Abstract
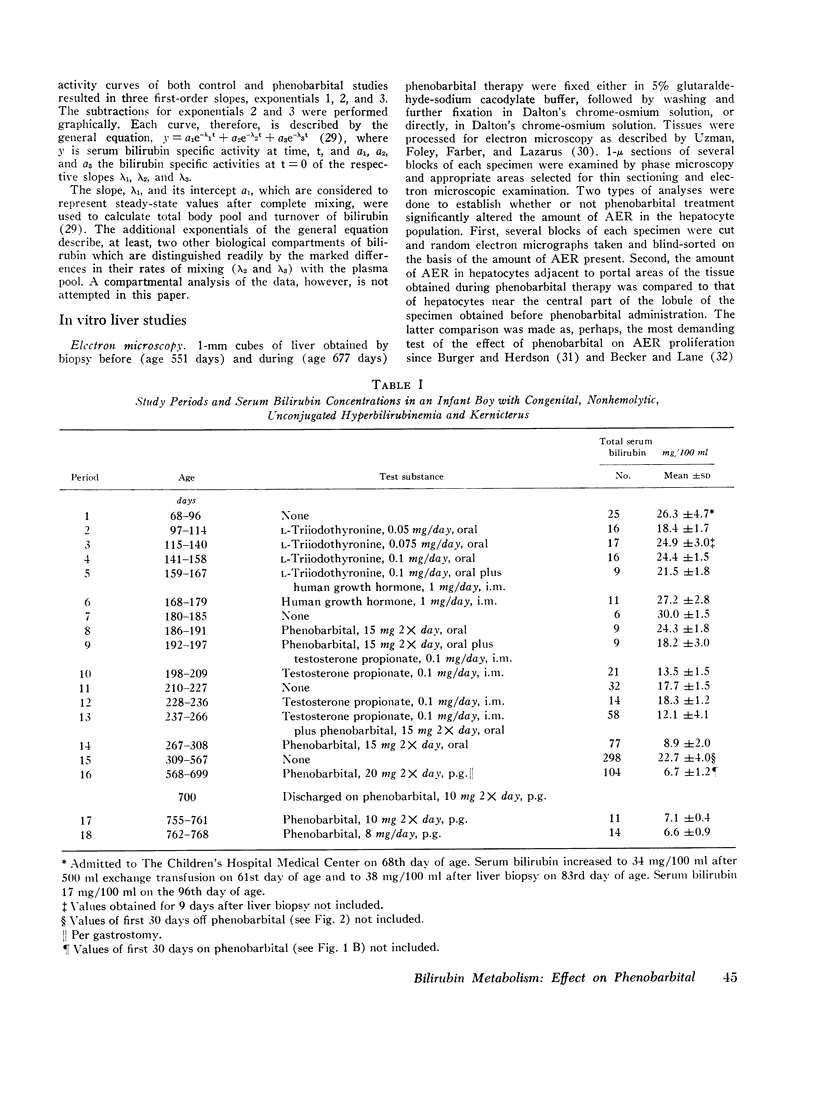
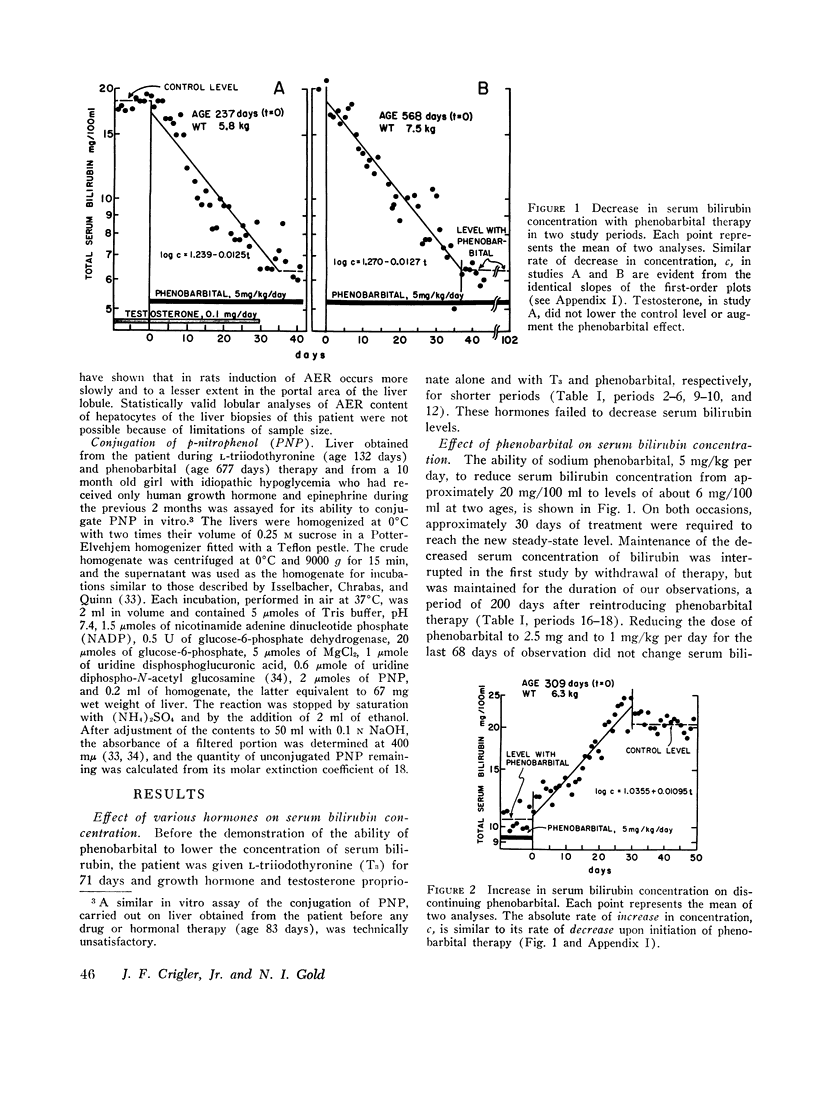
Sodium phenobarbital and various hormones, compounds capable of hepatic enzyme induction, were given to an infant boy with congenital, nonhemolytic, unconjugated, hyperbilirubinemia and severe kernicterus for prolonged periods between the ages of 2 and 25 months to determine their effect on serum bilirubin concentrations. Phenobarbital, 5 mg/day orally, on two occasions