Abstract
Introduction
The purpose of this study was to investigate the relationship among Pseudomonas aeruginosa acquisition on the intensive care unit (ICU), environmental contamination and antibiotic selective pressure against P. aeruginosa.
Methods
An open, prospective cohort study was carried out in a 16-bed medical ICU where P. aeruginosa was endemic. Over a six-month period, all patients without P. aeruginosa on admission and with a length of stay >72 h were included. Throat, nasal, rectal, sputum and urine samples were taken on admission and at weekly intervals and screened for P. aeruginosa. All antibiotic treatments were recorded daily. Environmental analysis included weekly tap water specimen culture and the presence of other patients colonized with P. aeruginosa.
Results
A total of 126 patients were included, comprising 1,345 patient-days. Antibiotics were given to 106 patients (antibiotic selective pressure for P. aeruginosa in 39). P. aeruginosa was acquired by 20 patients (16%) and was isolated from 164/536 environmental samples (31%). Two conditions were independently associated with P. aeruginosa acquisition by multivariate analysis: (i) patients receiving ≥3 days of antibiotic selective pressure together with at least one colonized patient on the same ward on the previous day (odds ratio (OR) = 10.3 ((% confidence interval (CI): 1.8 to 57.4); P = 0.01); and (ii) presence of an invasive device (OR = 7.7 (95% CI: 2.3 to 25.7); P = 0.001).
Conclusions
Specific interaction between both patient colonization pressure and selective antibiotic pressure is the most relevant factor for P. aeruginosa acquisition on an ICU. This suggests that combined efforts are needed against both factors to decrease colonization with P. aeruginosa.
Introduction
Pseudomonas aeruginosa infections on the ICU are a constant concern [1]. Colonization with this organism often precedes infection [2] and its prevention is, therefore, extremely important. P. aeruginosa colonization has been reported to originate from exogenous sources such as tap water [3], fomites and/or patient-to-patient transmission, or as an endogenous phenomenon related to antibiotic use. Some studies have highlighted the importance of exogenous colonization during hospitalization (50 to 70% of all colonizations) [4-9] whereas others have questioned its relative importance [10-13]. Molecular epidemiology techniques have given an insight into P. aeruginosa acquisition by demonstrating that the same pulsotypes may spread from the environment to patients [14,15], sometimes in an epidemic mode. This could explain the discrepancies between studies with different levels of exogenous acquisition [14-16]. Although genotyping methods are useful, they fail in giving a definitive result for the origin of bacteria. First, a strain shared by a patient and his/her environment has not necessarily been transmitted from the environment to the patient. Furthermore, acquisition of a strain not isolated from the environment does not necessarily mean that it is part of the patient's flora (the classical endogenous definition [17,18]). It could also have been acquired through previous healthcare procedures from undiscovered environmental sources (misdiagnosed exogenous acquisition). Whatever the mode of acquisition, the determinants of colonization remain unclear. In particular, the role of antibiotic selective pressure on P. aeruginosa colonization is an important issue.
In a previous study [3], we carried out a genotypic analysis on our medical ICU. This analysis eliminated an exogenous epidemic spread but showed that P. aeruginosa colonization was associated with tap water contamination over several weeks. It suggested, together with an overall incidence of 11.3 colonized/infected cases per 100 patients, an endemic P. aeruginosa context [3]. However, this study had several limitations. Only genotyping from one colony of each culture was performed so that only one-third of the strains were analysed. Thus, it was not possible to ascertain which acquisition mechanism predominated. More importantly, the potential role of antibiotic selective pressure on acquisition was not studied. Based on the same study population, the aim of the current study was to explore the respective roles of environment and antibiotic selective pressure on P. aeruginosa colonization during healthcare delivery in these endemic conditions.
Materials and methods
Study setting
The study was performed on a 16-bed medical ICU in a 1,624-bed university teaching hospital between April and November 2003 (29 weeks). Patients were treated in single rooms distributed on four wards of four rooms each. Other rooms such as a rest area, sterilization room (a room dedicated to sterilization of medical devices), toilet, equipment storage room, office and night duty bedroom were shared (Figure 1). Each room had its own water tap. The nurse:patient ratio was 1:4. The antibiotic policy and hygiene protocols were not modified during the study period. No digestive decontamination was used on the ICU. Twice monthly chlorine tap water disinfection was started in July (Week 11). Hygiene protocols consisted of contact barrier precautions for medical and nursing staff caring for patients colonized or infected with multi-resistant microorganisms (not including P. aeruginosa). These precautions were applied systematically on admission of previously hospitalized patients from other medical or surgical units for more than 48 h and for known carriers. P. aeruginosa carriers were identified on admission from rectal and oropharyngeal swabs. No screening was performed at discharge. Hand hygiene procedures were emphasized routinely.
Figure 1.
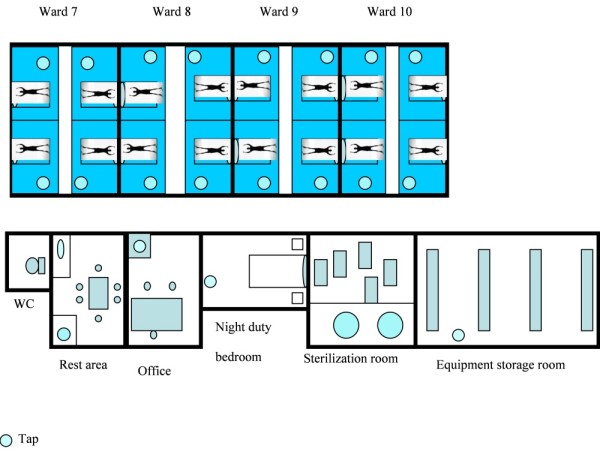
Schematic representation of the 16-bed medical ICU.
