Abstract
The primate amygdala is composed of multiple subnuclei that play distinct roles in amygdala function. While some nuclei have been areas of focused investigation, others remain virtually unknown. One of the more obscure regions of the amygdala is the paralaminar nucleus (PL). The PL in humans and non-human primates is relatively expanded compared to lower species. Long considered to be part of the basal nucleus, the PL has several interesting features that make it unique. These features include a dense concentration of small cells, high concentrations of receptors for corticotropin releasing hormone and benzodiazepines, and dense innervation of serotonergic fibers. More recently, high concentrations of immature-appearing cells have been noted in the primate PL, suggesting special mechanisms of neural plasticity. Following a brief overview of amygdala structure and function, this review will provide an introduction to the history, embryology, anatomical connectivity, immunohistochemical and cytoarchitectural properties of the PL. Our conclusion based on the following information, is that the PL is a unique subregion of the amygdala that may yield important clues about the normal growth and function of the amygdala, particularly in higher species.
Keywords: basal nucleus, depression, anxiety, intercalated neurons, neurogenesis, ganglionic eminence, contextual fear learning, lateral paracapsular island, plasticity, stress, basolateral nucleus
1. Overview of emerging concepts of primate amygdala function
The overall function of the amygdala is to provide emotional salience to biologically relevant stimuli (review, Adolphs, 2010). The most extensively researched example of this function is fear learning, in which a neutral stimulus elicits a fear response after being paired multiple times with an aversive stimulus (reviews, LeDoux, 2000; Phelps and LeDoux, 2005). Fear learning studies in rodents reveal that the amygdala is necessary for the acquisition of a fear memory associated with the conditioning experience and is also necessary for the expression of a fear response (Blanchard and Blanchard, 1972; review, LeDoux, 2000). Similar findings have been reported in humans and monkeys through both lesion and neuroimaging studies (Buchel et al., 1998; Kalin et al., 2004; LaBar et al., 1998).
The amygdala also responds to positive affect, suggesting that the amygdala codes salience as a whole. Electrophysiological studies in monkeys indicate that amygdala neurons respond to rewarding, as well as aversive stimuli (Nishijo et al., 1988; Paton et al., 2006). The amygdala also participates in appetitive learning in which an object is given a value that is associated with the representation of a reward (review, Baxter and Murray, 2002 and O’Doherty et al., 2002). In this process, the amygdala is thought to maintain and rapidly update the value of an object, as its value changes (review, Baxter and Murray, 2002).
In primates, the amygdala also processes socially relevant stimuli (reviews, Adolphs, 2010; Amaral et al., 2003), a function especially important in species depending on a tight social order. Evidence for the amygdala’s role in social function was established by early temporal lobe resection studies documenting abnormal behavior including decreased fearfulness, hyperorality and alterations in sexual behavior in monkeys (Klüver and Bucy, 1939; Weiskrantz, 1956), which in some cases resulted in ostracization from the group, or a fall in social status (Dicks et al., 1969; Rosvold et al., 1954). More recently, selective fiber-sparing amygdala lesions have produced similar, albeit more complex results (Emery et al., 2001; Machado and Bachevalier, 2006). For instance, in dyadic relations‘, amygdalectomized monkeys are much more likely to approach other monkeys, resulting in increased grooming by their counterparts. When placed in larger social groups however, lesioned monkeys actually exhibit decreased social interactions and more avoidance behaviors compared to their conspecifics. These data suggest that amygdalectomized monkeys are actually capable of the full repertoire of social behaviors‘, but display an impaired ability to recognize the biological relevance of social stimuli‘, resulting in an inability to deploy behaviors in a contextually appropriate manner (reviews, Adolphs, 2010; Amaral et al., 2003 and Emery et al., 2001; Machado et al., 2008).
Given its role in social responses, it is not surprising that facial expressions, which convey critical social information, activate the amygdala. A subset of neurons in the monkey amygdala selectively responds to faces (Brothers and Ring, 1993; Gothard et al., 2007; Heit et al., 1988; Leonard et al., 1985; Rolls, 1984) and patients with amygdala damage are unable to recognize complex emotion in faces, particularly fear (Adolphs et al., 1994; Broks et al., 1998). While many early neuroimaging studies suggested that the human amygdala responds mainly to fearful faces (Breiter et al., 1996; Morris et al., 1996; Whalen et al., 1998), more recent data indicate that it responds to a range of facial expressions, including neutral faces (review, Adolphs, 2010 and Fitzgerald et al., 2006; Yang et al., 2002). Taken together, studies in humans and nonhuman primates support the idea that the amygdala may be more involved in detecting overall emotional salience than any one type of emotion.
Finally, amygdala dysfunction has been consistently implicated in the pathophysiology of human mood and anxiety disorders (e.g., Drevets, 2001; Phillips et al., 2003; Protopopescu et al., 2005; Schwartz et al., 2003; Stein et al., 2002). The hallmarks of these illnesses include social withdrawal, negative attentional bias (tendency to focus on negative versus positive environmental cues), and decreased ability to experience pleasure (Sadock and Sadock, 2007), suggesting that the amygdala’s ability to extract salience is biased or compromised. Evidence for the amygdala’s involvement in these disorders has been repeatedly documented in functional neuroimaging studies (review, Victor et al., 2010).
Ultimately, the overall role of the amygdala in coding emotional salience is achieved by its individual nuclei which participate in different aspects of information processing. Although we have described it as a single unit, the amygdala is actually comprised of several subnuclear regions. While extensive information exists on several of these subnuclear structures, other subnuclei remain virtually unknown. This is especially true for the relatively obscure paralaminar nucleus (PL). Our recent data and a close review of general studies on the primate amygdala however, suggest that the PL is positioned to play an important role in both amygdala function and dysfunction. In the following review, we give a comprehensive understanding of this subnuclear region and provide information on its distinctive features. We conclude by examining the potential implications of the PL for amygdala-specific psychopathologic processes in human psychiatric illnesses.
2. Overview of amygdala anatomy and connectivity
Before focusing on the PL, we set the stage by giving an overview of the main amygdala subnuclei and their basic connectivity (for a more complete review see Freese and Amaral, 2009). Through their extrinsic and intrinsic connections these subnuclei process a wealth of sensory and contextual information in order to code the salience of the stimuli, and effect appropriate responses.
While the nomenclature and boundaries of amygdalar subregions have changed over time, today, most investigators use nomenclature initially proposed by Johnston (Johnston, 1923) and further modified by Crosby and Humphrey (Crosby and Humphrey, 1941; Humphrey, 1968) and Price and Amaral (Price et al., 1987) and DeOlmos (De Olmos, 2004; DeOlmos, 1990) (Fig. 1). The cytoarchitectonic features and differential nuclear staining that delineate these regions, are visualized by using classic stains such as cresyl violet and acetylcholinesterase (AChE), respectively.
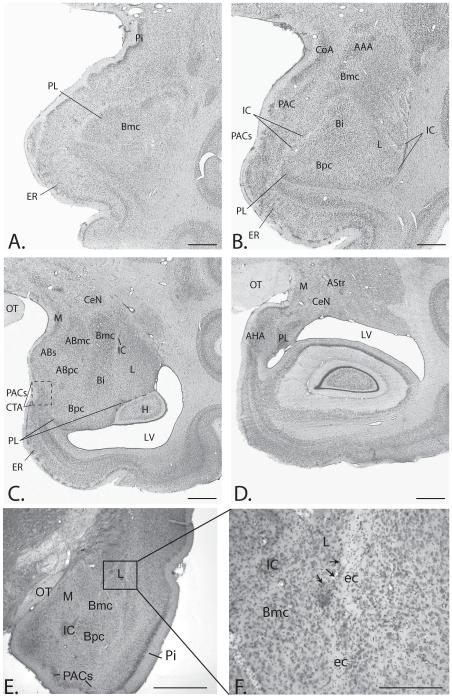
Figure 1.
A-D). Coronal sections through the rostrocaudal extent of the monkey amygdala depicting the main nuclear regions. Note that the paralaminar nucleus (PL) is closely related to the basal nucleus. The boxed area in C) is the cortico-amygdaloid transition area (CTA), where the PL merges with the sulcal division of the periamygdaloid cortex (PACs). E). Coronal section through the rat amygdala at the level of the putative PL. F). High power image showing position of the rat PL (arrows), interposed between the lateral nucleus (L) and the external capsule (ec). Scale bars in A-E = 1mm. Scale bar in F. = 100 μm. ABmc = accessory basal nucleus, magnocellular division; ABpc = accessory basal nucleus, parvicellular division; ABs = accessory basal nucleus, sulcal division; AHA = amygdalohippocampal area; Astr = amygdalostriatal area; Bi = basal nucleus, intermediate division; Bmc = basal nucleus, magnocellular division; Bpc = basal nucleus, parvicellular division; CeN = central nucleus; CoA = anterior cortical nucleus; CTA = cortico-amygdaloid transition area; ec = external capsule; ER = entorhinal cortex; H = hippocampus; IC =intercalated islands; L = lateral nucleus; M = medial nucleus; OT = optic tract; PAC = periamygdaloid cortex; PACs = periamygdaloid cortex, sulcal division; Pi = piriform cortex; PL = paralaminar nucleus; LV = lateral ventricle.
The amygdala is divided into the ‘superficial’ amygdala and the ‘deep’ amygdala (Fig. 1). The superficial amygdala is considered ‘allocortex’, a primitive 2-3 layered cortex that is highly conserved phylogenetically (Vogt and Vogt, 1919). It includes the anterior and posterior cortical nuclei, the medial nucleus, and periamygdaloid cortex. The periamygdaloid cortex (or ‘prepiriform’ cortex) contains a molecular layer (layer I), a deep pyramidal layer (layer II), and occasional cell clusters forming a rudimentary layer III (review, Gloor, 1997a). The sulcal subdivision of the periamygdaloid cortex (PACs) surrounds the annular sulcus and is the least differentiated. Importantly, the PACs merges with the medial PL. This entire area has also been referred to as the ‘corticoamygdaloid transition zone’ (CTA) (Fig 1C, boxed area) (Rosene and Van Hoesen, 1987) other terminologies for this unique region include the ‘cortical nucleus’ (Johnston, 1923), and ‘amygdalopiriform area’ (DeOlmos, 1990). We use the ‘CTA’ designation because it focuses on the transitional nature of this zone (Johnston, 1923). Caudally, the PACs and PL merge into yet another transition region, the ‘amygdalohippocampal area (AHA) (Fig. 1D). The entire superficial amygdala is generally associated with olfactory function, however inputs from other sensory modalities project here as well (Carmichael et al., 1994; Turner et al., 1980).
The ‘deep’ nuclei include the basal, accessory basal, and lateral nuclei. Unlike other amygdaloid regions, this entire group of structures, sometimes referred to as the ‘basolateral nuclear group’, is progressive across mammalian species, reaching its relatively greatest size in primates (Stephan and Andy, 1977). These nuclei are phylogenetically recent adaptations, and their development parallels the massive expansion of the temporal and frontal cortices from which they receive their main inputs (Jerison, 1973).
The lateral nucleus is the largest nucleus, reaching its greatest extent in the human (Stephan and Andy, 1977). It is the main receiving site of the amygdala, and is the main site where sensory information from visual and polysensory association cortices of the temporal lobe project (Aggleton et al., 1980; Amaral and Insausti, 1992; Stefanacci and Amaral, 2000; Turner et al., 1980). These projections are reciprocated but also channeled into the basal and accessory basal nuclei, following a topography based on sensory modality (Pitkanen and Amaral, 1998). The basal nucleus is also the recipient of additional extrinsic projections, many of which it returns. The medial and orbital prefrontal cortex, the insula, and the entorhinal cortex densely project to the basal nucleus (Amaral and Insausti, 1992; Carmichael and Price, 1995; Ghashghaei and Barbas, 2002; Van Hoesen et al., 1981). Additional inputs from CA1 and subicular regions of the hippocampus also terminate in the basal nucleus, but interestingly, specifically target the ventral basal nucleus including the PL in the primate (Rosene and Van Hoesen, 1977; Saunders et al., 1988). In addition, the thalamus also projects to these regions (Carmichael and Price, 1996; Ghashghaei and Barbas, 2002; Mehler, 1980; Van Hoesen et al., 1981). The basal nucleus is comprised of magnocellular (Bmc), intermediate (Bi) and parvicellular (Bpc) subregions, oriented dorsally to ventrally (Amaral and Bassett, 1989; Braak and Braak, 1983). The dorsal Bmc has the largest cells and the strongest AChE staining, while the ventral Bpc has the smallest cells and the lightest AChE staining. The PL, as discussed in detail later, is closely associated with the basal nucleus in primates and has historically been considered to be part of this nucleus by some authors (Barbas and de Olmos, 1990; Jimenez-Castellanos, 1949).
The accessory basal nucleus (AB), located medial to the basal nucleus, receives many of the same intrinsic and extrinsic connections as the basal nucleus, (Freese and Amaral, 2009). It is divided into magnocellular (ABmc), parvicellular (ABpc) and sulcal divisions (ABs), which have some important differences anatomically and connectionally. One notable distinction in primates is that the ABpc receives hippocampal inputs, whereas the ABmc does not (Saunders et al., 1988). The ABmc, along with the basal nucleus, form the bulk of the amygdalostriatal pathway; the parvicellular subdivision of the accessory basal nucleus has very sparse inputs to the striatum (Fudge and Haber, 2002).
