Abstract
Introduction
The aim of this study, performed in an intensive care unit (ICU) population with a normal serum creatinine, was to estimate urinary creatinine clearance (CLCR) in a population of polytrauma patients (PT) through a comparison with a population of non trauma patients (NPT).
Methods
This was a retrospective, observational study in a medical and surgical ICU in a university hospital. A total of 284 patients were consecutively included. Two different groups were studied: PT (n = 144) and NPT (n = 140). Within the second week after admission to the ICU, renal function was assessed using serum creatinine, 24 h urinary CLCR .
Results
Among the 106 patients with a CLCR above 120 mL minute-1 1.73 m-2, 79 were PT and 27 NPT (P < 0.0001). Only 63 patients had a CLCR below 60 mL minute-1 1.73 m-2 with 15 PT and 48 NPT (P < 0.0001). Patients with CLCR greater than 120 mL minute-1. 1.73 m -2 were younger, had a lower SAPS II score and a higher male ratio as compared to those having CLCR lower than 120 mL minute-1. 1.73 m -2. Through a logistic regression analysis, age and trauma were the only factors independently correlated to CLCR.
Conclusions
In ICU patients with normal serum creatinine, CLCR, is higher in PT than in NPT. The measure of CLCR should be proposed as routine for PT patients in order to adjust dose regimen, especially for drugs with renal elimination.
Introduction
Early detection of renal dysfunction in intensive care unit (ICU) patients is important. Indeed, an increase of the glomerular filtration rate (GFR) was demonstrated in some ICU patients populations by using urinary creatinine clearance (CLCR) as a surrogate marker, and many of the drugs used in ICU patients need dose adjustment as a function of GFR [1]. Despite a normal serum creatinine measurement, a substantial number of burn patients demonstrated an increase in GFR with the need for increasing doses of renal elimination drugs to maintain therapeutic concentrations [1,2]. A study of our group showed that 42% of burn patients had a creatinine clearance greater than 120 mL minute-1 1.73 m2 [1]. Also, several studies performed in a general population of ICU patients suggested a poor correlation between serum creatinine concentration and GFR in polytrauma patients (PT) [3-5]. To the best of our knowledge, no study has specifically explored this population of PT patients.
The aim of this study, performed in a population of ICU patients with normal serum creatinine, was to estimate GFR, evaluated by measured CLCR, in a population of PT patients through a comparison with a population of non-trauma patients (NPT).
Materials and methods
Patients
This observational study was conducted in the ICU of Toulouse University hospital during a five-year period (November 2002 to December 2007). The study was performed according to the Declaration of Helsinki. No change in our current clinical practice (measured creatinine clearance monitoring, at least once a week, is a part of the routine medical care of the patients) and no randomization was performed. As it was an observational retrospective study, in accordance with French law, neither approval of the ethics committee nor informed consent was required.
Ten days, on average, after admission in ICU, consecutive critically ill patients meeting the inclusion criteria were included. Inclusion criteria were: patients older than 18 years, with an arterial catheter, a urinary bladder catheter, a diuresis over 500 mL d-1. All patients had a tracheal tube and were mechanically ventilated. Patients were hemodynamically stable presenting with a stable serum creatinine in a normal range (40 to 125 μmol L-1).
Patients were excluded from the study: if they were hemodynamically unstable or if they needed a high dose of catecholamine (norepinephrine > 1 mg h-1); if they were recovering from acute kidney injury (AKI) or developing AKI; if they received histamine-2-receptor antagonist due to its interference with tubular creatinine secretion [6]; and if they had a medical history of diabetes, of chronic hepatic disease, cirrhosis or ongoing liver dysfunction with hepatitis [7,8]. Patients treated with diuretics were also excluded.
Baseline characteristics of patients were recorded at enrolment in the study and the SAPS II was obtained at ICU admission. PT patients had an ISS (Injury Severity Score) > 16. SOFA (Sequential Organ Failure Assessment) score was obtained on the day the urine 24-hour measure was sampled [9-11].
Urine was sampled over 24 hours to measure urinary creatinine concentration. Serum creatinine was also measured during the urine collection period.
