Abstract
Diacylglycerol kinases (DGKs) metabolize diacylglycerol (DAG) to phosphatidic acid (PA). In T lymphocytes, DGKα acts as a negative regulator of TCR signaling by decreasing diacylglycerol levels and inducing anergy. Here, we show that upon co-stimulation of the TCR with CD28 or SLAM, DGKα, but not DGKζ, exit from the nucleus and undergoes rapid negative regulation of its enzymatic activity. Inhibition of DGKα is dependent on the expression of SAP, an adaptor protein mutated in X-linked lymphoproliferative disease (XLP), which is essential for SLAM-mediated signaling and contributes to TCR/CD28-induced signaling and T cell activation. Accordingly, over-expression of SAP is sufficient to inhibit DGKα, while SAP mutants unable to bind either phospho-tyrosine residues or SH3 domain are ineffective. Moreover phospholipase C activity and calcium, but not Src-family tyrosine kinases, are also required for negative regulation of DGKα. Finally, inhibition of DGKα in SAP-deficient cells partially rescues defective TCR/CD28 signaling, including Ras and ERK-1/2 activation, PKCθ membrane recruitment, induction of NF-AT transcriptional activity and IL-2 production. Thus SAP-mediated inhibition of DGKα sustains diacylglycerol signaling, thereby regulating T cell activation and may represent a novel pharmacological strategy for XLP treatment.
Introduction
In T lymphocytes, engagement of the TCR by specific antigens, along with stimulation by co-stimulatory receptors such as CD28, leads to T cell activation, cytokine production and differentiation. Moreover, several other receptors influence cell activation by quantitatively or qualitatively modifying immunoreceptor-derived signals. Conversely, stimulation via the TCR alone, while partially activating intracellular signaling pathways, is not sufficient to induce effector functions such as cytokine production and proliferation (1).
SLAM (CD150) is a homotypic transmembrane receptor expressed in T and B lymphocytes, dendritic cells and monocytes (2). Upon engagement, SLAM undergoes a conformational change leading to Fyn-mediated tyrosine phosphorylation and activation of several signaling pathways that modulate TCR-induced responses (2). Fyn recruitment to the activated SLAM is mediated by SAP, an adaptor protein comprising a single SH2 domain and a SH3 domain-binding sequence (3). In humans, SAP loss-of-function mutations cause X-linked lymphoproliferative disease (XLP), an immune disorder characterized by a deregulated immune response to Epstein-Barr virus, susceptibility to lymphoma and defective antibody production (4). Interestingly, SAP-deficient T lymphocytes from either XLP patients or SAP knock-out mice exhibit defective responses to TCR/CD28 co-stimulation in vitro: i) T cells from XLP patients feature reduced ERK-1/2 and NF-kB activation, decreased IL-2 production and impaired proliferation (5); ii) CD4+ T cells from XLP patients exhibit reduced ICOS expression and IL-10 production (6); iii) T cells from SAP knockout mice feature reduced PKCθ membrane recruitment, Bcl-10 phosphorylation and NF-kB activation, which are associated with defective IL-4 secretion and enhanced INFγ production (7).
Antigen-mediated activation of the TCR in the presence of other co-activating molecules triggers a complex signaling network leading to transcriptional activation of specific genes whose expression mediates T cell proliferation and differentiation. Activation of Ras and PKCθ triggers key signaling pathways, leading, among others, to the activation of NF-AT and NF-kB and contributing to transcription of the IL-2 gene (8, 9). In T cells, activation of Ras and PKCθ is dependent on the generation of diacylglycerol (DAG) through PLC-mediated hydrolysis of phosphatidylinositol-4,5-bis-phosphate. DAG recruits RasGRP, the Ras-GEF mainly responsible for TCR-induced Ras activation, and PKCθ to the plasma membrane (10, 11). Notably, engagement of TCR in the absence of co-stimulation results in a weak and transient activation of both Ras and PKCθ, which drives T cells into anergy, a hypo responsive status characterized by the inability to produce IL-2 and proliferate (12, 13).
DAG generated upon T cell activation is rapidly metabolized by Diacylglycerol kinases (DGKs), a multigenic family of enzymes responsible for phosphorylation of DAG to phosphatidic acid (PA). Consistently with the crucial role of DAG signaling in T cell activation, several pieces of evidence indicate that the DGKα and ζ isoforms, which are highly expressed in thymus and T cells, act as negative regulators of TCR signaling and immune cell function (14). Specifically i) genetic deletion of DGKα and ζ in T cells enhances TCR-induced activation of ERK-1/2 resulting in defective induction of anergy (15, 16); ii) DGKα is strongly induced in anergic T cells (13); iii) over-expression of either DGKα or DGKζ impairs CD3/CD28-induced activation of Ras signaling (17–19); iv) pharmacological inhibition of DGKs reverses the inability of anergic cells to produce IL-2 in response to TCR stimulation (13); v) DGKα expression is down-regulated within a few hours from T cell activation (19). Collectively, these data support the concept that second messengers signaling is highly dependent on the fine tuning of DAG synthesis and degradation rates. While there is no evidence for regulation of DGKζ upon T cell activation, TCR/CD28 co-stimulation of T cells results in rapid and sustained recruitment of DGKα to the plasma membrane (19), an event mediated by both Lck-dependent phosphorylation of tyrosine 335 and calcium binding to the EF hand domain of DGKα (20, 21).
Based on the role of DGKα as a negative regulator of T cell responses, we investigated the hypothesis that, upon T cell stimulation, DGKα activity might undergo negative regulation. Herein we show indeed that the enzymatic activity of DGKα is inhibited upon co-stimulation of TCR and CD28 through a SAP-mediated mechanism. Moreover, we found that, in SAP-deficient cells, defective TCR/CD28 signaling and T cell activation can be partially rescued by inhibition of DGKα.
Material and Methods
Cell Culture
Jurkat A3 cells (LGC) and 293FT (Life Technologies) were cultured respectively in RPMI GlutaMAX medium or DMEM GlutaMAX high glucose (Life Technologies), supplemented with 10% fetal bovine serum (FBS, Life Technologies) and antibiotic-antimycotic solution (Sigma-Aldrich), in humidified atmosphere with 5% CO2. Peripheral blood mononuclear cells (PBLs) were isolated by Lymphoprep gradient (Axis-Shield) of ACD (130 mM citric acid, 152 mM sodium citrate and 112 mM glucose)-treated venous blood obtained from healthy volunteers after informed consent. Briefly, blood was layered onto Ficoll–Hypaque separating media, after 20 min centrifugation at 300 RCF, cells were collected, washed and suspended in RPMI GlutaMAX medium supplemented with 10% heat inactivated FBS and antibiotic-antimycotic solution. Monocytes were depleted by plastic adherence at 37 °C for 1 h and the remaining PBLs were maintained for 18 hours in a humidified atmosphere with 5% CO2 before further stimulation. BI-141 TTS-SAP were a kind gift of A. Veillette (Montreal, Canada).
Jurkat/SAP-shRNA cells were obtained by infection of Jurkat cells with lentiviruses encoding SAP-specific shRNA in pLKO.1-Puro vector (82712 RNAi Consortium through Sigma-Genosys), sequence: CCGGCACAAGGTACTACAGGGATAACTCGAGTTATCCCTGTAGTACCTTGTGTTTTTG. Jurkat/control-shRNA were obtained by infection with lentiviruses encoding a shRNA specific for murine DGKα, in pLKO.1-Puro vector (24825 RNAi Consortium through Sigma-Genosys), sequence: CCGGGAGCTAAGTAAGGTGGTATATCTCGAGATATACCACCTTACTTACTTAGCTCTTTTT.
Lentivirus production and Jurkat infection were carried out according to the manufacturer’s instructions. Infected Jurkat cells were selected for 14 days in puromycin (1μg/ml) and used as a bulk population in all experiments.
Reagents
Antibodies used: Pan-Ras (Ab-4, Merck), H-Ras (F235, Cel Signaling), LAT (Santa Crutz), TAC (Abcam), CD3 agonist (OKT3, provided by U. Dianzani, Novara, Italy), CD28 agonist (ANC28.1/5D10, Ancell) except for Fig. 4D where anti-CD28 was from Pharmingen, SLAM agonistic antibody (A12, Biolegend), anti DGKζ antibodies (kind gift of M. Topham, Salt Lake City, USA), mixture of DGKα antibodies used for immunoprecipitation (22), DGKα (C-20) and PKCθ (Santa Cruz) were used for immunofluorescence, ERK1/2 and phospho-ERK1/2 from Cell Signalling for Supplementary material Fig. 2 and from Transduction Laboratories for Fig. 5C, SAP (FL-128, Upstate Biotech), α-tubulin (Sigma-Aldrich), secondary horseradish peroxidase-conjugated antibodies (PerkinElmer), secondary fluorescein isothiocyanate (FITC)-conjugated antibody (Dako), Alexa Flour 546-phalloidin (Life Technologies). In all experiments involving stimulation with antibodies, species-matched pre-immune serum (Santa Cruz) was used for controls in equal amount.
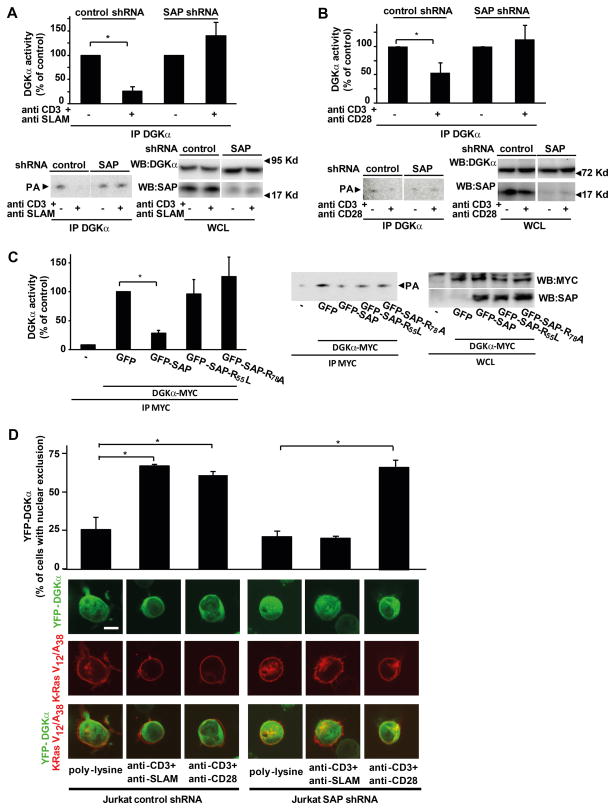
Figure 4. SAP negatively regulates DGKα activity.
