Abstract
IL-15, a promising cytokine for treating cancer and viral diseases, is presented in trans by the IL-15 receptor (IL-15R) alpha-chain to the IL-15Rβγc complex displayed on the surface of T cells and natural killer (NK) cells. We previously reported that an asparagine to aspartic acid substitution at amino acid 72 (N72D) of IL-15 provides a 4–5 fold increase in biological activity compared to the native molecule. In this report, we describe Chinese hamster ovary (CHO) cell expression of a soluble complex (IL-15N72D:IL-15RαSu/Fc) consisting of the IL-15 N72D superagonist and a dimeric IL-15Rα sushi domain-IgG1 Fc fusion protein. A simple but readily scalable affinity and ion exchange chromatography method was developed to highly purify the complex having both IL-15 binding sites fully occupied. The immunostimulatory effects of this complex were confirmed using cell proliferation assays. Treatment of mice with a single intravenous dose of IL-15N72D:IL-15RαSu/Fc resulted in a significant increase in CD8+ T cells and NK cells that was not observed following IL-15 treatment. Pharmacokinetic analysis indicated that the complex has a 25-hour half-life in mice which is considerably longer than <40-minute half-life of IL-15. Thus, the enhanced activity of the IL-15N72D:IL-15RαSu/Fc complex is likely the result of the increased binding activity of IL-15N72D to IL-15Rβγc, optimized cytokine trans-presentation by the IL-15RαSu domain, the dimeric nature of the cytokine domain and its increased in vivo half-life compared to IL-15. These findings indicate that this IL-15 superagonist complex could serve as a superior immunostimulatory therapeutic agent.
Keywords: Interleukin-15 superagonist, IL-15 receptor α fusion protein, CHO cell expression, NK cells, CD8+ T lymphocytes
1. Introduction
Interleukin-15 (IL-15) is a critical factor for the development, proliferation and activation of effector NK and CD8+ memory T cells [1]. IL-15 and IL-2 are related cytokines capable of stimulating immune cells via interactions with shared signaling receptor components, IL-2/IL-15Rβγc, and unique α chain subunits (IL-2Rα and IL-15Rα) that are required for high affinity binding to the βγc receptor complex. Based on its ability to provide durable immune responses in a subset of patients, IL-2 has been approved by the FDA for use in treatment of metastatic renal cell carcinoma and malignant melanoma. However, the broad effectiveness of IL-2 as an anti-cancer therapeutic has been questioned based on its pivotal role in the maintenance of CD4+CD25+ T-regulatory cells and in activation-induced cell death (AICD), a process that leads to the elimination of stimulated T cells and induction of T-cell tolerance, thereby limiting memory T cell responses. In contrast, IL-15 inhibits IL-2-induced AICD and supports long lasting CD8+ T cell memory and effector responses against diseased cells [2], suggesting that IL-15 may have significant advantages over IL-2 for the treatment of cancer [3]. Indeed, a recent NCI review listed IL-15 as the most promising product candidate among twelve immunotherapy drugs that could potentially cure cancer [4]. However, there are several limitations in developing IL-15-based approaches that include difficulty in producing large amounts of this cytokine in standard mammalian cell expression systems, low potency and short serum half-life. To contend with one of these shortcomings, we have identified a novel IL-15 mutant, IL-15N72D, with increased affinity to bind IL-15Rβγc and enhanced biological activity [5].
IL-15 has a novel mechanism of action in which IL-15 and IL-15Rα are coordinately expressed by antigen-presenting cells (monocytes and dendritic cells) [6], and IL-15 bound to IL-15Rα is presented in trans to neighboring NK or CD8+ T cells expressing only the IL-15Rβγc receptor [7]. Soluble IL-15Rα and IL-15 are able to form high-affinity heterodimeric complexes in solution with the N-terminal IL-15Rα fragments, containing the so-called “sushi” domain (Su), which bears most of the structural elements responsible for cytokine binding. In fact, we have found that the IL-15 and IL-15 Rα interaction domains could serve as a functional protein scaffold for generating multivalent or multispecific binding molecules [8]. Additionally, soluble IL-15:IL-15Rα complexes are fully capable of modulating immune responses via the IL-15Rβγc complex [9–11]. It has been shown in some studies that the biological activity of IL-15 could be increased 50-fold by administering preformed complexes of IL-15 and soluble IL-15Rα through a mechanism that is likely due in part to the longer half-life of the complex compared to IL-15 alone [9,11]. Recent studies in mouse tumor models have also demonstrated that the efficacy of IL-15 can be dramatically increased by pre-associating it with soluble IL-15Rα either in a single chain format or as an IL-15Rα/Fc fusion [12–14]. These responses were found to be mediated by either NK cell or T cells, including tumor-resident effector cells, depending on the tumor model [12–14].
Based on these findings, we evaluated whether it is possible to co-express the IL-15N72D superagonist and dimeric IL-15RαSu/Fc fusion protein to achieve a high level of production of an IL-15N72D:IL-15RαSu/Fc complex in recombinant CHO cells and to develop a simple approach to purify this IL-15 superagonist complex. The goal of these studies was to produce significant quantities of this complex in a biologically active form to support clinical development of this IL-15-based immunotherapy.
2. Materials and methods
2.1. Construction of vectors for protein complex expression
The IL-15RαSu/Fc fusion gene was constructed by overlap PCR amplification of DNA templates encoding the sushi domain of human IL-15Rα (aa1-66 of human IL-15Rα) and the human IgG1 Fc fragment. The signal peptide-IL-15RαSu coding region [8] and human IgG1-Fc gene fragment [15] were amplified using the primer pairs: BA494: 5’- GACTTCAAGCTTAATTAAGCCACCATGGACAGACTTACTTCTTC-3’; BA550R: 5’- GTGAGTTTTGTCACAAGATTTCGGCTCTCTAATGCATTTGAGACTGGGGGTTG-3’, and BA550F: 5’GAGCCGAAATCTTGTGACAAAACTCAC-3’; BA393R: 5’- GTAATATTCTAGACGCGTTCATTATTTACCAGGAGACAGGGAGAGGCTCTTC-3’, respectively. The resulting IL-15RαSu/Fc fusion gene was ligated into a puromycin-resistant expression vector pMSGV-1 [16] to construct the expression vector pMSGV-IL-15RαSu/Fc.
The coding sequence of IL-15N72D [5] was cloned into a modified retrovirus expression vector pMSGV-1 [16] that carries the neomycin resistance gene after an IRES region to construct the expression vector pMSGV-IL-15N72D.
2.2. Co-expression of IL-15N72D:IL-15RαSu/Fc fusion complex in CHO cells
To co-express IL-15N72D and IL-15RαSu/Fc fusion proteins (see Fig. 1), pMSGV-IL-15RαSu/Fc and pMSGV-IL-15N72D were co-transfected into CHO cells followed by selection in medium containing 2 mg/mL G418 (Hyclone, Logan, UT) and 10 µg/mL of puromycin (Hyclone, Logan, UT). The IL-15RαSu/Fc fusion protein was also expressed individually in CHO cells for use in loading of recombinant human wild-type IL-15 (IL-15wt) as a control. For production of the fusion proteins, the recombinant CHO cells were grown in serum free defined medium (SFM4CHO, Hyclone, Logan, UT) at 37°C. When the viable cell density of the cultures reached a maximum, the incubation temperature was shifted down to 30°C for accumulation of the soluble complex. Culture supernatants were then harvested when the viable cell density of the cultures reached approximately 10% viable cells.
