Abstract
Background and Aims
Wnt/β-catenin signaling is important in liver physiology. β-Catenin is also pivotal in adherens junctions (AJ). Here, we investigate hepatocyte-specific β-catenin-conditional null mice (KO), for any alterations in AJ and related tight junctions (TJ).
Materials and Methods
Using genearray, PCR, western blot, immunohistochemistry, immunofluorescence and coprecipitation studies, we compare and contrast the composition of AJ and TJ in KO and littermate wild-type (WT) control livers.
Results
We demonstrate E-cadherin-β-catenin association in epithelial cells in WT livers, which is lost in the KOs. While total levels of α-catenin, E-cadherin and F-actin were modestly decreased, KO livers show increased γ-catenin/plakoglobin. By co-precipitation, E-cadherin-β-catenin-F-actin association was observed in WT livers, but E-cadherin-γ-catenin-F-actin association was evident in KO livers. γ-Catenin was localized at the hepatocyte membrane at baseline in the KO liver. While γ-catenin gene expression remained unaltered, an increase in serine- and threonine-phosphorylated, but not tyrosine-phosphorylated γ-catenin was observed in KO livers by immunoprecipitation. A continued presence of γ-catenin at hepatocyte membrane without any nuclear localization was observed in liver regeneration after partial-hepatectomy at 40 and 72 hours in both KO and WT by immunofluorescence. Analysis of TJ revealed lack of claudin-2 and increased levels of JAM-A and claudin-1 in KO livers.
Conclusions
γ-Catenin adequately maintains AJ in the absence of β-catenin in hepatocytes, however it lacks nuclear localization. Also, β-catenin-claudin-2 may be an important mechanism of crosstalk between the AJ and TJ.
INTRODUCTION
Wnt/β-catenin signaling in liver pathobiology plays roles in regulating zonation, metabolism, development and regeneration [22]. Its aberrant activation due to mutations in β-catenin gene, or components of its degradation complex such as axin, results in stabilization and nuclear translocation of β-catenin that induces target genes critical to tumor growth and survival. β-Catenin is also important in cell-cell adhesion [5, 26], and is a constituent of Adherens Junctions (AJ) where it bridges E-cadherin to actin cytoskeleton [1]. Loss of E-cadherin-β-catenin association due to tyrosine-phoshorylation of E-cadherin and β-catenin by receptor-tyrosine kinases such as epidermal growth factor receptor, hepatocyte growth factor receptor, and others, results diminished intercellular adhesion, commonly seen in tumors [13, 14, 23, 35]. Other than AJ, tight junctions (TJ) between the basolateral and apical domains of hepatocytes, contribute to paracellular adhesion, cell polarity and serve as occluding barriers around the biliary canaliculi [21, 24]. TJ are multiprotein complexes composed chiefly of claudins, junctional adhesion molecules (JAM) and occludin [32]. Anomalies in TJ have been associated with an imperfect blood-bile barrier and implicated in various hepatic pathologies [11, 29]. A cross-talk between AJ and TJ, and among other plasma domains is reported [10].
The major goal of the current study was to understand the junctional organization in recently reported β-catenin-conditional knockout mice (KO) generated by interbreeding transgenic mice homozygous for floxed-β-catenin gene (Ex1-6) and transgenic mice expressing Cre-recombinase under the Albumin promoter [37]. The KO mice exhibit lower liver weight to body weight ratio, a modest intrahepatic cholestasis and anomalous biliary canalicular architecture [38]. No gross defects in cell polarity or adhesion are evident in KO livers. We wanted to explore the KO hepatic phenotype or lack thereof, through identification of compensatory mechanisms in AJ and TJ, which enable the maintenance of a near normal liver histology in the absence of β-catenin. Here we identify an important compensatory role of γ-catenin (plakoglobin) at AJ along with altered organization of TJ in the KO livers.
MATERIAL AND METHODS
Animals and Surgery
All animal studies were approved by University of Pittsburgh IACUC office. Homozygous floxed β-catenin mice (C57BL/6 strain), and albumin-cre transgenic mice were bred as described previously [37]. Mice with genotype Ctnnblox/lox;Alb-Cre+/− are referred to as knockouts (KO). Littermates with floxed β-catenin genotypes were used as wild-type controls (WT). Livers from age- and sex-matched 90–120 day-old KO and WT (n≥3) were paraffin embedded, cryo-fixed and frozen at −80°C until use. Partial-hepatectomy on KO and WT is described elsewhere [37].
Protein Extraction
Whole-cell lysates (WCL), were obtained from the WT and KO livers using radioimmunoprecipitation assay (RIPA) buffer (Santa Cruz Biotechnology) containing fresh protease and phosphatase inhibitor cocktails (Sigma) as described previously [37]. Cytoskeleton-associated proteins were also extracted as described by Hugh et al [16] to obtain Triton-insoluble or cytoskeletal-associated lysates (CAL). Protein assays were performed using BCA (Pierce).
Western Blot Analysis
At least 3–4 independent livers from KO or WT group were used to do this analysis and representative western blots (WBs) are shown. 18–50μg of the proteins were resolved on 5%, 7.5% or 12% Tris-HCl precast gels (Bio-Rad Laboratories) using SDS-PAGE. The resolved proteins were transferred to polyvinylidene difluoride (PVDF) membranes and signal detected by Super-Signal West Pico or Femto Chemiluminescent Substrate (Pierce). Primary antibodies used were against β-catenin (BD Transduction Laboratories); γ-catenin, E-cadherin (Cell Signaling); α-catenin (Abcam); E-cadherin (BD Transduction Laboratories); claudin-1, claudin-2 (Invitrogen); γ-catenin, JAM-A, occludin, GAPDH, p-Tyr, p-Ser, p-Thr (Santa Cruz); and actin (Chemicon). Horseradish-peroxidase–conjugated secondary antibodies were purchased from Chemicon. Densitometry was performed on scanned autoradiographs using Image J software (NIH). For statistical analysis of differences in protein expression between KO and WT livers, signal from each lane for a protein was normalized to GAPDH expression in that lane. The mean (+/− standard deviation or SD) normalized expression values of any protein in WT and KO livers, was compared for statistical significance by the two-tailed student t test and p<0.05 was considered significant.