(A) Jurkat control-shRNA or Jurkat SAP-shRNA cells were stimulated for 15 min with 10 μg/ml anti-CD3 and anti-SLAM antibodies and lysed. Anti-DGKα immunoprecipitates were assayed for DGK enzymatic activity while an aliquot of whole cell lysate was analyzed by western blot with anti-DGKα antibody to ensure equal loading and with anti-SAP antibody to verify the down-regulation of SAP expression. A representative experiment is shown together with a graph of the mean ± SE of three independent experiments shown as percentage of control, * t-test vs. control p<0,05.
(B) Jurkat control-shRNA or Jurkat SAP-shRNA were stimulated for 15 min with 10 μg/ml anti-CD3 and anti-CD28 antibodies and lysed. Anti DGKα immunoprecipitates were assayed for DGK enzymatic activity while an aliquot of whole cell lysate was analyzed by western blot with anti-DGKα antibody to ensure equal loading and with anti-SAP antibody to verify the down-regulation of SAP expression. A representative experiment is shown together with a graph of the mean ± SE of three independent experiments shown as percentage of control, * t-test vs. control p<0.05.
(C) Jurkat A3 cells were transiently co-transfected with myc-DGKα and the indicated GFP-SAP mutants. After 48 hours, cells were lysed and anti-myc immunoprecipitates were assayed for DGK enzymatic activity while an aliquot of whole cell lysate was assayed by western blot with anti-myc and anti-SAP antibodies to verify transfection efficiency. A representative experiment is shown along with a graph showing the mean ± SE of three independent experiments shown as percentage of control, * t-test vs. control p<0.05.
(D) Jurkat control-shRNA and Jurkat SAP-shRNA were transfected with YFP-DGKα (green) and DS-Red-K-Ras V12/A38 (red). After 24 hours, cells were serum starved for 2 hours, seeded for 1 hour on poly-L-lysine, anti-CD3+anti-SLAM or anti-CD3+antiCD28 agonistic antibodies (10 μg/ml each) coated glass-bottom dishes and images acquired. Representative pictures are shown together with a quantification from three independent experiments, * t-test vs. control p<0.05. Scale bar = 5 μM.
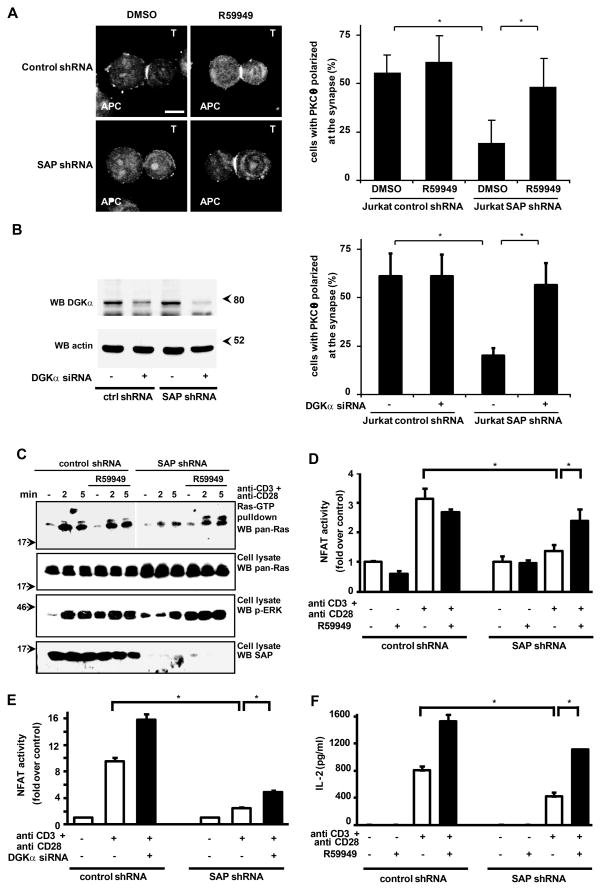
Figure 5. DGKα inhibition rescues defective TCR-induced diacylglycerol-dependent signaling and IL-2 production of SAP-deficient T lymphocytes.
(A) Jurkat control-shRNA and Jurkat SAP-shRNA cells (T) were pretreated with R59949 (10 μM 30 min) incubated with super antigen-loaded Raji cells (APC) for 15 min, fixed and stained for PKCθ. Representative pictures are shown. Scale bar = 5 μm. Cells displaying PKCθ at the immune synapse were counted. The histogram shows data from 3 independent experiments as mean +/− SE (* t-test p<0.05).
(B) Jurkat control-shRNA and Jurkat SAP-shRNA cells (T) were transfected with DGKα specific siRNA or control siRNA. After 72 hours cells were lysed and analyzed by western blot with anti DGKα and anti actin antibodies (left panel). At the same time cells were incubated with super antigen-loaded Raji cells (APC) for 15 min, fixed and stained for PKCθ. Cells displaying PKCθ at the synapse were counted (right panel). The histogram shows data from 3 independent experiments as mean +/− SE (* t-test p<0.05).
(C) Control shRNA Jurkat or SAP shRNA Jurkat cells were stimulated with 1 μg/ml anti-CD3 and 0,1 μg/ml anti-CD28 antibodies, in the presence or in absence of 1 μM R59949. After 15 min cells were lysed and Ras-GTP separated by pull-down with Raf-RBD and quantified by western blotting with anti pan-Ras antibody. Total Ras, phospho-ERK-1/2 and SAP content were revealed in whole cell lysates by western blotting.
(D) Jurkat control-shRNA and Jurkat SAP-shRNA were transfected with a dual-luciferase NF-AT reporter system. After 48 hours cells were stimulated with 1 μg/ml anti-CD3 and anti-CD28 antibodies in the presence or absence of R59949 1mM. After 16 hours of stimulation cells were lysed and analyzed for NF-AT-driven luciferase activity. Graph shows the mean ± SE of quadruplicates of a representative experiment, * t-test vs. control p<0.05.
(E) Jurkat control-shRNA or Jurkat SAP-shRNA were transfected with a siRNA targeting DGKα or a control siRNA and a dual-luciferase NF-AT reporter system. After 48 hours, cells were stimulated with 1 μg/ml anti-CD3 and anti-CD28 antibodies. After 16 hours of stimulation cells were lysed and analyzed for NF-AT driven luciferase activity. Graph shows the mean ± SE of quadruplicates of a representative experiment, * t-test vs. control p<0.05.
(F) Jurkat control-shRNA or Jurkat SAP-shRNA were stimulated with 1 μg/ml anti-CD3 and 0.1 μg/ml anti-CD28 antibodies. After 72 hours, cells were lysed and the amount of IL-2 released in the medium was measured by ELISA. Graph shows the mean ± SE of four replicates of a representative experiment, * t-test vs. control p<0.05.
Inhibitors used were from Sigma-Aldrich: R59949, DGKs inhibitor; PP2, Src-family inhibitor; U73122, PLC inhibitor; BAPTA-AM, cell permeable Calcium chelator; wortmannin, PI3Ks inhibitor; IPA-3, PAK-specific inhibitor. BAPTA-AM was dissolved in water while others inhibitors were dissolved in DMSO. DMSO was always used in control samples at the same dilution as the inhibitor tested.
Expression Vectors and Transfections
GFP-SAP-wt, GFP-SAP-R78A and GFP-SAP-R55L were a kind gift of P. Schwartzberg (NIH, Betesda, USA). N-terminal yellow fluorescent protein (YFP)-DGKα was obtained by cloning DGKα in pYFP-N-DEST (Life Technologies) using the Gateway kit (Life Technologies) according to manufacturer’s instructions. pNFAT-TA-luciferase reporter vector and pRL-TK normalization vector were for Clontech. SiRNA and negative control siRNA were from Ambion (Life Technologies): DGKα siRNA (23) sense GGUCAGUGAUGUCCUAAAGTT, antisense CUUUAGGACAUCACUGACCTT
Transient transfections in Fig. 5D and 5E were performed using Lipofectamine2000 reagent (Life Technologies) according to the manufacturer’s instructions. Microporation of Jurkat cells for imaging experiments were performed according to manufacturer’s instructions with the Microporator MP100 system–Digital Bio technology (Fig. 2 and supplemental Fig. 3B) or Gene pulser II from Biorad (Fig. 5B).
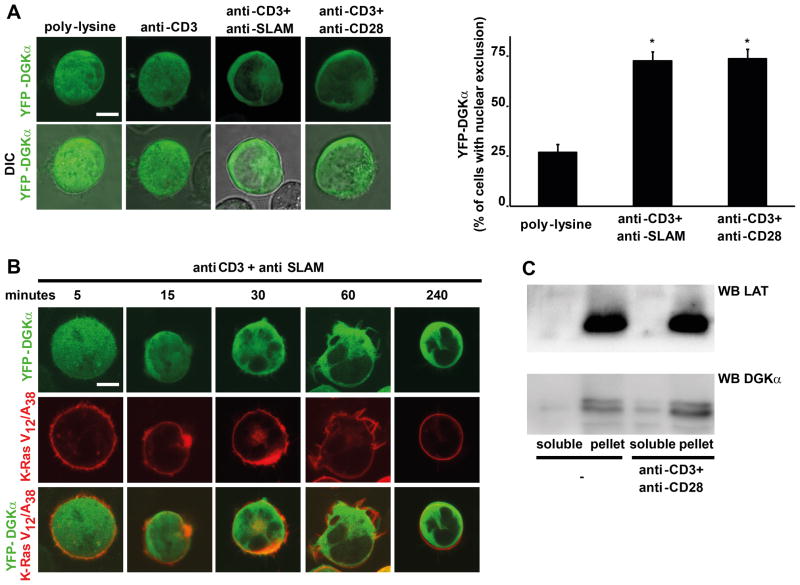
Figure 2. YFP-DGKα localization upon T cell stimulation.
