Abstract
Background
Primary care physicians (PCPs) care for the majority of non-dialysis-dependent chronic kidney disease (CKD) patients. Studies suggest that PCPs may deliver suboptimal CKD care. One means to improve PCP treatment of CKD is clinical decision support systems (CDSS).
Study Design
Cluster randomized controlled trial
Setting & Participants
Thirty PCPs in a university-based outpatient general internal medicine practice and their 248 moderate to advanced CKD patients who had not been referred to a nephrologist.
Intervention
Two CKD educational sessions were held for PCPs in both arms. The 15 intervention arm PCPs also received real-time automated electronic medical record alerts for patients with estimated glomerular filtration rates < 45 ml/min/1.73m2 recommending renal referral and urine albumin quantification if not done within the prior year.
Outcomes
Primary outcome was referral to a nephrologist; secondary outcomes were albuminuria/proteinuria assessment, CKD documentation, optimal blood pressure (i.e., < 130/80), and use of renoprotective medications.
Results
The intervention and control arms did not differ in renal referrals (9.7% vs. 16.5%, respectively; between group difference, −6.8% (95% CI, −15.5% to 1.8%; P=0.1)) or proteinuria assessments (39.3% vs. 30.1%, respectively; between group difference, 9.2% (95% CI, −2.7% to 21.1%; P=0.1)). Among intervention and control group patients without a baseline proteinuria assessment, 27.7% versus 16.3%, respectively had one at follow-up (P=0.06). After controlling for clustering, these findings were largely unchanged and no significant differences were apparent between the groups.
Limitations
Small single-center university based practice, use of a passive CDSS that required PCPs to trigger the electronic order set.
Conclusions
PCPs were willing to partake in a randomized trial of CDSS to improve outpatient CKD care. While CDSS may possess potential, larger studies are needed to further explore how best to deploy them to enhance CKD care.
Keywords: Chronic Kidney Disease, Primary Care Physician, Nephrologist, Quality of Care, Estimated Glomerular Filtration Rate, Renal Referral
Millions of Americans live with chronic kidney disease (CKD).1,2 With an ageing population and an increasing prevalence of obesity, diabetes, and hypertension,2–7 the prevalence of CKD is expected to rise. In the face of this growing public health burden, effective treatments to improve patient outcomes are available.8,9 Due to the high prevalence of CKD and the relative shortage of nephrologists,10–12 primary care physicians (PCPs) deliver the majority of care to patients with non–dialysis-dependent CKD.13–16 However, multiple studies have documented suboptimal CKD awareness and care by PCPs including underuse of angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARB), inadequate blood pressure control, and late nephrology referrals.17–19
Initiatives to improve CKD awareness and treatment are underway and include the continued development and dissemination of CKD guidelines (e.g., the KDOQI (Kidney Disease Outcomes Quality Initiative) guidelines from the National Kidney Foundation (NKF)) and the promotion of routine estimated glomerular filtration rate (eGFR) reporting.20,21 Despite these actions, only modest improvements in care have been realized to date.22,23 Changing physician practice patterns to adhere to standards of care remains a challenge in improving patient outcomes. One approach to enhancing physician care is the deployment of clinical decision support systems (CDSS).24–27 Rule-based CDSS allow the combination of programmed expert rules and available patient data to trigger patient specific recommendations that can enhance care by guiding patient-provider decision making.28,29
We conducted a cluster-randomized controlled trial to examine the feasibility of an educational intervention and CDSS versus an educational intervention alone to enhance referrals and quantitative proteinuria assessments in moderate to advanced CKD patients. We chose to intervene on these processes because there is literature documenting: 1) improved outcomes in association with timely renal referrals18,30,31 and 2) improved outcomes in proteinuric CKD patients treated with an ACE inhibitor/ARB.32–36
Methods
Setting and Participants
The study was conducted at a large academic hospital-based general internal medicine (GIM) clinic where more than 11,000 patients are cared for annually. We approached GIM faculty at regularly scheduled department meetings. Physician faculty members who were not present during those meetings were contacted via telephone or email. Faculty were eligible for study enrollment if they had at least a ½-day GIM continuity clinic per week. Faculty without a continuity clinic or who were planning to leave the department within 6 months were excluded. Housestaff were excluded to minimize contamination from attendings in one arm precepting residents in the other arm and potentially discussing the CKD alert recommendations. All participating faculty members provided written informed consent and the University of Pittsburgh Institutional Review Board approved this study.
Consented faculty were randomized to either the CDSS intervention or usual care. Randomization was stratified by the number of ½-day clinic sessions per week (i.e., 5 categories were used: 1–2, 3–4, 5–6, 7–8, and 9–10 sessions) and was performed using a computer generated random number sequence in SAS using blocks of 4. Participants were aware that the study was assessing and attempting to improve CKD care using an electronic medical record (EMR) CDSS intervention regarding proteinuria assessments and timely renal referrals. Patients seen by enrolled PCPs at an outpatient office visit during the study period were included if they had an eGFR < 45ml/min/1.73m2 in the 12 months prior to their visit and had never been evaluated by a university nephrologist. Patients seen by multiple providers during the trial were analyzed according to the study assignment of their PCP of record.
Intervention
Prior to consenting eligible physicians, we held two 15-minute CKD didactic sessions during scheduled monthly GIM faculty meetings. Nephrology faculty discussed blood pressure control, proteinuria quantification and suppression, monitoring for complications of CKD, and timely renal referrals. The presenters also answered occasional CKD related questions from the GIM faculty at the end of the session. These sessions were completed approximately 6 months prior to activation of the CDSS described below.
