Abstract
Mammalian matrix metalloproteinases (MMPs) which degrade extracellular matrix facilitate colon cancer cell invasion into the bloodstream and extra-colonic tissues; in particular, MMP1 expression correlates strongly with advanced colon cancer stage, hematogenous metastasis and poor prognosis. Likewise, muscarinic receptor signaling plays an important role in colon cancer; muscarinic receptors are over-expressed in colon cancer compared to normal colon epithelial cells. Muscarinic receptor activation stimulates proliferation, migration and invasion of human colon cancer cells. I n mouse intestinal neoplasia models genetic ablation of muscarinic receptors attenuates carcinogenesis. In the present work, we sought to link these observations by showing that MMP1 expression and activation plays a mechanistic role in muscarinic receptor agonist-induced colon cancer cell invasion. We show that acetylcholine, which robustly increases MMP1 expression, stimulates invasion of HT29 and H508 human colon cancer cells into human umbilical vein endothelial cell monolayers – this was abolished by pre-incubation with atropine, a non-selective muscarinic receptor inhibitor, and by pre-incubation with anti-MMP1 neutralizing antibody. Similar results were obtained using a Matrigel chamber assay and deoxycholyltaurine (DCT), an amidated dihydroxy bile acid associated with colon neoplasia in animal models and humans previously shown to interact functionally with muscarinic receptors. DCT treatment of human colon cancer cells resulted in time-dependent, 10-fold increased MMP1 expression, and DCT-induced cell invasion was also blocked by pre-treatment with anti-MMP1 antibody. This study contributes to understanding mechanisms underlying muscarinic receptor agonist-induced promotion of colon cancer and, more importantly, indicates that blocking MMP1 expression and activation has therapeutic promise to stop or retard colon cancer invasion and dissemination.
Keywords: Cell migration, Cell invasion, Colon cancer, Matrix metalloproteinases, Acetylcholine, Bile acids
1. Introduction
Mammalian matrix metalloproteinases (MMPs), a family of at least 23 zinc-dependent proteases, are key players in cancer cell migration and invasion [1,2]. Produced as secreted or membrane-bound pro-enzymes, MMPs are activated by proteolytic cleavage and classified into subgroups; collagenases, stromelysins, gelatinases, membrane-type-MMPs and others that cooperatively degrade all components of the extracellular matrix. MMP1, a collagenase, plays an important role in degrading extracellular matrix substrates, thereby facilitating colon cancer cell migration and invasion into the bloodstream and extra-colonic tissues. Hence, it is not surprising that MMP1 expression correlates strongly with advanced colon cancer stage, hematogenous metastasis and poor prognosis [3,4,5]. Nonetheless, little is known regarding the regulation of MMP1 expression or activity in colon cancer.
Muscarinic receptor (MR) signaling plays an important role in colon cancer cell proliferation and survival [6,7,8,9]. Further evidence for the importance of MR signaling in colon cancer was provided by work using mouse models wherein genetic ablation of type-3 MRs (Chrm3) and inhibition of MR activation with scopolamine butylbromide attenuated colon neoplasia [10,11]. In human colon cancer cells, we observed that MR agonists stimulate actin stress fiber formation and colon cancer cell migration and invasion [12]. Collectively, these observations suggested to us that MMPs, particularly MMP1, might play a key role in MR agonist-induced colon cancer cell invasion. In this context, it is relevant that we previously showed that MR agonists robustly stimulate MMP gene transcription; notably, acetylcholine treatment stimulated a 40- to 50-fold increase in MMP1 expression [13]. In control experiments, the half-life of MMP1 mRNA (~16 h) was not altered by MR agonist treatment, thereby confirming that these agents stimulate MMP1 expression at the transcriptional level [13].
Death from colon cancer is largely due to metastatic disease; cancer limited to the bowel is readily treated by surgery [14]. Given the importance of colon cancer invasion and the possible therapeutic potential of blocking MMP1 expression and activation, we investigated the role of MMP1 in mediating MR agonist-induced colon cancer cell invasion. To enhance the likelihood that our observations were generalizable, i.e. not idiosyncratic to one cell line, we used two well-characterized human colon cancer cell lines (HT29 and H508 cells) and two validated approaches to measure cell invasion, including a novel, physiologically-relevant electrical cell impedance sensing assay. Using these approaches, we demonstrate that MR agonists stimulate MMP1-dependent invasion of human colon cancer cells and that blocking MMP1 activation has therapeutic potential.
2. Materials and methods
2.1. Materials
Acetylcholine (ACh), atropine and deoxycholyltaurine (DCT) were from Sigma-Aldrich (St. Louis, MO). Anti-MMP1 neutralizing antibody was from R&D Systems (Minneapolis, MN). All other chemicals were obtained from Sigma-Aldrich or Fisher.
2.2. Cell culture
HT29 human colon cancer cells were grown in McCoy's 5A medium supplemented with 10% fetal bovine serum. H508 human colon cancer cells were grown in RPMI 1640 supplemented with 10% fetal bovine serum. Adherent cultures were passaged weekly at subconfluence after trypsinization. Cultures were maintained in incubators at 37°C in an atmosphere of 5% CO2 and 95% air.
