Abstract
Objective
To examine the prevalence and correlates of non-opioid and opioid analgesic use and descriptively evaluate potential undertreatment in a sample of community-dwelling elders with symptomatic knee and/or hip osteoarthritis (OA).
Design
Cross-sectional
Setting
Health, Aging and Body Composition Study
Patients
652 participants attending the year 6 visit (2002-03) with symptomatic knee and/or hip OA.
Outcome Measures
Analgesic use was defined as taking ≥ 1 non-opioid and/or ≥ 1 opioid receptor agonist. Non-opioid and opioid doses were standardized across all agents by dividing the daily dose used by the minimum effective analgesic daily dose. Inadequate pain control was defined as severe/extreme OA pain in the past 30 days from a modified Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC).
Results
Just over half (51.4%) reported taking at least one non-opioid analgesic and approximately 10% were taking an opioid, most (88.5%) of whom also took a non-opioid. One in five participants (19.3%) had inadequate pain control, 39% of whom were using < 1 standardized daily dose of either a non-opioid or opioid analgesic. In adjusted analyses, severe/extreme OA pain was significantly associated with both non-opioid (adjusted odds ratio [AOR]=2.44; 95% confidence interval [95% CI]=1.49-3.99) and opioid (AOR=2.64; 95% CI, 1.26-5.53) use.
Conclusions
Although older adults with severe/extreme knee and/or hip OA pain are more likely to take analgesics than those with less severe pain, a sizable proportion take less than therapeutic doses and thus may be undertreated. Further research is needed to examine barriers to optimal analgesic use.
Keywords: Aged, Analgesic, Osteoarthritis
Introduction
Osteoarthritis (OA), a chronic degenerative disorder characterized by cartilage loss, is the most common form of arthritis in the United States (US) [1,2]. The strongest risk factor for development of OA is advanced age. Therefore, as the US population ages, the prevalence of OA is expected to increase [3,4]. Increased OA in an aging population poses a public health problem due to significant morbidity, including decreased mobility and impaired quality of life, often the consequence of significant pain [3,4].
OA management includes both behavioral (e.g., weight loss, resistive and aerobic exercise) and pharmacologic approaches using analgesics such as acetaminophen [1,2,4]. Analgesic prescribing for older adults can be especially challenging due to the increased presence of co-morbid diseases, polypharmacy, and pharmacokinetic and pharmacodynamic changes. Furthermore, non-opioid analgesics (i.e., acetaminophen, non-steroidal anti-inflammatory drugs [NSAIDs]) are available over-the-counter (OTC) and are widely used by older adults [5].
Few studies have examined the use of analgesics by community-dwelling older adults with OA [6-8]. However, these studies lacked a generalizable population (e.g., limited to women or veterans), did not examine both prescription and OTC analgesics, or did not report detailed dosage information. In addition, although over-medication is a common concern in older adults, there is evidence of potential undertreatment [9,10]. In order to better understand current management of symptomatic lower extremity joint pain, this study examined the prevalence and correlates of non-opioid and opioid analgesic use and descriptively evaluated potential undertreatment in a large and diverse sample of community-dwelling older adults with symptomatic knee and/or hip OA.
Methods
Source of Data
This study uses data from the Health, Aging and Body Composition (Health ABC) study, a population-based, prospective, observational study of community-dwelling older adults [11]. The baseline sample included 3075 black and white men and women aged 70-79 years who reported no difficulty walking ¼ mile, climbing 10 steps, and who lived in specified zip codes surrounding Pittsburgh, PA and Memphis, TN. Individuals meeting the above criteria were enrolled between 1997 and 1998. This study was approved by the University of Pittsburgh and University of Tennessee Memphis Institutional Review Boards and informed consent was obtained from each participant prior to data collection.
Study Design, Sample, and Data Collection
This cross-sectional analysis uses data from a subset of 652 participants from the year 6 (2002-03) interview (N=2499) with symptomatic (e.g., reported pain, aching and/or stiffness) OA of the knee and/or hip most days for at least one month in the previous 12 months. The year 6 visit was selected to assure adequate numbers of participants with potentially debilitating OA symptoms from among an initially limitation-free cohort at baseline. Year 6 was also the most recent year-to-date in which both OTC and prescription medication dosages were collected. The relevant data collected included questionnaire material regarding demographic characteristics, multiple aspects of health status and access to healthcare, and medication use. For medications, participants were asked to bring to clinic all medications, including OTC agents, that they had recently taken. Trained examiners transcribed from each medication container the medication name, strength, dosage form, and number of times the respondent reported taking the product the previous day, week, or month. The medication data were categorized using the Iowa Drug Information System (IDIS) codes [12].
