Abstract
Recent research suggests a central role for inflammatory mechanisms in cognitive decline that may occur prior to evidence of neurodegeneration. Limited information exists, however, regarding the relationship between low-grade inflammation and cognitive function in healthy older adults. This study examined the relation between inflammation, verbal memory consolidation, and medial temporal lobe volumes in a cohort of older community-dwelling subjects. Subjects included 141 functionally intact, community dwelling older adults with detectable (n = 76) and undetectable (n= 65) levels of C-reactive protein. A verbal episodic memory measure was administered to all subjects, and measures of delayed recall and recognition memory were assessed. A semiautomated parcellation program was used to analyze structural MRI scans. On the episodic memory task, analysis of covariance revealed a significant CRP group by memory recall interaction, such that participants with detectable levels of CRP evidenced worse performance after a delay compared to those with undetectable levels of CRP. Individuals with detectable CRP also demonstrated lower performance on a measure of recognition memory. Imaging data demonstrated smaller left medial temporal lobe volumes in the detectable CRP group as compared with the undetectable CRP group. These findings underscore a potential role for inflammation in cognitive aging as a modifiable risk factor.
Keywords: inflammation, memory, aging, cognition, temporal lobe, c-reactive protein, cytokines
The systemic restructuring of the immune system may contribute to many age-associated disorders, including cognitive dysfunction and neurodegeneration (Glass et al., 2010, Ownby, 2010). Although inflammatory processes represent a normal response to pathogen invasion that are critical to maintaining homeostasis, sustained neuroinflammation has deleterious effects on neurological functioning that can disrupt cognition and influence brain structure. Recent research on inflammation and Alzheimer’s disease suggests a central role for inflammatory mechanisms in cognitive decline (Akiyama, 1994, Schmidt et al., 2002, Weaver et al., 2002, Holmes et al., 2003, Yaffe et al., 2004, Dik et al., 2005, Dimopoulos et al., 2006, Alley et al., 2008, Bermejo et al., 2008, Locascio et al., 2008, Forlenza et al., 2009) that may predate evidence of neurodegeneration (Schuitemaker et al., 2009); however, a paucity of information exists regarding the relationship between low-grade inflammation, cognitive functioning, and brain structure in healthy older adults (Ravaglia et al., 2005, Wersching et al., 2010, Simen et al., 2011).
More specifically, inflammation has been implicated in memory performance in older adults (Gunstad et al., 2006, Noble et al., 2010) and in animal models (Semmler et al., 2005, Hein et al., 2010). Chronic inflammation impairs hippocampal functioning by disrupting long-term potentiation and memory formation (Murray and Lynch, 1998, Semmler et al., 2005, Lin et al., 2009, Terrando et al., 2010), suggesting that protracted inflammatory processes may serve as a contributing factor to episodic memory dysfunction. Additionally, recent studies indicate that non-demented, older adults with higher levels of systemic inflammatory markers perform worse on verbal memory tests at baseline (Teunissen et al., 2003, Noble et al., 2010) and are at risk for future decline (Yaffe et al., 2004, Komulainen et al., 2007); however, the relationship between inflammation and memory is not consistent, as additional cross-sectional (Wersching et al., 2010) and longitudinal studies (Hoth et al., 2008) have failed to find an appreciable connection between inflammation and memory function. The variability in findings may stem from methodological reliance on composite scores, which may mask important nuances in encoding and consolidation processes, particularly in healthy older adult populations who do not evidence gross memory impairments. Furthermore, the relative lack of neuroimaging data renders it difficult to interpret prior evaluations of the relationship (or lack thereof) between verbal memory and laboratory indices of inflammation (Noble et al., 2010), as discrepancies in results may be due to changes in cognitive function that are not dependent on the medial temporal lobes. Clearly, the association between inflammation and cognitive functioning remains relatively understudied, and little is known about the mechanisms by which inflammation and age interact to affect memory functioning.
The purpose of the current study was to examine the relationship between inflammation, verbal memory, and medial temporal lobe volumes in a cohort of older adults. We hypothesized that individuals with detectable blood levels of C-reactive protein (CRP) would have poorer memory consolidation and smaller left medial-temporal lobes (MTL) than those with undetectable levels of CRP. We selected the left medial temporal lobe as an anatomic region of interest, as this area is known to mediate verbal memory consolidation (Schacter and Wagner, 1999, Squire et al., 2004). Considering the animal literature linking inflammation to perturbation in memory consolidation (Semmler et al., 2005, Terrando et al., 2010), we hypothesized that individuals with detectable levels of CRP would demonstrate worse performance on delayed free recall, even after accounting for their overall verbal learning. We also chose to evaluate an additional facet of episodic memory that has been linked with the ability to encode and consolidate verbal information, specifically recognition memory (Libon et al., 1998, Price et al., 2009).