2.3. Matrigel chamber invasion assay
Assays were performed using BD Biocoat Matrigel Invasion Chambers (BD Biosciences, Bedford, MA) as described previously [12]. HT29 human colon cancer cells were trypsinized and resuspended in serum-free McCoy's 5A medium, placed in the upper chamber (5 × 104 cells/chamber) and treated with indicated agents or vehicle (control). McCoy's 5A medium containing 10% FBS was placed in the lower chamber. Cells were incubated for 24 h in a humidified atmosphere with 95% air and 5% CO2 at 37°C. Invasive cells were fixed and stained using Hema 3 stain (Fisher). Non-invasive cells in the upper chamber were removed by wiping with a cotton swab. Cells on the lower surface of the insert which had penetrated Matrigel were counted in five randomly-selected high power fields (HPF) using a light microscope. Each experiment was performed in triplicate.
2.4 Electrical cell impedance sensing (ECIS) invasion assay
ECIS was performed as described previously [15]. Briefly, human umbilical vein endothelial cells (HUVEC) were plated (2.5 × 105 cells/well) on small gold electrodes (diameter, 250 μm) at the bottom of tissue culture wells To measure cell micromotion, in- and out-of-phase voltages across electrodes were recorded each second. Colon cancer cell movement into the HUVEC monolayer was measured by fluctuations in resistance recorded and analyzed using ECIS software (Applied BioPhysics, Troy, NY). After adding colon cancer cells (5 X 104 cells/well), invasion into the HUVEC monolayer reduced resistance because HUVEC cell-to-cell attachments were disrupted; this was confirmed by light microscopy.
2.5. Scratch-wound assay
Cell migration was measured using a monolayer wound closure assay as described previously [12]. Briefly, H508 colon cancer cells were grown as confluent monolayers in 6-well plates in RPMI 1640 supplemented with 10% fetal bovine serum. Cell monolayers were wounded by scraping with a disposable 200-μl pipette tip, washed twice with fresh serum-free medium, and incubated in serum-free medium in the absence or presence of test agents. Using an Axiovert 200 microscope (Carl Zeiss) (total magnification, 100X), rates of migration of cells at the anterior edges of wounded monolayers were determined from serial photographs taken 4 and 8 h after wounding. Measurements (12/field) were taken by an investigator masked to treatment. Results were expressed as mean ± SE distance migrated (arbitrary units). Cell proliferation, a potential confounding variable, was inhibited by pre-incubating cells for 1 h with mitomycin C (10 μg/ml) [12].
2.6. Quantitative real-time PCR (qPCR)
Measurement of MMP1 mRNA levels by qPCR was performed as described previously [13]. Cells were subcultured in six-well plates (106 cells/well). After 24 h incubation at 37°C, cells were serum-starved overnight in RPMI 1640 media and washed once with RPMI 1640 before adding test agents. Total cellular RNA was isolated using Trizol reagent (Invitrogen). First-strand cDNAs were synthesized using the Superscript III First Strand Synthesis System for RT-PCR (Invitrogen) and qPCR performed using 7900HT Fast System (ABI) with Power SYBR Green master mix (ABI), 20 ng primer and cDNA synthesized from 50 ng total RNA. PCR conditions were 5 min at 95°C, 35 cycles at 95°C for 15 sec, 60°C for 20 sec, 72°C for 40 sec and a final cycle at 95°C for 15 sec, 60°C for 15 sec and 95°C for 15 sec. PCR data were analyzed using ABI instrument software SDS 2.1 and relative levels of mRNA expression were calculated according to the standard ΔΔCt method. Specificity of amplifications was confirmed by melting-curve analysis. Expression values were normalized by comparison with glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Primers used (all 5′-3′) were - MMP1 forward primer sequence: aactgccaaatgggcttg, MMP1 reverse primer sequence: ccgtgtagcacattctgtcc; GAPDH forward primer sequence: ccccatggtgtctgagcg, GAPDH reverse primer sequence: cgacagtcagccgcatctt.
2.7 Statistical analysis
Bar graphs show mean ± SE of at least three independent experiments. Statistical analysis of cell migration data between control and treatment groups was performed using Student's un-paired t-test and by ANOVA with post tests (Tukey test) (SigmaPlot, Systat Software, San Jose, CA). Statistical significance is shown by the number of asterisks (*P < 0.05; **P < 0.01). P < 0.05 was considered statistically significant.
3. Results
3.1. Acetylcholine stimulates MMP1-dependent human colon cancer cell invasion
Recently, using a scratch-wound cell migration assay, we showed that the classical MR agonist acetylcholine (ACh) strongly stimulated migration of H508 human colon cells [12]. ACh-induced cell migration was mediated by a complex mechanism involving MMP7-catalyzed release of HBEGF, post-EGFR signaling via ERK and PI3K/AKT, and activation of RhoA and ROCK [12]. In the course of that work, preliminary experiments showed that ACh also stimulates HT29 human colon cancer cell invasion in Matrigel chambers [12]. Based on the rationale in the Introduction, in the following experiments we pursued a line of investigation seeking to identify a mechanistic role for MMP1 activation in MR agonist-induced colon cancer cell invasion.