Analgesic Use Outcomes
Analgesic use was defined as taking one or more agents from either or both of two major drug categories: 1) non-opioids (i.e., acetaminophen, prescription and OTC conventional NSAIDs, and COX-2 selective NSAIDs); and 2) opioid receptor agonists (e.g., morphine, propoxyphene, tramadol). We did not include OTC topical analgesics or nutriceuticals. For descriptive purposes only, we calculated the average daily dose of analgesics by multiplying the number of dosage forms taken the previous day, week, or month by medication strength. For opioids, this average daily dose was then converted to oral morphine equivalents (OME) [13,14]. Subsequently, the average daily dose was converted to a standardized daily dose for all analgesics by dividing it by the minimum effective analgesic daily dose recommended for older adults (e.g., 2000 mg for acetaminophen, 15 mg/day OME for opioids) [15,16]. Thus, a person taking 1.0 standardized analgesic unit will have taken the minimum recommended effective analgesic dose for older adults for one agent [16]. For example, an individual reporting an acetaminophen daily dose of 2000 mg would have taken 1.0 standardized daily dose (i.e., 2000 mg/2000 mg). The individual analgesic standardized daily doses were then summed to create an overall non-opioid and opioid standardized analgesic daily dosage for each participant. For instance, a patient reporting use of both morphine sustained-release 15 mg twice daily (30 OME) and acetaminophen/tramadol 37.5/325 mg one tablet daily (7.5 OME) would have taken a total of 37.5 OMEs for a standardized daily opioid dose of 2.5 (i.e., 37.5 OME/15 OME). In addition, this patient's standardized daily non-opioid dose would have been 0.16 (i.e., acetaminophen 325 mg/2000 mg). Dosage was then summarized by two clinically relevant categories: inadequate dose (< 1 standardized daily doses, or in the case of acetaminophen <2000 mg/day) and adequate dose (≥ 1 standardized daily doses).
Knee/Hip Osteoarthritis Pain Measure
Knee/hip OA pain severity was derived from a modified Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain subscale [17,18]. Among those participants reporting symptomatic knee/hip OA pain for at least a month in the previous 12 months, study interviewers assessed knee/hip symptoms at the year 6 visit by asking participants if they had “pain, aching or stiffness on most days” in the past 30 days in either knee/hip. Among those with knee/hip OA symptoms in the past 30 days, interviewers administered a modified WOMAC pain subscale with five response categories (i.e., none, mild, moderate, severe, extreme) to indicate knee/hip pain during six activities in the past 30 days: 1) walking on a flat surface; 2) going up or down stairs; 3) at night while in bed; 4) standing upright; 5) getting in or out of a chair; and 6) getting in or out of a car. For analysis purposes, pain severity was based on the participant's most severe response among the six activities. A three-level categorical variable was created for severity of knee/hip OA pain (none, mild/moderate, and severe/extreme).
Covariates
We adjusted for potential confounding variables that may influence the relationship between severity of knee/hip OA pain and non-opioid and opioid analgesic use [16, 19-21]. Demographic factors were represented by dichotomous variables for age, sex, race, site, and marital status. Race was self-reported as black or white and was included in the study to assess potential disparities. Categorical variables were created for education (less than high school graduate, high school graduate, and post secondary education) and health literacy (6th grade level and below, between 7th and 8th grade level, and at least 9th grade level) [22].
Health status factors were represented by dichotomous measures (present/absent) for self-reported health conditions and other factors, including osteoporosis, coronary heart disease (CHD), history of cancer (prevalent and incident cases up to the year 6 visit), falls, sleep disturbance, urinary problems, current smoking, self-rated health (poor/fair vs. good/very good/excellent), history of knee/hip arthroplasty, having depressive symptoms (score > 15 on Center for Epidemiologic Studies depression scale) and cognitive impairment (modified mini-mental state score < 80 calculated at the year 5 visit) [23,24]. Measured height and weight were used to obtain body mass index (BMI) (weight (kg)/height (m2)) and classification of underweight/normal <25.0, overweight 25.0-29.9, and obese ≥ 30.0 [25]. We also controlled for the use of any prescription and non-prescription medication. In addition, we controlled for physical function measures, including persistent lower extremity limitation, physical activity (>1000 kcal/week), and intensity of exercise (high/moderate intensity) [26,27].
Access to healthcare factors were represented by the presence/absence of home ownership, health insurance (other than Medicare), prescription medication insurance, having gone without medications in the past 12 months due to money problems, having a usual place to go for healthcare (private doctor/public clinic/health maintenance organization/hospital outpatient clinic vs. emergency room/other), having a recent hospitalization (past 6 months), needs being met by income (poorly vs. fairly well/very well), and having delayed medical care in the past 12 months due to money problems. Family income was categorized as < $10,000, ≥ $10,000 to ≤ $25,000, >$25,000 to < $50,000, and ≥ $50,000; other assets were categorized as none, 1-2, and 3-7 asset classes, including: 1) checking/savings accounts 2) money market accounts 3) CDs, savings bonds, or treasury bills 4) investment property or housing other than current residence 5) businesses or farms 6) stocks and mutual funds and 7) IRAs and KEOGH accounts [21].