Methods
Subjects
A sample of 141 neurologically healthy older adult participants was selected from the University of California, San Francisco Memory and Aging Center database based on the availability of laboratory blood work assessment (i.e. fasted blood draw); in addition, a subset of 133 individuals received a 3T structural MR scan. All 3 testing sessions occurred within a 90 day period. Participants were recruited from a larger umbrella study on healthy aging and cognition (i.e. Myelin and Aging study, Larry J. Hillblom foundation), and were between the ages 65 and 90 years. Participants were reviewed in a screening visit, which entailed an informant interview, neurological examination, and cognitive testing. Inclusion as a “neurologically healthy” participant was based on several criteria, including a Mini-Mental State Exam score of > 25, Clinical Dementia Rating score of 0, and no subject or informant report of significant cognitive decline during the previous year. Participants were excluded if they had a major psychiatric disorder, neurological conditions affecting cognition (e.g., Parkinson’s disease, epilepsy; large vessel infarct), dementia or mild cognitive impairment, substance abuse, significant systemic medical illnesses (e.g., cancer; renal failure), current medications likely to affect CNS functions (e.g., long-active benzodiazepines), significant sensory or motor deficits that would interfere with cognitive testing, current depression (Geriatric Depression Scale Score greater than 15 of 30)(Yesavage et al., 1982), or insulin-dependent diabetes. All available information regarding medical history and medication use was garnered via participant self-report. Demographic, health/lifestyle, and cognitive variables are reported in Table 1. The study was approved by the UCSF committee on human research, and all subjects provided written, IRB-approved informed consent before participating.
Table 1.
Demographics and Unadjusted Verbal Memory Performance by C-Reactive Protein Group
| Undetectable CRP n = 65 |
Detectable CRP n = 76 |
|
|---|---|---|
| Age (years) | 70.8 (5.3) | 71.8 (6.3) |
| Education (years) | 17.6 (2.1) | 17.4 (2.3) |
| Female Sex (%) | 53.8 | 59.2 |
| History of Hypertension (%) | 26.2 | 39.5 |
| Current Smoker (%)a | 6.2 | 0 |
| Ever Smoker (%) | 56.9 | 49.3 |
| Body Mass Indexb | 23.9 (3.2) | 25.9 (4.3) |
| Non-Aspirin NSAID Use (%) | 20.0 | 15.8 |
| Aspirin Use (%) | 50.8 | 46.1 |
| Statin Use (%) | 30.8 | 35.5 |
|
| ||
| MMSE | 29.2 (1.0) | 29.39 (.7) |
| Geriatric Depression Scale Total | 2.9 (2.8) | 2.7 (3.2) |
| CVLT-Trial 5 Correct | 13.2 (2.2) | 12.9 (2.5) |
| CVLT-Short Delay Recallc | 12.0 (3.1) | 10.7 (3.3) |
| CVLT-Recognition D′d | 3.4 (0.6) | 3.2 (0.7) |
Significant group difference (p < .05)
Significant group difference (p < .01)
Significant group by trial (learning vs short delay recall) interaction after controlling for covariates, p = .01
Significant group difference after controlling for covariates, p = .05
All values represent Mean (Standard Deviation). Dichotomous variables represent percents.
CVLT= California Verbal Learning Test-II
D′ = Recognition Discriminability
Memory Assessment
Participants were administered the California Verbal Learning Test, 2nd edition (CVLT-II), a widely used and well normed measure of episodic memory (Delis et al., 2000). Subjects were read a list of 16 words (List A) presented over five learning trials, and instructed to recall as many words as they could for each trial. They were then presented with an interference list (List B) composed of 16 new words. In order to assess a widely used index of episodic memory on the CVLT-II, we focused our primary analysis on the delayed recollection of List A words (i.e. Short Delay Free Recall), as this provides a relative pure measure of consolidation without categorical cueing. Considering that we were also interested in supplemental measures that have been associated with medial temporal lobe functioning, we included the recognition memory trial as a secondary dependent variable.
The recognition memory trial consists of the 16 target items and 32 distracters presented in a randomly ordered array; subjects were to respond “yes” if the item was from the target list and “no” if it was not. Level of performance on a yes–no recognition memory test is reflected in the number of correct hits and false-positive errors. These data yield a measure of recognition memory that has been adapted from signal detection theory: recognition discriminability. Discriminability refers to the ability to distinguish target words from distracter words, and it is widely considered to be the best measure of recognition memory accuracy. The recognition discriminability index, or d′, is analogous to a contrast z score reflecting the absolute difference in standard deviation units between the subjects hit rate and false-positive rate.