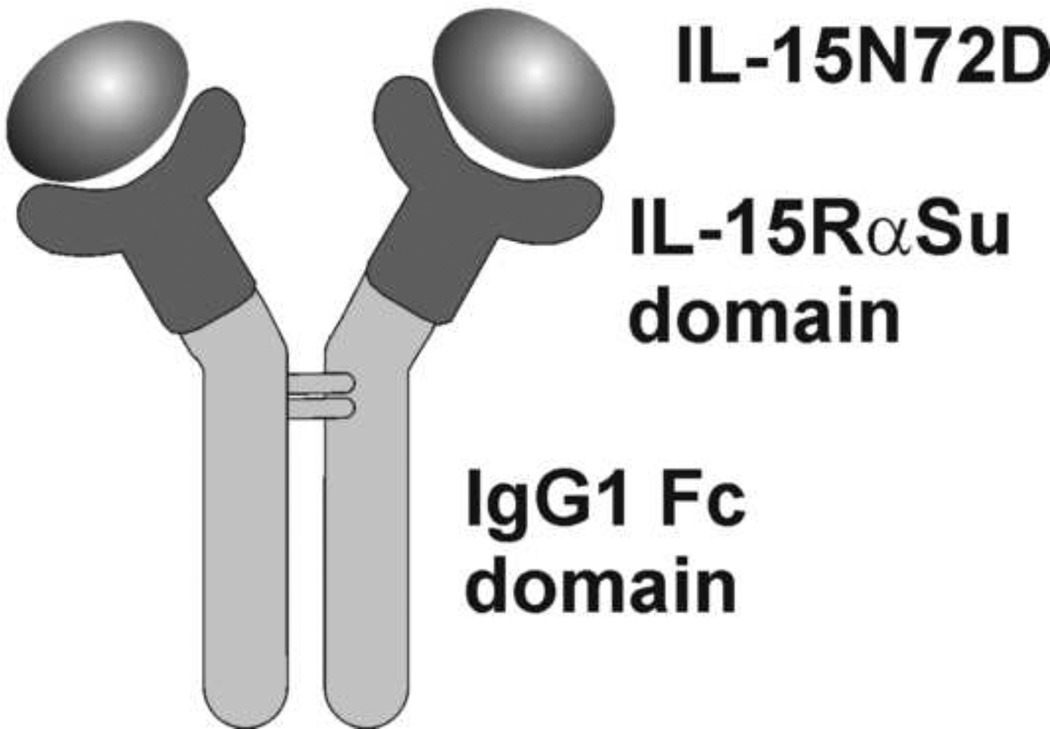
Fig. 1.
Schematic drawing of the IL-15N72D:IL-15RαSu/Fc complex consisting IL-15N72D noncovalently associated with the dimeric IL-15RαSu/Fc fusion protein.
2.3. Purification procedure
The recombinant CHO cell culture medium was centrifuged and filtered to remove cells and debris before the supernatant was adjusted to pH 8.0 with 1 M Tris-HCl, pH 8.0. The soluble IL-15N72D:IL-15RαSu/Fc fusion protein complex was purified using a two-step affinity and ion exchange chromatography-based process.
Since the IL-15N72D:IL-15RαSu/Fc complex contains the IgG1-Fc domain, an rProtein A Sepharose Fast Flow (GE Healthcare Life Sciences, Piscataway, NJ) column was used as the first step in the purification process. Prior to sample loading, the column was washed with 5 column volumes (CV) of 20 mM Tris-HCl, pH 8.0, sanitized with 5 CV of 0.1 N NaOH for 1 h, and then equilibrated with 7 CV of 20 mM Tris-HCl, pH 8.0. The supernatant was loaded onto the 11 mL column at 2 mL/min, and the column was then washed with 8 CV of 20 mM Tris-HCl, pH8.0, followed by 7 CV of washing buffer (0.1 M Na-citrate, pH 5.0) to remove non-specifically bound proteins. The protein was then eluted with 0.2 M Na-citrate, pH 4.0 and the pH of collected peak fractions was immediately adjusted to pH 3.5 using 0.2 M citric acid; the eluted protein was held at this low pH for 30 minutes as a standard viral clearance step. After the low pH hold step, the pH of the eluted preparation was adjusted to pH 7.7 by using 2 M Tris-HCl, pH 8.0. The preparation was concentrated and buffer exchanged into 20 mM Tris-HCl, pH 8.0 by using an Amicon Ultra-15 centrifugal concentrator (30 kDa cut-off, Millipore, Billerica, MA) before sterile filtration using a 0.22 µm filter (Corning Life Sciences, Lowell, MA).
The protein preparation was then applied to a Q Sepharose Fast Flow (QSFF; GE Healthcare Life Sciences, Piscataway, NJ) ion exchange column. A 5 mL column was washed with buffer A (20 mM Tris-HCl, pH 8.0), sanitized by 5 CV of 0.1 N NaOH for 1h, and then equilibrated with buffer A. The protein concentration in the preparation was first adjusted to <1 mg/mL with 20 mM Tris-HCl, pH 8.0 and was then loaded onto the QSFF column at a rate of 1 mL/min. The protein was then eluted from the column using a three-step-gradient process as follows: 20 mM Tris-HCl, pH 8.0, 130 mM NaCl for four CV as the first step, 20 mM Tris-HCl, pH 8.0, 300 mM NaCl for four CV for the second step and 20 mM Tris-HCl, pH 8.0, 1 M NaCl for two CV as the last step. Protein peak fractions were collected, buffer exchanged into PBS (Hyclone, Logan, UT), and filtered using a 0.22 µm filter. Protein concentration was determined by UV spectrophotometer at 280 nM using an extinction coefficient of 1 A280nm = 0.79 mg/mL. This extinction coefficient was calculated based on the deduced amino acid sequence of the IL-15N72D:IL-15RαSu/Fc complex.
Individually expressed IL-15RαSu/Fc was purified using rProtein A affinity chromatography as described above for assembling of complex in solution with IL-15N72D or IL-15wt produced in E. coli and refolded [5]. These in vitro assembled complexes were used as standards for biological activity evaluation and estimation of degree of occupancy of the IL-15 binding sites in co-expressed complexes.
2.4. Gel electrophoresis and size exclusion chromatography (SEC) analysis
Purified proteins were analyzed by different types of gel electrophoresis methods, which included NuPAGE 12% Bis-Tris gel (under reduced and non-reduced conditions), 4–20% Tris-glycine gel (native condition), and IEF pH3-10 gel (for pI determination). All supplies were from Invitrogen (Carlsbad, CA). Experimental methods were performed as described by the manufacturer. Superdex 200 HR 10/30 (GE Healthcare Life Sciences) chromatography with PBS (Hyclone, Logan, UT) as the running buffer was used to examine purity and to estimate molecular mass of the proteins.
2.5. N-terminal amino acid sequence and glycosylation analysis
Protein bands of interest were separated on SDS-PAGE gels, blotted onto PVDF membrane and stained by Ponceau S solution. N-terminal amino acids sequencing was performed using the Edman degradation method (Molecular Structure Facility, UC Davis, Davis, CA).