Statistical Analysis
OA pain severity, analgesic use, and covariate data were summarized by frequencies and percentages. Chi-square tests were used to compare non-opioid and opioid analgesic daily use by standardized dose as well as the severity of knee/hip OA pain. Logistic regression models were fit with each analgesic use measure as the dichotomous dependent variable and each measure of OA pain severity as the main predictor of interest. Analyses were performed with and without adjustment for covariates. Covariates were selected using a backward selection approach applied separately for each of three domains of covariates (demographic, health status, and access to healthcare). Specifically, covariates significant at the liberal α=0.15 criteria and those deemed important a priori (i.e., age, sex, and race) were included in the final multivariable logistic regression model to calculate adjusted odds ratios (AOR) and their 95% confidence intervals (95% CI). To retain individuals with missing data in the final analyses, we created dummy variables for a “missing” category. The underlying statistical assumption of collinearity was evaluated using variance inflation factors, and the regression diagnostic of goodness-of-fit was verified using Hosmer-Lemeshow testing. All statistical analyses were conducted using SAS® Version 9.2 (SAS Institute, Inc., Cary, North Carolina).
Results
Table 1 presents demographic and clinical characteristics for those participants with symptomatic knee/hip OA at the year 6 visit (n=652). The sample had a mean [±SD] age of 78.5 [±2.86] years; the majority were female and white and reported good to excellent self-rated health. Almost 70% were classified as overweight or obese.
Table 1. Characteristics of Health ABC Year 6 Knee/Hip Osteoarthritis Sample (N=652).
| Variable | N (%) |
|---|---|
| Demographic Factors | |
| Age ≥ 80 years | 230 (35.3) |
| Female | 427 (65.5) |
| Black | 262 (40.2) |
| Pittsburgh site | 356 (54.6) |
| Married | 319 (48.9) |
| Education | |
| < 12 years | 146 (22.4) |
| = 12 years | 216 (33.1) |
| > 12 years | 289 (44.3) |
| Missing | 1 (0.2) |
| Health Literacy [REALM] | |
| ≤ 6th grade (≤ 44) | 44 (6.8) |
| 7th-8th grade (45-60) | 68 (10.4) |
| ≥ 9th grade (>60) | 498 (76.4) |
| Missing | 42 (6.4) |
| Health Status Factors | |
| Osteoporosis | 130 (19.9) |
| Coronary heart disease | 163 (25.0) |
| History of cancer | 126 (19.3) |
| Falls | 197 (30.2) |
| Sleep disturbance | 324 (49.7) |
| Urinary problem | 72 (11.0) |
| Current smoking | 30 (4.6) |
| Excellent/very good/good self-rated health | 463 (71.0) |
| History of knee/hip arthroplasty | 49 (7.5) |
| Depression (CES-D >15) | 113 (17.3) |
| Cognitive impairment (3MS ≤ 80) | 50 (7.7) |
| BMI (kg/m2) | |
| Underweight/normal (<25) | 152 (23.3) |
| Overweight (25-30) | 251 (38.5) |
| Obese (>30) | 186 (28.5) |
| Missing | 63 (9.7) |
| Use of any prescription medications | 617 (94.6) |
| Health Status Factors | |
| Use of any non-prescription medications | 558 (85.6) |
| Persistent lower extremity limitation | 244 (37.4) |
| Physical activity (>1000 kcal/week) | 282 (43.2) |
| High/moderate intensity exercise | 133 (20.4) |
| Access to Healthcare Factors | |
| Home ownership | 453 (69.5) |
| Health insurance (i.e., other than Medicare) | 524 (80.4) |
| Prescription medication insurance | 386 (59.2) |
| Gone without medications in past 12 months due to money problems | 24 (3.7) |
| Usual place for healthcare | 547 (83.9) |
| Recent hospitalization (past 6 months) | 62 (9.5) |
| Needs poorly met by income | 37 (6.7) |
| Delayed medical care in past 12 months due to money problems | 24 (3.7) |
| Access to Healthcare Factors | |
| Family income | |
| < $10,000 | 73 (11.2) |
| ≥ $10,000 to ≤ $25,000 | 219 (33.6) |
| > $25,000 to < $50,000 | 190 (29.1) |
| ≥ $50,000 | 91 (14.0) |
| Missing | 79 (12.1) |
| Other assets | |
| 0 | 234 (35.9) |
| 1-2 | 201 (30.8) |
| 3-7 | 217 (33.3) |
Abbreviations: 3MS: modified mini-mental state exam; BMI: body mass index; CES-D: Center for Epidemiologic Studies Depression Scale; REALM: Rapid estimate of adult literacy in medicine
Overall, 51.4% of participants took at least one non-opioid, the majority of which were OTC agents with acetaminophen the most commonly used (158/652; 24.2%). In order of decreasing frequency, the most commonly used NSAIDs of any type (i.e., prescription or OTC) were ibuprofen (7.4%), celecoxib (7.2%), rofecoxib (6.9%), naproxen (5.2%), and valdecoxib (3.1%). Prescription opioids were taken by 9.4% of participants. Most (54/61; 88.5%) opioid users also took a non-opioid. In order of decreasing frequency, the most commonly used acetaminophen-containing combination opioids were as follows: 1.) propoxyphene (3.7% overall; 23/24 in combination with acetaminophen); 2.) hydrocodone (2.3% overall; 15/15 in combination with acetaminophen); 3.) oxycodone (1.4% overall; 6/9 in combination with acetaminophen); 4.) tramadol (1.4% overall; 4/9 in combination with acetaminophen); and 5.) codeine (0.8%; 4/5 in combination with acetaminophen). Morphine immediate-release was reported in 2 participants while use of morphine sustained-release was reported by 1 participant. Transdermal fentanyl was used by 2 participants.