Laboratory Biomarker of Inflammation Data and Analysis
Blood was collected into serum separator tubes and left to clot at room temperature for 30–60 min. The blood was then centrifuged at 2500 rpm (1300–1800 × g) at room temperature for 15 minutes. Serum was stored at −80 deg C until analysis. C-reactive protein (CRP), an acute-phase protein and marker of system low-grade inflammation, was measured by immunoturbidimetric assay (University of California, Davis Medical Center Clinical Laboratory, Davis, CA). This was not a high-sensitivity assay; thus, the lower detection limit was 0.1 mg/dL. Considering that a large number of our healthy adults did not evidence detectable levels of inflammatory markers, we divided the participants into two groups: detectable CRP and undetectable CRP (Kravitz et al., 2009). Of our 141 participants, 65 individuals were classified in the undetectable CRP group, and 76 were in the detectable CRP group (mean = 0.34; range = 0.1–2.4 mg/dL). Notably, elevations in CRP were under 3.0 mg/dL, which has been used as a benchmark for acute inflammation (as noted in Figure 1 of Wersching et al, 2010). Although participants were not extensively screened for acute inflammation, the range of CRP values suggests that acute inflammation or illness is unlikely. Furthermore, no significant between-group differences were noted in demographic variables, depression scores, or MMSE (p > .05 for all), as shown in Table 1.
Neuroimaging Data and Analysis
MRI scans were obtained on a 3.0 Tesla Siemens (Siemens, Iselin, NJ) TIM Trio scanner equipped with a 12-channel head coil located at the UCSF Neuroscience Imaging Center. Whole brain images were acquired using volumetric magnetization prepared rapid gradient-echo sequence (MPRAGE; TR/TE/TI = 2300/2.98/900 ms, α = 9°). The field of view was 240 × 256 mm, with 1 × 1 mm in-plane resolution and 1 mm slice thickness.
The T1 MPRAGE structural MR images were analyzed using Freesurfer, which is documented and freely available for download online at:http://surfer.nmr.mgh.harvard.edu/. Previous publications have provided detailed descriptions and validation of the software (Dale et al., 1999, Fischl et al., 2001, Segonne et al., 2004). Freesurfer is a surface-based structural MRI analysis tool that segments white matter and tessellates both gray and white matter surfaces. The procedure, in brief, involves the removal of non-brain tissue using a hybrid watershed/surface deformation procedure (Segonne et al., 2004) and intensity normalization (Sled et al., 1998), followed by automated Talairach transformation and volumetric segmentation of cortical and subcortical gray and white matter, subcortical limbic structures, basal ganglia and ventricles (Fischl et al., 2002, Fischl et al., 2004). Estimated total intracranial volume (ICV) is calculated via an atlas normalization procedure. The surfacing algorithm uses intensity and continuity data, and corrects topological defects to generate a continuous cortical ribbon used to calculate gray matter volume and thickness (Fischl and Dale, 2000, Fischl et al., 2001, Segonne et al., 2007), a procedure validated against histological analysis (Rosas et al., 2002) and manual measurements (Kuperberg et al., 2003). This cortical surface is then inflated and registered to a spherical atlas and parcellated into regions of interest (ROI) based on gyral and sulcal structure (Fischl et al., 1999, Desikan et al., 2006). Region of interest was the left medial temporal lobe (MTL), which was defined as the left hippocampus, parahippocampal gyrus, and entorhinal cortex. In order to assess whether our results were primarily related to medial temporal lobe volume, we also examined the left temporal neocortex (i.e. left banks of the superior temporal sulcus, inferior temporal gyrus, middle temporal gyrus, and superior temporal gyrus grey matter volume), as well as frontal and parietal cortical regions that have been shown to associate with episodic memory performance, specifically the left middle frontal (i.e. left caudal and rostral middle frontal gyri grey matter volume), left lateral frontal (i.e. left pars opercularis, pars orbitalis, and pars triangularis gyri grey matter volume) and left parietal neocortex (left inferior parietal, superior parietal, and supramarginal grey matter volume) (Kramer et al., 2005).