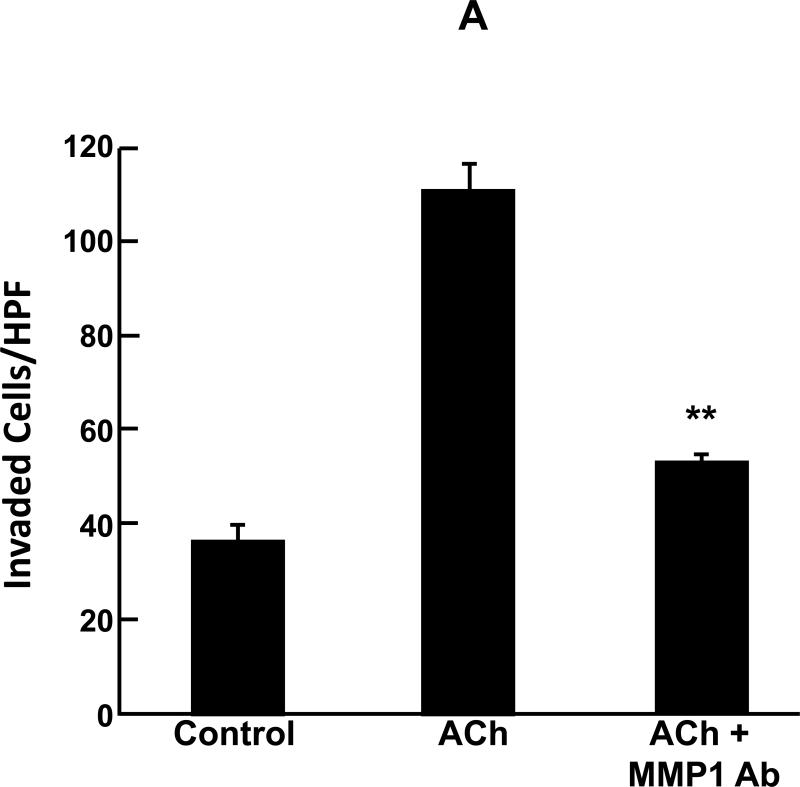
Using Matrigel chamber invasion assays, we examined the effects of adding anti-MMP1 neutralizing antibody on ACh-induced actions of HT29 human colon cancer cells. Our published work showed that the effects of ACh in this assay are blocked by pre-incubation with a non-selective MR antagonist, atropine [12]. As shown in Fig. 1A, ACh stimulated a 3-fold increase in HT29 cell invasion. Pre-incubation with 10 μg/ml anti-MMP1 antibody strikingly attenuated ACh-induced HT29 cell invasion (Fig. 1A). In control experiments, this concentration of anti-MMP1 antibody alone did not alter HT29 cell invasion (not shown). These findings provided the first direct evidence that ACh-induced colon cancer cell invasion is mediated by activation of MMP1.
Fig. 1.
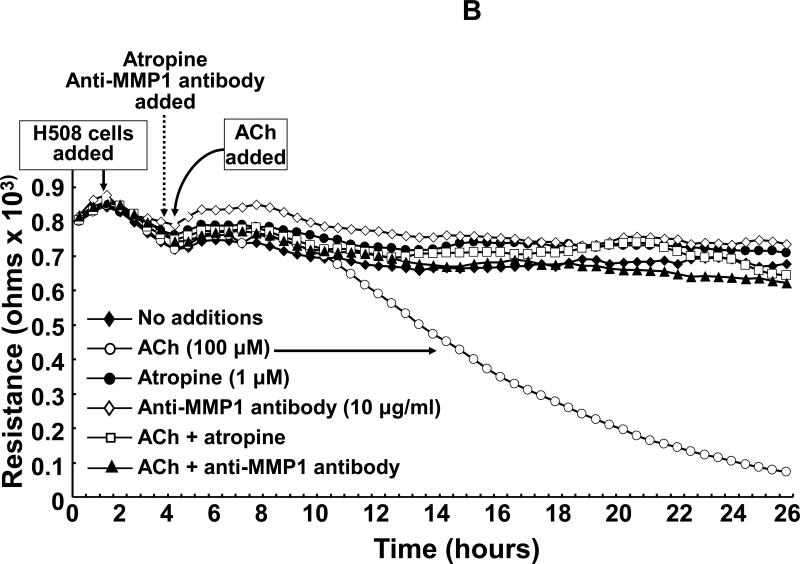
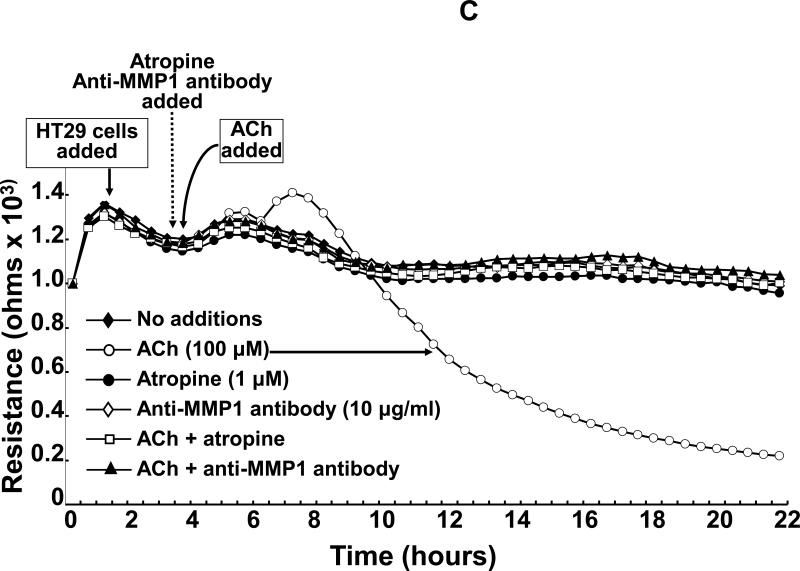
Acetylcholine (ACh) stimulates MMP1-dependent human colon cancer cell invasion. A: HT29 colon cancer cells were incubated for 24 h with ACh (100 μM), alone or in cells pre-incubated for 30 min with anti-MMP1 antibody (10 μg/ml), and invasion was measured using BD Biocoat Invasion Chambers with Matrigel inserts. Values represent mean ± SE (3 separate experiments). Anti-MMP1 antibody alone did not alter basal HT29 cell invasion (not shown). HPF, high power field. **, P < 0.01, Student's paired t-test. B: Electrical cell impedance sensing (ECIS) shows ACh-induced H508 colon cancer cell invasion of HUVEC monolayers. At the time shown, ACh (100 μM) was added to H508 cells, with or without pre-incubation for 30 min with atropine (1 μM) or anti-MMP1 antibody (10 μg/ml), and invasion into the HUVEC monolayer was determined by measuring trans-HUVEC monolayer resistance. ACh-induced H508 cell invasion into the HUVEC monolayer was blocked by both atropine and anti-MMP1 antibody. In additional control experiments, the agents tested had no effect on HUVEC monolayer resistance in the absence of added H508 cells (not shown). C: ECIS experiments were conducted with HT29 cells instead of H508 cells using the same experimental approach and controls described for Fig. 1B. For both H508 and HT29 cells, resistance (ohms) of the HUVEC monolayer over the course of the experiments (mean of duplicates at each time point) was representative of three separate experiments.
