Abstract
Increasing evidence suggests that mechanical factors play a critical role in fate decisions of stem cells. Recently we have demonstrated that a local force applied via Arg-Gly-Asp (RGD) peptides coated magnetic beads to mouse embryonic stem (ES) cells increases cell spreading and cell stiffness and decreases Oct3/4 (Pou5f1) gene expression. However, it is not clear whether the effects of the applied stress on these functions of ES cells can be extended to natural extracellular matrix proteins or cell-cell adhesion molecules. Here we show that a local cyclic shear force applied via fibronectin or laminin to integrin receptors increased cell spreading and stiffness, downregulated Oct3/4 gene expression, and decreased cell proliferation rate. In contrast, the same cyclic force applied via cell-cell adhesion molecule E-cadherin (Cdh1) had no effects on cell spreading, Oct3/4 gene expression, and the self-renewal of mouse ES cells, but induced significant cell stiffening. Our findings demonstrate that biological responses of ES cells to force applied via integrins are different from those to force via E-cadherin, suggesting that mechanical forces might play different roles in different force transduction pathways to shape early embryogenesis.
Keywords: mechanical stress, mechanotransduction, differentiation, cell stiffness, deformation
Introduction
Increasing evidence suggests that mechanical factors play a critical role in fate decisions of stem cells [1–3], in addition to soluble factors [4]. Recently, we have reported that a local cyclic force on a single mouse embryonic stem (ES) cell via Arg-Gly-Asp (RGD)-containing-peptides coated magnetic beads induced spreading, stiffening and downregulation of Oct3/4 gene (also known as Pou5f1) [5,6], a primary marker for pluripotency [7]; lowering endogenous forces of ES cells by seeding them on soft substrates that match ES cell’s intrinsic stiffness promotes the self-renewal and pluripotency of ES cells [8]. However, how ES cells sense mechanical forces via different adhesion molecules remains elusive. In addition, RGD-containing peptides are synthetic peptides and it is not clear how ES cells sense force applied via natural matrix molecules such as fibronectin or laminin. In this study, we demonstrate that a local cyclic stress applied to individual ES cells using magnetic beads coated with different ligands resulted in drastically different biological responses: force applied via integrin receptors but not E-cadherin (also known as Cdh1) resulted in cell spreading, downregulation of Oct3/4 gene expression, and a decrease in cell proliferation rate, although the ES cell stiffened in response to both cell-matrix and cell-cell mediated force application.
Materials and Methods
Mouse ES cell culture
Undifferentiated mouse ES cells (OGR1) that express enhanced GFP (EGFP) under the promoter of Oct3/4 (Oct3/4::EGFP) were cultured and maintained in feeder free conditions with leukaemia inhibitory factor (LIF; Chemicon) as previously described [5]. For live cell imaging, individual mouse ES cells were plated sparsely on the 0.6 kPa gel substrate (0.06% bis-acrylamide, 3% polyacrylamide) [9] for ~8 hours prior to experiment to match the substrate stiffness with the intrinsic mouse ES cell stiffness. The gel substrate was coated with collagen-I (200 μg/ml) 24 hours before plating cells. Gridded glass-bottomed dishes (MatTek) were used to track cells of interest over a long period (24 hours). To assess the general transcription status of the ES cells under stress, OGR1 cells transfected with pCAGGS DsRedT3_T2A_Puro were used [5]. The pluripotent state of cells was quantified by measuring the intensity of the EGFP expression. Cell spreading area was measured using an active contours algorithm through ImageJ (NIH).
Bead coating
Ferromagnetic microbeads (Fe3O4) ~4 μm in diameter were coated with different ligands. For Arg-Gly-Asp (RGD) or fibronectin coated beads, 50 μg of RGD peptide or 25 μg of fibronectin (Sigma-Aldrich) per milligram of beads in 1 ml of carbonate buffer (pH9.4) were used. The beads were incubated overnight at 4°C while being gently rotated. For laminin coated beads, 10 μg of beads and 20 μg of laminin (Sigma) were suspended in 100 μl of PBS buffer and incubated for 10 minutes. For E-cadherin coated beads, 1 mg of beads were suspended in 1 ml of coating buffer (20 mM HEPES buffer supplemented with 100 mM NaCl and 5 mM CaCl2 at pH 8.0). The bead solution was mixed with 100 μg of E-Cadherin/Fc Chimera (Sigma) and incubated overnight at 4°C while gently being rotated. After coating, RGD, fibronectin, laminin, or E-cadherin coated beads were suspended in serum-free medium or the coating buffer before storing at 4 °C or used immediately in the experiments.
