Abstract
Although the roles of dopaminergic signaling in learning and behavior are well established, it is not fully understood how the activity of dopaminergic neurons is dynamically regulated under different conditions in a constantly changing environment. Dopamine neurons must integrate sensory, motor, and cognitive information online to inform the organism to pursue outcomes with the highest reward probability. In this article, we provide an overview of recent advances on the intrinsic, extrinsic (i.e., synaptic), and plasticity mechanisms controlling dopamine neuron activity, mostly focusing on mechanistic studies conducted using ex vivo brain slice preparations. We also hope to highlight some unresolved questions regarding information processing that takes place at dopamine neurons, thereby stimulating further investigations at different levels of analysis.
Keywords: burst, pause, firing pattern, reward prediction error, disinhibition, synaptic plasticity, reinforcement learning
INTRODUCTION
Dopamine (DA) neurons in the substantia nigra pars compacta (SNc), ventral tegmental area (VTA) and retrorubal field (RRF) are part of a network of midbrain nuclei innervated primarily by other parts of the basal ganglia, but they apparently generate one of the most important neural signals for motivated behavior found anywhere in the brain. Recording studies in non-human primates and rodents, combined with human functional imaging and computational modeling studies, have suggested that their firing pattern can encode reward prediction error, which is thought to be a critical mediator of reinforcement learning (Fig. 1 Montague et al., 1996, Schultz, 1998, Pan et al., 2005, D'Ardenne et al., 2008) [but also see (Redgrave and Gurney, 2006, Bromberg-Martin et al., 2010 2010) for other functions of DA output]. It is likely the essential nature of this signal that links disruptions of DA function to many of the symptoms of drug addiction and a wide range of psychiatric disorders, and in the extreme case of the degeneration of these neurons, to Parkinson's disease. It is somewhat puzzling, however, that this signal should be generated by the DA neurons of the ventral midbrain, which do not seem to be in a position to directly receive all the sensory, motor, and cognitive information required to calculate the reward prediction error signal. How does the DA neuron get access to all that information? Is the cell simply a relay for a similar signal coming from elsewhere, or does it synthesize that signal from a number of independent inputs? As a first step toward answering these questions, it is necessary to understand the intrinsic properties that enable DA neurons to burst and pause. This will then direct future studies that will determine how DA neurons integrate their intrinsic properties with different inputs to encode reward prediction error.
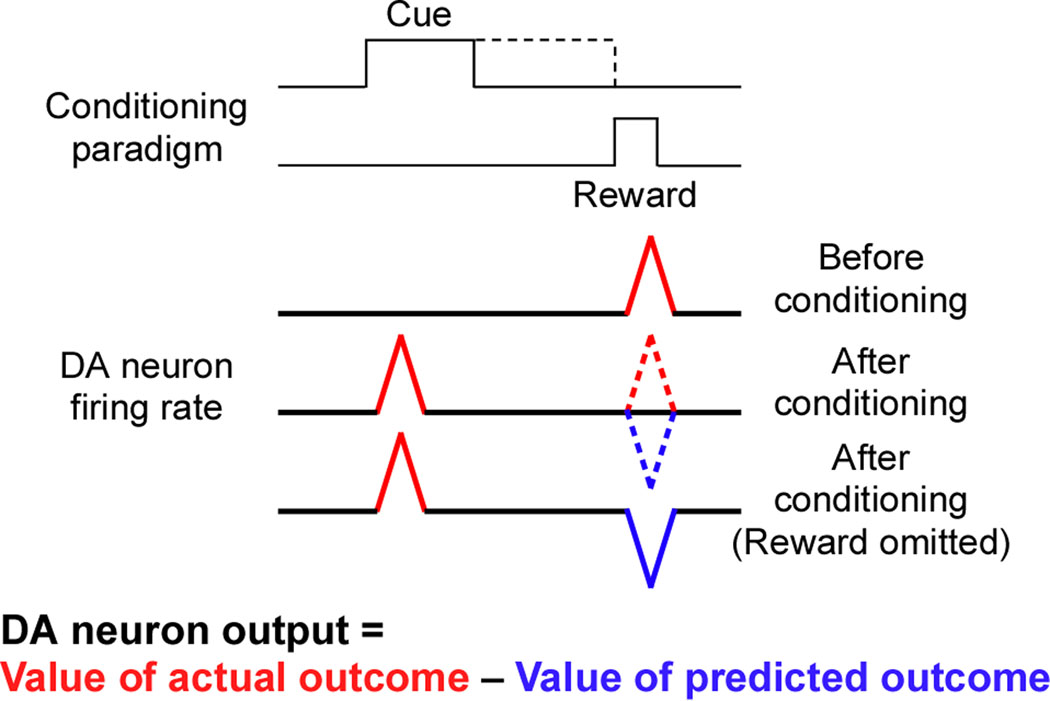
Figure 1. Schematic diagram illustrating the encoding of reward prediction error by DA neuron firing activity.
During cue-reward conditioning, the reward is delivered at the end of the cue or after a certain delay after the cue offset. After conditioning, the DA neuron burst response transfers from the time of reward delivery to the onset of the cue. Note that the depression (pause) response to the omission of expected reward after cue presentation is time-locked to the expected time of reward delivery.
Dopamine Neuron Populations
The most widely studied populations of DA neurons are 1) those in SNc that project to the dorsal striatum, giving rise to the nigrostriatal system, and 2) those in the VTA that project to limbic structures, mainly the ventral striatum [i.e., the nucleus accumbens (NAc)], and the prefrontal cortex, giving rise to mesolimbic and mesocortical pathways. Historically, the nigrostriatal system has been largely implicated in volitional control of movement, while the mesocorticolimbic system is thought to be more critical for reward-based learning and motivation. However, accumulating evidence indicates that this type of dichotomous (or trichotomous) distinction is oversimplified and that it can rather be described as a lateral-to-medial gradient, in which significant intermixing of DA neuron populations, having different projection targets, is observed (Bjorklund and Dunnett, 2007, Ikemoto, 2007, Wise, 2009). Furthermore, different parts of the VTA/SNc complex may have functional interactions, either through local connections (Ferreira et al., 2008) or through long-range feedback loops (Haber et al., 1990, Heimer et al., 1991, Haber et al., 2000, Paladini et al., 2003). In the latter case, there appears to be a cascading spiral of projections between the VTA/SNc and the NAc/striatum. Here, DA neurons in the medial part of the VTA innervate medium spiny projection neurons in the ventral striatum, which then project back to more lateral parts of the VTA/SNc complex that in turn send projections to more dorsal parts of the striatum. It has been suggested that this type of anatomical organization might underlie the transition from a goal-directed action to a stimulus-response habit during the progression of reward-based behavioral conditioning (e.g., drug addiction), in which more lateral parts of the VTA/SNc complex, together with more dorsal parts of the striatum, are gradually recruited (Everitt and Robbins, 2005, Belin and Everitt, 2008).
In line with the anatomical and functional heterogeneity of DA neurons in intact animals, studies using ex vivo brain slice preparations have described different electrophysiological and neurochemical properties of DA neurons depending on their location, e.g., lateral-to-medial gradients in the expression levels of certain ion channels (Wolfart et al., 2001, Neuhoff et al., 2002). It should also be noted that the definition of the SNc and VTA is somewhat arbitrary with different labs using different slice planes (e.g., horizontal vs. coronal). More recent studies have investigated electrophysiological and pharmacological profiles of DA neurons based on their projection targets (Ford et al., 2006, Margolis et al., 2006, Lammel et al., 2008, Margolis et al., 2008, Lammel et al., 2011). However, only a few laboratories have conducted this type of analysis that involves recording of DA neurons labeled with retrograde tracers, followed by post hoc tyrosine hydroxylase immunohistochemistry, and some conflicting results have been reported. This may be partly due to differences in the species/strain/age of animals examined, together with some difference in the site and volume used for retrograde tracer injection; and even differences in internal solution used for whole-cell recordings, which tends to dialyze the intracellular contents of the recorded cell (Margolis et al., 2010, Zhang et al., 2010). It is also possible that even DA neurons sharing the same projection target might be heterogeneous in terms of their intrinsic properties, or in terms of their specific inputs. Therefore, full characterization of different subpopulations of DA neurons still requires more time and effort to reach consensus in the field, hopefully with the aid of genetically engineered animals having different DA neuron subpopulations fluorescently labeled.
In the present article, we provide up-to-date knowledge regarding 1) the ionic mechanisms underlying intrinsic properties of DA neurons, 2) influences of neurotransmitter inputs on DA neuron activity, and 3) plasticity of these neurotransmitter inputs, mostly focusing on studies conducted ex vivo. We will frequently refer to DA neurons as “SNc DA neurons” or “VTA DA neurons”, with the understanding that this simple distinction may become obsolete in the future. Furthermore, identification of DA neurons in the vast majority of recording studies (both in vivo and ex vivo) has been based solely on electrophysiological criteria (e.g., action potential width, hyperpolarization-activated cationic conductance termed Ih, D2 DA receptor-mediated inhibition), and those neurons remain to be putative DA neurons without unequivocal confirmation of their neurochemical identify, especially in the medial VTA (Ungless et al., 2004, Lammel et al., 2008, Margolis et al., 2008, Zhang et al., 2010, Lammel et al., 2011).
1 - THE IONIC MECHANISMS UNDERLYING INTRINSIC PROPERTIES OF DOPAMINE NEURONS
1.1 - Cellular Mechanisms of Spontaneous Activity
Studies of the spontaneous tonic activity of DA neurons have already revealed much about their neurophysiological properties. SNc DA neurons are autonomous pacemakers with a particularly simple pacemaking mechanism. A low voltage-activated, but non-inactivating, Ca2+ channel (probably Cav 1.3, see Mercuri et al., 1994, Durante et al., 2004) and a persistent Na+ current are weakly activated in the subthreshold voltage range, and these depolarize the neuron gradually until Ca2+ concentration becomes adequate to activate a small conductance Ca2+-dependent K+ current (SK3) (Wolfart et al., 2001) sufficiently to overcome the inward currents. Subsequently, the membrane potential is hyperpolarized until Ca2+ levels are reduced enough to begin the cycle again. It should be noted that the hyperpolarization-activated cationic conductance (Ih) may also contribute to depolarization following SK-mediated hyperpolarization, although the reliance of pacemaking on Ih appears to vary depending on the age and species of animals used, as well as on the location and neurochemical properties of DA neurons recorded (Neuhoff et al., 2002, Okamoto et al., 2006, Chan et al., 2007, Puopolo et al., 2007, Guzman et al., 2009, Khaliq and Bean, 2010). Under the influence of this subthreshold oscillatory mechanism, DA neurons exhibit a low-frequency (<10 Hz) oscillation: a slow oscillatory potential (SOP), even in the absence of spiking (e.g. Paladini et al., 1999b, Wilson and Callaway, 2000). When spiking is present, DA neurons fire no more than one action potential on each cycle of the oscillation. There are a number of other currents that modulate the frequency of the oscillation and shape the membrane potential trajectory (e.g. Neuhoff et al., 2002, Wolfart and Roeper, 2002), but principally only Ca2+ channels and Ca2+-dependent K+ channels are required for the subthreshold oscillation that controls the rhythmic activity of SNc DA neurons in slices.
In contrast to SNc, DA neurons in the VTA have a different pacemaking mechanism. When exposed to TTX, the cells do not exhibit a SOP, but instead have a stable resting membrane potential. The pacemaking pattern of VTA DA cells is relatively insensitive to Ca2+ channel blockade when compared to SNc neurons (Chan et al., 2007, Khaliq and Bean, 2010). Rather, the spontaneous firing activity of VTA DA neurons is driven by a TTX-insensitive and non-voltage-dependent sodium current to begin depolarization in between spikes, followed later in the depolarization phase by a TTX-sensitive and voltage-dependent sodium current to bring the membrane potential to spike threshold (Khaliq and Bean, 2010). Interestingly, this difference in Ca2+ currents between SNc and VTA DA neurons has been suggested as a possible mechanism for the selective vulnerability of SNc DA neurons in Parkinson’s disease (Chan et al., 2007).
In vivo, many DA cells exhibit rhythmic background firing similar to that seen in slices, but about an equal number exhibit more irregular firing (Wilson et al., 1977b, Tepper et al., 1995, Paladini and Tepper, 1999, Hyland et al., 2002). The irregular pattern does not occur in slices, and has therefore been presumed to arise from synaptic inputs that interact with the subthreshold currents (but see Lobb et al., 2010).
1.2 - Cellular Mechanisms of Bursts
Encoding of the reward prediction error signal by DA neurons is relatively simple. These neurons fire constantly at a low rate, and speed up, firing a phasic burst when reward exceeds prediction (for example an unexpected reward) or pause when an expected reward does not occur (e.g. Tobler et al., 2003) [see Fig. 1]. Although the importance of bursts and pauses in DA neurons can only be appreciated from studies done in awake behaving animals, most of what we know about their mechanisms arise from studies in anesthetized animals or in reduced ex vivo preparations. DA neurons exhibit spontaneous bursts even in anesthetized animals. Much of the work on burst generation is derived from studies of these spontaneous bursts (e.g. Grace and Bunney, 1984, Chergui et al., 1993, Tepper et al., 1995, Tong et al., 1996a, Overton and Clark, 1997, Lokwan et al., 1999, Paladini and Tepper, 1999). While there is no guarantee that these arise from mechanisms identical to those acting in awake animals, there is some reason to think that they are similar. Spontaneous bursts are similar to reward-related bursts in duration, firing frequency, and other structural features (Hyland et al., 2002). Generally, bursts are considered to arise from a different mechanism than the slow subthreshold currents underlying the pacemaking mechanism. There are three general models of burst generation in DA neurons: first, the plateau potential model; second, the large EPSP idea (simple synaptic depolarization); and third, the interaction between synaptic events and intrinsic membrane properties of the DA neuron. For the experimentalist, this presents an opportunity for clarification because these models have all been clearly presented in published form, and they make a number of very different predictions.