We confirmed this important finding by examining the actions of a different human colon cancer cell line, H508 cells, using electrical cell impendance sensing (ECIS) – a more physiologically relevant cell invasion assay [15]. Moreover, we thought it important to generalize our observations to more than one human colon cancer cell line and, because of their larger size, H508 cells do not readily transit Matrigel inserts. Hence, using ECIS, an assay that depends less on cell size, offered the benefits of allowing us to study H508 cell invasion and provided a complementary invasion assay to confirm results observed in Matrigel chambers.
As shown in Fig. 1B, in the absence of additions, HUVEC monolayer resistance remained relatively stable over the course of the 26-h experiment. Adding H508 cells caused only minor fluctuations in resistance until the addition of 100 μM ACh; starting approximately 4 h after addition of ACh we observed progressive reduction in HUVEC monolayer resistance such that 20 h after adding ACh resistance was approximately 10% basal (Fig. 1B). Pre-incubating H508 cells with 1 μM atropine, a non-selective MR inhibitor, blocked ACh-induced H508 cell invasion into the HUVEC monolayer – there was no change in resistance (Fig. 1B). Likewise, pre-incubation with 10 μg/ml anti-MMP1 neutralizing antibody abolished the effects of ACh on cell invasion (Fig. 1B). In the absence of stimulation with ACh, atropine and anti-MMP1 antibody did not alter resistance in wells when H508 cells were added to the HUVEC cell monolayer (Fig. 1B). Moreover, in control experiments wherein H508 cells were not added, ACh, atropine and anti-MMP1 antibody did not alter HUVEC monolayer resistance (not shown).
As additional confirmation of these striking results, we repeated these experiments using HT29 cells instead of H508 cells in the ECIS assay. Approximately 2 h after adding ACh we observed a transient modest increase in resistance of unknown cause followed by a progressive reduction in monolayer resistance similar to that observed with H508 cells (Fig. 1C). Eighteen hours after adding ACh, HUVEC monolayer resistance was approximately 20% basal. Again, as shown in Fig. 1C, pre-incubation with atropine and anti-MMP1 antibody completely blocked ACh-induced HT29 cell invasion into the HUVEC monolayer. In the absence of stimulation with ACh, atropine and anti-MMP1 antibody did not alter resistance in wells when HT29 cells were added to the HUVEC cell monolayer (Fig. 1C). In control experiments wherein HT29 cells were not added, ACh, atropine and anti-MMP1 antibody did not alter HUVEC monolayer resistance (not shown). Finally, in parallel experiments, light microscopy was utilized to verify that changes in resistance were indeed associated with colon cancer cell invasion into monolayers. Collectively, these novel observations support the conclusion that ACh-induced colon cancer cell invasion is dependent on activation of both MR and MMP1.