Magnetic Twisting Cytometry (MTC)
The MTC technique has been reported before [10,11]. After placing the dish on the microscope, a brief and strong magnetic impulse (~1000G, <100 μs) was applied to magnetize the beads. A twisting torque with peak stress of 17.5 Pa was applied to the cells by a homogeneous sinusoidal magnetic field (0.3 Hz) perpendicular to the bead’s magnetic moment. This magnetic torque causes the bead to rotate, thus deforming the cells. The cell shear modulus was estimated by quantifying the embedded area of the bead and cell stiffness [12].
Staining
ES cells were fixed with 4 % paraformaldehyde and permeabilized with 0.5% Triton X-100. Cells were then incubated with 1 μg/ml Rhodamine-phalloidin for 30 minutes. Nuclei were counter-stained with 300 nM DAPI (Invitrogen) for 1 hour. Specimens were rinsed two times with cytoskeleton buffer solution and once with dH2O.
Bead binding specificity
Mouse ES cells were plated on 200 μg/ml collagen-I coated glassbottomed dish (MatTek) at a density of 6.5 × 103 cells/cm2. About 8 hours after incubation at 37 °C in 5% CO2, the culture medium was replaced with a serum free medium [10]. Cells were pre-treated for 10 minutes with soluble RGD, fibronectin or laminin at a final concentration of 10 or 50 μg/ml whereas EGTA (Sigma-Aldrich) was used at a concentration of 3 or 9 mM to determine binding specificity of the ligand-coated magnetic beads. The total number of beads left on the apical surface of cells after washing was counted. A two-tailed Student’s t-test was used for statistics.
Results
ES cells stiffen in response to stress via focal adhesions and E-cadherin
We first determined the binding specificity of different ligand-coated magnetic beads. Soluble ligands were added to the culture medium of ES cells for 10 minutes at different concentrations. Then ligand-coated beads were added to the culture for 15 minutes and unbound beads were washed away. Obviously beads bound to the cells were significantly decreased by the addition of soluble ligands. Binding of the ligand-coated beads to the cell surface showed dose-dependent competition with soluble ligands (Fig. 1A, B, C). Furthermore, binding of E-cadherin coated beads to the cells was decreased by increasing concentrations of EGTA, a calcium chelator (Fig. 1D). Cell-cell adhesions via homophilic E-cadherin protein-protein interactions are strongly dependent on the presence of calcium [13]. These results suggest that ligand-coated magnetic beads interact with specific cell surface receptors.
Figure 1. Binding of ligand-coated magnetic beads to embryonic stem cells showed ligand specificity.

The number of surface-bound beads coated with each ligand indicated was quantified before (0 minutes) and 10 minutes after addition of each soluble ligand (10 and 50 μg/ml for RGD, fibronectin and laminin, and 3 and 9 mM for EGTA). (A) RGD-bead: RGD-containing peptide-coated beads; p<0.003 between 0 and 10 μg/ml soluble RGD; p<0.033 between 10 and 50 μg/ml soluble RGD peptides. (B) FN-bead: fibronectin-coated beads; p<0.011 between 0 and 10 μg/ml soluble fibronectin; p<0.05 between 10 and 50 μg/ml fibronectin. (C) LN-bead: laminin-coated beads; p<0.015 between 0 and 10 μg/ml; p>0.25 between 10 and 50 μg/ml soluble laminin. (D) ECad-bead: E-cadherin coated beads; p<0.001 between 0 and 3 mM soluble EGTA; and p<0.002 between 3 and 9 mM EGTA. Data were collected from 3 biological replicates for each condition. Means ± SE are shown.
