Abstract
Background
We sought to determine the impact of naltrexone on hepatic enzymes and HIV biomarkers in HIV-infected patients.
Methods
We used data from the Veterans Aging Cohort Study-Virtual Cohort, an electronic database of administrative, pharmacy and laboratory data. We restricted our sample to HIV-infected patients who received an initial oral naltrexone prescription, of at least seven days duration. We examined aspartate aminotransferase (AST) and alanine aminotransferase (ALT) and HIV biomarker (CD4 and HIV RNA) values for the 365 days prior to, during, and for the 365 days post-naltrexone prescription. We also examined cases of liver enzyme elevation (LEE; defined as greater than 5 times baseline ALT or AST or greater than 3.5 times baseline if baseline ALT or AST was greater than or equal to 40 IU/L).
Results
Of 114 HIV-infected individuals, 97% were male, 65% white, 57% Hepatitis C co-infected, median age was 49 years; 89% of the sample had a history of alcohol dependence and 32% had opioid dependence. Median duration of naltrexone prescription was 49 (interquartile range 30–83) days, representing 9,525 person-days of naltrexone use. Mean ALT and AST levels remained below the upper limit of normal. Two cases of LEE occurred. Mean CD4 count remained stable and mean HIV RNA decreased after naltrexone prescription.
Conclusions
In HIV-infected patients, oral naltrexone is rarely associated with clinically significant ALT or AST changes and does not have a negative impact on biologic parameters. Therefore, HIV-infected patients with alcohol or opioid dependence can be treated with naltrexone.
1. Introduction
Naltrexone is an opioid antagonist that blocks activation of the μ-opioid receptor and moderates alcohol and opioid induced dopamine increases in the nucleus accumbens.(Anton, 2008) In the United States naltrexone is available as an oral tablet and a depot injection formulation (approved for the treatment of both alcohol and opioid dependence). Naltrexone is an effective treatment for patients with unhealthy alcohol use. It decreases alcohol consumption in patients with heavy drinking and prevents relapse in patients who have achieved abstinence.(Anton et al., 2006; Garbutt et al., 2005; Hernandez-Avila et al., 2006; Kranzler et al., 2009; O'Malley, 1996; Streeton and Whelan, 2001) Additionally, naltrexone has been used for relapse prevention in patients with opioid dependence (Comer et al., 2006; Hulse et al., 2009; Minozzi et al., 2006).
Alcohol and opioid use disorders are common among HIV-infected individuals. Approximately half of HIV-infected patients report current alcohol use; with 15% (two to three times that of non-infected individuals) of drinkers classified as heavy drinkers.(Galvan et al., 2002) Approximately 20–40% of HIV-infected individuals have alcohol problems.(Samet et al., 2004) In addition, roughly 20% of all HIV-infected individuals in the United States acquired the virus through injection opioid use.(Centers for Disease Control and Prevention, 2009) Both heavy drinking and opioid misuse have adverse consequences on antiretroviral adherence, HIV disease progression, development of viral drug resistance, risk taking behaviors and biologic parameters in HIV-infected patients.(Arnsten et al., 2002; Braithwaite et al., 2005; Celentano et al., 1998; Cook et al., 2001; Galvan et al., 2002; Lucas et al., 2001; Lucas et al., 2002; Samet et al., 2003; Stein et al., 2000) Therefore, it is imperative to screen for and treat unhealthy alcohol use and opioid use among HIV-infected patients.
Prior to investigation and widespread clinical use of naltrexone in HIV-infected patients, both the safety of naltrexone and its impact on HIV therapies should be evaluated. Common side-effects of naltrexone include nausea, vomiting, and abdominal discomfort. Hepatotoxicity, one of the more concerning side-effects, occurs in 1–2% of patients, generally at doses of naltrexone higher than used for the treatment of alcohol and opioid dependence—roughly 100 to 300 mg.(Anton, 2008) This finding has resulted in a “black box” warning, restricting the use of naltrexone to patients without severe hepatic dysfunction. HIV treatment, its associated comorbidities and treatment of associated comorbidities also confer additional risks of hepatotoxicity. For instance, certain antiretroviral medications used to treat HIV have known hepatotoxicity (e.g. ritonavir, efavirenz, nevirapine, tipranavir).(Panel on Antiretroviral Guidelines for Adults and Adolescents, 2009) Dyslipidemia is a common side effect of certain classes of antiretroviral medications and is often treated with cholesterol-lowering medications which may cause hepatotoxicity.(Panel on Antiretroviral Guidelines for Adults and Adolescents, 2009) In addition roughly 30% of HIV-infected patients are co-infected with Hepatitis C (HCV), and HIV/ HCV-co-infected patients have a more rapid progression to the development of cirrhosis than do patients with HCV monoinfection, increasing the concern for medication induced hepatotoxicity. (Soto et al., 1997; Sulkowski et al., 2000) However, the impact of naltrexone on hepatic function in HIV-infected patients has never been systematically assessed.