3.2. Deoxycholyltaurine (DCT), a dihydroxy secondary bile acid, stimulates human colon cancer cell migration
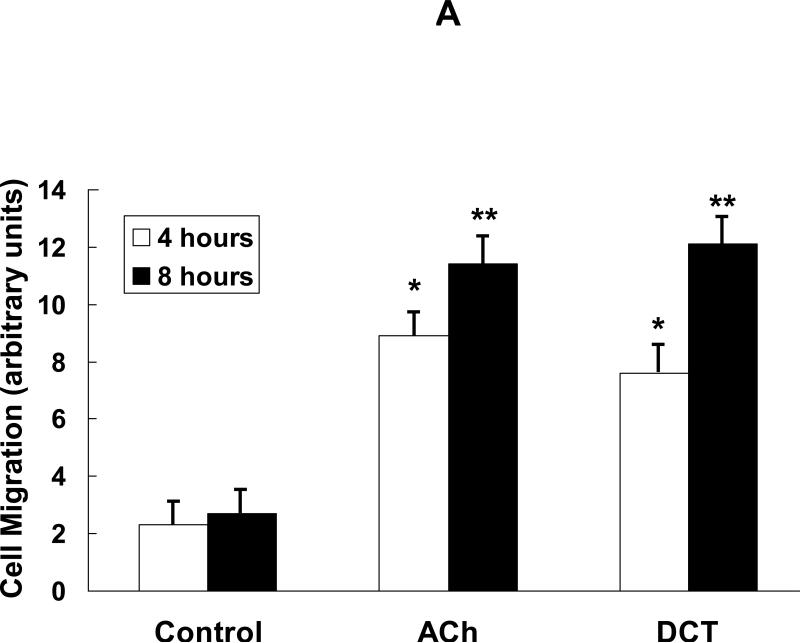
Our previous work indicates that in human colon cancer cells dihydroxy secondary bile acids associated with the development of neoplasia in animal models and humans [16,17] interact functionally with MR [7,18]. To determine whether bile acids have similar effects on human colon cancer cells as ACh, we started by investigating the actions of DCT on cell migration [12]. As shown in Fig. 2A, in a scratch-wound migration assay within 4 to 8 h after incubation with DCT we observed 4- to 6-fold increased H508 cell migration compared to control incubations with vehicle alone. The responses with DCT were of similar magnitude to those observed with the same concentration of ACh (Fig. 2A). These initial findings stimulated us to determine whether the bile acid also had similar effects as ACh on MMP1 expression and colon cancer cell invasion.
Fig. 2.
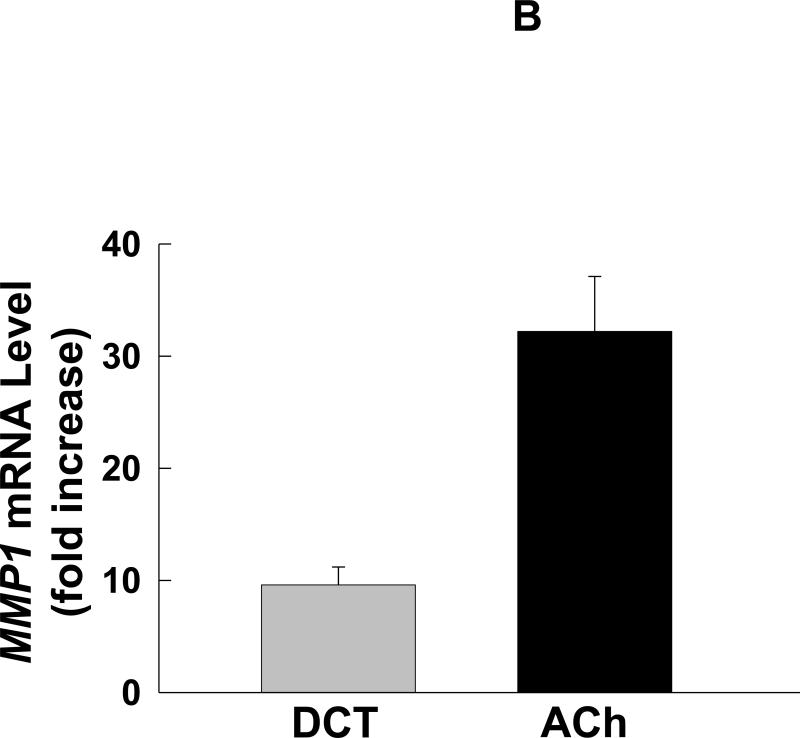
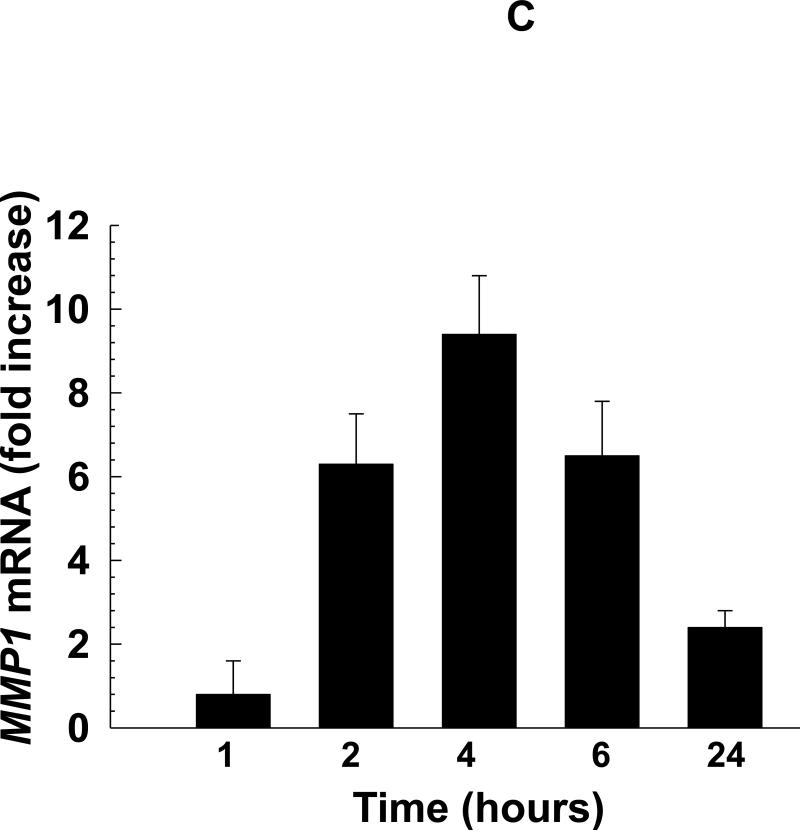
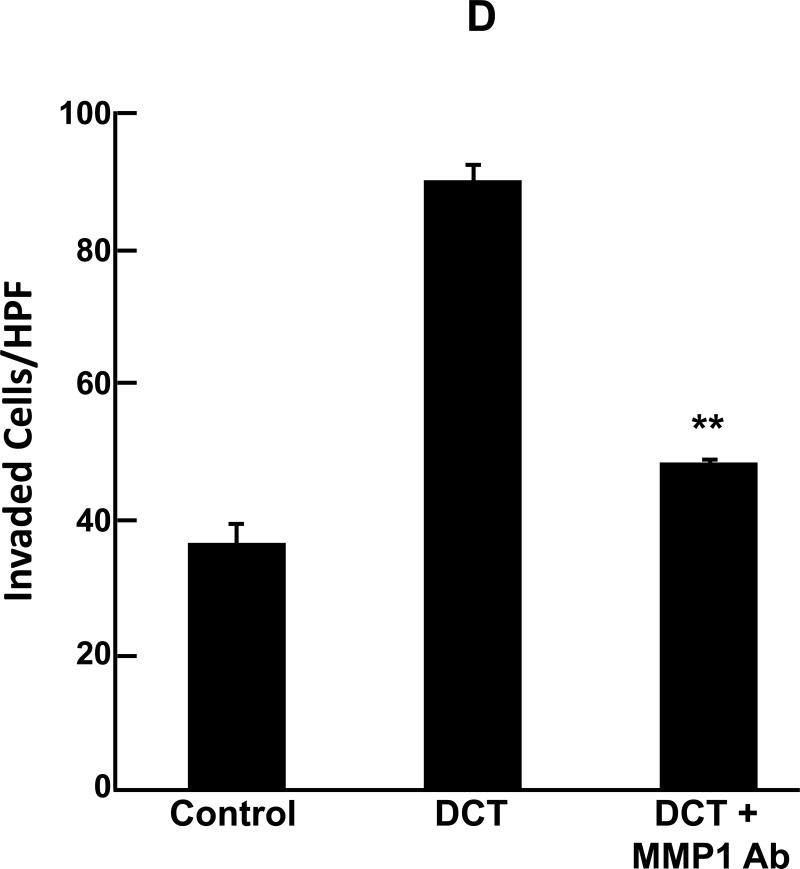
Deoxycholyltaurine (DCT), a dihydroxy bile acid that interacts functionally with muscarinic receptors, stimulates colon cancer cell migration and MMP1-dependent invasion. A: DCT (100 μM) stimulates H508 cell