Abstract
The LD50 for soman is 10 to 20-fold higher for a mouse than a human. The difference in susceptibility is attributed to the presence of carboxylesterase in mouse, but not in human plasma. Our goal was to make a mouse lacking plasma carboxylesterase. We used homologous recombination to inactivate the carboxylesterase ES1 gene on mouse chromosome 8 by deleting exon 5 and by introducing a frame shift for amino acids translated from exons 6 to 13. ES1−/− mice have no detectable carboxylesterase activity in plasma but have normal carboxylesterase activity in tissues. Homozygous ES1−/− mice and wild-type littermates were tested for response to a nerve agent model compound (soman coumarin) at 3 mg/kg sc. This dose intoxicated both genotypes, but was lethal only to ES1−/− mice. This demonstrated that plasma carboxylesterase protects against a relatively high toxicity organophosphorus compound. The ES1−/− mouse should be an appropriate model for testing highly toxic nerve agents and for evaluating protection strategies against the toxicity of nerve agents.
Keywords: carboxylesterase, soman coumarin
Introduction
Studies on the toxicology of organophosphorus compounds (OP), including pesticides and nerve agents, have been complicated by the absence of a suitable laboratory animal model. Inexpensive animal models that mimic humans are needed to test the effects of exposure to OP agents and to identify antidotes. Rodents are the traditional laboratory model animals. The principal problem with rodents is the presence of carboxylesterase in their blood. For example, mouse plasma contains four esterases: acetylcholinesterase (AChE), butyrylcholinesterase (BChE), carboxylesterase and paraoxonase. Human and monkey plasma contain BChE and paraoxonase, but no carboxylesterase (1) and only minute amounts of AChE. AChE, BChE, and carboxylesterase are all inhibited by OP toxicants. The LD50 of soman in mice is approximately 20 fold higher than in Rhesus monkeys (2). This difference in toxicity is attributed to the presence of carboxylesterase (ES1) in mouse plasma. ES1 acts as an OP bioscavenger, effectively reducing the amount of OP reaching biologically relevant targets.
In the past, the contribution of plasma carboxylesterase activity to nerve agent toxicity has been minimized by pretreating animals with the carboxylesterase inhibitor cresylbenzodioxaphosphorin oxide(3) (CBDP). This strategy potentiated the toxicity of OP nerve agents, so that the LD50 dose became similar in mouse, rat, guinea pig, and rabbit where these animals differ in the amounts of carboxylesterase in plasma (2–8). Use of CBDP may be problematic because this agent inhibits not only carboxylesterase but also a number of plasma and tissue enzymes to varying degrees (9), thereby confounding analysis of OP effects.
Our goal was to produce a mouse that lacks plasma carboxylesterase (ES1) but has normal levels of carboxylesterase in liver, intestine, lung and other organs. Plasma carboxylesterase deficient mice have been previously reported in a screen of progeny from triethylenemelamine-treated male mice (10, 11). The Jackson Laboratory bred ES1e/ES1e males to C57BL/6J females to produce heterozygous embryos for cryopreservation. Before we started the project to make the ES1−/− mouse, we purchased live mice (stock number 000785), strain B6; D2-aEs1e/J. The mice and their progeny had plasma alpha-naphthyl acetate activity levels that were indistinguishable from wild type. However, ES1 deficient mice produced from this stock by another laboratory had 26% of normal activity with o-nitrophenyl acetate and 1% of normal activity with irinotecan (12).
ES1 carboxylesterase is a 65 kDa glycoprotein synthesized by mouse liver. It constitutes the majority of esterase activity in mouse plasma (13). Inhibition of ES1 by OP agents is without apparent associated toxicity (9). It was anticipated that this animal model would more accurately mirror human response to OP exposure, because humans have no carboxylesterase in plasma, but do have carboxylesterase in liver, intestine, lung, and other organs (14, 15). We made an ES1 knockout mouse that is devoid of plasma carboxylesterase activity and tested its response to soman coumarin, a fluorogenic soman model compound. Soman coumarin was used because it is less toxic than authentic nerve agent and is therefore permitted in non-military laboratories. Soman coumarin inhibits human and bovine acetylcholinesterase(16, 17). Authentic soman, but not soman coumarin is hydrolyzed by human plasma paraoxonase and squid DFPase(16). Soman coumarin has been used to screen bacterial colonies for soman hydrolase activity where it was found to mimic authentic soman(18, 19). This work reports the phenotypes of wild-type ES1+/+, heterozygous (ES1+/−) and homozygous (ES1−/−) mice. The role of plasma carboxylesterase in protection against OP toxicity was tested by comparing the response of ES1−/− and ES1+/+ mice to soman coumarin.
Materials and Methods
Production of the ES1 knockout mouse
The ES1 knockout mouse was created by Ozgene Pty Ltd (Bentley WA, Australia) on a pure C57BL/6 background by standard homologous recombination techniques (20). The time from design of the targeting vector to founder animals was 3 years. Founder animals (3 female and 3 male ES1 heterozygous (ES1+/−) mice in strain C57BL6) were delivered to the University of Nebraska Medical Center.
The ES1 knockout mouse in strain C57BL/6 is available from The Jackson Laboratory Repository (Bar Harbor, ME) http://jaxmice.jax.org/query where it is listed as JAX Stock No. 014096 C57BL/6-Ces1ctm1.1Loc/J. An alternative name for the ES1 gene is Ces1c. The Jackson Laboratory provides heterozygous animals that must be bred to produce animals completely deficient in plasma carboxylesterase.
Breeding
Animal work was conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health. Formal approval to conduct the experiments was obtained from the Institutional Animal Care and Use Committee (IACUC) of the University of Nebraska Medical Center. ES1+/− founders were bred to C57BL/6 wild type mice (The Jackson Laboratory, Bar Harbor, Maine) to expand the colony. The designation ES1+/+ is for wild-type mice. ES1+/− mice were bred to create ES1+/+, ES1+/− and ES1−/− mice. This breeding protocol allowed siblings to be utilized in experiments, reducing the possibility of effects from genetic drift.