1.2.1 - The plateau potential model
In slices of the SNc or VTA, rhythmic bursting can sometimes be induced during experiments that employ apamin to block the SK current. It is well known that DA cells burst spontaneously in slices after treatment with apamin, frequently in conjunction with NMDA, but not with AMPA receptor agonists (Shepard and Bunney, 1991, Johnson and Wu, 2004). Although these bursts do not very much resemble those seen in vivo, apamin-induced bursting is clearly driven by rhythmic plateau potentials. This is evident by the fact that spiking usually fails due to depolarization block, leaving only the plateau potentials (Johnson and Wu, 2004). Spiking can be rescued after apamin by applying an appropriate constant current through the recording electrode. It should also be noted that firing during apamin-induced plateau potentials (in the absence of NMDA) usually does not exceed the maximum rate that can be obtained by depolarizing current injections. Still, it is possible that in the absence of SK channels, a spike repolarizing current, such as a delayed rectifier may be sufficient to establish a high frequency oscillation that can drive the cell above 10 – 15 Hz in the presence of NMDA. In combination with removal of the SK current (as after apamin), an inactivating A-type potassium current can also support phasic, NMDA-evoked bursting (Deister et al., 2009). This happens because of the help in repolarization (and de-inactivation) the potassium current receives by the Mg2+-block of the NMDA channel during the spike-afterhyperpolarization. That is, at the peak of a spike afterhyperpolarization, the depolarizing NMDA current is blocked, and therefore does not diminish the peak amplitude of the hyperpolarization, allowing for removal of inactivation of the A-type current.
1.2.2 - The large EPSP model
Under certain conditions, DA neurons can be made to burst by simple depolarizing current injection (Georges and Aston-Jones, 2002, Blythe et al., 2007, Blythe et al.) [(but see Richards et al., 1997)], indicating that any depolarizing synaptic input is capable of generating a burst. Other than differences in activation and inactivation kinetics, this model predicts that there is no difference between AMPA and NMDA receptor activation since both are capable of depolarizing DA neurons. Indeed, in the papers by Blythe et al (2007, 2009), both NMDA and AMPA antagonists are capable of decreasing intraburst frequency and number of spikes within synaptically driven bursts, and only a combination of AMPA with NMDA antagonists is capable of abolishing bursts.
1.2.3 - Interaction between synaptic events and intrinsic properties
Although the studies by Blythe et al (2007, 2009) have shown that under certain in vitro recording conditions bursting can be induced by simple depolarization, a peculiar feature of the DA neuron offers reason for suspecting that most bursts in DA cells have a common mechanism. The cells seem, under most conditions when studied in intracellular recording (both in vivo and in vitro), to be incapable of bursting in response to applied current pulses. Firing can be controlled by applied current over a range of frequencies of about 0–10 Hz, which is also the range of background (non-bursting) firing rates of the cell (Richards et al., 1997, Paladini et al., 1999b). During bursts, the cells attain rates of 20–50 Hz (Grace and Bunney, 1984, Tepper et al., 1995, Paladini and Tepper, 1999, Hyland et al., 2002, Schultz, 2002). Attempts to pass larger currents, to force the cell to fire at bursting rates, lead quickly to spike failure, apparently from sodium channel inactivation (Richards et al., 1997). Under these conditions, bursting must rely on a mechanism that cannot be mimicked by somatic constant current injection.
In the case of an interaction between synaptic events and intrinsic properties, bursting is initiated by the transient interaction of NMDA receptors and voltage-gated ion channels on the membrane of the DA neuron to induce a high-frequency, action potential-independent oscillation (Deister et al., 2009). AMPA receptors, or somatic current injection, do not initiate bursts in the same recordings. NMDA receptors have the unique capability to increase the peak-to-peak amplitude of the membrane oscillations, imparted by voltage-gated ion channels, because of their voltage dependence (Mayer et al., 1984, Nowak et al., 1984). During an oscillation period, the NMDA receptor undergoes Mg2+ block during the hyperpolarization phase, allowing for full repolarization; and unblocks during the depolarizing phase, increasing the peak amplitude of the depolarization. Thus, the current from the NMDA receptor varies in phase with the oscillation (Kuznetsov et al., 2006, Deister et al., 2009). This has the effect of postponing the sodium channel inactivation that is evident during constant depolarization.
1.3 - Cellular Mechanisms of Pauses
Spontaneous bursts in vivo, and experimenter-induced bursts in vitro, are often followed by a long pause, after which spontaneous activity resumes (e.g. Overton and Clark, 1997). This is in contrast to reward-prediction-related bursts seen in behaving animals, in which there is often no pause immediately following a burst (e.g. Hyland et al., 2002, Schultz, 2002). Despite its scarcity in behaving animals, researchers studying spontaneous bursts have long assumed that the post-burst pause is an essential consequence of the burst (e.g. an after-hyperpolarization). But in behaving animals, bursts and pauses can be evoked in different situations and have opposite behavioral significance (pauses signal the absence of an expected reward).
More recently, studies of DA neurons in slices have shown that the slow after-hyperpolarization and pause in spontaneous firing in response to synaptic excitation have entirely different mechanisms (Fiorillo and Williams, 1998, Paladini et al., 2001, Morikawa et al., 2003, Paladini et al., 2004, Paladini and Williams, 2004). Instead of relying on AMPA or NMDA receptors, the pause is mediated by Ca2+ release from intracellular stores and the resultant activation of Ca2+-dependent K+ channels after mGluR activation. The co-occurrence of bursts and pauses in spontaneous and glutamate-evoked bursts is thus due to sequential activation of AMPA/NMDA and mGlu receptors.
Furthermore, pauses can as well be activated by other phosphoinositide (PI)-coupled receptors that activate release of Ca2+ from stores, including the α-adrenergic receptor and muscarinic acetylcholine receptor. These also produce a powerful and long-lasting hyperpolarization by activation of an SK current (Fiorillo and Williams, 1998, Paladini et al., 2001, Morikawa et al., 2003, Paladini and Williams, 2004). In some cases, release of Ca2+ from stores is regenerative, and can lead to a Ca2+ wave that propagates throughout the dendritic field of the DA neuron at about 100 µm/second (Paladini and Williams, 2004). This raises the possibility of spatial interactions between pauses generated at different parts of the DA neuron that could contribute to the function of the cell. Receptors mediating the release of Ca2+ from stores share some intracellular signaling pathways. For example, mGluRs and α-adrenergic receptors both employ IP3 and cADPR signaling, and show some cross-desensitization (Morikawa et al., 2003, Paladini and Williams, 2004). This pause mechanism allows for pauses to be generated by the cooperative action from a number of different sources, including glutamatergic and DA activation. DA is the likely ligand for the α-adrenergic receptor in the SNc (Paladini et al., 2004) [although see (Grenhoff and Svensson, 1993)]. The insights obtained from studying the cooperative action of different neurotransmitters can provide the basis for future studies on the synaptic integration of neuromodulatory signals that may produce regenerative pauses in DA cells in vivo when an expected reward is withheld.
Another candidate mechanism for the pauses seen in vivo are IPSPs generated by GABAergic synaptic afferents from the rostromedial tegmental nucleus (RMTg), striatum, nucleus accumbens, globus pallidus, ventral pallidum, and substantia nigra pars reticulata (Tepper et al., 1998, Celada et al., Paladini et al., 1999a, Paladini and Tepper, Lee et al., 2004, Brazhnik et al., 2008, Jhou et al., 2009, Sesack and Grace, 2010) [see Fig. 2]. The majority of the afferents to SNc DA neurons induce a GABAA-mediated inhibition of firing (Paladini et al., 1999a). Although IPSPs are capable of delaying action potentials in a time-dependent way, the powerful somatic oscillatory currents of the DA cell ensure that the cell will fire again unless inhibition is strong and of long duration. The strength of the IPSP depends partly on the GABAA reversal potential in DA cells, which is Cl−-mediated, and occurs near the most hyperpolarized part of the DA cell’s oscillation (Gulacsi et al., 2003). As a result, GABAA currents are lowest at the most hyperpolarized point of the oscillation, making it unlikely that they will hyperpolarize the neuron much further. Therefore, it is likely that a pause induced by phasic activation of GABAA-mediated afferents will require the recruitment of several inputs to overcome the pacemaking currents (Lobb et al., 2010). Indeed, the cooperative action of tonic GABAergic and glutamatergic inputs may well have significant influence on bursts and pauses (see section 2.6 entitled “Afferent Control of the DA Neuron”, and Lobb et al., 2010). Additionally, GABAB-mediated K+ currents are capable of inhibiting DA neurons by hyperpolarization due to the more hyperpolarized reversal potential for K+ (Brazhnik et al., 2008). It is possible that in nature, pauses can be independently activated by GABAergic and PI-coupled receptors to produce the pause responses in the appropriate (usually different) circumstances. Pauses immediately following a burst may very well have a different mechanism from pauses occurring independently of a burst.
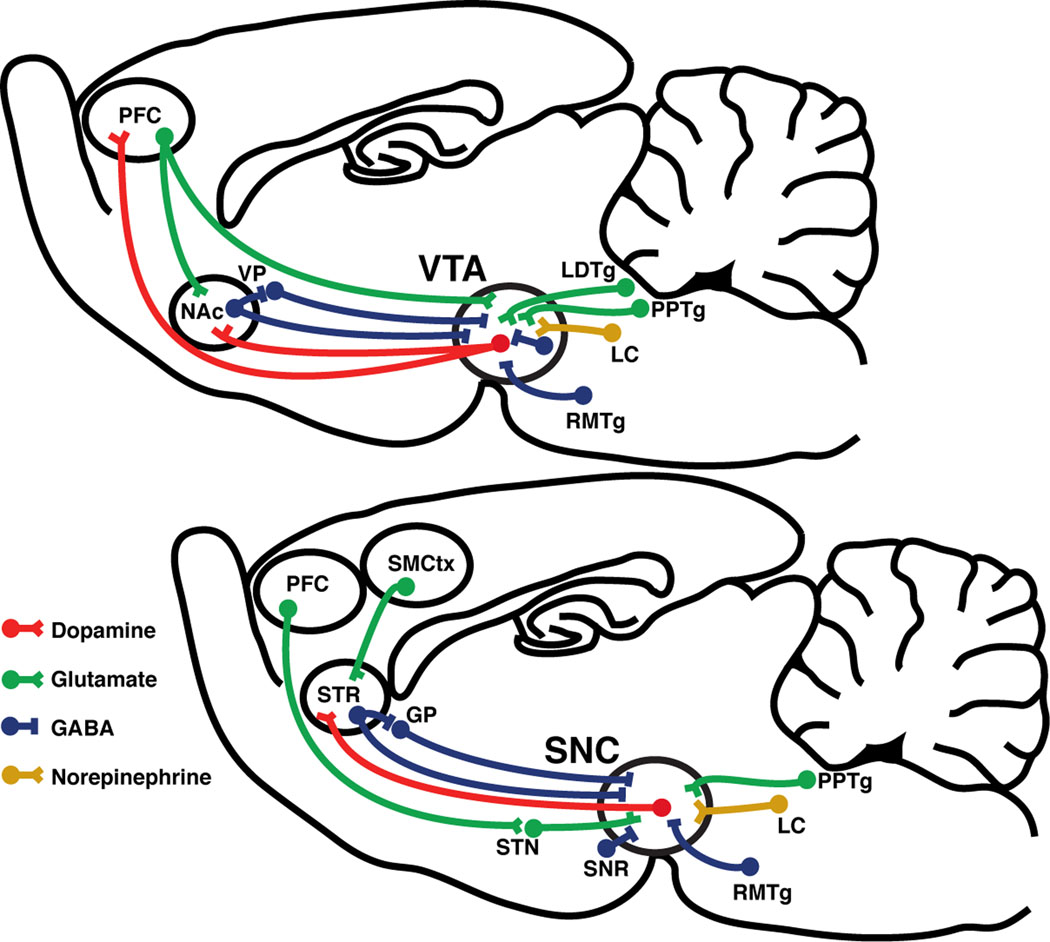
Figure 2. Summary of the major afferents to the VTA and SNc.
GP: globus pallidus, LC: locus coeruleus, LDTg: laterodorsal tegmental nucleus, NAc: nucleus accumbens, PFC: prefrontal cortex, PPTg: pedunculopontine tegmental nucleus, RMTg: rostromedial tegmental nucleus, SMCtx: sensorimotor cortex, SNc: substantia nigra pars compacta, SNr: substantia nigra pars reticulata, STR: striatum, VP: ventral pallidum, VTA: ventral tegmental area,
2 - INFLUENCES OF NEUROTRANSMITTER INPUTS ON DOPAMINE NEURON ACTIVITY (Fig. 2)
2.1 - Glutamate
Feedback glutamatergic input from the PFC provides the major cortical projection to the VTA (Sesack and Pickel, 1992, Tong et al., 1996b, Geisler et al., 2007). The excitatory PFC afferents preferentially target DA neurons that project back to the PFC and GABAergic neurons that project to the NAc, demonstrating considerable target specificity of PFC input into the VTA (Carr and Sesack, 2000).