Table 2 presents the severity distribution of knee and hip OA pain using a modified WOMAC pain subscale. Overall, either mild/moderate or severe/extreme knee pain was reported more frequently than any hip pain (34.1% vs. 19.8%, respectively). Combined, any knee or hip OA pain was reported by 42.9% (280/652) of the sample, of whom 45% (126/280) had severe/extreme pain. Furthermore, among those with knee OA only, hip OA only, and knee and hip OA, 16.7% (51/305), 16.3% (22/135), and 25% (53/212) reported severe/extreme OA pain (P=0.04). However, there was not a significant association between higher standardized daily analgesic doses of non-opioids and the presence of both knee and hip OA (P=0.41).
Table 2. Severity of Knee/Hip Osteoarthritis Pain in Community-Dwelling Older Adults* (N=652).
| Knee, N (%) | Hip, N (%) | Knee or Hip†, N (%) | |
|---|---|---|---|
| Mild/moderate Pain | 116 (17.8) | 85 (13.0) | 154 (23.6) |
| Severe/extreme Pain | 106 (16.3) | 44 (6.8) | 126 (19.3) |
Knee/hip pain defined as any pain during the last 30 days in association with ≥ 1 activity listed in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain subscale.
Knee or hip pain variable is measured on the participant level, thus it is not additive.
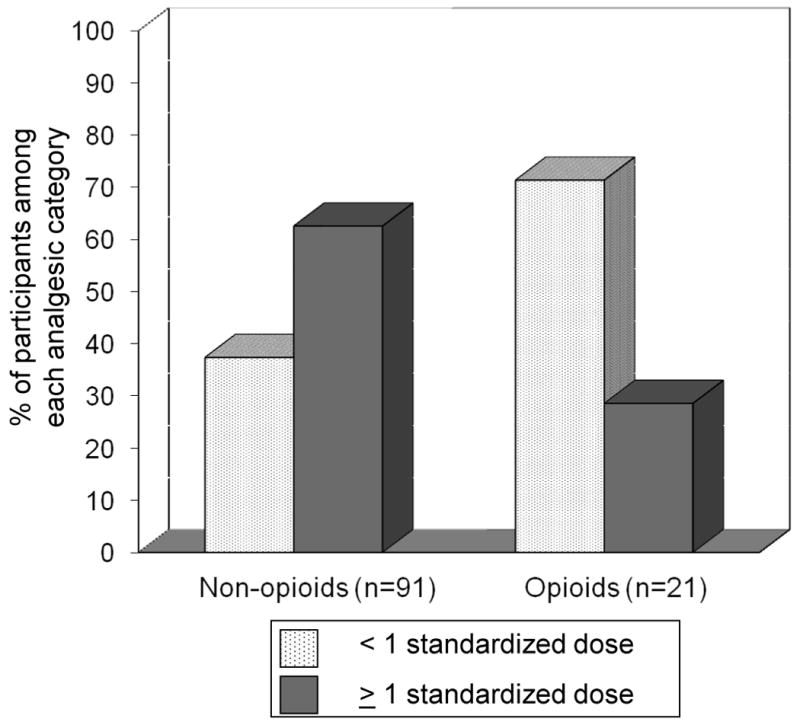
Overall, the mean (SD) standardized daily doses for non-opioid and opioid use were 1.21 (0.97) and 0.78 (0.74), respectively. Figure 1 shows the standardized daily dose of non-opioids and opioids in those with severe/extreme OA pain. More participants with severe/extreme OA pain used non-opioids compared to opioids (72.2% vs. 16.7%; p=0.01). Moreover, among the non-opioid users with severe/extreme OA pain (n=91), the majority took ≥ 1 standardized daily dose (62.6%); in contrast, opioid users with severe/extreme OA pain (n=21) more frequently took < 1 standardized daily dose (71.4%). Among those with severe/extreme OA pain, 39% were using < 1 standardized daily dose of either a non-opioid or opioid.
Figure 1. Use of Non-opioids and Opioids by Standardized Daily Dose among those with Severe/Extreme Osteoarthritis Pain.
Factors associated with non-opioid use are presented in Table 3. Non-opioid use was more common in those with severe/extreme OA pain (AOR=2.44; 95% CI=1.49-3.99) and in obese persons (AOR=1.76; 95% CI=1.08-2.85). Persons with symptomatic knee and/or hip OA who also had CHD were less likely to use a non-opioid analgesic (AOR=0.67; 95% CI=0.45-0.99).