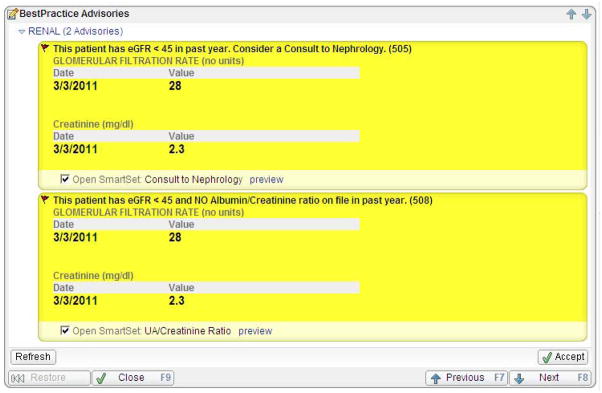
The CDSS intervention consisted of two separate alerts (Figure 1) within the ambulatory EMR, EpicCare (Epic Systems, www.epic.com), which has been in use by the GIM department for over a decade. Each alert was designed to be active for patients who met the following criteria: 1) an eGFR < 45ml/min/1.73m2 within 12 months of the patient’s office visit and 2) had not been seen by a university nephrologist. The first alert suggested a nephrology referral and provided the option to enter an order set containing an order for the referral. The second alert suggested ordering a spot urine albumin-creatinine ratio for patients who had not had a quantitative albuminuria/proteinuria assessment within the last year. This alert also provided the option to enter an order set containing an order for the urine test. These alerts did not interrupt physician work-flow. Rather they appeared in bright yellow on the Visit Navigator®, a list of tasks that are performed during a visit. Physicians could choose to view these alerts and activate the appropriate order sets at a time that best suited their workflow.
Figure 1.
Sample clinical decision support alert
On December 14, 2008, the alerts were turned on for participating GIM faculty members in the intervention group. The alerts were scheduled to continue for 6-months. However, due to the relatively small number of eligible patients (58 in the control group, 60 in the intervention group), the alerts were extended for a total duration of 10-months (i.e., through October 13, 2009). During the study, no limit was placed on the number of times the alerts could be triggered for each patient.
Data Collection
Kidney Function
For eligible patients, all serum creatinine and eGFR values from the 12 months preceding and following study initiation (i.e., 12/14/07 – 12/13/09) were abstracted from the EMR. The most recent serum creatinine/eGFR from the 12 months preceding and following study initiation was used to determine baseline and follow-up creatinine/eGFR, respectively. Isotope dilution mass spectrometry traceable serum creatinine assays were used by the local laboratory. The IDMS-traceable 4-variable Modification of Diet in Renal Disease Study equation37 was used by the local laboratory to calculate eGFR values. The following message accompanied eGFR results: “eGFR < 60mL/min/1.73m2 indicates kidney disease. eGFR < 15 mL/min/1.73m2 indicates kidney failure.”
Covariates
As previously described,38 we abstracted the following variables from the EMR on all eligible patients: age, sex, race, insurance status, diabetic status, and hypertensive status.
Outcomes
The primary outcome was the presence of an EMR order for a nephrology consultation (regardless of whether the patient kept the appointment) or the presence of a nephrology encounter in the EMR during the 10-month study period or within 2 months of the end of the study. This 2-month delay allowed time for patients evaluated at the end of the study to have laboratory tests completed, further orders placed, and specialist consults fulfilled.
The pre-specified secondary outcomes (described in further detail below) were measures of quality of CKD care. These included changes in the proportion of patients with annual albuminuria or proteinuria quantification, active ACE inhibitor or ARB use, optimal blood pressure control, non-steroidal anti-inflammatory drug (NSAID) use, and CKD documentation. We also examined hemoglobin, bicarbonate, calcium, phosphorus, and parathyroid hormone (PTH) levels and the proportion of patients with at least once a year monitoring of these labs.
Baseline and follow-up urinary albumin or protein quantification was determined by the presence of an EMR order or laboratory result of a quantitative assessment for albuminuria or proteinuria (including spot or 24-hour measurements) in the 12 months prior to or following study initiation, respectively. All blood pressure values from the 12 months before and after study initiation were obtained from office visit vital signs recorded in the EMR. The single most recent blood pressure value from the 12 months preceding and following study initiation was used to determine baseline and follow-up blood pressure, respectively. Optimal blood pressure control was defined as both a systolic blood pressure < 130mmHg and a diastolic blood pressure < 80mmHg. Baseline and follow-up use of an ACE inhibitor/ARB and NSAID was determined by the presence of an ACE inhibitor/ARB or NSAID on the patient’s EMR medication list at study onset or 12-months after study initiation, respectively. The EMR medication list contains all medications that are prescribed using the EMR and, based on the practice’s standards, all medications (i.e., over-the-counter and herbal medications) are added to the list for documentation purposes. As previously described,38 CKD documentation was determined by the presence of an International Classification of Disease, Ninth Revision code on the patient’s EMR problem list or as a billing diagnosis in a PCP outpatient encounter in the 12 months prior to (baseline status) or following (follow-up status) study initiation.
Baseline and follow-up lab testing for hemoglobin, bicarbonate, calcium, phosphorus, and PTH were determined by the presence of an EMR order or laboratory result for each respective test in the 12 months prior to or following study initiation, respectively. Each of the most recent laboratory measures from the 12 months preceding and following study initiation was used to determine baseline and follow-up values, respectively. A random sample was used to select 10% of patient charts for an EMR chart audit to verify the accuracy of abstracted data.
Statistical Analyses
Power Calculation
We estimated 30 PCPs would enroll in the study and care for 365 moderate to advanced CKD patients. Estimating a control group referral rate between 5 to 50% and provider intraclass correlation coefficients of 0.01 (as recommended for health services research when no preliminary data are available) provided 80% power to detect differences in renal referral rates (i.e., the primary outcome) ranging from 7% (control group referral rate of 5%) to 16% (control group referral rate of 50%).39
Data Analysis
Baseline differences between the groups in physician characteristics, patient demographics, clinical variables, and clinical care (nephrology referral, proteinuria quantification, etc) were assessed using a Student’s t-test or a Wilcoxon-Mann-Whitney test for continuous variables and a chi-square or Fisher’s exact test for categorical variables. Group differences at study completion and changes from baseline were assessed using a Student’s t-test for continuous variables and a chi-square or Fisher’s exact test for categorical variables. To examine patients who were most likely to benefit from the CDSS, we then stratified the analysis by baseline care status (i.e., proteinuria assessment, ACE inhibitor/ARB use, CKD documentation, blood pressure < 130/80, and NSAID use) to determine how patients who received suboptimal baseline care were affected. We conducted univariate logistic regression analyses using primary and secondary outcomes as dependent variables and treatment arm as the independent variable. We then controlled for clustering at the PCP level using generalized estimating equations with an exchangeable working correlation matrix.