To examine how ES cells respond mechanically to a local force via specific ligand-receptor interactions, a local cyclic shear stress (17.5 Pa at 0.3 Hz) was applied to ES cells bound with beads coated with each ligand for one hour and cell stiffness was quantified. When baseline cell stiffness was measured with application of a short, 10-second stress, stiffness of ES cells bound with RGD, fibronectin, laminin, or E-cadherin coated beads was similar and ~0.5 kPa (Fig. 2B), consistent with previously published results [5,6]. As the stress was applied for longer periods of duration to the ES cells via different ligands, cell stiffness increased for all conditions examined, although the cell stiffening rate varied depending on the ligand coating beads (Fig. 2A). Since cell stiffening in response to the same magnitude of applied stress is an index of mechanotransduction-dependent cytoskeletal remodeling [14], these results indicate that both integrin focal adhesion complexes and E-cadherin complexes are bona fide mechanosensors in ES cells, consistent with previously published results in other cells types [13,14].
Figure 2. Stress induced stiffening in embryonic stem cells bound with ligand-coated beads.
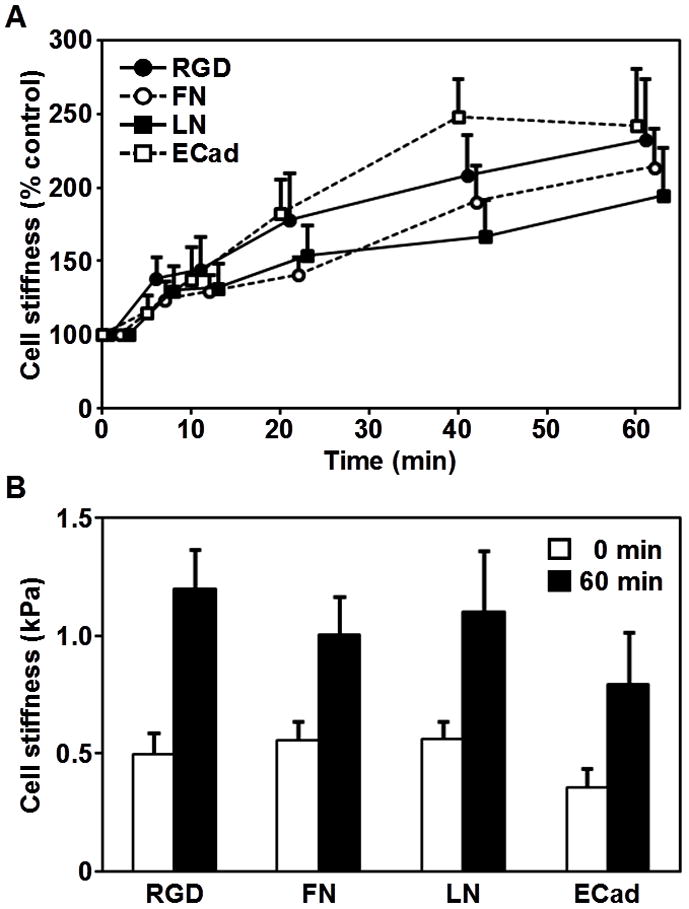
(A) Stiffening response is shown as a function of duration of stress application. The peak of cyclic stress is 17.5 Pa; the frequency of loading is 0.3 Hz. All stiffness measurements are normalized with respect to time zero. Means ± SE are shown. (B) Absolute magnitudes of cell stiffness (shear complex modulus) are shown before (0 minutes) and after (60 minutes) stress application. Each comparison of the cell stiffness before and after stress application showed significant differences; RGD, p < 0.012; fibronectin, p < 0.017; laminin, p < 0.041; and E-cadherin, p < 0.044. Data were collected from 5, 10, 9, and 7 cells for RGD, fibronectin (FN), laminin (LN), and E-cadherin (ECad) coated beads, respectively. Means ± SE are shown.
Force via integrins but not E-cadherin induces ES cell spreading and slows the cell proliferation rate
Previously we have shown that force applied via RGD-coated magnetic beads induces ES cell spreading [5]. However, it is not clear whether natural matrix ligands such as fibronectin or laminin and cell-cell adhesion molecule E-cadherin can mediate force-dependent cell spreading in ES cells. One hour after force application, magnetic beads coated with RGD, fibronectin, or laminin induced bleb formation and caused a ~50% increase in the ES cell projected area in 12 hours (Fig. 3B). These ES cells remained spread and flattened for up to 24 hours after stress was applied. In contrast, control cells that were not stressed or cells stressed via magnetic beads coated with E-cadherin ligands exhibited a 20% decrease in cell projected areas in 24 hours (Fig. 3B). It is known that normal pluripotent self-renewing mouse ES cells have a cell-doubling time of ~10.5 hours [6]. Comparing the number of nuclei in control stress-free cells or cells stressed via E-cadherin coated beads at 12 hours with that at 24 hours, it clearly demonstrate that these ES cells double normally (Fig. 3A). In sharp contrast, the cells stressed via RGD, FN, or LN coated beads remained as single cells at 24 hours (Fig. 3A), suggesting that the force applied via integrin pathways delayed cell proliferation rates in these ES cells, possibly by impeding the self-renewing capacity of the ES cells. These results demonstrate that not all force transduction pathways in mouse ES cells are the same: forces via integrin-mediated pathways induce cell spreading and slow down cell proliferation, whereas forces via E-cadherin-mediated pathways have no effect on either cell spreading or cell proliferation rate.