Determination of phenotype
Pups were phenotyped by assaying plasma carboxylesterase activity of blood collected from the saphenous vein. Body weight, body temperature, litter size, and Mendelian ratios were compared between the ES1 phenotypes.
Determination of carboxylesterase, AChE, BChE, and paraoxonase activity
Enzyme activity was measured at 25°C in a 2-mL reaction volume using a Gilford spectrophotometer interfaced with a MacLab data recorder (ADInstruments Pty Ltd, Castle Hill, Australia). Carboxylesterase activity was determined by monitoring the hydrolysis rate of alpha-naphthyl acetate (7). Plasma (3 μL) or tissue (10–100 μL of solubilized preparations) samples were added to a solution of 0.1 M potassium phosphate buffer pH 7.0 in the presence of 0.01 mM eserine to inhibit AChE and BChE, and 1.3 mM EDTA to inhibit paraoxonase. The samples were incubated at 25°C for 15 min. After incubation, 0.1 M α-naphthyl acetate in ethanol (100 μL) was added to the cuvette and the rate of hydrolysis was followed at 321 nm. The extinction coefficient for the reaction product was 2220 M−1cm−1. AChE and BChE activities were assayed in plasma (3 μL) according to the Ellman protocol (21) at 412 nm using an extinction coefficient of 13,600 M−1cm−1. AChE activity was assayed with 1 mM acetylthiocholine in 0.1 M potassium phosphate pH 7.0, 0.5 mM dithiobisnitrobenzoic acid, at 25°C in the presence of 0.01 mM ethopropazine to inhibit BChE. BChE activity was assayed with 1 mM butyrylthiocholine in 0.1 M potassium phosphate pH 7.0, 0.5 mM dithiobisnitrobenzoic acid at 25°C. Plasma paraoxonase activity (5 μL plasma) was assayed in 10 mM Tris buffer pH 8.0 containing 1.0 mM CaCl2 in the presence of 1 mM phenyl acetate. The hydrolysis rate was recorded at 270 nm and activity was calculated using an extinction coefficient of 1310 M−1cm−1. A unit of AChE, BChE, carboxylesterase, or paraoxonase activity was defined as one Pmole of substrate hydrolyzed per min. The concentrations of AChE, BChE, and carboxylesterase in mouse plasma are 0.2 mg/L, 2.6 mg/L, and 80 mg/L respectively (1). The subunit molecular weights are 70 kDa for AChE, 85 kDa for BChE, and 70 kDa for carboxylesterase. Mouse plasma AChE is predominantly a tetramer of 280 kDa, while plasma BChE is a tetramer of 340 kDa, and plasma carboxylesterase is a monomer.
Nondenaturing gradient gel electrophoresis and staining for carboxylesterase activity
4–30% polyacrylamide gradient gels, 0.75 mm thick were prepared in a Hoefer gel apparatus (SE600; Hoefer, Holliston, MA). Plasma samples (2 μL per lane) were mixed with an equal volume of 50% glycerol, 0.1 M TrisCl pH 6.8, 0.1% bromophenol blue before loading onto the gel. Gels were run at constant voltage for 5000 volt hours (200 volts for 25 hours) at 4°C. To visualize carboxylesterase activity nondenaturing gels were incubated in 100 mL buffer (Tris-Cl pH 8.0) containing α-naphthyl acetate (50 mg dissolved in 1 mL ethanol) and 50 mg of solid Fast Blue RR for 30 min until green bands of esterase activity appeared.
Challenge with Soman Coumarin
Caution: The nerve agent model compound soman coumarin is hazardous and is handled carefully. Adult male ES1+/+ and −/− mice (n=6 per genotype) were challenged with soman coumarin to determine differences in toxicity and in effects on plasma BChE, AChE and ES1 activities. The structures of the authentic compound and the model compound are shown in Figure 1. Dose finding experiments were conducted in ES1+/+ mice to find a non-lethal dose that would produce toxic signs. Soman coumarin (gift from Dr. Gareth Williams) (17) (Briseno-Roa 2006, Scheme 2, no. 11) was dissolved in ethanol and delivered subcutaneously (sc) at a dose of 3 mg/kg.
Figure 1.
Structural differences between the authentic nerve agent soman, and the nerve agent model compound soman coumarin.
Observations, body weights and surface body temperatures (Thermalert model TH-5, Physitemp Instruments, Inc., Clifton, New Jersey) were recorded prior to challenge, at 5, 10, 15, 30, 45 minutes, then hourly through 4–8 hours and finally at 24 hours post dosing. Blood was collected prior to challenge and at 1, 4, 20, 48, 72, and 96 hours post dosing via the saphenous vein (50 μL) into heparinized collection tubes. Plasma BChE, AChE and carboxylesterase activities were determined at each time point as described.
Functional observational battery
Mice were observed for toxic signs as described by McDaniel and Moser (22) including posture, involuntary motor movements, tremors, seizures, convulsions, palpebral closure, reactivity to being handled, lacrimation, salivation, piloerection, gait, mobility, arousal, and temperature.
Plasma and tissue AChE, BChE and carboxylesterase activity following challenge with soman coumarin
ES1+/+ and −/− mice (n=3 per group, males age 6 weeks) were injected subcutaneously with 3 mg/kg soman coumarin dissolved in ethanol. Observations of mouse behavior, body weights and temperatures were recorded prior to challenge and at 5, 10, 15, and 30 minutes post challenge. Plasma samples were collected prior to challenge and at 30 minutes post challenge. At 30 minutes post challenge mice were euthanized by CO2 asphyxiation, and perfused by transcardial wash with 60 mL of saline. Brain, diaphragm and quadriceps muscle were collected and homogenized for 10 s in 10 volumes of ice-cold 50 mM potassium phosphate, pH 7.4, 0.5% Tween 20, using a Polytron homogenizer (Brinkmann Instruments, Mississauga, ON, Canada). The homogenate was clarified by centrifuging 3 times in a microfuge to remove all turbid material. The supernatant was transferred to a clean tube and assayed for activity as described.
Statistics
Comparison of means was by two tailed t-test. Enzyme activity values are given ± SD. Analysis was conducted using SPSS software (IBM Corporation Chicago, IL).