Previous studies have identified a limited number of subcortical structures providing glutamatergic afferents into the VTA/SNc. These include the subthalamic nucleus (Kita and Kitai, 1987), the pedunculopontine tegmental (PPTg) and laterodorsal tegmental (LDTg) nuclei (Clements et al., 1991, Lavoie and Parent, 1994, Charara et al., 1996), the bed nucleus of the stria terminals (BNST) (Georges and Aston-Jones, 2002), and the superior colliculus (SC) (Comoli et al., 2003, Dommett et al., 2005). However, a more recent thorough analysis using retrograde tract-tracing combined with in situ hybridization for vesicular glutamate transporters has revealed a large number of previously unknown subcortical sources of glutamatergic inputs into the VTA, such as the lateral hypothalamic and preoptic areas, periaqueductal gray, and the dorsal and median raphe (Geisler et al., 2007), although whether these glutamatergic inputs innervate DA neurons and/or non-DA neurons was not examined in this study. Furthermore, glutamatergic synapses from these subcortical structures outnumber those from the PFC (Geisler et al., 2007, Omelchenko and Sesack, 2007). These diverse sources of glutamatergic afferents may provide DA neurons with an opportunity to integrate information arising from a wide range of environmental stimuli. For example, electrophysiological evidence implicates glutamatergic inputs primarily from the SC and PPTg in mediating DA neuron burst responses to visual and auditory cues, respectively (Comoli et al., 2003, Pan and Hyland, 2005).
Glutamatergic inputs activate AMPA- and NMDA-type ionotropic glutamate receptors in DA neurons. Accordingly, iontophoretic application of either AMPA or NMDA receptor agonists can stimulate DA neuron firing (Christoffersen and Meltzer, 1995, Zhang et al., 1997). A number of in vivo electrophysiological studies in anesthetized animals have reported that DA neuron bursts occurring spontaneously, or those triggered by electrical stimulation of the PFC, PPTg, or subthalamic nucleus, are suppressed by local application of NMDA, but not AMPA, receptor antagonists (Overton and Clark, 1992, Chergui et al., 1993, Chergui et al., 1994, Christoffersen and Meltzer, 1995, Tong et al., 1996a). In line with this, genetic inactivation of NMDA receptors selectively in DA neurons impairs spontaneous bursts as well as those evoked by PPTg stimulation (Zweifel et al., 2009). Furthermore, DA neuron bursts, triggered by electrical stimulation of glutamatergic afferents, or iontophoretic application of glutamate/aspartate in brain slices, are also blocked by NMDA receptor antagonists (Morikawa et al., 2003, Deister et al., 2009). These converging lines of evidence indicate the critical role of NMDA receptors in the generation of DA neuron bursts. Nevertheless, it should be noted that AMPA receptor antagonists can also attenuate DA neuron burst firing evoked by stimulation of glutamatergic inputs both in vivo and in vitro (Georges and Aston-Jones, 2002, Blythe et al., 2007). Therefore, the exact contribution of these two types of ionotropic glutamate receptors to DA neuron bursting under different conditions, and perhaps even in different populations of DA neurons (Lammel et al., 2011), remains to be fully determined.
Glutamate also activates metabotropic glutamate receptors (mGluRs), mainly type 1 mGluR (mGluR1), in DA neurons (Moraru et al., 1999, Hubert et al., 2001). Tonic activation of mGluRs produces membrane depolarization that is mediated, at least partly, by opening of a non-selective cationic conductance (Guatteo et al., 1999, Tozzi et al., 2003). In contrast, rapid activation of mGluRs leads to membrane hyperpolarization via opening of SK channels following Ca2+ release from intracellular stores (Fiorillo and Williams, 1998, Morikawa et al., 2003). Additionally, tonic mGluR activation causes amplification of AP-evoked Ca2+ signals, thereby driving activity-dependent synaptic plasticity of NMDA receptor-mediated transmission (Cui et al., 2007, Harnett et al., 2009).
2.2 - GABA
The powerful influence of GABAergic inhibition on DA neuron activity is illustrated by the observation that over 70% of synapses onto SNc DA neurons are GABAergic (Bolam and Smith, 1990, Tepper and Lee, 2007), although the proportion of GABAergic synapses seems to be lower in the VTA (Smith et al., 1996). There are major GABAergic feedback projections from the NAc/striatum and the ventral pallidum/globus pallidus (external segment) into the VTA/SNc (Conrad and Pfaff, 1976, Walaas and Fonnum, 1980, Zahm, 1989, Smith and Bolam, 1990, Kalivas et al., 1993). Additionally, although there is a rich literature demonstrating GABAergic afferent innervation of DA neurons (Juraska et al., 1977, Gerfen, 1985, Gerfen and Young, 1988, Bolam and Smith, 1990, Prensa and Parent, 2001, Saitoh et al., 2004, Matsuda et al., 2009, Fujiyama et al., 2011), recent evidence employing an optogenetic approach indicates that GABAergic feedback from the NAc/striatum projects more densely to non-DA neurons (Chuhma et al., 2011, Xia et al., 2011). DA neurons also receive GABAergic inputs from local GABA neurons within the VTA, or in the neighboring substantia nigra pars reticulata for SNc (Phillipson, 1979, Johnson et al., 1992, Tepper et al., 1995, Omelchenko and Sesack, 2009). Furthermore, a region located at the caudal tail of the VTA, termed rostromedial tegmental nucleus (RMTg), is an important source of GABAergic input to both the VTA and SNc (Jhou et al., 2009, Kaufling et al., 2009).
Both GABAA and GABAB receptors mediate the inhibitory action of GABA on DA neurons (Johnson and North, 1992, Brazhnik et al., 2008). GABAB receptor-mediated inhibition is achieved through activation of G protein-gated inwardly rectifying K+ (GIRK) channels (Lacey et al., 1988, Labouebe et al., 2007). Furthermore, activation of presynaptic GABAB receptors inhibits glutamate and GABA release onto DA neurons (Hausser and Yung, 1994, Manzoni and Williams, 1999). GABAergic inhibition via either GABAA or GABAB receptors can suppress burst firing of DA neurons (Paladini and Tepper, 1999, Erhardt et al., 2002), and recent evidence indicates that a disinhibitory mechanism (i.e., transient removal of GABAergic inhibition) may contribute to the generation of phasic DA neuron bursts in behaving animals (Matsumoto and Hikosaka, 2007, Tepper and Lee, 2007, Jhou et al., 2009, Lobb et al., 2010, 2011b).
2.3 - Dopamine (DA)
Dendrodendritic synapses, as well as a small number of axodendritic synapses, formed between DA neurons have been observed with electron microscopy in both the VTA and SNc (Wilson et al., 1977a, Bayer and Pickel, 1990). Vesicular monoamine transporter 2 (VMAT2)-positive vesicles, which would underlie Ca2+-dependent vesicular DA release from the somoatodendritic area (Rice et al., 1997, Chen and Rice, 2001, Kita et al., 2009), have also been detected in DA neuron dendrites (Nirenberg et al., 1996). Released DA exerts feedback inhibition of DA neuron activity via activation of D2 autoreceptors, causing GIRK-mediated hyperpolarization (Groves et al., 1975, Lacey et al., 1987). Indeed, D2 receptor-mediated IPSCs elicited by electrical stimulation of dendrodendritic synapses have been more recently reported in DA neurons of the SNc and VTA (Beckstead et al., 2004, Beckstead and Williams, 2007). In addition, DA inhibits glutamate release and facilitates GABA release onto DA neurons via activation of presynaptic D2 and D1 receptors, respectively (Cameron and Williams, 1993, Koga and Momiyama, 2000). Postsynaptic D5 receptor-mediated slow potentiation of NMDA EPSCs has also been reported, suggesting that DA may promote burst firing of DA neurons (Schilstrom et al., 2006).
2.4 - Norepinephrine (NE)
The locus coeruleus and other noradrenergic nuclei in the medulla give rise to norepinephrine (NE)-containing terminals in the VTA/SNc (Phillipson, 1979, Mejias-Aponte et al., 2009). Overall, NE exerts excitatory control over DA neuron activity via activation of α1 adrenergic receptors, causing membrane depolarization, or suppressing the mGluR-induced pause (Grenhoff et al., 1995, Paladini et al., 2001). However, transient activation of α1 adrenergic receptors produces SK-mediated membrane hyperpolarization (Paladini and Williams, 2004). NE can also bind to D2 DA receptors and cause membrane hyperpolarization in DA neurons (Grenhoff et al., 1995, Guiard et al., 2008).
2.5 - Acetylcholine (ACh)
Cholinergic inputs to the SNc and VTA display some topographical organization, with the PPTg and LDTg nuclei mainly projecting to the SNc and VTA, respectively (Cornwall et al., 1990, Semba and Fibiger, 1992, Oakman et al., 1995, Omelchenko and Sesack, 2005, Mena-Segovia et al., 2008). It has been shown that glutamate or GABA is co-released with ACh from these projections (Clements et al., 1991, Lavoie and Parent, 1994, Jia et al., 2003, Omelchenko and Sesack, 2006), although whether there is co-release from the same terminals remains controversial (Mena-Segovia et al., 2008). Cholinergic terminals in the VTA preferentially innervate DA neurons that project to the NAc (Omelchenko and Sesack, 2006).
DA neurons generally exhibit excitatory responses to ACh via activation of nicotinic and muscarinic ACh receptors. Different subtypes of nicotinic ACh receptors (nAChRs), including α3–7 and β2–4, are expressed in DA neurons, although β2-containing nAChRs mediate the majority of postsynaptic nicotinic responses (Azam et al., 2002, Marubio et al., 2003, Wooltorton et al., 2003). Activation of postsynaptic M5 muscarinic ACh receptors causes excitation of DA neurons (Lacey et al., 1990, Gronier and Rasmussen, 1998, Yeomans et al., 2001, Yamada et al., 2003), although rapid activation of these receptors can evoke SK-mediated membrane hyperpolarization via intracellular Ca2+ release (Fiorillo and Williams, 2000).
2.6 - Afferent Control of the DA Neuron
As mentioned above, in behaving animals DA neuron firing switches from tonic, single-spike activity to phasic bursts in response to the unexpected presentation of a primary reward (e.g., a drop of juice in the mouth). However, after conditioning with repeated cue-reward pairing, the burst response shifts in time to the onset of a reward-predicting cue, and DA neurons no longer respond to the primary reward when predicted by the preceding cue (Schultz, 1998, Pan et al., 2005). Furthermore, a transient pause in DA neuron firing is observed at the expected time of reward if the cue is presented alone within a single trial without subsequent reward (Fig. 1). The responses of DA neurons are correlated in time with the activity of other basal ganglia neurons such that they can be modulated within a single trial (e.g. Morris et al., 2004). These observations led to the widely publicized view that DA neuron firing represents the prediction error teaching signal used in reinforcement learning models, which encodes the difference in the actual and predicted outcomes [positive prediction error (burst) when the outcome is better than predicted, and negative prediction error (pause) when the outcome is worse than predicted] (Barto, 1995, Montague et al., 2004). Although beyond the scope of the current review, recent studies indicate that a subset of DA neurons exhibit phasic bursts in response to aversive stimuli (e.g., air puff to the face, foot shock, etc.) and also learn to respond to cues predicting aversive events (Brischoux et al., 2009, Matsumoto and Hikosaka, 2009, Zweifel et al., 2011).
As interesting as it is that DA neurons are able to generate a reward prediction signal, this finding raises some questions. Which afferent(s) provide(s) the DA neuron with the necessary input to drive bursts or pauses within the time of a trial in a given learning task? Phasic stimulation in vitro, either electrical or by local application of agonist, can produce bursts identical to those seen in vivo (e.g. Morikawa et al., 2003). The burst generated in this way is critically dependent upon NMDA receptor activation. And, there is a host of evidence connecting NMDA receptor activation to spontaneous burst generation in vivo (Charlety et al., 1991, Overton and Clark, 1997, Chergui et al., 1993, Christoffersen and Meltzer, 1995, Tong et al., 1996a). However, if it proves true that phasic activation of NMDA receptors is the only mechanism responsible for the reward-prediction bursts seen in behaving animals, it must still explain how the glutamatergic inputs are activated at the right times. It is especially challenging to understand how the response to a reward is suppressed when an identical reward is predicted by a previous stimulus. This suppression must occur on a timescale on the order of hundreds of milliseconds (e.g. Schultz, 2002). One class of explanations for this is based on the strong GABAergic innervation preventing bursts in the DA neuron (Bolam and Smith 1990, Paladini and Tepper, 1999). In this case, disinhibition models of burst generation are favored because they allow for control of burst timing by the inhibitory descending forebrain pathways (themselves under cortical influence), rather than glutamatergic brainstem inputs from the subthalamic nucleus and PPTg nucleus.