In order to examine the potential effects of the inclusion of patients with acute kidney injury or substantial serum creatinine/eGFR variability, sensitivity analysis was conducted excluding patients with any eGFR > 60ml/min/1.73m2 in the 12 months following study initiation. For all analyses, P values <0.05 were considered significant. Analyses were performed using SAS version 9.1 (SAS Institute Inc., http://www.sas.com/).
Results
Baseline Characteristics
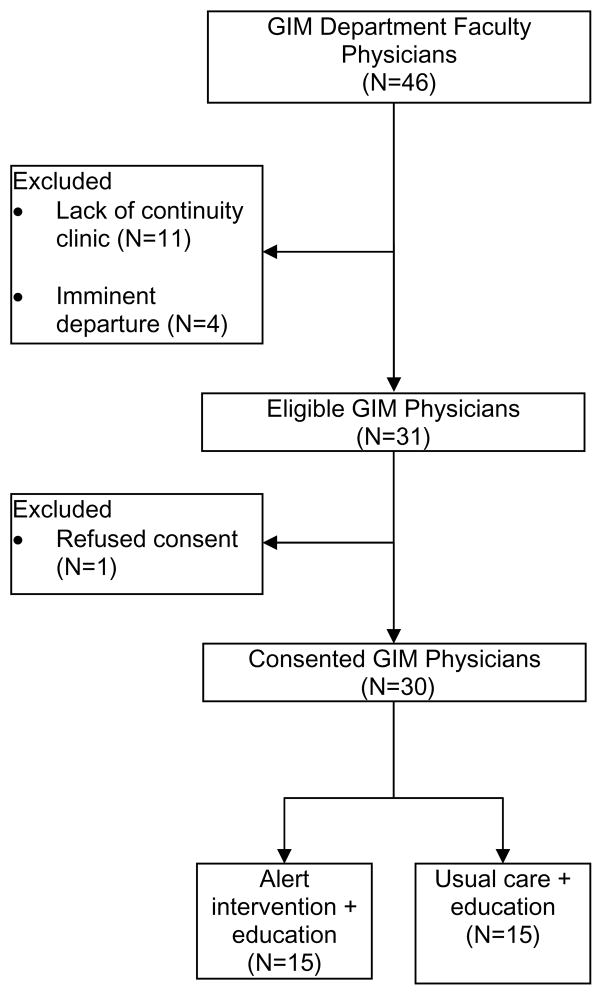
We screened 46 GIM faculty members (Figure 2). Fifteen were excluded due to lack of continuity clinics (N=11) or pending departure (N=4). Of the 31 eligible physicians, 30 consented to the study and 15 were randomized to the CDSS alert arm. There were no physician dropouts during the study. Provider characteristics in each arm were well matched at baseline (Table 1). Eligible patients in each arm were similar in sociodemographics and comorbidities at baseline (Table 1). However, a larger proportion of patients in the intervention group had CKD documented at baseline (Table 1). Otherwise, the patients in each arm were similar in terms of CKD processes of care and laboratory parameters including baseline kidney function (Table 1 and 2).
Figure 2.
Physician enrollment
GIM general internal medicine
Table 1.
Baseline Characteristics
| Control | Intervention | P-Value | |
|---|---|---|---|
| PCP Characteristics | |||
| No. | 15 | 15 | |
| Female | 8 (53.3) | 8 (53.3) | 0.9* |
| Weekly half-day clinics | 0.9* | ||
| 1–2 | 7 (46.7) | 7 (46.7) | |
| 3–4 | 6 (40.0) | 6 (40.0) | |
| 5–6 | 2 (13.3) | 2 (13.3) | |
| Academic rank | 0.9* | ||
| Assistant Professor | 10 (66.7) | 8 (53.3) | |
| Associate Professor | 2 (13.3) | 4 (26.7) | |
| Professor | 3 (20.0) | 3 (20.0) | |
| Years since medical school | 14.5 +/− 8.9 | 16.7 +/− 7.1 | 0.2† |
| Attended ≥ 1 CKD educational session | 12 (80.0) | 9 (60.0) | 0.2* |
| Patient Characteristics | |||
| No. | 103 | 145 | |
| Demographics | |||
| Age | 64.8 +/− 14.0 | 65.7 +/− 14.0 | 0.6 |
| Female | 61 (59.2) | 95 (65.5) | 0.3 |
| African-American | 41 (39.8) | 52 (35.9) | 0.5 |
| Insurance Status | 0.9* | ||
| Private | 52 (50.5) | 66 (45.5) | |
| Medicare | 40 (38.8) | 62 (42.8) | |
| Medical Assistance | 7 (6.8) | 12 (8.3) | |
| Self-pay | 4 (3.9) | 5 (3.4) | |
| Comorbid conditions | |||
| Diabetes | 33 (32.0) | 48 (33.1) | 0.9 |
| Hypertension | 69 (67.0) | 105 (72.4) | 0.4 |
| Quality of baseline care | |||
| Proteinuria assessment | 23 (22.3) | 33 (22.8) | 0.9 |
| ACEi/ARB | 59 (57.3) | 88 (60.7) | 0.6 |
| No ACEi/ARB or proteinuria assessment | 38 (36.9) | 52 (35.9) | 0.9 |
| CKD documentation | 10 (9.7) | 37 (25.5) | 0.002 |
| BP < 130/80 mmHg** | 47 (49.0) | 63 (48.5) | 0.9 |
| Systolic BP (mmHg) | 131.0 +/− 20.9 | 130.6 +/− 19.7 | 0.9 |
| Diastolic BP (mmHg) | 75.5 +/− 12.4 | 75.4 +/− 13.5 | 0.9 |
| NSAID use | 20 (19.4) | 18 (12.4) | 0.1 |
Continuous variables are presented as mean +/− standard deviation. Categorical variables are expressed as number (percentage).
P-values represent Student’s t-test for continuous variables and chi-square for categorical variables except as noted.
Fischer’s exact test
Wilcoxon-Mann-Whitney test
N = 96 for the control group, N = 130 for the intervention group.