Table 3. Predictors of Any Non-Opioid Use in Year 6 Health ABC Knee/Hip Osteoarthritis Sample* (N=652).
| Variable | Adjusted OR (95% CI) | P-value |
|---|---|---|
| Primary Independent Variable | ||
| Knee/Hip OA Pain | ||
| None | 1.00 (Reference) | -- |
| Mild/moderate | 1.20 (0.79-1.82) | 0.39 |
| Severe/extreme | 2.44 (1.49-3.99) | <0.001 |
| Demographic Factors | ||
| Age ≥ 80 years | 0.92 (0.64-1.32) | 0.64 |
| Female | 1.23 (0.85-1.80) | 0.27 |
| Black | 1.01 (0.70-1.46) | 0.96 |
| Memphis Site | 1.28 (0.91-1.81) | 0.16 |
| Health Status Factors | ||
| Coronary heart disease | 0.67 (0.45-0.99) | 0.04 |
| Sleep disturbance | 0.73 (0.52-1.03) | 0.07 |
| Urinary problem | 0.64 (0.38-1.08) | 0.09 |
| Current smoking | 1.96 (0.83-4.64) | 0.13 |
| Fair/poor self-reported health status | 1.28 (0.84-1.95) | 0.26 |
| Health Status Factors | ||
| BMI | ||
| Underweight/normal | 1.00 (Reference) | |
| Overweight | 1.03 (0.66-1.60) | 0.91 |
| Obese | 1.76 (1.08-2.85) | 0.02 |
| Use of any OTC medication | 1.66 (0.99-2.78) | 0.05 |
| Persistent lower extremity limitation | 1.45 (0.99-2.12) | 0.06 |
| Physical activity (>1000 kcal/week) | 0.74 (0.51-1.06) | 0.10 |
| Access to Healthcare Factors | ||
|
| ||
| Needs met by income | 0.71 (0.33-1.54) | 0.39 |
Hosmer and Lemeshow Goodness-of-Fit Test: p=0.68; x2=5.70; df=8
Abbreviations: BMI: body mass index; OA: osteoarthritis; OTC: over-the-counter
Factors associated with opioid use are presented in Table 4. Opioid use was more likely with severe/extreme OA pain (AOR=2.64; 95% CI=1.26-5.53) and history of cancer (AOR=2.11; 95% CI=1.11-3.99). In post-hoc analysis, we found that among those with severe/extreme OA pain who took an opioid, the majority (15/21; 71.4%) did not have a history of cancer. A sensitivity analysis excluding ‘history of cancer’ from the final model was also conducted, and the significance of the results was unchanged.
Table 4. Predictors of Any Opioid Use in Year 6 Health ABC Knee/Hip Osteoarthritis Sample* (N=652).
| Variable | Adjusted OR (95% CI) | P-value |
|---|---|---|
| Primary Independent Variable | ||
| Knee/Hip OA Pain | ||
| None | 1.00 (Reference) | -- |
| Mild/moderate | 1.50 (0.68-3.32) | 0.32 |
| Severe/extreme | 2.64 (1.26-5.53) | 0.01 |
| Demographic Factors | ||
| Age ≥ 80 | 0.72 (0.38-1.34) | 0.30 |
| Female | 0.93 (0.48-1.82) | 0.84 |
| Black | 0.57 (0.30-1.09) | 0.09 |
| Memphis Site | 1.73 (0.96-3.11) | 0.07 |
| Years of education | ||
| < 12 years | 1.00 (Reference) | -- |
| =12 years | 1.09 (0.52-2.26) | 0.82 |
| >12 years | 0.52 (0.24-1.13) | 0.10 |
| Health Status Factors | ||
| Osteoporosis | 1.84 (0.93-3.65) | 0.08 |
| History of cancer | 2.11 (1.11-3.99) | 0.02 |
| Health Status Factors | ||
| Urinary problem | 0.29 (0.07-1.26) | 0.10 |
| Fair/poor self-reported health status | 1.55 (0.82-2.92) | 0.17 |
| BMI | ||
| Underweight/normal | 1.00 (Reference) | -- |
| Overweight | 1.24 (0.52-2.97) | 0.62 |
| Obese | 1.97 (0.84-4.65) | 0.12 |
| Access to Healthcare Factors | ||
|
| ||
| Prescription medication insurance | 1.06 (0.55-2.05) | 0.86 |
Hosmer and Lemeshow Goodness-of-Fit Test: p=0.80; x2=4.62; df=8
Abbreviations: BMI: body mass index; OA: osteoarthritis
Discussion
In community-dwelling older adults, severe pain from knee and/or hip OA is common and may be frequently undertreated, especially with inadequate doses of opioids. This finding is consistent with studies of pain management in other conditions in older adults such as cancer pain and in other settings including the nursing home [9,10].
Despite guidelines that recommend considering a trial of opioids for moderate/severe persistent noncancer pain with clear therapeutic goals and diligent monitoring for pain relief and/or adverse effects, opioid use was low in this cohort [15]. In addition, a recent systematic review of opioid use in the treatment of persistent noncancer pain in older adults highlighted that short-term opioid use was associated with reduction in pain intensity and better physical function [28]. However, there is a lack of consensus among guidelines on the use of opioids for the management of knee and hip OA largely due to the unknown long-term safety and efficacy of opioids in older adults. The low opioid use observed may be partly related to this lack of clarity in the guidelines. Remarkably, the most common opioid used was propoxyphene, which has recently been removed from the market in the US due to increased risk of cardiotoxicity. It is important to note that much has changed in the area of analgesic prescribing since these data were collected (2002-03); however, there are still significant implications that can be drawn.