Results
Strategy to knockout the ES1 carboxylesterase gene
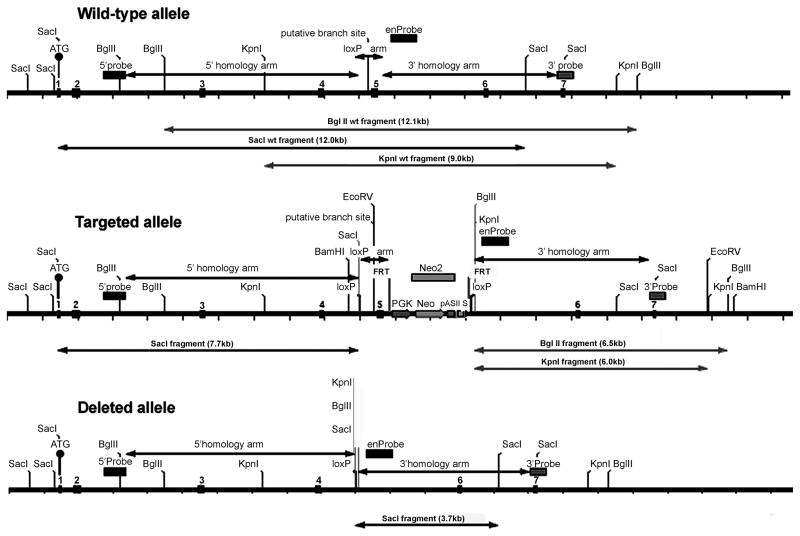
The National Center for Biotechnology Information map viewer shows 16 carboxylesterase genes and pseudogenes on mouse chromosome 8. The ES1 gene is located in ensEMBL gene ID: ENSMUSG00000057400. The intron/exon structure was confirmed by alignment of mRNA sequence NM_007954 (23) with the ensEMBL genomic sequence. The ES1 gene has 13 exons spread over 32.3 kb. The peptide that includes the active site serine is encoded by exon 5. The ES1 gene was inactivated by deleting exon 5, which introduced a frame shift for exons 6 to 13. This strategy deleted the Ser, Glu and His amino acid residues of the catalytic triad, leaving no possibility of an active ES1 carboxylesterase enzyme (accession# gi:6679689 updated to gi:247269929). ES1 targeted mice were bred to CRE deleter mice thereby removing the loxP flanked sequences in the genome and resulting in a complete knockout. The wild type, targeted and deleted alleles are shown in Figure 2.
Figure 2.
Targeting strategy to delete exon 5 of the ES1 gene. The top drawing shows the first 7 exons of the wild-type allele, as well as the location of the 5′ and 3′ homology arms in the gene targeting vector, the location of two probes (5′ probe and 3′ probe) outside the gene targeted region, and the location of one internal probe. Also shown are the restriction sites used for Southern blotting to distinguish wild-type, targeted, and deleted alleles. The middle drawing shows the targeted allele after recombination has inserted loxP sites and the Neo gene cassette. The bottom drawing shows the deleted allele produced by breeding to Cre recombinase-expressing mice, thus deleting exon 5 and the Neo gene cassette.
Phenotype of the ES1 knockout mouse
ES1+/+, +/− and −/− pups were born to ES1+/− parents at the expected Mendelian ratio. Litter sizes averaged 7.2 pups. Breeding of ES1−/− pairs demonstrated that the ES1−/− mice were fertile and produced healthy offspring. ES1+/− and −/− mice were found to be viable, healthy, and of comparable body weight and body temperature to ES1+/+ mice. ES1+/− and −/− mice had no observable phenotypes.
AChE, BChE, carboxylesterase, and paraoxonase plasma activity of ES1+/+, +/− and −/− mice
AChE, BChE, and paraoxonase activity levels in plasma of adult ES1+/+, +/− and −/− mice (n=20 per genotype) were found to be the same between genotypes (AChE 0.3±0.04 u/mL, BChE 1.2±0.06 u/mL, paraoxonase 75±6.5 u/mL). Carboxylesterase activity was determined in the plasma of 6-week old mice (n=20 per genotype) by hydrolysis of α-naphthyl acetate following inhibition of AChE, BChE and paraoxonase (Table 1). ES1+/+ male and female mice had 9–13 u/mL of carboxylesterase activity in plasma; ES1+/− had about 4–7 u/mL, and ES1−/− had 0.5–0.7 u/mL. The background activity in ES1−/− plasma was due to albumin hydrolysis of α-naphthyl acetate (14). This conclusion was arrived at by staining non-denaturing polyacrylamide gels for esterase activity (see the following section).
Table 1.
Plasma carboxylesterase activity in 6 week old mice
| Gender | ES1 Genotype | ES1 Activity (u/mL) |
|---|---|---|
| Male | +/+ | 10.9±1.6 |
| Male | +/− | 5.5±1.1 |
| Male | −/− | 0.7±0.4 |
| Female | +/+ | 11.8±1.7 |
| Female | +/− | 6.2±1.1 |
| Female | −/− | 0.5±0.7 |
±SD; n =20 mice per group; α-naphthyl acetate substrate
No detectable carboxylesterase observed in the plasma of ES1−/− mice
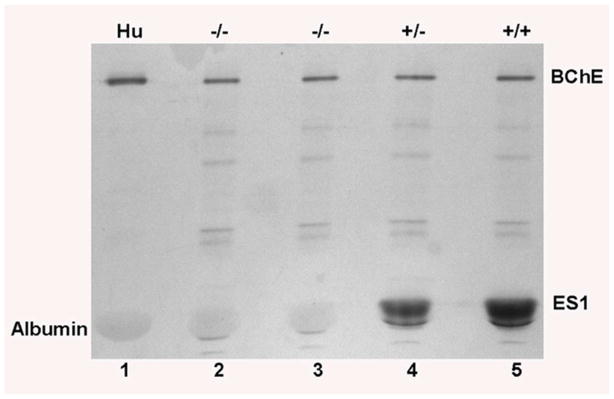
Plasma ES1 activity was visualized on a nondenaturing gradient gel stained for esterase activity with α-naphthyl acetate and Fast Blue RR. Lanes 2 and 3 in Figure 3 show no band of carboxylesterase activity in ES1−/− plasma. Similarly, human plasma in lane 1 has no band of carboxylesterase activity. Strong bands of activity appear at the position of ES1 in lanes 4 and 5, for ES1+/− and ES+/+ sera. All ES1 phenotypes (lanes 2–5), as well as human plasma in lane 1, had a BChE band near the top of the gel and an albumin band of esterase activity near the bottom of the gel. The albumin band is broad because of the large amount of albumin in 2 μl of plasma (80 μg). Albumin runs close to the ES1 carboxylesterase position. However, albumin activity is readily distinguished from the carboxylesterase activity in lanes 4 and 5. These results confirm the absence of carboxylesterase activity in ES1−/− plasma and the presence of a background pseudo-esterase activity from albumin. The pseudo-esterase activity of albumin is due to acetylation of multiple lysines on albumin by α-naphthyl acetate (14). The additional 4 faint bands of apparent esterase activity (lanes 2–5) probably reflect stable acetylation of lysines on unidentified proteins, similar to the acetylation of lysines in albumin.