(A) Jurkat A3 cells were transfected with YFP-DGKα (green) and after 24 hours were serum starved for 2 hours and seeded for 1 hour on either poly-L-lysine, anti-CD3, anti-CD3+anti-SLAM or anti-CD3+anti-CD28 (10 μg/ml each) coated glass-bottom dishes and microscope images acquired. Representative pictures are shown along with a quantification from three independent experiments, * t-test vs. control p<0.0005. Scale bar = 5 μm.
(B) Jurkat A3 cells were transfected with YFP-DGKα (green) and DS-Red-K-Ras V12/A38 (red), and after 72 hours were serum starved for 2 hours and seeded on anti-CD3+anti-SLAM agonistic antibodies (10 μg/ml each) coated glass-bottom dishes and images acquired at the indicated times. Representative pictures are shown, scale bar = 5 μm.
(C) Jurkat A3 cells were stimulated with anti-CD3 and anti-CD28 agonistic antibodies (10 μg/ml each) and homogenized 1 hour later. The post-nuclear and -mitochondrial fraction was separated by centrifugation (100,000 RCF) in a soluble fraction and in a membrane-associated fraction. 1/50 of the soluble fraction and the entire membrane-associated fraction were analyzed by western blotting for DGKα and LAT content.
Cell stimulation, preparation of cell lysates and homogenates, immunoprecipitation, western blotting, DGK assay
Cells (3×107/ml) were resuspended in RPMI and incubated for the indicated time with agonist antibodies or control species-matched pre-immune serum at 37°C. For immunoprecipitation, 3×107 cells were lysed in 1 ml of lysis buffer A (25mM Hepes (pH 8), 1% NP-40, 10% glycerol, 150mM NaCl, 5mM EDTA, 2mM EGTA, 1mM ZnCl2, 50mM ammonium molibdate, 10mM NaF, 1mM sodium orthovanadate and protease inhibitor cocktail from Sigma). An aliquot of cell lysate were retained for western blot analysis and the remaining was immunoprecipitated with a mixture of anti-DGKα antibodies as previously described (24). Whole cell homogenates were prepared by homogenizing 3×107 cells in 1 ml cold buffer B (buffer A without detergent) by 20 passages in a 23 G syringe. Protein concentration was determined by BCA (Pierce) and equal amounts of proteins were loaded in each lane. SDS–PAGE and western blots were performed as described previously (25). Western blot results were acquired with Versadoc system and quantified using QuantityOne software (Bio-rad).
DGKα activity in cell homogenates (25 μl) and anti-DGKα immunoprecipitates was assayed by measuring initial velocities (5 min at 30°C) as previously described (24). Radioactive signals were detected and quantified by GS-250 Molecular Imager and Phosphor Analyst Software (Bio-Rad).
Immunofluorescence
For immunofluorescence on fixed cells with antibody stimulation, cells were seeded on poly-L-Lysine-coated glass coverslips (Marienfeld) in 24-well plates for 1 hour, and then stimulated with 10 μg/ml of agonist antibodies for 1 hour in the presence or absence of the indicated inhibitors. Cells were then fixed with formaldehyde and stained as previously described (26). Confocal images were acquired with Leica confocal microscope TSP2 (objective 63x, numerical aperture 1,32) and analysed with LCS confocal software (Leica).
For the immunological synapse experiments, Raji cells (used as APC) were incubated for 2 h with 10 μg/ml staphylococcal enterotoxin E. Raji cells were washed, mixed with Jurkat control-shRNA or Jurkat SAP-shRNA (1:1) for 15 min and plated on polylysine-coated wells of diagnostic microscope slides (Erie Scientific Company). Cells were allowed to adhere for 15 min and then fixed in methanol at −20°C for 10 min. Samples were then washed for 5 min in PBS and incubated with anti-PKCθ antibody overnight at 4°C. After washing in PBS, samples were incubated for 1 h at room temperature with FITC-labeled anti-goat antibody. Images were taken using an Axio Imager Z1 microscope equipped with a HBO 50-W mercury lamp for epifluorescence and with an AxioCam HR cooled charge-coupled camera (Carl Zeiss).
For live cell imaging experiments, Jurkat A3 cells, Jurkat control-shRNA and Jurkat SAP-shRNA were microporated and serum starved in RPMI + 0.2% BSA + Hepes 50 mM for 2 hours. Cells were seeded on glass-bottom dishes coated with Poly-L-lysine or with the agonistic antibody anti-CD3, anti-CD3+anti-SLAM or anti-CD3+anti-CD28 at the final concentration of 10 μg/ml. Confocal images were acquired at the indicated times with a Zeiss LSM 510 (Carl Zeiss) inverted laser scanning microscope (LSM) using a C-Apochromat X63 water immersion objective lens (Zeiss). LSM image files were processed using the Zeiss “ZEN” LSM image browser software. When comparisons among images were to be made, the photos were taken in identical conditions and equally manipulated using Adobe Photoshop 7.0 software (Adobe Systems).
Cell fractionation
Cells (3×107/ml) were re-suspended in RPMI and incubated for the indicated time with agonist antibodies or control species-matched pre-immune serum at 37°C. Whole cell homogenates were prepared by homogenizing 3×107 cells as described above and sonicating the homogenates for 1 minute. Post-nuclear and -mitocondrial fraction (obtained by 10 min centrifugation at 10,000 RCF) was further separated by ultra-centrifugation (30 min at 10,0000 RCF). Surnatants (soluble cytoplasmic fraction) and pellet (insoluble membrane fraction) were collected and SDS–PAGE and western blots were performed as described previously (25) using anti-DGKα and anti-LAT antibodies.
Biochemical Ras activation assays
Recombinant GST-c-Raf-RBD protein was produced in E. Coli as described (27). Jurkat cells were serum deprived (2 h in RPMI supplemented with 0.2 % fatty-acid-free/endotoxin-low BSA and 50 mM HEPES pH 7.5). After stimulation, 1 ml of the cell suspension (107 cells) was lysed in 1 ml ice cold lysis buffer (50 mM HEPES pH 7.5, 140 mM NaCl, 5 mM MgCl2, 1 mM DTT, 1% NP-40, protease inhibitors) supplemented with 25 μg GST-RBD protein and 100 μM GDP, in order to quench post-lytic GTP-loading and GAP-dependent Ras-bound GTP hydrolysis, respectively. Cell extracts were cleared by centrifugation and GST-RBD/Ras-GTP complexes were collected on Glutatione-sepharose, washed once with lysis buffer and processed for SDS-PAGE analysis.
Mammalian two hybrid system
A modified Clontech MatchMaker (Becton-Dickinson) mammalian 2/3-hybrid assays was used. Full-length human SAP and its point-mutated variants were cloned into the pM series vectors as GAL4-binding domain fusions.
Either full length DGKα or the N-terminal DGKα fragment were cloned into a pVP vector to direct expression of VP16-activation domain fusion proteins. These were co-transfected into sub confluent HEK293 cells with a GAL4-luciferase reporter plasmid and a pVAX-based expression plasmid containing full-length human FynT. Luciferase activity was measured after 24h using a commercial kit (Promega).
NF-AT assay
Jurkat cells (4×106/ml) were co-transfected with pNF-AT-TA-luc and pRL-TK plasmids. After 48 h, cells were stimulated as indicated for 16 hours. Luciferase was assay with dual Luciferase reporter assay system (Promega) according to the manufacturer’s instructions and assessed using Victor3V multilabel counter (Perkin Elmer). NF-AT driven firefly luciferase activity was normalized for the reference renilla luciferase activity to take in account differences in transfection and expression efficiency and all values were expressed as fold increase upon unstimulated controls.
IL-2 assay
Jurkat cells (1×105) were plated in 100 μl medium supplemented with 10% FBS, and stimulated as indicated for 72 hours. IL-2 released in the media was measured by ELISA (GE Healthcare).
Statistical Analysis
The data were expressed as means ± SE. Statistical analysis was determined by student T test.