Slightly more than half of the sample took at least one non-opioid analgesic. It is promising that the most common individual agent used was acetaminophen, which is the recommended initial analgesic for OA pain [2,15]. However, consistent with previous studies, overall NSAID use was more common than acetaminophen use [6]. Among the NSAIDs, COX-2 selective agents were the most frequently used class. This analgesic use pattern is potentially problematic due to several deleterious adverse effects of chronic NSAID use in older adults, including gastrointestinal, renal, and cardiovascular effects [29]. Interestingly, two of the three COX-2 selective NSAIDs available during the study period (i.e., rofecoxib and valdecoxib) are no longer on the market due to the serious adverse effects of myocardial infarction and stroke.
As expected, severe/extreme OA pain was associated with both non-opioid and opioid use in this population. Among those reporting knee/hip OA pain, almost half (45%) had severe/extreme pain. Perhaps the most important finding is that among those with severe/extreme pain, 39% were using < 1 standardized daily dose of either a non-opioid or opioid. This low dosing was more pronounced with opioids than non-opioids, which is the opposite of what would be optimal for those with severe/extreme OA pain. Unfortunately, this finding is consistent with previous data from over a decade ago in which Pahor and colleagues reported that among those who had severe pain in their sample of older women with OA, 41.2% were using suboptimal doses (i.e., < 20% of the maximum analgesic dose) [8].
Several other findings from this study deserve mention. Previous studies have found racial disparities in OA treatment with analgesics [6-8]. In contrast, we did not find an association between race and analgesic use after accounting for several important confounders, such as education, health literacy, prescription medication insurance, and income. In assessing the fully adjusted multivariable model, the point estimate for the association between Black race and opioid use (AOR=0.57) suggests that there may be a differential use in opioids compared to White participants. However, this was not statistically significant. In a previous similar study of analgesic use and OA pain severity in older disabled women, White race was significantly associated with use of high analgesic doses [8]. Other studies have reported differential use among NSAIDs between Black and White participants [6,7]. Thus, it is unclear why our study did not find any differences in analgesic use between races.
Another encouraging finding is that participants with a history of CHD were significantly less likely to use non-opioids, the majority of which included NSAIDs. Of concern is that these patients are at an increased risk for future cardiac events, and thus should avoid/minimize the use of NSAIDs because of their potential to reduce the beneficial antiplatelet effect of aspirin [30-32].
Furthermore, obesity was significantly associated with non-opioid use, emphasizing the importance of behavioral interventions such as weight loss to manage OA and in turn decrease analgesic exposure. In addition, those with a history of cancer were significantly more likely to use an opioid. Post-hoc analysis suggests that those participants with the highest reported pain severity were likely taking the opioid for OA pain rather than cancer pain.
At the present time, there is a great need for quality evidence to enhance pain management guidelines.34 Until such time, we recommend the following basic strategies to manage older adults with OA pain. First, it is critical to consider the severity of the patient's OA pain and concomitant chronic conditions prior to initiating treatment. In addition, avoiding the use of specific agents (e.g., long half-life NSAIDs, such as oxaprozin and piroxicam, as well as certain other NSAIDs, such as indomethacin and ketorolac) and using preferred alternative agents (e.g., acetaminophen) is a prudent initial strategy.29 If acetaminophen is not deemed to be effective after adequate dosing and NSAID use is not contraindicated, a trial of analgesic dosing of a nonacetylated salicylate (e.g., salicylate) or ibuprofen or celecoxib may be acceptable. For those with moderate to moderately severe OA pain, a trial of a low-dose or short-acting opioid (e.g., codeine, tramadol) in combination with acetaminophen is another option. Finally, chronic opioid use should be closely monitored based on guideline-recommended practices, such as routinely reassessing pain as well as assessing pain-related function, opioid adverse effects, and treatment adherence.35
Importantly, the findings from this study are likely to be robust because they are obtained from a dataset that used comprehensive and state-of-the-art methods to capture both OTC and prescription agents and recorded detailed dosage information in a racially and gender-representative community sample of older adults with OA.
However, there are several potential limitations of this study. Because this was a cross-sectional study, the temporal relationship between medication use and OA pain cannot be established. However, we attempted to capture the most recent analgesic use data concurrent with OA pain symptoms. As is true with any non-randomized study, unmeasured factors may have confounded the results. To minimize this threat to validity, we controlled for numerous potential confounders, including BMI. We were unable to control for the use of non-pharmacologic interventions as this information was not collected in the Health ABC Study. We did, however, control for physical activity and exercise intensity. Another potential limitation is that we did not include radiographic confirmation to define those with OA. It is important to note that the requirement for x-rays to diagnose OA is controversial as elders can have radiographic evidence without OA pain [33].
Another limitation to this study is the relatively small sample size (N=652), which may have impacted our power in detecting statistically significant associations between mild/moderate OA pain severity and analgesic use. However, we believe that the point estimates reported are the best first approximations of the true association between mild/moderate OA pain severity and analgesic use. Another potential limitation is confounding by comorbidity. On post-hoc analysis, a comorbidity index score was created using a count of 5 conditions (i.e., osteoporosis, CHD, history of cancer, depression, and cognitive impairment) for all participants (range: 0-5; mean [SD] 0.89 [0.87]). In addition, as a proxy for comorbidities, we calculated the total number of prescription medications taken on a regular basis excluding analgesics (mean [SD] 4.4 [3.1]). These two additional variables were entered into each of the final multivariable models separately and combined, and the point estimate describing the association between the primary independent variable and the main outcomes was not substantively changed.
