Abstract
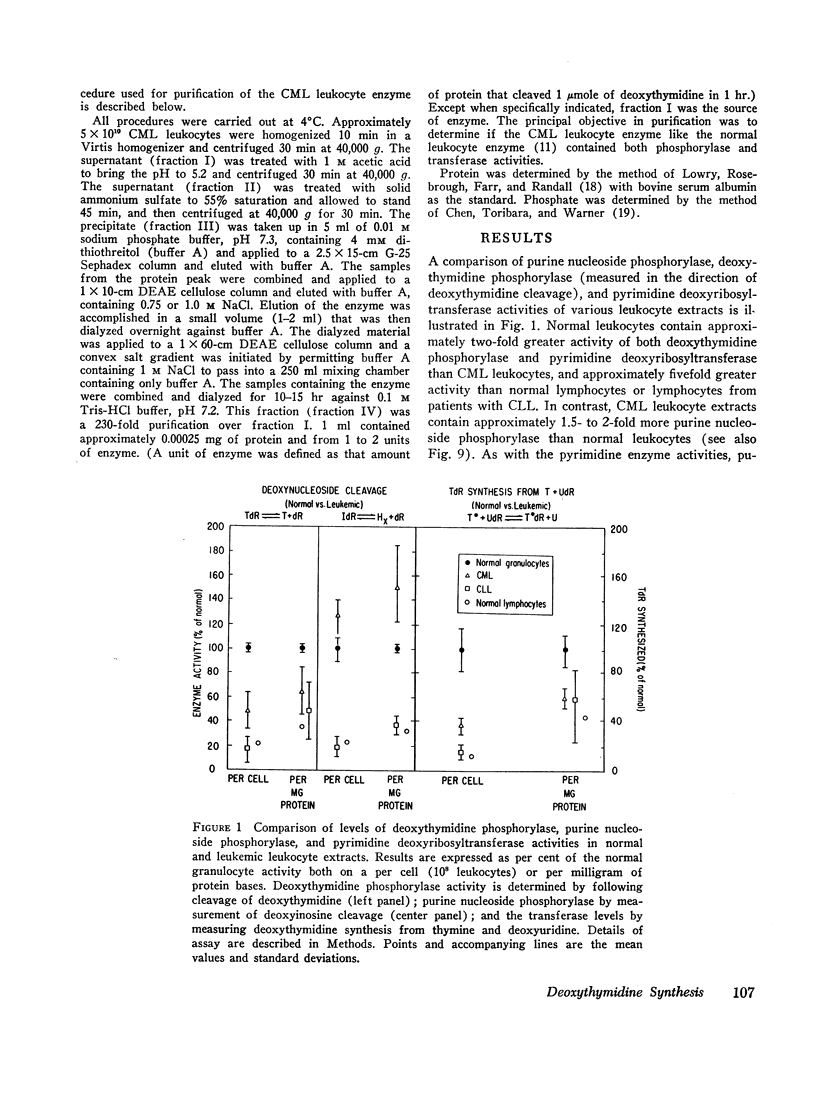
(1) Synthesis of deoxythymidine by either direct transfer of deoxyribosyl to thymine (pyrimidine deoxyribosyltransferase) or by a coupled deoxynucleoside phosphorylase mechanism is approximately twofold greater with normal leukocyte extracts (55 to 88% granulocytes) than with extracts prepared from leukocytes obtained from patients with chronic myelogenous leukemia. Activities in lymphocytes (normal or leukemic) are one-fifth the activity of normal granulocytes.
(2) The lower activity in chronic myelogenous leukemia remains at 50% of normal even when patients are in hematologic remission with a normal per cent mature granulocytes in the peripheral blood.