The central nucleus (CeN) is the main recipient of inputs from both the ‘superficial’ and ‘deep’ nuclei (Fudge and Tucker, 2009) and is a distinct structure composed of ‘striatal-like’ medium spiny neurons (Cassell et al., 1999; McDonald, 1982a). The central nucleus forms a continuum with the bed nucleus of the stria terminalis (BNST), a macrostructure known as the ‘extended amygdala’ (Alheid and Heimer, 1988; review, Heimer et al., 1999). Despite its location outside the amygdala (it resides caudal to the ventral striatum), the BNST is almost identical to the CeN cytoarchitecturally and histochemically. The CeN and BNST are linked by an almost continuous chain of cell islands that span the basal forebrain, hence, the designation ‘extended amygdala’. The full scope of input and output paths of the extended amygdala have been determined predominantly in rodent species (Alheid, 2003; Reynolds and Zahm, 2005), with relatively few studies in the primate.
The amygdala contains several other regions that are not easily grouped including, the anterior amygdaloid area (AAA), the amygdalo-striatal area (AStr), and the intercalated cell islands (IC). The ICs are a unique group of cells that have recently received attention because they inhibit amygdala output (Amano et al., 2010; Likhtik et al., 2008). The ICs are small heterogeneous cell clusters embedded in fiber tracts located between major amygdaloid nuclei. They are found in the rat and the primate, including the human. The morphology and chemical profile of the ICs indicate that they contain GABAergic, inhibitory neurons (Pitkanen and Amaral, 1994). Pertinent to this review, similarities between the IC and the PL have been reported (Amaral and Bassett, 1989; Braak and Braak, 1983; Krettek and Price, 1978; Millhouse, 1986), raising the idea that the PL may be either continuous with some ICs or developmentally linked to them. However, the low levels of GABA expression in the PL stand in contrast to the ICs, and are an important discontinuity, as we shall see.
Our own recent data and a close review of general studies on the primate amygdala, suggest that the PL is positioned to play an important role in both amygdala function and dysfunction. This may be particularly true in higher species such as the human and nonhuman primates, where the PL is significantly expanded compared to lower species such as rodents. For this reason, we focus primarily on the primate PL, including that in the human, and will conclude by examining the potential implications of the PL for amygdala-generated psychopathologic processes in psychiatric illnesses.
3. The paralaminar nucleus and its multiple descriptions
The PL has traditionally been identified in Nissl-stained sections (Fig. 2) (Amaral and Price, 1984; Barbas and de Olmos, 1990; Brockhaus, 1938; Crosby and Humphrey, 1941; DeOlmos, 1990; Jimenez-Castellanos, 1949; Price et al., 1987). Although there is no clear fiber bundle separating the PL from the neighboring Bpc and lateral nucleus, the cells of the PL appear more densely stained due to their relatively close packing, and small to medium-size (10-15 μm) (Braak and Braak, 1983) (Fig. 2B). The PL also contains dense neuropil, which has been attributed to a higher number of glial cells, compared to the overlying basal nucleus. However, multiple small neurons, including tiny ‘neurogliaform’ cells (tiny interneurons with glia-like morphologic characteristics (Kawaguchi et al., 1997; Ramón y Cajal, 1909) and nonpyramidal neurons also pack the PL, making its cellular composition highly heterogeneous (Amaral et al., 1992; Braak and Braak, 1983) (Fig. 2B-D). The PL wraps around the edges of the Bpc rostrally and caudally. Medially, the PL gradually transitions into the CTA (Rosene and Van Hoesen, 1987) (Fig. 1C). Caudolaterally, at the level of the amygdalohippocampal area, the PL lies adjacent to the subventricular zone (Fig.1D).
Figure 2.
A-D). Brightfield images of Nissl-stained sections depicting the cellular heterogeneity in the PL. A).Rostrocaudal level of the amygdala from which images were taken. B). Higher magnification of the boxed area in (A), shows that the PL is distinguished from the overlying Bpc by relatively dark staining, densely concentrated cells. C-D). At higher power, many cellular types are seen in the PL. Arrowheads at both magnifications indicate nuclei of putative oligodendrocytes. Small arrows point to small nonpyramidal neurons versus glia, and larger arrows show larger bipolar-type neurons. Scale bars = 25 um. WM= white matter.
A lack of consensus over its nomenclature has made the PL an elusive subregion to study (see Table 1 for a summary of alternative terminologies). Importantly, the PL varies significantly from species to species, both in its anatomical position and its overall prominence within the amygdala. Such species variations and multiple nomenclatures may account for why researchers have largely ignored the overall structure and function of the PL (see Species variations of the PL below).
Table 1.
Alternative terminologies for the PL nucleus
| Name | Authors | Species |
|---|---|---|
| Basal Nucleus (No distinction) |
Johnston, 1923 Jiminez- Castellanos, 1949 |
Human |
| Amygdaleum Profundum Ventrale | Brockhaus, 1938 | Human |
| Pars Medialis Superficialis |
Crosby and Humphrey, 1941
Lauer, 1945 |
Human Macaque |
| Granularis | Braak and Braak, 1983 | Human |
| Intercalated Islands | Millhouse, 1986 | Rat |
| Paralaminar Nucleus |
Amaral and Price 1984, Price et al., 1987 Flugge, 1994 |
Macaque, Cat, Rat Tree Shrew |
|
Medial: Basolateral Nucleus (paralaminar divison) Lateral: Glomerular Region |
De Olmos, 1990 | Human |
|
Medial: Basal Nucleus Lateral: Intercalated Islands |
Sims and Williams, 1990 | Human |
| Paralaminar nucleus (subdivided) Medial: Paralaminar division Lateral: Glomerular division |
De Olmos 2004 | Human |
The PL was first identified by Crosby and Humphrey in the human amygdala (Crosby and Humphrey, 1941). They proposed that this region was a distinct subdivision of the basal nucleus because of its higher cell density compared to the overlying Bpc and described the PL region as the most “superficial” (ventromedial) portion of the basal nucleus, referring to it as the “pars medialis superficialis of the basal nucleus”. The term “pars medialis superficialis” was maintained in the Macaque (Lauer, 1945). In some studies, the PL was identified as a subdivision of the basal nucleus (Barbas and de Olmos, 1990; Jimenez-Castellanos, 1949) while in other studies the PL was recognized as separate nucleus, and identified with several different names, including the ‘amygdaleum profundum ventrale’ (Brockhaus, 1938), the ‘granular nucleus’ (Braak and Braak, 1983), and ultimately, the ‘paralaminar nucleus’ (Amaral and Price, 1984; De Olmos, 2004; Price, 1981; Price et al., 1987).
Golgi and lipofuscin staining studies, in addition to Nissl staining, have been used as a justification for identifying the PL as separate nucleus from the basal nucleus (Braak and Braak, 1983; Tosevski et al., 2002). Golgi staining, which involves silver chromate impregnation, stains a limited sample of cells but allows visualization of the cell soma, its axon, and dendritic arborization in their entirety. Lipofuscin staining identifies a by-product of lysosome degradation and can be used to identify different classes of neurons. In contrast, Nissl staining detects broad cellular populations through the use of alkaline dyes that preferentially bind negatively charged RNA and proteins. All of these techniques have shown that the PL and the adjacent parvicellular basal nucleus have a much more complex array of cell types compared to the remaining subdivisions of the basal nucleus. Using these techniques in human samples, Braak and Braak found a clear structural distinction between the PL (their ‘granular’ nucleus), the basal nucleus, and the ‘transitory’ zone between them. This finding was echoed by Tosevski, who concluded that the degree of cellular heterogeneity and fascicular dendritic arrangements in the human PL warranted its classification as its own nuclear region (Tosevski et al., 2002).
In humans, the medial and lateral parts of the PL have sometimes been delineated based on differences in topology and cytoarchitectonics (review, De Olmos, 2004). The medial division of the PL covers the ventral aspect of the Bpc and can be distinguished by its smaller cells and higher cell density. In contrast, the lateral half of the PL is broken up into islands that cover the external surface of the lateral nucleus and are in proximity to the lateral ventricle. Here, cells are arranged in cell strands and shell-shaped islands which gives the lateral half of the human PL a unique appearance. The discontinuity of the PL laterally may explain why some authors propose that the lateral PL is part of the intercalated cell islands of the amygdala since PL cell islands, like other intercalated islands, are similarly interspersed along larger subnuclei (Sims and Williams, 1990). With respect to the medial part of the human PL, some authors have proposed that this region is part of the basal nucleus (Sims and Williams, 1990). Others have used alternative nomenclature, recognizing the medial portion of the PL as the “PL proper” and the lateral portion as the “granular islands of the PL” (De Olmos, 2004).
4. Species variations of the PL
The appearance of the PL varies across species, including the human (Crosby and Humphrey, 1941), monkey (Amaral and Price, 1984; Gloor, 1997b; Price, 1981; Price et al., 1987), tree shrew (a species included in the primate order, but with significant brain differences) (Flugge et al., 1994; Martin, 1990)) (Flugge et al., 1994), cat (Kamal and Tombol, 1975; Price et al., 1987), bat (Humphrey, 1936), and rat (Krettek and Price, 1978; McDonald, 1982b; Price et al., 1987). While Price and Amaral were the first to use the term “paralaminar nucleus” in rats and cats (Price et al., 1987), earlier studies noted this region to be distinct, describing it as the “shell” of the lateral nucleus in cats, or as part of the intercalated cell islands in rats (Krettek and Price, 1978; Millhouse, 1986).
The PL is progressive, increasing in relative size from lower species such as rats and cats and tree shrews to higher species such as Macaques and humans (Stephan and Andy, 1977). In lower species such as the rat and cat, the PL appears as a thin band of cells and makes up only a small proportion of the amygdala. In the rat, the PL is so small that it only appears in a few rostral sections, which may explain why many rat atlases and studies do not identify it (Fig. 1 E, F). It also may explain why some authors identify this region as intercalated islands. For example, Millhouse identified the region encompassing the PL as the ‘largest of the intercalated islands’ (Millhouse, 1986). More recently, the islands of cells interposed between the lateral nucleus and the external capsule has been termed the ‘lateral paracapsular island‘ (Fuxe et al., 2003; Marowsky et al., 2005). In contrast to its small appearance in rodents, the PL in humans and non-human primates is quite extensive as it extends around the basal, and to a lesser extent around the lateral nuclei as a large sheet of cells. The increasing relative size of the PL across phylogeny is suggestive of a greater functional role in higher species.
The anatomical location of the PL in human and non-human primates also differs from that in lower species such as rats and cats (Price et al., 1987). As discussed previously, in coronal sections of human and non-human primate amygdala, the PL is best seen ventral to the basal and lateral nucleus, extending caudally to the amygdala’s transition with the hippocampus and the lateral ventricle, and encircling the entire rostral and caudal poles of the basal nucleus (Fig. 1A-D). In contrast, in the rat (Fig. 1E, F) and cat, the PL is appreciated lateral to the lateral nucleus, wedged between it and the external capsule (Price et al., 1987). The basis for this anatomical difference is the greater rotation of the amygdala in primates, as discussed below.
5. Embryologic origin of the PL
The PL is the last nuclear region to form in both rats and higher primates, based on histologic and thymidine analog studies (Bayer, 1980; Kordower et al., 1992; Muller and O’Rahilly, 1990; Ulfig et al., 2003). Like other deep nuclei of the amygdala, it develops mainly from the ganglionic eminence (GE), a thickening of the telencephalic germinal zone arising from the lateral ventricles (Fig. 3). Although gene fate mapping studies in mice now indicate that several closely apposed neurogenetic zones in the region of the GE serve as sites of cell production in the amygdala during development, there is little information yet in higher primates (Bai et al., 2008; Cocas et al., 2009; Hirata et al., 2009; Zirlinger et al., 2001).
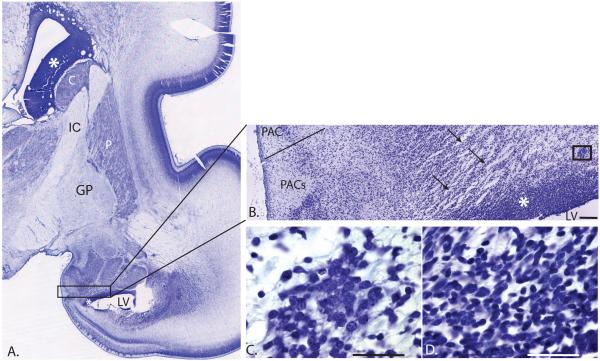
Figure 3.
A) Nissl-stained coronal section through the developing amygdala in the human fetal brain, at low power. The germinal zone of the ganglionic eminence lines the ventricles and is seen dorsal to the caudate nucleus and ventral to the amygdala (asterisks). B) At higher power, the germinal zone (inferior ganglionic eminence) (boxed area in A) is noted to be in the position of the future paralaminar nucleus (PL). The amygdala develops from columns of neuroblasts migrating out of this zone (arrows). Scale bar = 1 mm. C). Higher magnification of boxed area in (B) showing a cell cluster that has presumably migrated from the germinal zone. D) High magnification image of the germinal zone. Scale bars in C. and D. = 25 um. C = caudate nucleus; IC = internal capsule; LV = ventricle; P = putamen; PAC = periamygdaloid cortex; PACs = periamygdaloid cortex, sulcal division.
In humans, the inferior GE is situated ventral to the developing amygdala and persists throughout most of development (Ulfig et al., 2000) (Fig. 3). The GE is a source of neuronal, astrocytic and oligodendrocytic cell types (Birling and Price, 1998; Culican et al., 1990; Schmechel and Rakic, 1979; Ulfig et al., 1998). As a proliferative site, the human GE contains a number of densely packed cells that stain for markers found in proliferating and migrating cells such as PCNA (Ulfig et al., 2000), and the polysialylated form of neural cell adhesion marker (PSA-NCAM), as has been noted in the human (Ulfig and Chan, 2004). Immunoreactivity for PSA-NCAM is particularly apparent in the 5th gestational month and gradually diminishes after the 6-7th gestational months in humans (Ulfig and Chan, 2004; Ulfig et al., 1998).