Immunoprecipitation (IP)
IP studies were performed with 500μg of WCL or CAL as described elsewhere [23]. Antibodies used for IP were rabbit anti-γ-catenin (Cell Signaling), mouse anti-E-cadherin (BD Transduction Labs) or goat β-catenin-conjugated A/G agarose beads (Santa Cruz).
Immunohistochemistry (IHC) and Immunofluorescence (IF)
IHC was performed for β-catenin (Santa Cruz) or Ki-67 (Dako) as detailed elsewhere [37]. For IF, staining was performed using rabbit (Cell Signaling) or goat (Santa Cruz) anti-γ-catenin or E-cadherin (mouse monoclonal, BD Biosciences) and Alexa 488-conjugated donkey anti-rabbit (Molecular probes) and Cy3-conjugated donkey anti-mouse (Jackson Immunoresearch) secondary antibodies, as described elsewhere [23]. Sections were counterstained with DAPI and imaged with Olympus FV1000 Confocal Microscope with Fluoview 1.7A software.
Microarray Analysis
Livers from three KO and WT were used for Affymetrix genearray analysis as previously published [37]. The signals from KO and WT livers were compared and presented as fold-change.
Real-Time PCR
RNA was extracted from KO and WT mice using Trizol (Invitrogen) subjected to real-time PCR analysis as described elsewhere [2]. Comparative ΔΔCT was used for analysis of the data, and calculations were made without the StepOne software. The real-time PCR primer sequences are available in Table 1.
Table 1.
Primer Sequences.
| Primer Name | Sequence |
|---|---|
| JUP Fwd | 5′-ACG CCA TTG ATG CGG AGG GC -3′ |
| JUP Rev | 5′-CCC AGG CAG CTG GGT CAT GC -3′ |
| GAPDH Fwd | 5′-ACC CAG AAG ACT GTG GAT GG -3′ |
| GAPDH Rev | 5′-CAC ATT GGG GGT AGG AAC AC -3′ |
| PBGN Fwd | 5′-ATG TCC GGT AAC GGC GGC -3′ |
| PBGN Rev | 5′-CAA GGC TTT CAG CAT CGC CAC CA -3′ |
| Cyclophilin-A Fwd | 5′-CCC CAC CGT GTT CTT CGA CA -3′ |
| Cyclophilin-A Rev | TCC AGT GCT CAG AGC TCG AAA -3′ |
Real-time PCR was also performed with another JUP primer (SA Biosciences); two liver-specific reference genes (porphobilinogen synthase or PBGN and cyclophilin-A), and two reference genes-glyceraldehyde 3 phosphate dehydrogenase (GAPDH); and phosphoglycerate kinase 1 (PGK) from realtimeprimers.com.
Statistics
All statistical analysis was performed using the Microsoft Office Excel 2007 software.
RESULTS
β-Catenin is conditionally deleted from the parenchymal cells of the KO mice livers
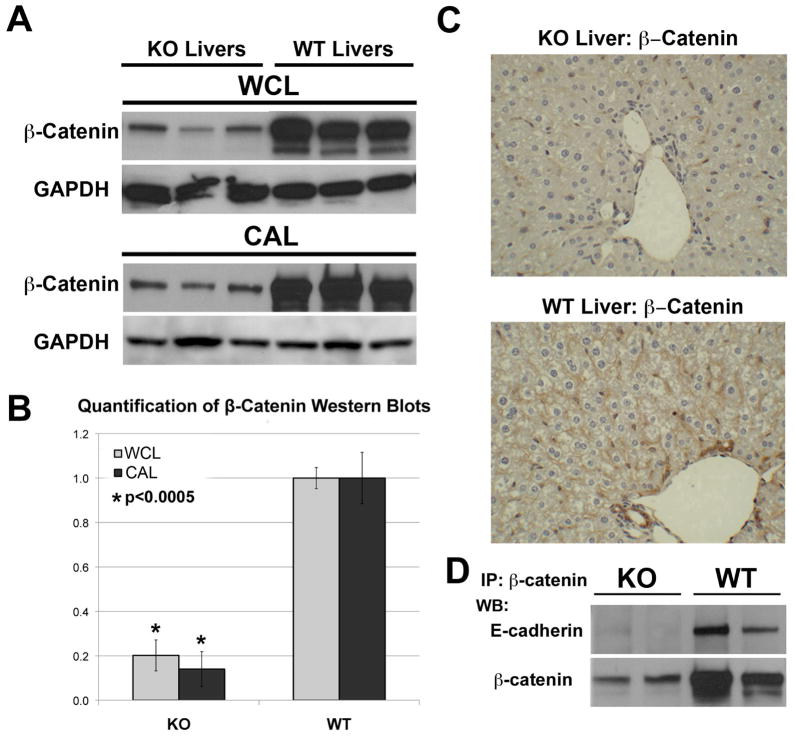
To confirm loss in KO livers, β-catenin levels were assessed. β-Catenin protein was significantly decreased in the WCL and CAL in KO livers as compared to WT (p<0.0005) (Fig. 1A–B). Some remnant expression evident in KO livers was due to existence of β-catenin in non-parenchymal cells unaffected by albumin-cre expression and due to a very small number of hepatocytes that spontaneously escape cre-mediated deletion [6 & unpublished observations]. Indeed IHC in KO shows loss of membranous β-catenin in hepatocytes and cytoplasmic β-catenin in cholangiocytes, whereas endothelial cells remained β-catenin-positive (Fig. 1C). IP studies for β-catenin were performed next. When blotted for E-cadherin, the association between β-catenin and E-cadherin was detected only in WT livers (Fig. 1D). In the KO livers, E-cadherin was not pulled down by β-catenin, confirming loss of β-catenin in epithelial compartment.