migration in a scratch-wound assay. Cells were plated at confluence before linear wounding. Photomicrographs were taken before adding test agents and at 4 and 8 hours after adding ACh and DCT. Values for ACh and DCT were greater than vehicle (control) at both time points (N > 3 expts; *, P < 0.05; **, P < 0.01, ANOVA with Tukey's test). B: Comparison of DCT- and ACh-induced MMP1 mRNA expression in human colon cancer cells. qPCR was performed using cDNA synthesized from total RNA prepared from H508 cells after incubation with MR agonists. MMP1 mRNA levels were measured after 4 h incubation with maximal concentrations of DCT (50 μM) and ACh (100 μM). Mean ± SE from at least 3 experiments. C: Time course for DCT-induced MMP1 expression. qPCR was performed using cDNA synthesized from total RNA prepared from H508 cells incubated for the times indicated with DCT (50 μM). Mean ± SE from at least 3 experiments. D: HT29 colon cancer cells were incubated for 24 h with DCT (100 μM), alone or in cells pre-incubated for 30 min with anti-MMP1 antibody (10 μg/ml). Cell invasion was measured using BD Biocoat Invasion Chambers with Matrigel inserts. Values represent mean ± SE (3 separate experiments). Anti-MMP1 antibody alone did not alter basal HT29 cell invasion (not shown). HPF, high power field. **, P < 0.01, Student's paired t-test.