decreased serum bilirubin concentrations approximately threefold over a period of 30 days. Withdrawal of phenobarbital after the first study resulted in a gradual (30 days) return of serum bilirubin to pretreatment levels. The lower serum bilirubin concentrations observed when phenobarbital therapy was reinstituted were maintained for 61 days on 2.5 mg/kg per day of the drug. Orally administered L-triiodothyronine, 0.05-0.1 mg/day for 71 days, intramuscular human growth hormone, 1 mg/day for 21 days, and testosterone propionate, 0.1 mg/day for 9 days, did not decrease serum bilirubin levels below lowest control values of 18 mg/100 ml.
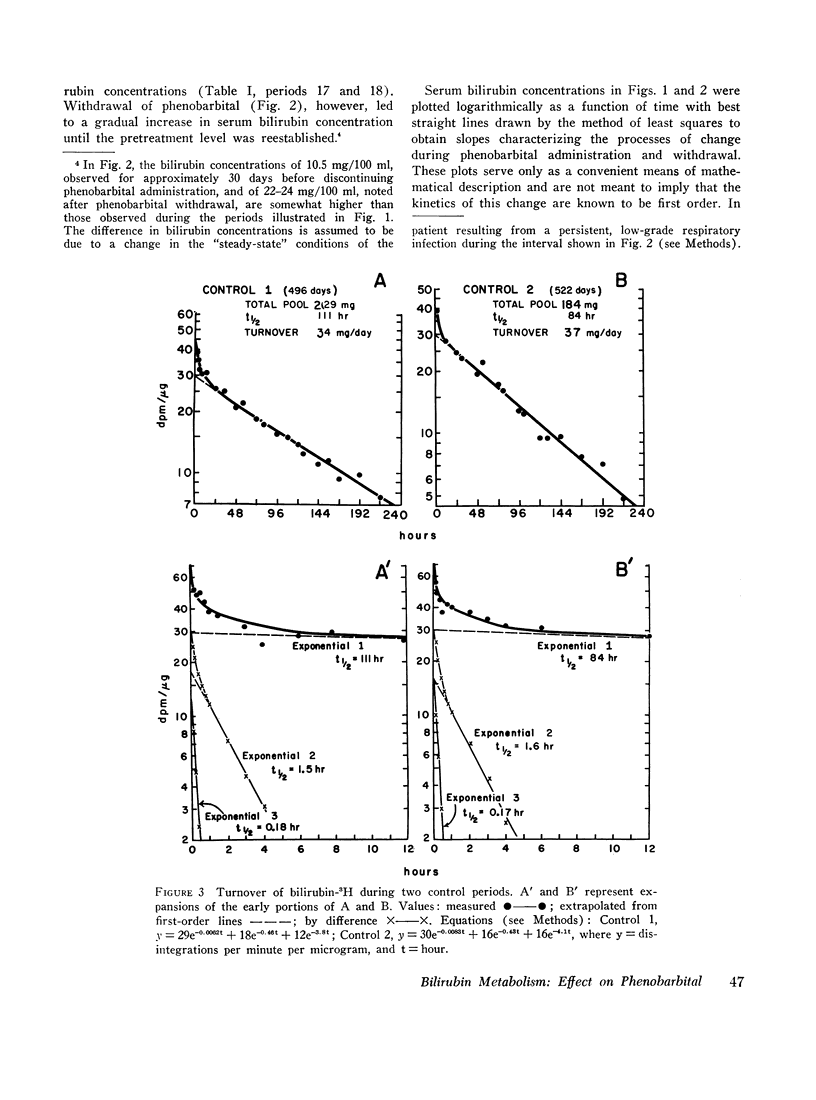
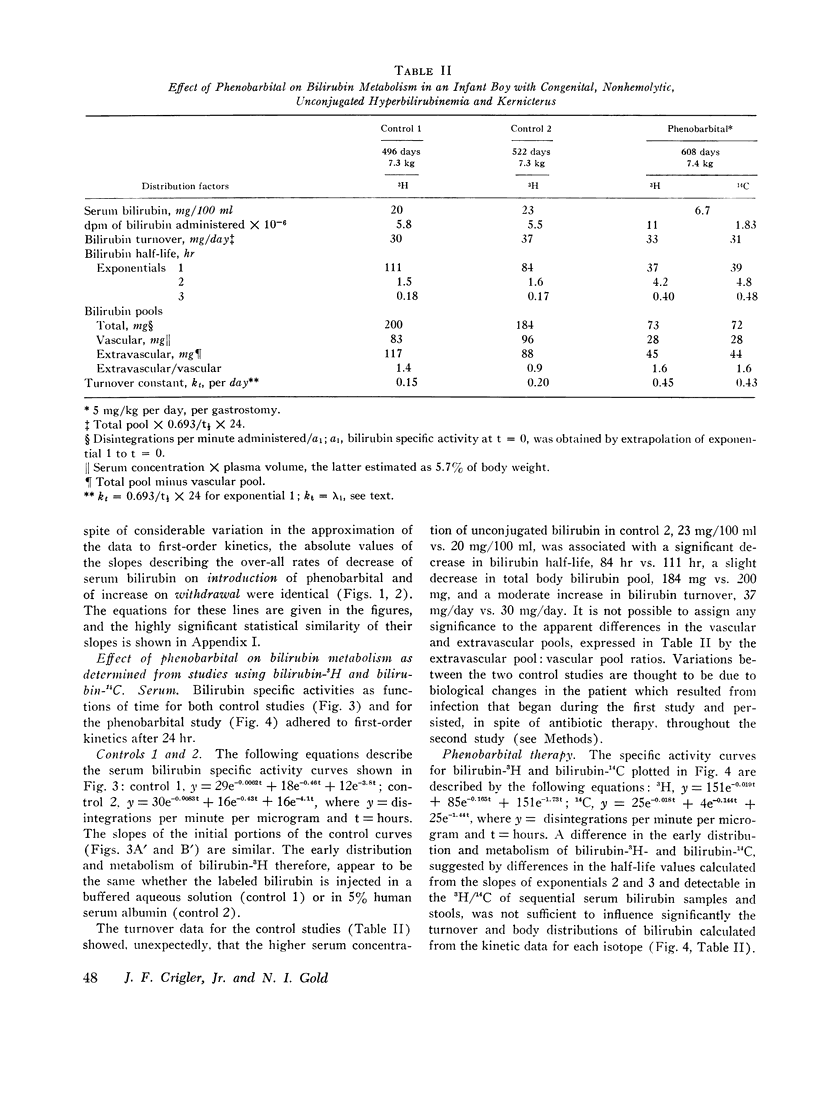
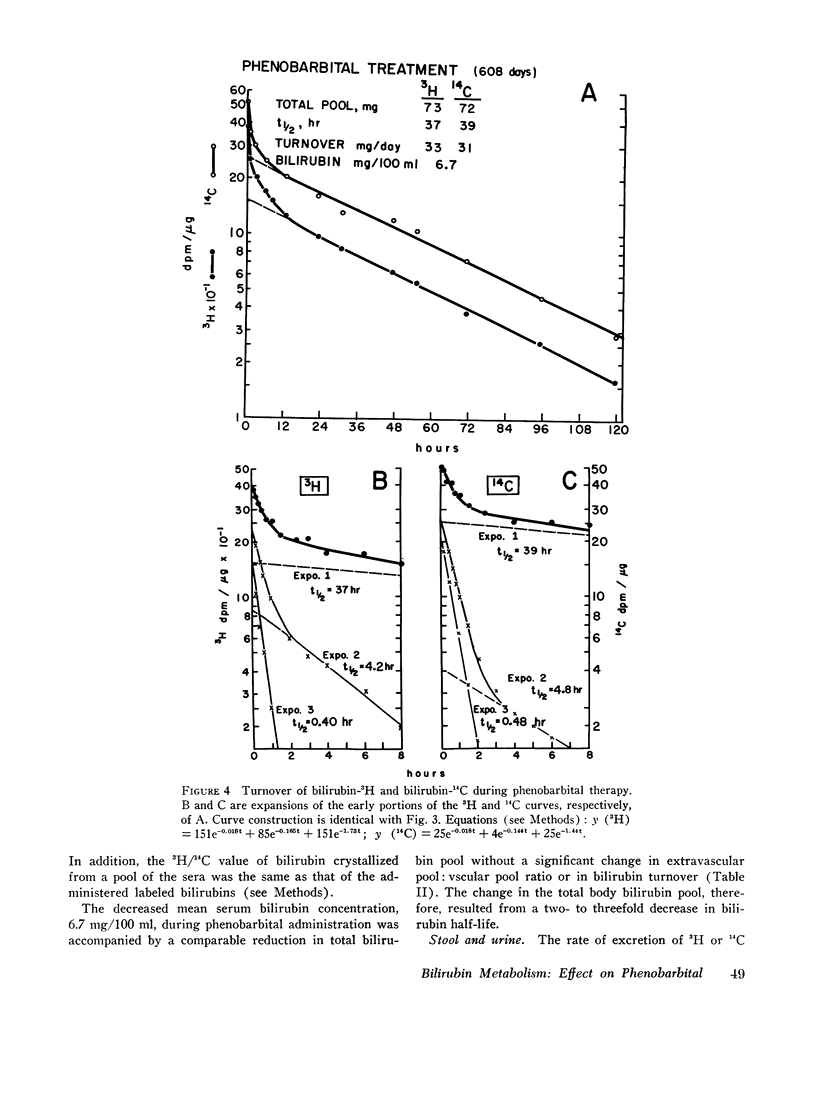
Bilirubin-3H was administered twice before and once with bilirubin-14C during phenobarbital therapy to study the kinetics of bilirubin metabolism. Results of the first and second control studies and of the bilirubin-3H and bilirubin-14C phenobarbital studies, respectively, were as follows: total body bilirubin pools, 200, 184, 73, and 72 mg; half-lives, 111, 84, 37, and 39 hr; and turnover, 30, 37, 33, and 31 mg/day. The data show that the approximate threefold decrease in serum bilirubin concentration and total body pool resulted from a comparable decrease in bilirubin half-life without a significant change in turnover.