Figure 1. Assessment of β-catenin in KO and WT livers.
A. β-Catenin (97kDa) and GAPDH (as loading control) protein levels in whole-cell lysates (WCL) and cytoskeleton-associated lysates (CAL) of three representative KO and age-and sex-matched WT livers by western blots (WB).
B. Normalized (to GAPDH) densitometric analysis reveals significantly lower (p <0.05) average (+/−SD) β-catenin protein expression in KO than WT livers (n=3).
C. Liver sections from KO and WT were analyzed for β-catenin expression by immunohistochemistry.
D. WCLs were immunoprecipitated with anti-β-catenin antibody and probed for E-cadherin (120kDa), by WB.
Assessment of AJ components in β-catenin KO livers
To investigate changes in gene expression in the components of AJ, we analyzed microarray data from KO and WT livers [37]. KO livers showed altered expression of α-catenin (+2.4), E-cadherin (+2.6) and γ-Catenin (+1.5) relative to WT (Table 2).
TABLE 2.
Gene expression changes in junctional protein components in KO livers.
| Gene/Protein | Cell-Cell Junction | GENE ID | Change in KO vs. WT (fold-change) |
|---|---|---|---|
| α-Catenin | AJ | Ctnna1 | +2.4 † |
| E-Cadherin | AJ | Cdh1 | +2.6 † |
| N-Cadherin | AJ | Cdh2 | NC * |
| Plakoglobin/γ-Catenin | Desmosome/AJ | JUP | +1.5 †‡ |
| Claudin-1 | TJ | Cldn1 | −2.3 † |
| Claudin-2 | TJ | Cldn2 | −8.0 † |
| Claudin-3 | TJ | Cldn3 | NC |
| JAM-A | TJ | JCAM1 | −2.0† |
| Occludin | TJ | Ocln | −1.3 † |
| ZO-1 | TJ | Tjp1 | NC |
| ZO-2 | TJ | Tjp2 | −1.2 |
NC = No change
Western blot included in this manuscript
Real-time PCR validation in this manuscript
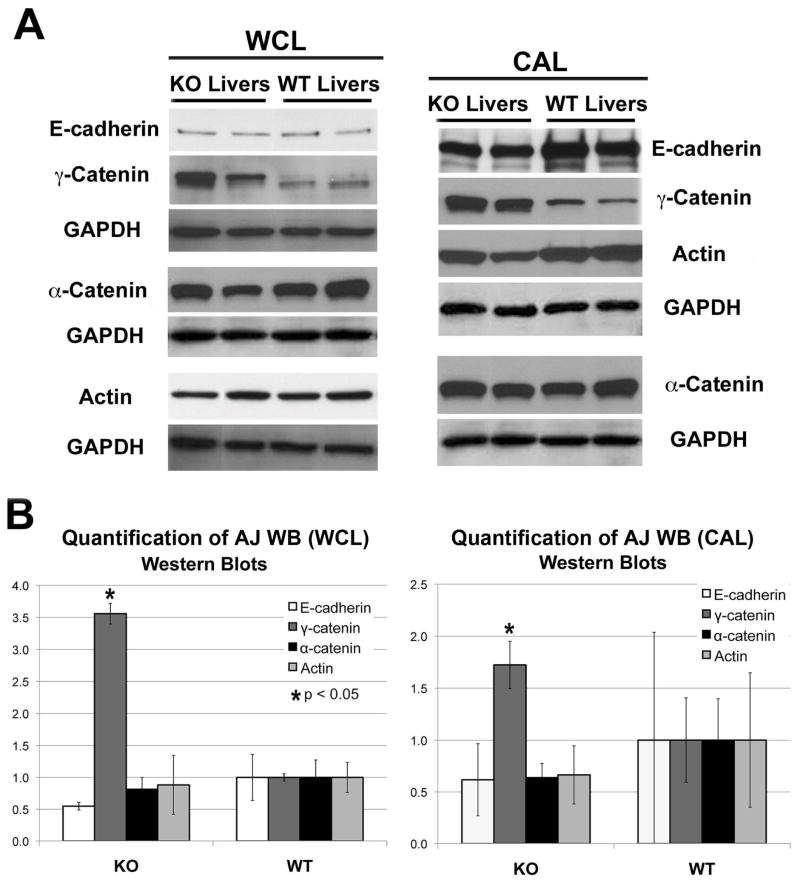
WB was performed to substantiate the array analysis. Insignificant differences in E-cadherin, α-catenin and F-actin protein levels were observed in CAL and WCL from KO and WT livers (Fig. 2A–B). Interestingly, a significant increase in γ-catenin protein expression was observed in KO (Fig. 2A–B).
Figure 2. Quantitative analysis of AJ proteins in KO and WT livers.
A-E-cadherin (120kDa), γ-catenin (83kDa), α-catenin (100kDa) and F-actin (42kDa) protein levels were detected in whole cell lysates (WCL) and cytoskeleton-associated lysates (CAL) from two representative KO and age-matched WT livers by WB. WB for GAPDH verified comparable loading.
B. Normalized (to GAPDH) densitometric analysis reveals significantly higher (P <0.05) average (+/−SD) protein expression of γ-catenin in KO than WT livers (n≥3), while other proteins showed insignificant differences between the two groups.