The normal limits of CLCR were estimated between 60 and 120 mL minute-1 1.73 m-2 [9,12].
Serum creatinine measurement and calibration
Creatinine measurements were performed in the same laboratory of the University Hospital of Toulouse. Blood samples were obtained simultaneously with the CLCR measurement. A modified kinetic Jaffe colorimetric method was used with a COBAS MIRA (ABX Diagnostics, Montpellier, France) analyzer. A two-point calibration was applied in each assay.
Before measurement, ultrafiltration of plasma through a 20 kD cutoff membrane (MPS-1; Amicon, Beverly, MA, USA) was performed to discard chromogens that were linked to albumin-like bilirubinemia and other heavy proteins. In the absence of an international standard for creatinine assay, the linearity of the measurements was verified by using plasma samples from normal subjects in which increasing amounts of desiccated creatinine hydrochloride (MW 149.6; Sigma Chemicals, Perth, Australia) had been added. Linear regression analysis showed that the relationship between measured and expected creatinine concentrations was 1.0008 ± 0.006 (95% confidence interval, 0.997 to 1.020) and that the Y-intercept was 0.014 ± 0.013 (95% confidence interval, -0.013 to 0.041). Squared Spearman rank coefficient of correlation was 0.998. Internal quality controls showed a coefficient of variation of 2.3% during the period.
Assessment of glomerular filtration rate
Creatinine clearance was measured according to the formula where urine creatinine (UCR) and serum creatinine (SCR) were expressed in μmol L-1 and V corresponded to the urinary rate (diuresis) in mL minute-1.
At the same time, the GFR was estimated using the Cockcroft Gault formula [13] for men, with age in years and weight in Kg. A correcting factor of 0.85 was used for women. The derivate formula proposed by Robert et al. [14] uses the ideal body weight and serum creatinine concentration corrected to 85 μmol L-1 when the actual value is lower than 85 μmol L-1. Ideal body weight was determined as 50 kg for men and 45.5 kg for women, plus 2.3 kg for each inch over five feet. The simplified formula of the Modification of Diet in Renal Disease index (sMDRD) [15] was also calculated according to sMDRD = 186.3 × SCR -1.154 × age-0.203 × (1.212 if black) × (0.742 if female) where serum creatinine was expressed in mg dL-1.
Statistical analysis
Statistical analyses were performed using StatView® software version 5.0 (SAS Institute Inc., Cary, NC, USA). Data are presented as mean ± standard deviation (SD) or ratio. Normal distribution of data was tested via Kolmogorov-Smirnov test. Chi-square test or Student's t-test was performed when appropriate. A logistic regression was performed to discriminate if trauma, age, SAPS II, ideal body weight and sex are independently correlated to the measured CLCR. A P-value < 0.05 was considered as statistically significant.
Results
Demographic and renal data are shown in Table 1. Two hundred, eighty-four patients were consecutively included in this observational study. The process of screening and inclusion in the study is shown in Figure 1 (flow chart).
Table 1.
Demographic data
| NPTa (n = 140) | PTb (n = 144) | P* | |
|---|---|---|---|
| Age (yr) | 58 ± 17 | 42 ± 18 | < 0.0001 |
| Weight (kg) | 72 ± 18 | 75 ± 14 | NS |
| Height (cm) | 170 ± 8 | 174 ± 9 | NS |
| Sex (F/M) | 52/88 | 36/108 | 0.03 |
| Ideal body weight (Kg) | 68 ± 11 | 72 ± 11 | 0.0006 |
| SAPS 2 | 52 ± 14 | 42 ± 15 | < 0.0001 |
| Total SOFA score | 3.7 ± 1.5 | 3.6 ± 1.4 | NS |
| Respiratory system | 0.3 ± 0.2 | 0.5 ± 0.6 | |
| Coagulation | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| Liver | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| Cardiovascular system | 1.3 ± 0.9 | 1.4 ± 0.9 | |
| Neurological system | 2.5 ± 1.5 | 2.4 ± 1.5 | |
| Renal system | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| Mean arterial blood pressure (mmHg) | 83 +/- 10 | 82 +/- 11 | NS |
| Systolic arterial blood pressure (mmHg) | 126 +/- 16 | 125 +/- 17 | NS |
| Diastolic arterial blood pressure (mmHg) | 63 +/- 10 | 63 +/- 11 | NS |
| Heart rate (bpm) | 91 +/- 17 | 92 +/- 16 | NS |
| Serum creatinine (μmol L-1) | 74 ± 26 | 72 ± 19 | NS |
| Measured creatinine clearance (mL minute-1 1.73 m-2) | 85 ± 5 | 131 ± 5 | < 0.0001 |
| Diuresis/24H | 2,700 ± 1,200 | 2,500 ± 1,200 | NS |
| Serum Urea | 8.7 ± 5 | 7 ± 3 | NS |
| Measured creatinine clearance < 60 (mL minute-1 1.73 m-2) | 48 | 15 | 0.0003 |
| Measured creatinine clearance > 120 (mL minute-1 .1.73 m-2) | 27 | 79 | < 0.0001 |
All data are expressed as ratio or mean ± SD.