To examine whether the fusion complex was glycosylated, 50 µg of the highly purified protein after the ion exchange chromatography was digested with 2 µL of N-Glycosidase F, C. meningosepticum (Calbiochem, La Jolla, CA) in a total volume of 50 µL in PBS at room temperature for 48 h and then was subjected to electrophoresis in NuPAGE 12% Bis-Tris gel under a reduced condition.
2.6. Determination of IL-15N72D occupancy of the purified IL-15N72D:IL-15RαSu/Fc complex
Purified IL-15RαSu/Fc was loaded with IL-15wt (produced in E. coli and refolded, provided by J. Yovandich, NCI, Fredrick, MD) at various ratios for 15 h at 4°C. After incubation, the IL-15wt:IL-15RαSu/Fc complex was purified using rProtein A affinity chromatography as described above. This purified complex was evaluated using two ELISA formats, one (anti-human IgG Fc capture and anti-IL-15 detection) which detects the intact complex and the other (anti-human IgG Fc capture and anti-human IgG Fc detection) which detects only the IL-15αSu/Fc fusion protein. The ratio between the intact IL-15wt: IL-15αSu/Fc complex and IL-15RαSu/Fc protein levels reflects the occupancy rate of the IL-15 binding sites of the complex. [Occupancy rate (%) = the intact complex (ng/mL) / IL-15RαSu/Fc (ng/mL) × 100%]. Fully occupied complex (pre-associated of IL-15RαSu/Fc and IL-15wt at a 1:3 ratio) was then used as a standard to quantitate the occupancy rate of purified IL-15N72D:IL-15RαSu/Fc fusion protein complexes after purification.
2.7. Determination of IL-15 biological activity
An in vitro cell proliferation assay using the IL-15-depended 32Dβ cell line was employed to assess the IL-15 biological activities of the purified complex and IL-15wt proteins as previously described [5].
2.8. Pharmacokinetic evaluation
The pharmacokinetic profile of IL-15N72D:IL-15RαSu/Fc complex and IL-15wt were evaluated in female CD-1 mice (4 mice/time point, Harlan, Indianapolis, IN) as previously described for IL-2 [17]. Serum levels of the IL-15N72D:IL-15RαSu/Fc complex were assessed with the two ELISA formats described above. IL-15wt levels were assessed by ELISA using anti-IL-15 capture (MAB647; R&D Systems, Minneapolis, MN) and anti-IL-15 detection (BAM247; R&D Systems, Minneapolis, MN). IL-15N72D:IL-15RαSu/Fc levels from each ELISA format were fit with a one-compartment model using PK Solution 2.0 (Summit Research Services, Montrose, CO). Data from mice treated with IL-15wt were best modeled as a two-compartment model.
2.9. Lymphocyte stimulation
C57BL/6 mice (male, 6 wks of age, Harlan, Indianapolis, IN) were injected intravenously with a single dose of IL-15N72D:IL-15RαSu/Fc fusion complex at 1 mg/kg or human IL-15wt at 0.28 mg/kg (molar equivalent dose), respectively, or PBS as a negative control. Four days after treatment, pooled blood (5 mice per group) and splenocytes were collected. PBMCs were isolated from the blood using histopaque (Sigma, St. Louis, MO). The PBMC and splenocytes were then stained with PE-labeled anti-CD19, PE-labeled anti-CD335 (NKp46), FITC-labeled anti-CD4 and FITC-labeled anti-CD8 antibodies (BioLegend, San Diego, CA). The stained cells were analyzed on a FACScan flow cytometer (BD Bioscience, San Jose, CA). All animal studies were performed following Altor’s IACUC approved protocols.
3. Results and discussion
We have previously reported that an asparagine to aspartic acid substitution at amino acid 72 of human IL-15 helix C (N72D) provided a 4–5 fold increase in IL-15 biological activity compared to the native molecule in proliferation assays with cells bearing the human IL-15Rβγc complex [5]. This increased activity is due to improved interactions of the IL-15N72D mutein with the human IL-15Rβ chain [5]. We also found that the activity of IL-15N72D following in vitro association with a soluble dimeric IL-15Rα/IgG fusion protein was further enhanced to 10–25 fold compared with that of native IL-15 [5], consistent with the results shown previously with other IL-15/IL-15Rα complexes [9–13]. These findings illustrate the potential of IL-15:IL-15Rα/IgG as an IL-15 based human therapeutic. However, production of such complexes has been largely limited to in vitro preassociation of E. coli-produced IL-15 and mammalian produced IL-15Rα molecules. Active IL-15 is currently generated as an insoluble, inclusion body in recombinant E. coli and requires a tedious and inefficient process of solublization, refolding and purification [18]. For clinical development purposes, such a production method is impractical and cost prohibitive. Thus, a better production and purification method for such a protein complex is needed to support the projected demand for its clinical development.
3.1. Co-expression of IL-15N72D and IL-15RαSu/Fc fusion gene in CHO cells
Previous studies have shown that recombinant IL-15 is poorly expressed by mammalian cells [18]. However, it has been reported that intracellular complex formation with IL-15Rα prevents IL-15 degradation in the ER [6]. Hence, we postulated that IL-15 could be produced at a higher level if it is co-expressed with IL-15Rα. It is known that soluble IL-15Rα fragment, containing the so-called “sushi” domain (Su) at the N terminus, bears most of the structural elements responsible for cytokine binding. Soluble IL-15RαSu (without its transmembrane domain) and IL-15 are able to form very stable heterodimeric complexes in solution (Kd of complex = 100 pM [19]) and these complexes are capable of modulating (i.e. either stimulating or blocking) immune responses via the IL-15Rβγc complex [9–11,19]. Thus, we chose to generate a complex consisting of IL-15N72D and an IL-15RαSu/Fc fusion protein (see Fig. 1). The IL-15RαSu domain was genetically fused to the human IgG1-Fc region to facilitate its purification and dimerization via interchain disulfide bonds. To co-express IL-15N72D and the IL-15RαSu/Fc, two individual retrovirus-based expression vectors, pMSGV-IL-15RαSu/Fc and pMSGV-IL-15N72D, were constructed and co-transfected into CHO cells. The recombinant CHO cells were selected based on the neomycin and puromycin resistance elements provided by the two expression vectors, and individual producing cell lines were then generated using limited dilution cloning. We were able to identify a clone that is capable of producing approximately 100 mg/L of IL-15N72D:IL-15RαSu/Fc complex, based on ELISA, in a serum-free, defined medium. This result demonstrated that IL-15 could be expressed at high levels in mammalian cells if it is co-expressed with the IL-15RαSu domain.
3.2. Purification and characterization of the IL-15N72D:IL-15RαSu/Fc complex
When IL-15RαSu/Fc and IL-15N72D were co-expressed and assembled intracellularly in recombinant CHO cells, four different forms of proteins were expected in the cell culture supernatants: 1) dimeric IL-15RαSu/Fc molecule fully occupied with two IL-15N72D subunits, 2) dimeric IL-15RαSu/Fc molecule partially occupied with one IL-15N72D subunit, 3) a small amount of free homodimeric IL-15RαSu/Fc molecule with no IL-15 bound, and 4) free IL-15N72D. Since IL-15N72D lacks an Fc region, we first used a rProtein A-based affinity purification step to separate the free IL-15N72D from all of the Fc-bearing fusion proteins in the culture supernatant.