During the initial phases of amygdalar development in both monkey and human, columns of neuroblasts from the GE migrate along radial glial fibers to form a primordial amygdala (Kordower et al., 1992; Muller and O’Rahilly, 1990; Ulfig et al., 2003) (Fig. 3B). In the monkey, the amygdala grows steadily in size throughout the second trimester, with its nuclei becoming more differentiated, medially to laterally (Kordower et al., 1992). Cell proliferation, as detected by the thymidine analogue, bromodeoxyuracil continues until the end of gestation. Late in fetal development, the GE recedes from a massive neurogenetic zone to a thin band of cells that borders the ventral portion of the maturing amygdala. In humans, the PL begins to form during the 8th and 9th gestational month, growing at the site of the remnant GE (Ulfig et al., 2003). Consequently, in postnatal monkeys and humans the PL resides in the same location as the former GE.
Across species, differences in the anatomical location of the late-forming PL are the result of varying degrees of amygdalar rotation and expansion that occur during neurodevelopment. During development, the temporal lobes gradually fold inward, causing the entire amygdala to rotate in a ventromedial direction. The extent of this rotation is much more dramatic in higher primate species compared to rodents, owing to the greater expansion of the temporal cortex (Jerison, 1973; Johnston, 1923). This difference in degree of rotation explains why much of the PL resides in a ventral position in humans and Macaques, while it is situated in a more lateral position in lower animals.
6. Functional connectivity of the PL
Because it is a thin laminar structure that is difficult to target, the full scope of PL connections is not yet known. The primate PL projects to the CeN and to the Bi, as well as to the shell of the ventral striatum based on available retrograde studies (Amaral and Insausti, 1992; Fudge, 2002; Fudge and Tucker, 2009). Future studies using small injections of anterograde tracers are needed to determine the full extent of efferent projections from the PL.
With regards to its major afferent inputs, the primate PL receives converging projections from the lateral nucleus of the amygdala and from the hippocampus. Polysensory information is channeled via in the ventral subdivision of the lateral nucleus, the main intra-amygydaloid input to the PL (Pitkanen and Amaral, 1998). Hippocampal inputs arising from the rostral CA1 and subiculum are also an important input (deCampo et al., 2009; Rosene and Van Hoesen, 1977; Saunders et al., 1988). These latter projections terminate in a relatively discrete pattern over the PL and its contiguous transition zones, in contrast to the situation in rodents, where the hippocampus broadly targets several amygdaloid nuclei including the basal, lateral, and central nucleus (Canteras and Swanson, 1992).
Hippocampal input to the amygdala is essential for the phenomenon of ‘contextual fear learning’ (Phillips and LeDoux, 1992), a finding supported by human fMRI studies (Grillon et al., 2006). In this form of learning, a negative stimulus becomes associated with the physical features of the surrounding environment, so that future exposure to the environment can itself elicit the fear response (Phillips and LeDoux, 1992). The anatomic circuit underlying this phenomena in primates thus may involve hippocampal inputs to the PL and its contiguous transition regions such as the CTA and AHA. The fact that the ventral subdivision of the lateral nucleus, which processes complex polysensory information, also projects to the PL, lends support to the PL’s potential role in contextual learning.
7. Immunohistochemical profile of the PL
Given the variations in anatomy and nomenclature, it is easy to understand why at present, there are no existing reports focusing on the PL. Instead, knowledge about the PL is solely derived from more general studies that examine the amygdala as a whole. Here we provide a brief summary of the distribution of several neurotransmitters, receptors and proteins in the PL and adjacent CTA and AHA transition areas in human and nonhuman primates, based on available literature (Table 2) (see De Olmos for an excellent review, De Olmos, 2004).
Table 2.
Markers in the Human and non-Human Primate PL
| CTA | Medial PL | Lateral PL | CTA | Medial PL | Lateral PL | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cells | Fibers | Cells | Fibers | Cells | Fibers | Autoradiography | |||
| GABA | ++ | ++ | + | ||||||
| Zinc | ++++ | ++++ | +++ | ||||||
| Calcium binding proteins | |||||||||
| Calbindin | +++ | +++ | +/++ | +++ | +/++ | +++ | |||
| Parvalbumin | − /+ | −/+ | − | − | − | − | |||
| Calretinin | ++ | +++ | ++ | ++ | + | +++ | |||
| Neuropeptides | |||||||||
| Neurotensisn | − | ++ | − | ++ | − | ++ | |||
| Somatostatin | ++++ | ++++ | ++ | ++++ | + | ++++ | |||
| Neuropeptide Y | +++ | +++ | ++ | +++ | ++ | +++ | |||
| Monoamines | |||||||||
| Dopamine | +++ | ++++ | ++++ | ||||||
| NE | ++ | + | + | + | + | + | |||
| 5HT/5HTT | ++++ | +++ | +++ | ||||||
| Receptors | |||||||||
| ERα | ++/+++ | + | + | ||||||
| ERβ | +/++ | −/+ | −/+ | ||||||
| Androgen | ++++ | + | + | ++ | + | + | |||
| CRF | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | |||
| BZD | ++++ | ++++ | ++++ | ||||||
| μ opiate | ++++ | ++++ | ++++ | ||||||
| Immature Neuronal Markers | |||||||||
| Class III beta tubulin |
|||||||||
| PSA-NCAM | ++++ | ++++ | +++ | ||||||
| Doublecortin | ++++ | ++++ | +++ | ||||||
| Phosphorylat ed CREB |
|||||||||
| Bcl-2 | ++++ | ++++ | +++ | ||||||
− negligible, + low, ++ moderate, +++ high, ++++ very high
7.1 Inhibitory and excitatory neurotransmitters
The primate PL has low numbers of GABAergic neurons compared to other amygdala regions, as indicated by immunohistochemistry with glutamic acid decarboxylase (GAD) and in-situ hybridization studies (Pitkanen and Amaral, 1994). GAD-positive cell bodies in the PL are oriented in a medial-lateral pattern with the majority of labeled cells found in the medial half. The highest numbers of cell bodies immunoreactive for GAD are present in the two PL transition areas, the CTA and AHA (Pitkanen and Amaral, 1994).
In contrast, excitatory amino acids can be found in relatively high concentrations in the PL and are suggestive of the presence of excitatory neurons within this region (Amaral and Insausti, 1992). These findings are based on a study using injections of D-[3H]-aspartate, a retrograde tracer that is selectively taken up by cells expressing glutamate and aspartate (Streit, 1980). Retrograde labeling with this compound following injections into the intermediate subdivision of the basal nucleus in monkeys demonstrates a high concentration of putative excitatory projection neurons uniformly distributed throughout the PL (Amaral and Insausti, 1992).
A high density of staining for zinc (Zn) in the PL suggests the presence of excitatory terminals. Generally, Zn is stored in synaptic terminals of excitatory neurons, where it may play a role in plasticity and learning (Nakashima and Dyck, 2009; Takeda et al., 2010). Zn is distributed in the amygdala along an increasing dorsal to ventral gradient, and it is striking that the PL contains the highest concentrations of Zn in the amygdala (Ichinohe and Rockland, 2005).
7.2 Calcium binding proteins
The calcium binding proteins, parvalbumin (PV), calbindin (CB) and calretinin (CR) are found in different subpopulations of interneurons, and are often used as markers of unique types of interneurons in the cortex (Baimbridge et al., 1992). It is commonly held that calcium-binding proteins act as buffers against high internal calcium concentrations, possibly conferring resistance to excitotoxic cell death (Sloviter, 1989; Weiss et al., 1990), although the specific role associated with individual binding proteins is not known.
PV-immunoreactive neurons and fibers are negligible in the human PL, CTA and AHA (Sorvari et al., 1995). Monkeys display a similar pattern in the PL (Pitkanen and Amaral, 1993b), however unlike humans, the monkey CTA and AHA contain relatively higher numbers of PV-cells and fibers, although these numbers are still low to moderate compared to the rest of the amygdala.
The calcium binding proteins calbindin (CB) and calretinin (CR) exist in relatively higher levels in both human and monkey PL compared to parvalbumin (see Fig. 4A for CB-positive cell distribution). In general, CB- and CR-immunoreactive neurons increase in density along the medial half of the PL and are abundant in the CTA and AHA transition areas (although CB-cell populations are somewhat lower in humans) (McDonald, 1994; Pitkanen and Amaral, 1993a; Sorvari et al., 1996a). CB-immunoreactive fiber distribution in the human PL is moderate, with heavier innervation occurring medially (Sorvari et al., 1996a). CR fiber distribution in the human parallels that of CB, however, precise details of CR fiber distribution in the monkey PL has not been reported (Sorvari et al., 1996b).
Figure 4.
A-C). Select histologic features of the non-human primate paralaminar nucleus (PL). A). Photomicrograph of calbindin-immunoreactive cells in the PL. Note the increased density of cells along the medial half of the PL (mPL). Scale Bar = 250 μm B). Distribution of serotonin transporter (5HTT) positive fibers in the PL, with higher densities in the medial half. 5HT-1A labeled cells are also present in this region of the PL as shown in the boxed inset (scale bar for inset = 25 μm.) C). A relatively high density of tyrosine hydroxylase (TH) positive fibers concentrated over the PL, compared to the Bpc. Scale bars = 250 μm. Bpc = basal nucleus, parvicellular division; lPL = lateral paralaminar nucleus; LV = lateral ventricle; lPL= lateral paralaminar nucleus; mPL = medial paralaminar nucleus.
7.3 Monoamines
7.3.1 Serotonin
Serotonin (5-hydroxytryptamine, 5-HT) participates in the modulation of amygdala circuits (Rainnie, 1999; Stutzmann et al., 1998; Wang and Aghajanian, 1977), as well as in maturation and development of immature neurons (review, Gaspar et al., 2003 and Whitaker-Azmitia, 2001). The PL contains one of the highest densities of 5-HT immunoreactive fibers in the amygdala (Bauman and Amaral, 2005). This extensive 5-HT innervation exhibits an increasing rostral-to-caudal and lateral-to-medial gradient, with the most dense innervation existing in the medial portions of the caudal PL. Fibers labeled for the serotonin transporter (5-HTT) follow a similar pattern of innervation (Fig. 4B) (O’Rourke and Fudge, 2006).
The human PL also contains one of highest binding densities of 5-HT1A receptors in the amygdala based on binding studies using [3H]8-OH-DPAT (Pazos et al., 1987). While the 5HT1A receptor is important in the regulation of emotional states, localization of the receptor protein at a cellular level has been notoriously difficult (Azmitia et al., l996). Using a generous gift of 5HT1A receptor antibody (amino acids 176-180, gift of Dr. E.C. Azmitia) characterized in the primate (Azmitia et al., l996), we were able to detect cellular labeling in the PL with good specificity. Our data in the Macaque show that 5HT1A immunoreactive cells are aggregated in the PL and adjacent corticoamygdaloid transition area (Fig. 4B, inset), similar to the distribution seen in ligand binding studies in human (Pazos et al., 1987). Interestingly, this pattern of distribution may not exist in lower species (Flugge et al., 1994). 5HT-1C receptors exhibit intermediate binding densities in the human PL when labeled with [3H]mesulergine (Hoyer et al., 1986b). Unlike 5-HT1A and 5-HT1C, 5-HT1B receptors are not found in the human amygdala through autoradiographic techniques (Hoyer et al., 1986a; Pazos et al., 1987). Information on the distribution of other 5-HT1 subtypes in the PL is lacking.
7.3.2 Dopamine
Immunocytochemical studies using antibodies to tyrosine hydroxylase (TH), the enzyme that catalyzes the synthesis of the dopamine precursor DOPA, indicate that dense TH immunoreactive fibers overlap the region of the caudal PL in both human and Macaque (Akil and Lewis, 1993; Akil and Lewis, 1994). Our personal observations are consistent with this finding, showing tight overlap of TH-positive fibers in the monkey PL (Fig. 4C). A study in the squirrel monkey (which occupies a different suborder than the Macaque) shows high TH-positive fiber densities in a region that may encompass the lateral PL, although the PL was not delineated in this study. Immunohistochemical localization of the dopamine transporter (DAT) indicates that there are many DAT+ fibers in the macaque ‘amygdalopiriform’ zone (CTA), which merges with the PL (although the PL was not specifically examined) (Ciliax et al., 1999; Smith and Porrino, 2008). In our material, there are no DAT-positive fibers in the Macaque PL or CTA (personal observations, not shown). Interpretation of results from DAT radioligand binding studies are complicated by the fact that the PL is not specifically delineated (Smith and Porrino, 2008). While several authors have reported moderate to high levels of DAT binding in the region of the PL (Klimek et al., 2002; Little et al., 1995; Staley et al., 1994), others have found negligible to background levels (Kaufman et al., 1991; Smith and Porrino, 2008; Staley et al., 1994; Staley et al., 1995).
7.3.3 Norepinephrine
Dopamine beta hydroxylase (DBH), the enzyme that converts dopamine to norepinephrine (NE), has been used to map NE-positive fibers in the squirrel monkey, although the PL was not delineated (Sadikot and Parent, 1990). Judging from the distribution of NE-positive fibers, there is a light innervation by DBH immunoreactive fibers in the region associated with the PL, with a slightly heavier distribution occurring medially (Sadikot and Parent, 1990). The distribution of NE in the Macaque has only been described using [3H]nisoxetine, a selective ligand for the NE transporter; levels in the PL appear low to moderate, although specific reference to the PL was not included (Smith et al., 2006). With regards to NE receptors in the PL, an autoradiographic study in the tree shrew reveals that the PL contains a high number of binding sites for α1 and α2 receptors and a low number of β binding sites (Flugge et al., 1994). However, because the PL in the tree shrew shows several discontinuities with Macaque and human, it is difficult to translate these findings.
