harvested and replated at 5×104 cells/well in flat-bottom 96-well plates in quadruplicates. Cells were then pulsed with either ovalbumin protein or OVA323–329 peptide at the indicated concentrations for 4h. To control for contaminating peptide in the ovalbumin protein, some DCs were pre-fixed with paraformaldehyde prior to addition of protein. Where indicated, mannose receptors were blocked by incubation with 3 mg/ml mannan from S. Cerevisiae (Sigma) for 1h prior to addition of soluble ovalbumin. For presentation of phagocytosed ovalbumin, cells were incubated for 4h with ovalbumin/BSA-coated beads at a ratio of 60 beads per cell. Antigen-pulsed DCs were washed and co-cultured with 2 × 105 CD4+ T cells for 24h. Cells and supernatants were lysed together, and IL-2 was measured by ELISA (R&D Systems). Presentation on MHC Class I molecules was performed similarly, except that BMDCs were pulsed with N1–355-VSV8 complex or free VSV8 peptide for 3–4h, and then an equal number of N15 hybridoma cells was added to each well. IL-2 released after 24h was measured by ELISA.
Biochemical analysis of receptor-mediated endocytosis
75–100 µg of GRP94 N1–355, mouse transferrin or ovalbumin (US Biological) were each labeled with 1.0 mCi of 125I-Iodine (Amersham) in Tris buffered saline, pH 7.2, using IODOBEADS (Amersham) according to the manufacturer’s recommendations. The unbound 125I-Iodine was removed on Biogel P10 columns (Biorad, Hercules, CA). Final specific activity was 2000–6000 CPM/ng protein. To assay receptor-mediated endocytosis, BMDCs were harvested, washed with HBSS, 0.1 % BSA and adjusted to 1×107/ml. Cells were then loaded with 125I-GRP94, 125I-transferrin or 125I-ovalbumin (10µg/ml) for 1 hour at 4°C. After washing with ice-cold HBSS/BSA, cells were resuspended in the same buffer, and aliquots of 1×106 cells each were warmed to 37°C for the indicated times before returning to 4°C. Surface-bound transferrin was stripped by mixing cells with an equal volume of acid (0.25 M acetic acid, 0.5 M NaCl, pH 2.3) for 6–10 seconds, followed by immediate neutralization with 50 µl 1M sodium acetate, pH 8.0. Surface-bound GRP94 or ovalbumin was stripped by treatment with 1.25 mg/ml pronase (Roche) in DMEM for 45 min at 4°C. After stripping, cells were washed, and cell-associated radioactivity was measured on a gamma counter.
Electron microscopy
WT and HS1−/− BMDCs were grown on 35mm tissue culture dishes. To inhibit dynamin, some dishes were treated with 80µM of the dynamin inhibitor dynasore monohydrate (Sigma), for 60min at 37°C, in serum-free media. Cells were then washed with HBSS and fixed as monolayers with 2.5% glutaraldehyde, 50mM sodium cacodylate, pH 7.4, containing 1.25 mM MgCl2 and 1.25 mM CaCl2. Cells were washed with 100 mM sodium cacodylate, post-fixed in 2% aqueous OsO4 followed by 2% aqueous uranyl acetate, and dehydrated in graded EtOH. Dishes were then processed using a modification of Griffiths et al. (60) to release cell monolayers and a thin layer of polystyrene, and cell sheets were embedded in EPON-812 (Electron Microscopy Sciences). Ultrathin cross-sections of the cell sheets were contrasted with aqueous 1.5% uranyl acetate followed by Reynold’s lead citrate, and viewed using a JEOL 1011 electron microscope operated at 100kV. Images were acquired with an Orius CCD camera using Digital Micrograph software (Gatan).
To label receptor-mediated endocytic compartments, cells were allowed to internalize 50 µg/ml mouse transferrin-horseradish peroxidase (HRP, Thermo Scientific) for 15min at 37°C. To label the fluid-phase pathway, cells were allowed to internalize 15 mg/ml soluble HRP (Sigma) for 30 min at 37°C. In both cases, cells were washed and fixed with glutaraldehyde as described above. Following fixation, cells were incubated for 30 min with 1mg/ml 3,3'-diaminobenzidine (DAB, Sigma) and 0.01% H2O2 in 200 mM sodium cacodylate, pH 7.4. Cells were then washed with 100 mM sodium cacodylate, and incubated with 2% aqueous OsO4 to generate an electron-dense HRP-DAB reaction product and post-fix the cells. Subsequent processing was as described above.
To determine the number of endocytic invaginations, cell profiles were examined at random at ≥60,000x magnification and structures were counted by an individual blinded to experimental conditions. Shallow, wide neck pits were distinguished from non-pit regions of the membrane on the basis of clearly defined edges, relatively regular size, and, in some cases, an obvious clathrin coat. Pits and wide-neck invaginations were collectively defined as structures where the width of the structure at the plasma membrane was larger than its depth. Narrow-neck invaginations were defined as structures where the width of the mouth was less than the diameter of the invagination. Structures of an unclear nature were discarded from analysis. To determine density of these structures as a function of plasma membrane length, low magnification (5,000X) micrographs were captured, and the total length of the plasma membrane in each cell profile was measured using ImageJ v.1.42 (NIH). Filopodia were excluded from analysis, as these were difficult to measure accurately, and were almost never observed to have endocytic invaginations.
Results
HS1 is not required for differentiation or maturation of dendritic cells
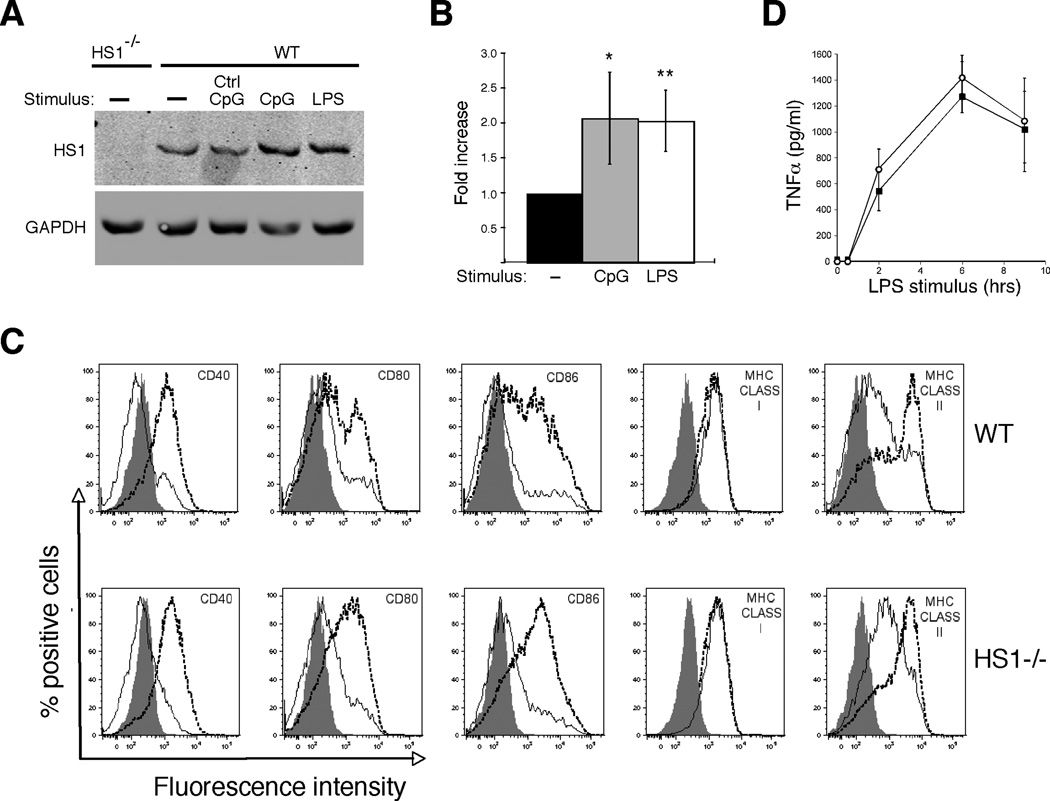
One of the key changes occurring during dendritic cell maturation is the re-programming of actin regulatory pathways (13, 61). This involves changes in expression patterns of actin regulatory proteins (62, 63), and functional downregulation of Rho family GTPases that drive the formation of branched actin filaments. We recently showed that DCs express HS1, a protein that promotes the formation of branched actin filaments in a Rho GTPase independent manner (28). To ask if HS1 expression is also downregulated upon DC maturation, we cultured BMDCs from wild type mice for 24h with LPS or CpG DNA to induce maturation, and analyzed cell lysates by western blotting for HS1. As shown in Fig. 1A and B, mature DCs do not downregulate HS1 expression. Instead, these cells express approximately 2-fold more HS1 than immature DCs, indicating that HS1 expression is upregulated as part of the maturation program.
Fig. 1. HS1−/− dendritic cells differentiate normally in response to LPS stimulation.
(A) HS1 expression in immature and mature DCs. BMDCs from WT and HS1−/− mice were stimulated with 100 ng/ml of LPS, 500 nM CpG or control oligonucleotides. After 24h of stimulation, cell lysates were made and blotted with anti-HS1. GAPDH was used as a loading control. (B) Quantitation of HS1 expression in DCs. Bands from blots as in (A) were scanned and the intensity of HS1 was normalized to that of GAPDH. Data represent means +/− StDev from at least 3 independent experiments. **, p<0.01; *, p<0.05 relative to untreated WT DCs. (C) BMDCs from WT or HS1−/− mice were stimulated for 24h with 100 ng/ml LPS to induce maturation. The cells were then analyzed by flow cytometry for expression of the indicated surface proteins. Filled profiles, isotype control; solid lines, unstimulated immature DC; dashed lines, LPS-stimulated DCs. (D) WT and HS1−/− BMDCs were stimulated with LPS for the indicated times and TNFα in the culture supernatants was measured by ELISA. Data represent means +/− StDev from replicate wells of one representative experiment.
We showed previously that BMDCs from HS1−/− mice lack both HS1 and the related protein cortactin (28). To ask if HS1 is required for DC development and/or maturation, BMDCs generated from WT and HS1−/− mice were cultured for 24h with or without LPS or CpG DNA, and surface marker expression was analyzed by flow cytometry. While the profiles of individual surface markers varied somewhat between DC preparations, cultures from HS1−/− and WT mice generated equivalent numbers of CD11c+ cells, and maturation-induced upregulation of MHC molecules and costimulatory proteins was similar in the two populations (Fig. 1C and data not shown). Secretion of TNFα was also comparable in the two populations of cells (Fig. 1D). Taken together, these results show that HS1 is not required for development or maturation of BMDCs.