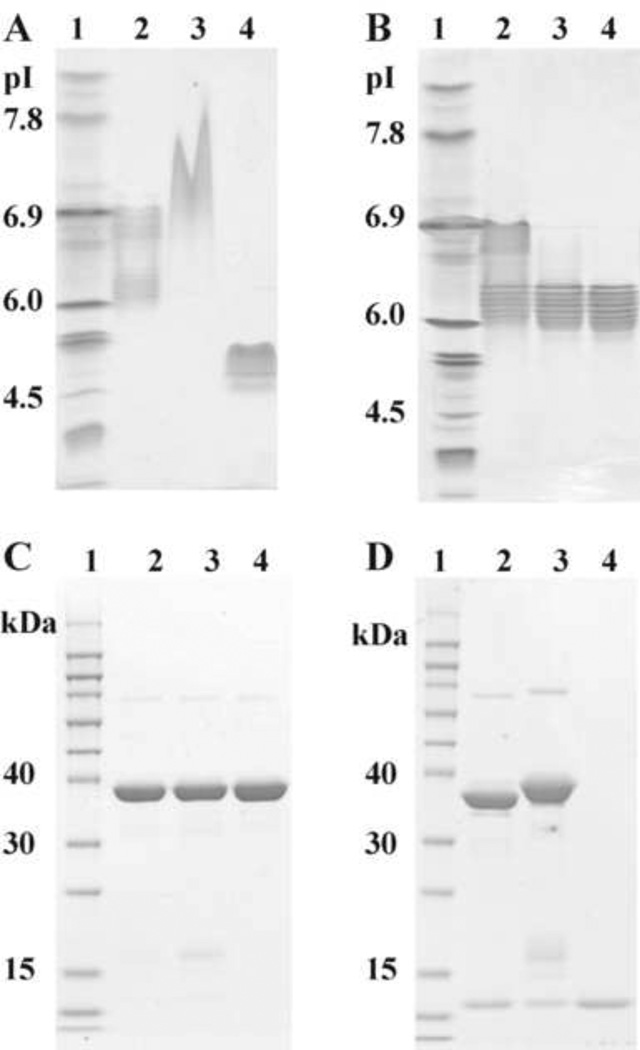
An ion exchange chromatography method was then developed to separate various forms of the IL-15RαSu/Fc complex. The calculated isoelectric point (pI) of the IL-15RαSu/Fc dimeric molecule is 8.5. As expected, this protein in 20 mM Tris-HCl, pH 8.0 solution was subsequently found to not bind to QSFF resin. Additionally, the calculated pI of IL-15N72D is 4.5. We, therefore, predicted that the overall charge of the partially occupied IL-15N72D:IL-15RαSu/Fc (i.e. dimeric IL-15RαSu/Fc + one IL-15N72D molecule) and the fully occupied IL-15N72D:IL-15RαSu/Fc (dimeric IL-15RαSu/Fc + two IL-15N72D molecules) are different. This is consistent with IEF gel analysis of the Protein-A-purified preparations, which showed two major groups of complexes with pIs between 5.8–6.3 and 6.6–6.9 corresponding with the expected pIs of the fully occupied and partially occupied complexes, respectively (Fig. 2A). The heterogeneity among pI bands of each protein group is likely due to the degree of subunit glycosylation (see below) and C-terminal lysine variants in the IgG1 chain [20]. Thus, buffers with different ionic strengths were employed to separately elute the partially occupied and fully occupied complexes from the QSFF. Using 130 mM NaCl, 20 mM Tris-HCl, pH 8.0, a single protein fraction (Q step 1) was eluted from QSSF and found to contain mainly the partially occupied complex based on ELISAs determining the fractional occupancy of the IL-15RαSu/Fc molecule (see Section 2.6). In the subsequent step using 300 mM NaCl, 20 mM Tris-HCl, pH 8.0, two protein fractions designated as Q1c and Q2C were further eluted from the QSFF. ELISA analyses performed on these preparations indicated that Q1c fraction contained a mixture of partially occupied (10% of total) and fully occupied (90%) complexes whereas Q2c fraction contained only the fully occupied complex (data not shown). These findings are consistent with IEF gel analysis of the purified protein preparations (Fig. 2B). Proteins eluted from Q step 1 have broad pIs ranging from 5.8 to 6.9; proteins of pIs 6.6 to 6.9 representing the partially occupied complex. Fraction Q1c of Q step 2 elution mainly contained protein with pIs ranging from 5.8 to 6.3 (i.e. fully occupied complex) but with small amounts of contaminant protein with pIs of 6.6 to 6.9. The Q2c fraction contained only proteins with pIs ranging from 5.8 to 6.3.
Fig. 2.
Gel electrophoresis profiles of IL-15N72D:IL-15RαSu/Fc preparations. (A) IEF pH 3–10 gel analysis. Lane 1, IEF Marker. Lane 2, IL-15N72D:IL-15RαSu/Fc complex purified by rProtein A column. Lane 3, IL-15RαSu/Fc. Lane 4, IL-15wt. (B) IEF pH3-10 gel analysis. Lane 1, IEF Marker. Lane 2, IL-15N72D:IL-15RαSu/Fc complex purified by Q step 1 elution. Lane 3, Q1c by Q step 2 elution. Lane 4, Q2c by Q step 2 elution. (C) SDS-PAGE (reduced) analysis. Lane 1, MW maker. Lane 2, IL-15N72D:IL-15RαSu/Fc complex purified by rProtein A column. Lane 3, IL-15N72D:IL-15RαSu/Fc (Q2c) by Q step 2 elution. Lane 4, IL-15RαSu/Fc (from Q flow through). (D) SDS-PAGE (reduced) analysis showing protein deglycosylation. Lane 1, MW markers. Lanes 2 and 3 show N-Glycosidase F digested and undigested IL-15N72D:IL-15RαSu/Fc protein, respectively. Lane 4, IL-15wt.