An additional concern is whether naltrexone impacts the effectiveness of HIV antiretroviral treatment. There are limited data on the pharmacokinetic and pharmacodynamic interactions between naltrexone and antiretroviral medications. Based on studies outlining the metabolism of naltrexone, these interactions appear to be minimal. The majority of antiretroviral medications are metabolized via the cytochrome P-450 system (CYP450). This metabolic pathway is known to lead to drug interactions with a number of medication classes. Naltrexone, however, is not completely metabolized via CYP450 and therefore significant interactions are not expected.(Faragon and Piliero, 2003) One study found that naltrexone had no significant effect on the area under the curve, maximal concentration or half-life of AZT (McCance-Katz et al., 2001). Another study suggested that naltrexone may actually potentiate the antiviral effects of AZT and a protease inhibitor, indinavir.(Gekker et al., 2001) One in vitro study, that demonstrated naltrexone inhibits alcohol induced HIV-viral replication in human lymphocytes, provides preliminary evidence that naltrexone may improve HIV disease progression in HIV-infected alcohol users.(Wang et al., 2006)
In preparation for the expanded use of naltrexone in HIV-infected individuals, the purpose of the current study was to assess the impact of naltrexone treatment on hepatic enzymes and HIV biomarkers in this patient population.
2. Materials and Methods
2.1 Study design and data source
The data source for this nested case series was the Veterans Aging Cohort Study-Virtual Cohort (VACS-VC). The VACS-VC is an electronic database of longitudinal administrative, pharmacy, and laboratory data of HIV-infected and HIV-uninfected matched controls. Full details of the VACS-VC have been described elsewhere.(Fultz et al., 2006) The study includes data from 41,783 HIV-infected veterans from 128 sites nationwide who were identified between October 1997 and September 2008. Of the HIV-infected veterans in the VACS-VC, 28% have alcohol dependence and 11% have opioid dependence.
2.2 Patients
Study inclusion criteria were HIV infection, first prescription of oral naltrexone after January 1, 2003, and duration of naltrexone prescription of at least 7 days. We restricted our analysis to patients' first prescription of naltrexone to avoid enriching our sample with patients who had prior exposure to naltrexone. We chose the cut-off date to assure complete information on both inpatient and outpatient use of naltrexone.
2.3 Variables
The variables included in our analysis included gender, age, and race/ethnicity; use of antiretroviral treatment (ART) at the time of naltrexone initiation; Hepatitis C virus (HCV) status by ICD-9 coding, positive antibody testing or evidence of HCV viral load; and hepatic enzymes: alanine aminotransferase (ALT) and aspartate aminotransferase (AST). We also examined cases of liver enzyme elevation (LEE) which we defined as an increase of greater than 5 times baseline (baseline defined as average of all available lab data in the year prior to naltrexone prescription) in ALT or AST or greater than 3.5 times baseline if baseline was greater than 40 IU/L (normal range for
ALT =9–60, normal range for AST 10–40).(Cicconi et al., 2007) Finally we included the HIV biomarkers CD4 count and HIV RNA.
2.4 Data Analyses
Summary statistics were used to describe the sample characteristics. We then performed mixed-model, random intercept regression to estimate levels of ALT, AST, CD4 count and HIV RNA as a function of naltrexone status, accounting for multiple measurements per patient (within a period and among periods). Because ALT, AST and HIV RNA are not normally distributed, log transformations were used in the analysis. Three time periods were considered: pre-naltrexone prescription, the 365 prior to first naltrexone prescription; during naltrexone prescription; and post-naltrexone, the 365 days following the end of the last naltrexone prescription. We used the coefficients from the regression equations to calculate adjusted means for the pre-, during- and post-naltrexone periods. We compared mean values from the during naltrexone and post-naltrexone periods to the pre-naltrexone period. We ran two separate analyses for ALT and AST, first using all available data for each of the time periods and second using only those patients with data available in all time periods. We also ran two separate analyses for CD4 count and HIV RNA, first using all available data for each of the time periods and second using data available from the pre-naltrexone and data from either the during or post-naltrexone periods. Because the number of available lab values for each patient at each of the three time points varies, we chose to use a mixed model analysis to properly account for the repeated measures on each patient. Differences were considered statistically significant if p value for the comparison was < 0.05. Because we were most interested in identifying clinically significant hepatotoxicity in our sample of HIV-infected patients prescribed naltrexone, we also individually examined cases of LEE (defined above). All analyses were performed with SAS software, version 9.1.3.