PCP primary care physician, CKD chronic kidney disease, ACEi angiotensin converting enzyme inhibitor, ARB angiotensin receptor blocker, BP blood pressure, NSAID non-steroidal anti-inflammatory drug.
Table 2.
Laboratory processes of care at baseline and study completion.
| Control (n=103) | Baseline Intervention (n=145) | P-Value | Control (n=103) | Study Completion Intervention (n=145) | P-Value | |
|---|---|---|---|---|---|---|
| Kidney Function | ||||||
| eGFR (ml/min/1.73m2) | 50.5 +/− 21.1 † | 48.5 +/− 17.4 † | 0.5 | 42.6 +/− 9.3 | 42.1 +/− 16.4 | 0.8 |
| Creatinine (mg/dL) | 1.8 +/− 1.9 † | 1.7 +/− 1.6 † | 0.7 | 2.1 +/− 1.9 | 2.0 +/− 1.9 | 0.7 |
| Lab values | ||||||
| Hemoglobin* (g/dL) | 12.3 +/− 1.8 | 12.5 +/− 1.7 | 0.5 | 12.4 +/− 1.8 | 12.3 +/− 1.9 | 0.9 |
| Bicarbonate* (mEq/L) | 26.0 +/− 3.8 | 26.3 +/− 4.1 | 0.6 | 26.3 +/− 3.7 | 26.2 +/− 3.6 | 0.8 |
| Calcium* (mg/dL) | 9.5 +/− 0.6 | 9.6 +/− 0.6 | 0.4 | 9.3 +/− 0.5 | 9.4 +/− 0.6 | 0.2 |
| Phosphorus* (mg/dL) | 3.9 +/− 0.7 | 3.9 +/− 1.0 | 0.9 | 4.1 +/− 0.9 | 4.0 +/− 1.1 | 0.9 |
| PTH* (pg/mL) | 163.8 +/− 206.2 | 113.6 +/− 107.1 | 0.5 | 207.9 +/− 450.5 | 154.0 +/− 208.8 | 0.7 |
| Lab variable assessed in the prior 12 mo | ||||||
| Hemoglobin | 71 (68.9) | 102 (70.3) | 0.8 | 92 (89.3) | 129 (89.0) | 0.9 |
| Bicarbonate | 86 (83.5) | 121 (83.4) | 0.9 | 103 (100.0) | 145 (100.0) | 1.0 |
| Calcium | 73 (70.9) | 111 (76.6) | 0.3 | 103 (100.0) | 145 (100.0) | 1.0 |
| Phosphorus | 16 (15.5) | 25 (17.2) | 0.7 | 35 (34.0) | 43 (29.7) | 0.5 |
| PTH | 5 (4.9) | 13 (9.0) | 0.2 | 12 (11.7) | 18 (12.4) | 0.9 |
Continuous variables are presented as means +/− standard deviations; categorical variables are expressed as number (percentage).
P-values represent Student’s t-test for continuous variables and chi-square for categorical variables except as noted.
N = 88 for control arm, N = 122 for intervention arm.
N for each variable is provided with each respective lab variable further below in the table
eGFR estimated glomerular filtration rate, PTH parathyroid hormone; Lab, laboratory
Conversion factors for units: serum creatinine in mg/dL to mol/L, ×88.4; hemoglobin in g/dL to g/L, ×10; calcium in mg/dL to mmol/L, ×0.2495; phosphorus in mg/dL to mmol/L, ×0.3229; no conversion necessary for serum bicarbonate in mEq/L and mmol/L, PTH in pg/mL and ng/L.
Quality of Care at Study Completion
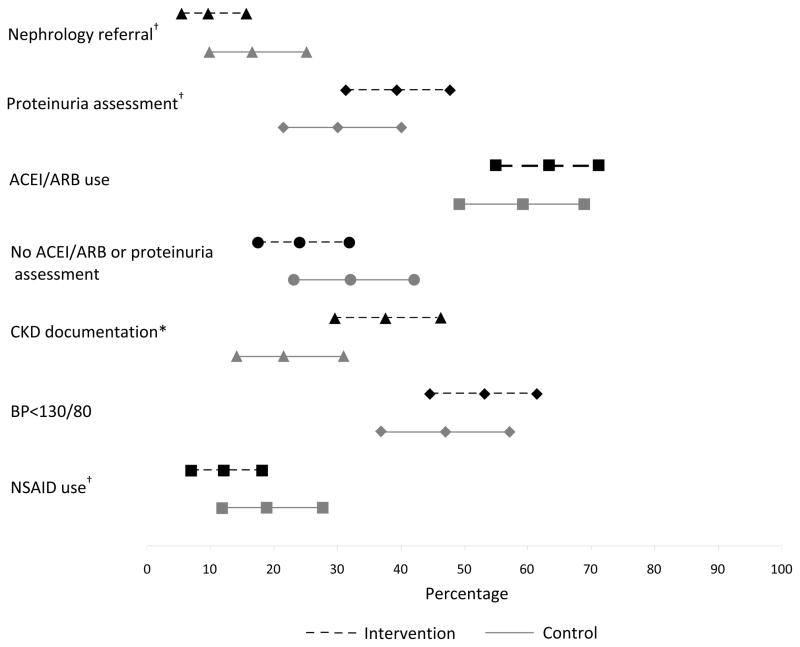
Approximately 10% of patients in the alert group were referred to a nephrologist versus 17% in the control group (P=0.1; Figure 3). Just over 39% of patients in the intervention arm had a proteinuria assessment versus 30.1% in the control arm (P=0.1; Figure 3). CKD was documented in the EMR in 37% of patients in the alert group versus 21% in the control group (P=0.008; Figure 3). No significant differences in ACE inhibitor/ARB use, optimal blood pressure management, or NSAID use were observed between groups (Figure 3). Systolic and diastolic blood pressure was similar between arms (systolic, 129.0 ± 16.0 in control vs. 129.0 ± 18.1 in intervention (P=0.9); diastolic, 73.6 ± 10.3 in control vs. 72.6 ± 11.9 in intevention (P=0.5)).
Figure 3.
Process of care outcomes at study completion, showing percentage and 95% confidence intervals.