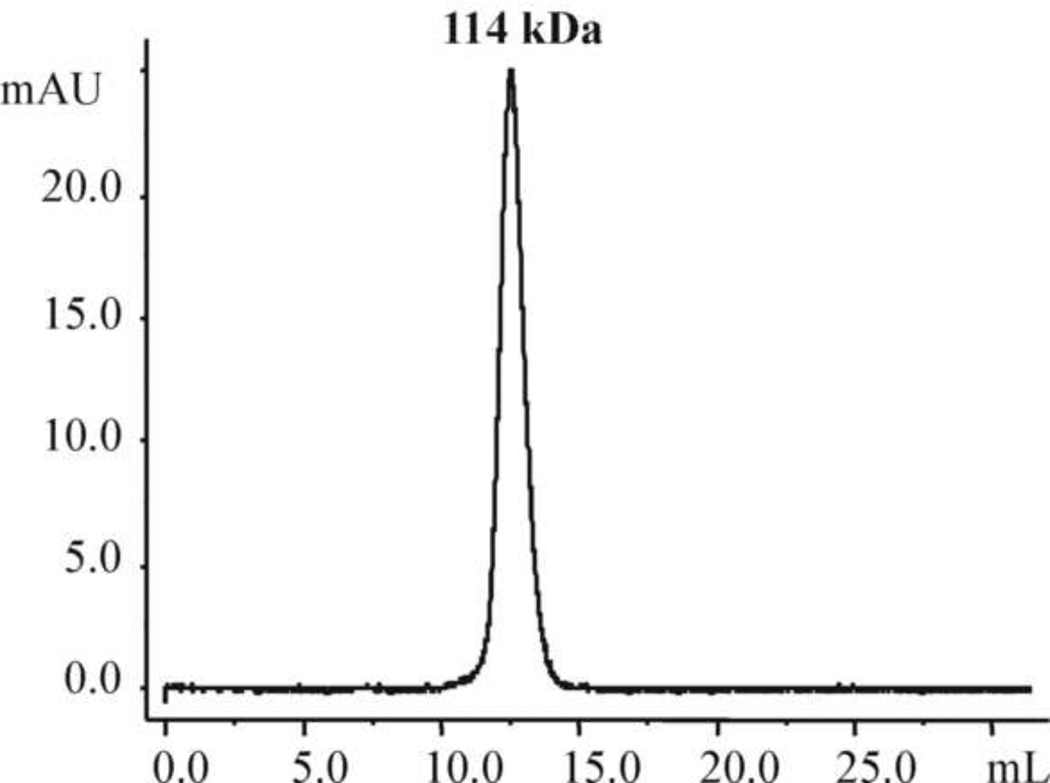
In SEC analysis, the purified IL-15N72D:IL-15RαSu/Fc Q2c preparation was found to elute as a single molecule with high purity (Fig. 3). The estimated molecular weight of the homodimer was approximately 114 kDa, which was larger than the 92 kDa molecular weight calculated based on the deduced amino acid sequence of IL-15N72D and IL-15RαSu/Fc fusion proteins. This is likely due to the glycosylation of the proteins produced by mammalian cells. This is supported by the results from our de-glycosylation studies as described below.
Fig. 3.
SEC chromatogram using Superdex 200 HR 10/30 gel filtration column. The purified IL-15N72D:IL-15Rα/Fc complex was eluted as a single peak.
In reducing SDS-PAGE (Fig. 2C), the purified IL-15N72D:IL-15RαSu/Fc preparation was found to contain three proteins with molecular weights of 40 kDa, 16 kDa and 13 kDa. However, after a digestion with N-Glycosidase F, only two proteins, with molecular weights of ~37 kDa and 13 kDa, were detected (Fig. 2D). These molecular weights closely match the calculated molecular weights of IL-15RαSu/Fc and IL-15 or IL-15N72D. This suggests that these two proteins were glycosylated during mammalian cell production and the IL-15N72D was produced in two major glycosylation forms with molecular weights of 13 kDa and 16 kDa. Based on IEF gel analysis, deglycosylation also reduced the number of the charge variants in the purified complex (data not shown). The relative abundance of these IL-15N72D species in the different purification fractions shown in Fig. 2C is consistent with levels of complex occupancy determined by ELISA and IEF gel analysis.
The IL-15N72D and IL-15RαSu/Fc were separated in reducing SDS-PAGE and the N-terminus amino acid sequences of these proteins were determined using the Edman degradation method. Approximately 15 N-terminal amino acid sequences were obtained for IL-15RαSu/Fc and IL15N72D, respectively. The determined N-terminal amino acid sequences of these proteins matched their amino acid sequences deduced from the coding regions of the two genes. The amino acid sequences for the two major bands that appeared on reduced SDS-PAGE at 13 and 16 kDa were confirmed to be IL-15N72D. This sequence confirmation again provided the evidence of glycosylation of IL-15N72D in mammalian cells.
3.3. Pharmacokinetics and in vitro and in vivo biological activities of the IL-15N72D:IL-15RαSu/Fc complex
3.4.1 Pharmacokinetic properties
It has previously reported that IL-15 and in vitro assembled IL15:IL-15Rα/Fc complex had a 1 h and 20 h serum half-life, respectively, in mice when these proteins were injected intraperitoneally [11]. To assess whether IL-15 and the co-expressed, purified IL-15:IL-15αSu/Fc complex behaved similarly when administered intravenously, their pharmacokinetic parameters were determined in CD-1 mice. Intravenous administration was chosen because this is likely the route of drug delivery to be used for the IL-15:IL-15αSu-Fc complex in humans. Female mice were injected intravenously with 1.0 mg/kg IL-15:IL-15αSu/Fc or 0.28 mg/kg IL-15 (a molar equivalent dose) and blood was collected at various time points from 15 min to 8 h for IL-15 and 30 min to 72 h for IL-15N72D:IL-15αSu/Fc post injection. Serum concentrations of IL-15N72D:IL-15αSu/Fc were evaluated using two ELISA formats, one (anti-IL-15 Ab detection) which detects the intact complex and the other (anti-human IgG Fc Ab detection) which detects only the IL-15αSu/Fc fusion protein (see Section 2.6). Concentrations of IL-15 were evaluated with a standard IL-15-specific ELISA.
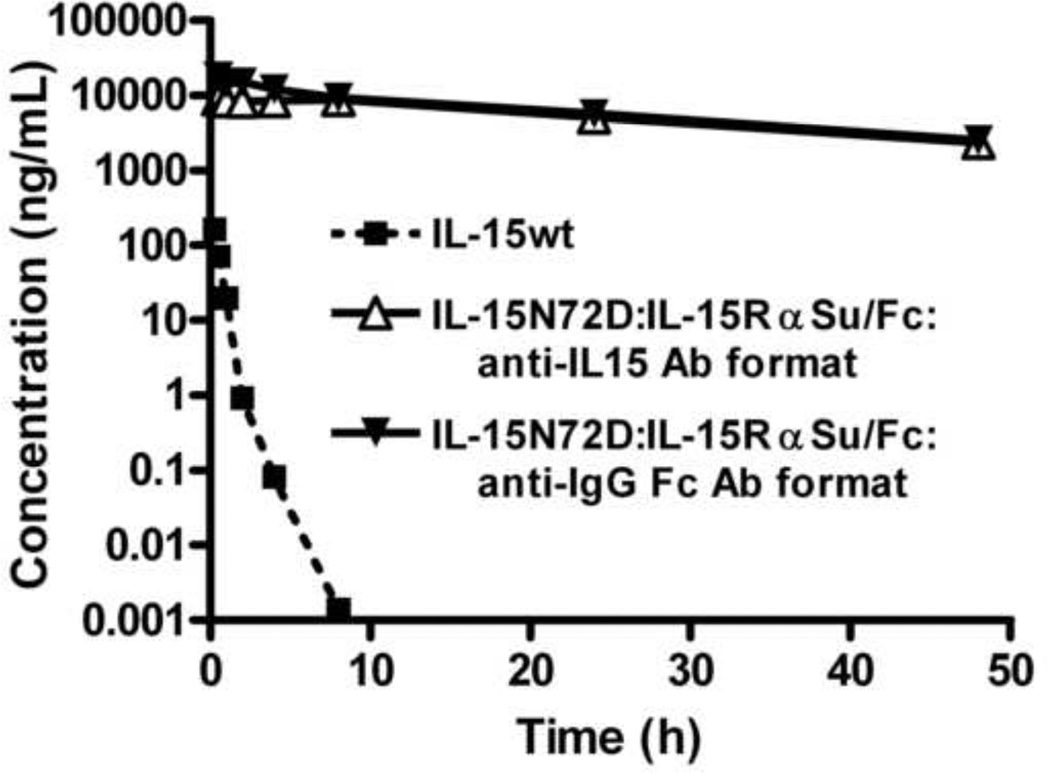
The predicted fit and actual data for IL-15:IL-15αSu/Fc and IL-15 following the single intravenous bolus injections are shown in Figure 4. The estimated half-life of IL-15:IL-15αSu/Fc using anti-IL-15 Ab-based or anti-human IgG Fc Ab-based ELISAs was about 25 or 18 h, respectively. These results indicate that the fusion protein was not cleaved and the IL-15 did not significantly disassociate from the IL-15RαSu/Fc molecule in vivo. The clearance (Cl) of IL-15:IL-15αSu/Fc ranged from 0.059 to 0.051 mL/h and the volume of distribution at steady state (Vss) ranged from 2.1 to 1.3 mL depending on the assay format. In comparison, IL-15 had an absorption half-life of 0.24 h and a terminal half-life of 0.64 h. The Cl of IL-15 was 49 mL/h, and the Vss was 18.4 mL. These results indicate that IL-15:IL-15αSu/Fc displays a >24 fold longer terminal half-life and is cleared >800 fold slower than IL-15.