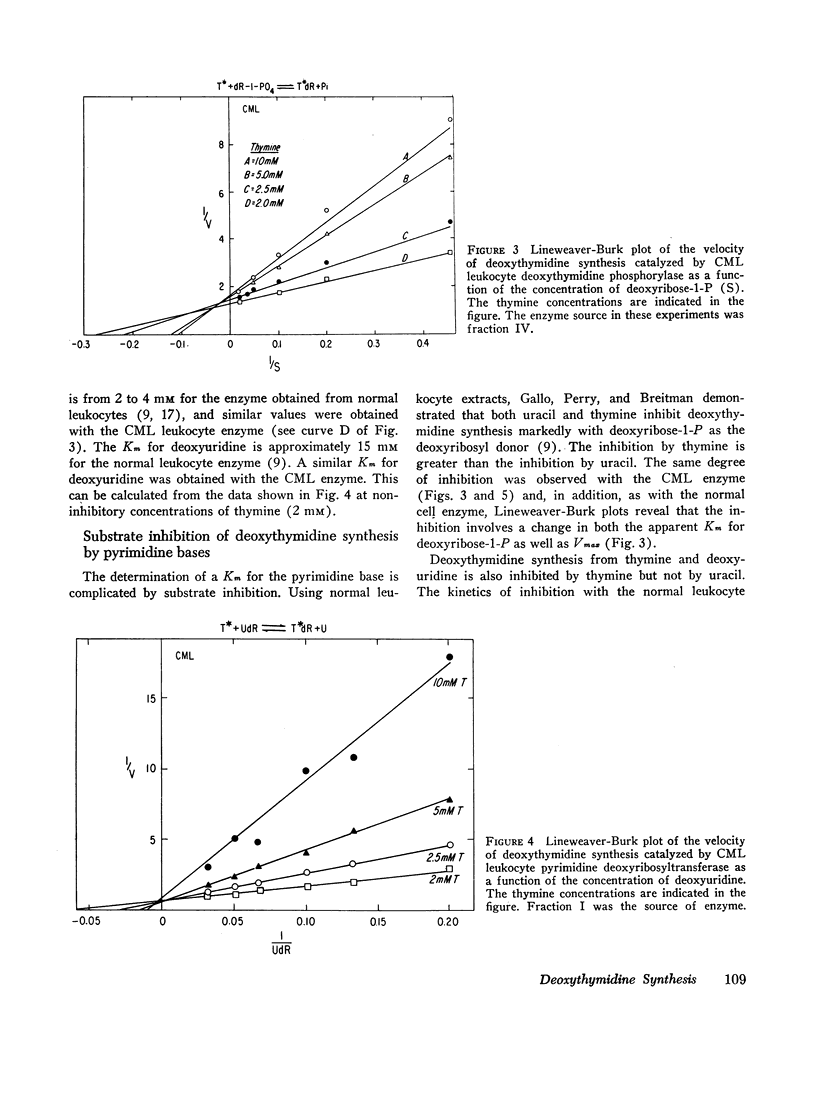
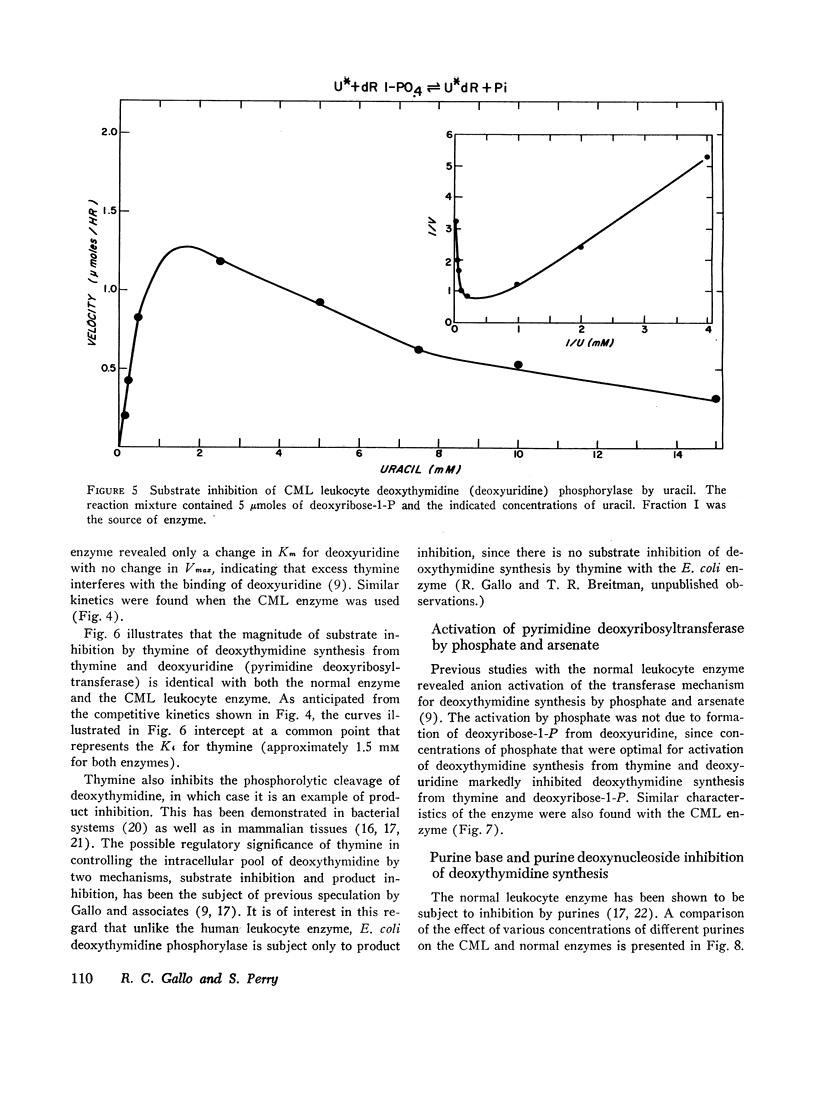
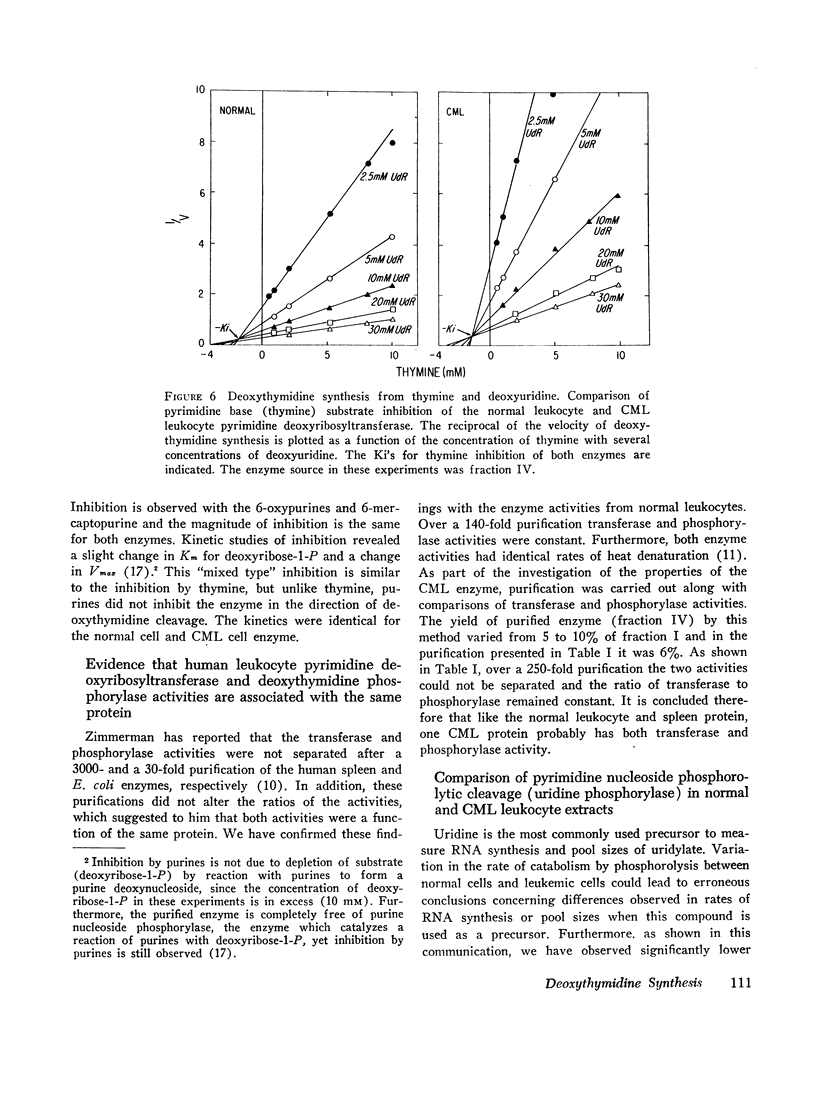
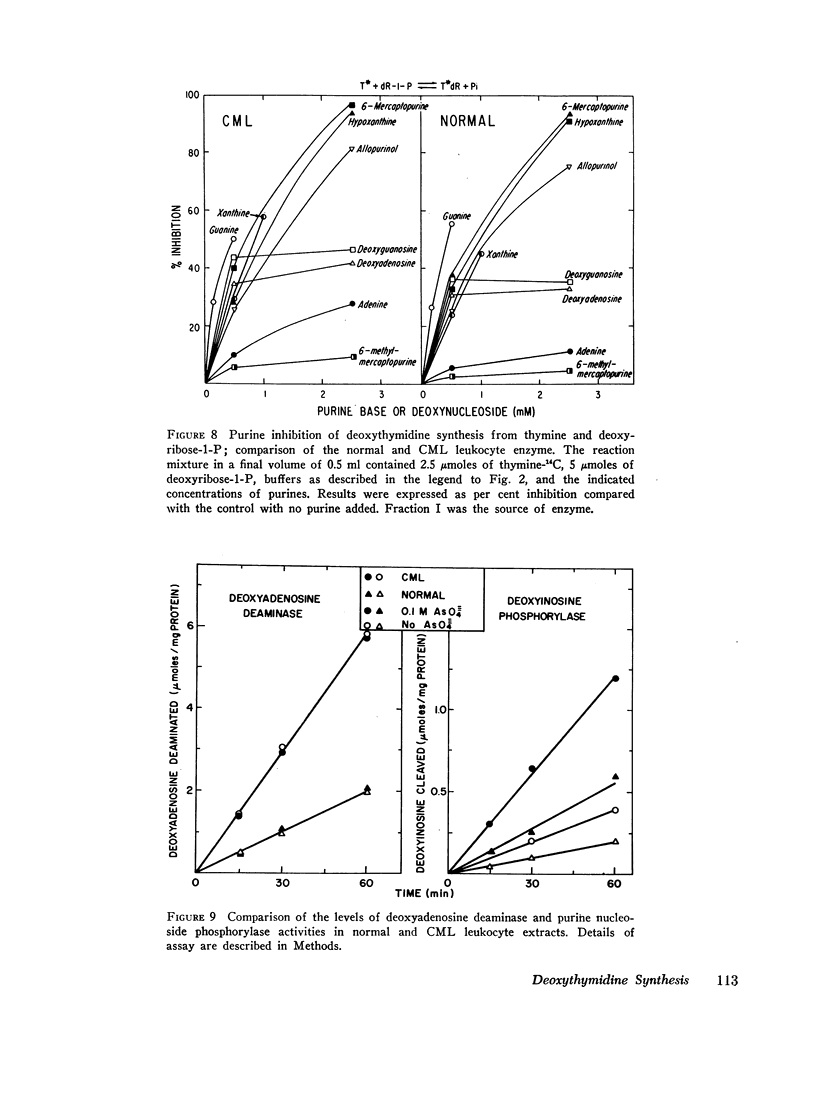
(3) The leukemic enzyme could not be distinguished from the normal by pH optima, thermal stability, or kinetic properties. The Km's for the deoxyribosyl acceptor and deoxyribosyl donors were identical for both enzymes. Both are subject to substrate inhibition by thymine and to inhibition by purine bases with similar Ki's. In addition, the transferase component of both the leukemic and the normal cell enzyme is activated by phosphate and arsenate. It appears, therefore, that there is no qualitative difference between the enzyme obtained from leukocytes of patients with chronic myelogenous leukemia and the enzyme obtained from normal leukocytes, suggesting that the difference in total cell activity is due to an actual decrease in amount of enzyme in chronic myelogenous leukemia or to a mixed cell population, one with a normal quantity of enzyme and the other with little or no active enzyme.
