Abstract
This study examined ontogenetic differences in anticipatory 50 kHz ultrasonic vocalization (USV) production to social interactions in male Sprague-Dawley rats. Adults increased USVs across days when tested socially but not when left alone (Exp 1), and displayed anticipatory USVs to return to the cage-mate (Exp 2). Adolescents did not display evidence of anticipatory USVs. To the extent that anticipatory USVs index incentive salience, this suggests an adolescent attenuation of incentive salience of social interactions.
Keywords: adolescent, ontogeny, ultrasonic vocalization, anticipatory response, rat
Adolescents may exhibit some degree of anhedonia which may underlie adolescent susceptibility to drug abuse (see [1] for references and review). There have been several studies both refuting (e.g. [2] [3] [4] [5]) and supporting (e.g. [6]) this hypothesis. As such, this series of experiments sought to further investigate this hypothesis by measuring ultrasonic vocalizations in anticipation of social interactions.
Under certain circumstances, ultrasonic vocalizations (USVs) produced by rats have long been used as an index of affective states (see [7] for review), with relatively long (300-3,000ms), low frequency (around 22 kHz) USVs associated with negative affective states [8], [9], whereas shorter (20-80 ms) high frequency (around 50 kHz) USVs have been associated with positive affective states [10, 11]. Rats produce 50 kHz USVs in contexts involving potential reward, including play fighting [12], social exploration [10][6], male agonistic behaviors [13], and during sexual approach, copulation and ejaculation [14][15]. Increases in 50 kHz USVs are also seen in experimental situations both in anticipation of, and during reward exposure, including tickling by the experimenter [16],[17][18], electrical stimulation of the reward pathway [19], and administration of drugs of abuse [20][12][21][22][23][24]. Although not all data support the use of 50 kHz USVs as an index of positive affect, with for instance USV production also increased during aggressive encounters [9], USV production under these circumstances have been posited to signal non-aggression or an attempt to evoke a more positive affective state in the aggressor (see [25] for review). In support of the use of 50 kHz USVs as an index of positive affective states in rats, they are associated with dopamine activity in reward-related brain areas [26],[12][7][27], and in a rewarding situation can be inhibited by aversive stimuli [26][28][25]. Some experiments supporting 50 kHz USV production as an index of positive affect were conducted utilizing adolescent animals [28] whereas others were conducted in adults [19, 24, 26]. Few studies conducted to date have compared USV production between adolescent and adult animals [6].
Interactions with peers are thought to be of principal importance for human adolescents, with individuals spending more time with peers during adolescence than at any other developmental period [11][29]. Adolescent rats likewise show high levels of social behavior, with these social interactions being essential for developing the ability to express and understand intraspecific communication signals [30]. Interactions with peers during adolescence provide a substantial source of positive experiences for humans [29][31], and, in the same way, an opportunity to interact with peers has been shown to be more rewarding for adolescent rats than for their more mature counterparts using a place conditioning task [3]. Yet, in prior work using USV production during social interactions as an index of positive affect, socially housed adolescents emitted substantially fewer 50 kHz USVs than did their adult counterparts [6]. It does not appear that the attenuated USV production during adolescence simply reflects developmental immaturity in the capacity to express USVs, given that isolate housed adolescents exposed to social peers have been previously reported to exhibit substantial 50 kHz USV production [28], suggesting that under the circumstances of chronic social deprivation (or the stress it produces – e.g. [33]), adolescents are capable of notable 50 kHz USV production, although no age comparisons were possible in that study where only adolescent animals were examined.
To the extent that 50 kHz USV production during social encounters reflects a positive affective state, the attenuated USV production seen by adolescents during social interactions in our earlier study [6] could be interpreted to reflect that socially housed adolescents may exhibit less positive affect during these social interactions than adults, even though they engage in more social behavior when compared to adults, and appear to find these interactions particularly reinforcing [3]. Such data hint that there may be marked age differences in the hedonic experiences of adolescents and adults. Given evidence that hedonic “liking” of a given reward can be experimentally dissociated from incentive salience (“wanting”) of that same reward [32], the question arises as to whether adolescents, though showing an apparent attenuated hedonic response to the presence of social stimuli, might show an increase in incentive salience for social rewards, with this increased “wanting” driving their elevated social interactions.