3.1 Sample characteristics
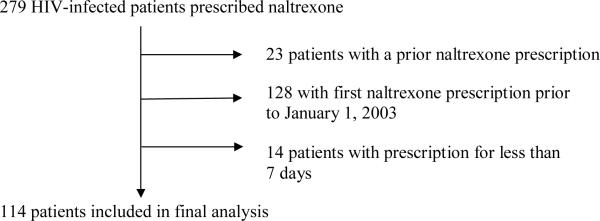
Of 279 HIV-infected patients prescribed naltrexone, 114 patients were included in the final analysis (Figure 1). The sample was predominantly male (97%), with a median age of 49 years, and 45% were white (Table 1). HCV co-infection was identified in 57% of patients. At the time of naltrexone initiation 52% were on ART, 89% had a diagnosis of alcohol dependence and 32% had opioid dependence (categories not mutually exclusive).
Figure 1.
Patient flow diagram
Table 1.
Patient characteristics, N=114
| Characteristic, n (%) | Value |
|---|---|
| Male | 111 (97) |
| Age, median (IQR) | 49 (45–53) |
| Race/Ethnicity | |
| White | 51 (45) |
| Black | 46 (53) |
| Other | 6 (7) |
| Antiretroviral use at initiation of naltrexone | 59 (52) |
| Protease Inhibitor (PI) based regimen | 21/59 (36) |
| Non-Nucleoside Reverse Transcriptase | |
| Inhibitor (NNRTI) based regimen | 29 (49) |
| Lamivudine | 37 (62) |
| Zidovudine | 31 (53) |
| Efavirenz | 23 (39) |
| Tenofovir | 22 (37) |
| Ritonavir | 19 (32) |
| Emtricitabine | 12 (20) |
| Abacavir | 10 (17) |
| Lopinavir | 10 (17) |
| Atazanavir | 8 (14) |
| Stavudine | 7 (12) |
| Nevirapine | 6 (10) |
| Nelfinavir | 4 (7) |
| Indinavir | 3 (5) |
| Didanosine | 2 (3) |
| Hepatitis C | 65 (57) |
| Alcohol dependence | 102 (89) |
| Opioid dependence | 37 (32) |
| Days of naltrexone prescription, median (IQR)* | 49 (30–83) |
| Naltrexone dose of 50 mg/day | 99 (87) |
| Person-days of naltrexone use | 9525 |
Naltrexone dose of 50mg/day is most common dose used to treat alcohol and opioid dependence.
3.2 Liver enzyme changes pre-, during, and post-naltrexone prescription
The median duration of naltrexone prescription was 49 days, representing 9,525 person-days of naltrexone prescription; 10 patients were on naltrexone for 6 months or more. Table 2a shows the adjusted mean ALT and AST levels (from regression equations) using all available data on patients pre-, during, and post-naltrexone prescription. Mean ALT and AST levels decreased both during and post-naltrexone prescription. In table 2b, we show these changes with the sample restricted to patients (n=58) who had lab data available at all three time points. For both ALT and AST, mean levels decreased and remained stable or increased during and post-naltrexone prescription; however, these values remained below the upper limit of normal.
Table 2a.
Liver enzymes pre-, during, and post- naltrexone prescription
| ALT (IU/L) | AST (IU/L) | |||||
|---|---|---|---|---|---|---|
| n | Mean | p | n | Mean | p | |
| Pre- | 113 | 41 | 112 | 41 | ||
| During | 65 | 36 | 0.02 | 66 | 37 | 0.02 |
| Post- | 100 | 37 | 0.002 | 101 | 39 | 0.03 |
Table 2b.
Liver enzymes pre-, during, and post-naltrexone prescription. Restricted to patients with complete data available at all three time points.
| ALT (IU/L) | AST (IU/L) | |||||
|---|---|---|---|---|---|---|
| n | Mean | p | n | Mean | p | |
| Pre- | 58 | 40 | 58 | 42 | ||
| During | 58 | 35 | 0.01 | 58 | 36 | 0.008 |
| Post- | 58 | 35 | 0.002 | 58 | 38 | 0.01 |
3.3 Cases of liver enzyme elevation (LEE)
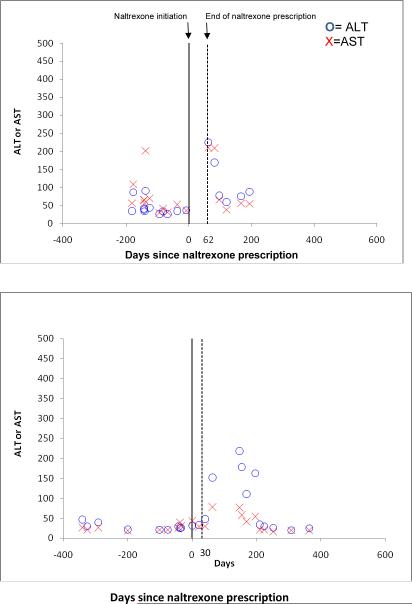
Out of 114 HIV-infected patients exposed to naltrexone, eight cases of LEE occurred; however, in only two cases did the LEE temporally coincide with the naltrexone prescription (2%, 95% CI 0–6%). Figure 2 shows the two cases where LEE occurred close to the time period covered by a naltrexone prescription. In the first HIV-infected patient with comorbid alcohol abuse/dependence and HCV, ALT increased to 225 at day 62 at which point naltrexone prescription ended and liver enzymes subsequently returned to baseline levels at day 82. In the second HIV-infected patient, naltrexone prescription ended at day 30 but ALT increased to 152 at day 63.