* The P-values correspond to comparison between NPT and PT groups.
a NPT, non-polytrauma patients.
b PT, polytrauma patients.
Figure 1.
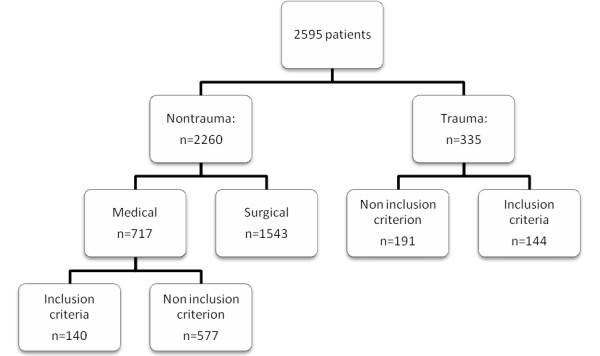
Flow chart showing the process of recruitment.
The group of 144 PT patients was compared with the group of 140 NPT patients. No difference was found concerning hemodynamic data (Table 1). No difference was found concerning ventilation pressure. All the patients were ventilated with a tidal volume of 6 to 8 ml/kg, the PEP value was set at 5.8 ± 3 in PT vs 5.5 ± 3 in NPT patients (NS). FiO2 was 45 +/- 16% in PT vs 45 +/- 15 in NPT (NS), with Ph = 7.38 +/- 0.8 vs 7.39 +/- 0.8 (NS), PaO2 = 107 +/- 16 in PT vs 108 +/- 15 in NPT (NS), PaCO2 = 39 +/- 8 in PT vs 40 +/- 9, SaO2 = 97 +/- 3 vs 97 +/- 3 (NS). Glycemia was not different between groups (6.2 +/- 1.7 vs 6.1 +/- 1.8; NS). Twenty-three percent of PT vs 24% of NPT received norepinephrine (NS).
The overall results show that serum creatinine was normal (73 ± 22 μmol L-1) and serum urea (8 ± 4 mmol L-1) was slightly higher than the normal limits, but with no difference between groups. One hundred, six patients had a CLCR above 120 mL minute-1 1.73 m-2, including 79 PT and 27 NPT (P < 0.0001). Only 63 patients had a CLCR below 60 mL minute-1 1.73 m-2 with 15 PT and 48 NPT (P < 0.0001), whereas nine patients had a CLCR below 30 mL minute-1 1.73 m-2, including two PT.
The overall urinary creatinine excretion was 929 ± 678 mg 24 h-1 1.73 m-2 for women and 1,369 ± 685 mg 24 h-1 1.73 m-2 for men. There was a significant difference between the urinary creatinine excretion of PT and NPT patients (1,489 ± 639 vs 969 ± 688 mg 24 h-1 1.73 m-2 respectively, P < 0.001). In the PT group, males had significantly higher urinary creatinine excretion than females (1,630 ± 644 vs 1,067 ± 392 mg 24 h-1 1.73 m-2, P < 0.001).