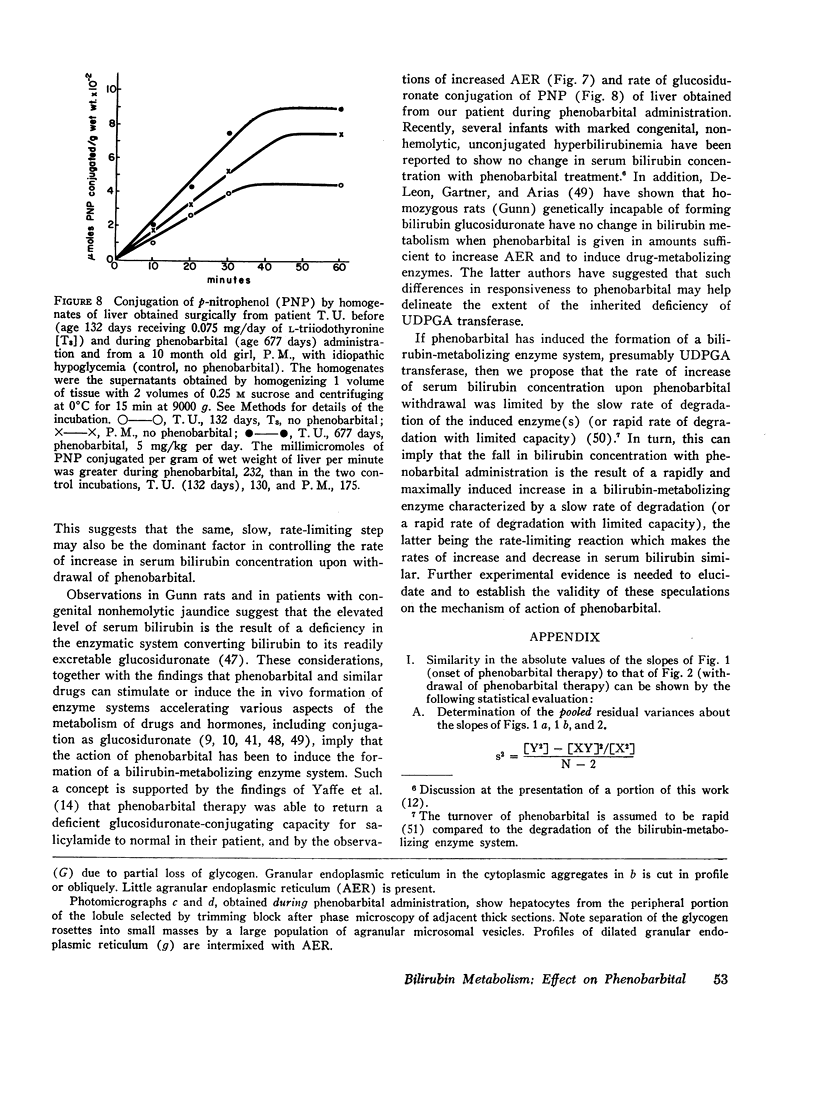
In vitro histological (electron microscopy) and enzymological studies of liver obtained by surgical biopsies before and during phenobaribtal administration showed that both the hepatocyte content of agranular endoplasmic reticulum (AER) and the ability of liver homogenate to conjugate p-nitrophenol were significantly increased during phenobarbital treatment.
The observations suggest that phenobarbital affects bilirubin metabolism by the induction of an enzyme(s) with a slow rate(s) of degradation (or rapid rate of degradation with limited capacity).