Figure 3.
Visualization of ES1 activity in the plasma of ES1+/+, +/− and −/− mice using α-NA as the substrate. Plasma was loaded at 2 μl/well onto a nondenaturing gradient polyacrylamide gel. Lane 1) Human plasma has BChE and albumin pseudo-esterase activity, but no carboxylesterase. Lanes 2 and 3) Plasma from ES1−/− mice demonstrates the complete absence of carboxylesterase activity in these mice. Lane 4) ES1+/− plasma. Lane 5) ES1+/+ plasma. The ES1 band intensity is greater in lane 5 than lane 4, corresponding to the higher carboxylesterase activity in +/+ than +/− plasma.
ES1−/− mice have normal carboxylesterase activity in tissues
Mice, rats, and humans have substantial carboxylesterase activity in liver, intestine, lungs and other tissues (15). The carboxylesterases in tissues are transcribed from the CES1 and CES2 gene clusters on mouse chromosome 8 and not from the ES1 gene. Our goal was to specifically knock out plasma carboxylesterase activity while leaving the tissue carboxylesterases at normal levels. The functional activity of tissue carboxylesterase was tested with α-naphthyl acetate as substrate. No significant differences in the carboxylesterase activity levels were found in the perfused tissues of ES1+/+, +/− and −/− adult female mice (n=6 per genotype) (Table 2). The single exception was the carboxylesterase activity in plasma, which was reduced in ES1 knockout mice. It was concluded that gene targeting specifically deleted the ES1 gene and had no effect on other carboxylesterase genes.
Table 2.
Carboxylesterase activity in perfused tissues from 6 week old female mice
| Genotype | Plasma | Brain | Lungs | Heart | Liver | Diaphragm | Kidney | Intestine | Quadriceps Muscle | Fat | Skin |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ES1+/+ | 13.1±1.3 | 4.4±0.4 | 36.5±2.4 | 4.6±0.3 | 188.7±10.1 | 32.4±2.7 | 43.7±11.1 | 109.7±10.8 | 4.3±0.3 | 80.3±3.5 | 10.4±2.8 |
| ES1+/− | 7.0±0.7 | 4.3±0.2 | 36.1±3.5 | 4.9±0.3 | 197.9±15.3 | 38.0±4.7 | 43.7±10.3 | 111.2±3.5 | 4.3±0.5 | 83.3±11.6 | 9.3±2.1 |
| ES1−/− | 0.5±0.4 | 4.1±0.2 | 36.5±4.5 | 4.8±0.2 | 194.8±8.9 | 34.5±4.8 | 41.3±15.1 | 109.6±11.7 | 4.2±1.4 | 83.5±8.7 | 9.7±3.2 |
(units/mL plasma; units/g wet weight tissue)
± SD; n=6 per genotype.
Plasma carboxylesterase protects against soman coumarin challenge
Signs of toxicity
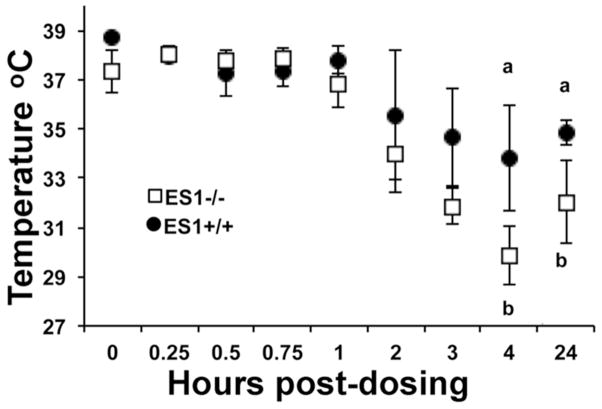
ES1+/+ and −/− mice (n=6 per genotype) were challenged with 3.0 mg/kg soman coumarin delivered subcutaneously (sc). The structure of soman coumarin is shown in Figure 1. Toxic signs in both genotypes included hunched posture, decreased arousal, muscle fasciculations (mild in ES1+/+ mice, more severe in ES1−/− mice), piloerection, and decreased handling reaction. In addition, ES1−/− mice displayed ataxic gait, mucus in the eyes, and flattened posture. None of the mice had seizures, convulsions, or salivation. Toxic signs started to appear at 1 to 3 h after subcutaneous injection. The delay is attributed to the time it takes a drug to be absorbed from the tissue beneath the skin. ES1−/− mice had significantly lower body temperatures at 4 and 24 hours post dosing compared to ES1+/+ mice (Figure 4). At 4 h, the temperature of ES1−/− mice was an average of 30°C while that of wild type mice was 34°C. At 20 h post dosing, 3 ES1−/− mice were moribund and 2 were dead while all ES1+/+ mice appeared to be recovering with minimal toxic signs. At 24 h, the temperature of the surviving ES1−/− mice averaged 32°C, while that of wild type mice averaged 35°C. At 48 hours post dosing 2 additional ES1−/− mice had died and the remaining ES1−/− mice were moribund while none of the 6 ES1+/+ mice had toxic signs.