Fig. 4.
Comparison of the pharmacokinetic profile of IL-15wt and IL-15N72D:IL-15RαSu/Fc complex following intravenous administration in CD-1 mice. The anti-IL-15 Ab ELISA measures the concentration of IL-15wt (■). The anti-IL-15 Ab ELISA measures the concentration of the intact IL-15N72D:IL-15RαSu/Fc molecule (△), whereas the anti-human IgG Fc Ab ELISA measures serum concentration of the IL-15RαSu/Fc fusion protein (▼). The observed concentrations are represented by symbols and the model-fitted curves are represented by lines.
3.4.2. In vitro and in vivo biological activities
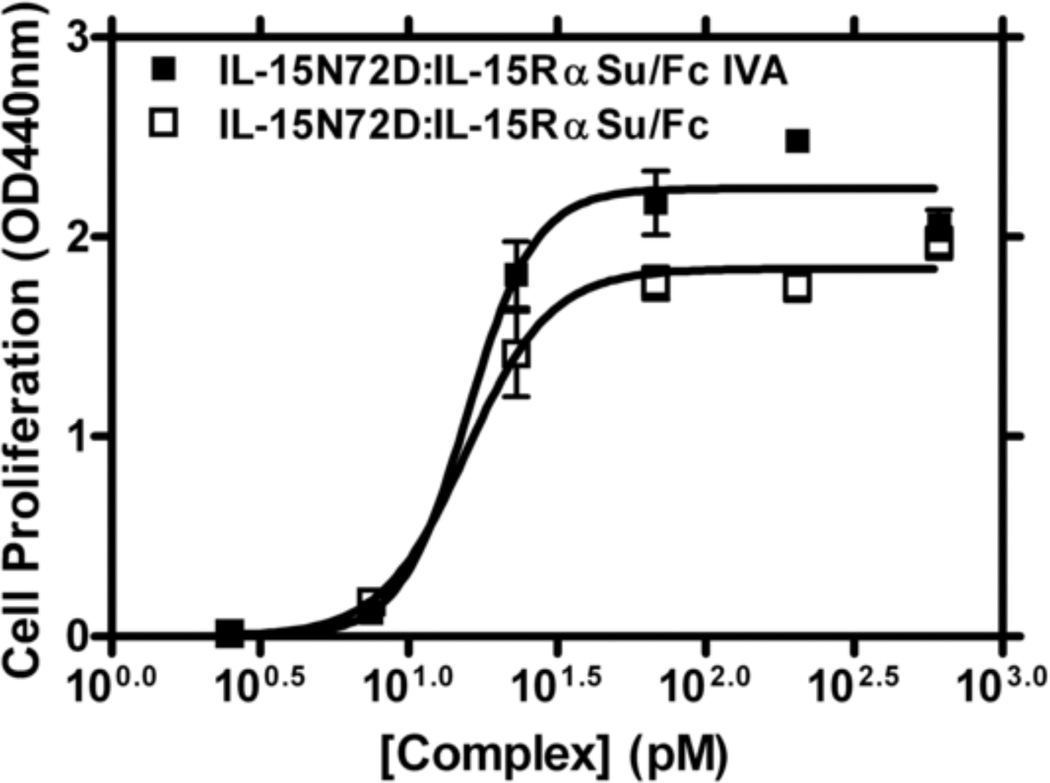
The biological activity of the co-expressed and purified IL-15N72D:IL-15RαSu/Fc complex was evaluated using an IL-15 dependent 32Dβ cell proliferation assay. For this assay we also generated an in vitro assembled (IVA) IL-15N72D:IL-15RαSu/Fc complex (IL-15N72D:IL-15RαSu/Fc IVA) by mixing IL-15N72D and IL-15RαSu/Fc at a 1:1 ratio for 30 min at 4°C. As shown in Figure 5, the IL-15N72D:IL-15RαSu/Fc complex had equivalent biological activity as IL-15N72D:IL-15RαSu/Fc IVA to support growth of 32Dβ cells. The IL-15N72D:IL-15RαSu/Fc complex exhibited an EC50 of 15.61 pM and the IL-15N72D:IL-15RαSu/Fc IVA displayed an EC50 of 15.83 pM. This demonstrates that the co-expressed IL-15N72D:IL-15RαSu/Fc complex is appropriately processed intracellularly and retains full IL-15 activity after purification. Thus, our method represents a better approach for generating cGMP-grade clinical material than current strategies employing in vitro assembly individually produced and in some cases refolded proteins.
Fig. 5.
Comparison of the biological activity of the in vitro assembled IL-15N72D:IL-15RαSu/Fc (IL-15N72D:IL-15RαSu/Fc IVA) with IL-15N72D:IL-15RαSu/Fc. 32Dβ cells were incubated with increasing concentrations of the in vitro assembled IL-15N72D:IL-15RαSu/Fc (■) or IL-15N72D:IL-15RαSu/Fc (□) for 72 h prior to addition of WST-1 for 4 h. Cell proliferation was quantitated by absorbance reading at 440 nm to assess formazan levels. The data points shown are means (± standard error) of triplicate samples and the lines represent sigmoidal dose-response curve fit for EC50 determination. The results are representative of at least three experiments.