Results
Negative regulation of DGKα during T cell activation
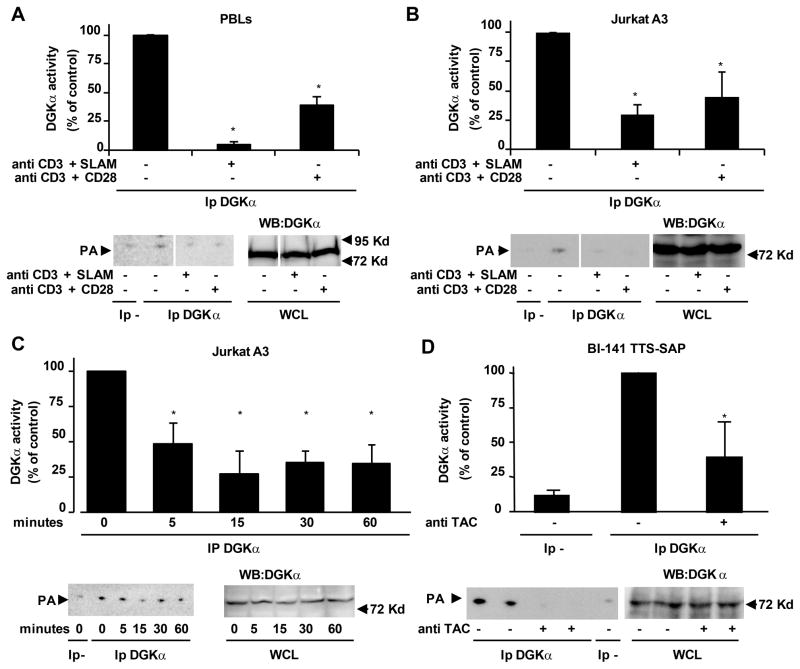
Since DGKα negatively regulates T cell activation (17, 19), we set out to investigate whether it is regulated in the early phase of lymphocyte activation. To this purpose we assayed the enzymatic activity and sub cellular localization of DGKα upon activation of primary lymphocytes (PBLs) and Jurkat leukemic T cells. DGKα activity was measured in vitro in the presence of exogenous substrates in anti-DGKα immunoprecipitates obtained from either control or stimulated lymphocytes. Following 15 min of co-stimulation of PBLs with agonistic anti-CD3 and anti-CD28 antibodies, the enzymatic activity of DGKα was reduced by about 60% as compared to unstimulated cells (Fig. 1A), without any change in DGKα protein content (Fig. 1A lower right panel). Stimulation of PBLs with anti-CD3 antibody alone did not significantly affect DGKα activity (data not shown). Since activation of SLAM family receptors was reported to enhance TCR signaling (28, 29), we investigated whether SLAM might regulate the enzymatic activity of DGKα. Indeed, 15 min of co-stimulation of PBLs with anti-CD3 and anti-SLAM agonist antibodies resulted in an even stronger inhibition of DGKα activity without affecting DGKα protein content (Fig. 1A). We then measured DGKα activity in anti-DGKα immunoprecipitates from Jurkat leukemia cells following co-stimulation with anti-CD3 and either anti-CD28 or anti-SLAM agonist antibodies. Similar to the data in PBLs, DGKα enzymatic activity was strongly reduced upon 15 min of co-stimulation via the TCR and either SLAM or CD28 and lasted for at least 1 hour, without changes in DGKα protein content (Fig. 1B, 1C). Finally, in order to address the reported ambiguity of how anti-SLAM antibodies may affect SLAM signaling, we used an alternative approach to induce SLAM signaling. We used a chimeric receptor featuring SLAM intracellular domain and the extracellular and transmembrane regions of the human IL-2 receptor α chain co-expressed with SAP in BI-141 lymphocytes (30). Crosslinking of the chimeric receptor with anti IL-2 α–receptor antibody (TAC) triggers SLAM signaling (30) and it was sufficient to induce a strong decrease of DGKα activity without changes in DGKα protein content (Fig. 1D). This result indicates that signals originating from the intracellular domain of SLAM lead to DGKα inhibition. Taken together, these observations indicate that upon co-stimulation of the TCR with either CD28 or SLAM, the enzymatic activity of DGKα undergoes a negative regulation, which likely contributes to the accumulation of DAG required for RasGRP-mediated activation of Ras and full T cell activation.
Figure 1. DGKα is inhibited upon T cell activation.
PBL (A) and Jurkat A3 cells (B) were stimulated for 15 min with 10 μg/ml of the indicated antibodies and lysed. Anti-DGKα immunoprecipitates were assayed for DGK enzymatic activity while an aliquot of whole cell lysate was analyzed by western blot with anti-DGKα antibody to ensure equal loading. A representative experiment is shown (lower panel) together with a graph showing the mean ± SE of four independent experiments shown as percentage of control (upper panel), *t-test vs. control p<0.05. (C) Jurkat A3 cells were stimulated with 10 μg/ml anti-CD3 and anti-SLAM antibodies and lysed at the indicated times. Anti-DGKα immunoprecipitates were assayed for DGK enzymatic activity while an aliquot of whole cell lysate was analyzed by western blot with anti-DGKα antibody to ensure equal loading. A representative experiment is shown (lower panel) together with a graph showing the mean ± SE of four independent experiments shown as percentage of control (upper panel), * t-test vs. control p<0.05. (D) BI-141 TTS-SAP cells were stimulated for 15 min with 5 mg/ml anti-TAC and 4 mg/ml anti-IgG and lysed. Anti DGKα immunoprecipitates were assayed for DGK enzymatic activity while an aliquot of whole cell lysate was analyzed by western blot with anti DGKα antibody to ensure equal loading. A representative experiment is shown (lower panel) together with a graph showing the mean ± SE of three independent experiments shown as percentage of control (upper panel), * t-test vs. control p<0.05.
To verify whether this regulation was specific to DGKα we first examined whether anti-CD3 co-stimulation with either anti-CD28 or anti-SLAM antibodies regulated DGKζ, which, along with DGKα is highly expressed in T cells. We observed that neither CD3/CD28 nor CD3/SLAM co-stimulation of T cells did affect the enzymatic activity of DGKζ in anti-DGKζ immunoprecipitates from either control or co-stimulated cells (Supplemental Fig. 1). These observations indicate that TCR activation specifically regulates DGKα enzymatic activity, while not affecting DGKζ. In order to verify the contribution of DGKα regulation to the total cellular DGK activity, we measured DGK activity in whole lymphocytes homogenates using exogenous substrates. Following 15 min of TCR/CD28 co-stimulation of either PBLs or Jurkat cells, total DGK activity was not significantly affected, even if the co-stimulation was sufficient to activate ERK-1/2 (Supplemental Fig. 2A and 2B). Conversely, upon 15 min TCR/SLAM co-stimulation, total DGK activity was significantly reduced (Supplemental Fig. 2C and 2D). Given the specific sub-cellular localization of DGK isoforms, these observations suggest that DGKα inhibition does not affect the bulk of DAG metabolism, while selectively promoting DAG accumulation at specific compartments.
As DGKα recruitment from the cytoplasm to the plasma membrane is highly regulated both upon growth factor stimulation of epithelial cells and TCR/CD28-mediated co-stimulation of lymphocytes (20, 24), we assessed DGKα localization following co-stimulation of the TCR with either CD28 or SLAM. Both endogenous DGKα in CD3+ PBLs and YFP-DGKα transiently expressed in Jurkat cells localize diffusely in the nucleus and in the cytoplasm of unstimulated or TCR-stimulated cells. Upon 1 hour co-stimulation of the TCR with either CD28 or SLAM, DGKα was almost entirely excluded from the nucleus and recruited to the cell periphery in both PBLs and Jurkat cells (Fig. 2A, 2B and Supplemental Fig. 3A and 3B). While inhibition of DGKα enzymatic activity was an early event, starting 5 min following co-stimulation, reaching maximal inhibition at 15 min and lasting up to 1 hour (Fig. 1C), translocation of DGKα became detectable 15 min after co-stimulation, reached its maximum at 30 min and lasted for several hours (Fig. 2B).
In order to distinguish between plasma membrane and cytoplasmic localization, we labeled plasma membrane with either K-Ras-V12/A28 (31) or wheat germ agglutinin (WGA). Upon T cell co-stimulation, DGKα only partially co-localized with K-Ras-V12/A28 (Fig. 2B, 4D) or with WGA (Supplemental Fig. 3B). Accordingly approximately 10% of cytoplasmic DGKα sedimented in the 100,000 RCF fraction of CD3/CD28 co-stimulated Jurkat cells (Fig. 2C). These findings indicate that, upon lymphocytes activation, DGKα undergoes both negative regulation of its enzymatic activity and translocation from the nucleus to the cell periphery, although with different kinetics.
Regulation of DGKα inhibition and recruitment to the cell periphery
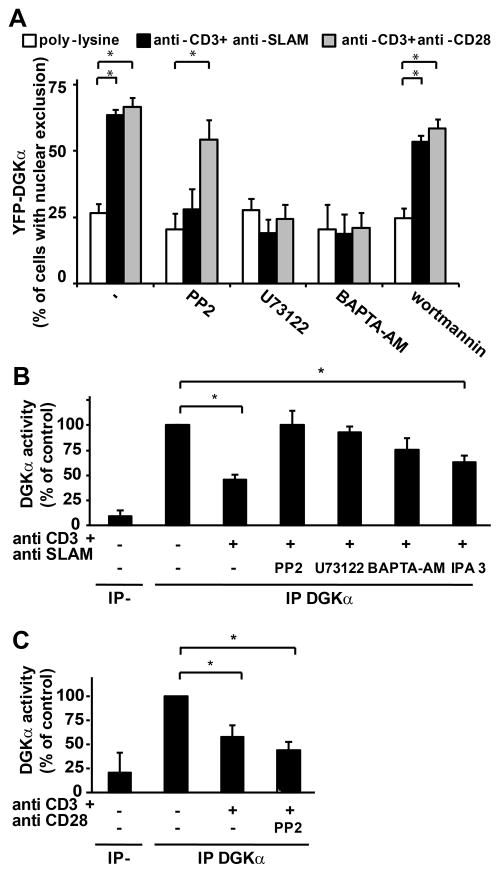
We explored whether translocation to the cell periphery and negative regulation of DGKα were regulated by common signaling pathways. DGKα activity and localization are regulated by Src-mediated tyrosine phosphorylation (21, 24, 25), calcium binding (17, 32), and D-3 phosphoinositides (33). Pharmacological inhibition of PLC by U73122 and calcium chelation by BAPTA-AM blunted DGKα translocation from the nucleus to the cell periphery induced by co-stimulation of TCR with either SLAM or CD28 (Fig. 3A). Interestingly, PP2-mediated inhibition of Src family tyrosine kinases (SFK) inhibited only CD3/SLAM-induced translocation. Conversely, wortmannin did not affect DGKα localization, indicating that phosphoinositides 3-kinases are not involved (Fig. 3A). Similarly, pharmacological inhibition of PLC and calcium signaling prevented the negative regulation of DGKα activity induced by TCR/SLAM co-stimulation (Fig. 3B). These data indicate that PLC activity and calcium release mediate both inhibition of DGKα activity and its translocation to the cell periphery. Conversely, inhibition of SFKs impaired specifically negative regulation of DGKα activity and its translocation to the cell periphery induced by TCR/SLAM co-stimulation, but not by TCR/CD28 co stimulation (Fig. 3A, 3C), indicating that SFKs requirement is restricted to SLAM-induced regulation of DGKα. Despite SAP overexpression regulates cdc42, IPA3-mediated inhibition of PAK, a cdc42 effector, does not affect DGKα activity (Fig. 3B).
Figure 3. Phospholipase C and calcium mediate TCR-induced regulation of DGKα.
(A) Jurkat A3 cells were transfected with YFP-DGKα and after 24 hours were serum starved for 2 hours, seeded for 1 hour on poly-L-lysine, CD3+SLAM or CD3+CD28 agonistic antibodies (10μg/ml each) coated glass-bottom dishes in the presence or absence of the indicated inhibitors (50μM PP2, 5 μM U73122, 10 μM BAPTA-AM or 100 nM Wortmannin) and images acquired. The quantification of three independent experiments is shown, * t-test vs. control p<0.0005.