Figure 4.
Average surface body temperatures of ES1−/− (open squares) and +/+ (closed circles) mice post-challenge with soman coumarin (± SD). a is significantly different than b by two tailed t-test p≤0.05.
Determination of plasma inhibition
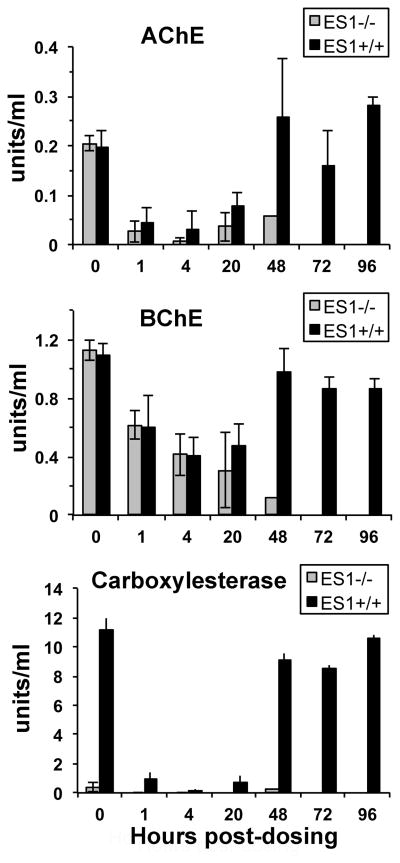
AChE activity was reduced to 10% of normal in ES1−/− plasma at 4 h, but had returned to 25% of normal at 48 h post dosing (p<0.01) (Figure 5). This low level of activity corresponded to the ES1−/− animals’ moribund status. BChE activity was reduced to 10% of normal in the ES1−/− mice at 48 h (Figure 5). AChE was inhibited to a greater degree than BChE in both ES1−/− and ES1 +/+ mice at 4 h. Both AChE and BChE activities recovered to baseline levels in ES1+/+ mice by 48 h post dosing. Plasma carboxylesterase activity was inhibited 90% in the wild type mice through 20 h, with recovery to baseline by 96 h (Figure 5). Overall, the wild-type mice fared better than the ES1−/− mice. It was concluded that plasma carboxylesterase was associated with protection from challenge by soman coumarin.
Figure 5.
Average AChE, BChE and ES1 carboxylesterase activity in the plasma of ES1−/− (grey bars) and ES1+/+ (black bars) mice pre- and post-challenge sc with 3.0 mg/kg soman coumarin. By 48 h esterase activities returned to baseline in wild-type, but not in ES1−/− mice. (± SD).
AChE activity is irreversibly inhibited by soman (24, 25). The return to normal plasma AChE activity in mice in 48 h is explained by synthesis of new molecules of AChE. Others have found that AChE activity recovers by 48–72 h after treatment of rats with soman (26). Gene expression studies have shown that mRNA for AChE in brain increases when mice are treated with sarin (27).
Determination of tissue inhibition
ES1+/+ and −/− mice (n=3 per genotype) were treated sc with 3 mg/kg soman coumarin. At 30 minutes post dosing the mice were euthanized, tissues were perfused, collected, homogenized and the supernatants were analyzed for carboxylesterase, AChE, and BChE activity. The results are shown in Table 3. After 30 minutes, carboxylesterase activities in the diaphragm of ES1−/− mice were significantly more inhibited than in the ES1+/+ mice. Relatively little carboxylesterase was inhibited in the brain of either genotype. Diaphragm AChE activity was significantly more inhibited in the ES1−/− mice than in the ES1+/+ mice. Brain AChE was inhibited approximately 70% in both genotypes. BChE inhibition was comparable between the genotypes, with no inhibition in the brain. It was concluded that plasma carboxylesterase protected mice from the toxicity of soman coumarin by significantly reducing inhibition of AChE and carboxylesterase in the diaphragm.
Table 3.
Carboxylesterase, AChE, and BChE activities pre-dose and 30 minutes after subcutaneous treatment with 3.0 mg/kg soman coumarin
| Enzyme | genotype | plasma pre | plasma post | brain pre | brain post | muscle pre | muscle post | diaphragm pre | diaphragm post |
|---|---|---|---|---|---|---|---|---|---|
| Carboxylesterase | ES1+/+ | 13.5±1.5 | 1.3±1.3 | 4.1±0.2 | 3.8±0.7 | 4.4±0.2 | 1.6±0.3 | 35.1±2.9 | 6.1±1.6a |
| Carboxylesterase | ES1−/− | 0.041±0.01 | 0.02±0.01 | 4.0±0.1 | 3.5±0.3 | 4.0±0.2 | 1.2±0.2 | 39.4±1.8 | 2.2±1.5b |
| AChE | ES1+/+ | 0.2±0.04 | 0.05±0.02 | 1.56±0.08 | 0.48±0.2 | 0.37±0.01 | 0.10±0.05 | 0.2±0.01 | 0.09±0.03c |
| AChE | ES1−/− | 0.2±0.06 | 0.03±0.02 | 1.39±0.06 | 0.39±0.2 | 0.38±0.03 | 0.06±0.01 | 0.2±0.01 | 0.04±0.01d |
| BChE | ES1+/+ | 1.7±0.2 | 0.75±0.4 | 0.10±0.01 | 0.10±0.03 | 0.18±0.01 | 0.17±0.02 | 0.4±0.01 | 0.19±0.1 |
| BChE | ES1−/− | 1.6±0.2 | 0.83±0.3 | 0.10±0.01 | 0.10±0.03 | 0.17±0.02 | 0.15±0.02 | 0.4±0.01 | 0.12±0.1 |
Values are given in unit/mL ± standard deviation for plasma and units/g wet weight for tissues.
n = 3 per genotype
is significantly different than b p=0.006 by two tailed t-test.
is significantly different than d p=0.03 by two tailed t-test.