The overall measured CLCR was 108 ± 57 mL minute-1 1.73 m-2. The CLCR was higher in PT patients than in NPT patients when using measured CLCR (131 ± 56 vs 85 ± 48 mL minute-1 1.73 m-2 respectively, P < 0.001).
Most patients with increased CLCR (above 120 mL minute-1 1.73 m-2) were PT patients as shown in Table 2. On the opposite, most patients with decreased CLCR (below 60 mL minute-1 1.73 m-2) were NPT patients.
Table 2.
Comparison of patients with different measured creatinine clearance (CLCR)
| ClCR < 120 (n = 178) | ClCR > 120 (n = 106) | P * | ClCR < 60 (n = 63) | ClCR > 60 (n = 221) | P # | P§ | |
|---|---|---|---|---|---|---|---|
| Age (yr) | 56 ± 18 | 40 ± 16 | < 0.0001 | 63 ± 15 | 46 ± 18 | < 0.0001 | < 0.0001 |
| Weight (Kg) | 74 ± 17 | 74 ± 14 | NS | 73 ± 19 | 74 ± 15 | NS | NS |
| Sex (F/M) | 64/114 | 24/82 | 0.03 | 35/28 | 161/60 | NS | 0.0032 |
| Ideal body weight (Kg) | 69 ± 12 | 72 ± 10 | 0.01 | 65 ± 11 | 71 ± 11 | 0.0002 | < 0.0001 |
| SAPS 2 | 50 ± 15 | 43 ± 14 | 0.0002 | 54 ± 14 | 45 ± 15 | < 0.0001 | < 0.0001 |
| Serum creatinine (μmol L-1) | 76 ± 24 | 67 ± 19 | 0.0001 | 85 ± 25 | 69 ± 20 | < 0.0001 | < 0.0001 |
| Diuresis/24H | 2,400 ± 100 | 2,800 ± 1200 | < 0.0001 | 584 ± 224 | 1,418 ± 694 | 0.0019 | < 0.0001 |
| Measured creatinine clearance (mL min-1 .1.73 m-2) | 74 ± 30 | 166 ± 50 | < 0.0001 | 43 ± 11 | 127 ± 50 | < 0.0001 | < 0.0001 |
| Simplified MDRD (mL minute-1 1.73 m-2) | 96 ± 39 | 119 ± 45 | < 0.0001 | 81 ± 34 | 112 ± 43 | < 0.0001 | < 0.0001 |
| Cockcroft-Gault (mL minute-1 1.73 m-2) | 98 ± 39 | 130 ± 34 | 0.0005 | 80 ± 39 | 118 ± 36 | < 0.0001 | < 0.0001 |
| Robert (mL minute-1 1.73 m-2) | 72 ± 23 | 94 ± 23 | < 0.0001 | 59 ± 2 | 85 ± 2 | < 0.0001 | < 0.0001 |
| PT b/NPT a | 65/113 | 79/27 | < 0.0001 | 15/48 | 129/92 | < 0.0001 | < 0.0001 |
All data are expressed as ratio or mean ± SD.
* The P-values correspond to comparison between subgroup with CLCR greater than 120 mL minute-1 1.73 m-2 versus subgroup with CLCR lower than 120 mL minute-1 1.73 m-2.
# The P-values correspond to comparaison between subgroup with CLCR greater than 60 mL minute-1 1.73 m-2 versus subgroup with CLCR lower than 60 mL minute-1 1.73 m-2.
§ The P-values correspond to comparison between less than 60 mL minute-1 1.73 m-2 more than 120 mL minute-1 1.73 m-2.
a NPT = non-polytrauma patients.
b PT = polytrauma patients.
Patients with CLCR greater than 120 mL minute-1 1.73 m -2 were younger (40 ± 16 years vs 56 ± 18 years), had a lower SAPS II score (43 ± 14 vs 50 ± 15) and a higher male ratio as compared with patients presenting a CLCR lower than 120 mL minute.
All factors presenting a statistical difference between hyperfiltration and hypofiltration subgroups (Table 2) were analyzed. Through a logistic regression analysis, including goodness of fit of the model, age and trauma were the only factors independently correlated to CLCR (Table 3).