7.4 Neuropeptides
The amygdala contains numerous neuropeptides that are involved in modulation of emotional states, particularly anxiety. The distribution of several neuropeptides, including somatostatin (SST), neurotensin (NT), neuropeptide Y (NPY), and receptors for corticotropin releasing factor (CRF1) have been noted in several descriptions of the primate amygdala. Studies that include information about the PL are reviewed below. Other peptides including vasoactive intestinal peptide (VIP), enkephalins, cholecystokinin, and galanin, which are present in the amygdala (Price et al., 1987; Roberts et al., 1983), are not included, since at present almost nothing is known about their organization in the primate PL.
7.4.1 Neurotensin
NT is a peptide that has been closely linked to dopamine system dynamics based on evidence that stress increases NT content in dopaminergic cells (Burgevin et al., 1992; Kilts et al., 1992). In general, NT immunoreactivity in the PL is due to a moderate density of coarse and fine varicose fibers that are uniform throughout both medial and lateral divisions (Benzing et al., 1992; Mai et al., 1987). Interestingly, this distribution is similar to the pattern of TH-positive fibers in the PL (reviewed above), suggesting possible co-localization. NT cell bodies in this region are negligible (Mai et al., 1987; Zech et al., 1986)
7.4.2 Somatostatin
SST is found in developing neurons (Le Verche et al., 2009; Yacubova and Komuro, 2002) and is also a potent neuromodulator (Epelbaum et al., 1994). In the monkey PL, immunoreactivity for biologically active SST peptides, SS14 and SS28, is found in both cell bodies and fibers in moderate to high densities, particularly in caudal sections of the PL (Amaral et al., 1989; McDonald et al., 1995). Although there are sparse numbers of SST-positive cells present in the lateral half of the PL, increasingly high concentrations are seen in the medial PL and CTA regions. SST-containing fibers are uniformly distributed throughout medial and lateral PL in moderate to high densities (Amaral et al., 1989; McDonald et al., 1995).
7.4.3 Neuropeptide Y
Like SST, NPY plays a role in neurodevelopmental processes and plasticity (Hansel et al., 2001; Real et al., 2009; Sajdyk et al., 2008; Uylings and Delalle, 1997), and the two peptides are frequently co-localized (McDonald et al., 1995; Schwartzberg et al., 1990). NPY reduces anxiety-like behavior via post-synaptic amygdala receptors in adult rodents (Heilig et al., 1993; Tasan et al., 2010). The PL contains moderate densities of NPY-immunoreactive neurons, particularly in the medial portions (McDonald et al., 1995; Walter et al., 1990; Zech et al., 1986). NPY fiber density has been reported to parallel that of SST-positive fibers, with dense innervation along the length of the PL (McDonald et al., 1995).
7.5 Hormones
7.5.1 Estrogen receptors
Estrogen is now understood to have a widespread influence on central nervous system plasticity in addition to its role in neural signaling and reproductive function (review, Tobet et al., 2009 and Taber et al., 2001). Early radioligand studies with [3H] estradiol, report strong binding densities in the periamygdaloid cortex region of the amygdala (Bonsall et al., 1986; Pfaff et al., 1976). However, these early studies do not distinguish between ERα and ERβ isoforms, as these isoforms had not yet been discovered.
Several reports have now characterized the distribution of ERα and ERβ receptor mRNA and proteins in the human and monkey amygdala (Gundlah et al., 2000; Osterlund and Hurd, 2001; Osterlund et al., 2000; Perlman et al., 2004). Comparison of mRNA transcripts indicates that the amygdala contains higher levels of ERα expression with lower levels of ERβ receptor mRNA (Osterlund and Hurd, 2001; Osterlund et al., 2000). Judging from the distribution of silver grains, ERα receptor mRNA in the PL appears low, although CTA and AHA transition zones exhibit moderate to high hybridization signals (Osterlund and Hurd, 2001; Perlman et al., 2004). Closer examination with counter-staining to delineate the PL, CTA, and AHA is necessary to understand these relationships. Consistent with mRNA data, immunhistochemical findings suggest that the region encompassing the PL contains low to moderate concentrations of ERα labeled cells, but that there are relatively high numbers of labeled cells in the periamygdaloid cortex and AHA (Blurton-Jones et al., 1999). ERβ receptor mRNA and binding in the amygdala overall has been described as ‘weak’; however nuclear-specific labeling is heterogeneous with at least moderate levels of mRNA in the region of the monkey PL (Gundlah et al., 2000; Osterlund and Hurd, 2001).
7.5.2 Androgen receptors
Mapping of testosterone receptors in monkeys using tritiated dihydrotestosterone (DHT) (Michael and Rees, 1982), and androgen receptor mRNA (Roselli et al., 2001) have not specifically addressed the PL. However, images from these studies suggest that androgen receptor levels, is negligible. In contrast, the CTA/AHA transition zones contain relatively higher levels of androgen receptor, although the precise concentration varies depending on methodology. For example, autoradiographic methods show relatively weak binding in the CTA region, while in-situ hybridization studies show higher message levels in the PACs and AHA areas (Michael and Rees, 1982; Roselli et al., 2001). Immunocytochemical data support mRNA findings, showing a higher density of androgen-receptor positive cells in the PACs and AHA (Michael et al., 1995). Based on in-situ hybridization and immunocytochemical data, therefore, the distribution of androgen receptor appears to roughly parallel the distribution of ERα receptor mRNA (above) with low levels in the PL, but relatively high levels in the transition region of the CTA/AHA.
7.6 Other receptors
7.6.1 Corticotropin releasing factor receptor 1(CRFR1)
Corticotropin releasing factor (CRF) is important in stress responses not only in the well-known hypophyseal-pituitary-adrenal axis, but also throughout the brain. Overexpression of CRF at specific developmental time points results in persistent depressive and anxiogenic phenotypes (Kolber et al., 2010). Conversely disruption of CRF 1 receptor, the main receptor for CRF in the forebrain, decreases anxiety-like behaviors (Muller et al., 2003). The primate PL is remarkable for its exceptionally high density of CRF1 receptors (Sanchez et al., 1999). Both autoradiography and in-situ hybridization studies indicate that the PL contains the highest densities of type 1 CRF receptors relative to all other amygdaloid nuclei. In contrast, the concentration of type 2 CRF receptors in the PL is low (Sanchez et al., 1999).
7.6.2 Glucocorticoids: Glucocorticoid receptor (GR) and Mineralcorticoid receptor (MR)
Despite numerous reports characterizing the function of glucocorticoids in the amygdala, detailed mapping studies on the receptors GR and MR in the human and monkey amygdala are surprisingly scarce. Sanchez notes relatively low levels of GR and MR messenger RNA and protein in the monkey amygdala overall (Sanchez et al., 2000). An autoradiographic study in the human by Sarrieau indicates that the amygdala contains a high concentration of GR based on ligand binding with the synthetic steroid RU38486; however, localization of these receptors to individual subnuclei was not performed and only one section in the amygdala was shown (Sarrieau et al., 1986). Nonetheless binding levels in the region encompassing the caudal PL appear high based on this single image.
7.6.3 Benzodiazepine Receptors
Benzodiazepines are well known for their anxiolytic actions and the therapeutic role they play in mood and anxiety disorders. Benzodiazepines act on allosteric binding sites on GABA-A receptors, and when GABA is also bound, enhance inhibition (Pritchett et al., 1989). Human autoradiographic studies using [3H]flunitrazepam, a ligand that binds to this allosteric site, indicate that the PL has high binding densities for the benzodiazpine site (Niehoff and Whitehouse, 1983; Zezula et al., 1988). According to Zezula (Zezula et al., 1988), the PL, along with the intercalated islands and lateral nucleus, exhibits one of highest binding densities in the forebrain.
7.6.4 Opiates: μ receptor
The PL also contains one of the highest concentrations of μ opiate receptors in the primate brain, as indicated by autoradiographic studies using [3H]-DAMGO to measure μ receptor binding, and [35S]GTPγS to measure G protein activation (Daunais et al., 2001; LaMotte et al., 1978; review, Mansour et al., 1988). In a detailed study by Daunais, relatively high levels of [3H]-DAMGO binding in the PL reached their greatest levels in the medial PL. There was also high binding in the overlying Bpc, with little differentiation between it and the PL (Daunais et al., 2001).
In summary, available evidence indicates that, compared to the amygdala as a whole, the PL contains a relatively strong innervation by the dopamine and serotonin system, and contains relatively high concentrations of cells and fibers for selected neuropeptides (NT, NPY, and SST). Neurons that are modulated by gonadal steroid receptors, and receptors involved in responses to CRF, serotonin, benzodiazepines, and endogenous opiates are also high in the PL.
8. Immature cells in the amygdala: a unique population in the PL
In addition to its unique cytoarchitectonics and diverse histochemical properties, the primate PL stands apart from the other amygdala nuclei in containing a subpopulation of immature-appearing neurons (Bernier et al., 2002; Bernier and Parent, 1998; Fudge, 2004; Yachnis et al., 2000; Zhang et al., 2009). In both the human and monkey, dense collections of small cells with thin leading processes, or leading and trailing processes, are found. Similar-sized cells, which have very thin processes and few dendrites, have been previously noted in Golgi studies (Braak and Braak, 1983; Millhouse, 1986; Tosevski et al., 2002). Immature-appearing cells in our material visualized with immunohistochemical markers found in postmitotic neurons, are similar in size and morphology to the small bipolar nonpyramidal cells previously described in human PL (Millhouse, 1986; Tosevski et al., 2002; Ulfig et al., 1999).
In humans and monkeys, immature-appearing cells in the PL contain protein markers typical of immature neurons such as class III beta-tubulin (TUJ1), doublecortin (DCX), and polysialyated neural cell adhesion molecule (PSA-NCAM) (Bernier et al., 2002; Bernier and Parent, 1998; deCampo et al., 2009; Draberova et al., 1998; Fudge, 2004; Meyer et al., 2002; Seki and Arai, 1993; Yachnis et al., 2000; Zhang et al., 2009). In one study examining neural proliferation using bromodeoxyuracil (BrdU) labeling in the primate, some of these cells were noted to have recently divided (Bernier et al., 2000). While the idea of neurogenesis outside the subgranular zone of the hippocampus or subventricular zone is controversial, some evidence supports this idea (reviews, Gould, 2007 and Rakic, 2002). As noted above, much of the primate PL does indeed occupy the region directly apposed to the temporal horn of the lateral ventricle (Fig. 1C, D), and in fact, occupies the position of the former ganglionic eminence (Fig. 3). This anatomic position makes it plausible that proliferating cells arising in the caudal subventricular zone might migrate into the amygdala. An alternative possibility is that immature neurons in the adult PL arise much earlier, perhaps during fetal life, and persist in an immature form post-natally, similar to Cajal-Retzius cells (Marin-Padilla, 1998; Meyer et al., 1999). This latter idea was advanced early on by Johnston (Johnston, 1923), and then by Sanides, who noted an abundance of immature-appearing neurons in the human amygdala (Sanides and Sas, 1970).
In our own material DCX, class III beta-tubulin, and PSA-NCAM, are co-localized in immature-appearing neurons in adult animals (deCampo et al., 2009) (Fig. 5A-D), although they decline in senescence (unpublished observations). Immature-appearing neurons also strongly express bcl-2 protein (Fudge, 2004) and its activated transcription factor, phosphorylated cyclic AMP response element binding protein (pCREB) (Fig. 6). Bcl-2 is significant in young neurons because it influences their rate of neuronal differentiation, including the timing at which neuron-specific markers are expressed and the rate of neurite extension (Middleton et al., 1998; Suzuki and Tsutomi, 1998; Zhang et al., 1996).
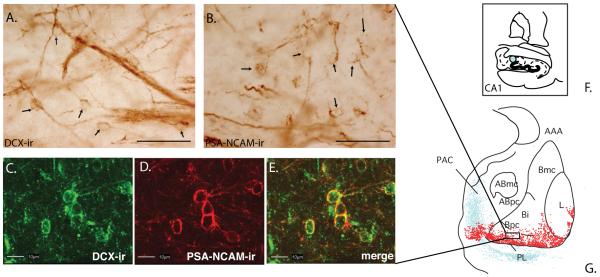
Figure 5.
A-B). Arrows pointing to DCX A) and polysialylated-neural cell adhesion molecule PSA-NCAM B) positive cells and their processes in the paralaminar nucleus (PL). Scale bars = 50 μm. C-E). Double-immunofluorescent labeling for DCX-immunoreactivity (green) and PSA-NCAM immunoreactivity (red) in the PL. Labeling can be seen in thin cytoplasmic rims and processes. Scale bar = 10 μm. F-G). Schematic of an anterograde tracer injection into the CA1 region of the hippocampus, resulting in labeled fibers (blue) in the PAC and medial PL, where a high density of DCX-labeled cells (red) are found. AAA= anterior amygdaloid area; ABmc = accessory basal nucleus, magnocellular division; ABpc = accessory basal nucleus, parvicellular division; Bi = basal nucleus; intermediate division; bmc = Basal nucleus, magnocellular division; Bpc = basal nucleus, parvicellular division; DCX = doublecortin; L = lateral nucleus; PAC = periamygdaloid cortex; PSA-NCAM = polysialylated-neural cell adhesion molecule.