Figure 2.
Cases of liver enzyme elevation occurring temporally related to naltrexone prescription
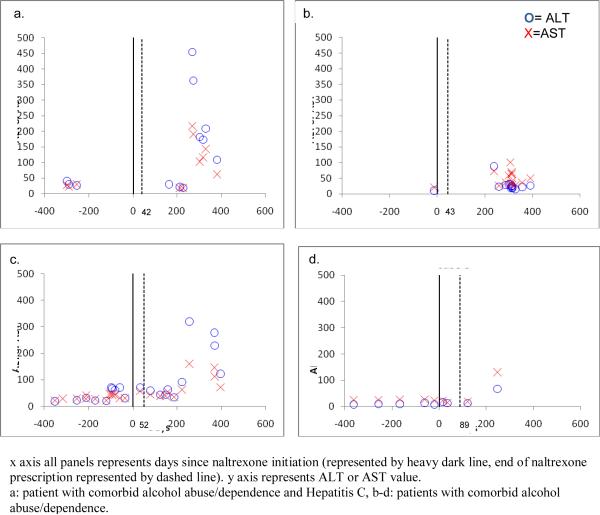
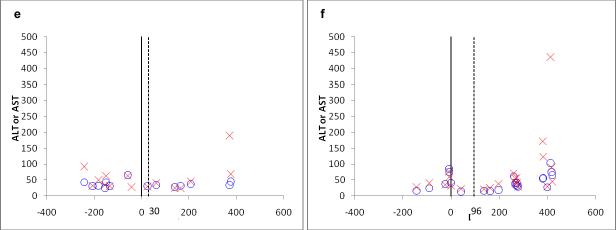
Figure 3a–d shows the remaining four cases of LEE. As can be seen in the figure, none of these cases temporally coincided with a naltrexone prescription, with elevations occurring between 100 to 200 days after naltrexone prescription had ended.
Figure 3.
Six remaining cases of liver enzyme elevation; not temporally related to naltrexone prescription.
x axis all panels represents days since naltrexone initiation (represented by heavy dark line, end of naltrexone prescription represented by dashed line). y axis represents ALT or AST value.
a: patient with comorbid alcohol abuse/dependence and Hepatitis C, b-d: patients with comorbid alcohol abuse/dependence.
e and f: patient with comorbid alcohol abuse/dependence and Hepatitis C
3.4 HIV biomarker changes pre-, during, and post-naltrexone prescription
The HIV biomarker changes pre-, during, and post-naltrexone prescription are shown in table 3a. There was no change in adjusted mean CD4 count during and post-naltrexone prescription compared with pre-naltrexone prescription levels. However, adjusted mean HIV RNA decreased from 1597 copies/mL pre-naltrexone prescription to 695 copies/mL post-naltrexone prescription (p<0.0001). In table 3b, we show the HIV biomarker changes pre- and either during or post-naltrexone prescription for the patients (N=61) who had lab data available pre-naltrexone and results either during or post-naltrexone prescription. Again, adjusted mean CD4 counts remained stable but HIV RNA decreased from 2,565 to 1,144 (p<0.0001). We did perform an exploratory analysis adjusting the models for ART use and this did not change the results.
Table 3a.
HIV biomarker changes pre-, during, and post-naltrexone prescription
| CD4 | HIV RNA | |||||
|---|---|---|---|---|---|---|
| n | Mean | p | n | Mean | p | |
| Pre- | 79 | 455 | 83 | 1597 | ||
| During | 33 | 475 | 0.4 | 42 | 971 | 0.07 |
| Post- | 66 | 449 | 0.7 | 78 | 695 | <0.001 |
Table 3b.
