Abstract
The mammalian target of rapamycin (mTOR) signaling pathway integrates environmental cues, promotes cell growth / differentiation and regulates immune responses. While inhibition of mTOR with rapamycin has potent immunosuppressive activity, mixed effects have been reported in ovalbumin-induced models of allergic asthma. We investigated the impact of two different rapamycin treatment protocols on the major characteristics of allergic asthma induced by the clinically relevant allergen, house dust mite (HDM). In protocol 1, Balb/c mice were exposed to 10 intranasal HDM doses over a period of 24 days and treated with rapamycin simultaneously during the sensitization/exposure period. In protocol 2, rapamycin was administered after the mice had been sensitized to HDM (I.P. injection) and prior to initiation of two intranasal HDM challenges over 4 days. Airway hyperreactivity (AHR), IgE, inflammatory cells, cytokines, leukotrienes, goblet cells, and activated T cells were assessed. In protocol 1, rapamycin blocked HDM-induced increases in AHR, inflammatory cell counts, IgE, and attenuated goblet cell metaplasia. In protocol 2, rapamycin blocked increases in AHR, IgE, T cell activation, and reduced goblet cell metaplasia, but had no effect on inflammatory cell counts. Increases in IL-13 and leukotrienes were also blocked by rapamycin, although increases in IL-4 were unaffected. These data demonstrate that rapamycin can inhibit cardinal features of allergic asthma including increases in AHR, IgE, and goblet cells most likely due to its ability to reduce the production of two key mediators of asthma, IL-13 and leukotrienes. These findings highlight the importance of the mTOR pathway in allergic airway disease.
Introduction
Asthma prevalence has increased substantially in recent years, especially in children (1–3). Allergic asthma is the most common form and is characterized by airway inflammation, airway hyperreactivity (AHR), goblet cell metaplasia, and increases in IgE and Th2 cytokines (1, 4, 5). Although glucocorticoids and bronchodilators are the mainstay of asthma treatment, these therapies are not effective in all asthmatics (1).
The discovery of the drug rapamycin (6, 7) has led to intense study of its target, the mammalian target of rapamycin (mTOR). mTOR is downstream of the phosphoinositide 3-kinase signaling cascade and signals via two complexes: mammalian TOR complex 1 (mTORC1) and mammalian TOR complex 2 (mTORC2) (8, 9). Activation of mTORC1, which is sensitive to rapamycin, leads to phosphorylation and activation of the ribosome S6 kinase and, subsequently, S6 ribosomal protein (S6) which promotes ribosomal protein synthesis (8). Although several reports indicate that mTORC2 is not inhibited by rapamycin, there is evidence showing that rapamycin can inhibit mTORC2 activity, depending on the specific cell type, duration, and dose of rapamycin treatment (10). mTOR is known to play a major role in regulating cell metabolism, growth/differentiation, and survival in many different cell types (8, 11). Dysregulation of this pathway has been implicated in various diseases, including cancer and type 2 diabetes (9, 12, 13). Rapamycin is currently used as an immunosuppressant drug to prevent transplant rejection (14, 15); however, the effects of rapamycin on inflammation in ovalbumin (OVA)-induced models of asthma are mixed (16–18). In addition, studies in OVA models (16–18) did not address whether mTOR inhibition alters IL-13 and leukotrienes, which are important mediators of allergic asthma responses, including AHR and goblet cell metaplasia.
The goal of our study was to determine if rapamycin would attenuate key characteristics of allergic asthma (AHR, inflammation, goblet cell metaplasia, IgE) and important mediators, IL-13 and cysteinyl leukotrienes, in a clinically relevant model induced by exposure to house dust mite (HDM). We hypothesized that inhibition of mTOR with rapamycin would attenuate allergic airway disease via reductions in these key mediators. To test this hypothesis, mice were either exposed to HDM and treated with rapamycin simultaneously, or first sensitized to HDM by systemic injection and then treated with rapamycin during subsequent intranasal HDM challenges. Multiple endpoints were assessed including sensitization, AHR, inflammation, goblet cells, T cells, cytokines and leukotrienes.
Methods
Animals
Animal protocols and procedures were approved by the Animal Care and Use Committee at the Cincinnati Children’s Hospital Research Foundation (Cincinnati, OH). Six to eight week old female Balb/c mice were purchased from Charles River Laboratories (Wilmington, MA). The treatment protocols used in these studies are described below.
Protocol 1
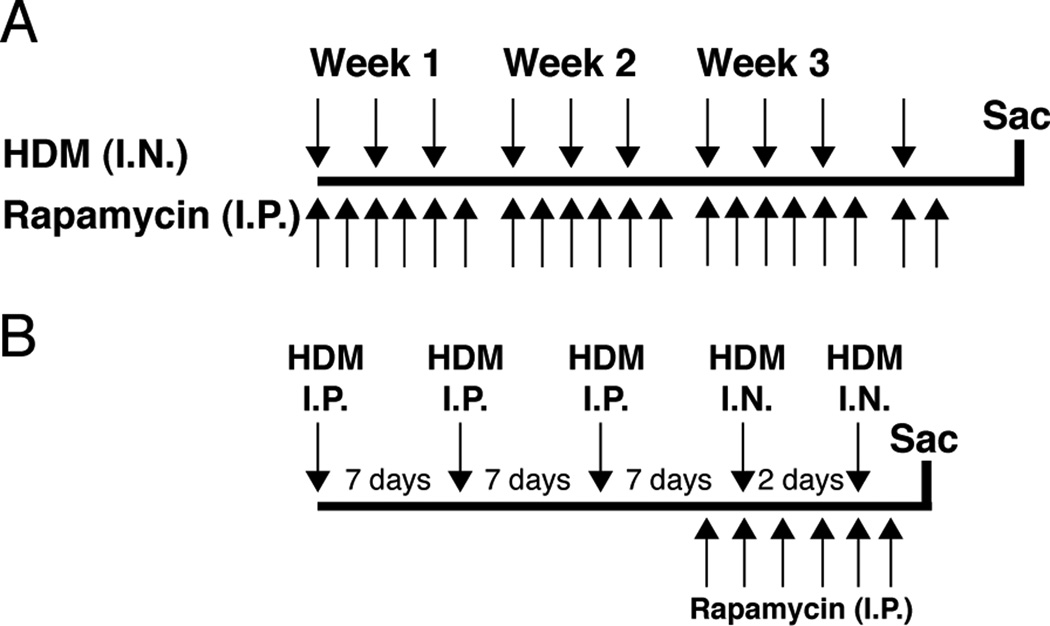
Mice were exposed to 10 intranasal (I.N.) doses of HDM (50µg in 20µl saline; Greer Laboratories, Lenoir, NC) or saline (0.9% NaCl, 20µl; control group) over 24 days (Fig. 1A). In a third study group, mice were exposed to HDM and treated with rapamycin. Rapamycin (4mg/kg) (LC Laboratories, Woburn, MA) was administered by intraperitoneal (I.P.) injection, six times a week starting with the first HDM exposure and continuing until one day after the last HDM exposure for a total of 20 treatments.
FIGURE 1.
Study protocols. A: Protocol 1: Mice were exposed to 10 intranasal (I.N.) HDM doses over 24 days. Rapamycin treatment by intraperitoneal injection (I.P.) was started simultaneously with HDM exposure and continued 6 days per week until one day after the last HDM exposure for a total of 20 injections. B: Protocol 2: Mice were sensitized to HDM by 3 I.P. injections before I.N. challenge with saline or HDM. Rapamycin treatment was started after the last I.P. injection and one day prior to I.N. HDM exposure.
Protocol 2
Mice were sensitized to HDM by three I.P. injections of HDM (50µg in 100µl saline) at 7-day intervals (Fig. 1B). Seven days after the last I.P. injection, two I.N. challenges of HDM (50µg in 20µl saline) were given 2 days apart. Another group was treated with rapamycin (4mg/kg, I.P.), starting one day prior to the first I.N. HDM challenge and continued until one day after the second HDM challenge for a total of 6 rapamycin treatments. Study groups were mice given I.P. Saline + I.N. Saline (Saline/Saline), I.P. HDM + I.N. Saline (HDM/Saline), I.P. HDM + I.N. HDM (HDM/HDM), and I.P. HDM + I.N. HDM + I.P. Rapamycin (HDM/HDM + Rapa).