HS1 is required for presentation of protein antigens
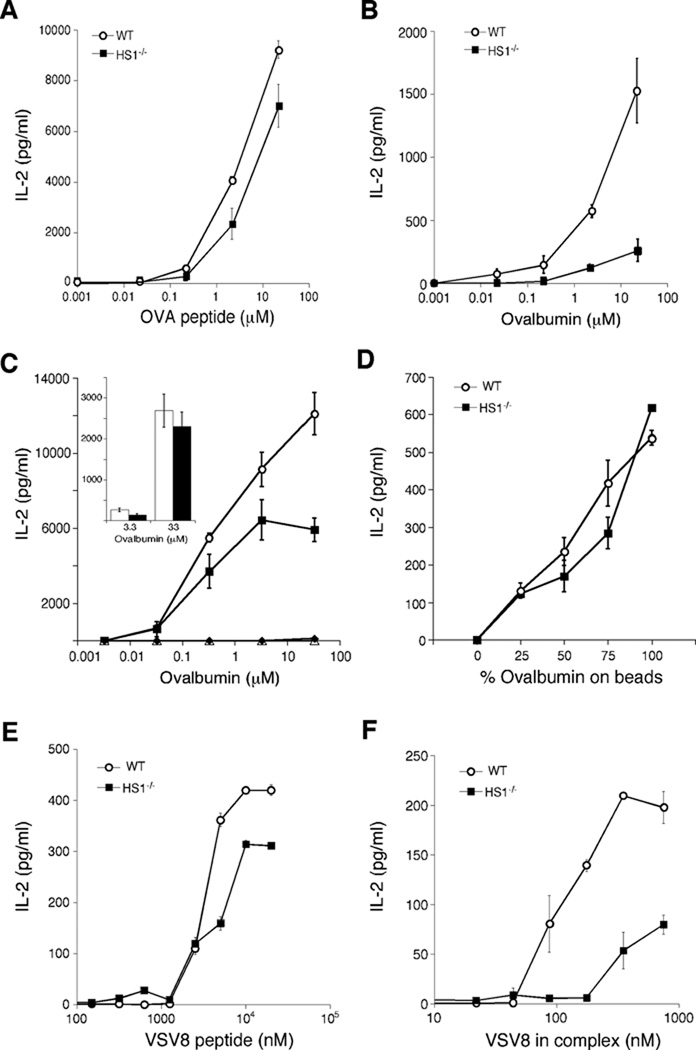
We next asked if HS1 is required for antigen presentation. As shown in Fig 2A, HS1−/− DCs could present OVA peptide efficiently to CD4+ T cells, as measured by IL-2 production by T cells from DO-11.10 mice. However, if HS1−/− DCs were pulsed instead with intact ovalbumin protein, they activated T cells much less efficiently than WT DCs. Although antigen insolubility precluded our ability to reach plateau levels of T cell activation, comparison of the antigen dose-response shows that HS1−/− DCs required 30–100 fold more protein than WT DCs to achieve comparable levels of T cell activation (Fig. 2B). Similar results were obtained with OTII cells and HS1−/− BMDCs on the C57Bl/6 background (Fig, 2C). To analyze antigen presentation on MHC I molecules, we took advantage of our experience working with the endoplasmic reticulum chaperone GRP94. We showed previously that GRP94 binds to various peptides, and the complex is taken up by DCs through a receptor-mediated process and transported to degradative organelles (57). GRP94-bound peptides then escape the endocytic pathway and are loaded onto MHC I molecules in the ER. As shown in Fig. 2E and F, HS1−/− DCs presented VSV8 peptide efficiently to the H2-Kb–restricted hybridoma N15, whose TCR is specific for VSV8. However, presentation of GRP94-VSV8 peptide complexes to this hybridoma was 10-fold less efficient than by WT DCs. Taken together, these results indicate that HS1 is required for the uptake or processing of protein antigens, and thereby for their presentation, but is not needed for later events leading to productive T cell activation.
Fig. 2. HS1 is required for presentation of protein antigens.
(A–B) BMDCs cultured from WT and HS1−/− on the Balb/c background were pulsed with the class II-restricted OVA323–339 peptide (A) or with the whole ovalbumin protein (B) at the indicated doses for 4h. They were then co-cultured with OVA-specific DO-11.10 T cells and 24h later, IL-2 levels in the culture supernatants were measured by ELISA. (C) BMDCs cultured from WT and HS1−/− mice on the C57Bl/6 background were incubated in the presence or absence of mannan to block mannose receptors, and then ovalbumin protein was added, so that any residual uptake would be by macropinocytosis. Antigen presentation to OTII T cells was then assayed as in panels A and B. In this experiment (representing 2 of 4 replicates), mannan completely blocked the response, indicating that even very high doses of ovalbumin are taken up almost exclusively by receptor-mediated endocytosis.  , WT DCs without mannan; ■, HS1−/− DCs without mannan; Δ, WT DCs with mannan; ◆, HS1−/− DCs with mannan. Inset, data from a separate experiment (representing 2 of 4 replicates) where T cell responses to mannan-blocked DCs were measurable at high ovalbumin doses. Open bars, WT DCs with mannan, filled bars, HS1−/− DCs with mannan; differences between WT and HS1−/− DCs in the presence of mannan were not statistically significant at any dose of ovalbumin. (D) WT and HS1−/− BMDCs (C57Bl/6 background) were allowed to phagocytose latex beads coated with ovalbumin and serum albumin in the indicated ratios, holding total protein and bead number constant. OTII T cell responses were then measured. (E–F) WT and HS1−/− BMDCs were pulsed with free VSV8 peptide (E) or VSV8 peptide pre-bound to GRP94 (F) at the indicated doses for 4h. VSV8-specific N15 T hybridoma cells were incubated with antigen-pulsed BMDCs for 24h and IL-2 levels in the supernatants were measured by ELISA. Data represent means +/− StDev from replicate wells of one representative experiment.
, WT DCs without mannan; ■, HS1−/− DCs without mannan; Δ, WT DCs with mannan; ◆, HS1−/− DCs with mannan. Inset, data from a separate experiment (representing 2 of 4 replicates) where T cell responses to mannan-blocked DCs were measurable at high ovalbumin doses. Open bars, WT DCs with mannan, filled bars, HS1−/− DCs with mannan; differences between WT and HS1−/− DCs in the presence of mannan were not statistically significant at any dose of ovalbumin. (D) WT and HS1−/− BMDCs (C57Bl/6 background) were allowed to phagocytose latex beads coated with ovalbumin and serum albumin in the indicated ratios, holding total protein and bead number constant. OTII T cell responses were then measured. (E–F) WT and HS1−/− BMDCs were pulsed with free VSV8 peptide (E) or VSV8 peptide pre-bound to GRP94 (F) at the indicated doses for 4h. VSV8-specific N15 T hybridoma cells were incubated with antigen-pulsed BMDCs for 24h and IL-2 levels in the supernatants were measured by ELISA. Data represent means +/− StDev from replicate wells of one representative experiment.
HS1 is required for efficient receptor-mediated endocytosis
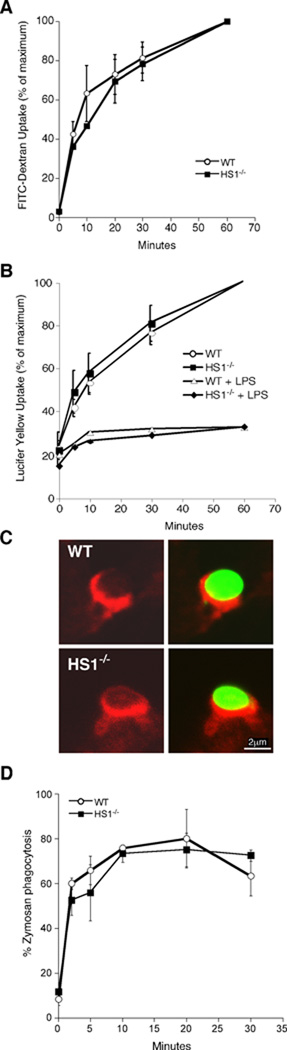
DCs take up antigen by multiple mechanisms, including phagocytosis, macropinocytosis and receptor-mediated endocytosis. Since phagocytosis and macropinocytosis are heavily dependent on actin polymerization to mobilize large pseudopodial extensions, we first asked if HS1 is required for antigen uptake through these pathways. To assess macropinocytosis, immature WT and HS1−/− DCs were allowed to take up FITC-Dextran (Fig. 3A) or Lucifer Yellow (Fig. 3B, closed symbols), for various times, and uptake was assessed by flow cytometry. In both cases, the time course of fluid phase uptake was identical in control and HS1-deficient cells. To ask if the maturation-associated downregulation of macropinocytic activity is intact in HS1−/− DCs, mature DCs were generated by culturing overnight in the presence of LPS. As reported previously (13), WT DCs showed diminished fluid phase endocytosis upon maturation (Fig. 3B, open circles). Mature HS1−/− DCs (open squares) behaved similarly, indicating that HS1 is not required for reprogramming of the macropinocytic pathway.
Fig. 3. HS1 is not required for macropinocytosis or phagocytosis.
(A) Immature WT and HS1−/− BMDCs were incubated with 1 mg/ml FITC-Dextran for the indicated times at 37°C and fluorescence intensity was assessed by flow cytometry.
(B) WT and HS1−/− BMDCs were cultured for 24h in the absence or presence of 100 ng/ml of LPS to induce maturation. The cells were then incubated at 37°C in the presence of 1 mg/ml Lucifer Yellow for the indicated times, and fluorescence intensity was assessed by flow cytometry. (C) WT and HS1−/− BMCDs were incubated with FITC-Zymosan particles (green) at 4°C and warmed to 37°C to induce uptake. Cells were fixed and stained with Alexa-594 Phalloidin to reveal the actin distribution (red). (D) The percentage of cells containing FITC-Zymosan particles over time was determined. Data represent means +/− StDev from replicate coverslips of one representative experiment.
To test the role of HS1 in phagocytosis, WT and HS1−/− DCs were incubated with FITC-conjugated zymosan particles at 4°C, warmed to 37°C to allow uptake, and analyzed by immunofluorescence microscopy. As shown in Fig. 3C, both WT and HS1−/− DCs exhibited F-actin-rich phagocytic cups at early times of internalization. To assess the kinetics of phagocytosis, cells fixed at various times after warming were labeled with fluorescent phalloidin to define cell boundaries, and the percentage of cells with internalized FITC-zymosan was determined. As shown in Fig. 3D, the kinetics of zymosan internalization were unaffected by HS1-deficiency. Moreover, labeling for the lysosomal protein LAMP-1 showed that internalized particles were transported to lysosomal compartments with normal kinetics (data not shown). Similarly, phagocytosis of apoptotic cell debris or of fluorescent Listeria were not impaired in HS1−/− DCs (data not shown). Taken together, these studies show that HS1 is not required for macropinocytosis or phagocytosis by DCs.