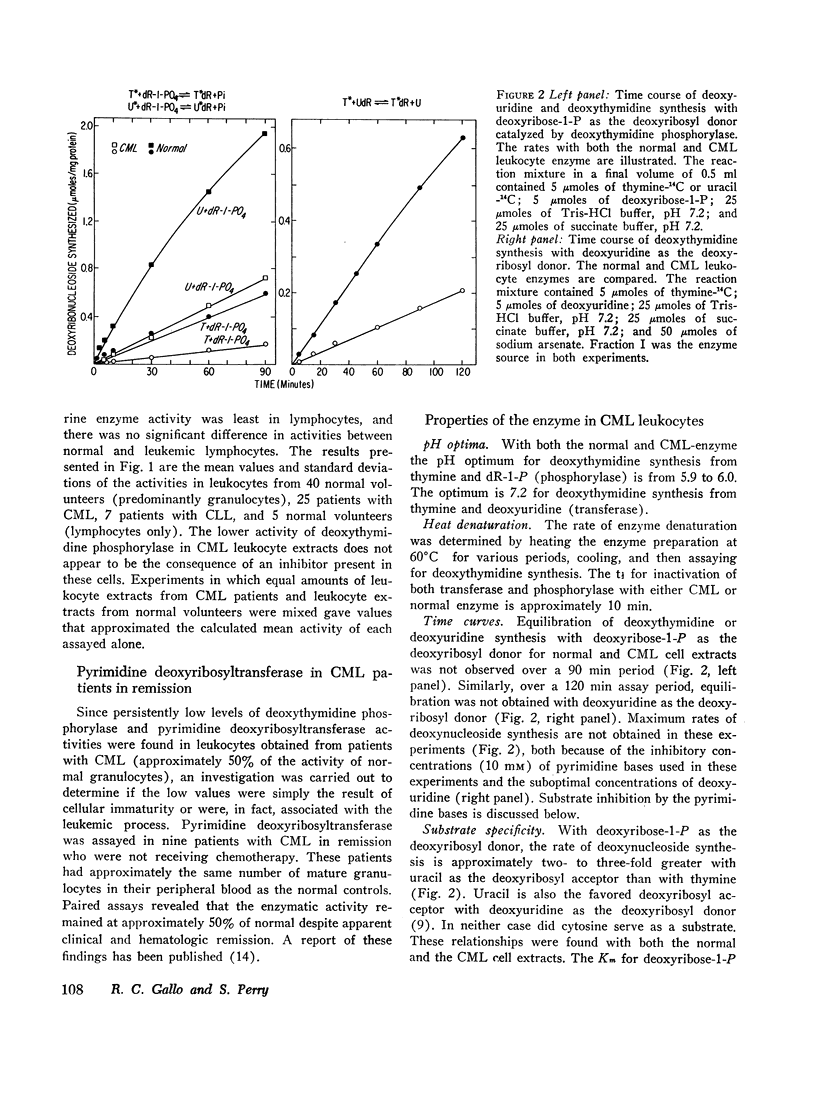
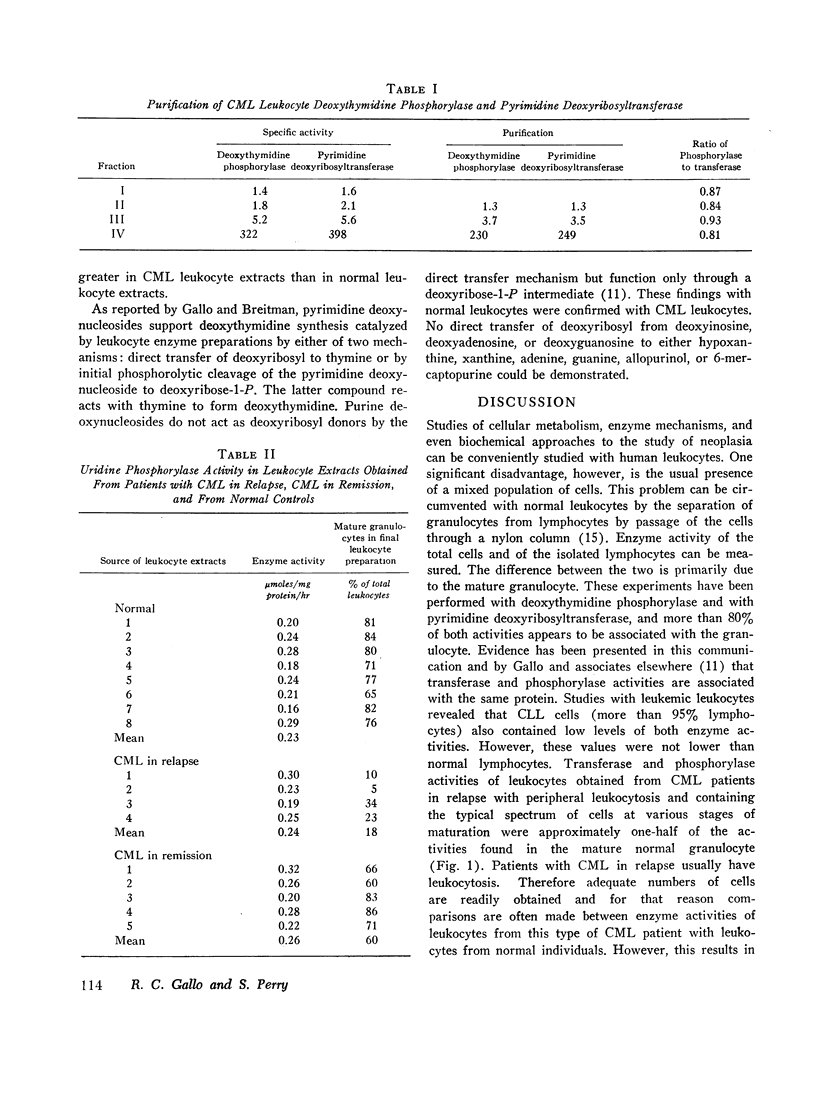
(4) In both the normal cell and the leukemic cell extracts, transferase and phosphorylase activities could not be separated. The ratio of the two activities remained constant over a 140- and a 230-fold purification in normal and leukemic cell extracts, respectively. These and other observations indicate that transferase and phosphorylase activities are associated with the same protein.
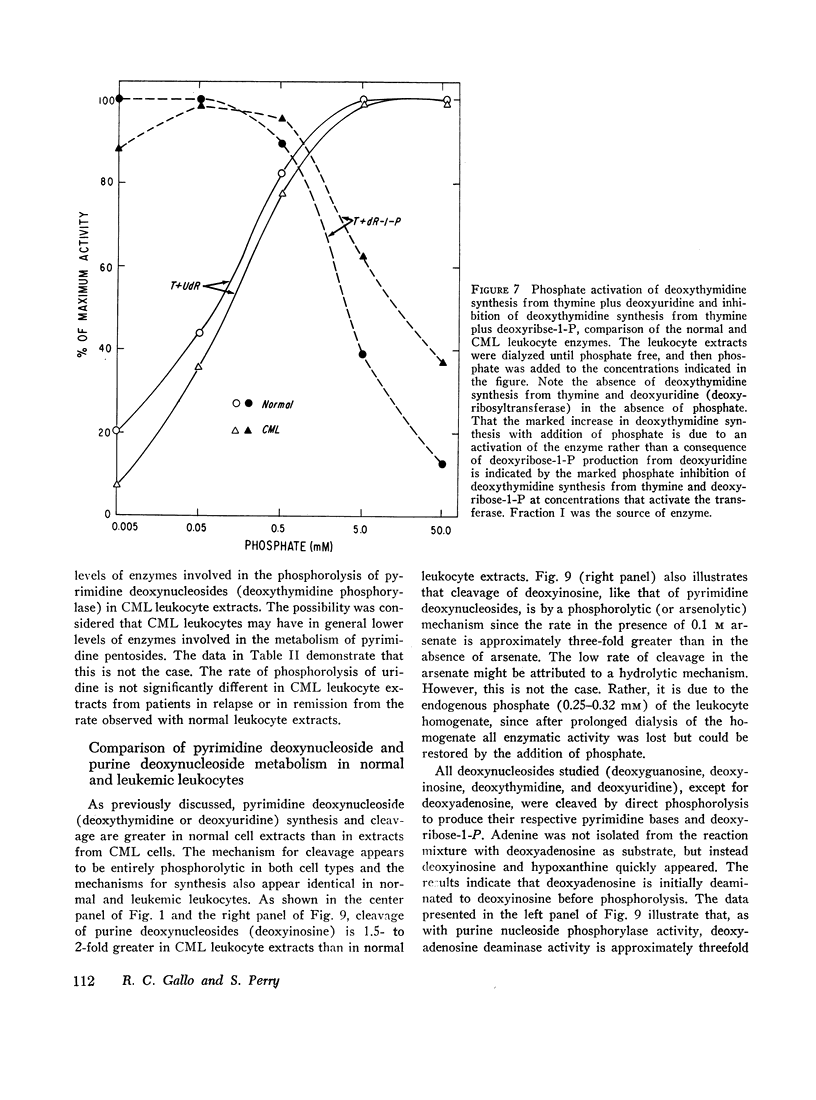
(5) The metabolism of pyrimidine and purine deoxynucleosides is similar for normal and leukemic cells. Catabolism of all deoxynucleosides tested was by direct phosphorolysis, except for deoxyadenosine which required initial deamination to deoxyinosine before phosphorolysis. In contrast to the greater rates of pyrimidine deoxynucleoside synthesis and cleavage with normal leukocyte extracts, the rates of purine deoxynucleoside synthesis and cleavage were approximately twofold greater with extracts prepared from cells of patients with chronic myelogenous leukemia. There was no significant difference in the rate of phosphorolytic cleavage of pyrimidine nucleosides (uridine) between the CML and normal leukocyte extracts.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECK W. S., LEVIN M. Purification, kinetics, and repression control of bacterial trans-N-deoxyribosylase. J Biol Chem. 1963 Feb;238:702–709. [PubMed] [Google Scholar]