Statistical Analysis
To evaluate group differences in episodic memory performance, a repeated measures analyses of covariance was conducted for delayed recall and univariate analysis of covariance for recognition d′, with alpha set a p = .05. For delayed recall, Trial 5 was included as a repeated measure (i.e. Trial 5 vs. Short Delay Free Recall) in order to account for differences in verbal learning. To assess the relationship between inflammation and structural imaging variables (including our region of interest: medial temporal lobe volume), separate univariate analyses of covariance were conducted, controlling for intracranial volume (ICV; i.e. head size). In terms of general study covariates, several demographic and health/lifestyle variables have been shown to affect the relationship between inflammation and cognitive functioning, including gender and cardiovascular risk factors (Ravaglia et al., 2005, Wersching et al., 2010, Simen et al., 2011). Thus, the decision to control for specific confounds was based on two factors. Covariates were included in analyses if one of the following two criteria were met: a) significant between-group (Detectable vs. Undetectable CRP) differences or b) significant correlations with primary dependent variables were found. Significance was leniently defined as p ≤ .1 to account for all possible confounds. Based on this criteria, between group differences were found for current tobacco use (p = .04), Body Mass Index (BMI; p = .01), and history of hypertension (p = .1); correlations with primary dependent variables were found for age (CVLT recall: r = −..36, p < .01; medial temporal lobe: age, r = −.30, p < .01), gender (CVLT recall: r = .26, p < .01; medial temporal lobe: r = −.20, p < .05), and MMSE (CVLT recall: r = .14, p = .10). Thus, the following covariates were used for all analyses: age, gender, MMSE, BMI, current tobacco use, and history of hypertension.
Additional demographic and health variables are reported in Table 1, including use of non-aspirin non-steroidal anti-inflammatory drugs (NSAID)1, aspirin, and statins, but were not related to outcome variables and did not significantly differ between groups (p’s > .1); as such, these variables were not controlled for in analyses. In addition, several lifestyle and health covariates were available only for a subsection of the sample (137 out of 141 participants for memory measures; 129 out of 133 participants for imaging analyses); as such, analyses are presented separately for variables available for all participants (age, gender, MMSE) vs. the subsection of participants (BMI, current tobacco use, history of hypertension). Effect sizes for all analyses were estimated using Cohen’s d calculations (Cohen, 1988).
Results
Primary Indices of Episodic Memory: Delayed Recall
After controlling for significant demographic covariates, there was a significant Group x Recall trial interaction (F (1, 136)=5.42, p =.02; d= .56), such that participants with detectable levels of CRP evidenced worse performance after a delay (Short Delay Free Recall Mean=10.7, SD=3.3) compared to those with undetectable levels of CRP (Mean=12.0, SD=3.1; See Figure 1). After controlling for all significant covariates, the Group × Recall trial interaction remained (F (1, 129)= 6.15, p = .01; d =.63).
Figure 1.
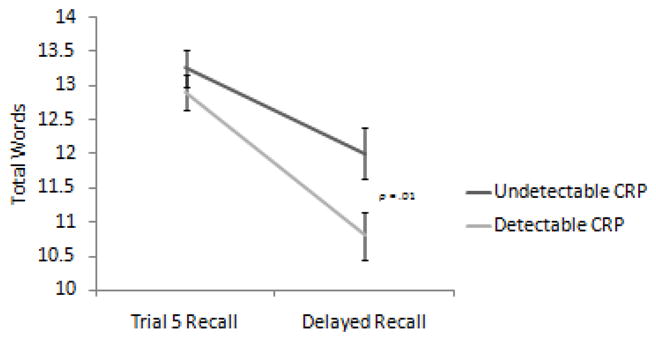
Significant interaction between learning and delayed recall by CRP group (detectable vs. undetectable). Error bars represent standard error of the mean, and values are based on estimated marginal means.
Supplementary Indices of Episodic Memory: Recognition Memory
After controlling for significant demographic covariates, participants’ performance on a recognition trial revealed a significant disparity in their ability to discriminate between target words and foils (recognition d′: F (1,136) = 4.11, p = .05; d=.35); specifically, individuals with detectable levels of CRP performed worse (recognition d′ = 3.2, SD =.7) on this measure than those with undetectable levels (recognition d′ = 3.4, SD = .6), suggesting poorer verbal recognition memory. The noted group differences could not be accounted for by response bias (p = .54), and remained even when controlling for all significant covariates (p = .04; d =.38) and total learning on Trial 5 (p = .05; d=.36).
Inflammation and Neuroimaging Index of Episodic Memory
Univariate analysis of covariance revealed that participants with detectable CRP had smaller left medial temporal lobes (7.71 cc, SD = 0.7) than individuals with undetectable levels (8.02 cc, SD = 0.8; F(1, 127) = 4.48, p = .04; d = .38), but no group differences in left temporal neocortex (32.05 cc vs 32.54 cc, p = .29; d=.20), left middle frontal (20.22 cc vs. 20.21 cc, p = .60; d=.10), left lateral frontal (9.71 cc vs 9.66 cc, p = .81; d=.05), or left parietal neocortex volume (45.20 cc vs. 45.80 cc, p = .34; d=.15) were noted after controlling for significant demographic covariates. Group differences in medial temporal lobe volume remained after controlling for all significant covariates (F (1, 120)= 4.87, p = .03; d =.42), with no changes in results for left temporal neocortex, middle frontal gyrus, lateral gyrus, or parietal volume.