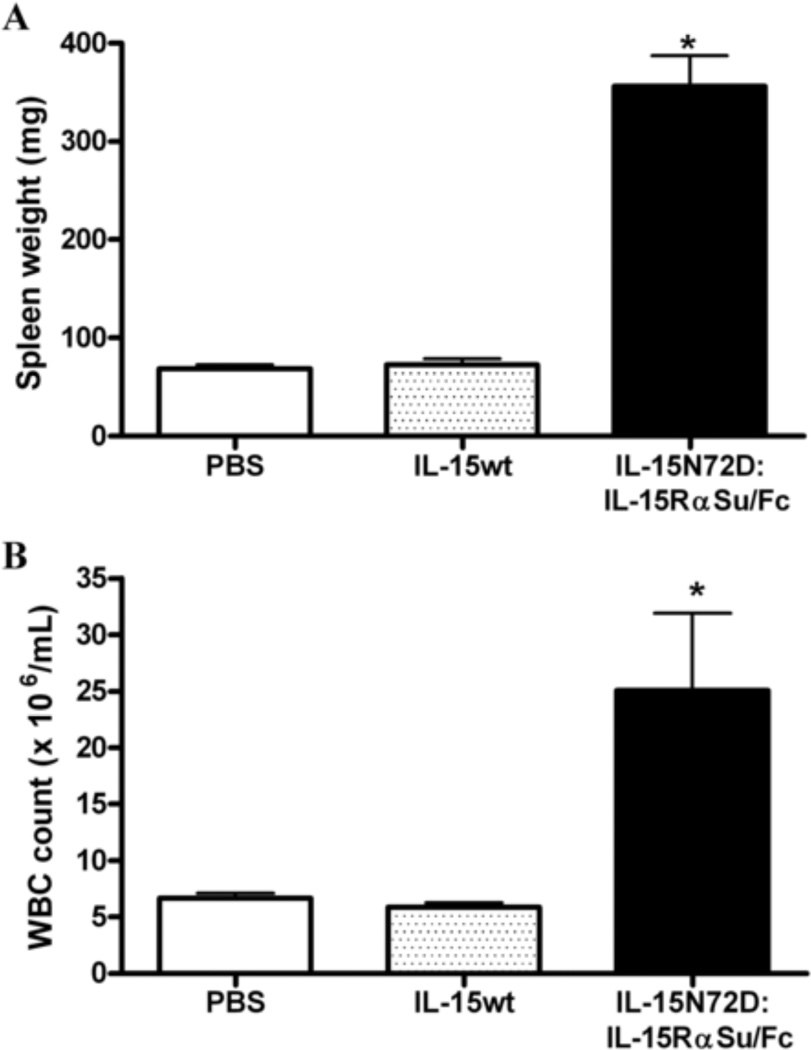
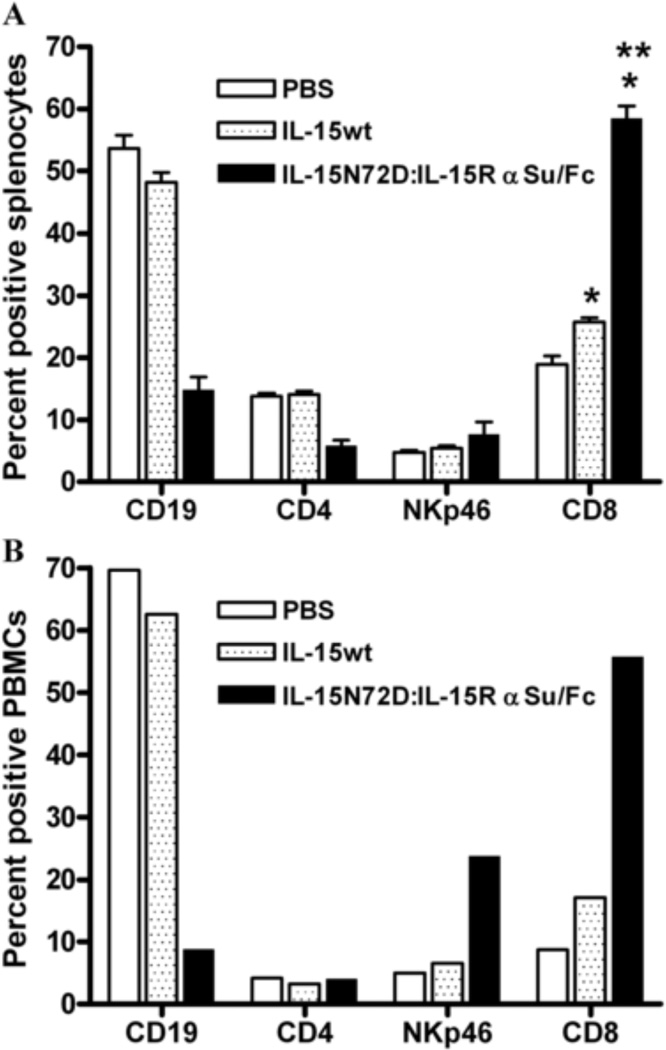
The IL-15N72D:IL-15RαSu/Fc complex and IL-15wt were also compared for their ability to induce the expansion of NK cells and CD8+ T cells in C57BL/6 mice. As shown in Figure 6, IL-15wt has no significant effect on the expansion of NK and CD8+ cells four days after a single intravenous dose of 0.28 mg/kg. In contrast, the IL-15N72D:IL-15RαSu/Fc complex significantly promoted NK and CD8+ T cell proliferation in the blood and spleen, which led to lymphocytosis in blood and splenomegaly (Figs. 6 and 7). These findings are consistent with previous reports that IL-15:IL-15Rα complexes significantly increased the biological activities of IL-15 in vivo [10–14]. This enhanced activity of the IL-15N72D:IL-15RαSu/Fc complex is likely the result of a combination of the increased binding activity of the N72D mutein to the IL-15Rβγc complex [5], optimized cytokine trans-presentation by the IL-15Rα chain in vivo (through the FcR receptors on dendritic cells and macrophage), the dimeric nature of the cytokine domain (increased avidity of binding to IL-15Rβγc) and its increased in vivo half-life compared to IL-15 (25 h vs. <40 min).
Fig. 6.
Effects of IL-15wt and IL-15N72D:IL-15RαSu/Fc complex on spleen weight and white blood cell levels. C57BL/6 mice (5 mice per group) were injected intravenously with a single dose of IL-15N72D:IL-15RαSu/Fc fusion complex at 1 mg/kg IL-15wt at 0.28 mg/kg (molar equivalent dose), or PBS as a negative control. Spleen weights (Panel A) and white blood cell counts in blood (Panel B) were determined 4 days after injection. The bars represent the mean ± standard error (n = 5). * P > 0.05 compared to PBS and IL-15wt. The results are representative of at least two experiments.
Fig. 7.
Effects of IL-15wt and IL-15N72D:IL-15RαSu/Fc complex on mouse lymphocytes. C57BL/6 mice (5 mice per group) were injected intravenously with a single dose of IL-15N72D:IL-15RαSu/Fc fusion complex at 1 mg/kg, IL-15wt at 0.28 mg/kg (molar equivalent dose), or PBS as a negative control. The percentage of B cells (CD19), CD4 T cells (CD4), NK cells (NKp46) and CD8 T cells (CD8) were determined in splenocytes (Panel A: mean ± standard error (n = 5)) and PBMCs (Panel B: levels in pooled blood (n = 5)) 4 days after injection. * P > 0.05 compared to PBS, ** P > 0.05 compared to IL-15wt. The results are representative of at least two experiments.