HIV biomarker changes with data restricted to pre, and either during or post naltrexone prescription.
| CD4 | HIV RNA | |||||
|---|---|---|---|---|---|---|
| n | Mean | p | n | Mean | p | |
| Pre- | 61 | 426 | 61 | 2565 | ||
| During | 30 | 452 | 0.3 | 36 | 1670 | 0.2 |
| Post- | 59 | 427 | 0.9 | 59 | 1144 | <0.001 |
4. Discussion
These findings represent the largest sample of HIV-infected patients who have received naltrexone and been evaluated longitudinally. Our results revealed no substantial changes in AST or ALT during naltrexone treatment in HIV-infected patients, the majority of whom were also HCV-infected and receiving ART. We witnessed two cases among 114 patients of LEE and in this both cases, the LEE reversed after naltrexone discontinuation. Additionally, HIV biomarkers do not appear to be adversely affected by naltrexone prescription.
Several studies investigating naltrexone for the treatment of alcohol and opioid dependence in HIV uninfected patients have concluded, as we have, that oral naltrexone treatment does not appear to cause hepatotoxicity, though monitoring of liver enzymes is prudent. (Berg et al., 1996; Brahen et al., 1988; Brewer and Wong, 2004; Croop et al., 1997; Pini et al., 1991; Yen et al., 2006) The hepatic safety of extended-release naltrexone for the treatment of alcohol dependence has also been assessed and reached similar conclusions.(Lucey et al., 2008) Although most studies investigating the use of naltrexone for the treatment of alcohol or opioid dependence conclude that naltrexone is safe, isolated cases of liver enzyme elevation did occur in some of these trials. As presented here, once naltrexone was discontinued, however, liver enzymes promptly returned to baseline.(Anton et al., 2006; Yen et al., 2006) Concerns over hepatotoxicity initially arose from studies of naltrexone treatment for patients with obesity.(Atkinson et al., 1985; Malcolm et al., 1985) However, in these studies, doses of naltrexone exceeded 100 mg daily, compared with 25–50 mg daily for the treatment of alcohol or opioid dependence.(Pfohl et al., 1986) Additionally, obesity itself is a known risk factor for increased liver enzymes and may have conferred additional risk in these studies.(Boppidi and Daram, 2008; Diehl, ; Fabbrini et al.) More recent studies on the use of high dose (greater than 100 mg) naltrexone for the treatment of impulse control disorders suggested that naltrexone is safe provided intake of other known hepatotoxic medications (i.e., acetaminophen, ibuprofen) is limited.(Kim et al., 2006; Marrazzi et al., 1997)
Our investigation differs from other studies as it focuses on hepatic safety of naltrexone in HIV-infected patients and also studies the clinical impact of potential drug-drug interactions with ART. Prior work has focused on the in-vitro effects of naltrexone on HIV-infected cells, but clinical studies of naltrexone in HIV-infected patients have been lacking.(Gekker et al., 2001; Wang et al., 2006) One prospective clinical trial investigated the hepatic safety of naltrexone in 116 patients with HCV and found no evidence of increased hepatic risk in this population.(Lozano Polo et al., 1997)
Our study has limitations. Because the data is observational and routine serial blood work was not performed, we were only able to detect cases of LEE that were measured in the course of clinical care. It is possible that some elevations went undetected, but we think it unlikely that elevations that would have reached our threshold (5 times normal) were not detected. Additionally, while we observe a decrease in HIV RNA both in the original analysis and when adjusted for ART, we note that these observations are strictly associations and these data do not allow us to make causal inferences about the relationship between naltrexone prescription and CD4 count or HIV RNA. Also, because this cohort is assembled using VA data, very few women are represented in this sample. Moreover, because we measured naltrexone prescriptions, initiation of and adherence to naltrexone was not assessed. Finally, the number of patients with data available at all time points studied is small and therefore may limit our conclusions. It is notable that the sample size of patients with available liver function tests and HIV biologic parameter data decreased during and post-naltrexone prescription. This missing lab data may represent poor follow-up on the part of the physician, perception of clinical improvement on the part of the patient and a lack of need to follow up with the doctor, or relapse to alcohol or opioid use. Despite these limitations, our study is the first to investigate hepatic safety and HIV biologic parameters in a cohort of HIV-infected patients prescribed naltrexone for the treatment of alcohol and opioid dependence.
Our results suggest that naltrexone, the only medication approved to treat both alcohol and opioid dependence and one of only a few available pharmacotherapies available to treat these disorders, rarely causes hepatotoxicity and has no adverse effects on biologic parameters in HIV-infected patients. One major advantage of naltrexone, compared with other pharmacotherapies, is its availability in a depot formulation which maximizes adherence. The data presented here provide support to the efforts to integrate alcohol and opioid dependence treatment with HIV clinical care. In addition, the decreased HIV RNA in HIV-infected patients receiving naltrexone warrants further investigation. Further investigation should include larger samples, with routine serial assessments and include more women to fully evaluate the impact of naltrexone on liver enzymes and HIV biologic parameters in HIV-infected patients. In addition, given the role that alcohol and opioid use play in the transmission and progression of HIV disease internationally, there is a need to further investigate medication assisted treatments in HIV-infected patients to determine their impact on cogent substance use and HIV outcomes.