Figure 3. Force via integrins but not E-cadherin increased cell spreading and doubling time.
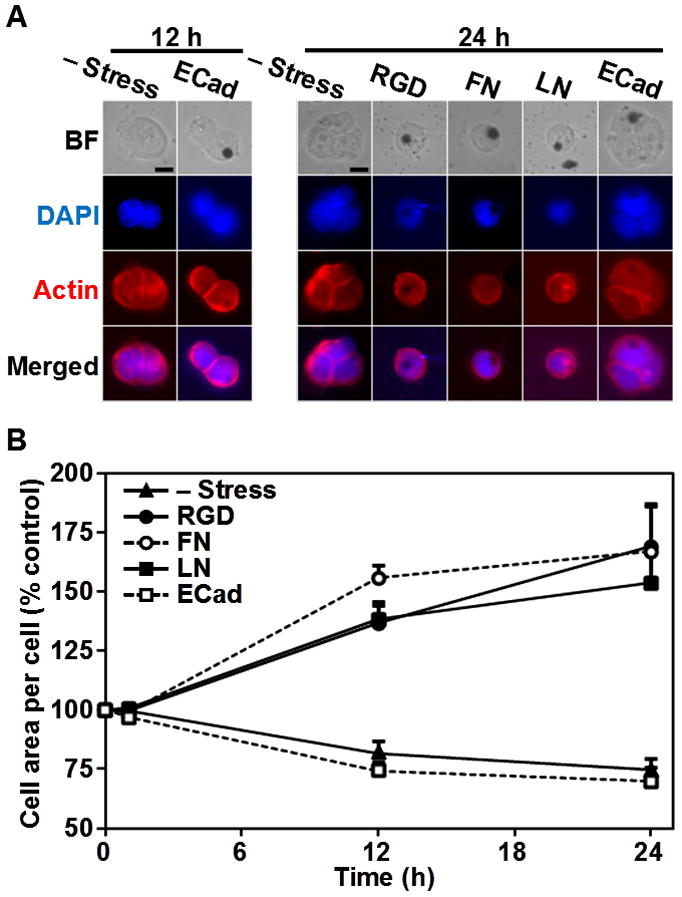
(A) Representative bright field (BF; top row) images of ES cells and fluorescent images indicating the presence of the nucleus (DAPI; 2nd row) and F-actin (Actin, 3rd row) are shown at 12 hours and 24 hours without stress (– Stress) or after stress was applied for 1 hour. Fluorescent images for DAPI and F-actin staining were merged on the bottom row. Cells stressed via RGD, fibronectin (FN), and laminin (LN) coated beads remained as single cells 24 hours after stress was applied. However, control cells (– Stress) and cells stressed with E-cadherin coated beads continued to proliferate, which is evident from the number of nuclei at 12 and 24 hours. Scale bars, 10 μm. (B) The cell area per cell was summarized as a function of time after stress. Cells stressed with RGD, FN, or LN-coated beads increased projected areas by 50–70% by. However, control cells or cells stressed with E-cadherin (ECad) coated beads decreased cell projected areas slightly due to cell division. Values for cell areas at 12 and 24 hours are statistically significant when compared with those before application of the stress (time 0; p<0.01 for all conditions). Values at 12 hours did not show statistical significance when compared with those at 24 hours (for all conditions, p>0.1). The cell projected area before stress application was ~230 μm2. Data were collected from 10, 8, 8, 7, and 8 for – Stress, RGD, FN, LN, and ECad-coated beads respectively. Means ± SE are shown.