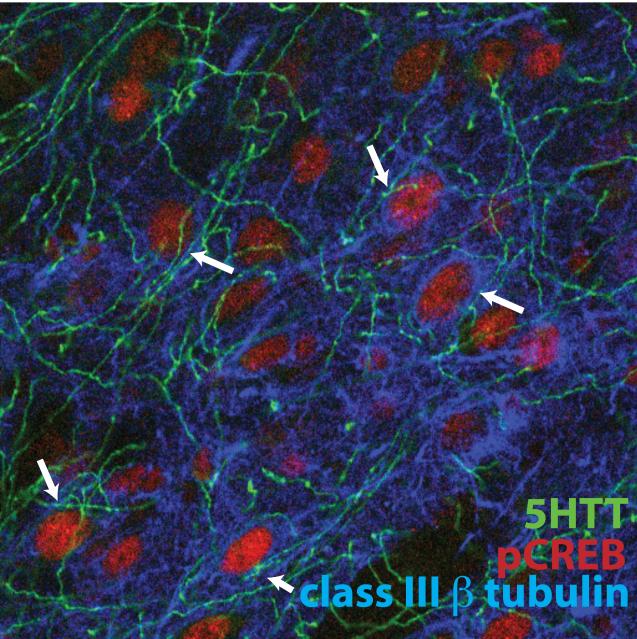
Figure 6.
Confocal image of cells in the PL, triple labeled for serotonin transporter (5HTT, green), class III/beta-tubulin (blue), and phosphorylated (activated) cAMP response element binding protein (pCREB). Immature-appearing cells containing class III-beta tubulin-positive cells have pCREB-labeled nuclei (arrows) and are surrounded by 5HTT fibers. (100x oil immersion).
The PL, with its immature neuronal component, is located at the crossroads of circuitry involved in contextual conditioning as mentioned earlier. Its main afferent inputs appear relatively specific, carrying information about temporal and spatial information from the past (via hippocampus) (Fig. 5F, G) that converges with polymodal sensory information occurring in the present (via the ventral lateral nucleus). This arrangement suggests that excitatory signals involved in the process of contextual learning or memory formation may shape the fate of immature neurons. In regions such as the dentate gyrus of the hippocampus, activity-dependent processes such as differentiation, axon and dendrite remodeling, and migration are mediated through activation of intracellular signaling pathways in immature neurons (reviews, Balu and Lucki, 2009; Kempermann et al., 2004).
Immature neurons in brain regions such as the dentate gyrus have also recently received much attention as substrates of mood and anxiety disorders, in the ‘neurotrophic hypothesis’ (Duman et al., 2000). This hypothesis may provide some insights into the role of immature-appearing neurons in the PL. Stress depletes, and antidepressant treatments support, the proliferation and growth of new neurons in the dentate gyrus by altering intracellular signaling pathways involving cyclic AMP response element binding protein (CREB), which in turn regulates processes including migration, differentiation, and synaptogenesis (Duman et al., 2000; Kempermann et al., 2004; Santarelli et al., 2003). We have found that immature-appearing cells in the monkey PL contain the activated form of the transcription factor CREB (Fig. 6), as occurs in the postmitotic cells of the dentate gyrus. While these findings are clearly only circumstantial evidence at present, they suggest CREB pathways may support growth processes in immature-appearing cells in the primate PL, as in they do in the dentate gyrus. Furthermore, the PL is also richly innervated by serotonin-containing fibers (Bauman and Amaral, 2005; O’Rourke and Fudge, 2006), as are the immature neurons of the dentate gyrus. Future studies are needed to examine whether serotonin manipulation affects the density of immature-appearing PL neurons, and whether it can induce morphologic changes, as it does in established neurogenetic zones (Banasr et al., 2004; Brezun and Daszuta, 1999; Wang et al., 2008).
9. Conclusions
This review provides an overview of what is currently known about the PL in humans and nonhuman primates. Due to frequent changes in its names and borders, information on this nucleus has been scarce. However, the PL represents a distinct region of the amygdala complex with unique connections as well as a wealth of proteins and neurotransmitters that have been implicated in the pathology of the mood and anxiety disorders. The PL also contains a population of immature-appearing cells. Their position in the PL, at the intersection of projections involved in contextual fear learning, may suggest new ways to understand the structural and functional changes that take place in these disorders.
Highlights.
▪ paralaminar nucleus is a subnucleus of the amygdala
▪ paralaminar nucleus is particularly prominent in human and non-human primates
▪ High densities of receptors and proteins linked to mood disorders are present in this structure
▪ Immature appearing cells in the paralaminar nucleus may be important in plastcitiy mediated processes
ACKNOWLEDGEMENTS
The authors thank Dr. Efrain Azmitia for his generous gift of 5HT1A receptor antibody. We also gratefully acknowledge Dr. Linda Callahan for assistance with confocal microscopy. Funding support was received from the University of Rochester Medical Scientist Training Program NIH T32 GM07356 (DD) and MH R0163291 (JF). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or NIH.
ABBREVIATIONS
- AAA
anterior amygdaloid area
- AB
accessory basal nucleus
- ABs
accessory basal nucleus, sulcal subdivision
- ABmc
accessory basal nucleus, magnocellular subdivision
- ABpc
accessory basal nucleus, parvicellular subdivision
- AChE
acetylcholinesterase
- AHA
amygdalohippocampal area
- Astr
amygdalostriatal area
- BDNF
brain-derived neurotrophic factor
- BDZ
benzodiazepine receptor
- Bi
basal nucleus, intermediate subdivision
- Bmc
basal nucleus, magnocellular subdivision
- Bpc
basal nucleus, parvicellular subdivision
- BrdU
bromodeoxyuracil analog
- CB
calbindin
- CeLcn
central nucleus, lateral core subdivision
- CeM
central nucleus, medial subdivision
- CeN
central nucleus
- CoA
anterior cortical nucleus
- CoP
posterior cortical nucleus
- CR
calretinin
- CRF
corticotropin releasing factor
- CTA
cortico-amygdaloid transition area
- DA
dopamine
- DAT
dopamine transporter
- DBH
dopamine beta hydroxylase
- DCX
doublecortin
- DHT
tritiated dihydrotestosterone
- EC
external capsule
- GAD
glutamic acid decarboxylase
- GE
ganglionic eminence
- GR
glucocorticoid receptor
- H
hippocampus
- IC
intercalated islands
- L
lateral nucleus
- LV
lateral ventricle
- M
medial nucleus
- MR
mineralcorticoid receptor
- NE
norepinephrine
- NPY
neuropeptide Y
- NT
neurotensin
- PAC
periamygdaloid cortex, subdivisions I-III
- PACs
periamygdaloid cortex, sulcal division
- pCREB
phosphorylated cyclic AMP response element binding protein
- PL
paralaminar nucleus
- PSA-NCAM
polysialylated neural cell adhesion marker
- PV
parvalbumin
- SST
somatostatin
- TH
tyrosine hydroxylase
- VIP
vasoactive intestinal peptide
- Zn
zinc
- 5-HT
serotonin
- 5-HTT
serotonin transporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- Adolphs R. What does the amygdala contribute to social cognition? Annals of the New York Academy of Sciences. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Burton MJ, Passingham RE. Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta) Brain Res. 1980;190:347–368. doi: 10.1016/0006-8993(80)90279-6. [DOI] [PubMed] [Google Scholar]
- Akil M, Lewis DA. The dopaminergic innervation of monkey entorhinal cortex. Cereb Cortex. 1993;3:533–550. doi: 10.1093/cercor/3.6.533. [DOI] [PubMed] [Google Scholar]
- Akil M, Lewis DA. The distribution of tyrosine hydroxylase-immunoreactive fibers in the human entorhinal cortex. Neuroscience. 1994;60:857–874. doi: 10.1016/0306-4522(94)90268-2. [DOI] [PubMed] [Google Scholar]
- Alheid GF. Extended amygdala and basal forebrain. Annals of the New York Academy of Sciences. 2003;985:185–205. doi: 10.1111/j.1749-6632.2003.tb07082.x. [DOI] [PubMed] [Google Scholar]
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Amano T, Unal CT, Pare D. Synaptic correlates of fear extinction in the amygdala. Nat Neurosci. 2010;13:489–494. doi: 10.1038/nn.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Avendano C, Benoit R. Distribution of somatostatin-like immunoreactivity in the monkey amygdala. J. Comp. Neurol. 1989;284:294–313. doi: 10.1002/cne.902840211. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Bassett JL. Cholinergic innervation of the monkey amygdala: An immunohistochemical analysis with antisera to choline acetyltransferase. J. Comp. Neurol. 1989;281:337–361. doi: 10.1002/cne.902810303. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Capitanio JP, Jourdain M, Mason WA, Mendoza SP, Prather M. The amygdala: is it an essential component of the neural network for social cognition? Neuropsychologia. 2003;41:235–240. doi: 10.1016/s0028-3932(02)00154-9. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Insausti R. Retrograde transport of D-[3H]-aspartate injected into the monkey amygdaloid complex. Experimental Brain Research. 1992;88:375–388. doi: 10.1007/BF02259113. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) J. Comp. Neurol. 1984;230:465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex, The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Wiley-Liss, Inc.; 1992. pp. 1–66. [Google Scholar]
- Bai J, Ramos RL, Paramasivam M, Siddiqi F, Ackman JB, LoTurco JJ. The role of DCX and LIS1 in migration through the lateral cortical stream of developing forebrain. Dev Neurosci. 2008;30:144–156. doi: 10.1159/000109859. [DOI] [PubMed] [Google Scholar]
- Baimbridge KG, Celio MR, Rogers JH. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992;15:303–308. doi: 10.1016/0166-2236(92)90081-i. [DOI] [PubMed] [Google Scholar]
- Balu DT, Lucki I. Adult hippocampal neurogenesis: regulation, functional implications, and contribution to disease pathology. Neurosci Biobehav Rev. 2009;33:232–252. doi: 10.1016/j.neubiorev.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Hery M, Printemps R, Daszuta A. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology. 2004;29:450–460. doi: 10.1038/sj.npp.1300320. [DOI] [PubMed] [Google Scholar]
- Barbas H, de Olmos J. Projections from the amygdala to basoventral and mediodorsal prefrontal regions in the Rhesus monkey. J. Comp. Neurol. 1990;300:549–571. doi: 10.1002/cne.903000409. [DOI] [PubMed] [Google Scholar]
- Bauman MD, Amaral DG. The distribution of serotonergic fibers in the macaque monkey amygdala: an immunohistochemical study using antisera to 5-hydroxytryptamine. Neuroscience. 2005;136:193–203. doi: 10.1016/j.neuroscience.2005.07.040. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nature reviews. Neuroscience. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Bayer SA. Quantitative 3H-thymidine radiographic analyses of neurogenesis in the rat amygdala. Journal of Comparative Neurology. 1980;194:845–875. doi: 10.1002/cne.901940409. [DOI] [PubMed] [Google Scholar]
- Benzing WC, Mufson EJ, Jennes L, Stopa EG, Armstrong DM. Distribution of neurotensin immunoreactivity within the human amygdaloid complex: a comparison with acetylcholinesterase- and Nissl-stained tissue sections. J Comp Neurol. 1992;317:283–297. doi: 10.1002/cne.903170306. [DOI] [PubMed] [Google Scholar]
- Bernier PJ, Bedard A, Vinet J, Levesque M, Parent A. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11464–11469. doi: 10.1073/pnas.172403999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier PJ, Parent A. Bcl-2 protein as a marker of neuronal immaturity in postnatal primate brain. Journal. Of Neuroscience. 1998 Apr. 118:2486–2497. doi: 10.1523/JNEUROSCI.18-07-02486.1998. 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier PJ, Vinet J, Cossette M, Parent A. Characterization of the subventricular zone of the adult human brain: evidence for the involvement of Bcl-2. Neuroscience Research - Supplement. 2000;37:67–78. doi: 10.1016/s0168-0102(00)00102-4. [DOI] [PubMed] [Google Scholar]
- Birling MC, Price J. A study of the potential of the embryonic rat telencephalon to generate oligodendrocytes. Dev Biol. 1998;193:100–113. doi: 10.1006/dbio.1997.8763. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. Innate and conditioned reaction to threat in rats with amygdaloid lesions. J. Comp. Physiol. Psychol. 1972;81:281–290. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- Blurton-Jones MM, Roberts JA, Tuszynski MH. Estrogen receptor immunoreactivity in the adult primate brain: neuronal distribution and association with p75, trkA, and choline acetyltransferase. J Comp Neurol. 1999;405:529–542. [PubMed] [Google Scholar]
- Bonsall RW, Rees HD, Michael RP. [3H]estradiol and its metabolites in the brain, pituitary gland, and reproductive tract of the male rhesus monkey. A combined autoradiographic and chromatographic study. Neuroendocrinology. 1986;43:98–109. doi: 10.1159/000124536. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuronal types in the basolateral amygdaloid nuclei of man. Brain Res Bull. 1983;11:349–365. doi: 10.1016/0361-9230(83)90171-5. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Brezun JM, Daszuta A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience. 1999;89:999–1002. doi: 10.1016/s0306-4522(98)00693-9. [DOI] [PubMed] [Google Scholar]
- Brockhaus H. Zur normalen aund pahologischen Anatomie des Mandelkerngebietes. J Psychol Neurol. 1938;49:1–136. [Google Scholar]
- Broks P, Young AW, Maratos EJ, Coffey PJ, Calder AJ, Isaac CL, Mayes AR, Hodges JR, Montaldi D, Cezayirli E, Roberts N, Hadley D. Face processing impairments after encephalitis: amygdala damage and recognition of fear. Neuropsychologia. 1998;36:59–70. doi: 10.1016/s0028-3932(97)00105-x. [DOI] [PubMed] [Google Scholar]
- Brothers L, Ring B. Mesial temporal neurons in the macaque monkey with responses selective for aspects of social stimuli. Behavioural brain research. 1993;57:53–61. doi: 10.1016/0166-4328(93)90061-t. [DOI] [PubMed] [Google Scholar]
- Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Burgevin M-C, Castel M-N, Quarteronet D, Chevet T, Laduron PM. Neurotensin increases tyrosine hydroxylase messenger RNA-positive neurons in substantia nigra after retrograde axonal transport. Neuroscience. 1992;49(3):627–633. doi: 10.1016/0306-4522(92)90232-q. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Swanson LW. Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: a PHAL anterograde tract-tracing study in the rat. Journal of Comparative Neurology. 1992;324:180–194. doi: 10.1002/cne.903240204. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Clugnet MC, Price JL. Central olfactory connections in the macaque monkey. J. Comp. Neurol. 1994;346:403–434. doi: 10.1002/cne.903460306. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. Journal of Comparative Neurology. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J. Comp. Neurol. 1996;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Freedman LJ, Shi C. The intrinsic organization of the central extended amygdala. Annals of the New York Academy of Sciences. 1999;877:217–241. doi: 10.1111/j.1749-6632.1999.tb09270.x. [DOI] [PubMed] [Google Scholar]
- Ciliax BJ, Drash GW, Staley JK, Haber S, Mobley CJ, Miller GW, Mufson EJ, Mash DC, Levey AI. Immunocytochemical localization of the dopamine transporter in human brain. Journal of Comparative Neurology. 1999;409:38–56. doi: 10.1002/(sici)1096-9861(19990621)409:1<38::aid-cne4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Cocas LA, Miyoshi G, Carney RS, Sousa VH, Hirata T, Jones KR, Fishell G, Huntsman MM, Corbin JG. Emx1-lineage progenitors differentially contribute to neural diversity in the striatum and amygdala. J Neurosci. 2009;29:15933–15946. doi: 10.1523/JNEUROSCI.2525-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby EC, Humphrey T. Studies of the vertebrate telencephalon: the nuclear pattern of the anterior olfactory nucleus, tuberculum olfactorium and the amygdaloid complex in adult man. Journal of Comparative Neurology. 1941;74:309–347. [Google Scholar]
- Culican SM, Baumrind NL, Yamamoto M, Pearlman AL. Cortical radial glia: identification in tissue culture and evidence for their transformation to astrocytes. J Neurosci. 1990;10:684–692. doi: 10.1523/JNEUROSCI.10-02-00684.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daunais JB, Letchworth SR, Sim-Selley LJ, Smith HR, Childers SR, Porrino LJ. Functional and anatomical localization of mu opioid receptors in the striatum, amygdala, and extended amygdala of the nonhuman primate. Journal of Comparative Neurology. 2001;433:471–485. doi: 10.1002/cne.1154. [DOI] [PubMed] [Google Scholar]
- De Olmos J. The Amygdala. In: Paxinos G, J. M, editors. The Human Nervous System. 2ed Elsevier Academic Press; San Diego: 2004. [Google Scholar]
- De Olmos JS. Amygdala. In: Paxinos G, editor. The Human Nervous System. Academic Press; San Diego: 1990. pp. 583–710. [Google Scholar]
- deCampo D, Becoats K, Fudge JL. Hippocampal inputs to immature neurons in the primate amygdala: novel substrate for plastic change. Society for Neuroscience; Chicago, IL: 2009. [Google Scholar]
- Dicks D, Myers RE, Kling A. Uncus and amiygdala lesions: effects on social behavior in the free-ranging rhesus monkey. Science. 1969;165:69–71. doi: 10.1126/science.165.3888.69. [DOI] [PubMed] [Google Scholar]
- Draberova E, Lukas Z, Ivanyi D, Viklicky V, Draber P. Expression of class III beta-tubulin in normal and neoplastic human tissues. Histochemistry & Cell Biology. 1998;109:231–239. doi: 10.1007/s004180050222. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Current Opinion in Neurobiology. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Nakagawa S, D’Sa C. Neuronal plasticity and survival in mood disorders. Biological Psychiatry. 2000;48:732–739. doi: 10.1016/s0006-3223(00)00935-5. [see comments.] [DOI] [PubMed] [Google Scholar]
- Emery NJ, Capitanio JP, Mason WA, Machado CJ, Mendoza SP, Amaral DG. The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 2001;115:515–544. [PubMed] [Google Scholar]
- Epelbaum J, Dournaud P, Fodor M, Viollet C. The neurobiology of somatostatin. Crit Rev Neurobiol. 1994;8:25–44. [PubMed] [Google Scholar]
- Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL. Beyond threat: amygdala reactivity across multiple expressions of facial affect. NeuroImage. 2006;30:1441–1448. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Flugge G, Ahrens O, Fuchs E. Monoamine receptors in the amygdaloid complex of the tree shrew (Tupaia belangeri) J Comp Neurol. 1994;343:597–608. doi: 10.1002/cne.903430409. [DOI] [PubMed] [Google Scholar]
- Freese JL, Amaral DG. Neuroanatomy of the Primate Amygdala. In: Whalen PJ, Phelps EA, editors. The Human Amygdala. Guilford Press; New York: 2009. pp. 3–42. [Google Scholar]
- Fudge J. Bcl-2 expression in specific subregions of the macaque temporal lobe. Soc for Neurosci Abstr. 2002 [Google Scholar]
- Fudge J, Haber S. Defining the caudoventral striatum in primates: cytoarchitectural and histochemical features. Journal of Neuroscience. 2002;22:10078–10082. doi: 10.1523/JNEUROSCI.22-23-10078.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JL. Bcl-2 immunoreactive neurons are differentially distributed in subregions of the amygdala and hippocampus of the adult macaque. Neuroscience. 2004;127:539–556. doi: 10.1016/j.neuroscience.2004.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JL, Tucker T. Amygdala Projections to Central Amygdaloid Nucleus Subdivisions and Transition Zones in the Primate. Neuroscience. 2009;159:819–841. doi: 10.1016/j.neuroscience.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Jacobsen KX, Hoistad M, Tinner B, Jansson A, Staines WA, Agnati LF. The dopamine D1 receptor-rich main and paracapsular intercalated nerve cell groups of the rat amygdala: relationship to the dopamine innervation. Neuroscience. 2003;119:733–746. doi: 10.1016/s0306-4522(03)00148-9. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nature Reviews Neuroscience. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Gloor P. The Amygdala, The Temporal Lobe and Limbic System. Oxford University Press; New York: 1997a. [Google Scholar]
- Gloor P. Comparative anatomy of the temporal lobe and of the limbic system, The Temporal Lobe and Limbic System. Oxford University Press; New York: 1997b. pp. 21–111. [Google Scholar]
- Gothard KM, Battaglia FP, Erickson CA, Spitler KM, Amaral DG. Neural responses to facial expression and face identity in the monkey amygdala. Journal of Neurophysiology. 2007;97:1671–1683. doi: 10.1152/jn.00714.2006. [DOI] [PubMed] [Google Scholar]
- Gould E. How widespread is adult neurogenesis in mammals? Nature Reviews Neuroscience. 2007;8:481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JM, Cornwell B, Johnson L. Context conditioning and behavioral avoidance in a virtual reality environment: effect of predictability. Biological Psychiatry. 2006;60:752–759. doi: 10.1016/j.biopsych.2006.03.072. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Kohama SG, Mirkes SJ, Garyfallou VT, Urbanski HF, Bethea CL. Distribution of estrogen receptor beta (ERbeta) mRNA in hypothalamus, midbrain and temporal lobe of spayed macaque: continued expression with hormone replacement. Brain Research. Molecular Brain Research. 2000;76:191–204. doi: 10.1016/s0006-8993(99)02475-0. [DOI] [PubMed] [Google Scholar]
- Hansel DE, Eipper BA, Ronnett GV. Neuropeptide Y functions as a neuroproliferative factor. Nature. 2001;410:940–944. doi: 10.1038/35073601. [DOI] [PubMed] [Google Scholar]
- Heilig M, McLeod S, Brot M, Heinrichs SC, Menzaghi F, Koob GF, Britton KT. Anxiolytic-like action of neuropeptide Y: mediation by Y1 receptors in amygdala, and dissociation from food intake effects. Neuropsychopharmacology. 1993;8:357–363. doi: 10.1038/npp.1993.35. [DOI] [PubMed] [Google Scholar]
- Heimer L, De Olmos JS, Alheid GF, Person J, Sakamoto N, Shinoda K, Marksteiner J, Switzer RC. The human basal forebrain. Part II. In: Bloom FE, Bjorkland A, Hokfelt T, editors. Handbook of Chemical Neuroanatomy. Elsevier; Amsterdam: 1999. pp. 57–226. [Google Scholar]
- Heit G, Smith ME, Halgren E. Neural encoding of individual words and faces by the human hippocampus and amygdala. Nature. 1988;333:773–775. doi: 10.1038/333773a0. [DOI] [PubMed] [Google Scholar]
- Hirata T, Li P, Lanuza GM, Cocas LA, Huntsman MM, Corbin JG. Identification of distinct telencephalic progenitor pools for neuronal diversity in the amygdala. Nat Neurosci. 2009;12:141–149. doi: 10.1038/nn.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D, Pazos A, Probst A, Palacios JM. Serotonin receptors in the human brain. I. Characterization and autoradiographic localization of 5-HT1A recognition sites. Apparent absence of 5-HT1B recognition sites. Brain Res. 1986a;376:85–96. doi: 10.1016/0006-8993(86)90902-9. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Pazos A, Probst A, Palacios JM. Serotonin receptors in the human brain. II. Characterization and autoradiographic localization of 5-HT1C and 5-HT2 recognition sites. Brain Res. 1986b;376:97–107. doi: 10.1016/0006-8993(86)90903-0. [DOI] [PubMed] [Google Scholar]
- Humphrey T. The telencephalon of the bat. J. Comp. Neurol. 1936;65:603–711. [Google Scholar]
- Humphrey T. The development of the human amygdala during early embryonic life. J. Comp. Neurol. 1968;132:135–166. doi: 10.1002/cne.901320108. [DOI] [PubMed] [Google Scholar]
- Ichinohe N, Rockland KS. Distribution of synaptic zinc in the macaque monkey amygdala. Journal of Comparative Neurology. 2005;489:135–147. doi: 10.1002/cne.20632. [DOI] [PubMed] [Google Scholar]
- Jerison HJ. Evolution of the Brain and Intelligence. Academic Press; New York: 1973. [Google Scholar]
- Jimenez-Castellanos J. The amygdaloid complex in monkey studies by reconstructional methods. J Comp Neurol. 1949;91:507–526. doi: 10.1002/cne.900910308. illust. [DOI] [PubMed] [Google Scholar]
- Johnston JB. Further contributions to the study of the evolution of the forebrain. J. Comp. Neuro. 1923;35:337–481. [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. Journal of Neuroscience. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal AM, Tombol T. Golgi studies on the amygdaloid nuclei of the cat. J Hirnforsch. 1975;16:175–201. [PubMed] [Google Scholar]
- Kaufman MJ, Spealman RD, Madras BK. Distribution of cocaine recognition sites in monkey brain: I. In vitro autoradiography with [3H]CFT. Synapse. 1991;9:177–187. doi: 10.1002/syn.890090304. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Aosaki T, Kubota Y. Cholinergic and GABAergic interneurons in the striatum. Nihon Shinkei Seishin Yakurigaku Zasshi. 1997;17:87–90. [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Coco ML, Ely TD, Bissette G, Nemeroff CB. Differential effects of conditioned and unconditioned stress on the neurotensin content of dopamine cell body groups of the ventral mesencephalon. Annals of the New York Academy of Sciences. 1992;668:266–276. doi: 10.1111/j.1749-6632.1992.tb27355.x. [DOI] [PubMed] [Google Scholar]
- Klimek V, Schenck JE, Han H, Stockmeier CA, Ordway GA. Dopaminergic abnormalities in amygdaloid nuclei in major depression: a postmortem study. Biological Psychiatry. 2002;52:740–748. doi: 10.1016/s0006-3223(02)01383-5. [DOI] [PubMed] [Google Scholar]
- Klüver H, Bucy PC. Preliminary analysis of functions of the temporal lobes in monkeys. Arch. Neurol. Psychiatry. 1939;42:979–997. doi: 10.1176/jnp.9.4.606. [DOI] [PubMed] [Google Scholar]
- Kolber BJ, Boyle MP, Wieczorek L, Kelley CL, Onwuzurike CC, Nettles SA, Vogt SK, Muglia LJ. Transient early-life forebrain corticotropin-releasing hormone elevation causes long-lasting anxiogenic and despair-like changes in mice. J Neurosci. 2010;30:2571–2581. doi: 10.1523/JNEUROSCI.4470-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Piecinski P, Rakic P. Neurogenesis of the amygdaloid nuclear complex in the rhesus monkey. Brain Research. Developmental Brain Research. 1992;68:9–15. doi: 10.1016/0165-3806(92)90242-o. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. A description of the amygdaloid complex in the rat and cat with observations on intra-amygdaloid axonal connections. Journal of Comparative Neurology. 1978;178:255–280. doi: 10.1002/cne.901780205. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- LaMotte CC, Snowman A, Pert CB, Synder SH. Opiate receptor binding in rhesus monkey brain: association with limbic structures. Brain Research. 1978;155:374–379. doi: 10.1016/0006-8993(78)91033-8. [DOI] [PubMed] [Google Scholar]
- Lauer EW. The nuclear pettern and fiber connections of certain basal telencephalic centers in the Macaque. The Journal of comparative Neurology. 1945;82:215–254. [Google Scholar]