In addition, the use of a minimum effective analgesic dose for each analgesic used to calculate the standardized daily analgesic dose has not been validated. We did, however, use evidence-based guidelines and drug information sources to derive this information. Another potential limitation is that participants may have reported analgesic use for painful conditions other than OA. However, the indication would not affect the relationship between analgesic use and severity of OA pain. Participants may have also had pain at other sites (e.g., hand, shoulder, back) since the recall period for pain and severity for these other sites in the Health ABC Study was different than that captured for lower extremity OA pain. Furthermore, we were unable to report other clinical outcomes (e.g., adverse effects from analgesics, changes in functional status). Finally, the Health ABC participants were from two regions of the US, and thus these findings may not generalize to all other community-dwelling older adults.
Conclusions
Severe/extreme OA pain may be undertreated and selection of agents may not be optimal. In order to improve management of persistent noncancer pain in older adults, future studies should examine barriers to optimal analgesic use.
Acknowledgments
This study was primarily supported by National Institute on Aging grants and contracts (R01AG027017, P30AG024827, T32 AG021885, K07AG033174, R01AG034056, N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106), a National Institute of Mental Health grant (R34 MH082682), a National Institute of Nursing Research grant (R01 NR010135), an Agency for Healthcare Research and Quality grant (R01 HS017695), and a VA Health Services Research grant (IIR-06-062). This research was also supported in part by the Intramural Research program of the NIH, National Institute on Aging. The authors would like to thank Yihuang Kang and Yan Zheng for their assistance with data programming.
Footnotes
None of the authors has any relevant conflict of interest/financial disclosures.
Presented as a poster at the Gerontological Society of America Annual Scientific Meeting in New Orleans, LA on Monday, November 22, 2010.
References
- 1.Richmond J, Hunter D, Irrgang J, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on the treatment of osteoarthritis (OA) of the knee. J Bone Joint Surg Am. 2010;92:990–3. doi: 10.2106/JBJS.I.00982. [DOI] [PubMed] [Google Scholar]
- 2.Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137–62. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Fitzcharles MA, Lussier D, Shir Y. Management of chronic arthritis pain in the elderly. Drugs Aging. 2010;27:471–90. doi: 10.2165/11536530-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Ling SM, Yvette LJ. Osteoarthritis. In: Halter JB, Ouslander JG, Tinetti ME, editors. Hazzard's Geriatric Medicine and Gerontology. 6th. New York: McGraw-Hill; 2009. pp. 289–302. [Google Scholar]
- 5.Hanlon JT, Guay DRP, Ives TJ. Oral analgesics: efficacy, mechanism of action, pharmacokinetics, adverse effects, drug interactions, and practical recommendations for use in older adults. In: Gibson SJ, Weiner DK, editors. Pain in Older Persons, Progress in Pain Research and Management. Seattle: IASP Press; 2005. pp. 205–22. [Google Scholar]
- 6.Albert SM, Musa D, Kwoh CK, Hanlon JT, Silverman M. Self-care and professionally guided care in osteoarthritis: racial differences in a population-based sample. J Aging Health. 2008;20:198–216. doi: 10.1177/0898264307310464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominick KL, Bosworth HB, Jeffreys AS, Grambow SC, Oddone EZ, Horner RD. Racial/ethnic variations in non-steroidal anti-inflammatory drug (NSAID) use among patients with osteoarthritis. Pharmacoepidemiol Drug Saf. 2004;13:683–94. doi: 10.1002/pds.904. [DOI] [PubMed] [Google Scholar]
- 8.Pahor M, Guralnick JM, Wan JY, et al. Lower body osteoarticular pain and dose of analgesic medications in older disabled women: the Women's Health and Aging Study. Am J Pub Health. 1999;89:930–4. doi: 10.2105/ajph.89.6.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernabei R, Gambassi G, Lapane K, et al. Management of pain in elderly patients with cancer. SAGE Study Group. Systematic Assessment of Geriatric Drug Use via Epidemiology. JAMA. 1998;279:1877–82. doi: 10.1001/jama.279.23.1877. [DOI] [PubMed] [Google Scholar]
- 10.Won AB, Lapane KL, Vallow S, Schein J, Morris JN, Lipsitz LA. Persistent nonmalignant pain and analgesic prescribing patterns in elderly nursing home residents. J Am Geriatr Soc. 2004;52:867–74. doi: 10.1111/j.1532-5415.2004.52251.x. [DOI] [PubMed] [Google Scholar]
- 11.Newman AB, Haggerty CL, Kritchevsky SB, Nevitt MC, Simonsick EM Health ABC Collaborative Research Group. Walking performance and cardiovascular response: associations with age and morbidity--the Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2003;(58):715–20. doi: 10.1093/gerona/58.8.m715. [DOI] [PubMed] [Google Scholar]