Discussion
Our results indicate that functionally intact, neurologically healthy older adults with detectable levels of the circulating inflammatory marker, CRP, evidenced poorer verbal memory consolidation and smaller left medial temporal lobes. Within the context of verbal episodic memory functioning, detectable levels of CRP were related to lower performance on a delayed recall task and a diminished ability to discriminate between target words and foils on a recognition memory trial. Additionally, detectable levels of CRP were associated with smaller left medial temporal lobes, a neuroanatomic area known to buttress verbal episodic memory consolidation. These group differences in neuroimaging findings could not be better accounted for by left temporal neocortex, left lateral or middle frontal grey matter volumes, or left parietal neocortical volumes. Overall, these findings support our hypotheses by associating inflammation with alterations in episodic memory function and brain structure.
The literature relating inflammatory biomarkers to cognitive function has primarily emphasized the downstream effects of neurodegenerative disease processes on postmortem evidence of inflammation (e.g. activated microglia). Early postmortem evaluations of Alzheimer’s disease patients identified increased inflammatory markers, including CRP, in the brain (McGeer and McGeer, 1995), as well as immunoreactivity of CRP in neurofibrillary tangles (Duong et al., 1997), suggesting an inflammatory state. Despite evidence that increased markers of inflammation predict later cognitive decline (Weaver et al., 2002, Yaffe et al., 2004, Dik et al., 2005, Alley et al., 2008), there is a dearth of research examining the relationship between inflammation and cognitive functioning in neurologically healthy older adults. In particular, evidence linking laboratory markers of inflammation and episodic memory have been contradictory (Dik et al., 2005, Wersching et al., 2010), signifying a possibly tenuous relationship between the two.
By contrast, the results of this study point to an association between episodic memory consolidation and recognition and a laboratory marker of inflammation. One explanation for the incongruous findings in the past is that characterization of memory in large samples is often cursory, with either one unitary score or several different measures collapsed to reflect the multi-faceted domain of verbal episodic memory. Given that neurologically healthy older adults generally perform well on commonly used composite indices of verbal memory, the more comprehensive appraisal utilized in the current work may have unearthed subtle changes in cognitive functioning not typically assessed in epidemiological studies.
Pursuant to the discussion of episodic memory, verbal recall is a heterogeneous construct consisting of several cognitive functions, including executive control, attention, and processing speed, as well as what we traditionally define as ‘memory’. As such, an important consideration is whether the findings of the current study reflect a consolidation (i.e. hippocampal dependent) vs. retrieval (i.e. dorsolateral frontal dependent) memory profile in the detectable CRP group. Memory profiles typified by consolidation difficulties are associated with poor free recall and minimal benefit from a recognition trial (Delis et al., 1991, Kramer et al., 2005, Price et al., 2009); in contrast, memory profiles typified by retrieval difficulties are associated with relatively better verbal recall and notable benefit from a recognition trial (Kramer et al., 1988, Lamar et al., 2010). Our results provide preliminary support for a consolidation profile in the detectable CRP group based on the cognitive data ascertained, as individuals with detectable levels of CRP demonstrated a greater decline in performance on delayed recall (relative to learning trials) and worse performance on a recognition memory trial, even after controlling for significant demographic, medication, and cardiovascular variables. A consolidation profile is further supported by the significant differences observed in brain structure, as individuals with detectable levels of CRP displayed smaller left medial temporal lobe volumes, with no concomitant differences in left temporal neocortex, parietal, or frontal lobe volumes. Of note, the participants in the current study were healthy older adults, with no evidence of clinically significant memory difficulties. Thus, this discussion is not to suggest that the participants evidenced impairments in this domain. However, considering that mechanistically, inflammation is purported to affect episodic memory by disrupting long-term potentiation in the medial temporal lobe, the current results provide preliminary support for an association between inflammation and a consolidation memory profile.
In evaluating the findings associating inflammation and memory function, it is important to highlight several salient considerations and limitations. First, the relationship between peripheral laboratory markers of inflammation and cognition is complex, and is likely moderated by lifestyle variables, vascular risk factors, and psychological functioning (e.g. stress, depression) (Black, 2002, Yaffe et al., 2004). Although we were able to incorporate and control for several participant-reported indices of lifestyle and cardiovascular risk, unearthing a linear, causal relationship between inflammation and memory function is complicated by our limited understanding of how these factors interact and modify each other. Although there is considerable evidence in animal models suggesting that inflammation directly impacts hippocampal functioning (Spulber and Schultzberg, 2010, Terrando et al., 2010), it is also plausible that inflammation plays a secondary, downstream role and is not necessarily the culpable factor driving these noted changes (Luciano et al., 2009). Given that the temporal relationship between elevations in inflammatory markers and cognitive change remains unclear, caution should be applied when attempting to make a causal association between the two. Second, the mild changes in episodic memory functioning beg the question of whether participants in the detectable CRP group are demonstrating early changes due to a neurodegenerative disease process. Given that the analyses were cross-sectional, it is currently unclear whether these participants will progress to develop MCI or later a dementia. However, while inflammation is clearly observed in neurodegenerative diseases, it is not pathognomonic nor is it specific in etiology. Mild changes in cognition do not necessarily suggest evidence of a pre-clinical neurodegenerative disease; thus, while we cannot definitively rule out an early, underlying process, all participants presented as “normal” healthy controls, and were screened for evidence of mild cognitive impairment or dementia.