The present study compared age differences in 50 kHz USV production in anticipation of social interactions in adolescent and adult socially housed rats, using these 50 kHz USVs as an index of anticipatory “wanting” affective states [25]. As there is variation across studies in the time during which USVs were measured during reward anticipation (e.g. [19][28]), the time course of USV production was analyzed across a 5 min anticipatory period prior to being placed with a social conspecific in the test context (Exp. 1) or prior to being returned to the home-cage and cage-mate (Exp. 2).
Male Sprague-Dawley rats bred and reared in our colony at Binghamton University were used in these studies. The focus in this study was on male rats, given that USV production in females has been reported to vary with stage of estrous cycle [34]. On the day after birth, all litters were culled to 8 -10 pups (6 males and 4 females when possible) and housed with their mother and father in standard breeding cages until weaning on postnatal day (P) 21. At that time, males were pair-housed with a same-sex littermate, and female offspring were assigned to other projects.
Animals were housed in a temperature-controlled (20-22° C) vivarium on a 14-/10-hr light/dark cycle (lights on at 0700) with ad libitum access to food (Purina Rat Chow, Lowell, MA) and tap water. All animals were treated in accordance with guidelines established by the National Institutes of Health using protocols approved by the Binghamton University Institutional Animal Care and Use Committee.
The social interaction testing chambers were located in a testing room adjacent to the colony room. Each social interaction test apparatus consisted of a Plexiglas (Binghamton Plate Glass, Binghamton, NY) chamber (30 × 20 × 20 cm for adolescents and 45 × 30 × 20 cm for adults) containing clean pine shavings. Each chamber was divided into two equally sized compartments by a Plexiglas partition containing an aperture (7 × 5 cm for adolescents and 9 × 7 cm for adults) to allow movement of the animals between compartments [35, 36]. After each testing session the social interaction chambers were wiped clean with a 3% hydrogen peroxide solution and refilled with clean shavings.
Test sessions were initiated on P29 (adolescents) or on P71-73 (adults) and were conducted daily between 1300 and 1700 hr under dim light for a total of 7 days. All experimental animals tested socially were assigned a partner animal of the same age from a different litter and home-cage. This partner remained constant throughout testing to avoid confounding age differences in social affect with previously reported age differences in novelty seeking assessed by conditioned place preference (CPP) [3]. Three days prior to the onset of testing, experimental subjects and partner animals were separated from their littermate cage mate and re-housed in a clean cage with a same-sex, same-age, non-littermate assigned to the same experimental condition. Subjects were assigned to these groups randomly, with the constraint that no more than one animal from a given litter was placed in any particular test group to avoid confounding litter with treatment effects [37][38]. Experimental and partner animals were weight matched as much as possible given the constraints of age and litter. The mean weight difference between play pairs was 9.39 ± 1.83 in adolescents and 25.25 ± 6.96 in adults.
On the day prior to test onset, all animals (experimental and partner) were placed individually in the test chamber for 30 min to habituate them to the testing situation, given that placement in a novel context notably suppresses social behavior [39]. On test days, experimental subjects and partners were individually isolated in holding cages in the colony room for 3 hr prior to testing in order to increase social motivation [40] while avoiding the stress of chronic isolate-housing. On each test day, experimental animals were taken from their holding cage 2.5 hrs into the pre-test isolation period, injected with saline (0.5 % body weight), and returned to their holding cages for 25 min prior to placement in the social interaction apparatus. Partners were retained in the holding cage for 30 min post-injection prior to placing them in the testing apparatus (to allow for the experimental animal's 5 min anticipatory period). Unless otherwise stated, all animals were returned to their home-cage immediately after testing on each of the 7 test days. Experimental animals were injected with saline in these studies for comparability with earlier work [6].
For testing, each experimental animal was placed alone into the social interaction chamber for a 5-min anticipatory period. For animals in the social group, the partner was then placed into the chamber with the experimental animal, and the dyad was allowed to interact for 10 min. Animals in the alone group simply remained in the chamber for an additional 10 min. USVs were recorded during the anticipatory period and the social interaction period on test days 1, 3, 5, and 7 for later analysis.