Airway Hyperreactivity
AHR was studied in anesthetized mice 48 hrs after the last HDM challenge. Anesthesia was delivered by intraperitoneal injection (I.P.) of Ketamine/Xylaxine/Acepromazine (4:1:1) solution (0.2mL/animal). Changes in airway resistance to methacholine were assessed as previously described (19). Briefly, a tracheostomy was performed and the mouse was connected to a flexiVent system (SCIREQ, Montreal, QC, Canada). Airway resistance was measured after nebulization of phosphate buffered saline (1 X PBS) (baseline) and then increasing doses of methacholine (6.25, 12.5, 25, and 50mg/mL; acetyl-β-methylcholine chloride, Sigma, St. Louis, MO). After the flexiVent studies, mice were killed by cutting the thoracic aorta. Blood was collected and serum was extracted for measurements of IgG1 and IgE.
Allergic Sensitization
Total and HDM-specific IgG1 and IgE levels were measured in serum by ELISA to assess allergic sensitization as previously described (20). Briefly, for total IgG1 and IgE, plates were coated overnight with 2µg/ml of anti-mouse IgG1 or IgE in PBS, respectively (Pharmingen, San Jose, CA). For HDM-specific IgG1 and IgE, the plates were coated with 0.01% HDM in PBS. The following day, plates were blocked with 1% BSA in PBS for 1 hour then coated with samples and IgG1 or IgE standards. Biotin-anti-mouse IgG1 or IgE (2.0µg/ml, Pharmingen) were used to capture IgG1 and IgE antibodies, respectively. Streptavidin-HRP (1:100, R & D, Minneapolis, MN) was added to detect biotin labeled antibodies and the reaction was developed by TMB substrate (1:1) (BD Biosciences, San Jose, CA).
Inflammatory cells and cytokines
Bronchoalveolar lavage fluid (BALF) was collected from mice to assess the inflammatory response as previously described (19). Briefly, the lungs were lavaged via a tracheostomy with 1ml of 1 X PBS containing BSA (1%) and EDTA (2mM) as previously described. The BALF was centrifuged (5000 RPM) and the supernatant frozen at −80°C for measurements of cytokines and leukotrienes. Cell pellets were resuspended and red blood cells were lysed using red blood cell lysing buffer (Sigma, St. Louis, MO). The remaining cells were resuspended in 1 X PBS after centrifugation. Total inflammatory cells were counted using a hemacytometer and cytospins were performed on the remaining cells for differential cell counts. Diff-Quick staining of cytospin slides (Shandon Lipshaw, Pittsburg, PA) was performed. Three hundred cells were counted per slide and the percentages of macrophages, lymphocytes, neutrophils, and eosinophils were calculated. Total differential inflammatory cell counts were also calculated. Cytokines in the BALF were measured using a multiplex biomarker panel (Chemokine Panel I) and Luminex xMAP technology (Millipore, Billerica, MA) following manufacturer’s instructions. Cysteinyl leukotriene (C4, D4, E4) levels were measured by a commercial company (ElisaTech, Aurora, CO) using ELISA kits (Cayman, Ann Arbor, Michigan), according to manufacturer’s instructions.
Western Blot Analysis
Western blot analysis was performed on lung homogenates to assess the levels of phosphorylated S6 ribosomal protein (P-S6, 1:1,000, Cell Signaling, Danvers, MA), total S6 (1:1,000, Cell Signaling), chloride channel, calcium activated, family member 3 (CLCA3, 1:2,000, Abcam, Cambridge, MA) (21, 22), and β-tubulin (1:1000, Cell Signaling). Secondary antibodies included goat anti-rabbit and goat anti-mouse (1:10,000, Calbiochem). ECL Plus system was used for chemiluminescence detection (GE Healthcare). An LAS4000 imaging system and Multi Gauge 3 software (Fujifilm, Japan) were used to image and quantitate the chemiluminescent signal for each Western blot. Values were normalized to β-tubulin to control for protein loading and transfer efficiency.
Muc5AC Immunohistochemistry
Lungs were inflation fixed at a constant pressure (25cmH2O) by tracheal installation of 4% paraformaldehyde, transferred to 70% ethanol after 24 hrs, and embedded in paraffin as previously described (19). Immunostaining for Mucin 5AC (Muc5AC) was performed on 5µm paraffin-embedded sections. Slides were incubated with a primary Muc5AC mouse monoclonal antibody (1:200, Thermo Scientific, Waltham, MA) overnight at 4°C, followed by a goat α mouse IgG1 secondary antibody (1:200, Southern Biotech, Birmingham, AL). Signal was detected using the DAB method of detection (19). Digital images of Muc5AC immunostaining were obtained using a Zeiss Axioplan 2 microscope and camera (Carl Zeiss Microimaging, Thornwood, NY).
Quantitative real-time PCR analysis
RNA for qRT-PCR was isolated using the SV Total RNA Isolation System (Promega, Madison, WI) and reverse transcribed into cDNA using an iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA) following the manufacturer’s instructions. cDNA samples were amplified with TaqMan and a primer/probe set specific for Muc5AC was used (assay ID: Mm01276725_g1) (Applied Biosystems, Carlsbad, CA). Quantification of gene expression was analyzed by a 7300 Real Time PCR System (Applied Biosystems) in triplicate and expression levels were normalized to β-actin mRNA levels.
Flow cytometry for activated and regulatory T cells
The upper right lung lobe was minced and incubated at 37°C for 25–30 minutes in 2ml of RPMI 1640 containing Liberase DL (0.5 mg/ml; Roche Diagnostics, Idianapolis, IN) and DNAse I (0.5 mg/ml; Sigma, St Louis, MO). Lung cells were passed through a 70µm cell strainer and the strainer washed with 5ml of RPMI+DNAse I media. Cells were centrifuged and resuspended in 2ml of RPMI before counting with a hemacytometer and viability confirmed by trypan blue exclusion. Approximately 500,000 lung cells were transferred to a 96 well plate with V shaped bottom on ice, centrifuged and resuspended in 1 X PBS containing FcBlock (2.4G2 mAb). To assess the levels of activated T cells, lung T cells were stained with CD3e–FITC, CD4-PE/Cy7, CD69-PE, CD25-APC (BioLegend, San Diego, CA). Live and dead cells were labeled with LIVE/DEAD Fixable Aqua Dead Cell Stain Kit according to manufacturer’s instructions (Invitrogen by Life Technologies, Carlsbad, CA). To assess the levels of regulatory T cells, intracellular staining for Foxp3-PerCP5.5 was performed using the classic protocol and reagents from eBioscience (San Diego,CA). Acquisition was done on a FACS Canto III (Becton Dickinson, Mountain View, CA) and analyzed used FlowJo software (Tree Star, Ashland, OR).
Statistical Analysis
Statistical analysis was performed with Prism 5 software (GraphPad Software, San Diego, CA). Unpaired t-tests, one-way ANOVA with Tukey’s post-hoc test, and two-way ANOVA tests with Bonferroni post-hoc test were used to make comparisons. P<0.05 was considered statistically significant.