(B) Jurkat A3 cells were treated with the indicated inhibitors (10 μM PP2, 5 μM U73122, 10 μM BAPTA-AM, 10 μM IPA-3) or vehicle for 30 min before stimulation with 10 μg/ml anti-CD3 and anti-SLAM antibodies. After 15 min cells were lysed and anti DGKα immunoprecipitates were assayed for DGK enzymatic activity. The graph shows the mean ± SE of at least three independent experiments for each inhibitor, * t-test vs. control p<0.05.
(C) Jurkat A3 cells were treated with 10 μM PP2 or vehicle for 30 min before stimulation with 10 μg/ml anti-CD3 and anti-CD28 antibodies. After 15 min cells were lysed and anti DGKα immunoprecipitates were assayed for DGK enzymatic activity. The graph shows the mean ± SE of at three independent experiments, * t-test vs. control p<0.05.
Upon SLAM engagement, SAP mediates the recruitment of Fyn, thereby promoting tyrosine phosphorylation of SLAM and activation of its downstream signaling (34). Thus, we investigated the role of SAP in negative regulation and membrane recruitment of DGKα. SAP expression was down-regulated in Jurkat cells by lentiviral-mediated stable expression of a SAP-specific shRNA (Fig. 4A and 4B). In SAP-deficient Jurkat cells, but not in control shRNA cells, DGKα activity was not inhibited following stimulation of TCR and SLAM (Fig. 4A), consistently with the essential role of SAP in SLAM-induced signaling. Surprisingly, in SAP-deficient cells, DGKα activity was not inhibited by TCR/CD28 co-stimulation. This observation suggests that SAP is not only required for SLAM signaling, but may also play a more direct role in promoting negative regulation of DGKα enzymatic activity (Fig. 4B). Indeed, over-expression of SAP and myc-DGKα in Jurkat cells resulted in the reduction of DGKα activity by 60% as measured in anti-myc immunoprecipitates, while myc-DGKα protein content was not affected (Fig. 4C). Conversely, in the same assay SAP mutants unable to bind either SH3-domains (SAP-R78A) or both tyrosine-phosphorylated proteins and SH3 domains (SAP-R55L) (3, 35) failed to inhibit DGKα (Fig. 4C). These findings indicate that SAP over-expression is sufficient to inhibit DGKα through a mechanism which requires SH3-binding ability of SAP.
The sequence surrounding tyrosine 335 of DGKα (SIY335PSV) features a high similarity to the SAP-SH2 binding motif on SLAM (TIY281AQV) (36), suggesting that DGKα might bind directly to SAP. However, we could not detect a direct physical association between SAP and DGKα in a mammalian two hybrid assay (Supplemental Fig. 4A) or in co-immunoprecipitation assays using transfected 293T cells (Supplemental Fig. 4B), even when the two proteins were co-expressed with SLAM and Fyn. Altogether these results indicate that SAP does not inhibit DGKα by directly binding to it, but through the SAP-mediated recruitment of a yet unidentified SH3-containing protein.
The role of SAP in DGKα membrane recruitment in Jurkat cells was investigated by shRNA-mediated stable knock down of SAP. SAP silencing selectively impaired the recruitment of DGKα to the cell periphery induced by TCR/SLAM co-stimulation, but not by TCR/CD28 co-stimulation (Fig. 4D). Similar results were obtained upon transient siRNA-mediated down-regulation of SAP in Jurkat cells (data not shown). Thus, following engagement of the TCR and SLAM, SAP is required for DGKα enzymatic inhibition and recruitment to the cell periphery. In contrast, while SAP is required for TCR/CD28-induced enzymatic inhibition of DGKα, it is not essential for translocation of DGKα to the cell periphery. These findings indicate that the localization and enzymatic activity of DGKα are regulated through distinct processes and kinetics, although the mechanisms involved are still partially unknown.
Inhibition of DGKα rescues the functional defects caused by SAP deficiency in XLP
Collectively, these data demonstrate that SAP is essential for regulation of DGKα activity upon T cell activation via the TCR/SLAM or TCR/CD28. We therefore reasoned that, similarly to SAP-deficient Jurkat cells, T cells of XLP patients lacking functional SAP might be defective in the negative regulation of DGKα, thereby contributing to the defective lymphocyte responses observed in both XLP patients and in SAP null mice. To address this hypothesis, we first characterized the signaling capacity of SAP-deficient Jurkat cells following stimulation via the TCR and CD28. We then assessed whether pharmacological inhibition of DGKα by R59949, or its siRNA-mediated down-regulation, might rescue those aberrant T cell responses.
DAG-dependent recruitment of PKCθ to the plasma membrane is defective in T cells from SAP null mice, it is potentiated upon SAP over-expression (7, 37) and it is negatively regulated by constitutive activation of DGKα (38). Consistently, in SAP-deficient Jurkat cells, PKCθ recruitment to the immune synapse with super antigen-loaded APC was impaired (Fig. 5A). Both pharmacological inhibition (Fig. 5A) and siRNA-mediated silencing of DGKα (Fig. 5B) nearly completely rescued the defective translocation of PKCθ to the immune synapse observed in SAP-deficient Jurkat cells, pointing to a rescue of DAG-mediated signaling.
Upon TCR/CD28 co-stimulation, both T cells from XLP patients and Jurkat cells made SAP-deficient by siRNA-mediated down regulation, exhibit defective ERK-1/2 activation (5, 39), suggesting that DAG-mediated Ras-GTP signaling is impaired. Indeed, upon TCR/CD28 co-stimulation, Jurkat SAP-shRNA cells showed both a decrease in ERK-1/2 phosphorylation and a marked reduction of Ras-GTP loading, as measured by Ras-GTP pull down with GST-RBD (Fig 5C). Pharmacological inhibition of DGKα with R59949 fully restored both ERK-1/2 phosphorylation and Ras-GTP loading (Fig. 5C). These findings confirm that SAP is required for Ras activation in human T cells and provide further support to the hypothesis that negative regulation of DGKα is a critical step in the activation of the Ras pathway downstream of TCR/CD28. Interestingly, R59949 raised basal levels of ERK-1/2 phosphorylation without significantly affecting Ras-GTP loading, suggesting that under these conditions ERK-1/2 phosphorylation may be enhanced through a Ras-independent mechanism, likely through DAG-dependent PKCθ activation (40).
Activation of PKCθ and Ras pathways upon TCR/CD28 co-stimulation triggers NF-AT transcriptional activity, which plays a central role in cytokine production (8, 41). Moreover, NF-AT is activated upon SAP over-expression in Jurkat cells (42). Consistently SAP down-regulation in Jurkat cells impaired TCR/CD28-induced stimulation of NF-AT activity, as measured by luciferase reporter system (Fig. 5D, 5E). In SAP-deficient cells, pharmacological inhibition of DGK with 1 μM R59949 fully restored TCR/CD28-induced activation of NF-AT without affecting basal NF-AT activity (Fig. 5D), while siRNA-mediated DGKα silencing resulted only in partial rescue (Fig. 5E). These data suggest that either DGKα along with other R59949-sensitive DGKs mediate NF-AT activation downstream from SAP, or that the low quantity of DGKα remaining after RNA interference may still transduce the signaling.
Upon T cell stimulation, activation of Ras, PKCθ and NF-AT signaling pathways leads to IL-2 production (41, 43, 44), which has been reported to be reduced in lymphocytes from XLP patients (5). Indeed, in Jurkat cells, shRNA-mediated SAP silencing reduced TCR/CD28-induced IL-2 secretion (Fig 5F). Pharmacological inhibition of DGKα by R59949 enhanced TCR/CD28-induced IL-2 production in control cells and fully rescued the defective IL-2 secretion of SAP-deficient Jurkat cells. These findings suggest that SAP-mediated negative regulation of DGKα is a key event in the modulation of T cell activation.
Discussion
In this paper we demonstrate that, within minutes following co-stimulation of the TCR with either CD28 or SLAM, the enzymatic activity of DGKα, as assayed in immunoprecipitates in the presence of saturating DAG substrate concentration, undergoes a strong negative regulation without protein down-regulation. This finding is surprising, given accumulating evidence that synthesis of PA is increased upon T cell stimulation (15, 16, 45) and that DGK activity is increased in whole cell lysates from in vivo activated T cells (19). However, increased PA synthesis through DAG phosphorylation may depend on both positive regulation of one or more DGK isoforms and on increased availability of DAG, whose production by PLCγ is increased upon TCR/CD28 co-stimulation (46). A parallel increase of DG and PA levels upon TCR stimulation has been indeed observed (45, 47). Several pieces of evidence suggest that most of the PA generated upon T cell activation derives from phospholipase D2-mediated phospholipids hydrolysis and from DGKζ-mediated phosphorylation of DAG, while deletion of DGKα does not significantly affect PA production upon T cell stimulation (15, 16, 45). Nevertheless, recent genetic and biochemical data indicating that DGKα is a negative regulator of DAG-mediated TCR signaling (15, 17) are highly consistent with our finding that enzymatic activity of DGKα is reduced upon TCR co-stimulation with either CD28 or SLAM. This regulation appears to be isoform-specific, as DGKζ activity is unaffected by TCR triggering (Supplemental Fig. 1). Interestingly, the previous finding that stimulation of the sole TCR is not sufficient to promote sustained DAG signaling (48) is consistent with our observation that TCR activation in PBLs is not sufficient to inhibit DGKα activity in absence of co-stimulation. Moreover, co-stimulation of TCR/CD28, compared to TCR alone, strongly enhances production of DAG but not of PA (15), suggesting a slowdown in the rate of DAG conversion to PA that is consistent with a negative regulation of DGK activity.
