Abstract
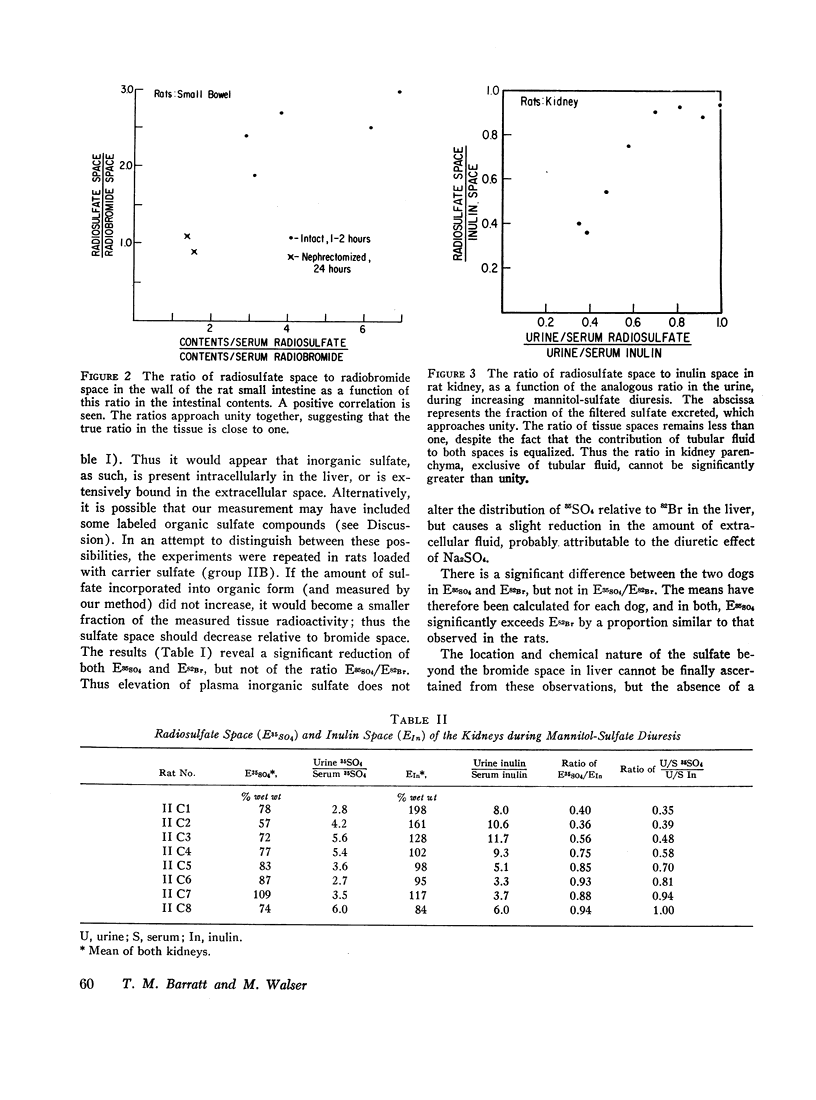
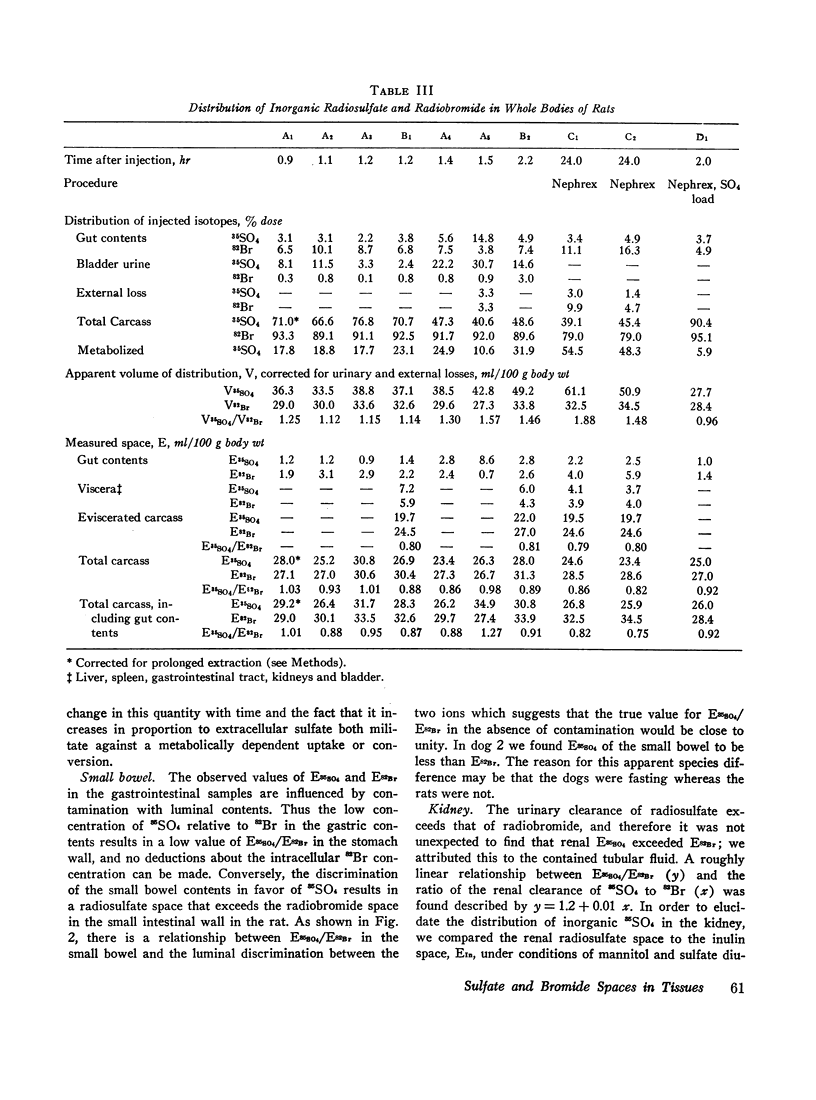
Radiosulfate, 35SO4, and radiobromide, 82Br, were administered simultaneously to rats and dogs. In rats, the apparent volume of distribution of 82Br averaged 30% of body weight and was constant between 0.5 and 35 hr after injection. The apparent volume of distribution of 35SO4, corrected for urinary loss, increased by 6% body weight/hr: the extrapolated volume at zero time was 88% of bromide space. Analysis of individual tissues and carcasses for 82Br and inorganic 35SO4 showed that equilibration of both isotopes in several organs and in the whole carcass was rapidly achieved within 1 to 2 hr: no further increase in measured spaces occurred in 24 hr. The carcass inorganic sulfate space was 92%±2% of the bromide space in intact rats, and showed no increase with time. However, a progressively greater fraction of the injected 35SO4 was not recovered, owing to metabolic alteration. In eviscerated rats, the inorganic sulfate space was a smaller and much more constant fraction (79.8% ±0.4%) of the bromide space, showing that at least 20% of body bromide (and hence chloride) is nonextracellular. The viscera chiefly responsible for the higher ratio of spaces in the intact animal were the liver, small bowel, and kidney. In the last two organs, excess inorganic 35SO4 (beyond the bromide space) was attributable to trapped transcellular fluid in which sulfate had been concentrated more than chloride (or bromide). Excess sulfate in liver and cartilage could not be explained in this manner: the results suggest passive binding of sulfate, but could reflect active cell uptake in these tissues. No excess sulfate was found in skin or tail. The implications of these observations with respect to the distribution of body chloride and the measurement of extracellular space are discussed. The extracellular volume of the rat is estimated to be 24% of body weight.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGLUND F., DEYRUP I. Sulfate content of rat kidney cortex in vitro. Am J Physiol. 1956 Nov;187(2):315–317. doi: 10.1152/ajplegacy.1956.187.2.315. [DOI] [PubMed] [Google Scholar]
- Barratt T. M., Walser M. Extracellular volume in skeletal muscle of the rat and dog: a comparison of radiosulphate and radiobromide spaces. Clin Sci. 1968 Dec;35(3):525–536. [PubMed] [Google Scholar]