- Le Verche V, Kaindl AM, Verney C, Csaba Z, Peineau S, Olivier P, Adle-Biassette H, Leterrier C, Vitalis T, Renaud J, Dargent B, Gressens P, Dournaud P. The somatostatin 2A receptor is enriched in migrating neurons during rat and human brain development and stimulates migration and axonal outgrowth. PLoS One. 2009;4:e5509. doi: 10.1371/journal.pone.0005509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual review of neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Rolls ET, Wilson FA, Baylis GC. Neurons in the amygdala of the monkey with responses selective for faces. Behavioural brain research. 1985;15:159–176. doi: 10.1016/0166-4328(85)90062-2. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [see comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little KY, Carroll FI, Cassin BJ. Characterization and localization of [125I]RTI-121 binding sites in human striatum and medial temporal lobe. J Pharmacol Exp Ther. 1995;274:1473–1483. [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2006;120:761–786. doi: 10.1037/0735-7044.120.4.761. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Emery NJ, Capitanio JP, Mason WA, Mendoza SP, Amaral DG. Bilateral neurotoxic amygdala lesions in rhesus monkeys (Macaca mulatta): consistent pattern of behavior across different social contexts. Behavioral neuroscience. 2008;122:251–266. doi: 10.1037/0735-7044.122.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai JK, Triepel J, Metz J. Neurotensin in the human brain. Neuroscience. 1987;22(No. 2):499–524. doi: 10.1016/0306-4522(87)90349-6. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Anatomy of CNS opioid receptors. Trends Neurosci. 1988;11:308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. Cajal-Retzius cells and the development of the neocortex. Trends in Neurosciences. 1998;21:64–71. doi: 10.1016/s0166-2236(97)01164-8. [DOI] [PubMed] [Google Scholar]
- Marowsky A, Yanagawa Y, Obata K, Vogt KE. A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron. 2005;48:1025–1037. doi: 10.1016/j.neuron.2005.10.029. [see comment] [DOI] [PubMed] [Google Scholar]
- Martin RD. Primate origins and evolution: a phylogenetic reconstruction. Princeton University Press; Princeton, NJ: 1990. [Google Scholar]
- McDonald AJ. Cytoarchitecture of the central amygdaloid nucleus of the rat. Journal of Comparative Neurology. 1982a;208:401–418. doi: 10.1002/cne.902080409. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Neurons of the lateral and basolateral amygdaloid nuclei: a Golgi study in the rat. J Comp Neurol. 1982b;212:293–312. doi: 10.1002/cne.902120307. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Calretinin immunoreactive neurons in the basolateral amygdala of the rat and monkey. Brain Res. 1994;667:238–242. doi: 10.1016/0006-8993(94)91501-6. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Augustine JR. Neuropeptide Y and somatostatin-like immunoreactivity in neurons of the monkey amygdala. Neuroscience. 1995;66:959–982. doi: 10.1016/0306-4522(94)00629-j. [DOI] [PubMed] [Google Scholar]
- Mehler WR. Subcortical afferent connections of the amygdala in the monkey. J. Comp. Neurol. 1980;190:733–762. doi: 10.1002/cne.901900407. [DOI] [PubMed] [Google Scholar]
- Meyer G, Goffinet AM, Fairen A. What is a Cajal-Retzius cell? A reassessment of a classical cell type based on recent observations in the developing neocortex. Cerebral Cortex. 1999;9:765–775. doi: 10.1093/cercor/9.8.765. [DOI] [PubMed] [Google Scholar]
- Meyer G, Perez-Garcia CG, Gleeson JG. Selective expression of doublecortin and LIS1 in developing human cortex suggests unique modes of neuronal movement. Cerebral Cortex. 2002;12:1225–1236. doi: 10.1093/cercor/12.12.1225. [DOI] [PubMed] [Google Scholar]
- Michael RP, Clancy AN, Zumpe D. Distribution of androgen receptor-like immunoreactivity in the brains of cynomolgus monkeys. J Neuroendocrinol. 1995;7:713–719. doi: 10.1111/j.1365-2826.1995.tb00813.x. [DOI] [PubMed] [Google Scholar]
- Michael RP, Rees HD. Autoradiographic localization of 3H-dihydrotestosterone in the preoptic area, hypothalamus, and amygdala of a male rhesus monkey. Life Sciences. 1982;30:2087–2093. doi: 10.1016/0024-3205(82)90450-7. [DOI] [PubMed] [Google Scholar]
- Middleton G, Pinon LG, Wyatt S, Davies AM. Bcl-2 accelerates the maturation of early sensory neurons. Journal of Neuroscience. 1998;18:3344–3350. doi: 10.1523/JNEUROSCI.18-09-03344.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millhouse OE. The intercalated cells of the amygdala. Journal of Comparative Neurology. 1986;247:246–271. doi: 10.1002/cne.902470209. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383(6603):812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Muller F, O’Rahilly R. The human brain at stages 21-23, with particular reference to the cerebral cortical plate and to the development of the cerebellum. Anatomy & Embryology. 1990;182:375–400. doi: 10.1007/BF02433497. [DOI] [PubMed] [Google Scholar]
- Muller MB, Zimmermann S, Sillaber I, Hagemeyer TP, Deussing JM, Timpl P, Kormann MS, Droste SK, Kuhn R, Reul JM, Holsboer F, Wurst W. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat Neurosci. 2003;6:1100–1107. doi: 10.1038/nn1123. [DOI] [PubMed] [Google Scholar]
- Nakashima AS, Dyck RH. Zinc and cortical plasticity. Brain Res Rev. 2009;59:347–373. doi: 10.1016/j.brainresrev.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Niehoff DL, Whitehouse PJ. Multiple benzodiazepine receptors: autoradiographic localization in normal human amygdala. Brain Res. 1983;276:237–245. doi: 10.1016/0006-8993(83)90730-8. [DOI] [PubMed] [Google Scholar]
- Nishijo H, Ono T, Nishino H. Single neuron responses in amygdala of alert monkey during complex sensory stimulation with affective significance. J. Neurosci. 1988;8:3570–3583. doi: 10.1523/JNEUROSCI.08-10-03570.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [see comment] [DOI] [PubMed] [Google Scholar]
- O’Rourke HJ, Fudge JL. Distribution of serotonin-labeled fibers in the primate amygdala: implications for mood disorders. Biological Psychiatry. 2006;60:479–490. doi: 10.1016/j.biopsych.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund MK, Hurd YL. Estrogen receptors in the human forebrain and the relation to neuropsychiatric disorders. Progress in Neurobiology. 2001;64:251–267. doi: 10.1016/s0301-0082(00)00059-9. [DOI] [PubMed] [Google Scholar]
- Osterlund MK, Keller E, Hurd YL. The human forebrain has discrete estrogen receptor alpha messenger RNA expression: high levels in the amygdaloid complex. Neuroscience. 2000;95:333–342. doi: 10.1016/s0306-4522(99)00443-1. [DOI] [PubMed] [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazos A, Probst A, Palacios JM. Serotonin receptors in the human brain--III. Autoradiographic mapping of serotonin-1 receptors. Neuroscience. 1987;21:97–122. doi: 10.1016/0306-4522(87)90326-5. [DOI] [PubMed] [Google Scholar]
- Perlman WR, Webster MJ, Kleinman JE, Weickert CS. Reduced glucocorticoid and estrogen receptor alpha messenger ribonucleic acid levels in the amygdala of patients with major mental illness. Biol Psychiatry. 2004;56:844–852. doi: 10.1016/j.biopsych.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Gerlach JL, McEwen BS, Ferin M, Carmel P, Zimmerman EA. Autoradiographic localization of hormone-concentrating cells in the brain of the female rhesus monkey. Journal of Comparative Neurology. 1976;170:279–293. doi: 10.1002/cne.901700302. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biological Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Amaral DG. Distribution of calbindin-D28k immunoreactivity in the monkey temporal lobe: The amygdaloid complex. J. Comp. Neurol. 1993a;331:199–224. doi: 10.1002/cne.903310205. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Amaral DG. Distribution of parvalbumin-immunoreactive cells and fibers in the monkey temporal lobe: the amygdaloid complex. J Comp Neurol. 1993b;331:14–36. doi: 10.1002/cne.903310103. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Amaral DG. The distribution of GABAergic cells, fibers, and terminals in the monkey amygdaloid complex: an immunohistochemical and in situ hybridization study. Journal of Neuroscience. 1994;14:2200–2224. doi: 10.1523/JNEUROSCI.14-04-02200.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A, Amaral DG. Organization of the intrinsic connections of the monkey amygdaloid complex: projections originating in the lateral nucleus. Journal of Comparative Neurology. 1998;398:431–458. doi: 10.1002/(sici)1096-9861(19980831)398:3<431::aid-cne9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Price J. Toward a consistent terminology for the amygdaloid complex. In: Ben-Ari Y, editor. The Amygdaloid Complex. Elsevier/North-Holland Biomedical Press; Amsterdam: 1981. pp. 13–19. [Google Scholar]
- Price JL, Russchen FT, Amaral DG. The limbic region. II. The amygdaloid complex. In: Hokfelt BT, Swanson LW, editors. Handbook of Chemical Neuroanatomy. Elsevier; Amsterdam: 1987. pp. 279–381. [Google Scholar]
- Pritchett DB, Sonthelmer H, Shivers BD. Importance of a novel GABA receptor subunit for benzodiazepine pharmacology. Nature. 1989;338:582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldstein M, Engelien W, Epstein J, Yang Y, Gorman J, LeDoux J, Silbersweig D, Stern E. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biological Psychiatry. 2005;57:464–473. doi: 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Rainnie DG. Serotonergic modulation of neurotransmission in the rat basolateral amygdala. Journal of Neurophysiology. 1999;82:69–85. doi: 10.1152/jn.1999.82.1.69. [DOI] [PubMed] [Google Scholar]
- Rakic P. Adult neurogenesis in mammals: an identity crisis. Journal of Neuroscience. 2002;22:614–618. doi: 10.1523/JNEUROSCI.22-03-00614.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramón y Cajal S. Histologie du systéme nerveux de l’homme and des vertébrés. Maloine; Paris: 1909. [Google Scholar]
- Real MA, Heredia R, Labrador Mdel C, Davila JC, Guirado S. Expression of somatostatin and neuropeptide Y in the embryonic, postnatal, and adult mouse amygdalar complex. J Comp Neurol. 2009;513:335–348. doi: 10.1002/cne.21970. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Zahm DS. Specificity in the projections of prefrontal and insular cortex to ventral striatopallidum and the extended amygdala. J Neurosci. 2005;25:11757–11767. doi: 10.1523/JNEUROSCI.3432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts GW, Ferrier IN, Lee Y, Crow TJ, Johnstone EC, Owens DG, Bacarese-Hamilton AJ, McGregor G, O’Shaughnessey D, Polak JM. Peptides, the limbic lobe and schizophrenia. Brain Research. 1983;288:199–211. doi: 10.1016/0006-8993(83)90095-1. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Neurons in the cortex of the temporal lobe and in the amygdala of the monkey with responses selective for faces. Human Neurobiology. 1984;3:209–222. [PubMed] [Google Scholar]
- Roselli CE, Klosterman S, Resko JA. Anatomic relationships between aromatase and androgen receptor mRNA expression in the hypothalamus and amygdala of adult male cynomolgus monkeys. Journal of Comparative Neurology. 2001;439:208–223. doi: 10.1002/cne.1343. [DOI] [PubMed] [Google Scholar]
- Rosene D, Van Hoesen G. The hippocampal formation of the primate brain. In: Jones E, Peters A, editors. Cerebral Cortex: Further Aspects of Cortical Function, Including HIppocampus. Plenum Press; New York: 1987. pp. 345–456. [Google Scholar]
- Rosene DL, Van Hoesen GW. Hippocampal efferents reach widespread areas of cerebral cortex and amygdala in the rhesus monkey. Science. 1977;198:315–317. doi: 10.1126/science.410102. [DOI] [PubMed] [Google Scholar]
- Rosvold HE, Mirsky AF, Pribram KH. Influence of amygdalectomy on social behavior in monkeys. J Comp Physiol Psychol. 1954;47:173–178. doi: 10.1037/h0058870. [DOI] [PubMed] [Google Scholar]
- Sadikot AF, Parent A. The monoaminergic innervation of the amygdala in the squirrel monkey: an immunohistochemical study. Neuroscience. 1990;36:431–447. doi: 10.1016/0306-4522(90)90439-b. [DOI] [PubMed] [Google Scholar]
- Sadock BJ, Sadock VA. Kaplan and Sadock’s Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry. 10th ed Lippincott Williams and Wilkens; Philadelphia: 2007. [Google Scholar]