Ultrasonic vocalizations were measured using Avisoft UltraSoundGate CM16 microphones, recorded by an UltraSoundGate 416-200 recording device, digitized by Avisoft-Recorder USG, and stored as .wav-files for later analysis (Avisoft Bioacoustics, Berlin, Germany). The microphones were sensitive to frequency ranges between 10 and 125 kHz with a high sensitivity at 50 kHz and moderate directional properties. The microphones were mounted on the outside edge of the social interaction chambers straddling the center divider in order to record USVs from both sides of the chamber. Sound analysis was performed using Avisoft-SASLab Pro software (Avisoft Bioacoustics, Berlin, Germany). Sound files were converted to spectrograms for manual visual counts of calls. A sound was considered to be a 50 kHz USV if it fell between 35 and 70 kHz with a duration of 30-80 ms [see [25] for discussion]. Calls were considered in the 22 kHz category if they fell between 18 and 32 kHz with a duration of 300-3000 ms. Twenty two kHz calls were seen in very few animals, and thus were not included in the statistical analyses. All sound files were scored by trained experimenters without knowledge of test group assignment. Inter-rater reliability exceeded 90%.
Outliers were eliminated from analysis based on total USV production during the day 1 anticipatory period, with subjects removed if their USV production was 2 standard deviations above or below the group mean of their respective age/condition. This resulted in the elimination of a total of 3 adolescents in Exp 1, and 4 adolescents and 3 adults in Exp 2, with no more than 2 subjects eliminated from any age/condition. Repeated measures analyses of variance (ANOVAs) were used to analyze 50 kHz USV production during the anticipatory period, with test day and min serving as within-subjects factors, and age as a between-subjects measure in all experiments. Additional between-subjects measures included testing condition (Exp1), and post-test isolation condition (Exp 2). Due to the emergence of interactions of age with other variables, each age was subjected to a separate ANOVA to further examine these effects. Significant main effects and interactions were further investigated using Fisher's LSD post-hoc tests.
Experiment 1 investigated possible age-related differences in anticipation of social interactions using data from saline control animals presented in Willey et al., 2009 [6]. The design of the study was a 2 (age: adolescent, adult) × 2 (test condition: socially, alone) factorial. After the removal of outliers, there were 8 subjects in each of the adult conditions, 7 subjects in the adolescent social condition, and 6 subjects in the adolescent alone condition.
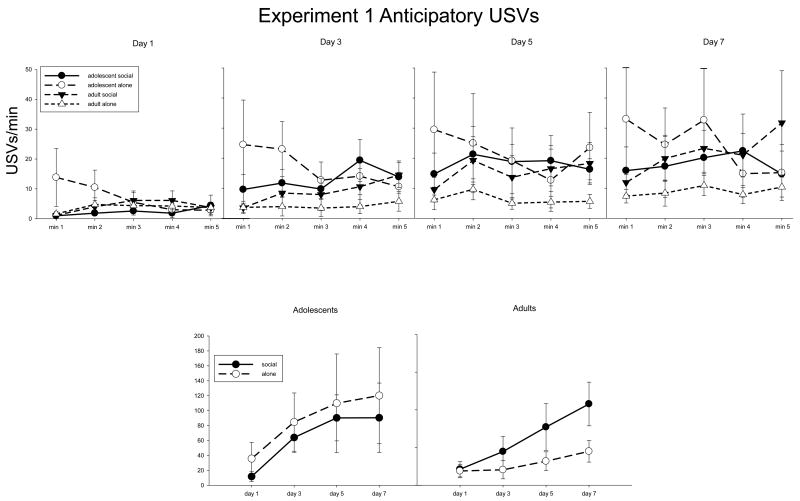
The data from Experiment 1 are shown in Fig 1. A 2 age × 2 test condition × 5 minute × 4 day ANOVA of anticipatory USV production revealed a significant main effect of day [F(3,75) = 12.35, p < 0.001], reflecting lower USV production on day 1 than on all subsequent test days, and on day 3 relative to day 7. Post-hoc analysis of the significant interaction of age and minute [F(4,100) = 2.61, p < 0.05] revealed that adolescents generally emitted more USVs during min 1 than did adults. Post-hoc analyses on data collapsed across age and day revealed that regardless of age or day, a significant interaction of test condition and min [F(4,100) = 3.98, p < 0.01] was associated with animals in the social group emitting more USVs during mins 4 and 5 than min 1, whereas animals in the alone group emitted more USVs during min 1 than min 4 (Fig 1 top panels).
Fig. 1.