The molecular mechanisms underlying the negative regulation of DGKα have not been elucidated yet. Herein we report that the adaptor function of SAP is required for DGKα inhibition induced by TCR co-stimulation with either SLAM or CD28. SAP is essential for SLAM tyrosine phosphorylation by recruiting the Src-related kinase FynT (3, 34), however, a growing body of evidence indicates that SAP is also involved in T cell responses to antigenic stimulation (2). Indeed SAP binds directly to ITAM sequences of CD3ζ subunit (29), while TCR activation promotes the recruitment of SAP and SLAM family receptors to the signalosome (28, 29, 49). Furthermore, genetic deletion of SAP in mice results in the impairment of TCR/CD28-induced DAG-mediated activation of PKCθ and of downstream signaling events (7). Moreover, TCR/CD28-induced ERK-1/2 activation and IL-2 production, which are both dependent on DAG-mediated activation of RasGRP, are impaired in T cells from SAP-deficient XLP patients (5). Intriguingly, SAP is physically associated to PKCθ and it has been demonstrated that SAP over-expression, which is sufficient to inhibit DGKα, promotes PKCθ recruitment to the immune synapse (37). Finally, we and others have shown that, in Jurkat cells, SAP silencing impairs TCR-induced Ras-GTP loading, ERK-1/2 activation, PKCθ recruitment, NF-AT activation and IL-2 production (5–7). Together, these observations suggest that, upon TCR/CD28 co-stimulation, SAP is required for optimal DAG signaling. The finding that SAP is required for inhibition of DGKα might provide a mechanistic link between SAP and the regulation of DAG signaling. Thus, we propose that, upon stimulation of T cells from either SAP-deficient XLP patients or SAP null mice, DGKα may inappropriately retain a high enzymatic activity, thereby converting DAG to PA and decreasing DAG signaling.
If this hypothesis holds true, we would expect that inhibition or down-regulation of DGKα would rescue, at least partially, the defective signaling of SAP-deficient T cells. Accordingly, we observed that the inhibition of DGKα enzymatic activity in SAP-deficient Jurkat cells rescued defective DAG-dependent PKCθ membrane recruitment, Ras-GTP loading, ERK-1/2 and NF-AT activation and IL-2 production. These findings indicate that the excess of DGKα activity contributes to the defective signaling of SAP-deficient cells, and, along with the demonstration that SAP over-expression inhibits DGKα, provide further support to the hypothesis that SAP negatively regulates DGKα. According to these findings, the negative regulation of DGKα activity represents a key event controlling the early phase of T cell activation by contributing to fine tuning of DAG levels required for appropriate signaling.
In here we observed that co-stimulation of the TCR with either SLAM or CD28 induces DGKα exit from the nucleus and accumulation in the cytoplasm with only partial localization at the plasma membrane. This finding appears to contrast previous studies reporting GFP-DGKα localization at the plasma membrane of CD3/CD28 co-stimulated Jurkat cells, however according to the same authors DGKα membrane translocation is rapid and transient and can be visualized in conditions that inhibit its relocalization to the cytoplasm (17, 21). Moreover, DGKα plasma membrane localization was clearly induced by stronger stimuli, such as the activation of ectopically over-expressed muscarinic receptor (20, 21, 50, 51), or antigen challenge in vivo (19). Further support to the hypothesis that enzymatic activity of DGKα regulates DAG level at the plasma membrane of T cells derives both from our finding that uncoupling of DGKα inhibition from TCR stimulation impairs PKCθ recruitment to the immune synapses (Fig. 5A) and from the observation that pharmacological inhibition of DGKα allows accumulation of DAG at the plasma membrane of T cells, thereby triggering activation of Ras signaling (52).
Interestingly, stimulation with either SLAM or TCR alone did not induce DGKα translocation from the nucleus, indicating that the concerted signaling via both receptors is required. Moreover, the finding that SAP, which is essential for SLAM tyrosine phosphorylation and signaling, is required for translocation induced exclusively by TCR/SLAM, but not by TCR/CD28, suggests that SAP may not directly regulate DGKα sub cellular localization. In addition, the fact that upon TCR/CD28 co-stimulation, SAP is required for inhibition of DGKα activity, but not for its exit from the nucleus, indicates that enzymatic activity and localization of DGKα are regulated independently of each other, as suggested also by the different kinetics of the two processes. Importantly, these findings also indicate that DGKα exit from the nucleus is not required for the inhibition of its enzymatic activity. The massive exit from the nucleus may reflect a potential increase in the availability of DGKα outside the nucleus for control of DAG signaling both at the plasma membrane and at intracellular vesicles. Indeed, several reports indicate a role of DGKs in T cells intracellular trafficking (50, 53, 54). Conversely, DGKα exit from the nucleus may contribute to regulate nuclear pools of DAG and PA acid. Indeed, several DGK isoforms have been reported to localize in the nucleus where they contribute to regulate transcription and cell cycle progression (55).
Previous evidence indicates that, SFK-induced phosphorylation of DGKα on tyrosine 335 mediates its activation and membrane localization upon growth factor stimulation of epithelial and large cell lymphoma cells (24, 56). Moreover in T cells DGKα phosphorylation by LCK on tyrosine 335 mediates CD3/CD28-induced recruitment of DGKα to the plasma membrane (21). Surprisingly, in our study pharmacological inhibition of SFKs did not affect neither TCR/CD28-induced inhibition of DGKα nor its exit from the nucleus, suggesting that both events are independent from SFKs-mediated tyrosine phosphorylation of DGKα. Conversely PP2 completely blocks DGKα inhibition and exit from the nucleus induced by CD3/SLAM, as SLAM signaling is dependent on Fyn tyrosine kinase.
The mechanism by which SAP regulates DGKα still remains to be elucidated. Based on the high similarity between the sequences surrounding Tyr 335 of DGKα and Tyr 281 of SLAM, we investigated the hypothesis that SAP may regulate DGKα by associating with it in a complex. However, we could not detect any direct or indirect physical interaction between the two proteins, even in a reconstituted association assay in a mammalian two-hybrid system. Our data demonstrate that SAP ability to inhibit DGKα requires the interaction with a yet unidentified SH3 domain-containing protein. The finding that inhibition of DGKα is independent of SFKs activity suggests that the SAP interactor required for DGKα inhibition is not Fyn. The previous observation that SAP over-expression activates Cdc42 signaling by interacting with SH3-containing β PIX and independently of Fyn, suggests that DGKα may be regulated by Cdc42-dependent PAK activation. However, the PAK-specific inhibitor IPA-3 did not affect the inhibition of DGKα following TCR/SLAM co-stimulation (Fig. 3B). Finally, upon TCR stimulation of Jurkat cells, SAP silencing results in defective tyrosine phosphorylation of several proteins, including LAT and SLP76 (39). As both LAT and SLP76 regulate PLCγ activation (57), it is possible to speculate that SAP regulates DGKα by controlling PLCγ activity. Consistently with this possibility, PLC activity and cytosolic free calcium are both required for DGKα inhibition and membrane recruitment. However, lack of SAP in T cells of both SAP null mice and XLP patients does not affect PLCγ-mediated intracellular calcium increase (5, 58). Moreover, cell stimulation with a calcium ionophore and phorbol ester failed to inhibit DGKα activity and to recruit it to the plasma membrane, indicating that activation of PLC is necessary but not sufficient to regulate both enzymatic activity and membrane localization (data not shown). Furthermore, the requirement for PLC activity in regulating DGKα suggests that the two enzymes may act as a bi-component unit able to finely modulate the extent and the duration of DAG signaling.
In conclusion our findings suggest that the coordinated, but independent, control of DGKα enzymatic activity and of its localization regulates both its access to DAG and its rate of conversion to PA. Upon T cell stimulation, such coordinated and complementary mechanism of regulation might finely tune the intensity and the duration of DAG-mediated signaling. Indeed, SAP silencing, by uncoupling TCR/CD28 co-stimulation from DGKα inhibition, results in the impairment of TCR/CD28-induced DAG-mediated signaling, providing further evidence that the SAP-mediated negative regulation of DGKα is crucial for the ability of T cells to trigger DAG-mediated responses.
Similarly to cAMP-signaling, which is triggered by G protein coupled receptors (GPCRs) by reciprocal regulation of both adenylate cyclase and phosphodiesterase activities (59), the findings presented here suggest that TCR/CD28 controls DAG-signaling both by means of PLCγ activation and DGKα inhibition. Similarly, genetic and biochemical studies in C. elegans motoneurons and murine hepatocytes showed that DAG-mediated signaling is controlled by GPCR-dependent reciprocal regulation of both PLC and DGKθ (60–62).
In summary, our findings demonstrate that SAP-mediated DGKα inhibition is an early event in TCR signaling, which might be required for efficient T cell activation. The impaired regulation of DGKα activity in SAP-deficient lymphocytes may contribute to their defective TCR-induced responses, suggesting that pharmacological inhibition of DGKα could be useful in the treatment of certain manifestations of XLP.
Supplementary Material
Acknowledgments
Grant support
This work was supported by Telethon grant GGP10034 to AG, Ricerca Sanitaria Finalizzata Regione Piemonte (to AG), Italian Ministry for University and Research (PRIN 2007 to AG) and (FIRB 2001 RBNE019J9W_003 to OP), Grant XRT/003 from the XLP Research Trust and NIH R01HL089745 to KN.
M.C. Zhong and A. Veillette (Montréal, Canada) provided BI-141 cells expressing IL-2R/SLAM chimera and SAP. M. Topham provided anti DGKζ antibodies. P. Schwartzberg (NIH, USA) provided GFP-SAP constructs. The authors declare no competing financial interest.