3.3. DCT stimulates MMP1 expression in human colon cancer cells
As anticipated, in H508 cells a maximal concentration of DCT stimulated time-dependent, 10-fold increased MMP1 mRNA levels (Fig. 2B and C). The response with DCT was not of the same magnitude as the 40-fold increase in MMP1 mRNA levels observed with ACh (Fig. 2B). Nonetheless, these findings showed that the bile acid was capable of stimulating a robust increase in MMP1 expression. Thus, we proceeded to determine whether the bile acid stimulated colon cancer cell invasion and whether this was MMP1-dependent.
3.4. DCT stimulates MMP1-dependent human colon cancer cell invasion
As shown in Fig. 2D, adding the bile acid stimulated reproducible, greater than 2-fold increased HT29 cell invasion; an effect of similar magnitude to that observed with ACh (Fig. 1A). Moreover, pre-incubating colon cancer cells with 10 μg/ml anti-MMP1 antibody significantly attenuated DCT-induced HT29 cell invasion to near basal levels (Fig. 2D). These findings provide strong evidence that a bile acid that is abundant in the fecal stream and is associated with increased colon cancer risk stimulates MMP1-dependent cancer cell invasion.
4. Discussion
In this study we investigated the actions of MR agonists on the invasive properties of human colon cells. We focused these studies on ACh, a classical MR agonist previously shown to stimulate human colon cancer migration in a scratch-wound assay and to selectively increase MMP expression, including that of MMP1, a collagenase associated with progression of human colon cancer. In addition to commonly-used Matrigel chamber invasion assays, we used a more physiologically relevant ECIS assay to show that ACh stimulates invasion of both HT29 and H508 human colon cancer cells into HUVEC monolayers. As anticipated, these effects were abolished by pre-incubation with atropine, a non-selective MR inhibitor. However, the more salient observation is that pre-incubating cells with anti-MMP1 neutralizing antibody also completely blocked the actions of ACh. Similar results were obtained with DCT, an amidated dihydroxy bile acid associated with colon neoplasia in animal models and humans, and previously shown to function by muscarinic mechanisms [7,18].
This study contributes to understanding the mechanisms underlying muscarinic agonist-induced promotion of colon cancer and, more importantly, indicates that a monoclonal antibody that blocks MMP1 activation shows promise as a therapeutic agent to stop or retard colon cancer invasion and dissemination. Chemical inhibitors of MMP1 and agents that specifically block MMP1 gene expression are likely to have similar effects on colon cancer cell invasion. Translational clinical studies of these agents must first await successful studies in animals using either murine intestinal neoplasia or xenograft models. Of course, in addition to demonstrating efficacy these studies must also show that off-target effects of such agents do not cause harm.
The results of this study demonstrate that agents that block MR and MMP1 activation prevent MR agonist-induced human colon cancer cells invasion. These findings highlight key roles in colon neoplasia for MR agonist-induced MMP1 gene expression and colon cancer cell invasion. Notably, these studies indicate that although it may be challenging, developing drugs to target MMP1 gene expression and activation in colon cancer is a therapeutic strategy worthy of further investigation to prevent invasion and metastasis, the primary causes of colon cancer morbidity and mortality.
Highlights.
Muscarinic receptor agonists stimulated robust human colon cancer cell invasion.
Anti-matrix metalloproteinase1 antibody pre-treatment blocks cell invasion.
Bile acids stimulate MMP1 expression, cell migration and MMP1-dependent invasion.