- CHEEK D. B., WEST C. D., GOLDEN C. C. The distribution of sodium and chloride and the extracellular fluid volume in the rat. J Clin Invest. 1957 Feb;36(2):340–351. doi: 10.1172/JCI103430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COTLOVE E. Mechanism and extent of distribution of inulin and sucrose in chloride space of tissues. Am J Physiol. 1954 Mar;176(3):396–410. doi: 10.1152/ajplegacy.1954.176.3.396. [DOI] [PubMed] [Google Scholar]
- DEYRUP I. J., USSING H. H. Accumulation of sulfate labelled with S35 by rat tissue in vitro. J Gen Physiol. 1955 May 20;38(5):599–612. doi: 10.1085/jgp.38.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNNING M. F., STEELE J. M., BERGER E. Y. Measurement of total body chloride. Proc Soc Exp Biol Med. 1951 Aug;77(4):854–858. doi: 10.3181/00379727-77-18947. [DOI] [PubMed] [Google Scholar]
- DZIEWIATKOWSKI D. D. Effect of age on some aspects of sulfate metabolism in the rat. J Exp Med. 1954 Mar;99(3):283–298. doi: 10.1084/jem.99.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DZIEWIATKOWSKI D. D. Some aspects of the metabolism of chondroitin sulfate-S35 in the rat. J Biol Chem. 1956 Nov;223(1):239–249. [PubMed] [Google Scholar]
- FORBES G. B., REID A., BONDURANT J., ETHERIDGE J. Estimation of total body chloride in young infants by radiobromide dilution. Proc Soc Exp Biol Med. 1953 Aug-Sep;83(4):871–872. doi: 10.3181/00379727-83-20518. [DOI] [PubMed] [Google Scholar]
- GALAMBOS J. T., CORNELL R. G. Mathematical models for the study of the metabolic pattern of sulfate. J Lab Clin Med. 1962 Jul;60:53–63. [PubMed] [Google Scholar]
- GAMBLE J. L., Jr, ROBERTSON J. S., HANNIGAN C. A., FOSTER C. G., FARR L. E. Chloride, bromide, sodium, and sucrose spaces in man. J Clin Invest. 1953 Jun;32(6):483–489. doi: 10.1172/JCI102764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAMBLE J. L., ROBERTSON J. S. Volume of distribution of radioactive chloride in dogs; comparisons with sodium, bromide and inulin spaces. Am J Physiol. 1952 Dec;171(3):659–667. doi: 10.1152/ajplegacy.1952.171.3.659. [DOI] [PubMed] [Google Scholar]
- GOLDBERG M. A., BARLOW C. F., ROTH L. J. ABNORMAL BRAIN PERMEABILITY IN CO2 NARCOSIS. Arch Neurol. 1963 Nov;9:498–507. doi: 10.1001/archneur.1963.00460110066007. [DOI] [PubMed] [Google Scholar]
- HELLERSTEIN S., KAISER C., DARROW D. D., DARROW D. C. The distribution of bromide and chloride in the body. J Clin Invest. 1960 Feb;39:282–287. doi: 10.1172/JCI104038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsura N., Davidson E. A. Metabolism of connective tissue polysaccharides in vivo. IV. The sulphate group. Biochim Biophys Acta. 1966 May 26;121(1):135–142. doi: 10.1016/0304-4165(66)90356-4. [DOI] [PubMed] [Google Scholar]