Many authors have presumed that the signal for the reward prediction error comes directly either from the cerebral cortex or the striatum. Direct inputs to the SNc from cortex are sparse, but more predominant to the VTA (e.g. Naito and Kita, 1994, Geisler and Zahm, 2005). Under some circumstances, bursts can be induced in SNc DA neurons by phasic cortical stimulation, but these are apparently relayed through the subthalamic nucleus (Overton and Clark, 1997). The most abundant known glutamate inputs to VTA/SNc DA neurons come from the subthalamic nucleus and PPTg nucleus (e.g. Kita and Kitai, 1987, Charara et al., 1996, Iribe et al., 1999), and these are tonically active inputs (Smith and Grace, 1992, Kreiss et al., 1997, Plenz and Kital, 1999, Datta et al., 2003), thus providing a tonic, rather than phasic, source of excitation.
The input from the striatum is plentiful and well documented (see review in Haber, 2003). Some striatal cells have been shown to fire in relation to reward expectation, or to other aspects of reward that could inform DA neurons (e.g. Cromwell and Schultz, 2003). However, the inputs from the striatum are all GABAergic and inhibitory (e.g. Paladini et al., 1999a). Is there a way that bursts could arise from striatal disinhibition? Striatonigral neurons are spiny neurons, and have very little tonic background activity. Also, striatal neurons receive input from DA cells, so the activity of the striatum could well be a consequence, rather than a cause, of DA responses. The reward-related responses of the spiny striatal neurons all consist of phasic increases in firing. On the face of it, if striatal spiny neurons only influence DA neurons through a direct connection, they would seem more likely as a source of the pause response, rather than the burst. However, the striatum can also influence DA cells via other connections within the basal ganglia. Optogenetic activation of the direct pathway of the basal ganglia results in inhibition of the GABAergic neurons of the substantia nigra pars reticulata (Kravitz et al., 2010). This has been posited as a potential source of disinhibition of SNc DA neurons, resulting in a burst (Lobb et al., 2011a). Indeed, appropriate electrical stimulation of the striatum excites dopaminergic neurons recorded in vivo (Grace and Bunney, 1985). Similarly, lateral habenula neurons, which inhibit VTA DA neurons, decrease their activity when an animal is presented with a stimulus-predicting cue (Matsumoto and Hikosaka, 2007).
While it is clear that a number of neurotransmitters control the firing pattern of DA neurons (see above), under the disinhibition model, the DA cell is subjected to a constant barrage of GABAergic and glutamatergic synaptic input from tonically active afferents. DA neurons undergo constant IPSPs in vivo (Grace and Bunney, 1985, Tepper and Lee, 2007), and local application of GABAA antagonists in vivo shifts the pattern of single DA neurons to fire in a bursty mode (Paladini and Tepper, 1999), indicating that a GABAergic tone is preventing DA neurons from bursting. Therefore, DA neurons are in a balanced state in vivo where the influence of tonic excitatory NMDA receptor activation is counterbalanced by the influence of tonic inhibitory GABA receptor activation, making disinhibition bursts possible. GABAA receptors are best suited for this balanced configuration since the Cl− reversal potential mediating GABAA inhibition of DA neurons is less hyperpolarized than the K+ reversal potential mediating GABAB inhibition. Again, the NMDA receptor is uniquely suited, this time for maintaining DA neurons in a balanced state between excitation and inhibition. This is because the negative slope conductance region on the IV plot of the NMDA receptor, imparted by Mg2+ block, produces an increase in input resistance (Koch, 1999). This increase in input resistance counteracts the decrease in input resistance caused by activation of GABAA receptors (Lobb et al., 2011b). Therefore, spiking still occurs in a balanced state between tonic NMDA and tonic GABAA activation. In contrast, activation of AMPA receptors increases a linear conductance which, when added with GABAA, eliminates spiking in DA neurons altogether (Lobb et al., 2011b).
Under this scheme, the firing pattern of DA neurons can be dynamically controlled on a milliseconds timescale. Bursts in firing are induced by phasic removal of tonic inhibition (i.e. disinhibition), whereas pauses in firing are initiated by a phasic increase in inhibition, or in a phasic removal of tonic excitation (Lobb et al., 2011b). With the rapid and dynamic control of burst timing and frequency by disinhibition (Lobb et al., 2010, 2011b), scaling of intraburst firing rate can occur according to the probability of reward associated with a stimulus within a single trial (i.e. within hundreds of milliseconds) (Morris et al., 2004), or on successive trials of a learning task (Pan et al., 2005). Nevertheless, accumulating evidence demonstrating various forms of activity-dependent plasticity at both excitatory and inhibitory synapses onto DA neurons suggest that synaptic plasticity within the VTA/SNc also contributes, at least in part, to the generation of conditioned DA neuron responses, as has been modeled previously (Contreras-Vidal and Schultz, 1999).
3 - SYNAPTIC PLASTICITY IN DOPAMINE NEURONS
Synaptic plasticity is widely accepted as the key neural substrate underlying memory formation in the CNS (Malenka and Bear, 2004). In the mesostriatal/mesocortical dopaminergic system, the current theory posits that phasic DA release, caused by burst firing of DA neurons in the VTA and SNc, triggers/promotes the induction of synaptic plasticity in projection areas, thereby forming memories of stimulus-reward associations and action-outcome contingencies (Montague et al., 2004, Wickens et al., 2007, Maia and Frank, 2011). In support of this idea, various forms of synaptic plasticity that require DA, or are modulated by DA, have been reported in neurons of DA projection areas (Kreitzer and Malenka, 2008, Surmeier et al., 2009, Wickens, 2009). Furthermore, it is generally assumed that acquisition of DA neuron responses during cue-reward conditioning, as illustrated in Fig. 1, is mediated by DA-dependent synaptic plasticity mostly in the striatum, which then affects DA neuron activity via feedback loops to the VTA/SNc (Houk et al., 1995, Schultz et al., 1998, Brown et al., 1999). However, studies over the past decade or so have revealed that synapses on DA neurons themselves also undergo activity/experience-dependent modifications in their strength (Kauer and Malenka, 2007, Chen et al., 2010). The vast majority of these studies have been performed in VTA DA neurons, which are generally more closely associated with reward-based learning than SNc neurons, especially during the early phase of learning, and by laboratories oriented toward investigations on drug addiction, which has a strong learning component in its etiology. In this section, we will review various forms of plasticity of synaptic inputs onto DA neurons, including the alterations observed after in vivo experience with addictive drugs.
3.1 - Glutamatergic synapses
3.1.1 - LTP of AMPAR-mediated transmission
Activity-dependent synaptic plasticity of glutamatergic synapses on DA neurons, as had been extensively studied in the hippocampus and other brain areas using brain slices, was first demonstrated over a decade ago (Bonci and Malenka, 1999, Overton et al., 1999). These studies were prompted by the idea that LTP-like processes at the level of DA neurons might mediate the development of behavioral sensitization to psychostimulants (Kalivas and Alesdatter, 1993, Tong et al., 1995, Wolf, 1998), which involves a contextual learning mechanism (Robinson and Berridge, 2003). The study by Bonci and Malenka induced LTP of AMPAR EPSCs in voltage clamp by pairing low-frequency (1 Hz) stimulation of glutamatergic inputs with large postsynaptic depolarization (+10 mV), a protocol that had been commonly used to induce LTP of AMPAR-mediated transmission in hippocampal CA1 pyramidal neurons. They found that LTP could be reliably induced in VTA DA neurons (Ih-positive neurons) but not in GABA neurons (Ih-negative neurons). Overton et al. (1999) induced LTP of EPSPs (mostly AMPAR-mediated) in current clamp by delivering high-frequency (100 Hz) stimulation of glutamatergic afferents from the subthalamic nucleus to the SNc while applying a constant depolarizing current to the recorded DA neuron. Both of these forms of AMPAR LTP were shown to be dependent on NMDAR activation for induction, as was known for LTP at glutamatergic synapses in other brain areas.
More recently, a spike timing-dependent (STD) protocol has been used in VTA DA neurons. In this case, LTP of glutamatergic EPSPs is induced by several repetitions of five EPSP-postsynaptic spike pairs at 10 Hz (mimicking burst firing), in which the onset of each EPSP precedes the peak of postsynaptic spike by ~5 ms (Liu et al., 2005, Luu and Malenka, 2008). This form of STD LTP is NMDAR-dependent (Zweifel et al., 2008), as in other forms of LTP described above. It further requires postsynaptic rise in Ca2+ and subsequent activation of PKC but not CaMKII (Luu and Malenka, 2008), and its expression involves membrane insertion of high-conductance, GluR2-lacking AMPARs (Argilli et al., 2008).
3.1.2 - LTD of AMPAR-mediated transmission
Two forms of LTD of AMPAR EPSCs have been described so far in VTA DA neurons. One form is induced by applying low-frequency (1 Hz) stimulation of glutamatergic afferents during modest postsynaptic depolarization (−40 mV) in voltage clamp (Jones et al., 2000, Gutlerner et al., 2002). The induction is independent of NMDAR or mGluR activation. Instead, it is thought to require Ca2+ entry through voltage-gated Ca2+ channels, causing PKA activation via Ca2+-sensitive adenylyl cyclase, which then leads to reduced membrane surface expression of AMPAR GluR1 subunits.
Another form of AMPAR LTD can be induced by simply stimulating glutamatergic inputs at high frequency (66 Hz) while the postsynaptic neurons are constantly held at −50 mV in voltage clamp (Bellone and Luscher, 2005). Conversely, similar high-frequency stimulation delivered in current clamp induces LTP of AMPAR EPSPs in SNc DA neurons (Overton et al., 1999), consistent with the idea that the magnitude of postsynaptic depolarizations, and hence the degree of postsynaptic Ca2+ rises, during induction gates the direction of synaptic plasticity (Lisman, 1989, Artola and Singer, 1993). Activation of mGluR1 is necessary and sufficient to mediate this form of LTD, and its expression involves membrane insertion of low-conductance, GluR2-containing AMPARs (Mameli et al., 2007).
3.1.3 - AMPAR plasticity triggered by in vivo exposure to addictive drugs
Since the initial finding that a single in vivo exposure to the psychostimulant cocaine produces long lasting (~5 days) and global potentiation of AMPAR-mediated transmission in VTA DA neurons (Ungless et al., 2001), numerous studies from different labs have reported similar observations using different classes of addictive drugs (opiates, nicotine, alcohol) or even after a single stressful experience (cold water swim) (Saal et al., 2003, Dong et al., 2004, Faleiro et al., 2004, Liu et al., 2005, Placzek et al., 2009). This AMPAR potentiation triggered by in vivo drug/stress experiences seems to share underlying mechanisms with the activity-dependent LTP of AMPARs observed ex vivo in brain slices: 1) the induction is dependent on NMDAR activation (Ungless et al., 2001, Saal et al., 2003, Zweifel et al., 2008), 2) the expression involves increased surface expression of high-conductance, GluR2-lacking AMPARs (Bellone and Luscher, 2006, Argilli et al., 2008) [but see (Faleiro et al., 2004)], and 3) previous in vivo drug exposure suppresses subsequent LTP induction in brain slices (Ungless et al., 2001, Chen et al., 2008, Luu and Malenka, 2008, Mameli et al., 2011) [but see (Liu et al., 2005)]. There are converging lines of evidence linking experience-dependent AMPAR potentiation to the associative learning mechanism involved in the development of drug addiction and also to reward-based learning in general. First, blocking NMDARs in the VTA suppresses both AMPAR potentiation and the learning of drug/reward-associated stimuli (Harris et al., 2004, Stuber et al., 2008, Zweifel et al., 2008, Zweifel et al., 2009), although certain studies have reported that NMDAR-dependent AMPAR plasticity in DA neurons may not be necessary for the development of behavioral sensitization to psychostimulants (Engblom et al., 2008, Luo et al., 2010). Next, AMPAR potentiation, which can be observed following intraperitoneal injection of cocaine by the experimenter or cocaine self-administration by animals, cannot be induced by non-contingent administration of cocaine through chronically implanted intravenous catheters (Chen et al., 2008). This observation indicates that the pharmacological action of cocaine by itself is not enough to produce AMPAR potentiation and that cocaine exposure presumably needs to be paired with certain explicit cues (e.g., being handled by the experimenter). Finally, AMPAR potentiation, although short-lived, is induced even during the learning of cue-natural reward (sucrose pellet) association (Stuber et al., 2008). Interestingly, the mGluR-dependent LTD mechanism, which causes increased surface expression of low-conductance, GluR2-containing AMPARs, can reverse the increase in high-conductance, GluR2-lacking AMPARs produced by in vivo cocaine exposure in VTA DA neurons (Bellone and Luscher, 2006, Mameli et al., 2007), and this acts to gate the persistence of cocaine-induced AMPAR depression observed in the NAc (Mameli et al., 2009). However, it is not entirely clear how this LTD mechanism during different phases of conditioning affects the learning of drug/reward-associated cues and behaviors.
It is generally assumed that AMPAR potentiation in VTA DA neurons initiates a cascade of events that lead to plastic/neuroadaptive changes in the NAc and other DA neuron projection areas necessary for the development and expression of behavioral sensitization and other behavioral models of drug addiction (Borgland et al., 2004, Luscher and Malenka, 2011). However, what exactly is that cascade of events? What is the impact of AMPAR potentiation on DA neuron activity? How do DA neuron projection areas sense AMPAR potentiation in DA neurons? One possibility is an increased contribution of AMPARs to DA neuron burst firing and hence to phasic DA release. Another important feature of high-conductance, GluR2-lacking AMPARs are their high Ca2+ permeability (Liu and Zukin, 2007). Indeed, a form of AMPAR LTP that is dependent on Ca2+-permeable AMPARs has been reported recently in VTA DA neurons after in vivo cocaine exposure (Mameli et al., 2011). Furthermore, Ca2+ influx through these AMPARs over time might exert some long-term influences (e.g., via alterations in gene expression) on DA neuron physiology (intrinsic excitability, neurotransmitter content, neurotransmitter release machinery, etc.), resulting in increased DA release from terminals.