Table 3.
Logistic regression for different measured creatinine clearance
| P * | Odd ratio (CI 95%) | |
|---|---|---|
| CLCR > 120 mL minute-1 1.73 m-2 | ||
| Age | < 0.0001 | 0.95 (0.93 to 0.97) |
| SAPS 2 | 0.56 | 1.00 (0.98 to 1.04) |
| Ideal body weight | 0.29 | 0.97 (0.93 to 1.02) |
| Gender | 0.564 | 0.41 (0.12 to 1.4) |
| PT | 0.0001 | 3.33 (1.8 to 6) |
| CLCR < 60 mL minute-1 1.73 m-2 | ||
| Age | < 0.0001 | 0.95 (0.93 to 0.97) |
| SAPS 2 | 0.36 | 0.98 (0.95 to 1.02) |
| Ideal body weight | 0.3 | 1.03 (0.97 to 1.09) |
| Gender | 0.8 | 0.84 (0.97 to 1.09) |
| NPT | 0.02 | 2.39 (1.15 to 4.97) |
PT = polytrauma patients, NPT non polytrauma patients.
* The P-values correspond to logistic regression with CLCR greater than 120 mL minute-11.73 m-2 (C index = 0.56).
# The P-values correspond to logistic regression with CLCR lower than 60 mL minute-1 1.73 m-2
(C index = 0.58).
Discussion
The present results comparing a population of hemodynamic stable PT patients to a population of hemodynamic stable NPT patients with steady state serum creatinine concentration with a normal creatinine serum value demonstrate that (i) PT patients exhibit dramatic variations of their CrCl; (ii) CrCl is higher in PT patients than in NPT patients; (iii) Age and trauma are independently correlated factors to CLCR in our study and in these study conditions.
Considering serum creatinine values, no significant difference was found between PT and NPT groups despite the variations of CrCl. These data demonstrated that a wide range of measured CLCR variations exists and, therefore, confirm Hoste data obtained in critically ill patients with serum creatinine within normal range [16]. These authors demonstrated that "serum creatinine has a low sensitivity for detection of renal dysfunction". Our results also revealed some opposite trends between CrCl and creatinine measurements, as some patients had significantly lower values of serum creatinine for CLCR > 60 mL minute-1 1.73 m-2 than for CLCR < 60 mL minute-1 1.73 m-2. These data underline the inaccuracy of serum creatinine values in estimating the renal function.
Fifty-five percent of PT patients, and only 19% of NPT patients presented a measured CLCR above 120 mL minute-1 1.73 m-2. In addition, only 10% of PT patients vs 34% of NPT patients presented a measured CLCR below 60 mL minute-1 1.73 m-2. In clinical practice, the diagnosis of increased CLCR as a surrogate marker of GFR is important and has largely been demonstrated in burn patients in the setting of antibiotics monitoring: ceftazidime, cefepime, vancomycin and amikacin [4,5,17,18]. In critically ill patients, high CLCR required high doses of drugs, which are eliminated by the kidneys to obtain therapeutic concentration. Recently we confirmed the need for CLCR monitoring in order to accurately monitor renal function and, therefore, to optimize the doses of antibiotics [4,19].
Our results demonstrate that age, gender, ideal body weight, severity index, trauma patients, and serum creatinine are factors for a CLCR above normal (> 120 mL minute-1 1.73 m-2), and for a moderate renal impairment (CLCR < 60 mL minute-1 1.73 m-2). Through a logistic regression analysis, only two factors (age and trauma patients) remained significantly correlated with a CLCR above normal and for a moderate renal impairment. In the current results, 12% of elderly patients (over 65 years) have a CLCR greater than 120 mL minute-1 1.73 m-2. The impact of age on CLCR is well known and this parameter was, therefore, introduced in the formulas estimating CLCR (Cockcroft-Gault, Robert and simplified MDRD) [13-15]. The decrease in glomerular filtration, the involution of nephronic units and the reduction of the renal blood flow explain the high frequency of renal impairement in elderly patients. However, it should be kept in mind that glomerular ageing is correlated to age in only two-thirds of the patients, and this phenomenon accounts for the inaccuracy of the CLCR estimated by calculated formulae [20].