Acknowledgments
Funding source: The Veterans Aging Cohort Study is funded by the National Institute on Alcohol Abuse and Alcoholism (U10 AA 13566) and the VHA Public Health Strategic Health Core Group. The material presented in this study is based upon work supported in part by funding from the National Institute on Alcohol and Alcohol Abuse (U01 AA 13566) and the National Institute on Drug Abuse (NIDA R01 DA019511-03, R01 DA025991, R01 DA020576-01A1, K23 DA024050-02). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of any of the funding agencies, including the Department of Veterans Affairs, or the United States government. The funding agencies had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
References
- Anton RF. Naltrexone for the management of alcohol dependence. New England Journal of Medicine. 2008;359(7):715–21. doi: 10.1056/NEJMct0801733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A, Group CSR. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003–17. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Arnsten JH, Demas PA, Grant RW, Gourevitch MN, Farzadegan H, Howard AA, Schoenbaum EE. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. Journal of General Internal Medicine. 2002;17(5):377–81. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson RL, Berke LK, Drake CR, Bibbs ML, Williams FL, Kaiser DL. Effects of long-term therapy with naltrexone on body weight in obesity. Clinical Pharmacology & Therapeutics. 1985;38(4):419–22. doi: 10.1038/clpt.1985.197. [DOI] [PubMed] [Google Scholar]
- Berg BJ, Pettinati HM, Volpicelli JR. A risk-benefit assessment of naltrexone in the treatment of alcohol dependence. Drug Safety. 1996;15(4):274–82. doi: 10.2165/00002018-199615040-00005. [DOI] [PubMed] [Google Scholar]
- Boppidi H, Daram SR. Nonalcoholic fatty liver disease: hepatic manifestation of obesity and the metabolic syndrome. Postgraduate Medicine. 2008;120(2):E01–7. doi: 10.3810/pgm.2008.07.1800. [DOI] [PubMed] [Google Scholar]
- Brahen LS, Capone TJ, Capone DM. Naltrexone: lack of effect on hepatic enzymes. Journal of Clinical Pharmacology. 1988;28(1):64–70. doi: 10.1002/j.1552-4604.1988.tb03102.x. [DOI] [PubMed] [Google Scholar]
- Braithwaite RS, McGinnis KA, Conigliaro J, Maisto SA, Crystal S, Day N, Cook RL, Gordon A, Bridges MW, Seiler JFS, Justice AC. A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcoholism: Clinical & Experimental Research. 2005;29(7):1190–7. doi: 10.1097/01.alc.0000171937.87731.28. [DOI] [PubMed] [Google Scholar]
- Brewer C, Wong VS. Naltrexone: report of lack of hepatotoxicity in acute viral hepatitis, with a review of the literature. Addiction Biology. 2004;9(1):81–7. doi: 10.1080/13556210410001674130. [DOI] [PubMed] [Google Scholar]
- Celentano DD, Vlahov D, Cohn S, Shadle VM, Obasanjo O, Moore RD. Self-reported antiretroviral therapy in injection drug users. JAMA. 1998;280(6):544–6. doi: 10.1001/jama.280.6.544. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention HIV/AIDS Surveillance Report. 2009 [Google Scholar]
- Cicconi P, Cozzi-Lepri A, Phillips A, Puoti M, Antonucci G, Manconi PE, Tositti G, Colangeli V, Lichtner M, Monforte Ada, Group ICS. Is the increased risk of liver enzyme elevation in patients co-infected with HIV and hepatitis virus greater in those taking antiretroviral therapy? AIDS. 2007;21(5):599–606. doi: 10.1097/QAD.0b013e328013db9c. [DOI] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Yu E, Rothenberg JL, Kleber HD, Kampman K, Dackis C, O'Brien CP. Injectable, sustained-release naltrexone for the treatment of opioid dependence: a randomized, placebo-controlled trial. Archives of General Psychiatry. 2006;63(2):210–8. doi: 10.1001/archpsyc.63.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RL, Sereika SM, Hunt SC, Woodward WC, Erlen JA, Conigliaro J. Problem drinking and medication adherence among persons with HIV infection. Journal of General Internal Medicine. 2001;16(2):83–8. doi: 10.1111/j.1525-1497.2001.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croop RS, Faulkner EB, Labriola DF. The safety profile of naltrexone in the treatment of alcoholism. Results from a multicenter usage study. The Naltrexone Usage Study Group. Archives of General Psychiatry. 1997;54(12):1130–5. doi: 10.1001/archpsyc.1997.01830240090013. [DOI] [PubMed] [Google Scholar]