Results
Protocol 1
Phosphorylated S6 ribosomal protein
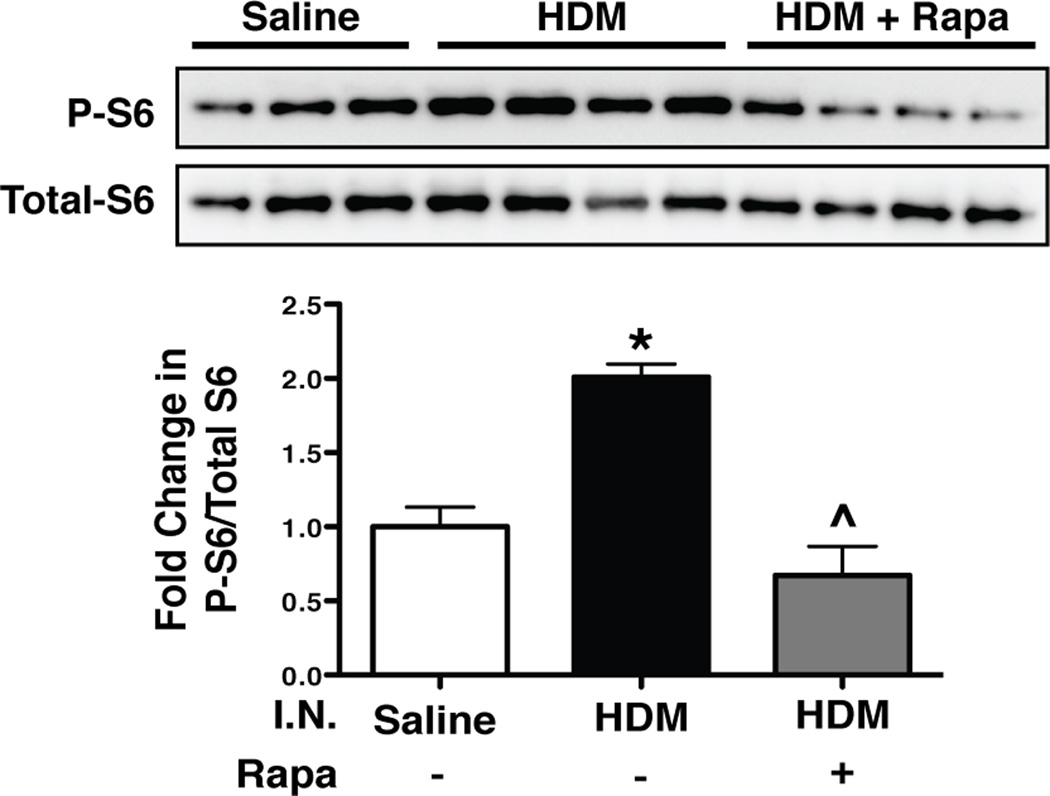
After 10 treatments with intranasal HDM (Fig. 1A), Western blot analysis for phosphorlyated-S6 (P-S6) was performed on lung homogenates from saline controls, HDM, and HDM + Rapamycin treated mice to verify that the dose of rapamycin was sufficient to inhibit signaling downstream of mTOR. Mice exposed to HDM had a 2-fold increase in P-S6 levels compared to saline controls (Fig. 2). Rapamycin treatment blocked this increase.
FIGURE 2.
Phosphorylated S6 levels corrected to total S6 levels in lung homogenates of mice exposed to intranasal (I.N.) HDM and treated with rapamycin. Western blot analysis of phosphorylated S6 (P-S6) protein, a downstream mediator of mTOR signaling, was increased after HDM exposure compared to saline controls. Rapamycin (rapa) prevented this increase in P-S6. n = 3 to 4 mice per group. *p<0.05 vs. Saline, ^p<0.05 vs. HDM.
AHR and goblet cell metaplasia
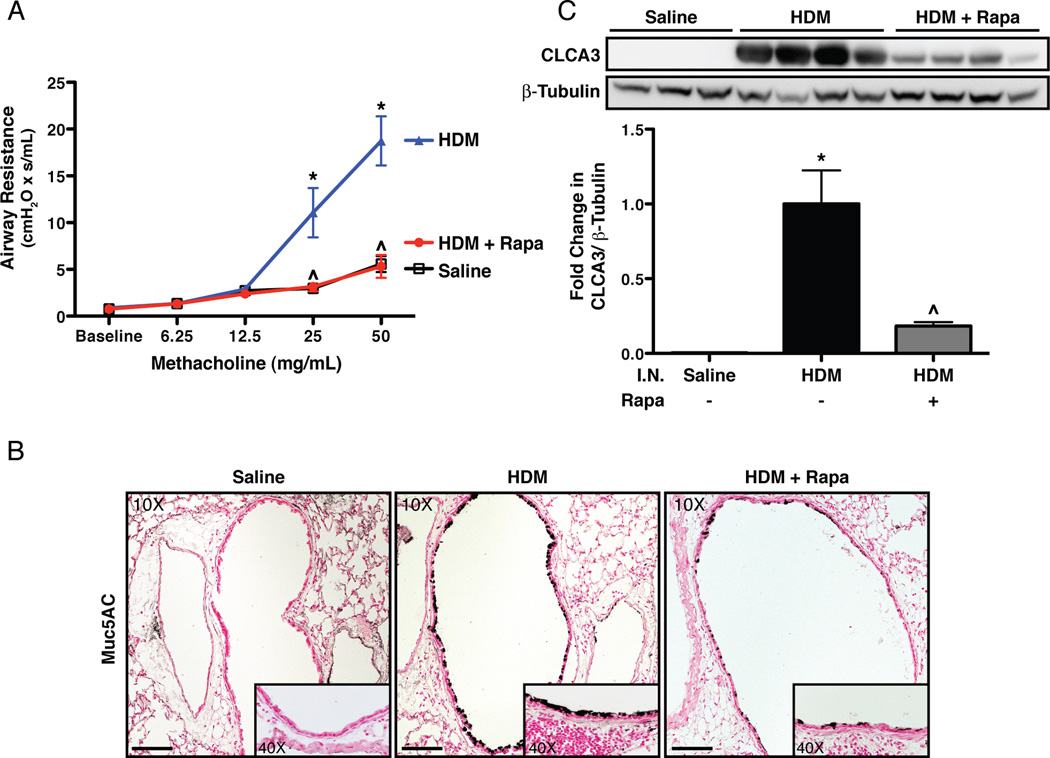
To determine if rapamycin treatment reduces HDM-induced AHR, methacholine challenges were performed using a flexiVent system. Increases in AHR were observed in HDM exposed mice at 25mg/mL (4.4-fold) and 50mg/mL (3.6-fold) methacholine compared to saline controls (Fig. 3A). AHR in HDM exposed mice treated with rapamycin was similar to saline controls. To assess changes in goblet cells, Muc5AC immunostaining was performed. Increased Muc5AC staining was observed in HDM exposed mice (Fig. 3B) compared to saline controls. Increases in another goblet cell protein, CLCA3, were observed in HDM exposed mice by Western blot (Fig. 3C). Rapamycin treatment reduced Muc5AC staining in the lungs and decreased CLCA3 protein levels by 80%.
FIGURE 3.
Airway hyperreactivity and goblet cells in mice exposed to intranasal (I.N.) HDM and rapamycin (rapa). A: Rapamycin prevented increases in airway resistance to methacholine in HDM exposed mice. n = 7 to 8 mice per group in two independent experiments. B: Rapamycin attenuated increases in Muc5AC immunostaining in airways after HDM exposure. On 10X panels, scale bar equals 100µm. C: Rapamycin attenuated increases in the goblet cell protein, CLCA3, by western blot in HDM exposed mice. n = 3 to 4 mice per group. *p<0.05 vs. Saline, ^p<0.05 vs. HDM.