Current evidence suggests that PT (mainly, young patients without significant comorbidities) present with a CLCR increase. However, this phenomenon as received little attention in the literature, and dose modification are therefore rarely considered. The present results clearly demonstrate for the first time that trauma is a major factor for CLCR increase. Several factors may explain this increase in CLCR in PT patients. First, urinary creatinine excretion may be involved in such a phenomenon. A higher creatinine urinary excretion was observed in PT compared with NPT patients whereas serum creatinine was similar in both groups. However, the higher creatinine urinary excretion observed in PT patients was within a normal range. Serum protein variations may impact our results. However, all the patients had a serum protein value between 50 and 55 gL-1. It is, therefore, very unlikely that serum protein variations interfere with the present results. Also, regarding hemodynamics, CLCR were studied and measured at a steady state in both groups (that is, distant from the admittance). It should be noted that our patients were hemodynamically stable at the time of data collection with no sign of dehydration. Although interference due to some cephalosporin has been described when the creatininemia was measured by using the Jaffe method [21]; no changes in this parameter were observed during the overall period of the study. Sepsis can also reduce creatinine production as described in mice [22]. Moreover, in critically-ill patients, a positive fluid balance may lead to underestimation of the severity of AKI and delay the recognition of a 50% relative increase in sCr [23]. Finally, it should be hypothesized that humoral and inflammatory mechanisms encountered after severe trauma [24] or burn [1] are involved in the observed CLCR increase.
The present study encountered some limitations. Increased CLCR is related with enhanced renal elimination of circulating drugs. However, describing this phenomenon in terms of current available measures of the GFR at the bedside is still debated. In our study, the GFR was estimated by measured creatinine clearance on a 24-hour urine collection. However, the gold standard for GFR assessment is the measure of inuline clearance [25] but the cost and complexity of this tool limits its application in routine. Another limitation is the lack of consensus regarding the upper limit of normal GFR. However, increasing data support the concept of increased GFR in PT patients, and several reports demonstrated subtherapeutic concentrations of drugs in PT patients [5,26]. Also, a cross-sectional single 24-hr measure of CLCR at 10 days in relatively stable patients was performed but fast modifications of kidney function may occur and there is a need for a continuous re-evaluation. Finally, some other factors may influence our results. In particular, fluid status, cardiac output may be significantly altered from baseline. However, whatever the causes of these alterations, the ATLS (Advanced Trauma Life support) principles are applied in our institution regarding resuscitation of PT patients. We, therefore, believe that the current results are broadly representative of the population of PT patients. It could be argued that the external validity of this single-center study may be limited. However, our findings may be relevant to the vast majority of level I trauma centers, provided that ATLS principles are applied in these institutions.
Conclusions
In hemodynamic ICU stable patients with steady state serum creatinine concentration, CLCR, which is a surrogate marker of GFR, is higher in polytrauma patients than in other critically ill patients. In ICU patients, the drug monitoring must take into account the glomerular filtration rate. The measure of CLCR should be routinely proposed for PT patients in order to adjust dose regimen, especially for drugs with renal elimination (betalactams, ceftazidime, cefepime, piperacillin, vancomycin, aminoglycosides, and so on).
Key messages
• In ICU patients with normal serum creatinine, CLCR, is higher in trauma than in non-trauma patients.
• The measure of CLCR should be proposed in routine for ICU patients in order to adjust dose regimen, especially for drugs with renal elimination.
• Age and trauma were the only factors independently correlated to CLCR.
• Glomerular filtration rate should be measured in ICU patient to detect renal filtration abnormalities.
• Serum creatinine is not a good marker for renal function estimation.