Acknowledgments
This work was supported by National Institutes of Health grants CA-107345, CA-120407 (J.-P. Raufman), DK-080843 (G. Xie), DK-081479 (S. Khurana) and DK-077137, DK-089130 (N. Saxena). A. Belo was supported by T32 DK-067872 (J.-P. Raufman).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Westermarck J, Kahari VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13:781–792. [PubMed] [Google Scholar]
- 2.Zucker S, Vacirca J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev. 2004;23:101–117. doi: 10.1023/a:1025867130437. [DOI] [PubMed] [Google Scholar]
- 3.Murray GI, Duncan ME, O'Neil P, Melvin WT, Fothergill JE. Matrix metalloproteinase-1 is associated with poor prognosis in colorectal cancer. Nat Med. 1996;2:461–462. doi: 10.1038/nm0496–461. [DOI] [PubMed] [Google Scholar]
- 4.Baker EA, Bergin FG, Leaper DJ. Matrix metalloproteinases, their tissue inhibitors and colorectal cancer staging. Br J Surg. 2000;87:1215–1221. doi: 10.1046/j.1365-2168.2000.01531.x. [DOI] [PubMed] [Google Scholar]
- 5.Sunami E, Tsuno N, Osada T, Saito S, Kitayama J, Tomozawa S, Tsuruo T, Shibata Y, Muto T, Nagawa H. MMP-1 is a prognostic marker for hematogenous metastasis of colorectal cancer. Oncologist. 2000;5:108–114. doi: 10.1634/theoncologist.5-2-108. [DOI] [PubMed] [Google Scholar]
- 6.Cheng K, Zimniak P, Raufman JP. Transactivation of the epidermal growth factor receptor mediates cholinergic agonist-induced proliferation of H508 human colon cancer cells. Cancer Res. 2003;63:6744–6750. [PubMed] [Google Scholar]
- 7.Cheng K, Raufman JP. Bile acid-induced proliferation of a human colon cancer cell line is mediated by transactivation of epidermal growth factor receptors. Biochem Pharmacol. 2005;70:1035–1047. doi: 10.1016/j.bcp.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Cheng K, Samimi R, Xie G, Shant J, Drachenberg C, Wade M, Davis RJ, Nomikos G, Raufman JP. Acetylcholine release by human colon cancer cells mediates autocrine stimulation of cell proliferation. Am J Physiol Gastrointest Liver Physiol. 2008;295:G591–597. doi: 10.1152/ajpgi.00055.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shant J, Cheng K, Marasa BS, Wang JY, Raufman JP. Akt-dependent NF-kappaB activation is required for bile acids to rescue colon cancer cells from stress-induced apoptosis. Exp Cell Res. 2009;315:432–450. doi: 10.1016/j.yexcr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raufman JP, Samimi R, Shah N, Khurana S, Shant J, Drachenberg C, Xie G, Wess J, Cheng K. Genetic ablation of M3 muscarinic receptors attenuates murine colon epithelial cell proliferation and neoplasia. Cancer Res. 2008;68:3573–3578. doi: 10.1158/0008-5472.CAN-07-6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raufman JP, Shant J, Xie G, Cheng K, Gao XM, Shiu B, Shah N, Drachenberg CB, Heath J, Wess J, Khurana S. Muscarinic receptor subtype-3 gene ablation and scopolamine butylbromide treatment attenuate small intestinal neoplasia in Apcmin/+ mice. Carcinogenesis. 2011;32:1396–1402. doi: 10.1093/carcin/bgr118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belo A, Cheng K, Chahdi A, Shant J, Xie G, Khurana S, Raufman JP. Muscarinic receptor agonists stimulate human colon cancer cell migration and invasion. Am J Physiol Gastrointest Liver Physiol. 2011;300:G749–760. doi: 10.1152/ajpgi.00306.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie G, Cheng K, Shant J, Raufman JP. Acetylcholine-induced activation of M3 muscarinic receptors stimulates robust matrix metalloproteinase gene expression in human colon cancer cells. Am J Physiol Gastrointest Liver Physiol. 2009;296:G755–763. doi: 10.1152/ajpgi.90519.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Markowitz SD, Dawson DM, Willis J, Willson JK. Focus on colon cancer. Cancer Cell. 2002;1:233–236. doi: 10.1016/s1535-6108(02)00053-3. [DOI] [PubMed] [Google Scholar]
- 15.Saxena NK, Sharma D, Ding X, Lin S, Marra F, Merlin D, Anania FA. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007;67:2497–2507. doi: 10.1158/0008-5472.CAN-06-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narisawa T, Magadia NE, Weisburger JH, Wynder EL. Promoting effect of bile acids on colon carcinogenesis after intrarectal instillation of N-methyl-N'-nitro-N-nitrosoguanidine in rats. J Natl Cancer Instit. 1974;53:1093–1097. doi: 10.1093/jnci/53.4.1093. [DOI] [PubMed] [Google Scholar]
- 17.Flynn C, Montrose DC, Swank DL, Nakanishi M, Ilsley JN, Rosenberg DW. Deoxycholic acid promotes the growth of colonic aberrant crypt foci. Mol Carcinog. 2007;46:60–70. doi: 10.1002/mc.20253. [DOI] [PubMed] [Google Scholar]
- 18.Raufman JP, Cheng K, Zimniak P. Activation of muscarinic receptor signaling by bile acids: physiological and medical implications. Digestive Diseases and Sciences. 2003;48:1431–1444. doi: 10.1023/a:1024733500950. [DOI] [PubMed] [Google Scholar]