Inflammatory cells and allergic sensitization
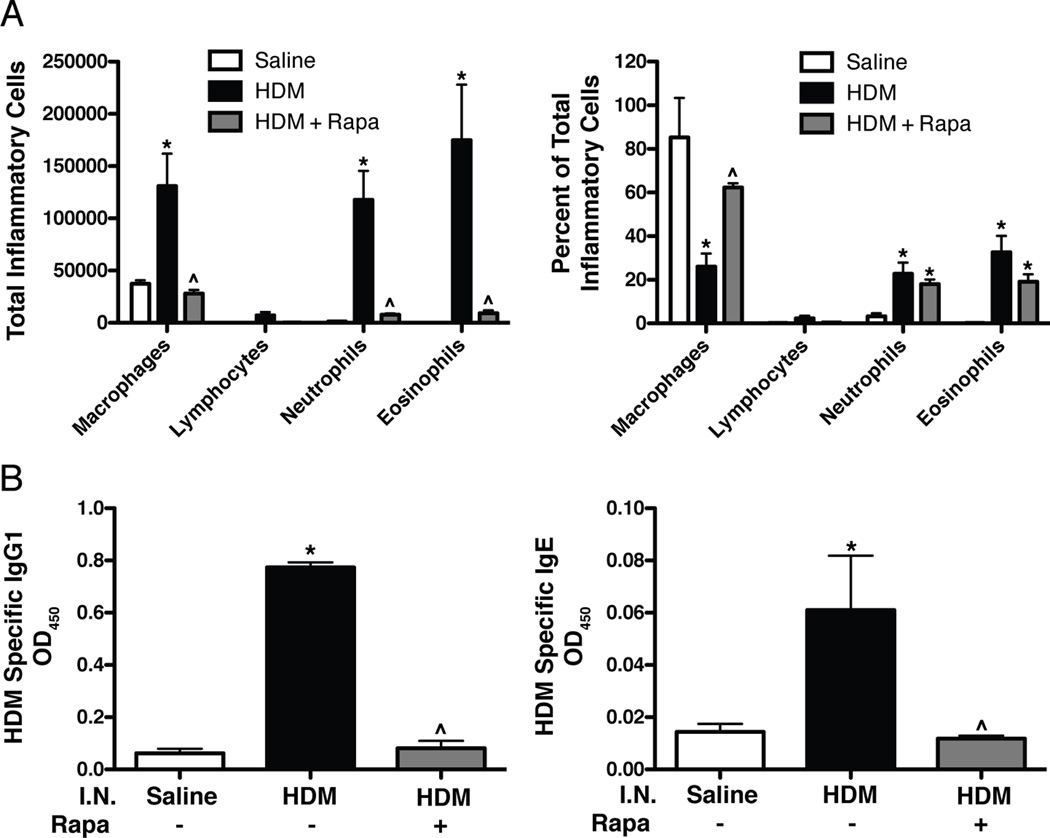
To determine the effects of rapamycin on allergic airway inflammation, total and differential inflammatory BALF cell counts were performed. Total BALF cell count increases with HDM exposure were prevented by rapamycin (data not shown). Specifically, increases in macrophages, neutrophils, and eosinophils were all prevented by rapamycin treatment (Fig. 4A). The percentage of macrophages, neutrophils, and eosinophils also changed with HDM exposure, however there was not a significant change in the percentage of neutrophils and eosinophils after rapamycin treatment (Fig. 4A). To assess allergic sensitization, HDM specific IgG1 and IgE levels were measured in serum and both were elevated in HDM exposed mice (Fig. 4B). Rapamycin prevented these increases, so that HDM specific IgG1 and IgE levels were similar to saline controls. Rapamycin also blocked increases in total IgG1 and IgE levels after HDM exposure (Supplemental Fig. S1).
FIGURE 4.
Inflammatory cell counts in BALF and HDM-specific IgG1 and IgE levels in serum. A: HDM exposure induced increases in total inflammatory cells and the percentage of neutrophils and eosinophils compared to saline controls. Rapamycin prevented HDM-induced increases in total inflammatory cell counts in the BALF. n = 6 mice per group. B: HDM specific IgG1 and IgE levels were increased after HDM exposure compared to saline controls and blocked by rapamycin. n = 9 mice per group. *p<0.05 vs. Saline, ^p<0.05 vs. HDM.
Protocol 2
IgG1 and IgE
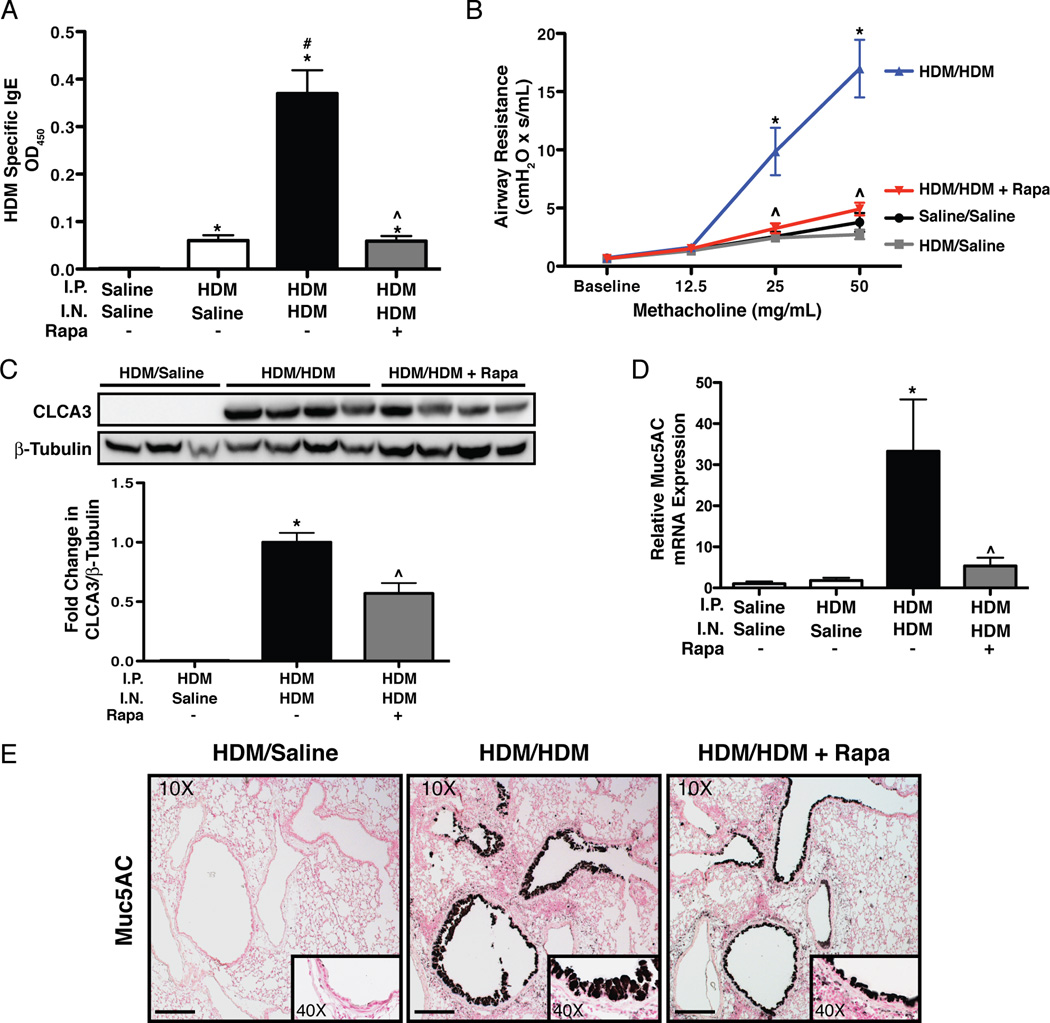
Data from protocol 1 demonstrates that administration of rapamycin simultaneously with HDM exposure blocks allergic sensitization and disease; however, the protective effect of rapamycin on these processes may be due to rapamycin preventing allergic sensitization. In protocol 2, allergic sensitization was first established by I.P. injection of HDM. Rapamycin treatment was initiated after the last I.P. injection of HDM and one day prior to I.N. HDM challenge (Fig. 1B). Control animals for these experiments were injected I.P. with either saline or HDM and then treated with I.N. saline (Saline/Saline or HDM/Saline, respectively). HDM/Saline mice showed increases in HDM specific IgE and IgG1 compared to Saline/Saline controls (Fig. 5A and Supplemental Fig. S2). HDM/HDM mice had higher levels of HDM-specific IgE (7-fold) and IgG1 (1.25-fold) compared to HDM/Saline control mice. In HDM/HDM+Rapa mice, HDM-specific IgE and IgG1 levels were similar to HDM/Saline controls. Total serum IgG1 and IgE were also increased after HDM sensitization (Supplemental Fig. S2). Rapamycin attenuated the increase in total IgE, but not total IgG1.
FIGURE 5.