Stress via interins but not E-cadherin induces ES cell differentiation
Recent reports show that mechanical forces influence ES cell proliferation and differentiation [2,3,5,15], but it is not known if different adhesion molecules mediate different responses to force. To address this question, expression of Oct3/4, the master regulator of pluripotency in ES cells [7], was monitored using the ES cell line that express enhanced green fluorescent protein (EGFP) under the Oct3/4 promoter [5]. The individual ES cells were healthy and pluripotent before stress was applied, as indicated by the round shape and high EGFP expression (Fig. 4A, 1st, 5th column). When a cyclic shear stress (17.5 Pa at 0.3 Hz) was applied to the cells for 1 hr using RGD, fibronectin or laminin coated beads (Fig. 4A 2nd, 3rd, 4th row), EGFP expression in these cells decreased by ~15 % in 12 hours and ~30 % by 24 hours (Fig. 4A, B). In sharp contrast, the cells that were stressed via E-cadherin coated beads did not have any decrease in EGFP expression in 12 or 24 hours, similar to control cells in the same culture dish that were not stressed or not bound with beads (Fig. 4A, B). It should be noted that the apparent increase in the projected area of control cells and stressed cells via E-cadherin coated beads at 12 hours and 24 hours are not due to cell spreading, but due to increased cell numbers as a result of cell proliferation (see Fig. 3). To make certain that the decrease in EGFP expression was not due to a reduction in the general transcription capacity of the cell, cells were simultaneously transfected with a construct that permits expression of DsRed under CAG, a constitutively active promoter [16]. Despite the decrease in EGFP expression by force applied via the RGD-coated beads, DsRed expression remained constant > 24 hours, indicating that the ES cells were healthy [5]. These results show that force applied via integrin pathways, but not E-cadherin pathways, is capable of downregulating Oct3/4 expression, leading to ES cell differentiation.
Figure 4. Force via integrins but not E-cadherin downregulated Oct3/4 expression.
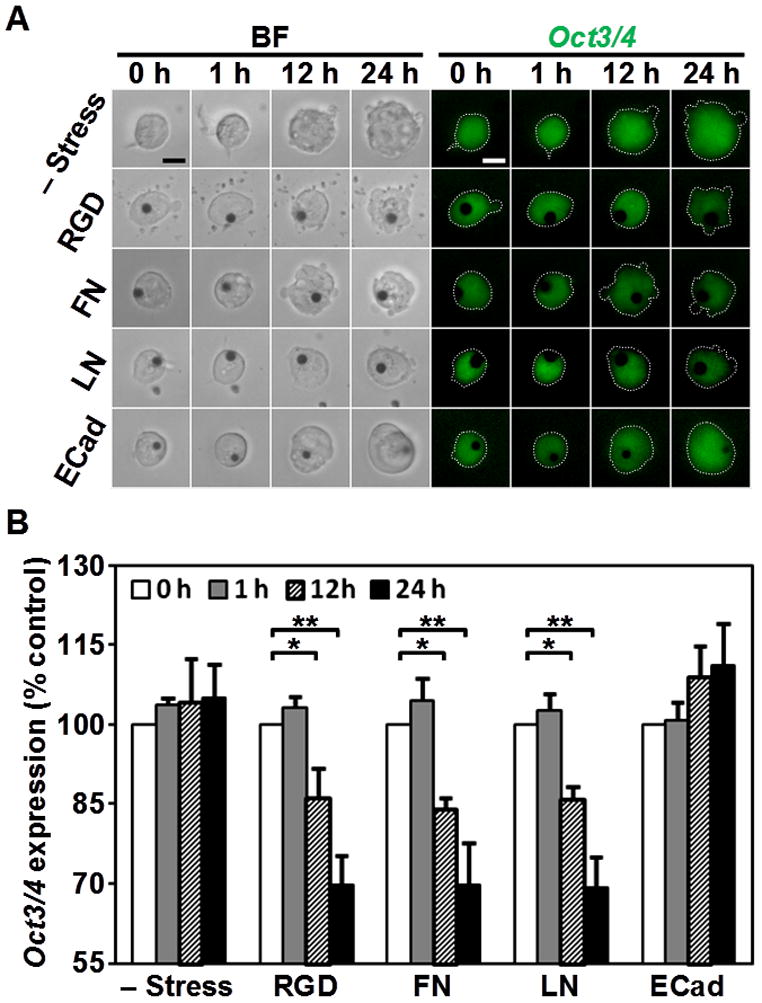
(A) Representative bright field (BF, left) and fluorescence (Oct3/4, right) images of mouse ES cells bound with beads coated with ligands as indicated on left at different times as indicated on top. Expression of Oct3/4 was shown by fluorescence of EGFP driven by the Oct3/4 promoter (EGFP-Oct3/4). Cyclic shear stress was applied for 1 hr (peak stress was 17.5 Pa at 0.3 Hz). Note that the EGFP-Oct3/4 fluorescence intensity decreased in cell stressed with RGD, fibronectin (FN), and laminin (LN) coated beads at 12 and 24 hours after stress was applied. Scale bars, 10 μm. (B) Expression levels of Oct3/4 were quantified up to 24 hours after stress was applied. Force via RGD (n=9), FN (n=10), or LN (n=10) coated beads decreased EGFP-Oct3/4 at 12 or 24 hours. *p<0.05; **p<0.01. However, force via E-cadherin coated beads has no effect on EGFP-Oct3/4 expression (n=8). Cells that had no beads bound were used as a control (– Stress; n = 8). Means ± SE are shown.