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AKERREN Y. Prolonged jaundice in the newborn associated with congenital myxedema; a syndrome of practical importance. Acta Paediatr. 1954 Sep;43(5):411–425. doi: 10.1111/j.1651-2227.1954.tb04049.x. [DOI] [PubMed] [Google Scholar]
- ARIAS I. M., GARTNER L., FURMAN M., WOLFSON S. Effect of several drugs and chemicals on hepatic glucuronide formation in newborn rats. Proc Soc Exp Biol Med. 1963 Apr;112:1037–1040. doi: 10.3181/00379727-112-28246. [DOI] [PubMed] [Google Scholar]
- BOOTH J., GILLETTE J. R. The effect of anabolic steroids on drug metabolism by microsomal enzymes in rat liver. J Pharmacol Exp Ther. 1962 Sep;137:374–379. [PubMed] [Google Scholar]
- BROWN H., ENGLERT E., Jr, WALLACH S. Metabolism of free and conjugated 17-hydroxycorticosteroids in subjects with thyroid disease. J Clin Endocrinol Metab. 1958 Feb;18(2):167–179. doi: 10.1210/jcem-18-2-167. [DOI] [PubMed] [Google Scholar]
- BURNS J. J., CUCINELL S. A., KOSTER R., CONNEY A. H. APPLICATION OF DRUG METABOLISM TO DRUG TOXICITY STUDIES. Ann N Y Acad Sci. 1965 Mar 12;123:273–286. doi: 10.1111/j.1749-6632.1965.tb12268.x. [DOI] [PubMed] [Google Scholar]
- BURSTEIN S., KLAIBER E. L. PHENOBARBITAL-INDUCED INCREASE IN 6-BETA-HYDROXYCORTISOL EXCRETION: CLUE TO ITS SIGNIFICANCE IN HUMAN URINE. J Clin Endocrinol Metab. 1965 Feb;25:293–296. doi: 10.1210/jcem-25-2-293. [DOI] [PubMed] [Google Scholar]
- Becker F. F., Lane B. P. Regeneration of the mammalian liver. VI. Retention of phenobarbital-induced cytoplasmic alterations in dividing hepatocytes. Am J Pathol. 1968 Jan;52(1):211–226. [PMC free article] [PubMed] [Google Scholar]
- Burger P. C., Herdson P. B. Phenobarbital-induced fine structural changes in rat liver. Am J Pathol. 1966 May;48(5):793–809. [PMC free article] [PubMed] [Google Scholar]
- CLARKE J. T. PURIFICATION AND ANALYSIS OF BILIRUBIN. Clin Chem. 1965 Jul;11:681–690. [PubMed] [Google Scholar]
- CONNEY A. H., SCHNEIDMAN K., JACOBSON M., KUNTZMAN R. DRUG-INDUCED CHANGES IN STEROID METABOLISM. Ann N Y Acad Sci. 1965 Mar 12;123:98–109. doi: 10.1111/j.1749-6632.1965.tb12248.x. [DOI] [PubMed] [Google Scholar]
- Conway W. D., Grace A. J., Rogers J. E. Simplification of oxygen-flask combustion procedure for preparation of samples for liquid scintillation counting. Anal Biochem. 1966 Mar;14(3):491–494. doi: 10.1016/0003-2697(66)90293-4. [DOI] [PubMed] [Google Scholar]
- DeLeon A., Gartner L. M., Arias I. M. The effect of phenobarbital on hyperbilirubinemia in glucuronyl transferase deficient rats. J Lab Clin Med. 1967 Aug;70(2):273–278. [PubMed] [Google Scholar]
- FOUTS J. R., HART L. G. HEPATIC DRUG METABOLISM DURING THE PERINATAL PERIOD. Ann N Y Acad Sci. 1965 Mar 12;123:245–251. doi: 10.1111/j.1749-6632.1965.tb12263.x. [DOI] [PubMed] [Google Scholar]
- GOLD N. I., CRIGLER J. F., Jr Influence of L-triodothyronine on steroid hormone metabolism: studies in a patient with adrenal hyperplasia (Cushing's syndrome). J Clin Endocrinol Metab. 1963 Feb;23:156–166. doi: 10.1210/jcem-23-2-156. [DOI] [PubMed] [Google Scholar]
- Gillette J. R. Biochemistry of drug oxidation and reduction by enzymes in hepatic endoplasmic reticulum. Adv Pharmacol. 1966;4:219–261. doi: 10.1016/s1054-3589(08)60100-3. [DOI] [PubMed] [Google Scholar]
- INSCOE J. K., AXELROD J. Some factors affecting glucuronide formation in vitro. J Pharmacol Exp Ther. 1960 Jun;129:128–131. [PubMed] [Google Scholar]
- ISSELBACHER K. J., CHRABAS M. F., QUINN R. C. The solubilization and partial purification of a glucuronyl transferase from rabbit liver microsomes. J Biol Chem. 1962 Oct;237:3033–3036. [PubMed] [Google Scholar]