Top panel: Experiment 1 anticipatory USVs across min and day. High levels of variability are due to the random assignment of animals to test groups. Inter-subject variability in USV production has been shown to be high while intra-subject USVs are generally highly stable [46]. Bottom panel: Sum of USVs produced during the 5 min anticipatory period.
When the data were separately analyzed at each age, analysis of the adolescent data revealed a significant main effect of day [F(3,33) = 5.16, p < 0.01], with USVs lower on day 1 than all subsequent days (Fig 1 bottom left panel). There was also a significant interaction of condition and min [F(4,44) = 3.11, p < 0.05], reflecting that adolescents subsequently left alone emitted more USVs during min 1 than mins 4 and 5, whereas no differences in USV production were seen across mins in adolescents who were then placed in a social situation (compare open and closed circles in Fig 1 top panels). The ANOVA of the adult data revealed a main effect of day [F(3,42) = 9.46, p < 0.001], with USVs on test day 5 higher than day 1, and test day 7 higher than days 1 and 3. As can be seen in Fig 1, bottom right panel, these increases in USV production across days tended to be greater in adults subsequently placed in a social situation than in adults left alone (p = 0.06 for interaction between day and condition).
Thus, overall anticipatory USVs generally increased across days, with adults tending to show a greater increase when subsequently placed in a social condition, whereas the adolescents groups did not differ from each other. Adolescents emitted more USVs during min 1 than did adults.
Experiment 2 was designed to investigate whether the general increases in USVs over test days seen during the anticipatory period in Exp 1 could be due to the animals anticipating their immediate post-test return to the home-cage and cage-mate; if so, this could have partially obscured differences between the “social” and “alone” conditions given that animals in both conditions were reunited with their cage-mates in their home-cages following the 15 min test period. To explore this possibility, animals in this experiment were isolated following the anticipatory period for different lengths of time prior to being returned to their cage-mate and home-cage. Each day, animals in the no-isolation group were reunited with their cage-mates in their home-cages immediately following the 5 min anticipatory period, whereas animals subjected to post-test isolation were returned to the same holding cages as used during the pre-test isolation period for 120 min prior to returning them to their home cages and housing partners. The design of this study was a 2 (age: adolescent, adult) × 2 (post-test isolation: none, 120 min) factorial. After removal of outliers, there were 10 adolescents in the post-test isolation group, and 11 adolescents not isolated post-test, whereas adult group sizes for these conditions were 7 and 8, respectively.
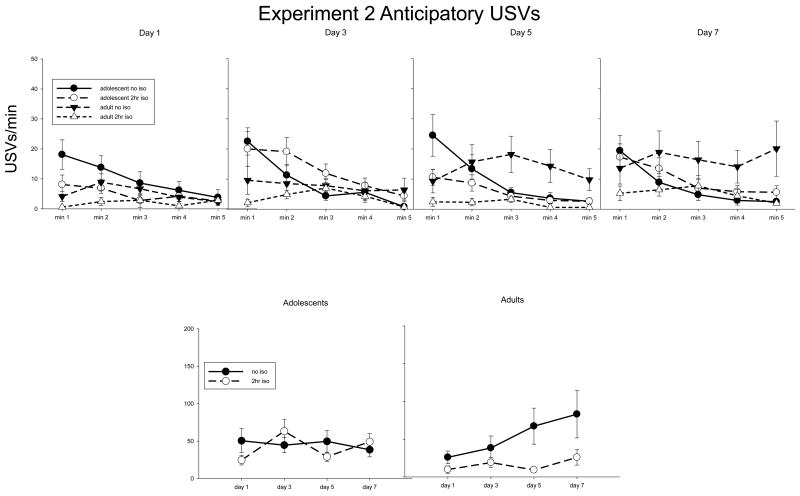
Data from experiment 2 data are shown in Fig 2. The 2 age × 2 post-test isolation conditions × 5 min × 4 days ANOVA of USVs revealed a significant interaction of day, age, and post-test isolation [F(3,93) = 2.81, p < 0.05], with adults not isolated post-test emitting more USVs than adults isolated for 2 hours post-test on days 5 and 7. Age and post-test isolation also interacted with min [F(4,124) = 3.58, p < 0.01], reflecting greater USV production among adolescents not isolated post-test than adolescents isolated for 2 hours post-test during min 1, and a similar pattern in adults during min 2. Both groups of adolescents emitted significantly more USVs than adults during min 1.