γ-Catenin associates with E-cadherin and F-actin in lieu of β-catenin in KO livers
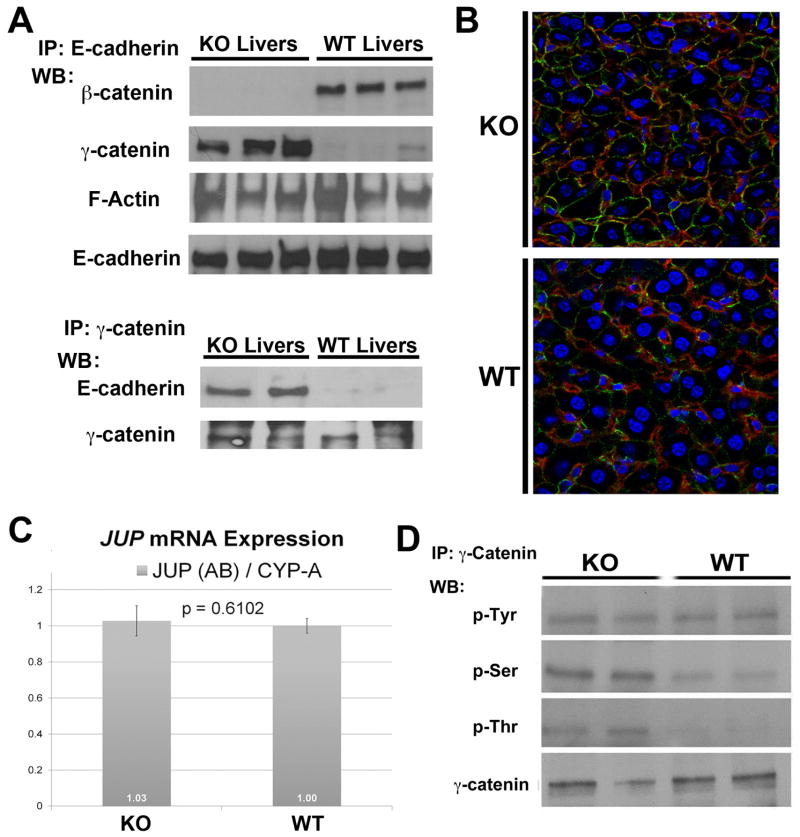
IP studies were performed next to see if β-catenin loss impacts association between AJ proteins. When E-cadherin was immunoprecipitated in WT and KO liver lysates, β-catenin association was detected only in WT (Fig. 3A). We could not detect α-catenin association with E-cadherin in WT or KO livers (data not shown). Interestingly, γ-catenin invariably associated with E-cadherin in KO while it was only minimally evident in complex with E-cadherin in the WT livers (Fig. 3A). When γ-catenin was immunoprecipitated in WT and KO livers, an association with E-cadherin was detected in KO livers only (Fig. 3A). Next, IF studies were utilized to examine co-localization of E-cadherin and γ-catenin that was detected in KO livers at the hepatocyte membrane as compared to the WT (Fig. 3B). We also detected F-actin association with E-cadherin by IP in both WT and KO livers in the same complex. Thus an intact connection of E-cadherin to actin cytoskeleton is maintained in WT and KO livers, albeit through γ-catenin and not β-catenin in the latter (Fig. 3A).
Figure 3. Mechanism of increased γ-catenin and its association with E-cadherin uniquely in KO livers.
A. Whole-cell lysates were immunoprecipitated with anti-E-cadherin or anti-γ-catenin antibodies. WB performed for β-catenin (97kDa), γ-catenin (83kDa) and F-Actin (42kDa) (upper panel) show differential co-precipitation of β-catenin and F-actin with E-cadherin in WT or γ-catenin and F-actin and E-cadherin in WT livers. The conditions were reversed to show that γ-catenin also co-precipitates with E-cadherin (120kDa) in KO (lower panel).
B. Increased membranous γ-catenin (green) associates with E-cadherin (red), prominently in the KO hepatocytes as shown by representative double immunofluorescence utilizing liver sections from KO and WT animals.
C. Real-time PCR shown for γ-catenin gene (JUP) and cyclophilin-A reference gene shows insignificant difference in average mRNA expression (+/−SD) in KO versus WT livers (n=3). Similar results were obtained for all JUP and reference gene primer combinations.
D. Whole-cell lysates immunoprecipitated with anti-γ-catenin antibody and western blotted for anti-phosphotyrosine, anti-phosphoserine, or anti-phosphothreonine show greater serine and threonine phosphorylation of γ-catenin in two representative KO livers as compared to WT, with no change in tyrosine phosphorylation.
Increase in γ-Catenin appears to be regulated by post-translational modifications
To begin to address the mechanism of γ-catenin increase in KO, additional validation of microarray analysis, which revealed an insignificant increase in JUP expression, we performed real-time PCR. 2-ΔΔCT values revealed insignificant differences in JUP expression between KO and WT livers in three independent experiments (Fig. 3C).
We next assessed the phosphorylation status of γ-catenin. IP studies using γ-catenin antibody, identified greater serine and threonine phosphorylation in KO when compared to WT (Fig. 3D). No differences were observed in tyrosine-phosphorylation of γ-catenin. Thus, increase in γ-catenin protein in KO livers coincided greater serine and threonine phosphorylation.
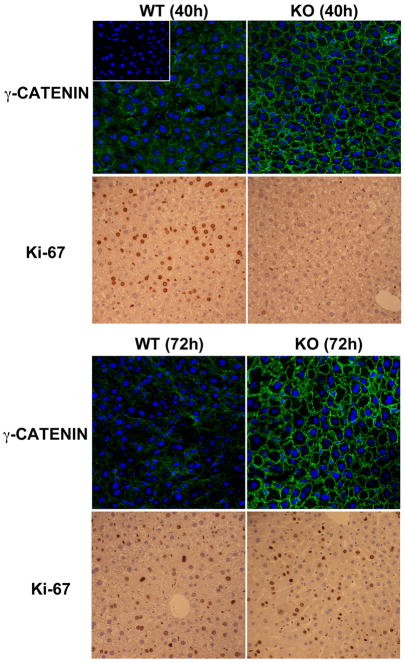
Lack of nuclear γ-catenin during hepatocyte proliferation in absence of β-catenin
Previously, others and we have reported a delay in liver regeneration after partial-hepatectomy in KO such that hepatocyte proliferation was evident at 72 instead of 40 hours, peak proliferation time in controls [33, 37]. We hypothesized that if γ-catenin was compensating for nuclear β-catenin function to induce hepatocyte proliferation, KO livers might show evidence of nuclear γ-catenin at 72 hours after hepatectomy. IF studies detected γ-catenin only at hepatocyte membrane and not nuclei at either 40 or 72 hours after hepatectomy in either group (Fig. 4). As expected, IHC for Ki-67 on corresponding sections reveal hepatocytes in S-phase of cell cycle in WT at 40 and KO at 72 hours (Fig. 4). Thus, γ-catenin is unlikely compensating for nuclear β-catenin function in the liver.