IgE, AHR and goblet cells in sensitized mice exposed to HDM and rapamycin. A: Rapamycin attenuated HDM-induced IgE. n = 4–11 mice per group. *p<0.05 vs. Saline/Saline, #p<0.05 vs. HDM/Saline, ^p<0.05 vs. HDM/HDM. B: Rapamycin blocked HDM-induced increases in AHR after methacholine challenge. n = 3–10 mice per group in two independent experiments. *p<0.05 vs. Saline/Saline, HDM/Saline, ^p<0.05 vs. HDM/HDM. C: Western blot analysis shows that rapamycin attenuated HDM-induced increases in CLCA3. n = 3 to 4 mice per group. *p<0.05 vs. HDM/Saline, ^p<0.05 vs. HDM/HDM. D: Q-RT-PCR analysis revealed decreased Muc5AC mRNA expression after HDM challenge in lungs of rapamycin treated mice. Gene expression was normalized to β-actin. n = 3–5 mice per group. Samples were run in triplicate. *p<0.05 vs. HDM/Saline, ^p<0.05 vs. HDM/HDM. E: Rapamycin reduced Muc5AC immunostaining in airways of HDM/HDM mice. On 10X panels, sale bar equals100µm.
AHR and goblet cells
AHR was increased in HDM/HDM mice at 25mg/mL (4.5-fold) and 50mg/mL (5.3-fold) methacholine compared to HDM/Saline controls (Fig. 5B). In HDM/HDM+Rapa mice, AHR was similar to both control groups (Saline/Saline, HDM/Saline). To assess goblet cells, CLCA3 protein levels were measured by western blot analysis. CLCA3 protein was not detectable in HDM/Saline control mice, but was readily detectable in lung homogenates from HDM/HDM mice. CLCA3 protein was reduced by 41% in HDM/HDM+Rapa mice compared to HDM/HDM mice (Fig. 5C). To further assess the goblet cell response, quantitative real-time RT-PCR was performed on lung RNA for the expression of Muc5AC. Muc5AC mRNA levels were normalized to β-actin mRNA. Muc5AC mRNA expression was increased 32-fold in HDM/HDM mice compared to Saline/Saline and HDM/Saline controls. Rapamycin reduced Muc5AC mRNA expression by 84% (Fig. 5D). Abundant goblet cells were also detected in the airways of HDM/HDM mice by Muc5AC immunostaining, whereas no staining was detectable in HDM/Saline controls (Fig. 5E). Muc5AC staining for goblet cells was reduced in HDM/HDM+Rapa mice.
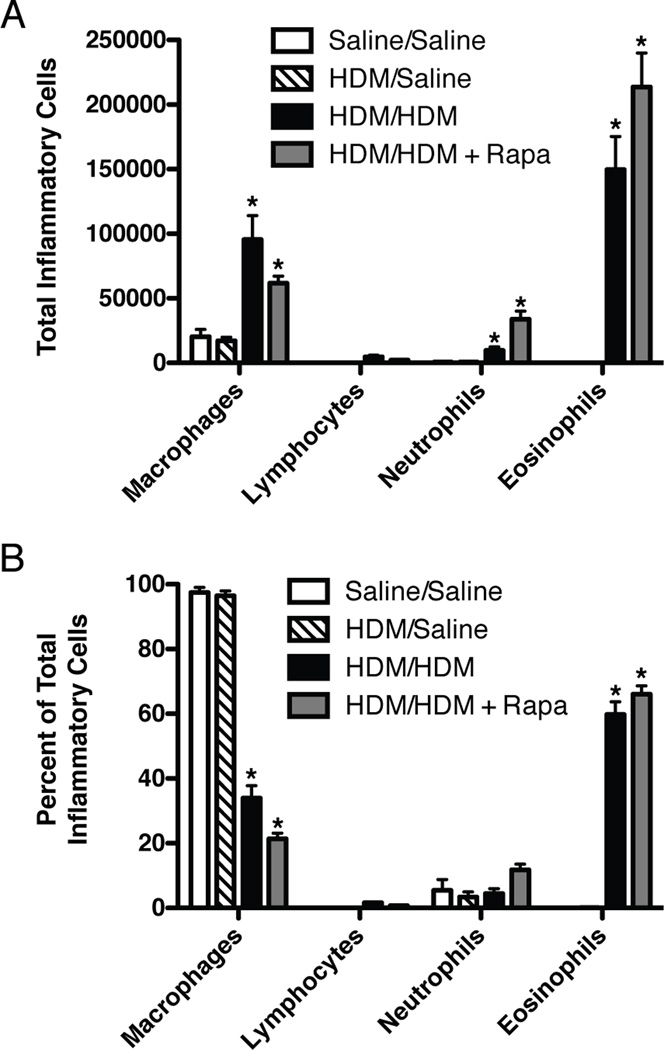
Inflammatory cell counts
Total BALF macrophages, neutrophils, and eosinophils (Fig. 6A) as well as the percentage of eosinophils (Fig. 6B) were increased in HDM/HDM mice compared to HDM/Saline controls and remained elevated in HDM/HDM+Rapa mice (Fig. 6A and 6B).
FIGURE 6.
Inflammatory cells in BALF from sensitized mice exposed to HDM and rapamycin. A: Total numbers of macrophages, neutrophils, and eosinophils were increased with HDM and unaltered by rapamycin treatment. n = 3–11 mice per group in two independent experiments. B: The percentage of eosinophils were increased after HDM challenge and unaffected by rapamycin treatment. n = 3–11 mice per group in two independent experiments. *p<0.05 vs. Saline/Saline, HDM/Saline.
T cells, cytokines, and leukotrienes
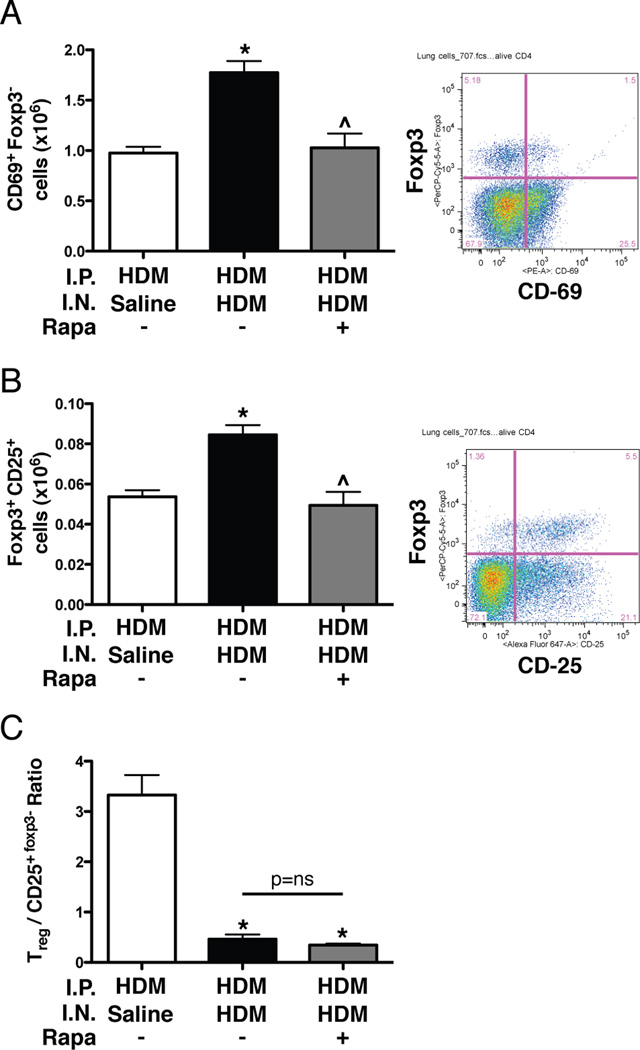
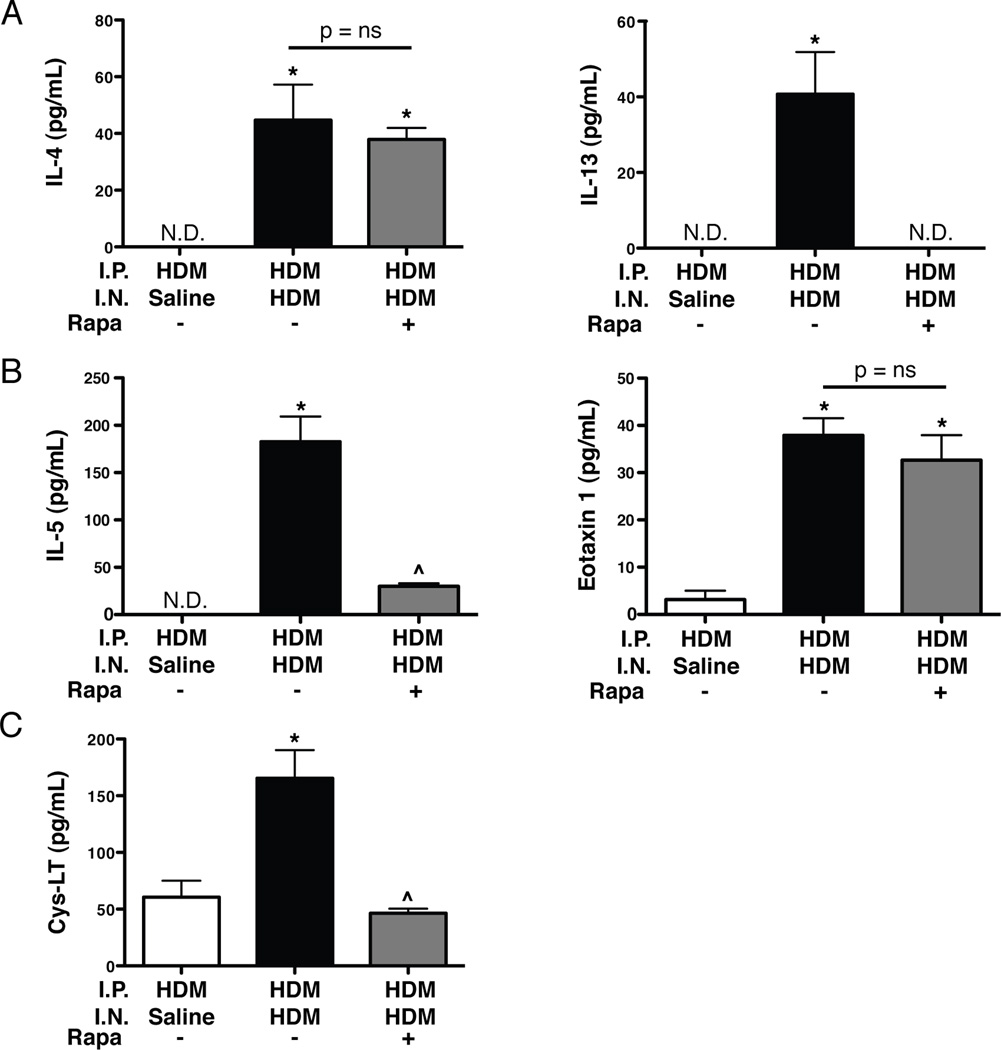
To examine the cellular and molecular mediators of the allergic response, T cells, cytokines, and leukotriene levels were assessed. The total number of CD69+Foxp3− CD4+ T cells was increased (1.7-fold) in the lungs of HDM/HDM mice compared to HDM/Saline controls (Fig. 7A and Supplemental Fig. S3). CD69+Foxp3− T cell numbers were reduced in HDM/HDM+Rapa mice compared to HDM/HDM mice, and were similar to HDM/Saline controls. The total number of Foxp3+CD25+ T regulatory cells in the lungs of HDM/HDM mice was increased (1.6 fold) compared to HDM/Saline controls. Foxp3+CD25+ T regulatory cell numbers in HDM/HDM+Rapa mice were reduced compared to HDM/HDM mice, and were similar to HDM/Saline controls (Fig. 7B and Supplemental Fig. S3). The ratio of Foxp3+CD25+ T regulatory cells to CD25+Foxp3− T cells was lower in HDM/HDM and HDM/HDM+Rapa mice compared to HDM/Saline controls (Fig. 7C). IL-4 levels were undetectable in the BALF of HDM/Saline controls, but were readily detectable in HDM/HDM mice (Fig. 8A). IL-4 levels were similar in HDM/HDM+Rapa mice compared to HDM/HDM mice. IL-13 levels were also undetectable in HDM/Saline control mice, but readily detectable in HDM/HDM mice (Fig. 8A). In rapamycin treated mice (HDM/HDM+Rapa) IL-13 levels were not detectable. IL-5 and eotaxin 1 levels were increased in HDM/HDM mice compared to HDM/Saline controls (Fig. 8B). Rapamycin treatment decreased IL-5; however, eotaxin 1 levels remained elevated in HDM/HDM+Rapa mice. Cysteinyl leukotriene levels were increased in BALF from HDM/HDM mice compared to HDM/Saline control mice (Fig. 8C). Leukotriene levels in HDM/HDM+Rapa mice were similar to HDM/Saline controls.