Fig. 2.
Top panel: Experiment 2 anticipatory USVs across min and day. Bottom panel: Sum of USVs produced during the 5 min anticipatory period.
Results of the ANOVA focused on the adolescent data revealed a significant interaction of post-test isolation × min [F(4,64) = 3.58, p < 0.05], in which adolescents returning home immediately post-test emitted more USVs during min 1 than adolescents isolated for 2 hours post-test; this effect, however, was present on the first day and did not vary across day and hence is unlikely to reflect a conditioning effect. Post-hoc testing of the interaction of post-test isolation condition and day [F(3,48) = 2.98, p < 0.05] revealed that adolescents isolated for 2 hours post-test emitted slightly but significantly more USVs on day 3 than days 1 or 5 (Fig 2 bottom left panel). Analysis of the adult data revealed a significant interaction of day × min × post-test isolation [F(12,180) = 1.81, p < 0.05]. As shown in Fig 2 bottom right panel, among adults isolated for 2 hours post-test, there were no significant differences in USV production across days, however adults not isolated post-test emitted significantly more USVs during all mins of days 5 and 7 when compared to day 1. Adults returned immediately to the home-cage post-test also emitted more USVs than adults isolated for 2 hours post-test during mins 2-4 on day 5 and min 5 on day 7.
Thus, the results of Exp 2 demonstrate that adults returned home immediately post-test emitted more anticipatory USVs than adults isolated 2 hours post-test, whereas no convincing evidence of anticipatory conditioning emerged in adolescent animals. Also, similar to Exp 1, adolescents again emitted more USVs than adults during min 1.
Overall, the results of these experiments revealed different temporal patterns of USV production between adolescent and adult male rats. Adolescents tended to emit more USVs than adults during the first min of the anticipatory session, whereas age differences were generally not seen during the later mins of the anticipatory session. This may have important implications for ontogenetic research on anticipatory responses, as assessment of an anticipatory period of only 1-2 mins would likely result in a greater USV responsiveness in adolescents compared to adults, whereas these age differences may not be seen when using a longer anticipatory period.
In these experiments, adult males developed a strong conditioned anticipatory response to the return to their home-cage and cage-mate and a similar trend during the anticipatory period prior to a social encounter with a conspecific, whereas little sign of anticipatory responding emerged among the adolescents.
The lack of development of anticipatory USV production among the adolescent males contrasts with the work of Knutson et al., [28] where both male and female P28 rats were found to emit more USVs in a chamber that had been previously paired with conspecific play than in a control chamber. Notable procedural differences across studies may account for the different findings. In the present study, chambers were cleaned following each testing session, whereas Knutson et al., [28] did not change apparatus bedding across test days, allowing a build-up of olfactory cues that may have stimulated USV production. The same play partner was used across days in the present study to avoid confounding age differences in novelty seeking (see [3]) and social affect, whereas Knutson et al., [28] utilized a novel play partner each day. Perhaps most notably, in contrast to the socially housed male rats assessed here, the adolescents utilized in [28] were isolate housed. Social deprivation has been shown to markedly elevate social behavior and USV production – perhaps due not only to notable increases in social motivation but also to the stressfulness of housing young animals in social isolation [33].
One possibility for the lack of apparent anticipatory conditioning in adolescents is that adolescents may require more than 7 days of conditioning to develop an anticipatory response. This possibility seems unlikely, however, given that socially tested adolescents were found to form a more pronounced CPP to a social stimulus than adults following 5 days of conditioning [3]. These contrasting findings across studies may reflect the differing nature of the tasks and measures used in place versus anticipatory conditioning. All incentive stimuli have three properties characterized by the ability to: elicit approach; strengthen instrumental conditioning; and serve as a conditional reinforcer during learning [41]. CPP and anticipatory USV production may reflect to differing extents these aspects of incentive stimuli. While it is a possibility that USVs may not be a good measure of anticipatory responding, anticipatory USVs have been seen previously in both male and female isolate housed adolescents when exposed to a chamber previously paired with conspecific exposure, and also when separated from a familiar play partner with a screen [28]. It is possible, however, that the 3 hrs of pre-test social deprivation utilized in the present experiments was not sufficient to provide the necessary social motivation to produce anticipatory USVs in socially housed adolescent males.