- Diehl AM. Hepatic complications of obesity. Gastroenterology Clinics of North America. 39(1):57–68. doi: 10.1016/j.gtc.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 51(2):679–89. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faragon JJ, Piliero PJ. Drug interactions associated with HAART: focus on treatments for addiction and recreational drugs. AIDS Reader. 2003;13(9):433–4. [PubMed] [Google Scholar]
- Fultz SL, Skanderson M, Mole LA, Gandhi N, Bryant K, Crystal S, Justice AC. Development and verification of a “virtual” cohort using the National VA Health Information System. Medical Care. 2006;44(8 Suppl 2):S25–30. doi: 10.1097/01.mlr.0000223670.00890.74. [DOI] [PubMed] [Google Scholar]
- Galvan FH, Bing EG, Fleishman JA, London AS, Caetano R, Burnam MA, Longshore D, Morton SC, Orlando M, Shapiro M. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: results from the HIV Cost and Services Utilization Study. Journal of Studies on Alcohol. 2002;63(2):179–86. doi: 10.15288/jsa.2002.63.179. [DOI] [PubMed] [Google Scholar]
- Garbutt JC, Kranzler HR, O'Malley SS, Gastfriend DR, Pettinati HM, Silverman BL, Loewy JW, Ehrich EW, Vivitrex Study G. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293(13):1617–25. doi: 10.1001/jama.293.13.1617. [Erratum appears in JAMA. 2005 Jun 15:293(23):2864].[Erratum appears in JAMA. 2005 Apr 27;293(16):1978] [DOI] [PubMed] [Google Scholar]
- Gekker G, Lokensgard JR, Peterson PK. Naltrexone potentiates anti-HIV-1 activity of antiretroviral drugs in CD4+ lymphocyte cultures. Drug & Alcohol Dependence. 2001;64(3):257–63. doi: 10.1016/s0376-8716(01)00140-5. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Song C, Kuo L, Tennen H, Armeli S, Kranzler HR. Targeted versus daily naltrexone: secondary analysis of effects on average daily drinking. Alcoholism: Clinical & Experimental Research. 2006;30(5):860–5. doi: 10.1111/j.1530-0277.2006.00101.x. [DOI] [PubMed] [Google Scholar]
- Hulse GK, Morris N, Arnold-Reed D, Tait RJ. Improving clinical outcomes in treating heroin dependence: randomized, controlled trial of oral or implant naltrexone. Archives of General Psychiatry. 2009;66(10):1108–15. doi: 10.1001/archgenpsychiatry.2009.130. [DOI] [PubMed] [Google Scholar]
- Kim SW, Grant JE, Yoon G, Williams KA, Remmel RP. Safety of high-dose naltrexone treatment: hepatic transaminase profiles among outpatients. Clinical Neuropharmacology. 2006;29(2):77–9. doi: 10.1097/00002826-200603000-00004. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Tennen H, Armeli S, Chan G, Covault J, Arias A, Oncken C. Targeted naltrexone for problem drinkers. Journal of Clinical Psychopharmacology. 2009;29(4):350–7. doi: 10.1097/JCP.0b013e3181ac5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano Polo JL, Gutierrez Mora E, Martinez Perez V, Santamaria Gutierrez J, Vada Sanchez J, Vallejo Correas JA. Effect of methadone or naltrexone on the course of transaminases in parenteral drug users with hepatitis C virus infection. Revista Clinica Espanola. 1997;197(7):479–83. [PubMed] [Google Scholar]
- Lucas GM, Cheever LW, Chaisson RE, Moore RD. Detrimental effects of continued illicit drug use on the treatment of HIV-1 infection. Journal of Acquired Immune Deficiency Syndromes: JAIDS. 2001;27(3):251–9. doi: 10.1097/00126334-200107010-00006. [DOI] [PubMed] [Google Scholar]
- Lucas GM, Gebo KA, Chaisson RE, Moore RD. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS. 2002;16(5):767–74. doi: 10.1097/00002030-200203290-00012. [DOI] [PubMed] [Google Scholar]