Discussion
Despite significant progress during the past decade in the field of mechanotransduction, the mechanisms of biological responses to force remain elusive. In particular, only very recently has it been shown that cell-cell adhesion molecule E-cadherin is a direct mechanosensor [13], similar to cell-matrix adhesion molecule integrins. Here we show that the biological responses to force in mouse ES cells depend on specific pathways through transmembrane adhesion molecules: both integrin and E-cadherin pathways can mediate force-dependent cell stiffening, whereas only integrins but not E-cadherin can mediate force-dependent cell spreading and Oct3/4 downregulation. These findings suggest that integrins and cadherins play different roles in directing embryonic stem cell fate decisions.
Recently we have shown that force applied via synthetic RGD-containing peptides can cause cell stiffening, spreading, and Oct3/4 downregulation [5,6]. In this study, we have extended these findings to investigating the effects of forces applied via natural ligands of integrins, which are fibronectin and laminin. It is known that fibronectin and laminin are essential for early embryogenesis and tissue organization [17,18]. Fibronectin interacts with cell surface via α5β1 and αvβ3 integrins [19]. Laminin, on the other hand, interact with cell surface via α6β1 or α7β1 [20]. Because fibronectin forms unique fibrils in vivo [21], it will be interesting to determine in the future whether fibronectin and laminin play different roles in lineage differentiation of embryonic stem cells.
Why do forces applied to mouse ES cells via integrins and cadherins elicit totally different responses in cell spreading and Oct3/4 expression although similar responses in cell stiffening were observed? At this time, it is not clear what the underlying mechanisms are but there are a few clues. Both integrin-based focal contacts and cadherin-based complexes provide anchoring support to cells through links to the actin cytoskeleton. Integrin clusters via cell-ECM interactions recruit proteins such as talin [22] and vinculin [23] that can be deformed, unfolded, and activated by forces of physiologic magnitudes. E-cadherin at cell-cell adhesion sites, on the other hand, recruits proteins such as α-catenin [24], β-catenin [25], and vinculin [13]. It has been shown that vinculin facilitates E-cadherin mediated mechanosensing and stiffening in response to shear force at cell-cell junctions [13]. Vinculin knockout cells display a reduction in the stress-induced cell stiffening response through E-cadherin whereas vinculin-reconstituted cells fully restore the force-dependent reinforcement of cadherin junctions [13]. Because vinculin is implicated in force-mediated strengthening of adhesion sites and is localized in both cell-matrix adhesions and cell-cell junctions [13,14], we hypothesize that vinculin is at least partly responsible for the stress-induced ES cell stiffening response in both integrin mediated pathways and cadherin mediated pathways. Cell spreading is a complicated process that involves plasma membrane protrusion mediated by coordinated actin polymerization, which, in turn, depends on activities of actin associated proteins such as Arp2/3 and WASP [26,27]. Stress-induced cell spreading also requires activated Src, cdc42, and myosin II [5]. When a force is applied via the integrin pathways, it induces cell membrane protrusion as early as ~30 sec of force application [5]. The ES cell continues to spread in response to the cyclic stress and the cell increases its projected area by ~50–70 % after 1 hr of force application [5] (Fig. 3). The significant downregulation of Oct3/4 expression, quantified by changes in EGFP fluorescence intensity in the cells, was only apparent at ~ 12 hours (Fig. 4). This suggests that cell spreading might precede downregulation of Oct3/4 expression and might be necessary for Oct3/4 downregulation and ES cell differentiation. However, this conclusion should be dealt with caution since the fluorescence intensity in these ES cells depends on the turnovers of EGFP, which might be much slower than the actual force-induced inhibitory action on the Oct3/4 gene. The relationship between ES cell spreading and ES cell differentiation needs to be examined carefully in the future. It will also be interesting to elucidate the mechanisms of why the same force results in totally different responses in spreading and Oct3/4 expression for integrins and E-cadherin.