Discussion
The role of carboxylesterase ES1 in the protection of rodents against OP intoxication
Mice and rats differ from monkeys and humans in that they are less sensitive toward intoxication by nerve agents (2). This difference has been attributed to the presence of carboxylesterase in the plasma of rodents and its absence from the plasma of humans and monkeys. Maxwell (28) measured the in vitro reactivities of various OP with rat carboxylesterase and rat AChE, and determined their toxicity in rats that had been depleted of carboxylesterase by treatment with CBDP and in rats that had not been depleted of carboxylesterase. He concluded that carboxylesterase “appears to perform the role of a high-affinity/low-capacity detoxication process” wherein it “is primarily important for detoxication of highly toxic OP compounds that achieve lower in vivo concentrations, for which the high affinity of the detoxication enzyme [carboxylesterase] is more important than its detoxication capacity”. The rationale for this conclusion is as follows.
Case 1) Inhibition of carboxylesterase has no clinical sequelae; therefore toxic effects following OP challenge can be ascribed to inhibition of AChE. OP that react readily with AChE will cause toxicity at low doses. If those OP react readily with carboxylesterase as well, then when carboxylesterase is present it will absorb a significant fraction of the toxin and provide protection. Case 2) If carboxylesterase reacts poorly with OP that react readily with AChE, then carboxylesterase will provide no protection. Case 3) OP that react less readily with AChE will cause toxicity only at high doses, i.e. doses that are much higher than the concentration of carboxylesterase in the subject. Under these conditions it makes no difference whether carboxylesterase reacts readily with the OP or not. If carboxylesterase reacts readily, it will be used up before it can absorb a significant fraction of the OP, thereby affording no protection. Case 4) If carboxylesterase reacts poorly, it simply will not afford protection.
Examples for each of these four scenarios can be easily found. Case 1) Pinacolyl-methylphosphonofluoridate (soman) is a neutral OP which reacts readily with both AChE (8.9×107 M−1min−1) and carboxylesterase (5.1×106 M−1min−1) (29). In a rat, carboxylesterase ameliorates the effects of soman (28). Case 2) O-ethyl S-[2(diisopropylamino)ethyl] methylphosphonothioate (VX) is a cationic OP which reacts readily with AChE (3.2×107 M−1min−1) but not with carboxylesterase (1.5×103 M−1min−1) (29). In a rat, carboxylesterase has no effect on the toxicity of VX (28). Case 3) Diisopropyl fluorophosphate (DFP) is a bulky OP that reacts poorly with AChE (2.9×104 M−1min−1) (29). Even though it reacts readily with carboxylesterase (3.5×106 M−1min−1) (29), carboxylesterase has no effect on the toxicity of DFP (28). Case 4) Tetraisopropylpyrophosphoramide (iso-OMPA) is a bulky OP that reacts poorly with both AChE (1.6×101 M−1min−1) and carboxylesterase (1.2×102 M−1min−1) (29). Carboxylesterase would be expected to have no effect on its toxicity.
Carboxylesterase protects mice by serving as a stoichiometric scavenger of OP. “Therefore, the capacity of carboxylesterase to detoxify OP compounds is quantitatively limited by the number of available carboxylesterase molecules” (28). Our carboxylesterase knock-out mice have been selectively depleted of serum carboxylesterase ES1. This makes the number of ES1 molecules in the wild type mouse a critical factor in determining how their ES1−/− brethren can be used for studies on OP toxicity. Estimation of the moles of carboxylesterase in a mouse can be made in more than one way. One method is to start with the concentration of ES1. ES1 carboxylesterase is found primarily in blood and lymph. A normal mouse has 80 mg of ES1 carboxylesterase per liter of blood. The molecular weight of carboxylesterase is 70,000 g/mole. This makes the concentration of ES1in blood about 1.2 μM. Assuming the blood volume for an average 25 g mouse is 1.75 mL, there would be 2 nmoles of ES1 in the blood. Another estimate can be made from the amount of OP that ES1 can protect against. The LD50 for soman is about 0.5 μM and ES1 can protect against an amount of soman equal to about 10 LD50, suggesting that there is about 5 μM ES1 in the blood. 1.75 mL of blood would contain about 9 nmoles of carboxylesterase. If ES1 were distributed throughout the lymph system as well as the blood, the total amount of carboxylesterase would be doubled to about 18 nmoles. Mice in this study were dosed with 200 nmoles soman coumarin.
ES1−/− mice show increased sensitivity to challenge with soman coumarin
For the first few hours following challenge with 200 nmoles of soman coumarin (17) the ES1−/− and wild-type mice showed very similar toxic signs. The major difference was that the ES1−/− mice had significantly lower body temperature compared to the ES1+/+ mice. These observations are consistent with the fact that at least 10-times more soman coumarin than carboxylesterase (maximum 18 nmoles) was present, making stoichiometric scavenging of soman coumarin by carboxylesterase an ineffective protection for the wild-type mice. After 20 hours, 2 of the ES1−/− mice were dead while the wild-type mice were clearly recovering. After 48 hours, 2 more ES1−/− mice had died and the remaining two were moribund, while the wild-type mice had recovered. It thus appears that carboxylesterase ameliorated the long term effects of soman coumarin without having a substantial effect on the short term effects. This strongly suggests that carboxylesterase was able to turnover the soman coumarin, albeit slowly. A half-time for spontaneous reactivation of 2.7 hours has been reported for soman-inhibited rat plasma carboxylesterase (30). The slow hydrolysis of soman coumarin reduced the soman coumarin concentration to a nontoxic level in wild-type mice. After 48 h, the amount of soman coumarin remaining in the wild-type mouse was so low that AChE activity was 100% normal (Figure 5). In conclusion, the absence of ES1 carboxylesterase and the loss of its bioscavenger effects made a significant difference in toxic signs and lethality after challenge by soman coumarin.