†P = 0.1, *P = 0.008
ACEI angiotensin converting enzyme inhibitor, ARB angiotensin receptor blocker, CKD chronic kidney disease, BP blood pressure, NSAID non-steroidal anti-inflammatory drug.
After stratifying by baseline care, a nonsignificant trend towards improved proteinuria assessments and blood pressure control was apparent in the intervention group while differences in CKD documentation were no longer apparent. In both arms, nearly 80% of patients with a proteinuria assessment at baseline also had one at follow-up (78.3% in control vs. 78.8% in intervention, P=0.9). However, among patients without a proteinuria assessment at baseline, 16.3% in the control group had one at follow-up versus 27.7% in the intervention group (P=0.06). In patients with optimal blood pressure control at baseline, there was little difference in follow-up blood pressure control with the majority remaining well controlled (70.2% in control vs. 74.6% in intevention, P=0.6). Yet, in patients with suboptimal blood pressure control at baseline, 37.3% of the intervention group achieved optimal blood pressure levels versus 22.4% in the control group (P=0.09). In contrast, when stratified by baseline status, differences in CKD documentation were no longer observed. In both arms, ≥80% of patients with CKD documentation at baseline also had documentation at study completion (80.0% in control vs. 86.5% in intervention, P=0.6). However, in patients without CKD documentation at baseline, 15% of the control arm had documentation at study completion versus 20% of the alert group (P=0.3).
After adjusting for clustering and stratifying by baseline care, our findings were unchanged; no significant differences in quality of care were apparent between groups though nonsignificant trends in proteinuria assessment and blood pressure control remained (Figure 4).
Figure 4.
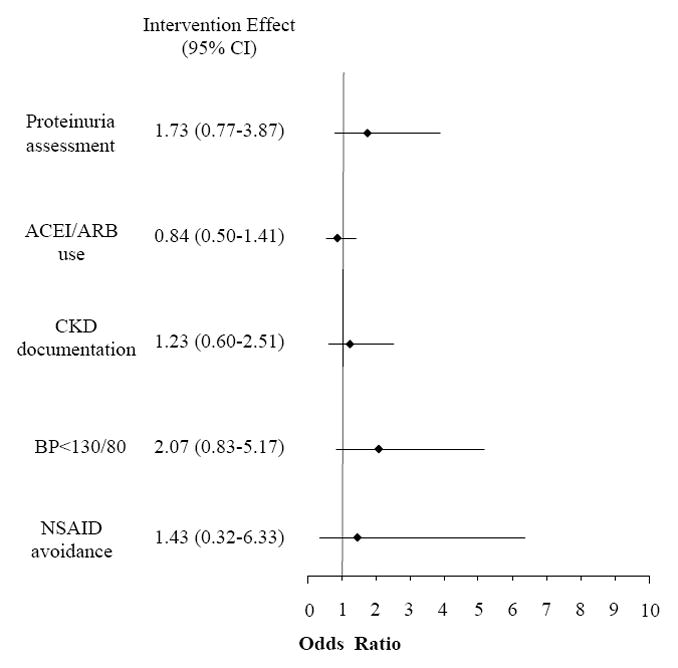
Intervention group odds of receiving specified care at study completion.*
*Analysis is restricted to patients in each arm who did not meet the optimal specified care parameter at baseline assessment.
CI confidence interval, ACEI angiotensin converting enzyme inhibitor, ARB angiotensin receptor blocker, CKD chronic kidney disease, BP blood pressure, NSAID non-steroidal anti-inflammatory drug
No differences between groups in laboratory values or the proportion of patients with at least annual monitoring of specified lab measures were apparent at study completion (Table 3). Universal calcium monitoring at study completion was likely due to inclusion of serum calcium in the local laboratory’s basic metabolic profile.
Sensitivity analyses that excluded patients with any recent eGFR > 60ml/min/1.73m2 resulted in the exclusion of 16 patients in the control arm and 17 patients in the intervention arm. No substantive differences in outcomes were apparent (data not shown).
Discussion
In this study, the overwhelming majority of PCPs at an outpatient GIM clinic were willing to participate in a randomized pilot trial assessing an automated clinical alert targeting patients with an eGFR < 45ml/min/1.73m2 for renal referrals and albuminuria/proteinuria assessments. Although the intervention did not increase renal referrals, it may have improved proteinuria assessments in patients who lacked one at baseline. A trend towards improved blood pressure control in patients with suboptimal baseline blood pressure was also observed. However, other downstream effects of improved CKD awareness including increased ACE inhibitor/ARB use were notably absent.
Multiple factors may have contributed to the lack of an observed effect from the nephrology referral alert. First, the implementation of eGFR reporting has already substantially increased the rate of renal referrals.40 For example, at our university’s GIM clinic, nearly 70% of patients with stage 4 CKD are already co-managed by a nephrologist.38 Patients who have not seen a nephrologist may represent a select population with appropriate reasons to defer specialist consultation or may have barriers to referral that are difficult to address (e.g., limited mobility, financial hardships). A clinical alert to refer CKD patients may be ineffective in this setting. Notably, less than 13% of our moderate to advanced CKD sample were referred in the 12 months following study initiation and this is qualitatively similar to larger studies examining PCP referral patterns following eGFR implementation.14 Future research is needed to examine why particular patients with moderate to advanced CKD are not referred to a nephrologist in the era of eGFR reporting. Second, the CDSS alert targeting nephrologist referrals may also have been ineffective if providers in the intervention arm felt more comfortable caring for patients with CKD and therefore did not feel that a renal consultation was likely to be helpful. While PCP characteristics and CKD care were well balanced at baseline, patients in the intervention group were significantly more likely to have CKD documented in their EMR at baseline. This may suggest greater provider interest in or awareness of CKD compared to control group PCPs.
Another factor potentially limiting the effectiveness of our referral alert was use of a passive alert. In order to view the alert, PCPs needed to scroll down to the appropriate EMR section (although they could always see that there was an unspecified patient care alert from the main screen). This minimized potential disruptions to workflow and subsequent alert fatigue.41–43 However, studies reveal that automated, system initiated CDSS are more effective than systems that require the user to activate or control the decision support.44–47 In the future, “stackable” alerts that remain in the provider’s immediate visual world but do not abruptly interrupt workflow and must be processed prior to the end of a patient encounter 48 may allow an improved compromise between active CDSS deployment and alert fatigue.