3.1.4 - LTP of NMDAR-mediated transmission
NMDARs on DA neurons are regulated by various neurotransmitters, including DA (Ungless et al., 2003, Borgland et al., 2006, Schilstrom et al., 2006). Recent studies further indicate that activity-dependent plasticity of NMDAR-mediated EPSCs in DA neurons of the SNc and VTA can be induced (Harnett et al., 2009, Ahn et al., 2010, Bernier et al., 2011). This form of LTP is induced by pairing sustained glutamatergic input stimulation with postsynaptic bursts of action potentials. LTP induction requires amplification of action potential-evoked Ca2+ signals by preceding synaptic activation of mGluR1s, which are coupled to the generation of inositol 1,4,5-trisphosphate (IP3) (Morikawa et al., 2003, Cui et al., 2007). Here, IP3 acts to increase the sensitivity of IP3 receptors to Ca2+-dependent activation (Taylor and Laude, 2002), thereby facilitating Ca2+-induced Ca2+ release triggered by burst-evoked Ca2+ influx. Therefore, for successful LTP induction, the burst needs to occur with a certain delay (>0.5 s) after the onset of sustained synaptic stimulation, reflecting the time required for synaptic activation of mGluRs to cause a sufficient rise in IP3 levels (Harnett et al., 2009).
The synaptic stimulation-burst pairing protocol used to induce NMDAR LTP may resemble the neural activity experienced during cue-reward pairing, in that cue presentation would give rise to working memory-type sustained glutamatergic input while the reward would elicit DA neuron burst firing (Funahashi et al., 1989, Brown et al., 1999). Importantly, LTP is induced in an input-specific manner, in that only those inputs activated during the induction will be potentiated. Thus, it is tempting to speculate that this form of NMDAR plasticity might mediate the acquisition of burst responses to the cue after repeated cue-reward pairing (Fig. 1), particularly in light of the prominent role of NMDARs in triggering DA neuron burst firing (Chergui et al., 1994, Tong et al., 1996a, Overton and Clark, 1997, Morikawa et al., 2003, Deister et al., 2009, Zweifel et al., 2009). However, the exact contribution of NMDAR LTP to conditioned DA neuron responses and to the learning of cue-reward association has yet to be determined.
3.2 - GABAergic synapses
LTP of GABAA IPSCs can be induced by activation of NMDARs, either by high-frequency (100 Hz) synaptic stimulation or simply by extracellular application of NMDA, in VTA DA neurons (Ih-positive neurons) but not in GABA neurons (Ih-negative neurons) (Nugent et al., 2007). This form of GABAergic synaptic plasticity does not require GABAAR activation during induction, and is thus induced in a purely heterosynaptic fashion by glutamatergic inputs activating NMDARs. Here, an NMDAR-mediated rise in postsynaptic Ca2+ levels causes Ca2+/calmodulin-dependent activation of nitric oxide synthase. Nitric oxide thus produced will then exit DA neurons and travel to GABAergic terminals, where it causes an increase in cGMP levels to mediate long-term enhancement of GABA release.
In contrast, activation of group I mGluRs, via low-frequency (10 Hz) stimulation of glutamatergic inputs or application of a selective agonist, induces LTD of GABAA IPSCs in VTA DA neurons (Pan et al., 2008a, b). mGluR-induced synthesis of endocannabinoids, which travel to GABAergic terminals to activate presynaptic CB1 receptors, is involved in LTD induction (Chevaleyre et al., 2006).
GABAAR-mediated inhibition can effectively counteract glutamatergic excitation of DA neurons (Tepper et al., 1998, Lobb et al., 2010). Recent experimental evidence, together with modeling studies in the past, suggests that GABAergic inhibition might mediate the transient pause in DA neuron firing when the expected reward is omitted after cue presentation (Fig. 1) (Brown et al., 1999, Contreras-Vidal and Schultz, 1999, Jhou et al., 2009, Lobb et al., 2010, Lobb et al., 2011a). In this scenario, GABAergic inhibition will cancel reward-induced glutamatergic excitation when the expected reward is presented after the cue, hence no change in DA neuron firing. Therefore, plasticity of GABAergic synapses at DA neurons might play an important role in gating phasic burst responses of DA neurons in behaving animals. However, one should be cautioned that input-specific plasticity requiring presynaptic GABAergic input activity for induction, as has been reported in other brain areas (Rodriguez-Duran et al., 2011), has not been demonstrated in DA neurons.
3.3 - Dopaminergic synapses
Plasticity of local dendrodendritic synapses among DA neurons has also been reported (Beckstead and Williams, 2007). Here, low-frequency (2 Hz) stimulation of these synapses induces LTD of D2 IPSCs through persistent desensitization of D2 autoreceptors. Resulting decreases in lateral inhibition among dopamine neurons might act to promote population burst responses.
CONCLUSION
This is an exciting time for DA aficionados. Direct and selective stimulation of DA neurons using an optogenetic method in behaving animals has unequivocally demonstrated that activation of these neurons is sufficient to shape animals’ preference for certain places (Tsai et al., 2009). Genetic deletion of NMDARs selectively in DA neurons has further confirmed the roles of phasic DA signaling in various types of learning and behavior (Zweifel et al., 2008, Zweifel et al., 2009). In this article, we started by asking one simple question. How do DA neurons process information coming from various sources? More specifically, we discussed how activation and/or removal of excitatory and inhibitory inputs, working through both ionotropic and metabotropic mechanisms, can mediate phasic excitation (burst) and inhibition (pause) of DA neuron firing, and further presented various forms of activity-dependent synaptic plasticity of these inputs that may occur during behavioral conditioning. The powerful tools described above (e.g., optogenetic stimulation of discrete inputs, cell type-specific knockout/knockin strategies) will hopefully enable us to tackle these issues with further precision in intact animals, and ex vivo preparations, under both physiological and pathological conditions.
Highlights.
This is a review of the current literature on the control of firing activity of dopaminergic neurons.
The review covers
The ionic mechanisms underlying intrinsic properties of dopamine neurons
Influences of neurotransmitter inputs on dopamine neuron activity
Synaptic plasticity in dopamine neurons
Acknowledgements
This manuscript was supported by NIH grants MH079276 and NS060658 to Carlos A. Paladini, and DA015687 and AA015521 to Hitoshi Morikawa.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahn KC, Bernier BE, Harnett MT, Morikawa H. IP3 receptor sensitization during in vivo amphetamine experience enhances NMDA receptor plasticity in dopamine neurons of the ventral tegmental area. J Neurosci. 2010;30:6689–6699. doi: 10.1523/JNEUROSCI.4453-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argilli E, Sibley DR, Malenka RC, England PM, Bonci A. Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area. J Neurosci. 2008;28:9092–9100. doi: 10.1523/JNEUROSCI.1001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artola A, Singer W. Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends Neurosci. 1993;16:480–487. doi: 10.1016/0166-2236(93)90081-v. [DOI] [PubMed] [Google Scholar]
- Azam L, Winzer-Serhan UH, Chen Y, Leslie FM. Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J Comp Neurol. 2002;444:260–274. doi: 10.1002/cne.10138. [DOI] [PubMed] [Google Scholar]
- Barto AG. Adaptive critics and the basal ganglia. In: Houk JC, et al., editors. Models of information processing in the basal ganglia. Cambridge, MA: MIT Press; 1995. pp. 215–232. [Google Scholar]
- Bayer VE, Pickel VM. Ultrastructural localization of tyrosine hydroxylase in the rat ventral tegmental area: relationship between immunolabeling density and neuronal associations. J Neurosci. 1990;10:2996–3013. doi: 10.1523/JNEUROSCI.10-09-02996.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron. 2004;42:939–946. doi: 10.1016/j.neuron.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Williams JT. Long-term depression of a dopamine IPSC. J Neurosci. 2007;27:2074–2080. doi: 10.1523/JNEUROSCI.3251-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Bellone C, Luscher C. mGluRs induce a long-term depression in the ventral tegmental area that involves a switch of the subunit composition of AMPA receptors. Eur J Neurosci. 2005;21:1280–1288. doi: 10.1111/j.1460-9568.2005.03979.x. [DOI] [PubMed] [Google Scholar]
- Bellone C, Luscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- Bernier BE, Whitaker LR, Morikawa H. Previous Ethanol Experience Enhances Synaptic Plasticity of NMDA Receptors in the Ventral Tegmental Area. J Neurosci. 2011;31:5205–5212. doi: 10.1523/JNEUROSCI.5282-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Blythe SN, Atherton JF, Bevan MD. Synaptic Activation of Dendritic AMPA and NMDA Receptors Generates Transient High-Frequency Firing in Substantia Nigra Dopamine Neurons In Vitro. J Neurophysiol. 2007;97:2837–2850. doi: 10.1152/jn.01157.2006. [DOI] [PubMed] [Google Scholar]
- Blythe SN, Wokosin D, Atherton JF, Bevan MD. Cellular mechanisms underlying burst firing in substantia nigra dopamine neurons. J Neurosci. 2009;29:15531–15541. doi: 10.1523/JNEUROSCI.2961-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam JP, Smith Y. The GABA and substance P input to dopaminergic neurones in the substantia nigra of the rat. Brain Res. 1990;529:57–78. doi: 10.1016/0006-8993(90)90811-o. [DOI] [PubMed] [Google Scholar]
- Bonci A, Malenka RC. Properties and plasticity of excitatory synapses on dopaminergic and GABAergic cells in the ventral tegmental area. J Neurosci. 1999;19:3723–3730. doi: 10.1523/JNEUROSCI.19-10-03723.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J Neurosci. 2004;24:7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Brazhnik E, Shah F, Tepper JM. GABAergic afferents activate both GABAA and GABAB receptors in mouse substantia nigra dopaminergic neurons in vivo. J Neurosci. 2008;28:10386–10398. doi: 10.1523/JNEUROSCI.2387-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci U S A. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Bullock D, Grossberg S. How the basal ganglia use parallel excitatory and inhibitory learning pathways to selectively respond to unexpected rewarding cues. J Neurosci. 1999;19:10502–10511. doi: 10.1523/JNEUROSCI.19-23-10502.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron DL, Williams JT. Dopamine D1 receptors facilitate transmitter release. Nature. 1993;366:344–347. doi: 10.1038/366344a0. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P, Paladini CA, Tepper JM. GABAergic control of rat substantia nigra dopaminergic neurons: role of globus pallidus and substantia nigra pars reticulata. Neuroscience. 1999;89:813–825. doi: 10.1016/s0306-4522(98)00356-x. [DOI] [PubMed] [Google Scholar]
- Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, Meredith GE, Surmeier DJ. 'Rejuvenation' protects neurons in mouse models of Parkinson's disease. Nature. 2007 doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- Charara A, Smith Y, Parent A. Glutamatergic inputs from the pedunculopontine nucleus to midbrain dopaminergic neurons in primates: Phaseolus vulgaris-leucoagglutinin anterograde labeling combined with postembedding glutamate and GABA immunohistochemistry. J Comp Neurol. 1996;364:254–266. doi: 10.1002/(SICI)1096-9861(19960108)364:2<254::AID-CNE5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Charlety PJ, Grenhoff J, Chergui K, De la Chapelle B, Buda M, Svensson TH, Chouvet G. Burst firing of mesencephalic dopamine neurons is inhibited by somatodendritic application of kynurenate. Acta Physiol Scand. 1991;142:105–112. doi: 10.1111/j.1748-1716.1991.tb09134.x. [DOI] [PubMed] [Google Scholar]
- Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, Chou JK, Bonci A. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron. 2008;59:288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Hopf FW, Bonci A. Synaptic plasticity in the mesolimbic system: therapeutic implications for substance abuse. Ann N Y Acad Sci. 2010;1187:129–139. doi: 10.1111/j.1749-6632.2009.05154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Rice ME. Novel Ca2+ dependence and time course of somatodendritic dopamine release: substantia nigra versus striatum. J Neurosci. 2001;21:7841–7847. doi: 10.1523/JNEUROSCI.21-19-07841.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chergui K, Akaoka H, Charlety PJ, Saunier CF, Buda M, Chouvet G. Subthalamic nucleus modulates burst firing of nigral dopamine neurones via NMDA receptors. Neuroreport. 1994;5:1185–1188. doi: 10.1097/00001756-199406020-00006. [DOI] [PubMed] [Google Scholar]
- Chergui K, Charlety PJ, Akaoka H, Saunier CF, Brunet JL, Buda M, Svensson TH, Chouvet G. Tonic activation of NMDA receptors causes spontaneous burst discharge of rat midbrain dopamine neurons in vivo. Eur J Neurosci. 1993;5:137–144. doi: 10.1111/j.1460-9568.1993.tb00479.x. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Christoffersen CL, Meltzer LT. Evidence for N-methyl-D-aspartate and AMPA subtypes of the glutamate receptor on substantia nigra dopamine neurons: possible preferential role for N-methyl-D-aspartate receptors. Neuroscience. 1995;67:373–381. doi: 10.1016/0306-4522(95)00047-m. [DOI] [PubMed] [Google Scholar]