- Jones A. L., Fawcett D. W. Hypertrophy of the agranular endoplasmic reticulum in hamster liver induced by phenobarbital (with a review on the functions of this organelle in liver). J Histochem Cytochem. 1966 Mar;14(3):215–232. doi: 10.1177/14.3.215. [DOI] [PubMed] [Google Scholar]
- LOUS P. Plasma levels and urinary excretion of three barbituric acids after oral administration to man. Acta Pharmacol Toxicol (Copenh) 1954;10(2):147–165. doi: 10.1111/j.1600-0773.1954.tb01332.x. [DOI] [PubMed] [Google Scholar]
- MacGillivray M. H., Crawford J. D., Robey J. S. Congenital hypothyroidism and prolonged neonatal hyperbilirubinemia. Pediatrics. 1967 Aug;40(2):283–286. [PubMed] [Google Scholar]
- ODELL G. B. The dissociation of bilirubin from albumin and its clinical implications. J Pediatr. 1959 Sep;55:268–279. doi: 10.1016/s0022-3476(59)80223-7. [DOI] [PubMed] [Google Scholar]
- OSTROW J. D., HAMMAKER L., SCHMID R. The preparation of crystalline bilirubin-C14. J Clin Invest. 1961 Aug;40:1442–1452. doi: 10.1172/JCI104375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSTROW J. D., SCHMID R. THE PROTEIN-BINDING OF C14-BILIRUBIN IN HUMAN AND MURINE SERUM. J Clin Invest. 1963 Aug;42:1286–1299. doi: 10.1172/JCI104813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrenius S., Ericsson J. L., Ernster L. Phenobarbital-induced synthesis of the microsomal drug-metabolizing enzyme system and its relationship to the proliferation of endoplasmic membranes. A morphological and biochemical study. J Cell Biol. 1965 Jun;25(3):627–639. doi: 10.1083/jcb.25.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POGELL B. M., LELOIR L. F. Nucleotide activation of liver microsomal glucuronidation. J Biol Chem. 1961 Feb;236:293–298. [PubMed] [Google Scholar]
- Robinson S. H., Lester R., Crigler J. F., Jr, Tsong M. Early-labeled peak of bile pigment in man. Studies with glycine-14C and delta-aminolevulinic acid-3H. N Engl J Med. 1967 Dec 21;277(25):1323–1329. doi: 10.1056/NEJM196712212772501. [DOI] [PubMed] [Google Scholar]
- SCHMID R., HAMMAKER L. METABOLISM AND DISPOSITION OF C14-BILIRUBIN IN CONGENITAL NONHEMOLYTIC JAUNDICE. J Clin Invest. 1963 Nov;42:1720–1734. doi: 10.1172/JCI104858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke R. T. Studies on the roles of synthesis and degradation in the control of enzyme levels in animal tissues. Bull Soc Chim Biol (Paris) 1966;48(10):1009–1030. [PubMed] [Google Scholar]
- Uzman B. G., Foley G. E., Farber S., Lazarus H. Morphologic variations in human leukemic lymphoblasts (CCRF-CEM cells) after long-term culture and exposure to chemotherapeutic agents. A study with the electron microscope. Cancer. 1966 Nov;19(11):1725–1742. doi: 10.1002/1097-0142(196611)19:11<1725::aid-cncr2820191142>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- WEBER A. P., SCHALM L. Quantitative separation and determination of bilirubin and conjugated bilirubin in human serum. Clin Chim Acta. 1962 Nov;7:805–810. doi: 10.1016/0009-8981(62)90063-3. [DOI] [PubMed] [Google Scholar]
- Yaffe S. J., Levy G., Matsuzawa T., Baliah T. Enhancement of glucuronide-conjugating capacity in a hyperbilirubinemic infant due to apparent enzyme induction by phenobarbital. N Engl J Med. 1966 Dec 29;275(26):1461–1466. doi: 10.1056/NEJM196612292752602. [DOI] [PubMed] [Google Scholar]
- Zeidenberg P., Orrenius S., Ernster L. Increase in levels of glucuronylating enzymes and associated rise in activities of mitochondrial oxidative enzymes upon phenobarbital administration in the rat. J Cell Biol. 1967 Feb;32(2):528–531. doi: 10.1083/jcb.32.2.528. [DOI] [PMC free article] [PubMed] [Google Scholar]