While the alert intervention did not increase renal referrals, a trend towards improved proteinuria assessments and blood pressure control were observed in patients who did not have proteinuria measurements or optimal blood pressure control at baseline. We hypothesize that the possible effectiveness of the automated reminder in improving proteinuria monitoring may relate to the relatively simple, inexpensive and non-invasive nature of this test. In future CKD studies, CDSS may be more effective when linked to interventions that are not perceived as burdensome to the patient. Interestingly, while the intervention arm tended to demonstrate a larger improvement in proteinuria assessments, both arms exhibited improvements in proteinuria monitoring. One explanation is that PCPs may have been cognizant that their CKD care was being scrutinized and this may have altered their usual behavior. Alternatively, our educational sessions may have improved baseline care. However, baseline care after the educational sessions was notably comparable to our published results of CKD practice characteristics in the same clinic in the months immediately preceding the didactics.38 Another possible explanation is that the implementation of a GIM physician quality reporting initiative targeting albuminuria assessments in diabetic patients during the study may have heightened provider awareness. In the future, combining physician incentives with CDSS may be a valuable strategy to improving care.
In addition to the direct effect of the clinical alert to check albuminuria/proteinuria, the trend towards enhanced blood pressure control may have been a downstream effect of measuring (and documenting the presence of) albuminuria/proteinuria. However, given our small study size and the lack of a significant impact in other secondary outcomes (e.g., ACE inhibitor/ARB use), caution is necessary in considering these findings. In the future, implementing automated CDSS that can risk stratify CKD patients based on commonly available prognostic markers (such as eGFR, proteinuria) and provide appropriate recommendations to enhance the care of patients receiving suboptimal care, may allow the efficient targeting of alerts for the highest-risk CKD patients. Additional studies are needed to determine if such an approach could improve care while minimizing alert fatigue. Unfortunately, low rates of proteinuria assessment may be a barrier to this approach in many settings.
The findings in this study should be considered in the context of several limitations. First, we only included patients with a documented eGFR < 45ml/min/1.73m2 during the intervention period. CKD patients without a documented serum creatinine/eGFR value or with milder stages of CKD were not included in this study. Future research examining interventions that can reliably target CKD patients at an earlier stage of disease or identify patients with substantial risks for CKD but without a confirmatory test may benefit larger numbers of patients. Second, our CDSS alert recommended renal referrals in the setting of an eGFR< 45ml/min/1.73m2. However, the current NKF CKD guidelines recommend a referral for an eGFR < 30 ml/min/1.73m2.49 Local PCPs may have disregarded the CDSS recommendation in patients who did not meet this criterion. However, we selected the eGFR < 45ml/min/1.73m2 threshold after discussions with the GIM department and based on literature documenting an increase in CKD related complications below this value.9,49 Third, the NKF definition of CKD stage 3 or greater requires a reduction in the eGFR to < 60 ml/min/1.73m2 for at least 3 months.49 Our use of a single eGFR < 45 ml/min/1.73m2 to identify patients may have resulted in misclassification. However, approximately 85% of patients had more than 1 eGFR value and excluding patients with any recent eGFR > 60ml/min/1.73m2 did not alter our findings. Fourth, this study was underpowered to detect potential differences between study arms. Finally, this was a small single center study conducted in an academic setting with a high baseline level of nephrology co-management, thereby limiting generalizability. While we excluded patients managed in resident clinics, we cannot exclude differences in academic physician practice patterns compared to their community peers.
This study also possesses a number of strengths. We randomized physicians in order to minimize contamination. Second, the EMR system used at our medical center is among the largest vendor-based ambulatory EMR products in the United States including an estimated 70 million patients, thereby enhancing generalizability.50 Third, the deployed CDSS gathered and processed information in an automated fashion to provide a tailored assessment and recommendation to the PCP in real-time (i.e., during the actual office visit). Finally, the CDSS required only one click to provide PCPs with immediate access to the action sequence necessary to execute the recommended goal.
In conclusion, enhancing physician care remains an important, complex challenge. Previous research indicates that disease management programs, electronic medical record tools, academic detailing, and multidisciplinary clinics can play an important role in improving CKD care.51–54 Studies in a variety of clinical settings suggest that multiple interventions are often necessary to produce incremental improvements in physician performance.24–27 Whereas didactics are generally ineffective,27 CDSS are among the most effective interventions.27,46 Indeed, CDSS have previously been used to improve care delivery to hospitalized patients with reduced kidney function.47,55,56 However, despite well-established shortcomings in care, there has been limited use or study of CDSS to enhance non-dialysis CKD care.53,57 Larger studies employing CDSS to improve CKD care are needed to further delineate the potential utility and ideal settings for deploying these tools.
Acknowledgments
Support: This work was supported by a NKF Foundation Clinical Research Fellowship, Ruth L. Kirschstein National Research Service Award Institutional Research Training Grants T32-DK061296 and Individual Postdoctoral Training Grant F32-DK084676, and by National Institutes of Health (NIH) Grant K23DK090304 (Dr Abdel-Kader); however, the study design, data collection, analysis, interpretation, and manuscript preparation and submission were determined by the authors alone. This publication was also made possible by Grant Number UL1 RR024153 from the National Center for Research Resources (NCRR), a component of the NIH, and NIH Roadmap for Medical Research.