Figure 4.
Sustained membranous localization of γ-catenin in WT and KO livers during liver regeneration. While an increase in membrane signal of γ-catenin (green) is evident in KO as compared to WT, it was localized to hepatocyte membrane only at both 40 and 72 hours after partial hepatectomy, when several Ki-67+ve hepatocytes are observed in WT and KO livers, respectively. No signal is detected in negative control when primary antibody is omitted in the reaction as shown in the inset in upper left panel.
Changes in mRNA and proteins of TJ components in the KO livers
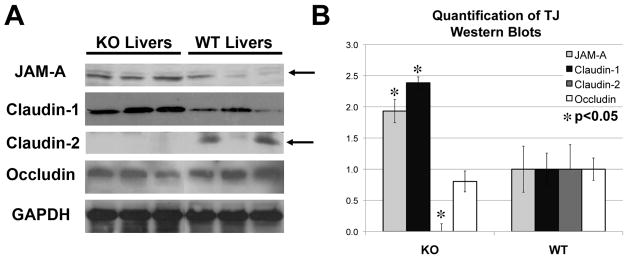
Based on the observed crosstalk between the components of AJ and TJ [17], we analyzed KO and WT livers for any alterations in gene expression of TJ components by microarray. KO livers showed altered expression of claudin-1 (−2.3), claudin-2 (−8.0) and JAM-A (−2.0) but insignificant changes in the expression of claudin-3, occludin, ZO-2 or ZO-1 (Table 2).
By WB, we assessed WCL from WT and KO livers for TJ proteins. Despite decreases in gene expression, JAM-A and claudin-1 protein levels were significantly elevated in KO (Fig. 5A–B). However, concordant with lower gene expression, a significant decrease in claudin-2 protein was observed in KO (Fig. 5A–B). Insignificant differences in occludin levels were evident (Fig. 5A–B).
Figure 5. Changes in TJ protein expression in the KO livers.
A. Examination of TJ protein expression by western blots using whole-cell lysates shows an increase in JAM-A (35kDa) and claudin-1 (22kDa), no change in occludin (60–82kDa) and absence of claudin-2 (22kDa) in KO livers. GAPDH served as the loading control.
B. Normalized (to GAPDH) densitometric analysis reveals significantly (P <0.05) lower average (+/−SD) protein expression of claudin-2 and higher average (+/−SD) expression of JAM-A and claudin-1 in KO than WT livers (n≥3), while occludin remained unchanged.
DISCUSSION
In adult liver, β-catenin is chiefly observed at the hepatocyte membrane regulating cell-cell adhesion and in the most perivenous hepatocytes displaying additional cytoplasmic and nuclear localization to regulate zonation [3]. Yet, conditional β-catenin loss in hepatocytes resulted in a mild phenotype [34, 37]. Here we address how β-catenin loss is compensated especially at AJ and how TJ might be impacted by changes in β-catenin.
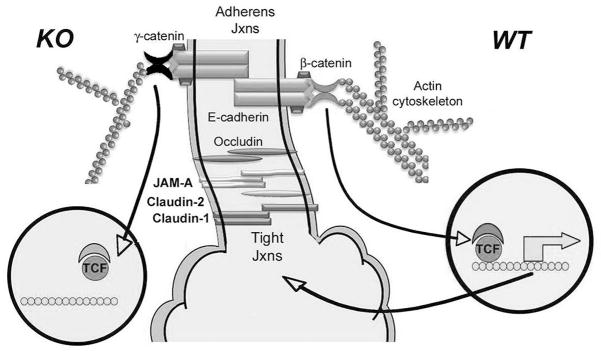
Hepatic architecture was well maintained in KO livers without any histological evidence of adhesion defect despite β-catenin being a critical component of AJ. γ-Catenin was identified to compensate for β-catenin loss to maintain AJ in KO. γ-Catenin is highly homologous to β-catenin with 79% sequence similarity in the armadillo domains, and 45% and 27% N- and C-terminal domain identities, respectively [8]. γ-Catenin is a unique component of desmosomes, [31]. Unlike the above paradigm however, in the γ-catenin KO, β-catenin, despite its presence at desmosomes, was insufficient to functionally compensate for γ-catenin, causing embryonic lethality due to faulty desmosomes [4]. In normal liver, we found a significant association of β-catenin and E-cadherin, but not of γ-catenin and E-cadherin, suggesting the former complex to be a major contributor of AJ. Based on nearly identical interactions with E-cadherin [8], it is conceivable that during steady state, these two catenins may be competing to bind E-cadherin and their relative levels may eventually determine their relative contribution to AJ. Our studies demonstrate that β-catenin is the predominant catenin in hepatocytes and thus the major form associating with E-cadherin. Indeed, increased levels of γ-catenin are known to displace β-catenin from AJ [30]. On the other hand, in the KO, in the absence of β-catenin, γ-catenin is the clear ‘winner’. In fact, an intact E-cadherin-γ-catenin-F-actin ternary complex was evident in KO livers in lieu of E-cadherin-β-catenin-F-actin in the WT (Fig. 6). A similar compensation by γ-catenin for β-catenin loss was reported to maintain normal cardiomyocyte structure and function [39].
Figure 6.