- Chuhma N, Tanaka KF, Hen R, Rayport S. Functional connectome of the striatal medium spiny neuron. J Neurosci. 2011;31:1183–1192. doi: 10.1523/JNEUROSCI.3833-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JR, Toth DD, Highfield DA, Grant SJ. Glutamate-like immunoreactivity is present within cholinergic neurons of the laterodorsal tegmental and pedunculopontine nuclei. Advances in experimental medicine and biology. 1991;295:127–142. doi: 10.1007/978-1-4757-0145-6_5. [DOI] [PubMed] [Google Scholar]
- Comoli E, Coizet V, Boyes J, Bolam JP, Canteras NS, Quirk RH, Overton PG, Redgrave P. A direct projection from superior colliculus to substantia nigra for detecting salient visual events. Nat Neurosci. 2003;6:974–980. doi: 10.1038/nn1113. [DOI] [PubMed] [Google Scholar]
- Conrad LC, Pfaff DW. Autoradiographic tracing of nucleus accumbens efferents in the rat. Brain Res. 1976;113:589–596. doi: 10.1016/0006-8993(76)90060-3. [DOI] [PubMed] [Google Scholar]
- Contreras-Vidal JL, Schultz W. A predictive reinforcement model of dopamine neurons for learning approach behavior. J Comput Neurosci. 1999;6:191–214. doi: 10.1023/a:1008862904946. [DOI] [PubMed] [Google Scholar]
- Cornwall J, Cooper JD, Phillipson OT. Afferent and efferent connections of the laterodorsal tegmental nucleus in the rat. Brain research bulletin. 1990;25:271–284. doi: 10.1016/0361-9230(90)90072-8. [DOI] [PubMed] [Google Scholar]
- Cromwell HC, Schultz W. Effects of expectations for different reward magnitudes on neuronal activity in primate striatum. J Neurophysiol. 2003;89:2823–2838. doi: 10.1152/jn.01014.2002. [DOI] [PubMed] [Google Scholar]
- Cui G, Bernier BE, Harnett MT, Morikawa H. Differential regulation of action potential- and metabotropic glutamate receptor-induced Ca2+ signals by inositol 1,4,5-trisphosphate in dopaminergic neurons. J Neurosci. 2007;27:4776–4785. doi: 10.1523/JNEUROSCI.0139-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ardenne K, McClure SM, Nystrom LE, Cohen JD. BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science. 2008;319:1264–1267. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- Datta S, Mavanji V, Patterson EH, Ulloor J. Regulation of rapid eye movement sleep in the freely moving rat: local microinjection of serotonin, norepinephrine, and adenosine into the brainstem. Sleep. 2003;26:513–520. doi: 10.1093/sleep/26.5.513. [DOI] [PubMed] [Google Scholar]
- Deister CA, Teagarden MA, Wilson CJ, Paladini CA. An intrinsic neuronal oscillator underlies dopaminergic neuron bursting. J Neurosci. 2009;29:15888–15897. doi: 10.1523/JNEUROSCI.4053-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dommett E, Coizet V, Blaha CD, Martindale J, Lefebvre V, Walton N, Mayhew JE, Overton PG, Redgrave P. How visual stimuli activate dopaminergic neurons at short latency. Science. 2005;307:1476–1479. doi: 10.1126/science.1107026. [DOI] [PubMed] [Google Scholar]
- Dong Y, Saal D, Thomas M, Faust R, Bonci A, Robinson T, Malenka RC. Cocaine-induced potentiation of synaptic strength in dopamine neurons: behavioral correlates in GluRA(−/−) mice. Proc Natl Acad Sci U S A. 2004;101:14282–14287. doi: 10.1073/pnas.0401553101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durante P, Cardenas CG, Whittaker JA, Kitai ST, Scroggs RS. Low-threshold L-type calcium channels in rat dopamine neurons. J Neurophysiol. 2004;91:1450–1454. doi: 10.1152/jn.01015.2003. [DOI] [PubMed] [Google Scholar]
- Engblom D, Bilbao A, Sanchis-Segura C, Dahan L, Perreau-Lenz S, Balland B, Parkitna JR, Lujan R, Halbout B, Mameli M, Parlato R, Sprengel R, Luscher C, Schutz G, Spanagel R. Glutamate receptors on dopamine neurons control the persistence of cocaine seeking. Neuron. 2008;59:497–508. doi: 10.1016/j.neuron.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Mathe JM, Chergui K, Engberg G, Svensson TH. GABA(B) receptor-mediated modulation of the firing pattern of ventral tegmental area dopamine neurons in vivo. Naunyn Schmiedebergs Arch Pharmacol. 2002;365:173–180. doi: 10.1007/s00210-001-0519-5. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Faleiro LJ, Jones S, Kauer JA. Rapid synaptic plasticity of glutamatergic synapses on dopamine neurons in the ventral tegmental area in response to acute amphetamine injection. Neuropsychopharmacology. 2004;29:2115–2125. doi: 10.1038/sj.npp.1300495. [DOI] [PubMed] [Google Scholar]
- Ferreira JG, Del-Fava F, Hasue RH, Shammah-Lagnado SJ. Organization of ventral tegmental area projections to the ventral tegmental area-nigral complex in the rat. Neuroscience. 2008;153:196–213. doi: 10.1016/j.neuroscience.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Williams JT. Glutamate mediates an inhibitory postsynaptic potential in dopamine neurons. Nature. 1998;394:78–82. doi: 10.1038/27919. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Williams JT. Cholinergic inhibition of ventral midbrain dopamine neurons. J Neurosci. 2000;20:7855–7860. doi: 10.1523/JNEUROSCI.20-20-07855.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CP, Mark GP, Williams JT. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J Neurosci. 2006;26:2788–2797. doi: 10.1523/JNEUROSCI.4331-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyama F, Sohn J, Nakano T, Furuta T, Nakamura KC, Matsuda W, Kaneko T. Exclusive and common targets of neostriatofugal projections of rat striosome neurons: a single neuron-tracing study using a viral vector. Eur J Neurosci. 2011;33:668–677. doi: 10.1111/j.1460-9568.2010.07564.x. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions. J Comp Neurol. 2005;490:270–294. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci. 2002;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic. I. Compartmental organization of projections from the striatum to the substantia nigra in the rat. J Comp Neurol. 1985;236:454–476. doi: 10.1002/cne.902360404. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Young WS., 3rd Distribution of striatonigral and striatopallidal peptidergic neurons in both patch and matrix compartments: an in situ hybridization histochemistry and fluorescent retrograde tracing study. Brain Res. 1988;460:161–167. doi: 10.1016/0006-8993(88)91217-6. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984;4:2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Opposing effects of striatonigral feedback pathways on midbrain dopamine cell activity. Brain Res. 1985;333:271–284. doi: 10.1016/0006-8993(85)91581-1. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, North RA, Johnson SW. Alpha 1-adrenergic effects on dopamine neurons recorded intracellularly in the rat midbrain slice. Eur J Neurosci. 1995;7:1707–1713. doi: 10.1111/j.1460-9568.1995.tb00692.x. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Svensson TH. Prazosin modulates the firing pattern of dopamine neurons in rat ventral tegmental area. Eur J Pharmacol. 1993;233:79–84. doi: 10.1016/0014-2999(93)90351-h. [DOI] [PubMed] [Google Scholar]
- Gronier B, Rasmussen K. Activation of midbrain presumed dopaminergic neurones by muscarinic cholinergic receptors: an in vivo electrophysiological study in the rat. Br J Pharmacol. 1998;124:455–464. doi: 10.1038/sj.bjp.0701850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves PM, Wilson CJ, Young SJ, Rebec GV. Self-inhibition by dopaminergic neurons. Science. 1975;190:522–528. doi: 10.1126/science.242074. [DOI] [PubMed] [Google Scholar]
- Guatteo E, Mercuri NB, Bernardi G, Knopfel T. Group I metabotropic glutamate receptors mediate an inward current in rat substantia nigra dopamine neurons that is independent from calcium mobilization. J Neurophysiol. 1999;82:1974–1981. doi: 10.1152/jn.1999.82.4.1974. [DOI] [PubMed] [Google Scholar]
- Guiard BP, El Mansari M, Merali Z, Blier P. Functional interactions between dopamine, serotonin and norepinephrine neurons: an in-vivo electrophysiological study in rats with monoaminergic lesions. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2008;11:625–639. doi: 10.1017/S1461145707008383. [DOI] [PubMed] [Google Scholar]
- Gulacsi A, Lee CR, Sik A, Viitanen T, Kaila K, Tepper JM, Freund TF. Cell type-specific differences in chloride-regulatory mechanisms and GABA(A) receptor-mediated inhibition in rat substantia nigra. J Neurosci. 2003;23:8237–8246. doi: 10.1523/JNEUROSCI.23-23-08237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutlerner JL, Penick EC, Snyder EM, Kauer JA. Novel protein kinase A-dependent long-term depression of excitatory synapses. Neuron. 2002;36:921–931. doi: 10.1016/s0896-6273(02)01051-6. [DOI] [PubMed] [Google Scholar]
- Guzman JN, Sanchez-Padilla J, Chan CS, Surmeier DJ. Robust pacemaking in substantia nigra dopaminergic neurons. J Neurosci. 2009;29:11011–11019. doi: 10.1523/JNEUROSCI.2519-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Lynd E, Klein C, Groenewegen HJ. Topographic organization of the ventral striatal efferent projections in the rhesus monkey: an anterograde tracing study. J Comp Neurol. 1990;293:282–298. doi: 10.1002/cne.902930210. [DOI] [PubMed] [Google Scholar]
- Harnett MT, Bernier BE, Ahn KC, Morikawa H. Burst-timing-dependent plasticity of NMDA receptor-mediated transmission in midbrain dopamine neurons. Neuron. 2009;62:826–838. doi: 10.1016/j.neuron.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Byrne R, Aston-Jones G. Glutamate-associated plasticity in the ventral tegmental area is necessary for conditioning environmental stimuli with morphine. Neuroscience. 2004;129:841–847. doi: 10.1016/j.neuroscience.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Hausser MA, Yung WH. Inhibitory synaptic potentials in guinea-pig substantia nigra dopamine neurones in vitro. The Journal of physiology. 1994;479(Pt 3):401–422. doi: 10.1113/jphysiol.1994.sp020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- Houk JC, Adams JL, Barto AG. A model of how the basal ganglia generate and use neural signals that predict reinforcement. In: Houk JC, et al., editors. Models of information processing in the basal ganglia. Cambridge, MA: MIT Press; 1995. pp. 249–270. [Google Scholar]
- Hubert GW, Paquet M, Smith Y. Differential subcellular localization of mGluR1a and mGluR5 in the rat and monkey Substantia nigra. J Neurosci. 2001;21:1838–1847. doi: 10.1523/JNEUROSCI.21-06-01838.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114:475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iribe Y, Moore K, Pang KC, Tepper JM. Subthalamic stimulation-induced synaptic responses in substantia nigra pars compacta dopaminergic neurons in vitro. J Neurophysiol. 1999;82:925–933. doi: 10.1152/jn.1999.82.2.925. [DOI] [PubMed] [Google Scholar]
- Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009;513:566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia HG, Yamuy J, Sampogna S, Morales FR, Chase MH. Colocalization of gamma-aminobutyric acid and acetylcholine in neurons in the laterodorsal and pedunculopontine tegmental nuclei in the cat: a light and electron microscopic study. Brain Res. 2003;992:205–219. doi: 10.1016/j.brainres.2003.08.062. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol. 1992;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, Seutin V, North RA. Burst firing in dopamine neurons induced by N-methyl-D-aspartate: role of electrogenic sodium pump. Science. 1992;258:665–667. doi: 10.1126/science.1329209. [DOI] [PubMed] [Google Scholar]
- Johnson SW, Wu YN. Multiple mechanisms underlie burst firing in rat midbrain dopamine neurons in vitro. Brain Res. 2004;1019:293–296. doi: 10.1016/j.brainres.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Jones S, Kornblum JL, Kauer JA. Amphetamine blocks long-term synaptic depression in the ventral tegmental area. J Neurosci. 2000;20:5575–5580. doi: 10.1523/JNEUROSCI.20-15-05575.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juraska JM, Wilson CJ, Groves PM. The substantia nigra of the rat: a Golgi study. J Comp Neurol. 1977;172:585–600. doi: 10.1002/cne.901720403. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Alesdatter JE. Involvement of N-methyl-D-aspartate receptor stimulation in the ventral tegmental area and amygdala in behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1993;267:486–495. [PubMed] [Google Scholar]
- Kalivas PW, Churchill L, Klitenick MA. GABA and enkephalin projection from the nucleus accumbens and ventral pallidum to the ventral tegmental area. Neuroscience. 1993;57:1047–1060. doi: 10.1016/0306-4522(93)90048-k. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. Afferents to the GABAergic tail of the ventral tegmental area in the rat. J Comp Neurol. 2009;513:597–621. doi: 10.1002/cne.21983. [DOI] [PubMed] [Google Scholar]
- Khaliq ZM, Bean BP. Pacemaking in dopaminergic ventral tegmental area neurons: depolarizing drive from background and voltage-dependent sodium conductances. J Neurosci. 2010;30:7401–7413. doi: 10.1523/JNEUROSCI.0143-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Kitai ST. Efferent projections of the subthalamic nucleus in the rat: light and electron microscopic analysis with the PHA-L method. J Comp Neurol. 1987;260:435–452. doi: 10.1002/cne.902600309. [DOI] [PubMed] [Google Scholar]