Alternative, non-degenerative reasons for the observed differences in the inflammatory marker, cognition, and medial temporal lobes include a low-grade, system wide response to the aging process, acute inflammation, or vascular disease/risk factors not assessed in the current study. Although we only included individuals whose CRP levels fell below 3.0 mg/dL, a benchmark for acute inflammation (Wersching et al., 2010), the range of values still include those at the higher end of risk (detectable CRP range: .1 to 2.4 mg/dL)(Pearson et al., 2003); as such, we cannot definitively rule out the contribution of underlying acute inflammation in some of our patients. While this does not directly answer why a large portion of our participants evidenced detectable levels of CRP while others did not, our study does imply a relationship between inflammation and cognition in a relatively healthy, aging population. Importantly, our study provides preliminary evidence for specific changes in episodic memory and medial temporal lobe volume that are supported in animal models (Terrando et al., 2010).
Finally, it is also important to highlight that the current study is cross-sectional in design and incorporated one commonly used peripheral index of inflammation; thus, the findings cannot be generalized to issues related to cognitive decline or protracted inflammatory processes, nor can they address relations between other inflammation analytes and cognitive functioning. Future studies should focus on elucidating the temporal role of inflammatory markers in aging and isolate modifiable factors that may confer risk for accelerated and pathological cognitive aging. In particular, longitudinal studies are crucial to our understanding of how inflammatory markers, cognition, and brain structure may or may not change in concert over time.
In summary, findings from the current study indicate that healthy, community-dwelling older adults with detectable levels of a peripheral inflammatory marker, C-reactive protein, demonstrate worse verbal memory consolidation and recognition memory, and smaller left medial temporal lobes than individuals with undetectable levels of CRP. This study offers the first detailed analysis of episodic memory function in relation to an inflammatory marker and medial temporal lobe volume in older adults. These results highlight the utility of examining numerous indices of memory when evaluating the role of inflammation in healthy older adults, and underscore a potential role for inflammation in cognitive aging.
Research Highlight.
Community-dwelling older adults with detectable levels of an inflammatory marker, CRP, evidence poorer verbal memory consolidation and smaller left medial temporal lobes.
Acknowledgments
Study Funding & Acknowledgements: The project described was supported by grant numbers P50 AG023501 and R01 AG032289 from NIH National Institute on Aging. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute on Aging or NIH. This work was also supported by the Larry L. Hillblom Foundation, and an anonymous private foundation. A portion of this work was presented at the 2011 American Academy of Neurology meeting. Dr. Bettcher had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We would like to thank Dr. Amanda K. LaMarre for her helpful comments on an earlier version of this manuscript.