4. Conclusions
Taken together, we have demonstrated that the IL-15N72D and IL-15RαSu/Fc genes can be co-expressed in recombinant CHO cells and a fully occupied IL-15N72D:IL-15RαSu/Fc complex can be highly purified from cell culture supernatants using a simple and readily scalable purification method. The purified complex retains full biological activities both in vitro and in vivo. We are currently employing this expression and purification approach to produce sufficient amounts of highly purified cGMP-grade IL-15N72D:IL-15RαSu/Fc material to support clinical development of IL-15-based immunotherapy.
Highlights.
> Soluble IL-15:IL-15Rα superagonist complex was produced at high levels in CHO cells.
> The highly purified superagonist complex has long serum half-life in mice.
> This complex exhibits potent immunomodulatory activities on NK and CD8+ T cells.
Acknowledgements
We would like to thank Dr. Dean Taylor for his careful review of this manuscript. This work was supported in part by an NIH/NCI SBIR grant (CA156740, PI: H. Wong).
Abbreviations
- IL-15N72D
Human IL-15 aa 72 N to D variant
- IL-15RαSu
human IL-15 receptor alpha-chain sushi domain
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
K. Han, X. Zhu, B. Liu, E. Jeng, L. Kong, W.D. Marcus, P.A. Chavaillaz, C.A. Romero, P.R. Rhode, and H.C. Wong are employees and/or shareholders of Altor BioScience Corporation.
References
- 1.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 2.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 3.Fehniger TA, Cooper MA, Caligiuri MA. Interleukin-2 and interleukin-15: immunotherapy for cancer. Cytokine Growth Factor Rev. 2002;13:169–183. doi: 10.1016/s1359-6101(01)00021-1. [DOI] [PubMed] [Google Scholar]
- 4.Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev. 2008;222:357–368. doi: 10.1111/j.1600-065X.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhu X, Marcus WD, Xu W, Lee HI, Han K, Egan JO, Yovandich JL, Rhode PR, Wong HC. Novel human interleukin-15 agonists. J Immunol. 2009;183:3598–3607. doi: 10.4049/jimmunol.0901244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergamaschi C, Rosati M, Jalah R, Valentin A, Kulkarni V, Alicea C, Zhang GM, Patel V, Felber BK, Pavlakis GN. Intracellular interaction of interleukin-15 with its receptor alpha during production leads to mutual stabilization and increased bioactivity. J Biol Chem. 2008;283:4189–4199. doi: 10.1074/jbc.M705725200. [DOI] [PubMed] [Google Scholar]
- 7.Huntington ND, Legrand N, Alves NL, Jaron B, Weijer K, Plet A, Corcuff E, Mortier E, Jacques Y, Spits H, Di Santo JP. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J Exp Med. 2009;206:25–34. doi: 10.1084/jem.20082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong RL, Liu B, Zhu X, You L, Kong L, Han KP, Lee HI, Chavaillaz PA, Jin M, Wang Y, Rhode PR, Wong HC. Interleukin-15:interleukin-15 receptor {alpha} scaffold for creation of multivalent targeted immune molecules. Protein Eng Des Sel. 2011;24:373–383. doi: 10.1093/protein/gzq116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mortier E, Quemener A, Vusio P, Lorenzen I, Boublik Y, Grotzinger J, Plet A, Jacques Y. Soluble interleukin-15 receptor alpha (IL-15R alpha)-sushi as a selective and potent agonist of IL-15 action through IL-15R beta/gamma. Hyperagonist IL-15 x IL-15R alpha fusion proteins. J Biol Chem. 2006;281:1612–1619. doi: 10.1074/jbc.M508624200. [DOI] [PubMed] [Google Scholar]
- 10.Rubinstein MP, Kovar M, Purton JF, Cho JH, Boyman O, Surh CD, Sprent J. Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha} Proc Natl Acad Sci U S A. 2006;103:9166–9171. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoklasek TA, Schluns KS, Lefrancois L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J Immunol. 2006;177:6072–6080. doi: 10.4049/jimmunol.177.9.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubois S, Patel HJ, Zhang M, Waldmann TA, Muller JR. Preassociation of IL-15 with IL-15R alpha-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. J Immunol. 2008;180:2099–2106. doi: 10.4049/jimmunol.180.4.2099. [DOI] [PubMed] [Google Scholar]
- 13.Epardaud M, Elpek KG, Rubinstein MP, Yonekura AR, Bellemare-Pelletier A, Bronson R, Hamerman JA, Goldrath AW, Turley SJ. Interleukin-15/interleukin-15R alpha complexes promote destruction of established tumors by reviving tumor-resident CD8+ T cells. Cancer Res. 2008;68:2972–2983. doi: 10.1158/0008-5472.CAN-08-0045. [DOI] [PubMed] [Google Scholar]
- 14.Bessard A, Sole V, Bouchaud G, Quemener A, Jacques Y. High antitumor activity of RLI, an interleukin-15 (IL-15)-IL-15 receptor alpha fusion protein, in metastatic melanoma and colorectal cancer. Mol Cancer Ther. 2009;8:2736–2745. doi: 10.1158/1535-7163.MCT-09-0275. [DOI] [PubMed] [Google Scholar]
- 15.Mosquera LA, Card KF, Price-Schiavi SA, Belmont HJ, Liu B, Builes J, Zhu X, Chavaillaz PA, Lee HI, Jiao JA, Francis JL, Amirkhosravi A, Wong RL, Wong HC. In vitro and in vivo characterization of a novel antibody-like single-chain TCR human IgG1 fusion protein. J Immunol. 2005;174:4381–4388. doi: 10.4049/jimmunol.174.7.4381. [DOI] [PubMed] [Google Scholar]
- 16.Hughes MS, Yu YY, Dudley ME, Zheng Z, Robbins PF, Li Y, Wunderlich J, Hawley RG, Moayeri M, Rosenberg SA, Morgan RA. Transfer of a TCR gene derived from a patient with a marked antitumor response conveys highly active T-cell effector functions. Hum Gene Ther. 2005;16:457–472. doi: 10.1089/hum.2005.16.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belmont HJ, Price-Schiavi S, Liu B, Card KF, Lee HI, Han KP, Wen J, Tang S, Zhu X, Merrill J, Chavillaz PA, Wong JL, Rhode PR, Wong HC. Potent antitumor activity of a tumor-specific soluble TCR/IL-2 fusion protein. Clin Immunol. 2006;121:29–39. doi: 10.1016/j.clim.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Ward A, Anderson M, Craggs RI, Maltby J, Grahames C, Davies RA, Finch D, Pattison D, Oakes H, Mallinder PR. E. coli expression and purification of human and cynomolgus IL-15. Protein Expr Purif. 2009;68:42–48. doi: 10.1016/j.pep.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Bouchaud G, Garrigue-Antar L, Sole V, Quemener A, Boublik Y, Mortier E, Perdreau H, Jacques Y, Plet A. The exon-3-encoded domain of IL-15ralpha contributes to IL-15 high-affinity binding and is crucial for the IL-15 antagonistic effect of soluble IL- 15Ralpha. J Mol Biol. 2008;382:1–12. doi: 10.1016/j.jmb.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 20.Liu H, Gaza-Bulseco G, Faldu D, Chumsae C, Sun J. Heterogeneity of monoclonal antibodies. J Pharm Sci. 2008;97:2426–2447. doi: 10.1002/jps.21180. [DOI] [PubMed] [Google Scholar]