- Kita JM, Kile BM, Parker LE, Wightman RM. In vivo measurement of somatodendritic release of dopamine in the ventral tegmental area. Synapse. 2009;63:951–960. doi: 10.1002/syn.20676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C. Biophysics of computation: information processing in single neurons. New York: Oxford University Press; 1999. [Google Scholar]
- Koga E, Momiyama T. Presynaptic dopamine D2-like receptors inhibit excitatory transmission onto rat ventral tegmental dopaminergic neurones. J Physiol. 2000;523 Pt 1:163–173. doi: 10.1111/j.1469-7793.2000.t01-2-00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiss DS, Mastropietro CW, Rawji SS, Walters JR. The response of subthalamic nucleus neurons to dopamine receptor stimulation in a rodent model of Parkinson's disease. J Neurosci. 1997;17:6807–6819. doi: 10.1523/JNEUROSCI.17-17-06807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60:543–554. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov AS, Kopell NJ, Wilson CJ. Transient high-frequency firing in a coupled-oscillator model of the mesencephalic dopaminergic neuron. J Neurophys. 2006;95:932–947. doi: 10.1152/jn.00691.2004. [DOI] [PubMed] [Google Scholar]
- Labouebe G, Lomazzi M, Cruz HG, Creton C, Lujan R, Li M, Yanagawa Y, Obata K, Watanabe M, Wickman K, Boyer SB, Slesinger PA, Luscher C. RGS2 modulates coupling between GABAB receptors and GIRK channels in dopamine neurons of the ventral tegmental area. Nat Neurosci. 2007;10:1559–1568. doi: 10.1038/nn2006. [DOI] [PubMed] [Google Scholar]
- Lacey MG, Calabresi P, North RA. Muscarine depolarizes rat substantia nigra zona compacta and ventral tegmental neurons in vitro through M1-like receptors. J Pharmacol Exp Ther. 1990;253:395–400. [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J Physiol. 1987;392:397–416. doi: 10.1113/jphysiol.1987.sp016787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. On the potassium conductance increase activated by GABAB and dopamine D2 receptors in rat substantia nigra neurones. J Physiol. 1988;401:437–453. doi: 10.1113/jphysiol.1988.sp017171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie B, Parent A. Pedunculopontine nucleus in the squirrel monkey: distribution of cholinergic and monoaminergic neurons in the mesopontine tegmentum with evidence for the presence of glutamate in cholinergic neurons. J Comp Neurol. 1994;344:190–209. doi: 10.1002/cne.903440203. [DOI] [PubMed] [Google Scholar]
- Lee CR, Abercrombie ED, Tepper JM. Pallidal control of substantia nigra dopaminergic neuron firing pattern and its relation to extracellular neostriatal dopamine levels. Neuroscience. 2004;129:481–489. doi: 10.1016/j.neuroscience.2004.07.034. [DOI] [PubMed] [Google Scholar]
- Lisman J. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proc Natl Acad Sci U S A. 1989;86:9574–9578. doi: 10.1073/pnas.86.23.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QS, Pu L, Poo MM. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437:1027–1031. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SJ, Zukin RS. Ca2+permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 2007;30:126–134. doi: 10.1016/j.tins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Lobb CJ, Troyer TW, Wilson CJ, Paladini CA. Disinhibition bursting of dopaminergic neurons. Frontiers in Systems Neuroscience. 2011a;5 doi: 10.3389/fnsys.2011.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobb CJ, Wilson CJ, Paladini CA. A dynamic role for GABA receptors on the firing pattern of midbrain dopaminergic neurons. J Neurophysiol. 2010;104:403–413. doi: 10.1152/jn.00204.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobb CJ, Wilson CJ, Paladini CA. High Frequency, Short Latency Disinhibition Bursting of Midbrain Dopaminergic Neurons. J Neurophysiol. 2011b doi: 10.1152/jn.01076.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokwan SJ, Overton PG, Berry MS, Clark D. Stimulation of the pedunculopontine tegmental nucleus in the rat produces burst firing in A9 dopaminergic neurons. Neuroscience. 1999;92:245–254. doi: 10.1016/s0306-4522(98)00748-9. [DOI] [PubMed] [Google Scholar]
- Luo Y, Good CH, Diaz-Ruiz O, Zhang Y, Hoffman AF, Shan L, Kuang SY, Malik N, Chefer VI, Tomac AC, Lupica CR, Backman CM. NMDA receptors on non-dopaminergic neurons in the VTA support cocaine sensitization. PLoS ONE. 2010;5:e12141. doi: 10.1371/journal.pone.0012141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu P, Malenka RC. Spike Timing-Dependent Long-Term Potentiation in Ventral Tegmental Area Dopamine Cells Requires PKC. J Neurophysiol. 2008 doi: 10.1152/jn.01384.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia TV, Frank MJ. From reinforcement learning models to psychiatric and neurological disorders. Nat Neurosci. 2011;14:154–162. doi: 10.1038/nn.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Mameli M, Balland B, Lujan R, Luscher C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science. 2007;317:530–533. doi: 10.1126/science.1142365. [DOI] [PubMed] [Google Scholar]
- Mameli M, Bellone C, Brown MT, Luscher C. Cocaine inverts rules for synaptic plasticity of glutamate transmission in the ventral tegmental area. Nat Neurosci. 2011 doi: 10.1038/nn.2763. [DOI] [PubMed] [Google Scholar]
- Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, Luscher C. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci. 2009;12:1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- Manzoni OJ, Williams JT. Presynaptic regulation of glutamate release in the ventral tegmental area during morphine withdrawal. J Neurosci. 1999;19:6629–6636. doi: 10.1523/JNEUROSCI.19-15-06629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Coker AR, Driscoll JR, Lemaitre AI, Fields HL. Reliability in the identification of midbrain dopamine neurons. PLoS ONE. 2010;5:e15222. doi: 10.1371/journal.pone.0015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL. Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc Natl Acad Sci U S A. 2006;103:2938–2942. doi: 10.1073/pnas.0511159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Mitchell JM, Ishikawa J, Hjelmstad GO, Fields HL. Midbrain Dopamine Neurons: Projection Target Determines Action Potential Duration and Dopamine D2 Receptor Inhibition. J Neurosci. 2008;28:8908–8913. doi: 10.1523/JNEUROSCI.1526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marubio LM, Gardier AM, Durier S, David D, Klink R, Arroyo-Jimenez MM, McIntosh JM, Rossi F, Champtiaux N, Zoli M, Changeux JP. Effects of nicotine in the dopaminergic system of mice lacking the alpha4 subunit of neuronal nicotinic acetylcholine receptors. Eur J Neurosci. 2003;17:1329–1337. doi: 10.1046/j.1460-9568.2003.02564.x. [DOI] [PubMed] [Google Scholar]
- Matsuda W, Furuta T, Nakamura KC, Hioki H, Fujiyama F, Arai R, Kaneko T. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J Neurosci. 2009;29:444–453. doi: 10.1523/JNEUROSCI.4029-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007 doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Mejias-Aponte CA, Drouin C, Aston-Jones G. Adrenergic and noradrenergic innervation of the midbrain ventral tegmental area and retrorubral field: prominent inputs from medullary homeostatic centers. J Neurosci. 2009;29:3613–3626. doi: 10.1523/JNEUROSCI.4632-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena-Segovia J, Winn P, Bolam JP. Cholinergic modulation of midbrain dopaminergic systems. Brain research reviews. 2008;58:265–271. doi: 10.1016/j.brainresrev.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Mercuri NB, Bonci A, Calabresi P, Stratta F, Stefani A, Bernardi G. Effects of dihydropyridine calcium antagonists on rat midbrain dopaminergic neurones. Br J Pharmacol. 1994;113:831–838. doi: 10.1111/j.1476-5381.1994.tb17068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, Dayan P, Sejnowski TJ. A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J Neurosci. 1996;16:1936–1947. doi: 10.1523/JNEUROSCI.16-05-01936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, Hyman SE, Cohen JD. Computational roles for dopamine in behavioural control. Nature. 2004;431:760–767. doi: 10.1038/nature03015. [DOI] [PubMed] [Google Scholar]
- Moraru II, Kaftan EJ, Ehrlich BE, Watras J. Regulation of type 1 inositol 1,4,5-trisphosphate-gated calcium channels by InsP3 and calcium: Simulation of single channel kinetics based on ligand binding and electrophysiological analysis. J Gen Physiol. 1999;113:837–849. doi: 10.1085/jgp.113.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa H, Khodakhah K, Williams JT. Two intracellular pathways mediate metabotropic glutamate receptor-induced Ca2+ mobilization in dopamine neurons. J Neurosci. 2003;23:149–157. doi: 10.1523/JNEUROSCI.23-01-00149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G, Arkadir D, Nevet A, Vaadia E, Bergman H. Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron. 2004;43:133–143. doi: 10.1016/j.neuron.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Naito A, Kita H. The cortico-pallidal projection in the rat: an anterograde tracing study with biotinylated dextran amine. Brain Res. 1994;653:251–257. doi: 10.1016/0006-8993(94)90397-2. [DOI] [PubMed] [Google Scholar]
- Neuhoff H, Neu A, Liss B, Roeper J. I(h) channels contribute to the different functional properties of identified dopaminergic subpopulations in the midbrain. J Neurosci. 2002;22:1290–1302. doi: 10.1523/JNEUROSCI.22-04-01290.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenberg MJ, Chan J, Liu Y, Edwards RH, Pickel VM. Ultrastructural localization of the vesicular monoamine transporter-2 in midbrain dopaminergic neurons: potential sites for somatodendritic storage and release of dopamine. J Neurosci. 1996;16:4135–4145. doi: 10.1523/JNEUROSCI.16-13-04135.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Nugent FS, Penick EC, Kauer JA. Opioids block long-term potentiation of inhibitory synapses. Nature. 2007;446:1086–1090. doi: 10.1038/nature05726. [DOI] [PubMed] [Google Scholar]
- Oakman SA, Faris PL, Kerr PE, Cozzari C, Hartman BK. Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to ventral tegmental area. J Neurosci. 1995;15:5859–5869. doi: 10.1523/JNEUROSCI.15-09-05859.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Harnett MT, Morikawa H. Hyperpolarization-activated cation current (Ih) is an ethanol target in midbrain dopamine neurons of mice. J Neurophysiol. 2006;95:619–626. doi: 10.1152/jn.00682.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Laterodorsal tegmental projections to identified cell populations in the rat ventral tegmental area. J Comp Neurol. 2005;483:217–235. doi: 10.1002/cne.20417. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Cholinergic axons in the rat ventral tegmental area synapse preferentially onto mesoaccumbens dopamine neurons. J Comp Neurol. 2006;494:863–875. doi: 10.1002/cne.20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Glutamate synaptic inputs to ventral tegmental area neurons in the rat derive primarily from subcortical sources. Neuroscience. 2007;146:1259–1274. doi: 10.1016/j.neuroscience.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Ultrastructural analysis of local collaterals of rat ventral tegmental area neurons: GABA phenotype and synapses onto dopamine and GABA cells. Synapse. 2009;63:895–906. doi: 10.1002/syn.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton P, Clark D. Iontophoretically administered drugs acting at the N-methyl-D-aspartate receptor modulate burst firing in A9 dopamine neurons in the rat. Synapse. 1992;10:131–140. doi: 10.1002/syn.890100208. [DOI] [PubMed] [Google Scholar]
- Overton PG, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res Brain Res Rev. 1997;25:312–334. doi: 10.1016/s0165-0173(97)00039-8. [DOI] [PubMed] [Google Scholar]
- Overton PG, Richards CD, Berry MS, Clark D. Long-term potentiation at excitatory amino acid synapses on midbrain dopamine neurons. Neuroreport. 1999;10:221–226. doi: 10.1097/00001756-199902050-00004. [DOI] [PubMed] [Google Scholar]
- Paladini CA, Celada P, Tepper JM. Striatal, pallidal, and pars reticulata evoked inhibition of nigrostriatal dopaminergic neurons is mediated by GABA(A) receptors in vivo. Neuroscience. 1999a;89:799–812. doi: 10.1016/s0306-4522(98)00355-8. [DOI] [PubMed] [Google Scholar]
- Paladini CA, Fiorillo CD, Morikawa H, Williams JT. Amphetamine selectively blocks inhibitory glutamate transmission in dopamine neurons. Nat Neurosci. 2001;4:275–281. doi: 10.1038/85124. [DOI] [PubMed] [Google Scholar]
- Paladini CA, Iribe Y, Tepper JM. GABAA receptor stimulation blocks NMDA-induced bursting of dopaminergic neurons in vitro by decreasing input resistance. Brain Res. 1999b;832:145–151. doi: 10.1016/s0006-8993(99)01484-5. [DOI] [PubMed] [Google Scholar]