Whereas adult but not adolescent male rats were found to display anticipatory responding, further studies are needed to determine whether similar age differences are seen in female animals. In a study of USV production in the presence of social stimuli, very few differences in USV production were seen between adolescent males and females [28]. Adolescent females, however, have been found to engage in less play behaviors than adolescent males [3], which could provide the basis for possible differences in USV production related to social stimuli under some circumstances. Adult females have been shown to emit varying levels of USVs across the estrous cycle [34] and hence, it would appear important to include cycle stage in analyses of adult females in order to assess whether differences in social hedonics emerge dependent on estrous phase.
To the extent that 50 kHz USVs reflect positive affect [20, 27] and that anticipatory responding can be used to index incentive salience (e.g., [42]) –an arguable position, as discussed earlier-- the results of the current experiments support the suggestion that adolescents experience less incentive salience to a social stimulus than adults. A similar conclusion was reached in work using a very different measure to index incentive salience for a non-social stimulus -- sign-tracking behavior during an autoshaping procedure (for food reward) [43]. Assessment of USV production during social interactions likewise found adolescents to emit fewer 50 kHz USVs than adults during social interactions [6]. In that study, adolescents elicited fewer USVs while at the same time engaging in more social behaviors, with no significant negative correlations between USVs and social behavior as would be expected if they were competing behaviors [6]. Upon initial evaluation, these apparent adolescent attenuations in incentive salience (as indexed via anticipatory USV production and sign-tracking) and hedonic value (assessed via USV production in the presence of reward) appear paradoxical, given the avidity with which adolescents often seek out social stimuli, and other rewards, including drugs (e.g. see [44]). Yet, these data are consistent with an adolescent anhedonia hypothesis in which adolescents are postulated to increase their consumption of rewards in part to counteract an adolescent-associated attenuation in hedonic value and incentive salience of social stimuli and other rewards [see [1] for further discussion]. Other work, however, utilizing different measures and rewarding stimuli, have reached opposite conclusions with adolescents sometimes showing evidence for enhanced hedonic responding ([45]; see [44] and [47] for reviews). Clearly future studies are needed that systematically vary the nature of the tests and rewards used to dissect which aspects of incentive processing differ between adolescents and adults and the neural substrates underlying these apparent developmental dissociations.
Research Highlights.
USVs were measured during anticipation of social interactions
Adults showed increasing anticipatory USVs over days, adolescents did not
Data suggest attenuated incentive salience in adolescence to social stimuli
Acknowledgments
The research presented in this article was supported by grants from the National Institute on Alcohol Abuse and Alcoholism (R01-AA017355 and R37-AA012525) to Linda P. Spear.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 2.Brenhouse HC, Andersen SL. Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behav Neurosci. 2008;122:460–5. doi: 10.1037/0735-7044.122.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Dev Psychobiol. 2004;45:153–62. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- 4.Torres OV, Tejeda HA, Natividad LA, O'Dell LE. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacol Biochem Behav. 2008;90:658–63. doi: 10.1016/j.pbb.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zakharova E, Leoni G, Kichko I, Izenwasser S. Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent male rats. Behav Brain Res. 2009;198:45–50. doi: 10.1016/j.bbr.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willey AR, Varlinskaya EI, Spear LP. Social interactions and 50 kHz ultrasonic vocalizations in adolescent and adult rats. Behav Brain Res. 2009;202:122–9. doi: 10.1016/j.bbr.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knutson B, Panksepp J. Effects of serotonin depletion on the play of juvenile rats. Ann N Y Acad Sci. 1997;807:475–7. doi: 10.1111/j.1749-6632.1997.tb51942.x. [DOI] [PubMed] [Google Scholar]