A cartoon representing the configuration of AJ and TJ in β-catenin KO liver (left) and normal liver (right). While β-catenin bridges E-cadherin to F-actin in the WT livers, this function is compensated by γ-catenin in absence of β-catenin. β-Catenin and γ-catenin are both able to translocate to the nucleus, however β-catenin has robust nuclear interactions with TCF/LEF and hence transactivates expression of genes such as claudin-2, which is a means of crosstalk between AJ proteins and TJ components. However in KO, loss of claudin-2 impacts JAM-A and claudin-1 expression, which may affect TJ functions.
γ-Catenin has also been shown to function as a downstream effector of the canonical Wnt pathway, translocating to the nucleus and regulating target gene expression, as reported in malignant mesothelioma (epithelial) cells albeit upon complete β-catenin loss [19]. γ-Catenin contains armadillo repeats, which weakly interact with TCF/LEF, regulating distinct Wnt signaling [36, 40]. At baseline, no nuclear γ-catenin is observed in the KO livers. Lack of nuclear compensation is also supported by absence in the KO of β-catenin transcriptional targets such as glutamine synthetase, CYP2E1 and CYP1A2, leading to defects in zonation, ammonia metabolism and xenobiotic metabolism [3, 7, 34, 37]. Similarly, regenerating livers at 40 and 72 hours after hepatectomy that represent times of peak hepatocyte proliferation in control and KO livers respectively, [33, 37], do not show any nuclear γ-catenin also arguing against nuclear compensation in absence of β-catenin.
Next we begin to address the mechanism of increased γ-catenin in KO. The protein levels of γ-catenin were disproportionately greater than its gene expression suggesting post-translational regulation. We identified increased serine and threonine phosphorylation of γ-catenin in KO livers along with greater γ-catenin-E-cadherin association. Changes in phosphorylation status of γ-catenin leading to its stabilization and altered subcellular localization have been reported previously [25]. Additional characterization is currently underway to determine signaling mechanisms regulating γ-catenin phosphorylation and its impact on association with E-cadherin.
β-Catenin activation due to multiple causes occurs in significant subsets of hepatocellular carcinoma (HCC) [18, 22]. While β-catenin is no doubt a therapeutic target, it remains unclear what impact β-catenin inhibition might have on the integrity of AJ and ultimately on tumor cell invasion and metastasis. From our current study, it appears that while β-catenin nuclear functions, which are more critical for tumor cell survival and proliferation may not be compensated, its functions at the AJ are effectively substituted by γ-catenin. Such pathways that may induce γ-catenin stabilization may need to be spared during anti-β-catenin therapies for cancer treatment to prevent concurrent γ-catenin suppression that may inadvertently increase tumor cell invasion.
The other components of AJ remained only minimally affected. There was an insignificant albeit consistent decrease in E-cadherin and α-catenin proteins in KO. E-cadherin-β-catenin complex from endoplasmic reticulum translocates to the membrane [12]. This complex prevents exposure of PEST domain in E-cadherin for recognition by the proteosomal degradation machinery [15]. It is conceivable, that loss of β-catenin may contribute to mild E-cadherin destabilization. However, as γ-catenin binds to E-cadherin identically [8], it is likely that excess degradation of E-cadherin is prevented. An increase in E-cadherin and α-catenin gene expression might be compensatory due to decreased proteins. We were unable to detect α-catenin in complex with E-cadherin in WT or KO livers. Recent studies have provided evidence that β-catenin and α-catenin interactions with F-actin might be mutually exclusive [27]. In our study we detect ternary complexes composed of E-cadherin-β-catenin-F-actin in the WT or E-cadherin-γ-catenin-F-actin in the KO livers.
We also identify changes in protein expression of various TJ components in the absence of β-catenin. TJ are a set of multi-protein complexes that contribute to cell-cell adhesion, polarity, segregating various domains of the hepatocytes, enabling proper hepatocyte secretory function [10, 21]. Claudin-2 was absent in KO livers. This is not surprising since it is a known Wnt target gene [20] and we also reported its downregulation in KO livers previously [37]. While gene expression of JAM-A and claudin-1 were decreased in the KO livers, their protein levels were increased, suggesting a post-transcriptional regulation. Interestingly, despite these changes, we reported lack of any major functional defect in the integrity of hepatic TJ in KO mice by assaying for biliary excretion of FITC-conjugated dextran after intravenous injection [38]. It is conceivable that loss of claudin-2 may in fact result in tighter TJ, since it is usually contributes to leakiness in epithelia [9, 28]. Changes in JAM-A might also have a compensatory impact on maintaining TJ in absence of claudin-2 and β-catenin. Despite its increased levels, claudin-1 is unlikely to compensate for the absence of claudin-2 since the former is a barrier forming whilst the latter, a pore forming claudin. While the notion of crosstalk between TJ and AJ exists, a recent study for the first time showed an inverse relationship between E-cadherin and JAM-A in hepatocytes [17]. Based on our observations, we suggest that β-catenin/claudin-2 may be another important means of cross-talk between AJ and TJ through which aberrations in one junction may be transmitted to the other (Fig. 6).
Acknowledgments
Funding support: This study was funded by NIH grants 1R01DK62277 and 1R01CA124414 to SPSM and by Rango’s Fund for the Enhancement of Pathology Research.