- BECK W. S. THE METABOLIC BASIS OF MEGALOBLASTIC ERYTHROPOIESIS. Medicine (Baltimore) 1964 Nov;43:715–726. doi: 10.1097/00005792-196411000-00015. [DOI] [PubMed] [Google Scholar]
- BRYANT B. J. IN VIVO REUTILIZATION OF THE DNA THYMIDINE OF NECROTIZED LIVER CELLS BY CELLS OF TESTIS AND INTESTINE. Exp Cell Res. 1963 Oct;32:209–212. doi: 10.1016/0014-4827(63)90094-6. [DOI] [PubMed] [Google Scholar]
- FREI E., 3rd, TJIO J. H., WHANG J., CARBONE P. P. STUDIES OF THE PHILADELPHIA CHROMOSOME IN PATIENTS WITH CHRONIC MYELOGENOUS LEUKEMIA. Ann N Y Acad Sci. 1964 Feb 28;113:1073–1080. doi: 10.1111/j.1749-6632.1964.tb40725.x. [DOI] [PubMed] [Google Scholar]
- FRIEDKIN M., ROBERTS D. The enzymatic synthesis of nucleosides. I. Thymidine phosphorylase in mammalian tissue. J Biol Chem. 1954 Mar;207(1):245–256. [PubMed] [Google Scholar]
- FRIEDKIN M., ROBERTS D. The enzymatic synthesis of nucleosides. II. Thymidine and related pyrimidine nucleosides. J Biol Chem. 1954 Mar;207(1):257–266. [PubMed] [Google Scholar]
- Feinendegen L. E., Bond V. P., Hughes W. L. Physiological thymidine reutilization in rat bone marrow. Proc Soc Exp Biol Med. 1966 Jun;122(2):448–455. doi: 10.3181/00379727-122-31159. [DOI] [PubMed] [Google Scholar]
- GREENWALT T. J., GAJEWSKI M., McKENNA J. L. A new method for preparing buffy coat-poor blood. Transfusion. 1962 Jul-Aug;2:221–229. doi: 10.1111/j.1537-2995.1962.tb00228.x. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Breitman T. R. The enzymatic mechanisms for deoxythymidine synthesis in human leukocytes. 3. Inhibition of deoxythymidine phosphorylase by purines. J Biol Chem. 1968 Oct 10;243(19):4943–4951. [PubMed] [Google Scholar]