Stereoisomers of soman coumarin
By analogy to soman (7), the soman coumarin used in the experiments was racemic at the phosphorus atom. It therefore existed as a pair of diastereoisomers – C(+)P(+), C(−)P(+) and C(+)P(−), C(−)P(−) – due to the presence of an additional chiral center at the secondary carbon atom of its pinacolyl side-chain. The rates of inhibition of AChE and hence LD50 values of the four different stereoisomers are expected to follow those of soman, being maximal for the P(−)isomers. The rate constants for inhibition of AChE at 25 °C by C(+)P(+), C(−)P(+), C(+)P(−) and C(−)P(−) soman are < 5 × 103, < 5 × 103, 2.8 × 108 and 1.8 × 108 M−1 min−1 respectively as reflected in the corresponding LD50 values to mice of > 5000, > 2000, 99 and 38 μg/kg (31). The bioscavenger effect of any enzyme will therefore be maximal if that enzyme shares the same stereoselectivity as AChE towards the nerve agent. The protective effect of mouse plasma carboxylesterase is due to selective targeting of the most toxic P(−) soman stereoisomers as shown in experiments with purified mouse plasma carboxylesterase where the P(−) stereoisomers where 100 times more potent inhibitors than the P(+) stereoisomers (32).
Conclusion
We have created a knockout mouse that is missing ES1 carboxylesterase from its blood, making it a reasonable human model for toxicity studies. We used this mouse to describe a new application of a fluorogenic nerve agent model compound that complements its established role as a tool for screening hydrolytic enzymes. The challenge study confirmed the conclusion that plasma carboxylesterase provides effective protection against highly toxic OP.
Acknowledgments
Funding Sources. This work was supported in part by the JSTO-Defense Threat Reduction Agency under project numbers I2_X005_04_RC_C and CBM.SCAV.01.10.RC.017. Part of the work was funded by the National Institutes of Health CounterACT Program through the National Institute of Neurological Disorders and Stroke (Award # U01 NS058038). The opinions, interpretations, conclusions, and recommendations in this manuscript are those of the authors and are not necessarily endorsed by the U.S. Army or the Department of Defense.
Abbreviations
- AChE
acetylcholinesterase
- BChE
butyrylcholinesterase
- CBDP
cresylbenzodioxaphosphorin oxide or cresyl saligenin phosphate
- DFP
diisopropyl fluorophosphate
- ES1
plasma carboxylesterase
- iso-OMPA
tetraisopropylpyrophosphoramide
- OP
organophosphorus agent
- VX nerve agent
O-ethyl S-[2(diisopropylamino)ethyl] methylphosphonothioate
References
- 1.Li B, Sedlacek M, Manoharan I, Boopathy R, Duysen EG, Masson P, Lockridge O. Butyrylcholinesterase, paraoxonase, and albumin esterase, but not carboxylesterase, are present in human plasma. Biochem Pharmacol. 2005;70:1673–1684. doi: 10.1016/j.bcp.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Maxwell DM, Brecht KM, O’Neill BL. The effect of carboxylesterase inhibition on interspecies differences in soman toxicity. Toxicol Lett. 1987;39:35–42. doi: 10.1016/0378-4274(87)90254-2. [DOI] [PubMed] [Google Scholar]
- 3.Boskovic B. The influence of 2-/o-cresyl/-4 H-1: 3: 2-benzodioxa-phosphorin-2-oxide (CBDP) on organophosphate poisoning and its therapy. Arch Toxicol. 1979;42:207–216. [PubMed] [Google Scholar]
- 4.Garrett TL, Rapp CM, Grubbs RD, Schlager JJ, Lucot JB. A murine model for sarin exposure using the carboxylesterase inhibitor CBDP. Neurotoxicology. 2010;31:502–508. doi: 10.1016/j.neuro.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Cohen O, Kronman C, Raveh L, Mazor O, Ordentlich A, Shafferman A. Comparison of polyethylene glycol-conjugated recombinant human acetylcholinesterase and serum human butyrylcholinesterase as bioscavengers of organophosphate compounds. Mol Pharmacol. 2006;70:1121–1131. doi: 10.1124/mol.106.026179. [DOI] [PubMed] [Google Scholar]
- 6.Yang ZP, Dettbarn WD. Prevention of tolerance to the organophosphorus anticholinesterase paraoxon with carboxylesterase inhibitors. Biochem Pharmacol. 1998;55:1419–1426. doi: 10.1016/s0006-2952(97)00650-3. [DOI] [PubMed] [Google Scholar]
- 7.Due AH, Trap HC, Van Der Wiel HJ, Benschop HP. Effect of pretreatment with CBDP on the toxicokinetics of soman stereoisomers in rats and guinea pigs. Arch Toxicol. 1993;67:706–711. doi: 10.1007/BF01973695. [DOI] [PubMed] [Google Scholar]
- 8.Casida JE, Baron RL, Eto M, Engel JL. Potentiation and neurotoxicity induced by certain organophosphates. Biochem Pharmacol. 1963;12:73–83. doi: 10.1016/0006-2952(63)90011-x. [DOI] [PubMed] [Google Scholar]
- 9.Clement JG. Importance of aliesterase as a detoxification mechanism for soman (pinacolyl methylphosphonofluoridate) in mice. Biochem Pharmacol. 1984;33:3807–3811. doi: 10.1016/0006-2952(84)90044-3. [DOI] [PubMed] [Google Scholar]
- 10.Soares ER. Identification of a new allele of Es-I segregating in an inbred strain of mice. Biochem Genet. 1979;17:577–583. doi: 10.1007/BF00502119. [DOI] [PubMed] [Google Scholar]
- 11.Soares ER. TEM-induced gene mutations at enzyme loci in the mouse. Environ Mutagen. 1979;1:19–25. doi: 10.1002/em.2860010108. [DOI] [PubMed] [Google Scholar]