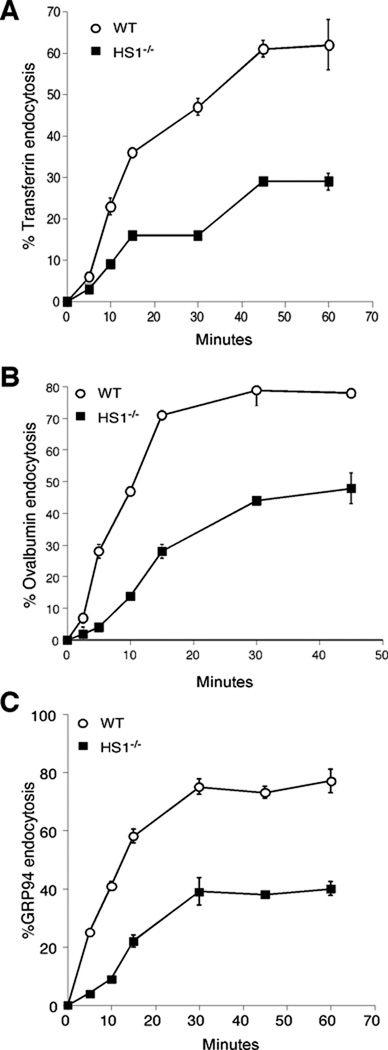
We next asked if receptor-mediated endocytosis is dependent on HS1. Initially, we tested endocytosis of transferrin, with is taken up by transferrin receptors via clathrin-coated vesicles (64). To obtain a quantitative measure of transferrin internalization, WT and HS1−/− DCs cells were surface-labeled with 125I-transferrin at 4°C, washed, and warmed to 37°C for various times. Residual surface-bound transferrin was then removed by washing at low pH, and cell-associated radioactivity was determined. As shown in Figure 4A, HS1−/− DCs internalized transferrin at only 30–40 % of the efficiency of WT cells. The diminished uptake was apparent both in terms of the rate of uptake and the overall amount of transferrin taken up. Although diminished ligand uptake can theoretically reflect either reduced endocytosis or enhanced ligand recycling, starting transferrin receptor levels were similar in WT and HS1−/− DCs, and the initial rates of endocytosis were already significantly different, arguing against a significant contribution from differential recycling. Thus, we conclude that the diminished overall uptake of transferrin by HS1−/− DCs reflects a defect in initial endocytic internalization. Ovalbumin is taken up in a receptor-mediated fashion by the mannose receptor (65, 66), and GRP94 is taken up by CD91 and other scavenger receptors (67–69, 57, 70). To ask if HS1 deficiency also leads to reduced endocytosis of these proteins, we analyzed their internalization using the same assay. As shown in Figures 4B and C, uptake of both ovalbumin and GRP94 was significantly reduced in HS1−/− DCs, indicating that the requirement for HS1 is a general one.
Fig. 4. HS1 is necessary for efficient receptor-mediated endocytosis.
WT and HS1−/− BMDCs were incubated with 125I-Transferrin (A), 125I-Ovalbumin (B) or 125I-GRP94 (C) at 4°C for 1h. Cells were then washed and warmed to 37°C for the indicated times. At each time point, residual surface-bound proteins were removed by acid wash (transferrin) or pronase treatment (GRP94 and ovalbumin). Radioactivity in the cell pellets was then determined and uptake calculated. Data represent means +/− StDev from independent experiments, each done in duplicate or triplicate. The number of binding sites for each protein did not differ between WT and HS1−/− BMDCs within experimental error. We calculate 6000–8500 binding sites for transferrin per cell, 20,000–40,000 sites for GRP94, and approximately 80,000 sites for ovalbumin.
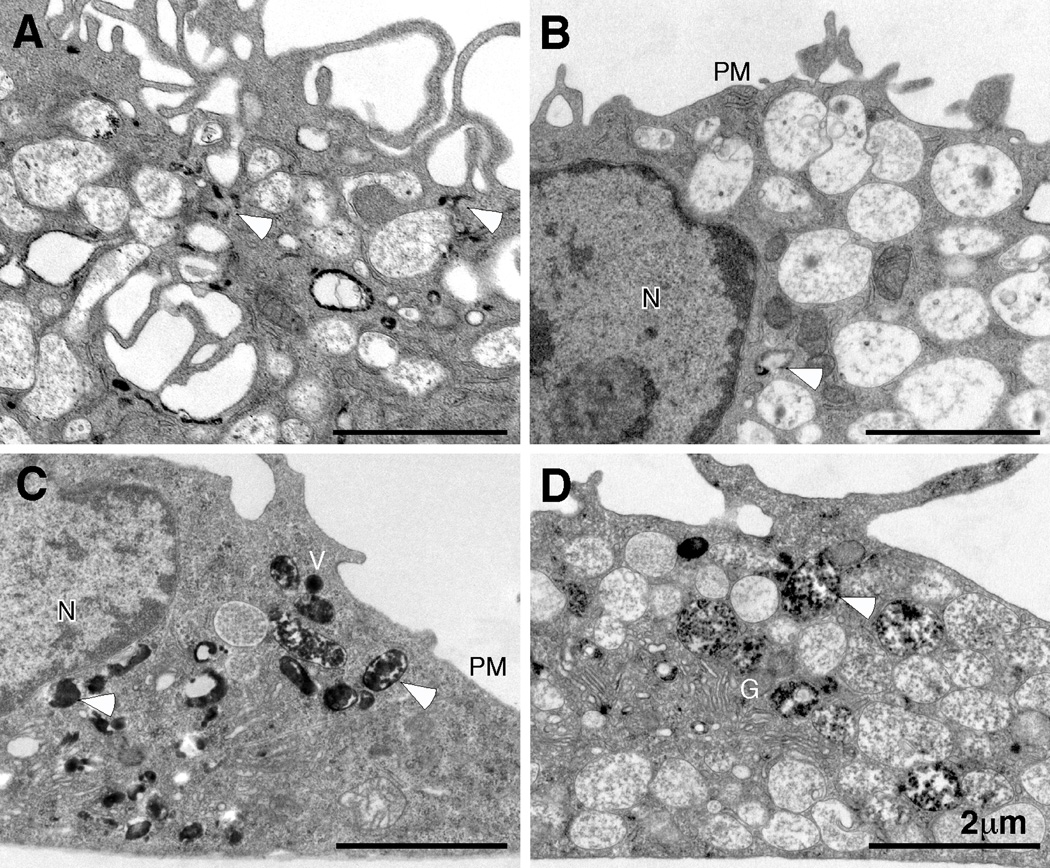
As an independent means of assessing endocytosis, HS1−/− DCs were incubated for 15 minutes in the presence of transferrin-HRP. Cells were then fixed and processed for cytochemistry and electron microscopy. As shown in Figure 5A, wild type BMDCs showed numerous vesicles and tubular endosomes filled with HRP reaction product. By comparison, HS1−/− BMDCs contained far fewer HRP+ endocytic structures (Fig. 5B). Indeed, most cell profiles lacked labeled endosomes; the image shown in Fig. 5B was selected because it contains one rare HRP-positive organelle (arrowhead). Importantly, HS1−/− DCs took up free HRP by fluid phase endocytosis as efficiently as WT cells. As shown in Fig. 5C and D, both cell types exhibited numerous macropinosomes containing electron dense reaction product. Together with the biochemical analysis of ligand internalization, these data show that HS1 is specifically required for efficient uptake of ligands via receptor-mediated endocytosis into early endocytic compartments.
Fig. 5. Analysis of fluid-phase and receptor-mediated endocytosis by electron microscopy.
WT (A, C) or HS1−/− (B, D) BMDCs were incubated with transferrin-HRP (A, B) for 15 min at 37°C to load endocytic compartments via receptor-mediated endocytosis. Alternatively, BMDCs were incubated with soluble HRP (C, D) for 30 min at 37°C, to load the endocytic pathway via fluid phase endocytosis (macropinocytosis). Cells were fixed and processed with DAB to generate an electron-dense reaction product and visualized by transmission electron microscopy. Representative images from 3 independent experiments are shown. Arrowheads indicate compartments (early endosomes and macropinosomes) filled with electron-dense HRP reaction product. Note the relative paucity of electron-dense deposits in the HS1−/− cells when HRP is delivered by receptor-mediated endocytosis (compare A and B), but not macropinocytosis (compare C and D). N, nucleus; PM, plasma membrane; G, Golgi complex; V, macropinocytic vacuoles.
On the basis of our finding that HS1 is selectively required for receptor-mediated endocytosis in DCs, we asked if the antigen presentation defects in HS1−/− DCs are limited to antigens taken up via this pathway. To facilitate comparisons, we targeted the same protein, ovalbumin, for uptake via different endocytic mechanisms. First, cells were incubated with soluble ovalbumin in the presence of excess mannan to inhibit receptor-mediated endocytosis and drive fluid phase uptake. As shown in Fig. 2C, mannan treatment strongly inhibited antigen presentation, confirming that ovalbumin is taken up by receptor-mediated endocytosis, even at very high doses. In two of four replicate experiments, no residual antigen presentation could be detected, making it impossible to assess the effects of HS1-deficiency on presentation of ovalbumin taken up by fluid-phase endocytosis. In two of four experiments, although T cell activation in response to mannan-treated DCs was low (~10% of that observed in the absence of mannan), it was measurable at the highest doses of ovalbumin (Fig 2C, inset). In such experiments, WT and HS1−/− DCs activated T cells equally well at all doses of ovalbumin where a response could be measured. We conclude that HS1−/− DCs are competent to present ovalbumin taken up by fluid phase endocytosis. We also tested the presentation of phagocytosed ovalbumin, using latex beads coated with varying ratios of ovalbumin protein and serum albumin. As shown in Fig. 2D, the response to phagocytosed ovalbumin was similar in T cells activated by WT and HS1−/− DCs. Taken together, these studies show that the requirement for HS1 in presentation of protein antigens is linked to its role in receptor-mediated endocytosis, and that HS1 is not needed for presentation of antigens taken up by other routes.