Footnotes
N section: Trial registration: www.ClinicalTrials.gov; study number: NCT00688285
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 3.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004;44(4):398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- 4.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288(14):1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 5.Gregg EW, Cheng YJ, Narayan KM, Thompson TJ, Williamson DF. The relative contributions of different levels of overweight and obesity to the increased prevalence of diabetes in the United States: 1976–2004. Prev Med. 2007;45(5):348–352. doi: 10.1016/j.ypmed.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 6.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291(23):2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 7.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 8.Taal MW. Slowing the progression of adult chronic kidney disease: therapeutic advances. Drugs. 2004;64(20):2273–2289. doi: 10.2165/00003495-200464200-00002. [DOI] [PubMed] [Google Scholar]
- 9.Abboud H, Henrich WL. Clinical practice. Stage IV chronic kidney disease. N Engl J Med. 2010;362(1):56–65. doi: 10.1056/NEJMcp0906797. [DOI] [PubMed] [Google Scholar]
- 10.Field M. Addressing the global shortage of nephrologists. Nat Clin Pract Nephrol. 2008;4(11):583. doi: 10.1038/ncpneph0951. [DOI] [PubMed] [Google Scholar]
- 11.Suki WN. Are physicians assistants the answer to a shortage of nephrologists? Am J Kidney Dis. 1999;33(4):796–797. doi: 10.1016/s0272-6386(99)70237-4. [DOI] [PubMed] [Google Scholar]
- 12.Estimating workforce and training requirements for nephrologists through the year 2010. Ad Hoc Committee on Nephrology Manpower Needs. J Am Soc Nephrol. 1997;8(5 Suppl 9):S9–13. i–xxii, 11–32. doi: 10.1681/ASN.V85s9. passim. [DOI] [PubMed] [Google Scholar]
- 13.Johnson CA, Levey AS, Coresh J, Levin A, Lau J, Eknoyan G. Clinical practice guidelines for chronic kidney disease in adults: Part I. Definition, disease stages, evaluation, treatment, and risk factors. Am Fam Physician. 2004;70(5):869–876. [PubMed] [Google Scholar]
- 14.Richards N, Harris K, Whitfield M, et al. The impact of population-based identification of chronic kidney disease using estimated glomerular filtration rate (eGFR) reporting. Nephrol Dial Transplant. 2008;23(2):556–561. doi: 10.1093/ndt/gfm839. [DOI] [PubMed] [Google Scholar]
- 15.Fox CH, Brooks A, Zayas LE, McClellan W, Murray B. Primary care physicians’ knowledge and practice patterns in the treatment of chronic kidney disease: an Upstate New York Practice-based Research Network (UNYNET) study. J Am Board Fam Med. 2006;19(1):54–61. doi: 10.3122/jabfm.19.1.54. [DOI] [PubMed] [Google Scholar]
- 16.Nissenson AR, Collins AJ, Hurley J, Petersen H, Pereira BJ, Steinberg EP. Opportunities for improving the care of patients with chronic renal insufficiency: current practice patterns. J Am Soc Nephrol. 2001;12(8):1713–1720. doi: 10.1681/ASN.V1281713. [DOI] [PubMed] [Google Scholar]
- 17.Philipneri MD, Rocca Rey LA, Schnitzler MA, et al. Delivery patterns of recommended chronic kidney disease care in clinical practice: administrative claims-based analysis and systematic literature review. Clin Exp Nephrol. 2008;12(1):41–52. doi: 10.1007/s10157-007-0016-3. [DOI] [PubMed] [Google Scholar]
- 18.Chan MR, Dall AT, Fletcher KE, Lu N, Trivedi H. Outcomes in patients with chronic kidney disease referred late to nephrologists: a meta-analysis. Am J Med. 2007;120(12):1063–1070. doi: 10.1016/j.amjmed.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 19.Boulware LE, Troll MU, Jaar BG, Myers DI, Powe NR. Identification and referral of patients with progressive CKD: a national study. Am J Kidney Dis. 2006;48(2):192–204. doi: 10.1053/j.ajkd.2006.04.073. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Andreoli SP, DuBose T, Provenzano R, Collins AJ. CKD: common, harmful, and treatable--World Kidney Day 2007. Am J Kidney Dis. 2007;49(2):175–179. doi: 10.1053/j.ajkd.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Schoolwerth AC, Burrows NR, Williams DE, Stith KR, McClellan W. Comprehensive public health strategies for preventing the development, progression, and complications of CKD: report of an expert panel convened by the Centers for Disease Control and Prevention. Am J Kidney Dis. 2009;53(3):522–535. doi: 10.1053/j.ajkd.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 22.Wyatt C, Konduri V, Eng J, Rohatgi R. Reporting of estimated GFR in the primary care clinic. Am J Kidney Dis. 2007;49(5):634–641. doi: 10.1053/j.ajkd.2007.02.258. [DOI] [PubMed] [Google Scholar]
- 23.Hemmelgarn BR, Zhang J, Manns BJ, et al. Nephrology visits and health care resource use before and after reporting estimated glomerular filtration rate. JAMA. 2010;303(12):1151–1158. doi: 10.1001/jama.2010.303. [DOI] [PubMed] [Google Scholar]
- 24.Balas EA, Weingarten S, Garb CT, Blumenthal D, Boren SA, Brown GD. Improving preventive care by prompting physicians. Arch Intern Med. 2000;160(3):301–308. doi: 10.1001/archinte.160.3.301. [DOI] [PubMed] [Google Scholar]
- 25.Weiner M, Callahan CM, Tierney WM, et al. Using information technology to improve the health care of older adults. Ann Intern Med. 2003;139(5 Pt 2):430–436. doi: 10.7326/0003-4819-139-5_part_2-200309021-00010. [DOI] [PubMed] [Google Scholar]
- 26.Bero LA, Grilli R, Grimshaw JM, Harvey E, Oxman AD, Thomson MA. Closing the gap between research and practice: an overview of systematic reviews of interventions to promote the implementation of research findings. The Cochrane Effective Practice and Organization of Care Review Group. BMJ. 1998;317(7156):465–468. doi: 10.1136/bmj.317.7156.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith WR. Evidence for the effectiveness of techniques To change physician behavior. Chest. 2000;118(2 Suppl):8S–17S. doi: 10.1378/chest.118.2_suppl.8s. [DOI] [PubMed] [Google Scholar]
- 28.Wyatt J, Spiegelhalter D. Field trials of medical decision-aids: potential problems and solutions. Proc Annu Symp Comput Appl Med Care. 1991:3–7. [PMC free article] [PubMed] [Google Scholar]