Abbreviations List
- XLP
X-linked lymphoproliferative disease
- DAG
diacylglycerol
- DGK
diacylglycerol kinase
- PA
phosphatidic acid
- WCL
whole cell lysate
- YFP
yellow fluorescent protein
- WGA
wheat germ agglutinin
- SFK
Src family kinase
- FBS
fetal bovine serum
- GPCR
G protein coupled receptor
- RCF
relative centrifugal force
References
- 1.Kroczek RA, Mages HW, Hutloff A. Emerging paradigms of T-cell co-stimulation. Curr Opin Immunol. 2004;16:321–327. doi: 10.1016/j.coi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Veillette A. SLAM-family receptors: immune regulators with or without SAP-family adaptors. Cold Spring Harb Perspect Biol. 2010;2:a002469. doi: 10.1101/cshperspect.a002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen R, Latour S, Shi X, Veillette A. Association between SAP and FynT: Inducible SH3 domain-mediated interaction controlled by engagement of the SLAM receptor. Mol Cell Biol. 2006;26:5559–5568. doi: 10.1128/MCB.00357-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rezaei N, Mahmoudi E, Aghamohammadi A, Das R, Nichols KE. X-linked lymphoproliferative syndrome: a genetic condition typified by the triad of infection, immunodeficiency and lymphoma. Br J Haematol. 2011;152:13–30. doi: 10.1111/j.1365-2141.2010.08442.x. [DOI] [PubMed] [Google Scholar]
- 5.Sanzone S, Zeyda M, Saemann MD, Soncini M, Holter W, Fritsch G, Knapp W, Candotti F, Stulnig TM, Parolini O. SLAM-associated protein deficiency causes imbalanced early signal transduction and blocks downstream activation in T cells from X-linked lymphoproliferative disease patients. J Biol Chem. 2003;278:29593–29599. doi: 10.1074/jbc.M300565200. [DOI] [PubMed] [Google Scholar]
- 6.Ma CS, Hare NJ, Nichols KE, Dupré L, Andolfi G, Roncarolo MG, Adelstein S, Hodgkin PD, Tangye SG. Impaired humoral immunity in X-linked lymphoproliferative disease is associated with defective IL-10 production by CD4+ T cells. J Clin Invest. 2005;115:1049–1059. doi: 10.1172/JCI23139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannons JL, Yu LJ, Hill B, Mijares LA, Dombroski D, Nichols KE, Antonellis A, Koretzky GA, Gardner K, Schwartzberg PL. SAP regulates T(H)2 differentiation and PKC-theta-mediated activation of NF-kappaB1. Immunity. 2004;21:693–706. doi: 10.1016/j.immuni.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Baldari CT, Heguy A, Telford JL. ras protein activity is essential for T-cell antigen receptor signal transduction. J Biol Chem. 1993;268:2693–2698. [PubMed] [Google Scholar]
- 9.Woodrow MA, Rayter S, Downward J, Cantrell DA. p21ras function is important for T cell antigen receptor and protein kinase C regulation of nuclear factor of activated T cells. J Immunol. 1993;150:3853–3861. [PubMed] [Google Scholar]
- 10.Altman A, Villalba M. Protein kinase C-theta (PKCtheta): it’s all about location, location, location. Immunol Rev. 2003;192:53–63. doi: 10.1034/j.1600-065x.2003.00027.x. [DOI] [PubMed] [Google Scholar]
- 11.Ebinu JO, Stang SL, Teixeira C, Bottorff DA, Hooton J, Blumberg PM, Barry M, Bleakley RC, Ostergaard HL, Stone JC. RasGRP links T-cell receptor signaling to Ras. Blood. 2000;95:3199–3203. [PubMed] [Google Scholar]
- 12.Asada A, Zhao Y, Komano H, Kuwata T, Mukai M, Fujita K, Tozawa Y, Iseki R, Tian H, Sato K, Motegi Y, Suzuki R, Yokoyama M, Iwata M. The calcium-independent protein kinase C participates in an early process of CD3/CD28-mediated induction of thymocyte apoptosis. Immunology. 2000;101:309–315. doi: 10.1046/j.1365-2567.2000.00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zha Y, Marks R, Ho AW, Peterson AC, Janardhan S, Brown I, Praveen K, Stang S, Stone JC, Gajewski TF. T cell anergy is reversed by active Ras and is regulated by diacylglycerol kinase-alpha. Nat Immunol. 2006;7:1166–1173. doi: 10.1038/ni1394. [DOI] [PubMed] [Google Scholar]
- 14.Wattenberg BW, Raben DM. Diacylglycerol kinases put the brakes on immune function. Sci STKE. 2007;2007:pe43. doi: 10.1126/stke.3982007pe43. [DOI] [PubMed] [Google Scholar]
- 15.Olenchock BA, Guo R, Carpenter JH, Jordan M, Topham MK, Koretzky GA, Zhong XP. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat Immunol. 2006;7:1174–1181. doi: 10.1038/ni1400. [DOI] [PubMed] [Google Scholar]
- 16.Zhong XP, Hainey EA, Olenchock BA, Jordan MS, Maltzman JS, Nichols KE, Shen H, Koretzky GA. Enhanced T cell responses due to diacylglycerol kinase zeta deficiency. Nat Immunol. 2003;4:882–890. doi: 10.1038/ni958. [DOI] [PubMed] [Google Scholar]
- 17.Sanjuán MA, Jones DR, Izquierdo M, Mérida I. Role of diacylglycerol kinase alpha in the attenuation of receptor signaling. J Cell Biol. 2001;153:207–220. doi: 10.1083/jcb.153.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong XP, Hainey EA, Olenchock BA, Zhao H, Topham MK, Koretzky GA. Regulation of T cell receptor-induced activation of the Ras-ERK pathway by diacylglycerol kinase zeta. J Biol Chem. 2002;277:31089–31098. doi: 10.1074/jbc.M203818200. [DOI] [PubMed] [Google Scholar]
- 19.Sanjuán MA, Pradet-Balade B, Jones DR, Martínez-A C, Stone JC, Garcia-Sanz JA, Mérida I. T cell activation in vivo targets diacylglycerol kinase alpha to the membrane: a novel mechanism for Ras attenuation. J Immunol. 2003;170:2877–2883. doi: 10.4049/jimmunol.170.6.2877. [DOI] [PubMed] [Google Scholar]
- 20.Merino E, Sanjuán MA, Moraga I, Ciprés A, Mérida I. Role of the diacylglycerol kinase alpha-conserved domains in membrane targeting in intact T cells. J Biol Chem. 2007;282:35396–35404. doi: 10.1074/jbc.M702085200. [DOI] [PubMed] [Google Scholar]
- 21.Merino E, Avila-Flores A, Shirai Y, Moraga I, Saito N, Mérida I. Lck-dependent tyrosine phosphorylation of diacylglycerol kinase alpha regulates its membrane association in T cells. J Immunol. 2008;180:5805–5815. doi: 10.4049/jimmunol.180.9.5805. [DOI] [PubMed] [Google Scholar]
- 22.Schaap D, van der Wal J, van Blitterswijk WJ, van der Bend RL, Ploegh HL. Diacylglycerol kinase is phosphorylated in vivo upon stimulation of the epidermal growth factor receptor and serine/threonine kinases, including protein kinase C-epsilon. Biochem J. 1993;289(Pt 3):875–881. doi: 10.1042/bj2890875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baldanzi G, Mitola S, Cutrupi S, Filigheddu N, van Blitterswijk WJ, Sinigaglia F, Bussolino F, Graziani A. Activation of diacylglycerol kinase alpha is required for VEGF-induced angiogenic signaling in vitro. Oncogene. 2004;23:4828–4838. doi: 10.1038/sj.onc.1207633. [DOI] [PubMed] [Google Scholar]
- 24.Baldanzi G, Cutrupi S, Chianale F, Gnocchi V, Rainero E, Porporato P, Filigheddu N, van Blitterswijk WJ, Parolini O, Bussolino F, Sinigaglia F, Graziani A. Diacylglycerol kinase-alpha phosphorylation by Src on Y335 is required for activation, membrane recruitment and Hgf-induced cell motility. Oncogene. 2008;27:942–956. doi: 10.1038/sj.onc.1210717. [DOI] [PubMed] [Google Scholar]
- 25.Cutrupi S, Baldanzi G, Gramaglia D, Maffè A, Schaap D, Giraudo E, van Blitterswijk W, Bussolino F, Comoglio PM, Graziani A. Src-mediated activation of alpha-diacylglycerol kinase is required for hepatocyte growth factor-induced cell motility. EMBO J. 2000;19:4614–4622. doi: 10.1093/emboj/19.17.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chianale F, Rainero E, Cianflone C, Bettio V, Pighini A, Porporato PE, Filigheddu N, Serini G, Sinigaglia F, Baldanzi G, Graziani A. Diacylglycerol kinase alpha mediates HGF-induced Rac activation and membrane ruffling by regulating atypical PKC and RhoGDI. Proc Natl Acad Sci U S A. 2010;107:4182–4187. doi: 10.1073/pnas.0908326107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubio I, Wetzker R. A permissive function of phosphoinositide 3-kinase in ras activation mediated by inhibition of GTPase-activating proteins. Curr Biol. 2000;10:R883. doi: 10.1016/s0960-9822(00)00842-3. [DOI] [PubMed] [Google Scholar]
- 28.Howie D, Simarro M, Sayos J, Guirado M, Sancho J, Terhorst C. Molecular dissection of the signaling and costimulatory functions of CD150 (SLAM): CD150/SAP binding and CD150-mediated costimulation. Blood. 2002;99:957–965. doi: 10.1182/blood.v99.3.957. [DOI] [PubMed] [Google Scholar]
- 29.Bida AT, Upshaw Neff JL, Dick CJ, Schoon RA, Brickshawana A, Chini CC, Billadeau DD. 2B4 utilizes ITAM-containing receptor complexes to initiate intracellular signaling and cytolysis. Mol Immunol. 2011;48:1149–1159. doi: 10.1016/j.molimm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Latour S, Gish G, Helgason CD, Humphries RK, Pawson T, Veillette A. Regulation of SLAM-mediated signal transduction by SAP, the X-linked lymphoproliferative gene product. Nat Immunol. 2001;2:681–690. doi: 10.1038/90615. [DOI] [PubMed] [Google Scholar]