Footnotes
Non-aspirin NSAID use included the following self-reported medications: diclofenac, etodolac, fenoprofen, flurbiprofen, ibuprofen, indomethacin, ketoprofen, ketorolac, meclofenamate, nabumetone, naproxen, oxaprozin, piroxicam, sulindac, or tolmetin.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiyama H. Inflammatory response in Alzheimer’s disease. Tohoku J Exp Med. 1994;174:295–303. doi: 10.1620/tjem.174.295. [DOI] [PubMed] [Google Scholar]
- Alley DE, Crimmins EM, Karlamangla A, Hu P, Seeman TE. Inflammation and rate of cognitive change in high-functioning older adults. J Gerontol A Biol Sci Med Sci. 2008;63:50–55. doi: 10.1093/gerona/63.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo P, Martin-Aragon S, Benedi J, Susin C, Felici E, Gil P, Ribera JM, Villar AM. Differences of peripheral inflammatory markers between mild cognitive impairment and Alzheimer’s disease. Immunol Lett. 2008;117:198–202. doi: 10.1016/j.imlet.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Black PH. Stress and the inflammatory response: a review of neurogenic inflammation. Brain Behav Immun. 2002;16:622–653. doi: 10.1016/s0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- Cohen J, editor. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. 2. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- Delis DC, Massman PJ, Butters N, Salmon DP, Cermak LS, Kramer JH. Profiles of demented and amnesic patients on the California Verbal Learning Test: Implications for the Assessment of Memory Disorders. Psychological Assessment: A Journal of Consulting and Clinical Psychology. 1991;3:19–26. [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dik MG, Jonker C, Hack CE, Smit JH, Comijs HC, Eikelenboom P. Serum inflammatory proteins and cognitive decline in older persons. Neurology. 2005;64:1371–1377. doi: 10.1212/01.WNL.0000158281.08946.68. [DOI] [PubMed] [Google Scholar]
- Dimopoulos N, Piperi C, Salonicioti A, Mitropoulos P, Kallai E, Liappas I, Lea RW, Kalofoutis A. Indices of low-grade chronic inflammation correlate with early cognitive deterioration in an elderly Greek population. Neurosci Lett. 2006;398:118–123. doi: 10.1016/j.neulet.2005.12.064. [DOI] [PubMed] [Google Scholar]
- Duong T, Nikolaeva M, Acton PJ. C-reactive protein-like immunoreactivity in the neurofibrillary tangles of Alzheimer’s disease. Brain Res. 1997;749:152–156. doi: 10.1016/s0006-8993(96)01359-5. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Forlenza OV, Diniz BS, Talib LL, Mendonca VA, Ojopi EB, Gattaz WF, Teixeira AL. Increased serum IL-1beta level in Alzheimer’s disease and mild cognitive impairment. Dement Geriatr Cogn Disord. 2009;28:507–512. doi: 10.1159/000255051. [DOI] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunstad J, Bausserman L, Paul RH, Tate DF, Hoth K, Poppas A, Jefferson AL, Cohen RA. C-reactive protein, but not homocysteine, is related to cognitive dysfunction in older adults with cardiovascular disease. J Clin Neurosci. 2006;13:540–546. doi: 10.1016/j.jocn.2005.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein AM, Stasko MR, Matousek SB, Scott-McKean JJ, Maier SF, Olschowka JA, Costa AC, O’Banion MK. Sustained hippocampal IL-1beta overexpression impairs contextual and spatial memory in transgenic mice. Brain Behav Immun. 2010;24:243–253. doi: 10.1016/j.bbi.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C, EI-Okl M, Williams AL, Cunningham C, Wilcockson D, Perry VH. Systemic infection, interleukin 1beta, and cognitive decline in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2003;74:788–789. doi: 10.1136/jnnp.74.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth KF, Haley AP, Gunstad J, Paul RH, Poppas A, Jefferson AL, Tate DF, Ono M, Jerskey BA, Cohen RA. Elevated C-reactive protein is related to cognitive decline in older adults with cardiovascular disease. J Am Geriatr Soc. 2008;56:1898–1903. doi: 10.1111/j.1532-5415.2008.01930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komulainen P, Lakka TA, Kivipelto M, Hassinen M, Penttila IM, Helkala EL, Gylling H, Nissinen A, Rauramaa R. Serum high sensitivity C-reactive protein and cognitive function in elderly women. Age Ageing. 2007;36:443–448. doi: 10.1093/ageing/afm051. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Delis DC, Blusewicz MJ, Brandt J, Ober BA, Strauss M. Verbal memory errors in Alzheimer’s and Hungtinton’s dementias. Developmental Neuropsychology. 1988;4:1–15. [Google Scholar]
- Kramer JH, Rosen HJ, Du AT, Schuff N, Hollnagel C, Weiner MW, Miller BL, Delis DC. Dissociations in hippocampal and frontal contributions to episodic memory performance. Neuropsychology. 2005;19:799–805. doi: 10.1037/0894-4105.19.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz BA, Corrada MM, Kawas CH. Elevated C-reactive protein levels are associated with prevalent dementia in the oldest-old. Alzheimers Dement. 2009;5:318–323. doi: 10.1016/j.jalz.2009.04.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SC, van der Kouwe AJ, Salat DH, Dale AM, Fischl B. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Lamar M, Price CC, Giovannetti T, Swenson R, Libon DJ. The dysexecutive syndrome associated with ischaemic vascular disease and related subcortical neuropathology: a Boston process approach. Behav Neurol. 2010;22:53–62. doi: 10.3233/BEN-2009-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libon DJ, Bogdanoff B, Cloud BS, Skalina S, Giovannetti T, Gitlin HL, Bonavita J. Declarative and procedural learning, quantitative measures of the hippocampus, and subcortical white alterations in Alzheimer’s disease and ischaemic vascular dementia. J Clin Exp Neuropsychol. 1998;20:30–41. doi: 10.1076/jcen.20.1.30.1490. [DOI] [PubMed] [Google Scholar]