Abbreviations
- KO
liver-specific-β-catenin conditional knockout
- WT
wild-type or controls
- WB
western blot
- IF
immunofluorescence
- AJ
adherens junctions
- TJ
tight junctions
- JUP
γ-catenin gene
- JAM
junctional adhesion molecules
- WB
western blot
- WCL
whole cell lysates
- CAL
cytoskeleton associated lysates
- RIPA
radioimmunoprecipitation assay
- PVDF
polyvinylidene difluoride
- IHC
immunohistochemistry
- IP
immunoprecipitation
- PCR
polymerase chain reaction
- ZO
zono occludens
Footnotes
Financial Conflict: None for any of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aberle H, Schwartz H, Kemler R. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61(4):514–523. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C514::AID-JCB4%3E3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 2.Behar J, Yeh TH, Krauland L, Otruba W, Cieply B, Hauth B, et al. Liver-specific beta-catenin knockout mice exhibit defective bile acid and cholesterol homeostasis and increased susceptibility to diet-induced steatohepatitis. Am J Pathol. 2010;176(2):744–753. doi: 10.2353/ajpath.2010.090667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benhamouche S, Decaens T, Godard C, Chambrey R, Rickman DS, Moinard C, et al. Apc tumor suppressor gene is the “zonation-keeper” of mouse liver. Dev Cell. 2006;10(6):759–770. doi: 10.1016/j.devcel.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Bierkamp C, Schwarz H, Huber O, Kemler R. Desmosomal localization of beta-catenin in the skin of plakoglobin null-mutant mice. Development. 1999;126(2):371–381. doi: 10.1242/dev.126.2.371. [DOI] [PubMed] [Google Scholar]
- 5.Birchmeier W, Weidner KM, Behrens J. Molecular mechanisms leading to loss of differentiation and gain of invasiveness in epithelial cells. J Cell Sci Suppl. 1993;17:159–164. doi: 10.1242/jcs.1993.supplement_17.23. [DOI] [PubMed] [Google Scholar]
- 6.Braeuning A, Singh Y, Rignall B, Buchmann A, Hammad S, Othman A, et al. Phenotype and growth behavior of residual beta-catenin-positive hepatocytes in livers of beta-catenin-deficient mice. Histochem Cell Biol. 2010 doi: 10.1007/s00418-010-0747-1. [DOI] [PubMed] [Google Scholar]
- 7.Burke ZD, Reed KR, Phesse TJ, Sansom OJ, Clarke AR, Tosh D. Liver zonation occurs through a beta-catenin-dependent, c-Myc-independent mechanism. Gastroenterology. 2009;136(7):2316–2324. e2311–2313. doi: 10.1053/j.gastro.2009.02.063. [DOI] [PubMed] [Google Scholar]
- 8.Choi HJ, Gross JC, Pokutta S, Weis WI. Interactions of plakoglobin and beta-catenin with desmosomal cadherins: basis of selective exclusion of alpha- and beta-catenin from desmosomes. J Biol Chem. 2009;284(46):31776–31788. doi: 10.1074/jbc.M109.047928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enck AH, Berger UV, Yu AS. Claudin-2 is selectively expressed in proximal nephron in mouse kidney. Am J Physiol Renal Physiol. 2001;281(5):F966–974. doi: 10.1152/ajprenal.2001.281.5.F966. [DOI] [PubMed] [Google Scholar]
- 10.Giepmans BN, van ljzendoorn SC. Epithelial cell-cell junctions and plasma membrane domains. Biochim Biophys Acta. 2009;1788(4):820–831. doi: 10.1016/j.bbamem.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Hadj-Rabia S, Baala L, Vabres P, Hamel-Teillac D, Jacquemin E, Fabre M, et al. Claudin-1 gene mutations in neonatal sclerosing cholangitis associated with ichthyosis: a tight junction disease. Gastroenterology. 2004;127(5):1386–1390. doi: 10.1053/j.gastro.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 12.Hinck L, Nathke IS, Papkoff J, Nelson WJ. Dynamics of cadherin/catenin complex formation: novel protein interactions and pathways of complex assembly. J Cell Biol. 1994;125(6):1327–1340. doi: 10.1083/jcb.125.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiscox S, Jiang WG. Association of the HGF/SF receptor, c-met, with the cell-surface adhesion molecule, E-cadherin, and catenins in human tumor cells. Biochem Biophys Res Commun. 1999;261(2):406–411. doi: 10.1006/bbrc.1999.1002. [DOI] [PubMed] [Google Scholar]
- 14.Hoschuetzky H, Aberle H, Kemler R. Beta-catenin mediates the interaction of the cadherin-catenin complex with epidermal growth factor receptor. J Cell Biol. 1994;127(5):1375–1380. doi: 10.1083/jcb.127.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huber AH, Stewart DB, Laurents DV, Nelson WJ, Weis WI. The cadherin cytoplasmic domain is unstructured in the absence of beta-catenin. A possible mechanism for regulating cadherin turnover. J Biol Chem. 2001;276(15):12301–12309. doi: 10.1074/jbc.M010377200. [DOI] [PubMed] [Google Scholar]
- 16.Hugh TJ, Dillon SA, O’Dowd G, Getty B, Pignatelli M, Poston GJ, et al. beta-catenin expression in primary and metastatic colorectal carcinoma. Int J Cancer. 1999;82(4):504–511. doi: 10.1002/(sici)1097-0215(19990812)82:4<504::aid-ijc6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 17.Konopka G, Tekiela J, Iverson M, Wells C, Duncan SA. Junctional adhesion molecule-A is critical for the formation of pseudocanaliculi and modulates E-cadherin expression in hepatic cells. J Biol Chem. 2007;282(38):28137–28148. doi: 10.1074/jbc.M703592200. [DOI] [PubMed] [Google Scholar]
- 18.Kundu JK, Choi KY, Surh YJ. beta-Catenin-mediated signaling: a novel molecular target for chemoprevention with anti-inflammatory substances. Biochim Biophys Acta. 2006;1765(1):14–24. doi: 10.1016/j.bbcan.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Maeda O, Usami N, Kondo M, Takahashi M, Goto H, Shimokata K, et al. Plakoglobin (gamma-catenin) has TCF/LEF family-dependent transcriptional activity in beta-catenin-deficient cell line. Oncogene. 2004;23(4):964–972. doi: 10.1038/sj.onc.1207254. [DOI] [PubMed] [Google Scholar]