- 29.Soman S, Zasuwa G, Yee J. Automation, decision support, and expert systems in nephrology. Adv Chronic Kidney Dis. 2008;15(1):42–55. doi: 10.1053/j.ackd.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Orlando LA, Owen WF, Matchar DB. Relationship between nephrologist care and progression of chronic kidney disease. N C Med J. 2007;68(1):9–16. [PubMed] [Google Scholar]
- 31.Martinez-Ramirez HR, Jalomo-Martinez B, Cortes-Sanabria L, et al. Renal function preservation in type 2 diabetes mellitus patients with early nephropathy: a comparative prospective cohort study between primary health care doctors and a nephrologist. Am J Kidney Dis. 2006;47(1):78–87. doi: 10.1053/j.ajkd.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 32.Jafar TH, Schmid CH, Landa M, et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med. 2001;135(2):73–87. doi: 10.7326/0003-4819-135-2-200107170-00007. [DOI] [PubMed] [Google Scholar]
- 33.Sarafidis PA, Khosla N, Bakris GL. Antihypertensive therapy in the presence of proteinuria. Am J Kidney Dis. 2007;49(1):12–26. doi: 10.1053/j.ajkd.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 34.Maschio G, Alberti D, Janin G, et al. Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. N Engl J Med. 1996;334(15):939–945. doi: 10.1056/NEJM199604113341502. [DOI] [PubMed] [Google Scholar]
- 35.Giatras I, Lau J, Levey AS. Effect of angiotensin-converting enzyme inhibitors on the progression of nondiabetic renal disease: a meta-analysis of randomized trials. Angiotensin-Converting-Enzyme Inhibition and Progressive Renal Disease Study Group. Ann Intern Med. 1997;127(5):337–345. doi: 10.7326/0003-4819-127-5-199709010-00001. [DOI] [PubMed] [Google Scholar]
- 36.Hou FF, Zhang X, Zhang GH, et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. 2006;354(2):131–140. doi: 10.1056/NEJMoa053107. [DOI] [PubMed] [Google Scholar]
- 37.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53(4):766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 38.Abdel-Kader K, Fischer GS, Johnston JR, Gu C, Moore CG, Unruh ML. Characterizing pre-dialysis care in the era of eGFR reporting: a cohort study. BMC nephrology. 2011;12:12. doi: 10.1186/1471-2369-12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murray DM. Design and analysis of group-randomized trials. New York: Oxford University Press; 1998. [Google Scholar]
- 40.Kagoma YK, Weir MA, Iansavichus AV, et al. Impact of Estimated GFR Reporting on Patients, Clinicians, and Health-Care Systems: A Systematic Review. Am J Kidney Dis. 2011;57(4):592–601. doi: 10.1053/j.ajkd.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 41.Ash JS, Sittig DF, Campbell EM, Guappone KP, Dykstra RH. Some unintended consequences of clinical decision support systems. AMIA Annu Symp Proc. 2007:26–30. [PMC free article] [PubMed] [Google Scholar]
- 42.Isaac T, Weissman JS, Davis RB, et al. Overrides of medication alerts in ambulatory care. Arch Intern Med. 2009;169(3):305–311. doi: 10.1001/archinternmed.2008.551. [DOI] [PubMed] [Google Scholar]
- 43.Shah NR, Seger AC, Seger DL, et al. Improving acceptance of computerized prescribing alerts in ambulatory care. J Am Med Inform Assoc. 2006;13(1):5–11. doi: 10.1197/jamia.M1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nies J, Colombet I, Degoulet P, Durieux P. Determinants of success for computerized clinical decision support systems integrated in CPOE systems: a systematic review. AMIA Annu Symp Proc. 2006:594–598. [PMC free article] [PubMed] [Google Scholar]
- 45.Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765. doi: 10.1136/bmj.38398.500764.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garg AX, Adhikari NK, McDonald H, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA. 2005;293(10):1223–1238. doi: 10.1001/jama.293.10.1223. [DOI] [PubMed] [Google Scholar]
- 47.McCoy AB, Waitman LR, Gadd CS, et al. A computerized provider order entry intervention for medication safety during acute kidney injury: a quality improvement report. Am J Kidney Dis. 2010;56(5):832–841. doi: 10.1053/j.ajkd.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wipfli R, Lovis C. Alerts in clinical information systems: building frameworks and prototypes. Stud Health Technol Inform. 2010;155:163–169. [PubMed] [Google Scholar]
- 49.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 50.Epic. [Accessed December 21, 2009]; http://www.epic.com/
- 51.Shahinian VB, Saran R. The role of primary care in the management of the chronic kidney disease population. Adv Chronic Kidney Dis. 2010;17(3):246–253. doi: 10.1053/j.ackd.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 52.Richards N, Harris K, Whitfield M, et al. Primary care-based disease management of chronic kidney disease (CKD), based on estimated glomerular filtration rate (eGFR) reporting, improves patient outcomes. Nephrol Dial Transplant. 2008;23(2):549–555. doi: 10.1093/ndt/gfm857. [DOI] [PubMed] [Google Scholar]
- 53.Fox CH, Swanson A, Kahn LS, Glaser K, Murray BM. Improving chronic kidney disease care in primary care practices: an upstate New York practice-based research network (UNYNET) study. J Am Board Fam Med. 2008;21(6):522–530. doi: 10.3122/jabfm.2008.06.080042. [DOI] [PubMed] [Google Scholar]
- 54.Rutkowski M, Mann W, Derose S, et al. Implementing KDOQI CKD definition and staging guidelines in Southern California Kaiser Permanente. Am J Kidney Dis. 2009;53(3 Suppl 3):S86–99. doi: 10.1053/j.ajkd.2008.07.052. [DOI] [PubMed] [Google Scholar]
- 55.Rind DM, Safran C, Phillips RS, et al. Effect of computer-based alerts on the treatment and outcomes of hospitalized patients. Arch Intern Med. 1994;154(13):1511–1517. [PubMed] [Google Scholar]
- 56.Chertow GM, Lee J, Kuperman GJ, et al. Guided medication dosing for inpatients with renal insufficiency. JAMA. 2001;286(22):2839–2844. doi: 10.1001/jama.286.22.2839. [DOI] [PubMed] [Google Scholar]
- 57.Patwardhan MB, Kawamoto K, Lobach D, Patel UD, Matchar DB. Recommendations for a clinical decision support for the management of individuals with chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(2):273–283. doi: 10.2215/CJN.02590508. [DOI] [PMC free article] [PubMed] [Google Scholar]