HS1 binds to dynamin 2 and promotes formation of plasma membrane invaginations
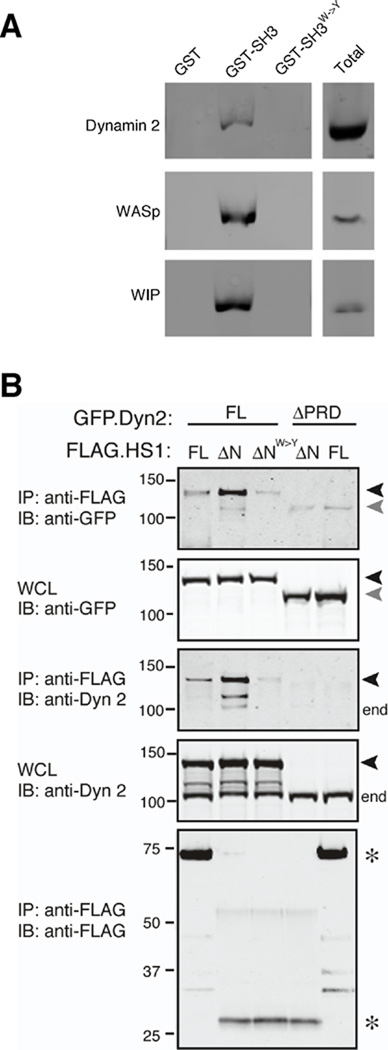
The HS1 homologue cortactin has been shown to interact through its SH3 domain with several proteins that promote endocytosis, including WASp, WIP and dynamin 2 (41, 71, 52). In particular, cortactin interacts physically and functionally with dynamin 2 to drive the internalization of endocytic vesicles (51, 72–74, 56, 75). We have shown that HS1 binds via its SH3 domain to the actin regulatory proteins WASp and WIP (28). To ask if HS1 interacts in a similar way with dynamin 2, we probed DC lysates with the GST-tagged HS1 SH3 domain and blotted the bound proteins for dynamin 2. WASp and WIP were used as positive controls. As shown in Fig. 6A, all three proteins associated with the HS1 SH3 domain. Mutation of tryptophan 465, a residue required for canonical binding to proline-rich ligands, disrupts binding, demonstrating the specificity of these interactions. To confirm that binding of HS1 and dynamin 2 takes place in intact cells and to map the relevant region of dynamin 2, we performed co-immunoprecipitation studies on cells co-expressing epitope-tagged HS1 and dynamin 2 constructs. As shown in Fig. 6B, full length HS1 and dynamin 2 could be co-immunoprecipitated from cells. This interaction does not require the Arp2/3- or actin-binding domains of HS1, since an N-terminal deletion mutant lacking these regions bound dynamin 2 efficiently. Indeed, on a mole-per-mole basis, this mutant showed over 200-fold more binding to dynamin 2 as compared with full length HS1. The enhanced binding ability of this HS1 truncation mutant also allowed detection of binding to endogenous dynamin 2 (Fig. 6B, end). Mutation of W465 within the SH3 domain of the HS1 ΔN mutant dramatically diminished dynamin 2 binding, confirming that the SH3 domain of HS1 mediates interaction with dynamin 2. Conversely, a dynamin 2 mutant lacking the C-terminal proline-rich domain (41) failed to co-immunoprecipitate efficiently with either full-length HS1 or the HS1 deletion mutant (Fig. 6B, ΔPRD). Low levels of association of HS1 and dynamin 2 were detectable under conditions where SH3-PRD interactions were abrogated, suggesting that a secondary interaction outside these domains may exist. Nonetheless, our results indicate that HS1 and dynamin 2 interact primarily through binding of the HS1 SH3 domain to the proline-rich domain of dynamin 2.
Fig. 6. The SH3 domain of HS1 interacts with the proline-rich domain of dynamin 2.
(A) GST alone, the SH3 domain of HS1 fused to GST, or the inactive SH3W->Y mutant fused to GST were immobilized on glutathione resin and incubated with DC lysates. Bound proteins were analyzed by SDS-PAGE and immunoblotting with anti-dynamin 2, anti-WASp or anti-WIP. Total, protein expression in whole cell lysates. (B) 293T cells were transiently transfected with full length GFP-tagged dynamin 2 (GFP.Dyn2 FL, black arrowheads) or a mutant lacking the proline rich domain (ΔPRD, gray arrowheads), together with FLAG-tagged HS1 (FLAG.HS1 FL), an N-terminal deletion mutant of HS1 lacking the actin regulatory region (ΔN), or the ΔN mutant bearing a point mutation in the SH3 domain (ΔNW->Y). Cells were lysed, immunoprecipitated with anti-FLAG, and western blots were probed as indicated. Note that the ΔPRD mutant is not detected by the anti-dynamin 2 (which was generated against this C-terminal region of dynamin 2), but is readily detectable in the WCL using anti-GFP antibody. end, endogenous dynamin 2; *, HS1 variants; WCL, whole cell lysate from which immunoprecipitation was performed.
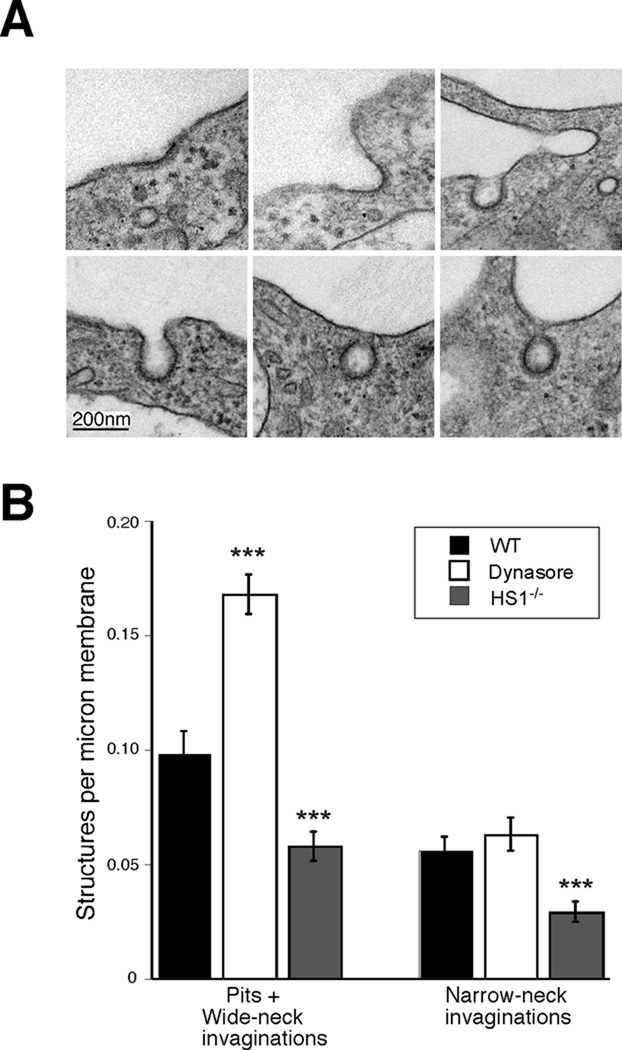
Dynamin is known to drive the scission of plasma membrane invaginations during receptor-mediated endocytosis, and cells expressing a dominant negative dynamin mutant or treated with the dynamin inhibitor dynasore generate increased numbers of deep, wide-neck (U-shaped) and narrow-neck (O-shaped) endocytic invaginations (76). To ask if HS1 is required at the same step in endocytic traffic, we analyzed plasma membrane invaginations in WT and HS1−/− BMDCs. Figure 7A shows examples of shallow pits and wide-neck invaginations (top row), as well as narrow-neck invaginations (bottom row) found in WT cells. The number of these structures per linear micron of plasma membrane was determined as described in Materials and Methods. As anticipated, treatment of WT cells with dynasore resulted in a significant increase in the surface density of endocytic invaginations (Fig 7B). These consisted primarily of deep invaginations with a wide-neck. In contrast, HS1−/− DCs showed only about half as many endocytic invaginations as WT cells, and this decrease was observed for both wide-neck and narrow-neck invaginations. These data indicate that HS1 and dynamin affect distinct steps of endocytosis in DCs, and support a model in which an HS1-dependent step occurs early in the formation of membrane invaginations, while dynamin is needed to complete later steps of membrane deformation and vesicle scission.
Figure 7. HS1 promotes endocytic invagination.
(A) Electron micrographs illustrating various stages in the process of the formation of forming coated vesicles. All images are from WT BMDCs; structures in HS1−/− BMDCs were comparable in morphology. Top row, examples of shallow pits and wide-necked invaginations. Bottom row, examples of narrow-neck invaginations. (B) Quantitation of the frequency of the structures shown in (A) in WT cells, WT cells treated with 80 µM dynasore and HS1−/− DCs. Cell profiles were photographed at random, and the number of structures in each category per linear micron of plasma membrane was determined as described in Materials and Methods. Values represent mean +/− SEM from 3–4 independent experiments, totaling 66–68 cells and 100–500 pits or invaginations per experimental condition. ***, p<0.001 relative to untreated WT cells.
Discussion
DC sentinel function depends on the ability of these cells to take up a wide variety of antigenic materials. This is achieved through a combination of phagocytosis, macropinocytosis, and receptor-mediated endocytosis (11, 1, 3, 4). We have found that HS1 plays an important role in this process, by promoting receptor-mediated endocytosis of protein antigens. DCs from HS1−/− mice show diminished receptor-mediated endocytosis of multiple proteins and impaired presentation of exogenous protein-derived antigens on both MHC Class I and MHC class II.
The requirement for HS1 in antigen trafficking and presentation appears to be restricted to receptor-mediated endocytic pathway. HS1−/− DCs presented peptide antigens efficiently, indicating that HS1 is not required for actin-dependent events at the DC side of the immunological synapse (77–79, 27). Moreover, while there is strong evidence that actin polymerization is important for macropinocytosis and phagocytosis (12, 14, 80), HS1 expression was not required for these processes. By extension, since we find no cortactin expression in murine BMDCs (28), cortactin family members generally are dispensible. Both macropinocytosis and phagocytosis are downregulated upon DC maturation (65, 13), while receptor-mediated endocytosis proceeds unabated in mature DCs (13, 81, 15). Phagocytosis and macropinocytosis depend on the actin nucleating proteins WASp and WAVE, and the downregulation of these processes is linked to diminished activation of the Rho family GTPase CDC42 upstream of these events (13). Interestingly, HS1 differs from WASp and WAVE in that it does not depend on activation by Rho GTPases, and so is not subject to downregulation at this level. Indeed, we find that HS1 expression is modestly upregulated upon DC maturation. Moreover, HS1 is enriched in podosomes, which are largely dissolved on DC maturation (28), presumably increasing the pool of HS1 available to support endocytic functions in mature DCs.