E-cadherin has been implicated in promoting the pluripotency and self-renewal of ES cells. When ES cells are plated on E-cadherin coated dishes, they maintain self-renewal and pluripotency [28]. These ES cells must adhere via E-cadherin molecules and should have generated tractions and transmitted forces from the cell to the substrate via E-cadherin [29]. Therefore the fact that they maintain self-renewal and pluripotency is completely consistent with our results that the force applied via E-cadherin maintains self-renewal and Oct3/4 expression.
The first recognized differentiation event occurs at the late morula stage when the outer layer of the compacted ball of totipotent cells adopts an epithelial structure. Similar epithelialization happens during the progression of embryoid body differentiation [30,31]. This may be due to the outer layer of cells experiencing different forces through the interaction with the surrounding microenvironment. It has been shown that forces at intercellular junctions regulate cell polarity, shape, movements, and germ cell migration during morphogenesiss [32,33]. Taken together, it is likely that forces mediated by pathways through integrins and cadherins are crucial in cell differentiation events during embryogenesis.
In conclusion, we have shown two distinct biological responses from mouse ES cells to a small local force of physiologic magnitudes through different ligands. Cell stiffening is elicited by force in both integrin and cadherin pathways; however, cell spreading and differentiation are induced by force only in the integrin but not in the E-cadherin pathway. It will be interesting to determine the effects of force magnitude, frequency, and modes on ES cell pluripotency and the specific germ-layer (endoderm, mesoderm or ectoderm) formation.
Highlights.
Force via integrins or cadherins induces similar cell stiffening responses.
Force via integrins but not cadherins induces cell spreading.
Force via integrins but not cadherins induces differentiation of embryonic stem cells.
Acknowledgments
We thank Russell Borduin for collecting and analyzing some early experimental results. We also thank Arash Tajik and Youhua Tan for discussions. This work was supported by NIH grant GM072744 (to NW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 2.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 3.Kurpinski K, Chu J, Hashi C, Li S. Anisotropic mechanosensing by mesenchymal stem cells. Proc Natl Acad Sci U S A. 2006;103:16095–16100. doi: 10.1073/pnas.0604182103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 5.Chowdhury F, Na S, Li D, Poh YC, Tanaka TS, Wang F, Wang N. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat Mater. 2010;9:82–88. doi: 10.1038/nmat2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poh YC, Chowdhury F, Tanaka TS, Wang N. Embryonic stem cells do not stiffen on rigid substrates. Biophys J. 2010;99:L19–21. doi: 10.1016/j.bpj.2010.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 8.Chowdhury F, Li Y, Poh YC, Yokohama-Tamaki T, Wang N, Tanaka TS. Soft substrates promote homogeneous self-renewal of embryonic stem cells via downregulating cell-matrix tractions. PLoS One. 2010;5:e15655. doi: 10.1371/journal.pone.0015655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 10.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 11.Fabry B, Maksym GN, Shore SA, Moore PE, Panettieri RA, Jr, Butler JP, Fredberg JJ. Selected contribution: time course and heterogeneity of contractile responses in cultured human airway smooth muscle cells. J Appl Physiol. 2001;91:986–994. doi: 10.1152/jappl.2001.91.2.986. [DOI] [PubMed] [Google Scholar]
- 12.Mijailovich SM, Kojic M, Zivkovic M, Fabry B, Fredberg JJ. A finite element model of cell deformation during magnetic bead twisting. J Appl Physiol. 2002;93:1429–1436. doi: 10.1152/japplphysiol.00255.2002. [DOI] [PubMed] [Google Scholar]