- 12.Morton CL, Wierdl M, Oliver L, Ma MK, Danks MK, Stewart CF, Eiseman JL, Potter PM. Activation of CPT-11 in mice: identification and analysis of a highly effective plasma esterase. Cancer Res. 2000;60:4206–4210. [PubMed] [Google Scholar]
- 13.Otto J, Ronai A, von Deimling O. Purification and characterization of esterase 1F, the albumin esterase of the house mouse (Mus musculus) Eur J Biochem. 1981;116:285–291. doi: 10.1111/j.1432-1033.1981.tb05331.x. [DOI] [PubMed] [Google Scholar]
- 14.Lockridge O, Xue W, Gaydess A, Grigoryan H, Ding SJ, Schopfer LM, Hinrichs SH, Masson P. Pseudo-esterase activity of human albumin: slow turnover on tyrosine 411 and stable acetylation of 82 residues including 59 lysines. J Biol Chem. 2008;283:22582–22590. doi: 10.1074/jbc.M802555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Satoh T, Hosokawa M. Structure, function and regulation of carboxylesterases. Chem Biol Interact. 2006;162:195–211. doi: 10.1016/j.cbi.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Blum MM, Timperley CM, Williams GR, Thiermann H, Worek F. Inhibitory potency against human acetylcholinesterase and enzymatic hydrolysis of fluorogenic nerve agent mimics by human paraoxonase 1 and squid diisopropyl fluorophosphatase. Biochemistry. 2008;47:5216–5224. doi: 10.1021/bi702222x. [DOI] [PubMed] [Google Scholar]
- 17.Briseno-Roa L, Hill J, Notman S, Sellers D, Smith AP, Timperley CM, Wetherell J, Williams NH, Williams GR, Fersht AR, Griffiths AD. Analogues with fluorescent leaving groups for screening and selection of enzymes that efficiently hydrolyze organophosphorus nerve agents. J Med Chem. 2006;49:246–255. doi: 10.1021/jm050518j. [DOI] [PubMed] [Google Scholar]
- 18.Briseno-Roa L, Timperley CM, Griffiths AD, Fersht AR. Phosphotriesterase variants with high methylphosphonatase activity and strong negative trade-off against phosphotriesters. Protein Eng Des Sel. 2010;24:151–159. doi: 10.1093/protein/gzq076. [DOI] [PubMed] [Google Scholar]
- 19.Gupta RD, Goldsmith M, Ashani Y, Simo Y, Mullokandov G, Bar H, Ben-David M, Leader H, Margalit R, Silman I, Sussman JL, Tawfik DS. Directed evolution of hydrolases for prevention of G-type nerve agent intoxication. Nat Chem Biol. 2011;7:120–125. doi: 10.1038/nchembio.510. [DOI] [PubMed] [Google Scholar]
- 20.Koentgen F, Suess G, Naf D. Engineering the mouse genome to model human disease for drug discovery. Methods Mol Biol. 2010;602:55–77. doi: 10.1007/978-1-60761-058-8_4. [DOI] [PubMed] [Google Scholar]
- 21.Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 22.McDaniel KL, Moser VC. Utility of a neurobehavioral screening battery for differentiating the effects of two pyrethroids, permethrin and cypermethrin. Neurotoxicol Teratol. 1993;15:71–83. doi: 10.1016/0892-0362(93)90065-v. [DOI] [PubMed] [Google Scholar]
- 23.Ovnic M, Tepperman K, Medda S, Elliott RW, Stephenson DA, Grant SG, Ganschow RE. Characterization of a murine cDNA encoding a member of the carboxylesterase multigene family. Genomics. 1991;9:344–354. doi: 10.1016/0888-7543(91)90263-e. [DOI] [PubMed] [Google Scholar]
- 24.Fleisher JH, Harris LW. Dealkylation as a mechanism for aging of cholinesterase after poisoning with pinacolyl methylphosphonofluoridate. Biochem Pharmacol. 1965;14:641–650. doi: 10.1016/0006-2952(65)90082-1. [DOI] [PubMed] [Google Scholar]
- 25.Michel HO, Hackley BE, Jr, Berkowitz L, List G, Hackley EB, Gillilan W, Pankau M. Ageing and dealkylation of Soman (pinacolylmethylphosphonofluoridate)-inactivated eel cholinesterase. Arch Biochem Biophys. 1967;121:29–34. doi: 10.1016/0003-9861(67)90006-9. [DOI] [PubMed] [Google Scholar]
- 26.Gupta RC, Patterson GT, Dettbarn WD. Biochemical and histochemical alterations following acute soman intoxication in the rat. Toxicol Appl Pharmacol. 1987;87:393–402. doi: 10.1016/0041-008x(87)90244-4. [DOI] [PubMed] [Google Scholar]
- 27.Damodaran TV, Jones KH, Patel AG, Abou-Donia MB. Sarin (nerve agent GB)-induced differential expression of mRNA coding for the acetylcholinesterase gene in the rat central nervous system. Biochem Pharmacol. 2003;65:2041–2047. doi: 10.1016/s0006-2952(03)00160-6. [DOI] [PubMed] [Google Scholar]
- 28.Maxwell DM. The specificity of carboxylesterase protection against the toxicity of organophosphorus compounds. Toxicol Appl Pharmacol. 1992;114:306–312. doi: 10.1016/0041-008x(92)90082-4. [DOI] [PubMed] [Google Scholar]
- 29.Maxwell DM, Brecht KM. Carboxylesterase: specificity and spontaneous reactivation of an endogenous scavenger for organophosphorus compounds. J Appl Toxicol. 2001;21(Suppl 1):S103–107. doi: 10.1002/jat.833. [DOI] [PubMed] [Google Scholar]
- 30.Jokanovic M, Kosanovic M, Maksimovic M. Interaction of organophosphorus compounds with carboxylesterases in the rat. Arch Toxicol. 1996;70:444–450. doi: 10.1007/s002040050297. [DOI] [PubMed] [Google Scholar]
- 31.Benschop HP, De Jong LPA. Nerve agent stereoisomers: analysis, isolation, and toxicology. Acc Chem Res. 1988;21:368–374. [Google Scholar]
- 32.Clement JG, Benschop HP, DeJong LP, Wolthuis OL. Stereoisomers of soman (pinacolyl methylphosphonofluoridate): inhibition of serum carboxylic ester hydrolase and potentiation of their toxicity by CBDP (2-(2-methylphenoxy)-4H-1,3,2-benzodioxaphosphorin-2-oxide) in mice. Toxicol Appl Pharmacol. 1987;89:141–143. doi: 10.1016/0041-008x(87)90184-0. [DOI] [PubMed] [Google Scholar]