Our analysis of HS1−/− DCs shows a 2-fold decrease in the maximal uptake of endocytosed proteins, yet these cells require 30–100 fold more protein antigen to achieve the same level of T cell activation as wild type DCs. The assay we used for receptor-mediated endocytosis measures only a single, synchronized round of protein uptake. When multiplied by the many rounds of endocytosis that take place within the 4-hour period of the antigen pulse, the defects we observe could readily account for at least 30–50 fold reduction in overall antigen uptake. Thus, the requirement for HS1 to facilitate endocytosis is likely to be the primary cause of the diminished antigen presentation that we observe in HS1-deficient DCs. The finding that antigens taken up by phagocytosis and macropinocytosis are presented efficiently further supports this conclusion. Nonetheless, it is possible that HS1 is also required for additional intracellular steps of antigen trafficking. Testing this possibility will require quantitative analysis of the fate of the endocytosed pool of protein.
While the DC literature tends to focus on phagocytosis and macropinocytosis, receptor-mediated endocytosis is also an important means of antigen uptake. Typically, it is argued that receptor-mediated endocytosis is particularly important at low ligand concentrations. While this is no doubt true, our side-by-side comparison of presentation of ovalbumin-derived peptides following uptake by receptor-mediated endocytosis and macropinocytosis shows that receptor-mediated uptake is dominant even at very high antigen doses. DCs express numerous cell surface receptors that preferentially bind non-self molecules and take them up in a highly efficient manner. Ovalbumin is taken up by the mannose receptor (65, 66 and Fig 2C), which is important for uptake of pathogen-derived glycoproteins (82–84). Uptake and cross-presentation of GRP94-peptide complexes also require receptor-mediated endocytosis, though the relevant receptor(s) remain controversial (67–69, 57, 70). Other receptors also play an important physiological role. Among these are the C-type lectin DC-SIGN, which promotes the endocytosis of HIV and other viruses and their presentation on MHC Class I (85–88). The multi-lectin receptor DEC205 plays a similar role (89, 90). Even Fcγ receptors, which are best known for their role in phagocytosis, carry out receptor-mediated endocytosis of small immune complexes (91–94). CD1b recycling occurs via receptor-mediated endocytosis, and continues in mature DCs (81), a phenomenon that has been proposed to be the basis of effective presentation of lipid antigens from mycobacteria, under conditions where presentation of antigens on MHC Class II fails (95). We show here that HS1 is required for receptor-mediated uptake via three distinct receptors, and it seems likely that this is a general requirement. Going forward, it will be interesting to ask if HS1 expression has an important impact on DC function in vivo, particularly in the context of viruses, lipid antigens, or DC-vaccines that rely on receptor-mediated uptake. Moreover, since HS1 is also expressed in B cells, it will be interesting to ask if BCR-dependent uptake of antigens is also HS1-dependent.
HS1 functions both as an actin regulatory protein and an adapter molecule, and it seems likely that both of these functions are called into play to promote receptor-mediated endocytosis. We show that HS1 interacts with dynamin 2 through binding of its C-terminal SH3 domain to the proline-rich domain of dynamin 2, the same mechanism used to mediate cortactin-dynamin 2 interaction (41). Interestingly, however, the endocytic phenotype of HS1−/− DCs does not mirror that of cells treated with the dynamin GTPase inhibitor dynasore. In keeping with studies in other cell types (76), we found that treatment of DCs with dynasore led to the accumulation of deep endocytic invaginations. The accumulation of wide-necked (U-shaped) structures was predominant, though narrow-necked (O-shaped) endocytic invaginations were also observed. The latter structures are also seen in cells expressing dominant negative dynamin mutants, and are thought to represent a requirement for dynamin in the fission of fully formed coated pits (9, 96). The U-shaped invaginations represent an earlier endocytic intermediate, and are proposed to represent an additional requirement for dynamin in driving membrane deformation (76). In contrast to dynamin-inhibited cells, HS1-deficient DCs exhibited abnormally low numbers of both U-shaped and O-shaped endocytic invaginations. The simplest interpretation of this finding is that HS1 is required for an early step in membrane invagination, preceding the deep invaginations formed by dynamin activity. These early intermediates are thought to be metastable (97, 98), perhaps explaining why no clear accumulation of shallow pits was observed in HS1-deficient DCs. This interpretation is consistent with a two-step model for dynamin function, in which dynamin first serves as regulatory GTPase to ensure vectorial coat assembly and enhance membrane curvature, and subsequently, in an assembled state, uses GTPase-driven mechano-chemical activity to drive vesicle scission (96). According to this model, SH3 domain containing proteins such as HS1 sense the status of cargo loading, membrane curvature and/or coat assembly, and regulate this functional switch.
In addition to binding dynamin 2, HS1 activates the Arp2/3 complex, binds actin filaments, and interacts with the actin regulatory proteins WASp and WIP (30, 28). Thus, it is also likely that HS1 promotes actin-dependent aspects of endocytosis. The literature regarding the requirement for actin polymerization in endocytosis is conflicting. In S. cerevisae, actin recruitment occurs after clathrin coat formation and provides an essential force for membrane deformation (99). In mammalian cells, however, results are variable and depend on the cell type and the nature of the endocytic structure (100–103). Recent work from the Kirchhausen group has shown that actin regulatory proteins are recruited to long-lived endocytic structures and are particularly important for internalization of large structures, such as clathrin plaques and some virus particles (104–106). On the basis of this work, it seems that actin polymerization provides the force needed to generate oversized clathrin-coated structures, and to overcome other obstacles, such as high membrane tension (107). Since HS1 promotes the formation of branched-actin filaments, it may play a key role in this process. Importantly, HS1’s interactions with the actin cytoskeleton and dynamin are likely to be coupled. Indeed, we find that interactions between HS1 and dynamin are dramatically enhanced by deletion of the N-terminal actin regulatory region of HS1. This, and similar observations regarding HS1 binding to WASp and WIP (28), are compatible with a model in which the N-terminus of HS1 partially restricts availability of the SH3 domain, such that interaction with the actin cytoskeleton releases the SH3 domain and promotes binding to dynamin 2 and other molecules. This model explains our inability to co-immunoprecipitate endogenous dynamin 2 and HS1, since the pool of active HS1 would be selectively associated with the insoluble actin cytoskeleton. A positive feedback loop may also be at play, since engagement of the dynamin proline-rich domain by SH3 domains has been shown to promote dynamin GTPase activity (108, 109). Such a mechanism would promote the coupled assembly of actin and dynamin at endocytic invaginations.
An important unresolved question is to what extent HS1 function is distinct from that of its more widely expressed homologue cortactin. In non-hematopoietic cells, the weight of the evidence indicates that cortactin plays a key role in endocytic uptake (51, 72–74, 110, 111, 56, 75), though some studies challenge this view (112, 113). Cortactin is recruited to long-lived clathrin-rich regions of the membrane, and kinetic analysis shows that cortactin recruitment peaks at a late time point, close to the time of dynamin recruitment (72, 104). Furthermore, phosphorylation of cortactin promotes its association with dynamin (74, 56) and the tyrosine phosphorylation sites that have been linked to regulated cortactin function are conserved in HS1. Given all the biochemical and cell biological similarities between cortactin and HS1, why did hematopoietic lineages evolve a distinct family member? There are, in fact, some important functional differences between HS1 and cortactin. The structural features necessary for F-actin binding are distinct (31). Moreover, HS1 is less efficient than cortactin at driving Arp2/3 complex-nucleated actin polymerization (30). Actin binding promotes cortactin interaction with dynamin (74), and our evidence suggests that the same is true for HS1. Thus, the difference in mode of actin binding between HS1 and cortactin may translate into important differences in the mechanisms by which these proteins link the actin cytoskeleton to dynamin function. Since BMDCs from HS1−/− mice lack both HS1 and cortactin, they provide an ideal experimental system in which to carry out comparative reconstitution studies to address this possibility.
Acknowledgements
The authors wish to thank Drs. H. Cao and M. McNiven for providing dynamin constructs, and Dr. T. Svitkina and the University of Pennsylvania Electron Microscopy Resource Laboratory for providing access to equipment for electron microscopy, and Dr. A. Mantegazza for help with analysis of macropinocytosed and phagocytosed antigens antigens. We thank Drs. Svitkina, M. Marks, T Laufer and members of the Burkhardt laboratory for helpful discussions.
Abbreviations
- BMDC
bone marrow-derived dendritic cell
- DAB
3,3'-diaminobenzidine
- DC
dendritic cell
- GRP94
glucose regulated protein of 94 kDa
- HRP
Horseradish peroxidase
- HS1
hematopoietic lineage cell-specific protein 1
- VSV
vesicular stomatitis virus
- WASp
Wiskott-Aldrich Syndrome protein
- WIP
WASp interacting protein
Footnotes
This work was supported by NIH grants T32AI05542806A1 and F32AI08006901 to DAKD, and R21AI088376 to JKB and YA.