- 20.Mankertz J, Hillenbrand B, Tavalali S, Huber O, Fromm M, Schulzke JD. Functional crosstalk between Wnt signaling and Cdx-related transcriptional activation in the regulation of the claudin-2 promoter activity. Biochem Biophys Res Commun. 2004;314(4):1001–1007. doi: 10.1016/j.bbrc.2003.12.185. [DOI] [PubMed] [Google Scholar]
- 21.Mitic LL, Van Itallie CM, Anderson JM. Molecular physiology and pathophysiology of tight junctions I. Tight junction structure and function: lessons from mutant animals and proteins. Am J Physiol Gastrointest Liver Physiol. 2000;279(2):G250–254. doi: 10.1152/ajpgi.2000.279.2.G250. [DOI] [PubMed] [Google Scholar]
- 22.Monga SP. Role of Wnt/beta-catenin signaling in liver metabolism and cancer. Int J Biochem Cell Biol. 2009 doi: 10.1016/j.biocel.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monga SP, Mars WM, Pediaditakis P, Bell A, Mule K, Bowen WC, et al. Hepatocyte growth factor induces Wnt-independent nuclear translocation of beta-catenin after Met-beta-catenin dissociation in hepatocytes. Cancer Res. 2002;62(7):2064–2071. [PubMed] [Google Scholar]
- 24.Nusrat A, Giry M, Turner JR, Colgan SP, Parkos CA, Carnes D, et al. Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc Natl Acad Sci USA. 1995;92(23):10629–10633. doi: 10.1073/pnas.92.23.10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasdar M, Li Z, Chlumecky V. Plakoglobin: kinetics of synthesis, phosphorylation, stability, and interactions with desmoglein and E-cadherin. Cell Motil Cytoskeleton. 1995;32(4):258–272. doi: 10.1002/cm.970320403. [DOI] [PubMed] [Google Scholar]
- 26.Peifer M. The product of the Drosophila segment polarity gene armadillo is part of a multi-protein complex resembling the vertebrate adherens junction. J Cell Sci. 1993;105 (Pt 4):993–1000. doi: 10.1242/jcs.105.4.993. [DOI] [PubMed] [Google Scholar]
- 27.Pokutta S, Drees F, Yamada S, Nelson WJ, Weis WI. Biochemical and structural analysis of alpha-catenin in cell-cell contacts. Biochem Soc Trans. 2008;36(Pt 2):141–147. doi: 10.1042/BST0360141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenthal R, Milatz S, Krug SM, Oelrich B, Schulzke JD, Amasheh S, et al. Claudin-2, a component of the tight junction, forms a paracellular water channel. J Cell Sci. 2010;123(Pt 11):1913–1921. doi: 10.1242/jcs.060665. [DOI] [PubMed] [Google Scholar]
- 29.Sakisaka S, Kawaguchi T, Taniguchi E, Hanada S, Sasatomi K, Koga H, et al. Alterations in tight junctions differ between primary biliary cirrhosis and primary sclerosing cholangitis. Hepatology. 2001;33(6):1460–1468. doi: 10.1053/jhep.2001.25086. [DOI] [PubMed] [Google Scholar]
- 30.Salomon D, Sacco PA, Roy SG, Simcha I, Johnson KR, Wheelock MJ, et al. Regulation of beta-catenin levels and localization by overexpression of plakoglobin and inhibition of the ubiquitin-proteasome system. J Cell Biol. 1997;139(5):1325–1335. doi: 10.1083/jcb.139.5.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt A, Heid HW, Schafer S, Nuber UA, Zimbelmann R, Franke WW. Desmosomes and cytoskeletal architecture in epithelial differentiation: cell type-specific plaque components and intermediate filament anchorage. Eur J Cell Biol. 1994;65(2):229–245. [PubMed] [Google Scholar]
- 32.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286(6):C1213–1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 33.Sekine S, Gutierrez PJ, Lan BY, Feng S, Hebrok M. Liver-specific loss of beta-catenin results in delayed hepatocyte proliferation after partial hepatectomy. Hepatology. 2007;45(2):361–368. doi: 10.1002/hep.21523. [DOI] [PubMed] [Google Scholar]
- 34.Sekine S, Lan BY, Bedolli M, Feng S, Hebrok M. Liver-specific loss of beta-catenin blocks glutamine synthesis pathway activity and cytochrome p450 expression in mice. Hepatology. 2006;43(4):817–825. doi: 10.1002/hep.21131. [DOI] [PubMed] [Google Scholar]
- 35.Shibamoto S, Hayakawa M, Takeuchi K, Hori T, Oku N, Miyazawa K, et al. Tyrosine phosphorylation of beta-catenin and plakoglobin enhanced by hepatocyte growth factor and epidermal growth factor in human carcinoma cells. Cell Adhes Commun. 1994;1(4):295–305. doi: 10.3109/15419069409097261. [DOI] [PubMed] [Google Scholar]
- 36.Simcha I, Shtutman M, Salomon D, Zhurinsky J, Sadot E, Geiger B, et al. Differential nuclear translocation and transactivation potential of beta-catenin and plakoglobin. J Cell Biol. 1998;141(6):1433–1448. doi: 10.1083/jcb.141.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan X, Behari J, Cieply B, Michalopoulos GK, Monga SP. Conditional deletion of beta-catenin reveals its role in liver growth and regeneration. Gastroenterology. 2006;131(5):1561–1572. doi: 10.1053/j.gastro.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 38.Yeh TH, Krauland L, Singh V, Zou B, Devaraj P, Stolz DB, et al. Liver-specific beta-catenin knockout mice have bile canalicular abnormalities, bile secretory defect, and intrahepatic cholestasis. Hepatology. 2010 doi: 10.1002/hep.23801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou J, Qu J, Yi XP, Graber K, Huber L, Wang X, et al. Upregulation of gamma-catenin compensates for the loss of beta-cateninin adult cardiomyocytes. Am J Physiol Heart Circ Physiol. 2007;292(1):H270–276. doi: 10.1152/ajpheart.00576.2006. [DOI] [PubMed] [Google Scholar]
- 40.Zhurinsky J, Shtutman M, Ben-Ze’ev A. Differential mechanisms of LEF/TCF family-dependent transcriptional activation by beta-catenin and plakoglobin. Mol Cell Biol. 2000;20(12):4238–4252. doi: 10.1128/mcb.20.12.4238-4252.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]