- 8.Brudzynski SM, Kehoe P, Callahan M. Sonographic structure of isolation-induced ultrasonic calls of rat pups. Dev Psychobiol. 1999;34:195–204. doi: 10.1002/(sici)1098-2302(199904)34:3<195::aid-dev4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 9.Sales GD, P D. Ultrasonic Communication by Animals. London: Chapman & Hall; 1974. [Google Scholar]
- 10.Blanchard RJ, Yudko EB, Blanchard DC, Taukulis HK. High-frequency (35-70 kHz) ultrasonic vocalizations in rats confronted with anesthetized conspecifics: effects of gepirone, ethanol, and diazepam. Pharmacol Biochem Behav. 1993;44:313–9. doi: 10.1016/0091-3057(93)90467-8. [DOI] [PubMed] [Google Scholar]
- 11.Harris JR. Where is the child's environment? A group socialization theory of development. Psychological Review. 1995;102:458–89. [Google Scholar]
- 12.Knutson B, Burgdorf J, Panksepp J. High-frequency ultrasonic vocalizations index conditioned pharmacological reward in rats. Physiol Behav. 1999;66:639–43. doi: 10.1016/s0031-9384(98)00337-0. [DOI] [PubMed] [Google Scholar]
- 13.Sales GD. Strain differences in the ultrasonic behavior of rats (Rattus norvegicus) American Journal of Zoology. 1979;19:513–27. [Google Scholar]
- 14.Bialy M, Rydz M, Kaczmarek L. Precontact 50-kHz vocalizations in male rats during acquisition of sexual experience. Behav Neurosci. 2000;114:983–90. doi: 10.1037//0735-7044.114.5.983. [DOI] [PubMed] [Google Scholar]
- 15.McIntosh TK, Vallano ML, Barfield RJ. Effects of morphine, beta-endorphin and naloxone on catecholamine levels and sexual behavior in the male rat. Pharmacol Biochem Behav. 1980;13:435–41. doi: 10.1016/0091-3057(80)90251-8. [DOI] [PubMed] [Google Scholar]
- 16.Burgdorf J, Panksepp J. Tickling induces reward in adolescent rats. Physiol Behav. 2001;72:167–73. doi: 10.1016/s0031-9384(00)00411-x. [DOI] [PubMed] [Google Scholar]
- 17.Mallo T, Matrov D, Herm L, Koiv K, Eller M, Rinken A, et al. Tickling-induced 50-kHz ultrasonic vocalization is individually stable and predicts behaviour in tests of anxiety and depression in rats. Behav Brain Res. 2007;184:57–71. doi: 10.1016/j.bbr.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 18.Panksepp J, Burgdorf J. 50-kHz chirping (laughter?) in response to conditioned and unconditioned tickle-induced reward in rats: effects of social housing and genetic variables. Behav Brain Res. 2000;115:25–38. doi: 10.1016/s0166-4328(00)00238-2. [DOI] [PubMed] [Google Scholar]
- 19.Burgdorf J, Knutson B, Panksepp J. Anticipation of rewarding electrical brain stimulation evokes ultrasonic vocalization in rats. Behav Neurosci. 2000;114:320–7. [PubMed] [Google Scholar]
- 20.Burgdorf J, Panksepp J. The neurobiology of positive emotions. Neurosci Biobehav Rev. 2006;30:173–87. doi: 10.1016/j.neubiorev.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Ma ST, Maier EY, Ahrens AM, Schallert T, Duvauchelle CL. Repeated intravenous cocaine experience: development and escalation of pre-drug anticipatory 50-kHz ultrasonic vocalizations in rats. Behav Brain Res. 2010;212:109–14. doi: 10.1016/j.bbr.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maier EY, Ahrens AM, Ma ST, Schallert T, Duvauchelle CL. Cocaine deprivation effect: cue abstinence over weekends boosts anticipatory 50-kHz ultrasonic vocalizations in rats. Behav Brain Res. 2010;214:75–9. doi: 10.1016/j.bbr.2010.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson B, Leonard KC, Brudzynski SM. Amphetamine-induced 50 kHz calls from rat nucleus accumbens: a quantitative mapping study and acoustic analysis. Behav Brain Res. 2006;168:64–73. doi: 10.1016/j.bbr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Wintink AJ, Brudzynski SM. The related roles of dopamine and glutamate in the initiation of 50-kHz ultrasonic calls in adult rats. Pharmacol Biochem Behav. 2001;70:317–23. doi: 10.1016/s0091-3057(01)00615-3. [DOI] [PubMed] [Google Scholar]