- Lin HB, Yang XM, Li TJ, Cheng YF, Zhang HT, Xu JP. Memory deficits and neurochemical changes induced by C-reactive protein in rats: implication in Alzheimer’s disease. Psychopharmacology (Berl) 2009;204:705–714. doi: 10.1007/s00213-009-1499-2. [DOI] [PubMed] [Google Scholar]
- Locascio JJ, Fukumoto H, Yap L, Bottiglieri T, Growdon JH, Hyman BT, Irizarry MC. Plasma amyloid beta-protein and C-reactive protein in relation to the rate of progression of Alzheimer disease. Arch Neurol. 2008;65:776–785. doi: 10.1001/archneur.65.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano M, Marioni RE, Gow AJ, Starr JM, Deary IJ. Reverse causation in the association between C-reactive protein and fibrinogen levels and cognitive abilities in an aging sample. Psychosom Med. 2009;71:404–409. doi: 10.1097/PSY.0b013e3181a24fb9. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Brain Res Rev. 1995;21:195–218. doi: 10.1016/0165-0173(95)00011-9. [DOI] [PubMed] [Google Scholar]
- Murray CA, Lynch MA. Evidence that increased hippocampal expression of the cytokine interleukin-1 beta is a common trigger for age- and stress-induced impairments in long-term potentiation. J Neurosci. 1998;18:2974–2981. doi: 10.1523/JNEUROSCI.18-08-02974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble JM, Manly JJ, Schupf N, Tang MX, Mayeux R, Luchsinger JA. Association of C-reactive protein with cognitive impairment. Arch Neurol. 2010;67:87–92. doi: 10.1001/archneurol.2009.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ownby RL. Neuroinflammation and cognitive aging. Curr Psychiatry Rep. 2010;12:39–45. doi: 10.1007/s11920-009-0082-1. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Price CC, Garrett KD, Jefferson AL, Cosentino S, Tanner JJ, Penney DL, Swenson R, Giovannetti T, Bettcher BM, Libon DJ. Leukoaraiosis severity and list-learning in dementia. Clin Neuropsychol. 2009;23:944–961. doi: 10.1080/13854040802681664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravaglia G, Forti P, Maioli F, Brunetti N, Martelli M, Servadei L, Bastagli L, Bianchin M, Mariani E. Serum C-reactive protein and cognitive function in healthy elderly Italian community dwellers. J Gerontol A Biol Sci Med Sci. 2005;60:1017–1021. doi: 10.1093/gerona/60.8.1017. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Ann Neurol. 2002;52:168–174. doi: 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- Schuitemaker A, Dik MG, Veerhuis R, Scheltens P, Schoonenboom NS, Hack CE, Blankenstein MA, Jonker C. Inflammatory markers in AD and MCI patients with different biomarker profiles. Neurobiol Aging. 2009;30:1885–1889. doi: 10.1016/j.neurobiolaging.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Segonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 2007;26:518–529. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- Semmler A, Okulla T, Sastre M, Dumitrescu-Ozimek L, Heneka MT. Systemic inflammation induces apoptosis with variable vulnerability of different brain regions. J Chem Neuroanat. 2005;30:144–157. doi: 10.1016/j.jchemneu.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Simen AA, Bordner KA, Martin MP, Moy LA, Barry LC. Cognitive dysfunction with aging and the role of inflammation. Therapeutic Advances in Chronic Disease. 2011;2:175–195. doi: 10.1177/2040622311399145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Spulber S, Schultzberg M. Connection between inflammatory processes and transmitter function-Modulatory effects of interleukin-1. Prog Neurobiol. 2010;90:256–262. doi: 10.1016/j.pneurobio.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Terrando N, Rei Fidalgo A, Vizcaychipi M, Cibelli M, Ma D, Monaco C, Feldmann M, Maze M. The impact of IL-1 modulation on the development of lipopolysaccharide-induced cognitive dysfunction. Crit Care. 2010;14:R88. doi: 10.1186/cc9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunissen CE, van Boxtel MP, Bosma H, Bosmans E, Delanghe J, De Bruijn C, Wauters A, Maes M, Jolles J, Steinbusch HW, de Vente J. Inflammation markers in relation to cognition in a healthy aging population. J Neuroimmunol. 2003;134:142–150. doi: 10.1016/s0165-5728(02)00398-3. [DOI] [PubMed] [Google Scholar]
- Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. lnterleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59:371–378. doi: 10.1212/wnl.59.3.371. [DOI] [PubMed] [Google Scholar]
- Wersching H, Duning T, Lohmann H, Mohammadi S, Stehling C, Fobker M, Conty M, Minnerup J, Ringelstein EB, Berger K, Deppe M, Knecht S. Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology. 2010;74:1022–1029. doi: 10.1212/WNL.0b013e3181d7b45b. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, Tylavsky FA, Newman AB. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]