Abbreviations
AKI: Acute Kidney Injury; CLCR: creatinine clearance; GFR: glomerular filtration rate; ICU: intensive care unit; NPT: non polytrauma patients; PT: polytrauma patients; sMDRD, Modification of Diet in Renal Disease index; SOFA: score, Sequential Organ Failure Assessment score.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AJ and IT carried out the serum creatinine measurement and calibration. SR, AB and TS carried out the patients' inclusions. KA helped to draft the manuscript and reviewed the intellectual content. BG, SS, OF and KS participated in the design of the study and helped to draft the manuscript. JMC and VM conceived of the study, and participated in its design and coordination and helped to draft the manuscript and performed the statistical analysis. All authors read and approved the final manuscript.
Contributor Information
Vincent Minville, Email: minville.v@chu-toulouse.fr.
Karim Asehnoune, Email: karim.asehnoune@chu-nantes.fr.
Stephanie Ruiz, Email: ruiz.stephanie@chu-toulouse.fr.
Audrey Breden, Email: breden.a@chu-toulouse.fr.
Bernard Georges, Email: seguin.t@chu-toulouse.fr.
Thierry Seguin, Email: georges.b@chu-toulouse.fr.
Ivan Tack, Email: tack.i@chu-toulouse.fr.
Acil Jaafar, Email: jaafar.a@chu-toulouse.fr.
Sylvie Saivin, Email: saivin.s@chu-toulouse.fr.
Olivier Fourcade, Email: fourcade.o@chu-toulouse.fr.
Kamran Samii, Email: samii.k@chu-toulouse.fr.
Jean Marie Conil, Email: conil.jm@chu-touloouse.fr.
References
- Conil JM, Georges B, Fourcade O, Seguin T, Lavit M, Samii K, Houin G, Tack I, Saivin S. Assessment of renal function in clinical practice at the bedside of burn patients. Br J Clin Pharmacol. 2007;63:583–594. doi: 10.1111/j.1365-2125.2006.02807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loirat P, Rohan J, Baillet A, Beaufils F, David R, Chapman A. Increased glomerular filtration rate in patients with major burns and its effect on the pharmacokinetics of tobramycin. N Engl J Med. 1978;299:915–919. doi: 10.1056/NEJM197810262991703. [DOI] [PubMed] [Google Scholar]
- Conil JM, Georges B, Breden A, Segonds C, Lavit M, Seguin T, Coley N, Samii K, Chabanon G, Houin G, Saivin S. Increased amikacin dosage requirements in burn patients receiving a once-daily regimen. Int J Antimicrob Agents. 2006;28:226–230. doi: 10.1016/j.ijantimicag.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Conil JM, Georges B, Lavit M, Laguerre J, Samii K, Houin G, Saivin S. A population pharmacokinetic approach to ceftazidime use in burn patients: influence of glomerular filtration, gender and mechanical ventilation. Br J Clin Pharmacol. 2007;64:27–35. doi: 10.1111/j.1365-2125.2007.02857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conil JM, Georges B, Mimoz O, Dieye E, Ruiz S, Cougot P, Samii K, Houin G, Saivin S. Influence of renal function on trough serum concentrations of piperacillin in intensive care unit patients. Intensive Care Med. 2006;32:2063–2066. doi: 10.1007/s00134-006-0421-1. [DOI] [PubMed] [Google Scholar]
- Larsson R, Bodemar G, Kagedal B, Walan A. The effects of cimetidine (Tagamet) on renal function in patients with renal failure. Acta Med Scand. 1980;208:27–31. doi: 10.1111/j.0954-6820.1980.tb01145.x. [DOI] [PubMed] [Google Scholar]
- Hull JH, Hak LJ, Koch GG, Wargin WA, Chi SL, Mattocks AM. Influence of range of renal function and liver disease on predictability of creatinine clearance. Clin Pharmacol Ther. 1981;29:516–521. doi: 10.1038/clpt.1981.72. [DOI] [PubMed] [Google Scholar]
- Papadakis MA, Arieff AI. Unpredictability of clinical evaluation of renal function in cirrhosis. Prospective study. Am J Med. 1987;82:945–952. doi: 10.1016/0002-9343(87)90156-2. [DOI] [PubMed] [Google Scholar]
- Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- Antonelli M, Moreno R, Vincent JL, Sprung CL, Mendoca A, Passariello M, Riccioni L, Osborn J. Application of SOFA score to trauma patients. Sequential Organ Failure Assessment. Intensive Care Med. 1999;25:389–394. doi: 10.1007/s001340050863. [DOI] [PubMed] [Google Scholar]
- Vincent JL, de Mendonca A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on "sepsis-related problems" of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26:1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- Froissart M, Rossert J, Jacquot C, Paillard M, Houillier P. Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol. 2005;16:763–773. doi: 10.1681/ASN.2004070549. [DOI] [PubMed] [Google Scholar]
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- Robert S, Zarowitz BJ, Peterson EL, Dumler F. Predictability of creatinine clearance estimates in critically ill patients. Crit Care Med. 1993;21:1487–1495. doi: 10.1097/00003246-199310000-00016. [DOI] [PubMed] [Google Scholar]
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- Hoste EA, Damen J, Vanholder RC, Lameire NH, Delanghe JR, Van den Hauwe K, Colardyn FA. Assessment of renal function in recently admitted critically ill patients with normal serum creatinine. Nephrol Dial Transplant. 2005;20:747–753. doi: 10.1093/ndt/gfh707. [DOI] [PubMed] [Google Scholar]
- Conil JM, Georges B, Lavit M, Seguin T, Tack I, Samii K, Chabanon G, Houin G, Saivin S. Pharmacokinetics of ceftazidime and cefepime in burn patients: the importance of age and creatinine clearance. Int J Clin Pharmacol Ther. 2007;45:529–538. doi: 10.5414/cpp45529. [DOI] [PubMed] [Google Scholar]
- Conil JM, Favarel H, Laguerre J, Brouchet A, Chabanon G, Cazal L, Bodnar M, Rouge D, Virenque C, Costagliola M. [Continuous administration of vancomycin in patients with severe burns] Presse Med. 1994;23:1554–1558. [PubMed] [Google Scholar]
- Conil JM, Georges B, de Lussy A, Khachman D, Seguin T, Ruiz S, Cougot P, Fourcade O, Houin G, Saivin S. Ciprofloxacin use in critically ill patients: pharmacokinetic and pharmacodynamic approaches. Int J Antimicrob Agents. 2008;32:505–510. doi: 10.1016/j.ijantimicag.2008.05.019. [DOI] [PubMed] [Google Scholar]
- O'Connell MB, Dwinell AM, Bannick-Mohrland SD. Predictive performance of equations to estimate creatinine clearance in hospitalized elderly patients. Ann Pharmacother. 1992;26:627–635. doi: 10.1177/106002809202600503. [DOI] [PubMed] [Google Scholar]
- Grotsch H, Hajdu P. Interference by the new antibiotic cefpirome and other cephalosporins in clinical laboratory tests, with special regard to the "Jaffe" reaction. J Clin Chem Clin Biochem. 1987;25:49–52. [PubMed] [Google Scholar]
- Doi K, Yuen PS, Eisner C, Hu X, Leelahavanichkul A, Schnermann J, Star RA. Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J Am Soc Nephrol. 2009;20:1217–1221. doi: 10.1681/ASN.2008060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo E, Bouchard J, Soroko SH, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, Mehta RL. Fluid accumulation, recognition and staging of acute kidney injury in critically-ill patients. Crit Care. 2010;14:R82. doi: 10.1186/cc9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoen S, Asehnoune K, Brailly-Tabard S, Mazoit JX, Benhamou D, Moine P, Edouard AR. Cortisol response to corticotropin stimulation in trauma patients: influence of hemorrhagic shock. Anesthesiology. 2002;97:807–813. doi: 10.1097/00000542-200210000-00010. [DOI] [PubMed] [Google Scholar]
- Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- Georges B, Conil JM, Seguin T, Ruiz S, Minville V, Cougot P, Decun JF, Gonzalez H, Houin G, Fourcade O, Saivin S. Population pharmacokinetics of ceftazidime in intensive care unit patients: influence of glomerular filtration rate, mechanical ventilation, and reason for admission. Antimicrob Agents Chemother. 2009;53:4483–4489. doi: 10.1128/AAC.00430-09. [DOI] [PMC free article] [PubMed] [Google Scholar]