References
- 1.Watts C, Amigorena S. Antigen traffic pathways in dendritic cells. Traffic. 2000;1:312–317. doi: 10.1034/j.1600-0854.2000.010404.x. [DOI] [PubMed] [Google Scholar]
- 2.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 3.Norbury CC. Drinking a lot is good for dendritic cells. Immunology. 2006;117:443–451. doi: 10.1111/j.1365-2567.2006.02335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savina A, Amigorena S. Phagocytosis and antigen presentation in dendritic cells. Immunol Rev. 2007;219:143–156. doi: 10.1111/j.1600-065X.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 5.Burgdorf S, Scholz C, Kautz A, Tampe R, Kurts C. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat Immunol. 2008;9:558–566. doi: 10.1038/ni.1601. [DOI] [PubMed] [Google Scholar]
- 6.May RC, Machesky LM. Phagocytosis and the actin cytoskeleton. J Cell Sci. 2001;114:1061–1077. doi: 10.1242/jcs.114.6.1061. [DOI] [PubMed] [Google Scholar]
- 7.Niedergang F, Chavrier P. Signaling and membrane dynamics during phagocytosis: many roads lead to the phagos(R)ome. Curr Opin Cell Biol. 2004;16:422–428. doi: 10.1016/j.ceb.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Kerr MC, Teasdale RD. Defining macropinocytosis. Traffic. 2009;10:364–371. doi: 10.1111/j.1600-0854.2009.00878.x. [DOI] [PubMed] [Google Scholar]
- 9.Schafer DA. Coupling actin dynamics and membrane dynamics during endocytosis. Curr Opin Cell Biol. 2002;14:76–81. doi: 10.1016/s0955-0674(01)00297-6. [DOI] [PubMed] [Google Scholar]
- 10.Kaksonen M, Toret CP, Drubin DG. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2006;7:404–414. doi: 10.1038/nrm1940. [DOI] [PubMed] [Google Scholar]
- 11.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 12.Cox D, Chang P, Zhang Q, Reddy PG, Bokoch GM, Greenberg S. Requirements for both Rac1 and Cdc42 in membrane ruffling and phagocytosis in leukocytes. J Exp Med. 1997;186:1487–1494. doi: 10.1084/jem.186.9.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrett WS, Chen LM, Kroschewski R, Ebersold M, Turley S, Trombetta S, Galan JE, Mellman I. Developmental control of endocytosis in dendritic cells by Cdc42. Cell. 2000;102:325–334. doi: 10.1016/s0092-8674(00)00038-6. [DOI] [PubMed] [Google Scholar]
- 14.West MA, Prescott AR, Eskelinen EL, Ridley AJ, Watts C. Rac is required for constitutive macropinocytosis by dendritic cells but does not control its downregulation. Curr Biol. 2000;10:839–848. doi: 10.1016/s0960-9822(00)00595-9. [DOI] [PubMed] [Google Scholar]
- 15.Platt CD, Ma JK, Chalouni C, Ebersold M, Bou-Reslan H, Carano RA, Mellman I, Delamarre L. Mature dendritic cells use endocytic receptors to capture and present antigens. Proc Natl Acad Sci U S A. 2010;107:4287–4292. doi: 10.1073/pnas.0910609107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allenspach EJ, Lemos MP, Porrett PM, Turka LA, Laufer TM. Migratory and lymphoid-resident dendritic cells cooperate to efficiently prime naive CD4 T cells. Immunity. 2008;29:795–806. doi: 10.1016/j.immuni.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lou Q, Conway TF, Jr, Egilmez NK, Loyall JL, Bernstein SH, Kelleher RJ, Jr, Bankert RB. B cell tumor vaccine enhanced by covalent attachment of immunoglobulin to surface proteins on dendritic cells. Clin Immunol. 2006;118:66–76. doi: 10.1016/j.clim.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Trumpfheller C, Finke JS, Lopez CB, Moran TM, Moltedo B, Soares H, Huang Y, Schlesinger SJ, Park CG, Nussenzweig MC, Granelli-Piperno A, Steinman RM. Intensified and protective CD4+ T cell immunity in mice with anti-dendritic cell HIV gag fusion antibody vaccine. J Exp Med. 2006;203:607–617. doi: 10.1084/jem.20052005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akutsu Y, Matsubara H, Urashima T, Komatsu A, Sakata H, Nishimori T, Yoneyama Y, Hoshino I, Murakami K, Usui A, Kano M, Ochiai T. Combination of direct intratumoral administration of dendritic cells and irradiation induces strong systemic antitumor effect mediated by GRP94/gp96 against squamous cell carcinoma in mice. Int J Oncol. 2007;31:509–515. [PubMed] [Google Scholar]
- 20.Nchinda G, Kuroiwa J, Oks M, Trumpfheller C, Park CG, Huang Y, Hannaman D, Schlesinger SJ, Mizenina O, Nussenzweig MC, Uberla K, Steinman RM. The efficacy of DNA vaccination is enhanced in mice by targeting the encoded protein to dendritic cells. J Clin Invest. 2008;118:1427–1436. doi: 10.1172/JCI34224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nchinda G, Amadu D, Trumpfheller C, Mizenina O, Uberla K, Steinman RM. Dendritic cell targeted HIV gag protein vaccine provides help to a DNA vaccine including mobilization of protective CD8+ T cells. Proc Nat Acad Sci USA. 2010;107:4281–4286. doi: 10.1073/pnas.1000621107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calle Y, Chou HC, Thrasher AJ, Jones GE. Wiskott-Aldrich syndrome protein and the cytoskeletal dynamics of dendritic cells. J Pathol. 2004;204:460–469. doi: 10.1002/path.1651. [DOI] [PubMed] [Google Scholar]
- 23.de Noronha S, Hardy S, Sinclair J, Blundell MP, Strid J, Schulz O, Zwirner J, Jones GE, Katz DR, Kinnon C, Thrasher AJ. Impaired dendritic-cell homing in vivo in the absence of Wiskott-Aldrich syndrome protein. Blood. 2005;105:1590–1597. doi: 10.1182/blood-2004-06-2332. [DOI] [PubMed] [Google Scholar]
- 24.Snapper SB, Meelu P, Nguyen D, Stockton BM, Bozza P, Alt FW, Rosen FS, von Andrian UH, Klein C. WASP deficiency leads to global defects of directed leukocyte migration in vitro and in vivo. J Leukoc Biol. 2005;77:993–998. doi: 10.1189/jlb.0804444. [DOI] [PubMed] [Google Scholar]
- 25.Chou HC, Anton IM, Holt MR, Curcio C, Lanzardo S, Worth A, Burns S, Thrasher AJ, Jones GE, Calle Y. WIP regulates the stability and localization of WASP to podosomes in migrating dendritic cells. Curr Biol. 2006;16:2337–2344. doi: 10.1016/j.cub.2006.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olivier A, Jeanson-Leh L, Bouma G, Compagno D, Blondeau J, Seye K, Charrier S, Burns S, Thrasher AJ, Danos O, Vainchenker W, Galy A. A partial down-regulation of WASP is sufficient to inhibit podosome formation in dendritic cells. Mol Ther. 2006;13:729–737. doi: 10.1016/j.ymthe.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Pulecio J, Tagliani E, Scholer A, Prete F, Fetler L, Burrone OR, Benvenuti F. Expression of Wiskott-Aldrich syndrome protein in dendritic cells regulates synapse formation and activation of naive CD8+ T cells. J Immunol. 2008;181:1135–1142. doi: 10.4049/jimmunol.181.2.1135. [DOI] [PubMed] [Google Scholar]
- 28.Klos Dehring DA, Clarke F, Ricart BG, Huang Y, Gomez TS, Williamson EK, Hammer DA, Billadeau DD, Argon Y, Burkhardt JK. Hematopoietic Lineage Cell-Specific Protein 1 Functions in Concert with the Wiskott-Aldrich Syndrome Protein To Promote Podosome Array Organization and Chemotaxis in Dendritic Cells. J Immunol. 2011;186:4805–4818. doi: 10.4049/jimmunol.1003102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Rossum AG, Schuuring-Scholtes E, van Buuren-van Seggelen V, Kluin PM, Schuuring E. Comparative genome analysis of cortactin and HS1: the significance of the F-actin binding repeat domain. BMC Genomics. 2005;6:15. doi: 10.1186/1471-2164-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uruno T, Zhang P, Liu J, Hao JJ, Zhan X. Haematopoietic lineage cell-specific protein 1 (HS1) promotes actin-related protein (Arp) 2/3 complex-mediated actin polymerization. Biochemical Journal. 2003;371:485–493. doi: 10.1042/BJ20021791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hao JJ, Zhu J, Zhou K, Smith N, Zhan X. The coiled-coil domain is required for HS1 to bind to F-actin and activate Arp2/3 complex. J Biol Chem. 2005;280:37988–37994. doi: 10.1074/jbc.M504552200. [DOI] [PubMed] [Google Scholar]
- 32.Burkhardt JK, Carrizosa E, Shaffer MH. The actin cytoskeleton in T cell activation. Annu Rev Immunol. 2008;26:233–259. doi: 10.1146/annurev.immunol.26.021607.090347. [DOI] [PubMed] [Google Scholar]
- 33.Higgs HN. Actin nucleation: cortactin caught in the act. Curr Biol. 2002;12:R593–595. doi: 10.1016/s0960-9822(02)01101-6. [DOI] [PubMed] [Google Scholar]
- 34.Weaver A, Heuser J, Karginov A, Lee W, Parsons J, Cooper J. Interaction of cortactin and N-wasp with arp2/3 complex. Curr Biol. 2002;12:1270. doi: 10.1016/s0960-9822(02)01035-7. [DOI] [PubMed] [Google Scholar]
- 35.Yamanashi Y, Okada M, Semba T, Yamori T, Umemori H, Tsunasawa S, Toyoshima K, Kitamura D, Watanabe T, Yamamoto T. Identification of HS1 protein as a major substrate of protein-tyrosine kinase(s) upon B-cell antigen receptor-mediated signaling. Proc Natl Acad Sci U S A. 1993;90:3631–3635. doi: 10.1073/pnas.90.8.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takemoto Y, Furuta M, Li XK, Strong-Sparks WJ, Hashimoto Y. LckBP1, a proline-rich protein expressed in haematopoietic lineage cells, directly associates with the SH3 domain of protein tyrosine kinase p56lck. Embo J. 1995;14:3403–3414. doi: 10.1002/j.1460-2075.1995.tb07346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruzzene M, Brunati AM, Marin O, Donella-Deana A, Pinna LA. SH2 domains mediate the sequential phosphorylation of HS1 protein by p72syk and Src-related protein tyrosine kinases. Biochemistry. 1996;35:5327–5332. doi: 10.1021/bi9528614. [DOI] [PubMed] [Google Scholar]
- 38.Takemoto Y, Sato M, Furuta M, Hashimoto Y. Distinct binding patterns of HS1 to the Src SH2 and SH3 domains reflect possible mechanisms of recruitment and activation of downstream molecules. Int Immunol. 1996;8:1699–1705. doi: 10.1093/intimm/8.11.1699. [DOI] [PubMed] [Google Scholar]