- Paladini CA, Mitchell JM, Williams JT, Mark GP. Cocaine self-administration selectively decreases noradrenergic regulation of metabotropic glutamate receptor-mediated inhibition in dopamine neurons. J Neurosci. 2004;24:5209–5215. doi: 10.1523/JNEUROSCI.1079-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladini CA, Robinson S, Morikawa H, Williams JT, Palmiter RD. Dopamine controls the firing pattern of dopamine neurons via a network feedback mechanism. Proc Natl Acad Sci U S A. 2003;100:2866–2871. doi: 10.1073/pnas.0138018100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladini CA, Tepper JM. GABA(A) and GABA(B) antagonists differentially affect the firing pattern of substantia nigra dopaminergic neurons in vivo. Synapse. 1999;32:165–176. doi: 10.1002/(SICI)1098-2396(19990601)32:3<165::AID-SYN3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Paladini CA, Williams JT. Noradrenergic inhibition of midbrain dopamine neurons. J Neurosci. 2004;24:4568–4575. doi: 10.1523/JNEUROSCI.5735-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Hillard CJ, Liu QS. D2 dopamine receptor activation facilitates endocannabinoid-mediated long-term synaptic depression of GABAergic synaptic transmission in midbrain dopamine neurons via cAMP-protein kinase A signaling. J Neurosci. 2008a;28:14018–14030. doi: 10.1523/JNEUROSCI.4035-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Hillard CJ, Liu QS. Endocannabinoid signaling mediates cocaine-induced inhibitory synaptic plasticity in midbrain dopamine neurons. J Neurosci. 2008b;28:1385–1397. doi: 10.1523/JNEUROSCI.4033-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WX, Hyland BI. Pedunculopontine tegmental nucleus controls conditioned responses of midbrain dopamine neurons in behaving rats. J Neurosci. 2005;25:4725–4732. doi: 10.1523/JNEUROSCI.0277-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WX, Schmidt R, Wickens JR, Hyland BI. Dopamine cells respond to predicted events during classical conditioning: evidence for eligibility traces in the reward-learning network. J Neurosci. 2005;25:6235–6242. doi: 10.1523/JNEUROSCI.1478-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson OT. Afferent projections to the ventral tegmental area of Tsai and interfascicular nucleus: a horseradish peroxidase study in the rat. J Comp Neurol. 1979;187:117–143. doi: 10.1002/cne.901870108. [DOI] [PubMed] [Google Scholar]
- Placzek AN, Zhang TA, Dani JA. Age dependent nicotinic influences over dopamine neuron synaptic plasticity. Biochem Pharmacol. 2009;78:686–692. doi: 10.1016/j.bcp.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenz D, Kital ST. A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus. Nature. 1999;400:677–682. doi: 10.1038/23281. [DOI] [PubMed] [Google Scholar]
- Prensa L, Parent A. The nigrostriatal pathway in the rat: A single-axon study of the relationship between dorsal and ventral tier nigral neurons and the striosome/matrix striatal compartments. J Neurosci. 2001;21:7247–7260. doi: 10.1523/JNEUROSCI.21-18-07247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puopolo M, Raviola E, Bean BP. Roles of subthreshold calcium current and sodium current in spontaneous firing of mouse midbrain dopamine neurons. J Neurosci. 2007;27:645–656. doi: 10.1523/JNEUROSCI.4341-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave P, Gurney K. The short-latency dopamine signal: a role in discovering novel actions? Nat Rev Neurosci. 2006;7:967–975. doi: 10.1038/nrn2022. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ, Greenfield SA. Characteristics of electrically evoked somatodendritic dopamine release in substantia nigra and ventral tegmental area in vitro. J Neurophysiol. 1997;77:853–862. doi: 10.1152/jn.1997.77.2.853. [DOI] [PubMed] [Google Scholar]
- Richards CD, Shiroyama T, Kitai ST. Electrophysiological and immunocytochemical characterization of GABA and dopamine neurons in the substantia nigra of the rat. Neuroscience. 1997;80:545–557. doi: 10.1016/s0306-4522(97)00093-6. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Duran LF, Castillo DV, Moguel-Gonzalez M, Escobar ML. Conditioned taste aversion modifies persistently the subsequent induction of neocortical long-term potentiation in vivo. Neurobiology of learning and memory. 2011;95:519–526. doi: 10.1016/j.nlm.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Saitoh K, Isa T, Takakusaki K. Nigral GABAergic inhibition upon mesencephalic dopaminergic cell groups in rats. Eur J Neurosci. 2004;19:2399–2409. doi: 10.1111/j.0953-816X.2004.03337.x. [DOI] [PubMed] [Google Scholar]
- Schilstrom B, Yaka R, Argilli E, Suvarna N, Schumann J, Chen BT, Carman M, Singh V, Mailliard WS, Ron D, Bonci A. Cocaine enhances NMDA receptor-mediated currents in ventral tegmental area cells via dopamine D5 receptor-dependent redistribution of NMDA receptors. J Neurosci. 2006;26:8549–8558. doi: 10.1523/JNEUROSCI.5179-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward prediction in primate basal ganglia and frontal cortex. Neuropharmacology. 1998;37:421–429. doi: 10.1016/s0028-3908(98)00071-9. [DOI] [PubMed] [Google Scholar]
- Semba K, Fibiger HC. Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: a retro- and antero-grade transport and immunohistochemical study. J Comp Neurol. 1992;323:387–410. doi: 10.1002/cne.903230307. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol. 1992;320:145–160. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- Shepard PD, Bunney BS. Repetitive firing properties of putative dopamine-containing neurons in vitro: regulation by an apamin-sensitive Ca(2+)-activated K+ conductance. Exp Brain Res. 1991;86:141–150. doi: 10.1007/BF00231048. [DOI] [PubMed] [Google Scholar]
- Smith ID, Grace AA. Role of the subthalamic nucleus in the regulation of nigral dopamine neuron activity. Synapse. 1992;12:287–303. doi: 10.1002/syn.890120406. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bolam JP. The output neurones and the dopaminergic neurones of the substantia nigra receive a GABA-containing input from the globus pallidus in the rat. J Comp Neurol. 1990;296:47–64. doi: 10.1002/cne.902960105. [DOI] [PubMed] [Google Scholar]
- Smith Y, Charara A, Parent A. Synaptic innervation of midbrain dopaminergic neurons by glutamate-enriched terminals in the squirrel monkey. J Comp Neurol. 1996;364:231–253. doi: 10.1002/(SICI)1096-9861(19960108)364:2<231::AID-CNE4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Klanker M, de Ridder B, Bowers MS, Joosten RN, Feenstra MG, Bonci A. Reward-predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons. Science. 2008;321:1690–1692. doi: 10.1126/science.1160873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Plotkin J, Shen W. Dopamine and synaptic plasticity in dorsal striatal circuits controlling action selection. Curr Opin Neurobiol. 2009;19:621–628. doi: 10.1016/j.conb.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CW, Laude AJ. IP3 receptors and their regulation by calmodulin and cytosolic Ca2+ Cell Calcium. 2002;32:321–334. doi: 10.1016/s0143416002001859. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Lee CR. GABAergic control of substantia nigra dopaminergic neurons. Progress in brain research. 2007;160:189–208. doi: 10.1016/S0079-6123(06)60011-3. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Martin LP, Anderson DR. GABAA receptor-mediated inhibition of rat substantia nigra dopaminergic neurons by pars reticulata projection neurons. J Neurosci. 1995;15:3092–3103. doi: 10.1523/JNEUROSCI.15-04-03092.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Paladini CA, Celada P. GABAergic control of the firing pattern of substantia nigra dopaminergic neurons. Adv Pharmacol. 1998;42:694–699. doi: 10.1016/s1054-3589(08)60843-1. [DOI] [PubMed] [Google Scholar]
- Tobler PN, Dickinson A, Schultz W. Coding of predicted reward omission by dopamine neurons in a conditioned inhibition paradigm. J Neurosci. 2003;23:10402–10410. doi: 10.1523/JNEUROSCI.23-32-10402.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong ZY, Overton PG, Clark D. Chronic administration of (+)-amphetamine alters the reactivity of midbrain dopaminergic neurons to prefrontal cortex stimulation in the rat. Brain Res. 1995;674:63–74. doi: 10.1016/0006-8993(94)01439-o. [DOI] [PubMed] [Google Scholar]
- Tong ZY, Overton PG, Clark D. Antagonism of NMDA receptors but not AMPA/kainate receptors blocks bursting in dopaminergic neurons induced by electrical stimulation of the prefrontal cortex. J Neural Transm. 1996a;103:889–904. doi: 10.1007/BF01291780. [DOI] [PubMed] [Google Scholar]
- Tong ZY, Overton PG, Clark D. Stimulation of the prefrontal cortex in the rat induces patterns of activity in midbrain dopaminergic neurons which resemble natural burst events. Synapse. 1996b;22:195–208. doi: 10.1002/(SICI)1098-2396(199603)22:3<195::AID-SYN1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Tozzi A, Bengtson CP, Longone P, Carignani C, Fusco FR, Bernardi G, Mercuri NB. Involvement of transient receptor potential-like channels in responses to mGluR-I activation in midbrain dopamine neurons. Eur J Neurosci. 2003;18:2133–2145. doi: 10.1046/j.1460-9568.2003.02936.x. [DOI] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303:2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Walaas I, Fonnum F. Biochemical evidence for gamma-aminobutyrate containing fibres from the nucleus accumbens to the substantia nigra and ventral tegmental area in the rat. Neuroscience. 1980;5:63–72. doi: 10.1016/0306-4522(80)90071-8. [DOI] [PubMed] [Google Scholar]
- Wickens JR. Synaptic plasticity in the basal ganglia. Behav Brain Res. 2009;199:119–128. doi: 10.1016/j.bbr.2008.10.030. [DOI] [PubMed] [Google Scholar]
- Wickens JR, Horvitz JC, Costa RM, Killcross S. Dopaminergic mechanisms in actions and habits. J Neurosci. 2007;27:8181–8183. doi: 10.1523/JNEUROSCI.1671-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Callaway JC. Coupled oscillator model of the dopaminergic neuron of the substantia nigra. J Neurophysiol. 2000;83:3084–3100. doi: 10.1152/jn.2000.83.5.3084. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Groves PM, Fifkova E. Monoaminergic synapses, including dendrodendritic synapses in the rat substantia nigra. Exp Brain Res. 1977a;30:161–174. doi: 10.1007/BF00237248. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Young SJ, Groves PM. Statistical properties of neuronal spike trains in the substantia nigra: cell types and their interactions. Brain Res. 1977b;136:243–260. doi: 10.1016/0006-8993(77)90801-0. [DOI] [PubMed] [Google Scholar]
- Wise RA. Roles for nigrostriatal--not just mesocorticolimbic--dopamine in reward and addiction. Trends Neurosci. 2009;32:517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Wolfart J, Neuhoff H, Franz O, Roeper J. Differential expression of the small-conductance, calcium-activated potassium channel SK3 is critical for pacemaker control in dopaminergic midbrain neurons. J Neurosci. 2001;21:3443–3456. doi: 10.1523/JNEUROSCI.21-10-03443.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfart J, Roeper J. Selective coupling of T-type calcium channels to SK potassium channels prevents intrinsic bursting in dopaminergic midbrain neurons. J Neurosci. 2002;22:3404–3413. doi: 10.1523/JNEUROSCI.22-09-03404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooltorton JR, Pidoplichko VI, Broide RS, Dani JA. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J Neurosci. 2003;23:3176–3185. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Driscoll JR, Wilbrecht L, Margolis EB, Fields HL, Hjelmstad GO. Nucleus accumbens medium spiny neurons target non-dopaminergic neurons in the ventral tegmental area. J Neurosci. 2011;31:7811–7816. doi: 10.1523/JNEUROSCI.1504-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Basile AS, Fedorova I, Zhang W, Duttaroy A, Cui Y, Lamping KG, Faraci FM, Deng CX, Wess J. Novel insights into M5 muscarinic acetylcholine receptor function by the use of gene targeting technology. Life sciences. 2003;74:345–353. doi: 10.1016/j.lfs.2003.09.022. [DOI] [PubMed] [Google Scholar]
- Yeomans J, Forster G, Blaha C. M5 muscarinic receptors are needed for slow activation of dopamine neurons and for rewarding brain stimulation. Life sciences. 2001;68:2449–2456. doi: 10.1016/s0024-3205(01)01038-4. [DOI] [PubMed] [Google Scholar]
- Zahm DS. Evidence for a morphologically distinct subpopulation of striatipetal axons following injections of WGA-HRP into the ventral tegmental area in the rat. Brain Res. 1989;482:145–154. doi: 10.1016/0006-8993(89)90552-0. [DOI] [PubMed] [Google Scholar]
- Zhang TA, Placzek AN, Dani JA. In vitro identification and electrophysiological characterization of dopamine neurons in the ventral tegmental area. Neuropharmacology. 2010;59:431–436. doi: 10.1016/j.neuropharm.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XF, Hu XT, White FJ, Wolf ME. Increased responsiveness of ventral tegmental area dopamine neurons to glutamate after repeated administration of cocaine or amphetamine is transient and selectively involves AMPA receptors. J Pharmacol Exp Ther. 1997;281:699–706. [PubMed] [Google Scholar]
- Zweifel LS, Argilli E, Bonci A, Palmiter RD. Role of NMDA receptors in dopamine neurons for plasticity and addictive behaviors. Neuron. 2008;59:486–496. doi: 10.1016/j.neuron.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS, Fadok JP, Argilli E, Garelick MG, Jones GL, Dickerson TM, Allen JM, Mizumori SJ, Bonci A, Palmiter RD. Activation of dopamine neurons is critical for aversive conditioning and prevention of generalized anxiety. Nat Neurosci. 2011;14:620–626. doi: 10.1038/nn.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Darvas M, Kim MJ, Mizumori SJ, Paladini CA, Phillips PE, Palmiter RD. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci U S A. 2009;106:7281–7288. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]