FIGURE 7.
Activated T cells and regulatory T cells in lungs of sensitized mice exposed to HDM and rapamycin. A: Increases in the total number of CD69+Foxp3−T cells with HDM was attenuated by rapamycin. n = 3–11 mice per group in two independent experiments. *p<0.05 vs. HDM/Saline, ^p<0.05 vs. HDM/HDM. B: The total number of Foxp3+CD25+ regulatory T cells was increased with HDM and back to the level of HDM/Saline control mice with rapamycin treatment. n = 3–11 mice per group in two independent experiments. *p<0.05 vs. HDM/Saline, ^p<0.05 vs. HDM/HDM. C: The ratio of Foxp3+CD25+ regulatory T cells to CD25+Foxp3−T cells is lower in HDM/HDM and HDM/HDM+Rapa mice compared to HDM/Saline controls. n = 3–11 mice per group in two independent experiments. *p<0.05 vs. HDM/Saline.
FIGURE 8.
Cytokines and cysteinyl leukotrienes levels in BALF of sensitized mice exposed to HDM and rapamycin. A: Rapamycin blocked HDM-induced increases in IL-13, but had no effect on IL-4 levels. n = 4–12 mice per group. *p<0.05 vs. HDM/Saline, ^p<0.05 vs. HDM/HDM. B: IL-5 and eotaxin 1 levels were increased in HDM/HDM mice. Rapamycin reduced IL-5 levels in the BALF, but had no affect on eotaxin 1 levels. n = 4–12 mice per group. *p<0.05 vs. HDM/Saline, ^p<0.05 vs. HDM/HDM. C: Rapamycin blocked HDM-induced increases in cysteinyl leukotrienes (C4, D4, E4). n = 5–10 mice per group. *p<0.05 vs. HDM/Saline, ^p<0.05 vs. HDM/HDM.
Discussion
The goal of our study was to determine whether the mTOR inhibitor, rapamycin, would suppress key characteristics and mediators in a clinically relevant asthma model induced by the aeroallergen HDM. We utilized two different protocols to investigate the effects of rapamycin on allergic responses to HDM, and while they cannot be directly compared, each protocol was designed to address specific questions. In the first protocol, we administered rapamycin to mice simultaneously with intranasal HDM to determine whether rapamycin could prevent HDM-induced allergic responses. Increases in AHR and inflammation were prevented by rapamycin treatment. In addition, increases in HDM-specific IgG1 and IgE levels were prevented by rapamycin, indicating that simultaneous treatment with rapamycin prevented allergic sensitization. Therefore, it was unclear whether inhibition of AHR and inflammatory cell influx into the lungs was due to direct effects on these processes or because allergic sensitization was prevented. Hence, we used a second protocol to address the question of whether rapamycin could suppress pulmonary responses to allergen exposure after allergic sensitization had already been established. In this protocol, we sensitized mice to HDM (by I.P. injections) before exposing the lung to the allergen and treating with rapamycin. In these sensitized mice, AHR, inflammatory cell influx (especially eosinophils), and IgE increased dramatically with intranasal HDM exposure. Rapamycin treatment, given after sensitization and just prior to HDM exposure, prevented these exacerbations, including the large increase in AHR and IgE. To explore potential mechanisms, Th2 cytokines and cysteinyl leukotrienes in BALF were measured. While IL-4 and eotaxin 1 levels were unaffected by rapamycin treatment, increases in IL-13 and leukotrienes with HDM exposure were completely suppressed. Decreases in IL-13 and leukotriene levels, both important mediators of AHR and goblet cell metaplasia, provides insight into a potential mechanism for the effects of rapamycin on allergic airway disease. The suppressive effects of rapamycin on allergen-induced IL-13 and leukotriene levels, to our knowledge, have not been previously reported in studies using allergic asthma models.