- 39.Gomez TS, McCarney SD, Carrizosa E, Labno CM, Comiskey EO, Nolz JC, Zhu P, Freedman BD, Clark MR, Rawlings DJ, Billadeau DD, Burkhardt JK. HS1 Functions as an Essential Actin-Regulatory Adaptor Protein at the Immune Synapse. Immunity. 2006;24:741–752. doi: 10.1016/j.immuni.2006.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carrizosa E, Gomez TS, Labno CM, Klos Dehring DA, Liu X, Freedman BD, Billadeau DD, Burkhardt JK. Hematopoietic lineage cell-specific protein 1 is recruited to the immunological synapse by IL-2-inducible T cell kinase and regulates phospholipase Cgamma1 Microcluster dynamics during T cell spreading. J Immunol. 2009;183:7352–7361. doi: 10.4049/jimmunol.0900973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McNiven MA, Kim L, Krueger EW, Orth JD, Cao H, Wong TW. Regulated interactions between dynamin and the actin-binding protein cortactin modulate cell shape. J Cell Biol. 2000;151:187–198. doi: 10.1083/jcb.151.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sawabe T, Horiuchi T, Koga R, Tsukamoto H, Kojima T, Harashima S, Kikuchi Y, Otsuka J, Mitoma H, Yoshizawa S, Niho Y, Watanabe T. Aberrant HS1 molecule in a patient with systemic lupus erythematosus. Genes & Immunity. 2003;4:122–131. doi: 10.1038/sj.gene.6363932. [DOI] [PubMed] [Google Scholar]
- 43.Otsuka J, Horiuchi T, Yoshizawa S, Tsukamoto H, Sawabe T, Kikuchi Y, Himeji D, Koyama T, Mitoma H, Watanabe T, Harada M. Association of a four-amino acid residue insertion polymorphism of the HS1 gene with systemic lupus erythematosus: molecular and functional analysis. Arthritis & Rheumatism. 2004;50:871–881. doi: 10.1002/art.20192. [DOI] [PubMed] [Google Scholar]
- 44.Scielzo C, Ghia P, Conti A, Bachi A, Guida G, Geuna M, Alessio M, Caligaris-Cappio F. HS1 protein is differentially expressed in chronic lymphocytic leukemia patient subsets with good or poor prognoses. J Clin Invest. 2005;115:1644–1650. doi: 10.1172/JCI24276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muzio M, Scielzo C, Frenquelli M, Bachi A, De Palma M, Alessio M, Ghia P, Caligaris-Cappio F. HS1 complexes with cytoskeleton adapters in normal and malignant chronic lymphocytic leukemia B cells. Leukemia. 2007;21:2067–2070. doi: 10.1038/sj.leu.2404744. [DOI] [PubMed] [Google Scholar]
- 46.Scielzo C, Bertilaccio MT, Simonetti G, Dagklis A, Ten Hacken E, Fazi C, Muzio M, Caiolfa V, Restuccia U, Bachi A, Rocchi M, Ponzoni M, Ghia P, Caligaris-Cappio F. HS1 has a central role in the trafficking and homing of leukemic B cells. Blood. 2010 doi: 10.1182/blood-2009-12-258814. [DOI] [PubMed] [Google Scholar]
- 47.Fukuda T, Kitamura D, Taniuchi I, Maekawa Y, Benhamou LE, Sarthou P, Watanabe T. Restoration of surface IgM-mediated apoptosis in an anti-IgM-resistant variant of WEHI-231 lymphoma cells by HS1, a protein-tyrosine kinase substrate. Proc Natl Acad Sci U S A. 1995;92:7302–7306. doi: 10.1073/pnas.92.16.7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taniuchi I, Kitamura D, Maekawa Y, Fukuda T, Kishi H, Watanabe T. Antigen-receptor induced clonal expansion and deletion of lymphocytes are impaired in mice lacking HS1 protein, a substrate of the antigen- receptor-coupled tyrosine kinases. Embo J. 1995;14:3664–3678. doi: 10.1002/j.1460-2075.1995.tb00036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Butler B, Kastendieck DH, Cooper JA. Differently phosphorylated forms of the cortactin homolog HS1 mediate distinct functions in natural killer cells. Nat Immunol. 2008;9:887–897. doi: 10.1038/ni.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schafer DA, Weed SA, Binns D, Karginov AV, Parsons JT, Cooper JA. Dynamin2 and cortactin regulate actin assembly and filament organization. Curr Biol. 2002;12:1852–1857. doi: 10.1016/s0960-9822(02)01228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cao H, Orth JD, Chen J, Weller SG, Heuser JE, McNiven MA. Cortactin is a component of clathrin-coated pits and participates in receptor-mediated endocytosis. Mol Cell Biol. 2003;23:2162–2170. doi: 10.1128/MCB.23.6.2162-2170.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kowalski JR, Egile C, Gil S, Snapper SB, Li R, Thomas SM. Cortactin regulates cell migration through activation of N-WASP. J Cell Sci. 2005;118:79–87. doi: 10.1242/jcs.01586. [DOI] [PubMed] [Google Scholar]
- 53.Luxenburg C, Parsons JT, Addadi L, Geiger B. Involvement of the Src-cortactin pathway in podosome formation and turnover during polarization of cultured osteoclasts. J Cell Sci. 2006;119:4878–4888. doi: 10.1242/jcs.03271. [DOI] [PubMed] [Google Scholar]
- 54.Webb BA, Eves R, Mak AS. Cortactin regulates podosome formation: roles of the protein interaction domains. Exp Cell Res. 2006;312:760–769. doi: 10.1016/j.yexcr.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 55.Buday L, Downward J. Roles of cortactin in tumor pathogenesis. Biochim Biophys Acta. 2007;1775:263–273. doi: 10.1016/j.bbcan.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 56.Zhu J, Yu D, Zeng XC, Zhou K, Zhan X. Receptor-mediated endocytosis involves tyrosine phosphorylation of cortactin. J Biol Chem. 2007;282:16086–16094. doi: 10.1074/jbc.M701997200. [DOI] [PubMed] [Google Scholar]
- 57.Biswas C, Sriram U, Ciric B, Ostrovsky O, Gallucci S, Argon Y. The N-terminal fragment of GRP94 is sufficient for peptide presentation via professional antigen-presenting cells. Int Immunol. 2006;18:1147–1157. doi: 10.1093/intimm/dxl049. [DOI] [PubMed] [Google Scholar]
- 58.Liu J, Tse AG, Chang HC, Wang J, Hussey RE, Chishti Y, Rheinhold B, Spoerl R, Nathenson SG, Sacchettini JC, Reinherz EL. Crystallization of a deglycosylated T cell receptor (TCR) complexed with an anti-TCR Fab fragment. J Biol Chem. 1996;271:33639–33646. doi: 10.1074/jbc.271.52.33639. [DOI] [PubMed] [Google Scholar]
- 59.Vogen S, Gidalevitz T, Biswas C, Simen BB, Stein E, Gulmen F, Argon Y. Radicicol-sensitive peptide binding to the N-terminal portion of GRP94. J Biol Chem. 2002;277:40742–40750. doi: 10.1074/jbc.M205323200. [DOI] [PubMed] [Google Scholar]
- 60.Griffiths G, Warren G, Quinn P, Mathieu-Costello O, Hoppeler H. Density of newly synthesized plasma membrane proteins in intracellular membranes. I. Stereological studies. J Cell Biol. 1984;98:2133–2141. doi: 10.1083/jcb.98.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burns S, Hardy SJ, Buddle J, Yong KL, Jones GE, Thrasher AJ. Maturation of DC is associated with changes in motile characteristics and adherence. Cell Motil Cytoskeleton. 2004;57:118–132. doi: 10.1002/cm.10163. [DOI] [PubMed] [Google Scholar]
- 62.Al-Alwan MM, Rowden G, Lee TD, West KA. Fascin is involved in the antigen presentation activity of mature dendritic cells. J Immunol. 2001;166:338–345. doi: 10.4049/jimmunol.166.1.338. [DOI] [PubMed] [Google Scholar]
- 63.Pereira SR, Faca VM, Gomes GG, Chammas R, Fontes AM, Covas DT, Greene LJ. Changes in the proteomic profile during differentiation and maturation of human monocyte-derived dendritic cells stimulated with granulocyte macrophage colony stimulating factor/interleukin-4 and lipopolysaccharide. Proteomics. 2005;5:1186–1198. doi: 10.1002/pmic.200400988. [DOI] [PubMed] [Google Scholar]
- 64.Dautry-Varsat A. Receptor-mediated endocytosis: the intracellular journey of transferrin and its receptor. Biochimie. 1986;68:375–381. doi: 10.1016/s0300-9084(86)80004-9. [DOI] [PubMed] [Google Scholar]
- 65.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burgdorf S, Lukacs-Kornek V, Kurts C. The mannose receptor mediates uptake of soluble but not of cell-associated antigen for cross-presentation. J Immunol. 2006;176:6770–6776. doi: 10.4049/jimmunol.176.11.6770. [DOI] [PubMed] [Google Scholar]
- 67.Wassenberg JJ, Dezfulian C, Nicchitta CV. Receptor mediated and fluid phase pathways for internalization of the ER Hsp90 chaperone GRP94 in murine macrophages. J Cell Sci. 1999;112:2167–2175. doi: 10.1242/jcs.112.13.2167. [DOI] [PubMed] [Google Scholar]
- 68.Singh-Jasuja H, Toes RE, Spee P, Munz C, Hilf N, Schoenberger SP, Ricciardi-Castagnoli P, Neefjes J, Rammensee HG, Arnold-Schild D, Schild H. Cross-presentation of glycoprotein 96-associated antigens on major histocompatibility complex class I molecules requires receptor-mediated endocytosis. J Exp Med. 2000;191:1965–1974. doi: 10.1084/jem.191.11.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berwin B, Hart JP, Rice S, Gass C, Pizzo SV, Post SR, Nicchitta CV. Scavenger receptor-A mediates gp96/GRP94 and calreticulin internalization by antigen-presenting cells. Embo J. 2003;22:6127–6136. doi: 10.1093/emboj/cdg572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jockheck-Clark AR, Bowers EV, Totonchy MB, Neubauer J, Pizzo SV, Nicchitta CV. Re-examination of CD91 function in GRP94 (glycoprotein 96) surface binding, uptake, and peptide cross-presentation. J Immunol. 2010;185:6819–6830. doi: 10.4049/jimmunol.1000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kinley AW, Weed SA, Weaver AM, Karginov AV, Bissonette E, Cooper JA, Parsons JT. Cortactin interacts with WIP in regulating Arp2/3 activation and membrane protrusion. Curr Biol. 2003;13:384–393. doi: 10.1016/s0960-9822(03)00107-6. [DOI] [PubMed] [Google Scholar]
- 72.Merrifield CJ, Perrais D, Zenisek D. Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell. 2005;121:593–606. doi: 10.1016/j.cell.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 73.Sauvonnet N, Dujeancourt A, Dautry-Varsat A. Cortactin and dynamin are required for the clathrin-independent endocytosis of gammac cytokine receptor. J Cell Biol. 2005;168:155–163. doi: 10.1083/jcb.200406174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu J, Zhou K, Hao JJ, Liu J, Smith N, Zhan X. Regulation of cortactin/dynamin interaction by actin polymerization during the fission of clathrin-coated pits. J Cell Sci. 2005;118:807–817. doi: 10.1242/jcs.01668. [DOI] [PubMed] [Google Scholar]