- Gallo R. C., Breitman T. R. The enzymatic mechanisms for deoxythymidine synthesis in human leukocytes. II. Comparison of deoxyribosyl donors. J Biol Chem. 1968 Oct 10;243(19):4936–4942. [PubMed] [Google Scholar]
- Gallo R. C., Perry S., Breitman T. R. Inhibition of human leukocyte pyrimidine deoxynucleoside synthesis by allopurinol and 6-mercaptopurine. Biochem Pharmacol. 1968 Oct;17(10):2185–2191. doi: 10.1016/0006-2952(68)90193-7. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Perry S., Breitman T. R. The enzymatic mechanisms for deoxythymidine synthesis in human leukocytes. I. Substrate inhibition by thymine and activation by phosphate or arsenate. J Biol Chem. 1967 Nov 10;242(21):5059–5068. [PubMed] [Google Scholar]
- Gallo R. C., Perry S. Enzyme abnormality in human leukaemia. Nature. 1968 May 4;218(5140):465–466. doi: 10.1038/218465a0. [DOI] [PubMed] [Google Scholar]
- KALCKAR H. M., MACNUTT W. S., HOFF-JØRGENSEN E. Trans-N-glycosidase studied with radioactive adenine. Biochem J. 1952 Jan;50(3):397–400. doi: 10.1042/bj0500397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MACNUTT W. S. The enzymically catalysed transfer of the deoxyribosyl group from one purine or pyrimidine to another. Biochem J. 1952 Jan;50(3):384–397. doi: 