- Sajdyk TJ, Johnson PL, Leitermann RJ, Fitz SD, Dietrich A, Morin M, Gehlert DR, Urban JH, Shekhar A. Neuropeptide Y in the amygdala induces long-term resilience to stress-induced reductions in social responses but not hypothalamic-adrenal-pituitary axis activity or hyperthermia. Journal of Neuroscience. 2008;28:893–903. doi: 10.1523/JNEUROSCI.0659-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM, Insel TR. Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. Journal of Comparative Neurology. 1999;408:365–377. [PubMed] [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM, Insel TR. Distribution of corticosteroid receptors in the rhesus brain: relative absence of glucocorticoid receptors in the hippocampal formation. Journal of Neuroscience. 2000;20:4657–4668. doi: 10.1523/JNEUROSCI.20-12-04657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanides F, Sas E. Persistence of horizontal cells of the Cajal foetal type and of the subpial granular layer in parts of the mammalian paleocortex. Z Mikrosk Anat Forsch. 1970;82:570–588. [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [see comment] [DOI] [PubMed] [Google Scholar]
- Sarrieau A, Dussaillant M, Agid F, Philibert D, Agid Y, Rostene W. Autoradiographic localization of glucocorticosteroid and progesterone binding sites in the human post-mortem brain. Journal of Steroid Biochemistry. 1986;25:717–721. doi: 10.1016/0022-4731(86)90300-6. [DOI] [PubMed] [Google Scholar]
- Saunders RC, Rosene DL, Van Hoesen GW. Comparison of the efferents of the amygdala and the hippocampal formation in the rhesus monkey: II. Reciprocal and non-reciprocal connections. J. Comp. Neurol. 1988;271:185–207. doi: 10.1002/cne.902710203. [DOI] [PubMed] [Google Scholar]
- Schmechel DE, Rakic P. Arrested proliferation of radial glial cells during midgestation in rhesus monkey. Nature. 1979;277:303–305. doi: 10.1038/277303a0. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Whalen PJ, McMullin KG, Rauch SL. Differential amygdalar response to novel versus newly familiar neutral faces: a functional MRI probe developed for studying inhibited temperament. Biological Psychiatry. 2003;53:854–862. doi: 10.1016/s0006-3223(02)01906-6. [DOI] [PubMed] [Google Scholar]
- Schwartzberg M, Unger J, Weindl A, Lange W. Distribution of neuropeptide Y in the prosencephalon of man and cotton-head tamarin (Saguinus oedipus): colocalization with somatostatin in neurons of striatum and amygdala. Anat Embryol (Berl) 1990;181:157–166. doi: 10.1007/BF00198955. [DOI] [PubMed] [Google Scholar]
- Seki T, Arai Y. Distribution and possible roles of the highly polysialylated neural cell adhesion molecule (NCAM-H) in the developing and adult central nervous system. Neuroscience Research. 1993;17:265–290. doi: 10.1016/0168-0102(93)90111-3. [DOI] [PubMed] [Google Scholar]
- Sims KS, Williams RS. The human amygdaloid complex: a cytologic and histochemical atlas using Nissl, myelin, acetylcholinesterase and nicotinamide adenine dinucleotide phosphate diaphorase staining. Neuroscience. 1990;36:449–472. doi: 10.1016/0306-4522(90)90440-f. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Calcium-binding protein (calbindin-D28k) and parvalbumin immunocytochemistry: localization in the rat hippocampus with specific reference to the selective vulnerability of hippocampal neurons to seizure activity. J Comp Neurol. 1989;280:183–196. doi: 10.1002/cne.902800203. [DOI] [PubMed] [Google Scholar]
- Smith HR, Beveridge TJ, Porrino LJ. Distribution of norepinephrine transporters in the non-human primate brain. Neuroscience. 2006;138:703–714. doi: 10.1016/j.neuroscience.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Smith HR, Porrino LJ. The comparative distributions of the monoamine transporters in the rodent, monkey, and human amygdala. Brain Structure & Function. 2008;213:73–91. doi: 10.1007/s00429-008-0176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorvari H, Soininen H, Paljärvi L, Karkola K, Pitkänen A. Distribution of parvalbumin-immunoreactive cells and fibers in the human amygdaloid complex. J. Comp. Neurol. 1995;360:185–212. doi: 10.1002/cne.903600202. [DOI] [PubMed] [Google Scholar]
- Sorvari H, Soininen H, Pitkanen A. Calbindin-D28k-immunoreactive cells and fibres in the human amygdaloid complex. Neuroscience. 1996a;75:421–443. doi: 10.1016/0306-4522(96)00296-5. [DOI] [PubMed] [Google Scholar]
- Sorvari H, Soininen H, Pitkanen A. Calretinin-immunoreactive cells and fibers in the human amygdaloid complex. J. Comp. Neurol. 1996b;369:188–208. doi: 10.1002/(SICI)1096-9861(19960527)369:2<188::AID-CNE2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Staley JK, Basile M, Flynn DD, Mash DC. Visualizing dopamine and serotonin transporters in the human brain with the potent cocaine analogue [125I]RTI-55: in vitro binding and autoradiographic characterization. J Neurochem. 1994;62:549–556. doi: 10.1046/j.1471-4159.1994.62020549.x. [DOI] [PubMed] [Google Scholar]
- Staley JK, Boja JW, Carroll FI, Seltzman HH, Wyrick CD, Lewin AH, Abraham P, Mash DC. Mapping dopamine transporters in the human brain with novel selective cocaine analog [125I]RTI-121. Synapse. 1995;21:364–372. doi: 10.1002/syn.890210412. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Amaral DG. Topographic organization of cortical inputs to the lateral nucleus of the macaque monkey amygdala: a retrograde tracing study. Journal of Comparative Neurology. 2000;421:52–79. doi: 10.1002/(sici)1096-9861(20000522)421:1<52::aid-cne4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry. 2002;59:1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- Stephan H, Andy OJ. Quantitative comparison of the amygdala in insectivores and primates. Acta Anat (Basel) 1977;98:130–153. doi: 10.1159/000144789. [DOI] [PubMed] [Google Scholar]
- Streit P. Selective retrograde labeling indicating the transmitter of neuronal pathways. J. Comp. Neurol. 1980;191:429–463. doi: 10.1002/cne.901910308. [DOI] [PubMed] [Google Scholar]
- Stutzmann GE, McEwen BS, LeDoux JE. Serotonin modulation of sensory inputs to the lateral amygdala: dependency on corticosterone. Journal of Neuroscience. 1998;18:9529–9538. doi: 10.1523/JNEUROSCI.18-22-09529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Tsutomi Y. Bcl-2 accelerates the neuronal differentiation: new evidence approaching to the biofunction of bcl-2 in the neuronal system. Brain Research. 1998;801:59–66. doi: 10.1016/s0006-8993(98)00523-x. [DOI] [PubMed] [Google Scholar]
- Taber KH, Murphy DD, Blurton-Jones MM, Hurley RA. An update on estrogen: higher cognitive function, receptor mapping, neurotrophic effects. J Neuropsychiatry Clin Neurosci. 2001;13:313–317. doi: 10.1176/jnp.13.3.313. [DOI] [PubMed] [Google Scholar]
- Takeda A, Tamano H, Imano S, Oku N. Increases in extracellular zinc in the amygdala in acquisition and recall of fear experience and their roles in response to fear. Neuroscience. 2010;168:715–722. doi: 10.1016/j.neuroscience.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Tasan RO, Nguyen NK, Weger S, Sartori SB, Singewald N, Heilbronn R, Herzog H, Sperk G. The central and basolateral amygdala are critical sites of neuropeptide Y/Y2 receptor-mediated regulation of anxiety and depression. J Neurosci. 2010;30:6282–6290. doi: 10.1523/JNEUROSCI.0430-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobet S, Knoll JG, Hartshorn C, Aurand E, Stratton M, Kumar P, Searcy B, McClellan K. Brain sex differences and hormone influences: a moving experience? J Neuroendocrinol. 2009;21:387–392. doi: 10.1111/j.1365-2826.2009.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosevski J, Malikovic A, Mojsilovic-Petrovic J, Lackovic V, Peulic M, Sazdanovic P, Alexopulos C. Types of neurons and some dendritic patterns of basolateral amygdala in humans--a golgi study. Ann Anat. 2002;184:93–103. doi: 10.1016/S0940-9602(02)80042-5. [DOI] [PubMed] [Google Scholar]
- Turner BH, Mishkin M, Knapp M. Organization of the amygdalopetal projections from modality-specific cortical association areas in the monkey. Journal of Comparative Neurology. 1980;191:515–543. doi: 10.1002/cne.901910402. [DOI] [PubMed] [Google Scholar]
- Ulfig N, Chan WY. Expression patterns of PSA-NCAM in the human ganglionic eminence and its vicinity: role of PSA-NCAM in neuronal migration and axonal growth? Cells Tissues Organs. 2004;177:229–236. doi: 10.1159/000080136. [DOI] [PubMed] [Google Scholar]
- Ulfig N, Neudorfer F, Bohl J. Transient structures of the human fetal brain: subplate, thalamic reticular complex, ganglionic eminence. Histol Histopathol. 2000;15:771–790. doi: 10.14670/HH-15.771. [DOI] [PubMed] [Google Scholar]
- Ulfig N, Setzer M, Bohl J. Transient architectonic features in the basolateral amygdala of the human fetal brain. Acta Anat (Basel) 1998;163:99–112. doi: 10.1159/000046489. [DOI] [PubMed] [Google Scholar]
- Ulfig N, Setzer M, Bohl J. Distribution of GAP-43-immunoreactive structures in the human fetal amygdala. European Journal of Histochemistry. 1999;43:19–28. [PubMed] [Google Scholar]
- Ulfig N, Setzer M, Bohl J. Ontogeny of the human amygdala. Ann N Y Acad Sci. 2003;985:22–33. doi: 10.1111/j.1749-6632.2003.tb07068.x. [DOI] [PubMed] [Google Scholar]
- Uylings HBM, Delalle I. Morphology of neuropeptide Y-immunoreactive neurons and fibers in human prefrontal cortex during prenatal and postnatal development. J. Comp. Neurol. 1997;379:523–540. doi: 10.1002/(sici)1096-9861(19970324)379:4<523::aid-cne5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Yeterian EH, Lavizzo-Mourney R. Widespread corticostriate projections from temporal cortex of the rhesus monkey. J. Comp. Neurol. 1981;1981:205–219. doi: 10.1002/cne.901990205. [DOI] [PubMed] [Google Scholar]
- Victor TA, Furey ML, Fromm SJ, Ohman A, Drevets WC. Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Arch Gen Psychiatry. 2010;67:1128–1138. doi: 10.1001/archgenpsychiatry.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt O, Vogt C. Ergebnisse unserer Hirnforschung. J. Psychol. Neurol. 1919;25:277–462. [Google Scholar]
- Walter A, Mai JK, Jimenez-Hartel W. Mapping of neuropeptide Y-like immunoreactivity in the human forebrain. Brain Res Bull. 1990;24:297–311. doi: 10.1016/0361-9230(90)90084-d. [DOI] [PubMed] [Google Scholar]
- Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. Journal of Neuroscience. 2008;28:1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RY, Aghajanian GK. Inhibition of neurons in the amygdala by dorsal raphe stimulation: mediation through a direct serotonergic pathway. Brain Research. 1977;120:85–102. doi: 10.1016/0006-8993(77)90499-1. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L. Behavioral changes associated with ablation of the amygdaloid complex in monkeys. J. Comp. Physiol. Psychol. 1956;49:381–391. doi: 10.1037/h0088009. [DOI] [PubMed] [Google Scholar]
- Weiss JH, Koh J, Baimbridge KG, Choi DW. Cortical neurons containing somatostatin- or parvalbumin-like immunoreactivity are atypically vulnerable to excitotoxic injury in vitro. Neurology. 1990;40:1288–1292. doi: 10.1212/wnl.40.8.1288. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal. Of Neuroscience. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM. Serotonin and brain development: role in human developmental diseases. Brain Research Bulletin. 2001;56:479–485. doi: 10.1016/s0361-9230(01)00615-3. [DOI] [PubMed] [Google Scholar]
- Yachnis AT, Roper SN, Love A, Fancey JT, Muir D. Bcl-2 immunoreactive cells with immature neuronal phenotype exist in the nonepileptic adult human brain. Journal of Neuropathology & Experimental Neurology. 2000;59:113–119. doi: 10.1093/jnen/59.2.113. [DOI] [PubMed] [Google Scholar]
- Yacubova E, Komuro H. Stage-specific control of neuronal migration by somatostatin. Nature. 2002;415:77–81. doi: 10.1038/415077a. [DOI] [PubMed] [Google Scholar]
- Yang TT, Menon V, Eliez S, Blasey C, White CD, Reid AJ, Gotlib IH, Reiss AL. Amygdalar activation associated with positive and negative facial expressions. Neuroreport. 2002;13:1737–1741. doi: 10.1097/00001756-200210070-00009. [DOI] [PubMed] [Google Scholar]
- Zech M, Roberts GW, Bogerts B, Crow TJ, Polak JM. Neuropeptides in the amygdala of controls, schizophrenics and patients suffering from Huntington’s chorea: an immunohistochemical study. Acta Neuropathol. 1986;71:259–266. doi: 10.1007/BF00688048. [DOI] [PubMed] [Google Scholar]
- Zezula J, Cortes R, Probst A, Palacios JM. Benzodiazepine receptor sites in the human brain: autoradiographic mapping. Neuroscience. 1988;25:771–795. doi: 10.1016/0306-4522(88)90036-x. [DOI] [PubMed] [Google Scholar]
- Zhang KZ, Westberg JA, Holtta E, Andersson LC. BCL2 regulates neural differentiation. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:4504–4508. doi: 10.1073/pnas.93.9.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XM, Cai Y, Chu Y, Chen EY, Feng JC, Luo XG, Xiong K, Struble RG, Clough RW, Patrylo PR, Kordower JH, Yan XX. Doublecortin-expressing cells persist in the associative cerebral cortex and amygdala in aged nonhuman primates. Front Neuroanat. 2009;3:17. doi: 10.3389/neuro.05.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirlinger M, Kreiman G, Anderson DJ. Amygdala-enriched genes identified by microarray technology are restricted to specific amygdaloid subnuclei. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5270–5275. doi: 10.1073/pnas.091094698. [DOI] [PMC free article] [PubMed] [Google Scholar]