- 75.Cao H, Chen J, Krueger EW, McNiven MA. SRC-mediated phosphorylation of dynamin and cortactin regulates the "constitutive" endocytosis of transferrin. Mol Cell Biol. 2010;30:781–792. doi: 10.1128/MCB.00330-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 77.Al-Alwan MM, Rowden G, Lee TD, West KA. The dendritic cell cytoskeleton is critical for the formation of the immunological synapse. J Immunol. 2001;166:1452–1456. doi: 10.4049/jimmunol.166.3.1452. [DOI] [PubMed] [Google Scholar]
- 78.Al-Alwan MM, Liwski RS, Haeryfar SM, Baldridge WH, Hoskin DW, Rowden G, West KA. Cutting edge: dendritic cell actin cytoskeletal polarization during immunological synapse formation is highly antigen-dependent. J Immunol. 2003;171:4479–4483. doi: 10.4049/jimmunol.171.9.4479. [DOI] [PubMed] [Google Scholar]
- 79.Rothoeft T, Balkow S, Krummen M, Beissert S, Varga G, Loser K, Oberbanscheidt P, van den Boom F, Grabbe S. Structure and duration of contact between dendritic cells and T cells are controlled by T cell activation state. Eur J Immunol. 2006;36:3105–3117. doi: 10.1002/eji.200636145. [DOI] [PubMed] [Google Scholar]
- 80.Park H, Cox D. Cdc42 regulates Fc gamma receptor-mediated phagocytosis through the activation and phosphorylation of Wiskott-Aldrich syndrome protein (WASP) and neural-WASP. Mol Biol Cell. 2009;20:4500–4508. doi: 10.1091/mbc.E09-03-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van der Wel NN, Sugita M, Fluitsma DM, Cao X, Schreibelt G, Brenner MB, Peters PJ. CD1 and major histocompatibility complex II molecules follow a different course during dendritic cell maturation. Mol Biol Cell. 2003;14:3378–3388. doi: 10.1091/mbc.E02-11-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Apostolopoulos V, McKenzie IF. Role of the mannose receptor in the immune response. Curr Mol Med. 2001;1:469–474. doi: 10.2174/1566524013363645. [DOI] [PubMed] [Google Scholar]
- 83.Taylor PR, Gordon S, Martinez-Pomares L. The mannose receptor: linking homeostasis and immunity through sugar recognition. Trends Immunol. 2005;26:104–110. doi: 10.1016/j.it.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 84.Li J, Jiang H, Wen W, Zheng J, Xu G. The dendritic cell mannose receptor mediates allergen internalization and maturation involving notch 1 signalling. Clin Exp Immunol. 2010;162:251–261. doi: 10.1111/j.1365-2249.2010.04244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alvarez CP, Lasala F, Carrillo J, Muniz O, Corbi AL, Delgado R. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J Virol. 2002;76:6841–6844. doi: 10.1128/JVI.76.13.6841-6844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Engering A, Geijtenbeek TB, van Vliet SJ, Wijers M, van Liempt E, Demaurex N, Lanzavecchia A, Fransen J, Figdor CG, Piguet V, van Kooyk Y. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J Immunol. 2002;168:2118–2126. doi: 10.4049/jimmunol.168.5.2118. [DOI] [PubMed] [Google Scholar]
- 87.Moris A, Nobile C, Buseyne F, Porrot F, Abastado JP, Schwartz O. DC-SIGN promotes exogenous MHC-I-restricted HIV-1 antigen presentation. Blood. 2004;103:2648–2654. doi: 10.1182/blood-2003-07-2532. [DOI] [PubMed] [Google Scholar]
- 88.Cambi A, Beeren I, Joosten B, Fransen JA, Figdor CG. The C-type lectin DC-SIGN internalizes soluble antigens and HIV-1 virions via a clathrin-dependent mechanism. Eur J Immunol. 2009;39:1923–1928. doi: 10.1002/eji.200939351. [DOI] [PubMed] [Google Scholar]
- 89.Mahnke K, Guo M, Lee S, Sepulveda H, Swain SL, Nussenzweig M, Steinman RM. The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II-positive lysosomal compartments. J Cell Biol. 2000;151:673–684. doi: 10.1083/jcb.151.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kamphorst AO, Guermonprez P, Dudziak D, Nussenzweig MC. Route of antigen uptake differentially impacts presentation by dendritic cells and activated monocytes. J Immunol. 2010;185:3426–3435. doi: 10.4049/jimmunol.1001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hedin U, Stenseth K, Thyberg J. Receptor-mediated endocytosis of immunoglobulin-coated colloidal gold particles in cultured mouse peritoneal macrophages. Eur J Cell Biol. 1984;35:41–48. [PubMed] [Google Scholar]
- 92.Tse SM, Furuya W, Gold E, Schreiber AD, Sandvig K, Inman RD, Grinstein S. Differential role of actin, clathrin, and dynamin in Fc gamma receptor-mediated endocytosis and phagocytosis. J Biol Chem. 2003;278:3331–3338. doi: 10.1074/jbc.M207966200. [DOI] [PubMed] [Google Scholar]
- 93.Huang ZY, Barreda DR, Worth RG, Indik ZK, Kim MK, Chien P, Schreiber AD. Differential kinase requirements in human and mouse Fc-gamma receptor phagocytosis and endocytosis. J Leukoc Biol. 2006;80:1553–1562. doi: 10.1189/jlb.0106019. [DOI] [PubMed] [Google Scholar]
- 94.Andersson LI, Hellman P, Eriksson H. Receptor-mediated endocytosis of particles by peripheral dendritic cells. Hum Immunol. 2008;69:625–633. doi: 10.1016/j.humimm.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 95.Hava DL, van der Wel N, Cohen N, Dascher CC, Houben D, Leon L, Agarwal S, Sugita M, van Zon M, Kent SC, Shams H, Peters PJ, Brenner MB. Evasion of peptide, but not lipid antigen presentation, through pathogen-induced dendritic cell maturation. Proc Natl Acad Sci U S A. 2008;105:11281–11286. doi: 10.1073/pnas.0804681105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mettlen M, Pucadyil T, Ramachandran R, Schmid SL. Dissecting dynamin's role in clathrin-mediated endocytosis. Biochem Soc Trans. 2009;37:1022–1026. doi: 10.1042/BST0371022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ehrlich M, Boll W, Van Oijen A, Hariharan R, Chandran K, Nibert ML, Kirchhausen T. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004;118:591–605. doi: 10.1016/j.cell.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 98.Loerke D, Mettlen M, Yarar D, Jaqaman K, Jaqaman H, Danuser G, Schmid SL. Cargo and dynamin regulate clathrin-coated pit maturation. PLoS Biol. 2009;7:e57. doi: 10.1371/journal.pbio.1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kaksonen M, Toret CP, Drubin DG. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell. 2005;123:305–320. doi: 10.1016/j.cell.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 100.Merrifield CJ, Qualmann B, Kessels MM, Almers W. Neural Wiskott Aldrich Syndrome Protein (N-WASP) and the Arp2/3 complex are recruited to sites of clathrin-mediated endocytosis in cultured fibroblasts. Eur J Cell Biol. 2004;83:13–18. doi: 10.1078/0171-9335-00356. [DOI] [PubMed] [Google Scholar]
- 101.Yarar D, Waterman-Storer CM, Schmid SL. A dynamic actin cytoskeleton functions at multiple stages of clathrin-mediated endocytosis. Mol Biol Cell. 2005;16:964–975. doi: 10.1091/mbc.E04-09-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boucrot E, Saffarian S, Massol R, Kirchhausen T, Ehrlich M. Role of lipids and actin in the formation of clathrin-coated pits. Exp Cell Res. 2006;312:4036–4048. doi: 10.1016/j.yexcr.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Batchelder EM, Yarar D. Differential requirements for clathrin-dependent endocytosis at sites of cell-substrate adhesion. Mol Biol Cell. 2010;21:3070–3079. doi: 10.1091/mbc.E09-12-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cureton DK, Massol RH, Saffarian S, Kirchhausen TL, Whelan SP. Vesicular stomatitis virus enters cells through vesicles incompletely coated with clathrin that depend upon actin for internalization. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000394. e1000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Saffarian S, Cocucci E, Kirchhausen T. Distinct dynamics of endocytic clathrin-coated pits and coated plaques. PLoS Biol. 2009;7 doi: 10.1371/journal.pbio.1000191. e1000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cureton DK, Massol RH, Whelan SP, Kirchhausen T. The length of vesicular stomatitis virus particles dictates a need for actin assembly during clathrin-dependent endocytosis. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu AP, Loerke D, Schmid SL, Danuser G. Global and local regulation of clathrin-coated pit dynamics detected on patterned substrates. Biophys J. 2009;97:1038–1047. doi: 10.1016/j.bpj.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gout I, Dhand R, Hiles ID, Fry MJ, Panayotou G, Das P, Truong O, Totty NF, Hsuan J, Booker GW, et al. The GTPase dynamin binds to and is activated by a subset of SH3 domains. Cell. 1993;75:25–36. [PubMed] [Google Scholar]
- 109.Scaife RM, Margolis RL. The role of the PH domain and SH3 binding domains in dynamin function. Cellular signalling. 1997;9:395–401. doi: 10.1016/s0898-6568(97)00041-7. [DOI] [PubMed] [Google Scholar]
- 110.Chen L, Wang ZW, Zhu JW, Zhan X. Roles of cortactin, an actin polymerization mediator, in cell endocytosis. Acta Biochim Biophys Sin (Shanghai) 2006;38:95–103. doi: 10.1111/j.1745-7270.2006.00141.x. [DOI] [PubMed] [Google Scholar]
- 111.Williams MR, Markey JC, Doczi MA, Morielli AD. An essential role for cortactin in the modulation of the potassium channel Kv1.2. Proc Natl Acad Sci U S A. 2007;104:17412–17417. doi: 10.1073/pnas.0703865104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Barroso C, Rodenbusch SE, Welch MD, Drubin DG. A role for cortactin in Listeria monocytogenes invasion of NIH 3T3 cells, but not in its intracellular motility. Cell Motility Cytoskeleton. 2006;63:231–243. doi: 10.1002/cm.20119. [DOI] [PubMed] [Google Scholar]
- 113.Lai FP, Szczodrak M, Oelkers JM, Ladwein M, Acconcia F, Benesch S, Auinger S, Faix J, Small JV, Polo S, Stradal TE, Rottner K. Cortactin promotes migration and platelet-derived growth factor-induced actin reorganization by signaling to Rho-GTPases. Mol Biol Cell. 2009;20:3209–3223. doi: 10.1091/mbc.E08-12-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]