- 12.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–11. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 13.Palliative Medicine Handbook [Internet] Wales: Palliative Medicine Handbook On-line Edition. c2006-2010 [cited 2010 November 5] Available at: http://book.pallcare.info/
- 14.Abramowicz M, editor. Treatment Guidelines from The Medical Letter. Vol. 5. 2007. Drugs for Pain; pp. 23–32. [PubMed] [Google Scholar]
- 15.American Geriatrics Society Panel on Persistent Pain in Older Persons. The management of persistent pain in older persons. J Am Geriatr Soc. 2002;50:S205–S224. doi: 10.1046/j.1532-5415.50.6s.1.x. [DOI] [PubMed] [Google Scholar]
- 16.Hanlon JT, Schmader KE, Landerman LR, et al. Relation of prescription nonsteroidal antiinflammatory drug use to cognitive function among community-dwelling elderly. Ann Epidemiol. 1997;(7):87–94. doi: 10.1016/s1047-2797(96)00124-x. [DOI] [PubMed] [Google Scholar]
- 17.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes in antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–40. [PubMed] [Google Scholar]
- 18.Hunter DJ, Zhang YQ, Niu JB, et al. Patella management, pain and patellofemoral progression: the Health ABC Study. Osteoarthritis Cartilage. 2007;15:1120–7. doi: 10.1016/j.joca.2007.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersen RM. Revisiting the behavioral model and access to medical care: does it matter? J Health Soc Behav. 1995;36:1–10. [PubMed] [Google Scholar]
- 20.Rooks RN, Simonsick EM, Miles T, et al. The association of race and socioeconomic status with cardiovascular disease indicators among older adults in the health, aging, and body composition study. J Gerontol B Psychol Sci Soc Sci. 2002;57:S247–56. doi: 10.1093/geronb/57.4.s247. [DOI] [PubMed] [Google Scholar]
- 21.Rooks RN, Simonsick EM, Klesges LM, Newman AB, Ayonayon HN, Harris TB. Racial disparities in health care access and cardiovascular indicators in Black and White older adults in the Health ABC Study. J Aging Health. 2008;20:599–614. doi: 10.1177/0898264308321023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis TC, Long SW, Jackson RH, et al. Rapid estimate of adult literacy in medicine: a shortened screening instrument. Fam Med. 1993;25:391–5. [PubMed] [Google Scholar]
- 23.Radloff LS. The CES-D scale: a self-report depression scale for research use in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 24.Teng EL, Chui HC. The Modified Mini-Mental state (3MS) examination. J Clin Pyschiatry. 1987;48:314–8. [PubMed] [Google Scholar]
- 25.National Heart, Lung, and Blood Institute (NHLBI) [Internet] Bethesda: The Practical Guide: Identification, Evaluation, and Treatment of Overweight and Obesity in Adults; c2000 [cited 2010 November 5] Available from: http://www.nhlbi.nih.gov/guidelines/obesity/practgde.htm.
- 26.Delmonico MJ, Harris TB, Lee JS, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc. 2007;55:769–74. doi: 10.1111/j.1532-5415.2007.01140.x. [DOI] [PubMed] [Google Scholar]
- 27.Peterson MJ, Giuliani C, Morey MC, et al. Physical activity as a preventative factor for frailty: the health, aging, and body composition study. J Gerontol A Biol Sci Med Sci. 2009;64:61–8. doi: 10.1093/gerona/gln001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papaleontiou M, Henderson CR, Jr, Turner BJ, et al. Outcomes associated with opioid use in the treatment of chronic noncancer pain in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2010;58:1353–69. doi: 10.1111/j.1532-5415.2010.02920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcum ZA, Hanlon JT. Recognizing the risks of chronic nonsteroidal anti-inflammatory drug use in older adults. Annals of Long-Term Care: Clinical Care and Aging. 2010;18:24–7. [PMC free article] [PubMed] [Google Scholar]
- 30.Capone M, Sciulli MG, Tacconelli S, et al. Pharmacodynamic interaction of naproxen with low-dose aspirin in healthy subjects. J Am Coll Cardiol. 2005;45:1295–1301. doi: 10.1016/j.jacc.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 31.Patrono C, Baigent C, Hirsh J, Roth G American College of Chest Physicians. Chest. 8th. Vol. 133. 2008. Antiplatelet drugs: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines; pp. 199S–233S. [DOI] [PubMed] [Google Scholar]
- 32.Renda G, Tacconelli S, Capone ML, et al. Celecoxib, ibuprofen, and the antiplatelet effect of aspirin in patients with osteoarthritis and ischemic heart disease. Clin Pharmacol Ther. 2006;80:264–74. doi: 10.1016/j.clpt.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Zhang W, Doherty M, Peat G, et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann Rheum Dis. 2010;69:483–89. doi: 10.1136/ard.2009.113100. [DOI] [PubMed] [Google Scholar]
- 34.Reid MC, Bennett DA, Chen WG, et al. Improving the pharmacologic management of pain in older adults: identifying the research gaps and methods to address them. Pain Med. 2011 doi: 10.1111/j.1526-4637.2011.01211.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krebs EE, Ramsey DC, Miloshoff JM, et al. Primary care monitoring of long-term opioid therapy among veterans with chronic pain. Pain Med. 2011;12:740–6. doi: 10.1111/j.1526-4637.2011.01099.x. [DOI] [PubMed] [Google Scholar]