Previous studies have used SAR 943, a derivative of rapamycin, and rapamycin in the OVA-induced allergic asthma model. In Brown-Norway rats sensitized to OVA, SAR 943 given prior to OVA challenge to the lung reduced the number of CD4+ T cells recruited into the lung; however, AHR and eosinophil infiltration were unaffected (16). A similar study in mice showed attenuation of eosinophils, IL-4, goblet cells, and AHR responses after SAR 943 treatment (17), but no effect on serum IgE levels. In a third study, mice sensitized with OVA and treated with rapamycin showed reductions in IgE levels; however, no changes in eosinophils or AHR were observed (18). Hence, these OVA studies reported mixed effects of mTOR inhibitors on allergic responses and as mentioned earlier did not assess IL-13 or leukotrienes.
Prior studies have demonstrated an important role for inflammation in the development of AHR, with increased inflammatory cells typically correlating with increases in AHR (23). However, in our second protocol we observed a marked disconnect between inflammatory cell influx and AHR. Despite no effect on inflammatory cell influx, rapamycin blocked the increase in AHR following HDM challenge to the lung. This dissociation between inflammatory cells and AHR has been observed previously. For example, in patients with allergic asthma, the number of inflammatory cells in the lung did not correlate with the degree of AHR (23, 24). In our second protocol, eotaxin 1 levels were increased in HDM/HDM mice, but unaffected by rapamycin treatment which is consistent with the lack of a change in eosinophils levels after rapamycin treatment, despite decreases in IL-5. This maintenance of elevated eosinophils is in contrast to the suppressive effects of rapamycin treatment on AHR, although there are conflicting reports on the role of eosinophils in AHR (25–28).
Another interesting observation in our study was that rapamycin treatment did not affect IL-4 levels in BALF, despite a major reduction in IL-13 levels. It is unclear which cell types are responsible for the differences in IL-4 and IL-13 levels we observed since a number of cell types are known to secrete these cytokines (29). Although mast cells can produce both IL-4 and IL-13, it has been reported that rapamycin did not affect degranulation and cytokine release from mouse mast cells (30). It is unlikely that eosinophils are the primary source of IL-4 and IL-13 in our model since only IL-13 levels are affected by rapamycin, whereas no changes in IL-4 and eosinophil numbers were observed. In addition, although both mast cells and eosinophils can release Th2 cytokines, the primary source of cytokines like IL-4 and IL-13 is most likely Th2 lymphocytes (29). Interestingly, it has been reported that when T cells were co-stimulated under Th2 skewing conditions and treated with rapamycin, IL-13 levels were more severely decreased and only a modest decrease in IL-4 levels were observed (31). It is possible that other cellular sources are contributing to sustained levels of IL-4 in our model and future studies will address this. Interestingly, despite no changes in IL-4, which is known to be an important mediator of the IgE response, the large increase in IgE detected after allergen challenge was blocked by rapamycin. In addition, AHR was still completely suppressed by rapamycin suggesting that reductions in IL-13 and/or leukotrienes were responsible, as these are known to play important roles in allergic asthma (32–36). However, it has also been reported that rapamycin can inhibit contractile proteins in airway smooth muscle cells (37). This raises the possibility that reductions in AHR in our second protocol could be due to direct effects of rapamycin on airway smooth muscle contractility.
The importance of mTOR in regulating immune responses is becoming increasingly apparent. Currently, rapamycin is used in patients after organ transplant as an immunosuppressant drug (12, 14). Early studies demonstrated a role for mTOR in regulating T cell proliferation, especially after cytokine stimulation (38). More recent studies have shown that T cells deficient in mTOR fail to become Th1, Th2, or Th17 effector cells under skewing conditions and instead default to Foxp3+ regulatory T cells (39). These data and others highlight the importance of mTOR in T cell differentiation (39, 40). Similar to other reports (41, 42), we demonstrated reductions in activated T cells in the lung with rapamycin treatment. However, in contrast to in vitro studies (39, 43, 44), a decrease in the total number of T regulatory cells with rapamycin treatment was seen in our in vivo study. This may be due to the fact that total T cell numbers were reduced by rapamycin treatment. Although T regulatory cell numbers were decreased with rapamycin, they did not fall below HDM/Saline controls. In addition, although the total number of T regulatory cells were decreased with rapamycin, the ratio of T regulatory cells to conventional T cells was not different compared to HDM/HDM mice.
Asthma is a complex disease that manifests differently among individual patients. Currently, inhaled glucocorticosteroids are the most effective anti-inflammatory therapies for the treatment of asthma (1), although some patients are refractory to glucocorticosteroids. Omalizumab, a relatively new therapy, is being used in the treatment of asthma and targets IgE. Omalizumab has been shown to be an effective treatment for allergic asthma as assessed by decreased asthma exacerbations and hospitalizations in patients receiving this therapy (45, 46). In our studies, rapamycin treatment suppressed or attenuated key characteristics of the asthmatic response including AHR, IgE, and goblet cell metaplasia, as well as important mediators including IL-13 and leukotrienes. Additional studies are necessary to elucidate the potential contribution of each of these mediators to the effects of rapamycin on the allergic response and AHR. Understanding these mechanisms may provide further insight into the role of mTOR in allergic disease.
Supplementary Material
Acknowledgments
The authors would like to thank Patricia Pastura for technical assistance in these studies and Dr. Satish Madala for critical review of the manuscript.
Abbreviations used in this article
- HDM
house dust mite
- mTOR
mammalian target of rapamycin
- mTORC1
Mammalian TOR complex 1
- mTORC2
Mammalian TOR complex 2
- AHR
airway hyperreactivity
- CLCA3
Chloride channel, calcium activated, family member 3
- I.P.
intraperitoneal
- I.N.
intranasal
- S6
S6 ribosomal protein
- BALF
Bronchoalveolar lavage fluid
Footnotes
This research was funded by National Institutes of Health Grants U19A170235 (G.K.Khurana Hershey), HL097135 (T. D. Le Cras and G. K. Khurana Hershey), NIEHS T32 ES010957 (E. B. Brandt), and American Heart Association Established Investigator Grant 740069N (T. D. Le Cras)
The online version of this article contains supplemental material
Disclosures: The authors have no disclosures or conflicts of interest to declare.