10.1042/bj0500384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAZZELL W. E., CASSHYAP P. SUBSTRATE SPECIFICITY AND INDUCTION OF THYMIDINE PHOSPHORYLASE IN ESCHERICHIA COLI. J Biol Chem. 1964 Jun;239:1789–1793. [PubMed] [Google Scholar]
- ROBINSON S. H., BRECHER G. DELAYED INCORPORATION OF TRITIATED THYMIDINE INTO DNA. Science. 1963 Oct 18;142(3590):392–393. doi: 10.1126/science.142.3590.392. [DOI] [PubMed] [Google Scholar]
- ROUSH A. H., BETZ R. F. Purification and properties of trans-N-deoxyribosylase. J Biol Chem. 1958 Aug;233(2):261–266. [PubMed] [Google Scholar]
- VALENTINE W. N., FOLLETTE J. H., SOLOMON D. H., REYNOLDS J. The relationship of leukocyte alkaline phosphatase to stress, to ACTH, and to adrenal 17-OH-corticosteroids. J Lab Clin Med. 1957 May;49(5):723–737. [PubMed] [Google Scholar]
- XEFTERIS E., MITUS W. J., MEDNICOFF I. B., DAMESHEK W. Leukocytic alkaline phosphatase in busulfan-induced remissions of chronic granulocytic leukemia. Blood. 1961 Aug;18:202–206. [PubMed] [Google Scholar]
- ZIMMERMAN M. DEOXYRIBOSYL TRANSFER. II. NUCLEOSIDE:PYRIMIDINE DEOXYRIBOSYLTRANSFERASE ACTIVITY OF THREE PARTIALLY PURIFIED THYMIDINE PHOSPHORYLASES. J Biol Chem. 1964 Aug;239:2622–2627. [PubMed] [Google Scholar]
- ZIMMERMAN M., SEIDENBERG J. DEOXYRIBOSYL TRANSFER. I. THYMIDINE PHOSPHORYLASE AND NUCLEOSIDE DEOXYRIBOSYLTRANSFERASE IN NORMAL AND MALIGNANT TISSUES. J Biol Chem. 1964 Aug;239:2618–2621. [PubMed] [Google Scholar]