- 31.Augsten M, Pusch R, Biskup C, Rennert K, Wittig U, Beyer K, Blume A, Wetzker R, Friedrich K, Rubio I. Live-cell imaging of endogenous Ras-GTP illustrates predominant Ras activation at the plasma membrane. EMBO Rep. 2006;7:46–51. doi: 10.1038/sj.embor.7400560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang Y, Qian W, Hawes JW, Walsh JP. A domain with homology to neuronal calcium sensors is required for calcium-dependent activation of diacylglycerol kinase alpha. J Biol Chem. 2000;275:34092–34099. doi: 10.1074/jbc.M004914200. [DOI] [PubMed] [Google Scholar]
- 33.Ciprés A, Carrasco S, Merino E, Díaz E, Krishna UM, Falck JR, Martínez-A C, Mérida I. Regulation of diacylglycerol kinase alpha by phosphoinositide 3-kinase lipid products. J Biol Chem. 2003;278:35629–35635. doi: 10.1074/jbc.M305635200. [DOI] [PubMed] [Google Scholar]
- 34.Chan B, Lanyi A, Song HK, Griesbach J, Simarro-Grande M, Poy F, Howie D, Sumegi J, Terhorst C, Eck MJ. SAP couples Fyn to SLAM immune receptors. Nat Cell Biol. 2003;5:155–160. doi: 10.1038/ncb920. [DOI] [PubMed] [Google Scholar]
- 35.Li C, Iosef C, Jia CY, Gkourasas T, Han VK, Shun-Cheng Li S. Disease-causing SAP mutants are defective in ligand binding and protein folding. Biochemistry. 2003;42:14885–14892. doi: 10.1021/bi034798l. [DOI] [PubMed] [Google Scholar]
- 36.Hwang PM, Li C, Morra M, Lillywhite J, Muhandiram DR, Gertler F, Terhorst C, Kay LE, Pawson T, Forman-Kay JD, Li SC. A “three-pronged” binding mechanism for the SAP/SH2D1A SH2 domain: structural basis and relevance to the XLP syndrome. EMBO J. 2002;21:314–323. doi: 10.1093/emboj/21.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cannons JL, Wu JZ, Gomez-Rodriguez J, Zhang J, Dong B, Liu Y, Shaw S, Siminovitch KA, Schwartzberg PL. Biochemical and genetic evidence for a SAP-PKC-theta interaction contributing to IL-4 regulation. J Immunol. 2010;185:2819–2827. doi: 10.4049/jimmunol.0902182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carrasco S, Merida I. Diacylglycerol-dependent binding recruits PKCtheta and RasGRP1 C1 domains to specific subcellular localizations in living T lymphocytes. Mol Biol Cell. 2004;15:2932–2942. doi: 10.1091/mbc.E03-11-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li C, Schibli D, Li SS. The XLP syndrome protein SAP interacts with SH3 proteins to regulate T cell signaling and proliferation. Cell Signal. 2009;21:111–119. doi: 10.1016/j.cellsig.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 40.Witte V, Laffert B, Gintschel P, Krautkrämer E, Blume K, Fackler OT, Baur AS. Induction of HIV transcription by Nef involves Lck activation and protein kinase C theta raft recruitment leading to activation of ERK1/2 but not NF kappa B. J Immunol. 2008;181:8425–8432. doi: 10.4049/jimmunol.181.12.8425. [DOI] [PubMed] [Google Scholar]
- 41.Pfeifhofer C, Kofler K, Gruber T, Tabrizi NG, Lutz C, Maly K, Leitges M, Baier G. Protein kinase C theta affects Ca2+ mobilization and NFAT cell activation in primary mouse T cells. J Exp Med. 2003;197:1525–1535. doi: 10.1084/jem.20020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gu C, Tangye SG, Sun X, Luo Y, Lin Z, Wu J. The X-linked lymphoproliferative disease gene product SAP associates with PAK-interacting exchange factor and participates in T cell activation. Proc Natl Acad Sci U S A. 2006;103:14447–14452. doi: 10.1073/pnas.0606624103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baldari CT, Macchia G, Telford JL. Interleukin-2 promoter activation in T-cells expressing activated Ha-ras. J Biol Chem. 1992;267:4289–4291. [PubMed] [Google Scholar]
- 44.Altman A, Villalba M. Protein kinase C-theta (PKC theta): a key enzyme in T cell life and death. J Biochem. 2002;132:841–846. doi: 10.1093/oxfordjournals.jbchem.a003295. [DOI] [PubMed] [Google Scholar]
- 45.Stewart SJ, Cunningham GR, Strupp JA, House FS, Kelley LL, Henderson GS, Exton JH, Bocckino SB. Activation of phospholipase D: a signaling system set in motion by perturbation of the T lymphocyte antigen receptor/CD3 complex. Cell Regul. 1991;2:841–850. doi: 10.1091/mbc.2.10.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonvini E, DeBell KE, Verí MC, Graham L, Stoica B, Laborda J, Aman MJ, DiBaldassarre A, Miscia S, Rellahan BL. On the mechanism coupling phospholipase Cgamma1 to the B- and T-cell antigen receptors. Adv Enzyme Regul. 2003;43:245–269. doi: 10.1016/s0065-2571(02)00033-x. [DOI] [PubMed] [Google Scholar]
- 47.Ticchioni M, Aussel C, Breittmayer JP, Manié S, Pelassy C, Bernard A. Suppressive effect of T cell proliferation via the CD29 molecule. The CD29 mAb 1 “K20” decreases diacylglycerol and phosphatidic acid levels in activated T cells. J Immunol. 1993;151:119–127. [PubMed] [Google Scholar]
- 48.Mor A, Campi G, Du G, Zheng Y, Foster DA, Dustin ML, Philips MR. The lymphocyte function-associated antigen-1 receptor costimulates plasma membrane Ras via phospholipase D2. Nat Cell Biol. 2007;9:713–719. doi: 10.1038/ncb1592. [DOI] [PubMed] [Google Scholar]
- 49.Snow AL, Marsh RA, Krummey SM, Roehrs P, Young LR, Zhang K, van Hoff J, Dhar D, Nichols KE, Filipovich AH, Su HC, Bleesing JJ, Lenardo MJ. Restimulation-induced apoptosis of T cells is impaired in patients with X-linked lymphoproliferative disease caused by SAP deficiency. J Clin Invest. 2009;119:2976–2989. doi: 10.1172/JCI39518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alonso R, Mazzeo C, Rodriguez MC, Marsh M, Fraile-Ramos A, Calvo V, Avila-Flores A, Merida I, Izquierdo M. Diacylglycerol kinase α regulates the formation and polarisation of mature multivesicular bodies involved in the secretion of Fas ligand-containing exosomes in T lymphocytes. Cell Death Differ. 2011 doi: 10.1038/cdd.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alonso R, Rodríguez MC, Pindado J, Merino E, Mérida I, Izquierdo M. Diacylglycerol kinase alpha regulates the secretion of lethal exosomes bearing Fas ligand during activation-induced cell death of T lymphocytes. J Biol Chem. 2005;280:28439–28450. doi: 10.1074/jbc.M501112200. [DOI] [PubMed] [Google Scholar]
- 52.Rubio I, Grund S, Song SP, Biskup C, Bandemer S, Fricke M, Förster M, Graziani A, Wittig U, Kliche S. TCR-induced activation of Ras proceeds at the plasma membrane and requires palmitoylation of N-Ras. J Immunol. 2010;185:3536–3543. doi: 10.4049/jimmunol.1000334. [DOI] [PubMed] [Google Scholar]
- 53.Alonso R, Mazzeo C, Mérida I, Izquierdo M. A new role of diacylglycerol kinase alpha on the secretion of lethal exosomes bearing Fas ligand during activation-induced cell death of T lymphocytes. Biochimie. 2007;89:213–221. doi: 10.1016/j.biochi.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 54.Rincón E, Santos T, Avila-Flores A, Albar JP, Lalioti V, Lei C, Hong W, Mérida I. Proteomics identification of sorting nexin 27 as a diacylglycerol kinase zeta-associated protein: new diacylglycerol kinase roles in endocytic recycling. Mol Cell Proteomics. 2007;6:1073–1087. doi: 10.1074/mcp.M700047-MCP200. [DOI] [PubMed] [Google Scholar]
- 55.Raben DM, Tu-Sekine B. Nuclear diacylglycerol kinases: regulation and roles. Front Biosci. 2008;13:590–597. doi: 10.2741/2704. [DOI] [PubMed] [Google Scholar]
- 56.Bacchiocchi R, Baldanzi G, Carbonari D, Capomagi C, Colombo E, van Blitterswijk WJ, Graziani A, Fazioli F. Activation of alpha-diacylglycerol kinase is critical for the mitogenic properties of anaplastic lymphoma kinase. Blood. 2005;106:2175–2182. doi: 10.1182/blood-2005-01-0316. [DOI] [PubMed] [Google Scholar]
- 57.Wange RL. LAT, the linker for activation of T cells: a bridge between T cell-specific and general signaling pathways. Sci STKE. 2000;2000:re1. doi: 10.1126/stke.2000.63.re1. [DOI] [PubMed] [Google Scholar]
- 58.Cannons JL, Yu LJ, Jankovic D, Crotty S, Horai R, Kirby M, Anderson S, Cheever AW, Sher A, Schwartzberg PL. SAP regulates T cell-mediated help for humoral immunity by a mechanism distinct from cytokine regulation. J Exp Med. 2006;203:1551–1565. doi: 10.1084/jem.20052097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grady EF. Cell signaling. Beta-arrestin, a two-fisted terminator. Science. 2007;315:605–606. doi: 10.1126/science.1138505. [DOI] [PubMed] [Google Scholar]
- 60.Baldanzi G, Alchera E, Imarisio C, Gaggianesi M, Dal Ponte C, Nitti M, Domenicotti C, van Blitterswijk WJ, Albano E, Graziani A, Carini R. Negative regulation of diacylglycerol kinase theta mediates adenosine-dependent hepatocyte preconditioning. Cell Death Differ. 2010;17:1059–1068. doi: 10.1038/cdd.2009.210. [DOI] [PubMed] [Google Scholar]
- 61.McMullan R, Hiley E, Morrison P, Nurrish SJ. Rho is a presynaptic activator of neurotransmitter release at pre-existing synapses in C. elegans. Genes Dev. 2006;20:65–76. doi: 10.1101/gad.359706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nurrish S, Ségalat L, Kaplan JM. Serotonin inhibition of synaptic transmission: Galpha(0) decreases the abundance of UNC-13 at release sites. Neuron. 1999;24:231–242. doi: 10.1016/s0896-6273(00)80835-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.