References
- 1.Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. The Eur Respir J. 2008;31:143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 2.Hesselmar B, Aberg B, Eriksson B, et al. Asthma in children: prevalence, treatment, and sensitization. Pediatr Allergy Immunol. 2000;11:74–79. doi: 10.1034/j.1399-3038.2000.00070.x. [DOI] [PubMed] [Google Scholar]
- 3.Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 4.Hamid Q, Tulic M. Immunobiology of asthma. Annu Rev Physiol. 2009;71:489–507. doi: 10.1146/annurev.physiol.010908.163200. [DOI] [PubMed] [Google Scholar]
- 5.Holgate ST. Pathogenesis of asthma. Clin Exp Allergy. 2008;38:872–897. doi: 10.1111/j.1365-2222.2008.02971.x. [DOI] [PubMed] [Google Scholar]
- 6.Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplantation. 2003;35:7S–14S. doi: 10.1016/s0041-1345(03)00211-2. [DOI] [PubMed] [Google Scholar]
- 7.Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. The J Antibiot. 1975;28:721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- 8.Reiling JH, Sabatini DM. Stress and mTORture signaling. Oncogene. 2006;25:6373–6383. doi: 10.1038/sj.onc.1209889. [DOI] [PubMed] [Google Scholar]
- 9.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 11.Powell JD, Delgoffe GM. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity. 2010;33:301–311. doi: 10.1016/j.immuni.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsang CK, Qi H, Liu LF, et al. Targeting mammalian target of rapamycin (mTOR) for health and diseases. Drug Discov Today. 2007;12:112–124. doi: 10.1016/j.drudis.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Saunders RN, Metcalfe MS, Nicholson ML. Rapamycin in transplantation: a review of the evidence. Kidney Int. 2001;59:3–16. doi: 10.1046/j.1523-1755.2001.00460.x. [DOI] [PubMed] [Google Scholar]
- 15.Saemann MD, Haidinger M, Hecking M, et al. The multifunctional role of mTOR in innate immunity: implications for transplant immunity. Am J Transplant. 2009;9:2655–2661. doi: 10.1111/j.1600-6143.2009.02832.x. [DOI] [PubMed] [Google Scholar]
- 16.Eynott PR, Salmon M, Huang TJ, et al. Effects of cyclosporin A and a rapamycin derivative (SAR943) on chronic allergic inflammation in sensitized rats. Immunology. 2003;109:461–467. doi: 10.1046/j.1365-2567.2003.01672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujitani Y, Trifilieff A. In vivo and in vitro effects of SAR 943, a rapamycin analogue, on airway inflammation and remodeling. Am J Respir Crit Care Med. 2003;167:193–198. doi: 10.1164/rccm.200205-455OC. [DOI] [PubMed] [Google Scholar]
- 18.Nagai H, Maeda Y, Tanaka H. The effect of anti-IL-4 monoclonal antibody, rapamycin and interferon-gamma on airway hyperreactivity to acetylcholine in mice. Clin Exp Allergy. 1997;27:218–224. [PubMed] [Google Scholar]
- 19.Kramer EL, Mushaben EM, Pastura PA, et al. Early growth response-1 suppresses epidermal growth factor receptor-mediated airway hyperresponsiveness and lung remodeling in mice. Am J Respir Cell Mol Biol. 2009;41:415–425. doi: 10.1165/rcmb.2008-0470OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Lewkowich IP, Kohl G, et al. A protective role for C5a in the development of allergic asthma associated with altered levels of B7-H1 and B7-DC on plasmacytoid dendritic cells. J Immunol. 2009;182:5123–5130. doi: 10.4049/jimmunol.0804276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Cras TD, Acciani TH, Mushaben EM, et al. Epithelial EGF receptor signaling mediates airway hyperreactivity and remodeling in a mouse model of chronic asthma. Am J Physiol Lung Cell Mol Physiol. 2011;300:L414–421. doi: 10.1152/ajplung.00346.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leverkoehne I, Gruber AD. The murine mCLCA3 (alias gob-5) protein is located in the mucin granule membranes of intestinal, respiratory, and uterine goblet cells. J Histochem Cytochem. 2002;50:829–838. doi: 10.1177/002215540205000609. [DOI] [PubMed] [Google Scholar]
- 23.Haley KJ, Drazen JM. Inflammation and airway function in asthma: what you see is not necessarily what you get. Am J Respir Crit Care Med. 1998;157:1–3. doi: 10.1164/ajrccm.157.1.ed-19. [DOI] [PubMed] [Google Scholar]
- 24.Crimi E, Spanevello A, Neri M, et al. Dissociation between airway inflammation and airway hyperresponsiveness in allergic asthma. Am J Respir Crit Care Med. 1998;157:4–9. doi: 10.1164/ajrccm.157.1.9703002. [DOI] [PubMed] [Google Scholar]
- 25.Denzler KL, Borchers MT, Crosby JR, et al. Extensive eosinophil degranulation and peroxidase-mediated oxidation of airway proteins do not occur in a mouse ovalbumin-challenge model of pulmonary inflammation. J Immunol. 2001;167:1672–1682. doi: 10.4049/jimmunol.167.3.1672. [DOI] [PubMed] [Google Scholar]
- 26.Humbles AA, Lloyd CM, McMillan SJ, et al. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 27.Lee JJ, Dimina D, Macias MP, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 28.Siegle JS, Hansbro N, Herbert C, et al. Airway hyperreactivity in exacerbation of chronic asthma is independent of eosinophilic inflammation. American journal of respiratory cell and molecular biology. 2006;35:565–570. doi: 10.1165/rcmb.2006-0135OC. [DOI] [PubMed] [Google Scholar]
- 29.Gessner A, Mohrs K, Mohrs M. Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. J Immunol. 2005;174:1063–1072. doi: 10.4049/jimmunol.174.2.1063. [DOI] [PubMed] [Google Scholar]
- 30.Kim MS, Kuehn HS, Metcalfe DD, et al. Activation and function of the mTORC1 pathway in mast cells. J Immunol. 2008;180:4586–4595. doi: 10.4049/jimmunol.180.7.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung U, Foley JE, Erdmann AA, et al. Ex vivo rapamycin generates Th1/Tc1 or Th2/Tc2 Effector T cells with enhanced in vivo function and differential sensitivity to post-transplant rapamycin therapy. Biol Blood Marrow Transplant. 2006;12:905–918. doi: 10.1016/j.bbmt.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 32.Barrett NA, Austen KF. Innate cells and T helper 2 cell immunity in airway inflammation. Immunity. 2009;31:425–437. doi: 10.1016/j.immuni.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finkelman FD, Hogan SP, Hershey GK, et al. Importance of cytokines in murine allergic airway disease and human asthma. J Immunol. 2010;184:1663–1674. doi: 10.4049/jimmunol.0902185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuperman DA, Huang X, Koth LL, et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med. 2002;8:885–889. doi: 10.1038/nm734. [DOI] [PubMed] [Google Scholar]
- 35.Montuschi P, Sala A, Dahlen SE, et al. Pharmacological modulation of the leukotriene pathway in allergic airway disease. Drug Discov Today. 2007;12:404–412. doi: 10.1016/j.drudis.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Vargaftig BB, Singer M. Leukotrienes, IL-13, and chemokines cooperate to induce BHR and mucus in allergic mouse lungs. Am J Physiol Lung Cell Mol Physiol. 2003;284:L260–269. doi: 10.1152/ajplung.00226.2002. [DOI] [PubMed] [Google Scholar]
- 37.Halayko AJ, Kartha S, Stelmack GL, et al. Phophatidylinositol-3 kinase/mammalian target of rapamycin/p70S6K regulates contractile protein accumulation in airway myocyte differentiation. American journal of respiratory cell and molecular biology. 2004;31:266–275. doi: 10.1165/rcmb.2003-0272OC. [DOI] [PubMed] [Google Scholar]
- 38.Dumont FJ, Staruch MJ, Koprak SL, et al. Distinct mechanisms of suppression of murine T cell activation by the related macrolides FK-506 and rapamycin. J Immunol. 1990;144:251–258. [PubMed] [Google Scholar]
- 39.Delgoffe GM, Kole TP, Zheng Y, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu G, Yang K, Burns S, et al. The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nat Immunol. 2010;11:1047–1056. doi: 10.1038/ni.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 42.Kang J, Huddleston SJ, Fraser JM, et al. De novo induction of antigen-specific CD4+CD25+Foxp3+ regulatory T cells in vivo following systemic antigen administration accompanied by blockade of mTOR. J Leukocyte Biol. 2008;83:1230–1239. doi: 10.1189/jlb.1207851. [DOI] [PubMed] [Google Scholar]
- 43.Strauss L, Whiteside TL, Knights A, et al. Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. J Immunol. 2007;178:320–329. doi: 10.4049/jimmunol.178.1.320. [DOI] [PubMed] [Google Scholar]
- 44.Valmori D, Tosello V, Souleimanian NE, et al. Rapamycin-mediated enrichment of T cells with regulatory activity in stimulated CD4+ T cell cultures is not due to the selective expansion of naturally occurring regulatory T cells but to the induction of regulatory functions in conventional CD4+ T cells. J Immunol. 2006;177:944–949. doi: 10.4049/jimmunol.177.2.944. [DOI] [PubMed] [Google Scholar]
- 45.Bousquet J, Cabrera P, Berkman N, et al. The effect of treatment with omalizumab, an anti-IgE antibody, on asthma exacerbations and emergency medical visits in patients with severe persistent asthma. Allergy. 2005;60:302–308. doi: 10.1111/j.1398-9995.2004.00770.x. [DOI] [PubMed] [Google Scholar]
- 46.D'Amato G, Salzillo A, Piccolo A, et al. A review of anti-IgE monoclonal antibody (omalizumab) as add on therapy for severe allergic (IgE-mediated) asthma. Ther Clin Risk Manag. 2007;3:613–619. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.