- 25.Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychol Bull. 2002;128:961–77. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- 26.Burgdorf J, Wood PL, Kroes RA, Moskal JR, Panksepp J. Neurobiology of 50-kHz ultrasonic vocalizations in rats: electrode mapping, lesion, and pharmacology studies. Behav Brain Res. 2007;182:274–83. doi: 10.1016/j.bbr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Panksepp J. Neuroevolutionary sources of laughter and social joy: modeling primal human laughter in laboratory rats. Behav Brain Res. 2007;182:231–44. doi: 10.1016/j.bbr.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 28.Knutson B, Burgdorf J, Panksepp J. Anticipation of play elicits high-frequency ultrasonic vocalizations in young rats. J Comp Psychol. 1998;112:65–73. doi: 10.1037/0735-7036.112.1.65. [DOI] [PubMed] [Google Scholar]
- 29.La Greca AM, Prinstein MJ, Fetter MD. Adolescent peer crowd affiliation: linkages with health-risk behaviors and close friendships. J Pediatr Psychol. 2001;26:131–43. doi: 10.1093/jpepsy/26.3.131. [DOI] [PubMed] [Google Scholar]
- 30.Vanderschuren LJ, Niesink RJ, Van Ree JM. The neurobiology of social play behavior in rats. Neurosci Biobehav Rev. 1997;21:309–26. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- 31.Larson R, Richards MH. Daily companionship in late childhood and early adolescence: changing developmental contexts. Child Dev. 1991;62:284–300. doi: 10.1111/j.1467-8624.1991.tb01531.x. [DOI] [PubMed] [Google Scholar]
- 32.Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–13. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 33.Weintraub A, Singaravelu J, Bhatnagar S. Enduring and sex-specific effects of adolescent social isolation in rats on adult stress reactivity. Brain Res. 1343:83–92. doi: 10.1016/j.brainres.2010.04.068. [DOI] [PubMed] [Google Scholar]
- 34.Matochik JA, White NR, Barfield RJ. Variations in scent marking and ultrasonic vocalizations by Long-Evans rats across the estrous cycle. Physiol Behav. 1992;51:783–6. doi: 10.1016/0031-9384(92)90116-j. [DOI] [PubMed] [Google Scholar]
- 35.Varlinskaya EI, Spear LP, Spear NE. Social behavior and social motivation in adolescent rats: role of housing conditions and partner's activity. Physiol Behav. 1999;67:475–82. doi: 10.1016/s0031-9384(98)00285-6. [DOI] [PubMed] [Google Scholar]
- 36.Varlinskaya EI, Spear LP, Spear NE. Acute effects of ethanol on behavior of adolescent rats: role of social context. Alcohol Clin Exp Res. 2001;25:377–85. [PubMed] [Google Scholar]
- 37.Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–8. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- 38.Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol. 1997;30:141–50. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 39.Vanderschuren LJ, Niesink RJ, Spruijt BM, Van Ree JM. Influence of environmental factors on social play behavior of juvenile rats. Physiol Behav. 1995;58:119–23. doi: 10.1016/0031-9384(94)00385-i. [DOI] [PubMed] [Google Scholar]
- 40.Vanderschuren LJ, Stein EA, Wiegant VM, Van Ree JM. Social isolation and social interaction alter regional brain opioid receptor binding in rats. Eur Neuropsychopharmacol. 1995;5:119–27. doi: 10.1016/0924-977X(95)00010-M. [DOI] [PubMed] [Google Scholar]
- 41.Robinson TE, Flagel SB. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry. 2009;65:869–73. doi: 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flagel SB, Watson SJ, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to a reward-related cue: influence on cocaine sensitization. Behav Brain Res. 2008;186:48–56. doi: 10.1016/j.bbr.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson RI, Spear LP. Autoshaping in adolescence enhances sign-tracking behavior in adulthood: impact on ethanol consumption. Pharmacol Biochem Behav. 2011;98:250–60. doi: 10.1016/j.pbb.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 2010;72:114–23. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilmouth CE, Spear LP. Hedonic sensitivity in adolescent and adult rats: taste reactivity and voluntary sucrose consumption. Pharmacol Biochem Behav. 2009;92:566–73. doi: 10.1016/j.pbb.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwarting RK, Jegan N, Wohr M. Situational factors, conditions and individual variables which can determine ultrasonic vocalizations in male adult Wistar rats. Behav Brain Res. 2007;182:208–22. doi: 10.1016/j.bbr.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 47.Spear LP. Rewards, aversions and affect in adolescence: Emerging convergences across laboratory animal and human data. Dev Cogn Neurosci. 2011;1:390–403. doi: 10.1016/j.dcn.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]