- 13.le Duc Q, Shi Q, Blonk I, Sonnenberg A, Wang N, Leckband D, de Rooij J. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J Cell Biol. 2010;189:1107–1115. doi: 10.1083/jcb.201001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- 15.Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, Healy KE. Substrate modulus directs neural stem cell behavior. Biophys J. 2008;95:4426–4438. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–9. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 17.George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 18.Leivo I, Vaheri A, Timpl R, Wartiovaara J. Appearance and distribution of collagens and laminin in the early mouse embryo. Dev Biol. 1980;76:100–114. doi: 10.1016/0012-1606(80)90365-6. [DOI] [PubMed] [Google Scholar]
- 19.Danen EH, Sonneveld P, Brakebusch C, Fassler R, Sonnenberg A. The fibronectin-binding integrins alpha5beta1 and alphavbeta3 differentially modulate RhoA-GTP loading, organization of cell matrix adhesions, and fibronectin fibrillogenesis. J Cell Biol. 2002;159:1071–1086. doi: 10.1083/jcb.200205014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterk LM, Geuijen CA, van den Berg JG, Claessen N, Weening JJ, Sonnenberg A. Association of the tetraspanin CD151 with the laminin-binding integrins alpha3beta1, alpha6beta1, alpha6beta4 and alpha7beta1 in cells in culture and in vivo. J Cell Sci. 2002;115:1161–1173. doi: 10.1242/jcs.115.6.1161. [DOI] [PubMed] [Google Scholar]
- 21.Singh P, Carraher C, Schwarzbauer JE. Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol. 2010;26:397–419. doi: 10.1146/annurev-cellbio-100109-104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323:638–641. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, Yang MT, McLean MA, Sligar SG, Chen CS, Ha T, Schwartz MA. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 2010;466:263–266. doi: 10.1038/nature09198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol. 2010;12:533–542. doi: 10.1038/ncb2055. [DOI] [PubMed] [Google Scholar]
- 25.Obama H, Ozawa M. Identification of the domain of alpha-catenin involved in its association with beta-catenin and plakoglobin (gamma-catenin) J Biol Chem. 1997;272:11017–11020. doi: 10.1074/jbc.272.17.11017. [DOI] [PubMed] [Google Scholar]
- 26.DeMali KA, Barlow CA, Burridge K. Recruitment of the Arp2/3 complex to vinculin: coupling membrane protrusion to matrix adhesion. J Cell Biol. 2002;159:881–891. doi: 10.1083/jcb.200206043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Partridge MA, Marcantonio EE. Initiation of attachment and generation of mature focal adhesions by integrin-containing filopodia in cell spreading. Mol Biol Cell. 2006;17:4237–4248. doi: 10.1091/mbc.E06-06-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagaoka M, Koshimizu U, Yuasa S, Hattori F, Chen H, Tanaka T, Okabe M, Fukuda K, Akaike T. E-cadherin-coated plates maintain pluripotent ES cells without colony formation. PLoS One. 2006;1:e15. doi: 10.1371/journal.pone.0000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ladoux M, Anon E, Lambert M, Rabodzey A, Hersen P, Buguin A, Silberzan P, Mège RM. Strength dependence of cadherin-mediated adhesions. Biophys J. 2010;98:534–42. doi: 10.1016/j.bpj.2009.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ralston A, Rossant J. Genetic regulation of stem cell origins in the mouse embryo. Clin Genet. 2005;68:106–112. doi: 10.1111/j.1399-0004.2005.00478.x. [DOI] [PubMed] [Google Scholar]
- 31.Wobus AM, Boheler KR. Embryonic stem cells: prospects for developmental biology and cell therapy. Physiol Rev. 2005;85:635–678. doi: 10.1152/physrev.00054.2003. [DOI] [PubMed] [Google Scholar]
- 32.Desprat N, Supatto W, Pouille PA, Beaurepaire E, Farge E. Tissue deformation modulates twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev Cell. 2008;15:470–477. doi: 10.1016/j.devcel.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Rauzi M, Lenne PF, Lecuit T. Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature. 2010;468:1110–1114. doi: 10.1038/nature09566. [DOI] [PubMed] [Google Scholar]