- Lucey MR, Silverman BL, Illeperuma A, O'Brien CP. Hepatic safety of once-monthly injectable extended-release naltrexone administered to actively drinking alcoholics. Alcoholism: Clinical & Experimental Research. 2008;32(3):498–504. doi: 10.1111/j.1530-0277.2007.00593.x. [DOI] [PubMed] [Google Scholar]
- Malcolm R, O'Neil PM, Sexauer JD, Riddle FE, Currey HS, Counts C. A controlled trial of naltrexone in obese humans. International Journal of Obesity. 1985;9(5):347–53. [PubMed] [Google Scholar]
- Marrazzi MA, Wroblewski JM, Kinzie J, Luby ED. High-dose naltrexone and liver function safety. American Journal on Addictions. 1997;6(1):21–9. doi: 10.3109/10550499708993159. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Rainey PM, Friedland G, Kosten TR, Jatlow P. Effect of opioid dependence pharmacotherapies on zidovudine disposition. American Journal on Addictions. 2001;10(4):296–307. [PubMed] [Google Scholar]
- Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database of Systematic Reviews. 2006;(1):CD001333. doi: 10.1002/14651858.CD001333.pub2. [DOI] [PubMed] [Google Scholar]
- O'Malley SS. Opioid antagonists in the treatment of alcohol dependence: clinical efficacy and prevention of relapse. Alcohol & Alcoholism. 1996;31(Suppl 1):77–81. [PubMed] [Google Scholar]
- Panel on Antiretroviral Guidelines for Adults and Adolescents . In: Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services, editor. 2009. pp. 1–161. [Google Scholar]
- Pfohl DN, Allen JI, Atkinson RL, Knopman DS, Malcolm RJ, Mitchell JE, Morley JE. Naltrexone hydrochloride (Trexan): a review of serum transaminase elevations at high dosage. NIDA Research Monograph. 1986;67:66–72. [PubMed] [Google Scholar]
- Pini LA, Ferretti C, Trenti T, Ferrari A, Sternieri E. Effects of long-term treatment with naltrexone on hepatic enzyme activity. Drug Metabolism & Drug Interactions. 1991;9(2):161–74. doi: 10.1515/dmdi.1991.9.2.161. [DOI] [PubMed] [Google Scholar]
- Samet JH, Horton NJ, Traphagen ET, Lyon SM, Freedberg KA. Alcohol consumption and HIV disease progression: are they related? Alcoholism: Clinical & Experimental Research. 2003;27(5):862–7. doi: 10.1097/01.ALC.0000065438.80967.56. [DOI] [PubMed] [Google Scholar]
- Samet JH, Phillips SJ, Horton NJ, Traphagen ET, Freedberg KA. Detecting alcohol problems in HIV-infected patients: use of the CAGE questionnaire. AIDS Research & Human Retroviruses. 2004;20(2):151–5. doi: 10.1089/088922204773004860. [DOI] [PubMed] [Google Scholar]
- Soto B, Sanchez-Quijano A, Rodrigo L, del Olmo JA, Garcia-Bengoechea M, Hernandez-Quero J, Rey C, Abad MA, Rodriguez M, Sales Gilabert M, Gonzalez F, Miron P, Caruz A, Relimpio F, Torronteras R, Leal M, Lissen E. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. Journal of Hepatology. 1997;26(1):1–5. doi: 10.1016/s0168-8278(97)80001-3. [DOI] [PubMed] [Google Scholar]
- Stein MD, Rich JD, Maksad J, Chen MH, Hu P, Sobota M, Clarke J. Adherence to antiretroviral therapy among HIV-infected methadone patients: effect of ongoing illicit drug use. American Journal of Drug & Alcohol Abuse. 2000;26(2):195–205. doi: 10.1081/ada-100100600. [DOI] [PubMed] [Google Scholar]
- Streeton C, Whelan G. Naltrexone, a relapse prevention maintenance treatment of alcohol dependence: a meta-analysis of randomized controlled trials. Alcohol & Alcoholism. 2001;36(6):544–52. doi: 10.1093/alcalc/36.6.544. [DOI] [PubMed] [Google Scholar]
- Sulkowski MS, Mast EE, Seeff LB, Thomas DL. Hepatitis C virus infection as an opportunistic disease in persons infected with human immunodeficiency virus. Clinical Infectious Diseases. 2000;30(Suppl 1):S77–84. doi: 10.1086/313842. [DOI] [PubMed] [Google Scholar]
- Wang X, Douglas SD, Peng J-S, Metzger DS, O'Brien CP, Zhang T, Ho W-Z. Naltrexone inhibits alcohol-mediated enhancement of HIV infection of T lymphocytes. Journal of Leukocyte Biology. 2006;79(6):1166–72. doi: 10.1189/jlb.1105642. [DOI] [PubMed] [Google Scholar]
- Yen M-H, Ko H-C, Tang F-I, Lu R-B, Hong J-S. Study of hepatotoxicity of naltrexone in the treatment of alcoholism. Alcohol. 2006;38(2):117–20. doi: 10.1016/j.alcohol.2006.05.003. [DOI] [PubMed] [Google Scholar]