- Lacroix M., Busset R., Mach R. S. Utilisation comparative du soufre-35 et du brome-82 pour la détermination du volume de l'eau extracellulaire. Helv Med Acta. 1965 Jun;32(2):87–134. [PubMed] [Google Scholar]
- MILLER E., HLAD C. J., Jr, LEVINE S., HOLMES J. H., ELRICK H. The use of radioisotopes to measure body fluid constituents. I. Plasma sulfate. J Lab Clin Med. 1961 Oct;58:656–661. [PubMed] [Google Scholar]
- Mellick R., Cavanagh J. B. Extracellular space of normal peripheral nerve and normal skin as measured by radioactive sulfate in the chicken. Exp Neurol. 1967 Jun;18(2):224–227. doi: 10.1016/0014-4886(67)90043-x. [DOI] [PubMed] [Google Scholar]
- NICHOLS G., Jr, NICHOLS N., WEIL W. B., WALLACE W. M. The direct measurement of the extracellular phase of tissues. J Clin Invest. 1953 Dec;32(12):1299–1308. doi: 10.1172/JCI102858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAHILL W. J., WALSER M. A SIMPLE METHOD FOR SOFT TISSUE MAGNESIUM AND CALCIUM. Anal Biochem. 1964 Sep;9:119–122. doi: 10.1016/0003-2697(64)90091-0. [DOI] [PubMed] [Google Scholar]
- ROBBINS P. W., LIPMANN F. Isolation and identification of active sulfate. J Biol Chem. 1957 Dec;229(2):837–851. [PubMed] [Google Scholar]
- SWEET N. J., NADELL J., EDELMAN I. S. Gastrointestinal water and electroyltes. III. The equilibration of radiobromide in gastrointestinal contents and the proportion of exchangeable chloride (Cle) in the gastrointestinal tract. J Clin Invest. 1957 Feb;36(2):279–288. doi: 10.1172/JCI103422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALSER M., BODENLOS L. J. Composition of skin as compared with muscle. Am J Physiol. 1954 Jul;178(1):91–96. doi: 10.1152/ajplegacy.1954.178.1.91. [DOI] [PubMed] [Google Scholar]
- WALSER M., DAVIDSON D. G., ORLOFF J. The renal clearance of alkali-stable inulin. J Clin Invest. 1955 Oct;34(10):1520–1523. doi: 10.1172/JCI103204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALSER M., SELDIN D. W., BURNETT C. H. Blood volume and extracellular fluid volume during administration of ACTH and cortisone. Am J Med. 1955 Mar;18(3):454–461. doi: 10.1016/0002-9343(55)90226-6. [DOI] [PubMed] [Google Scholar]
- WALSER M., SELDIN D. W., GROLLMAN A. An evaluation of radiosulfate for the determination of the volume of extracellular fluid in man and dogs. J Clin Invest. 1953 Apr;32(4):299–311. doi: 10.1172/JCI102739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALSER M. Volume of distribution of radiosulfate as a measure of the extracellular fluid. Proc Soc Exp Biol Med. 1952 Mar;79(3):372–375. doi: 10.3181/00379727-79-19382. [DOI] [PubMed] [Google Scholar]