Patients
All patients admitted during the study period were systematically included in a prospective cohort. Secondary exclusion criteria included: length of ICU stay <72 h and carriage of P. aeruginosa on admission. These patients were, however, considered as potential P. aeruginosa environmental sources as they were present in the ICU. Data were recorded prospectively each day until P. aeruginosa colonization/infection, death, discharge to another unit, or end of the study period. The variables examined for all patients included demographic data (age, gender), underlying conditions (immunosuppression as defined by cancer, AIDS with CD4 T-lymphocytes <100, haemopathy, or corticotherapy >0.5 mg/kg/day, diabetes mellitus, end-stage renal disease, chronic liver disease, chronic heart or respiratory failure) and severity evaluated by the Simplified Acute Physiology Score (SAPS II) [19]. Data regarding the use of intravascular catheters, nasogastric or endotracheal tubes were also collected daily.
This study was approved by our local ethics committee (Comité de Protection des Personnes Sud-Ouest et Outre Mer III, reference number: DC2010/38). The need to obtain informed consent was waived because no change was done to our ICU's usual practices (the endemic context of the ICU justified an intense surveillance procedure), but patients and/or their proxies were informed of the study's purpose.
Microbiological screening
As a routine surveillance procedure, throat, nasal and rectal swabs as well as sputum and urine samples were collected on admission and weekly thereafter on predefined days. Other specimens were taken when clinically indicated. Environmental screening included weekly tap water samples from the patients' rooms and tap water samples from shared rooms every three weeks. The methods of specimen collection and culture have been described previously [3].
Definition of acquired P. aeruginosa colonization/infection
Acquired colonization/infection was defined as the isolation of P. aeruginosa from at least one surveillance or clinical culture from patients not colonized or infected at ICU admission. P. aeruginosa infection was defined as a positive culture with clinical and biological manifestations of infection. In cases of lower respiratory tract infection, quantitative cultures were positive if a threshold of ≥107 colony-forming units (CFU)/ml for tracheal aspirates or ≥104 CFU/ml for bronchoalveolar lavage were obtained.
Risk factors for P. aeruginosa colonization/infection
Antibiotics
Antibiotic treatment was recorded daily and classified according to P. aeruginosa susceptibility (no antibiotic treatment, inactive or active against P. aeruginosa including ureido and carboxypenicillins, antipseudomonal cephalosporins, carbapenems, fluoroquinolones, aminoglycosides, colimycin, fosfomycin). If a patient was treated simultaneously with both active and non-active antibiotics, the patient was considered to have been treated with active antibiotics.
Environmental factors
Systematic environmental screening included other patients from the ward on which the patient was hospitalized, other patients on the ICU, tap water from the same ward, tap water from the ICU and tap water from shared rooms. Daily indices of environmental pressure were calculated as assessed in other studies of patient-induced colonization pressure [11]. Briefly, for each study day, the number of patients and tap water samples colonized with P. aeruginosa on the ward/ICU where the patient was hospitalized was estimated. Two variables were then described: (i) the colonization of patients or tap water samples on the previous day (called previous patient/tap water colonization pressure); and (ii) the number of patients or tap water samples colonized since the patient's admission (called cumulative patient/tap water colonization pressure). Environmental exposure was assumed to be constant between two screenings. Hence, patients who acquired P. aeruginosa had several environmental pressure profiles (including patient colonization pressure and tap water colonization pressure) allowing a comparison with patients who did not acquire P. aeruginosa.
Statistical analysis
Quantitative variables were compared using the Student's t-test or Wilcoxon test according to the distribution of data. Qualitative variables were compared using the Chi2 or Fisher's exact test. A marginal logistic regression model accounting for repeated measurements [20] was used to assess the relationship between environment, antibiotic pressure and P. aeruginosa acquisition each day, and the results were expressed as odds ratios (OR) and 95% confidence intervals (CI). Univariate analysis of P. aeruginosa acquisition included: (i) fixed variables for patient characteristics at admission; (ii) longitudinal data on patient/tap water colonization pressures, as described above, on the cumulative number of days since admission with a nasogastric tube (which was selected to represent invasive devices as it is strongly associated with the use of other invasive devices in our clinical practice) or with antibiotics classified as active or inactive against P. aeruginosa. Selection of the environmental exposure index (previous or cumulated colonization pressure) was based on Akaike criteria [21]: patient/tap water colonization pressure on the previous day was finally introduced in the multivariate analysis. Quantitative data were analyzed as categorical variables when the log-linearity assumption was not followed. All factors with a P-value < 0.20 in univariate analysis were selected for multivariate analysis. In multivariate analysis, the factors related to patient/tap water colonization pressures, that is, "patients on the same ward", "tap water from the ICU", "tap water from the shared rooms" or antibiotics were first introduced together and forced in the model. Because wards are included in the ICU, only the most significant index among colonization pressure onto the ward or the ICU was selected for analysis purpose. Other factors were then introduced in a stepwise manner to control for confounding. According to our main objective, the final model looked for interactions between each of the three patient/tap water colonization pressures and antibiotic variables. A P-value of <0.05 was considered significant. Data were recorded prospectively with Epidata (3.1; Odense, Denmark). The model was fitted using the GENMOD procedure on SAS software (SAS Institute, Inc., Cary, NC, USA).
Results
Study population
Of the 415 patients admitted to the ICU during the 29-week study period, 262 were excluded because their length of stay was <72 h and 27 were excluded because screening at admission revealed P. aeruginosa. Finally, 126 patients were included, comprising 1,345 patient-days. The demographic and clinical characteristics of these patients are shown in Table 1.
Table 1.
Demographic and clinical characteristics of the study population (n = 126)
| Characteristic | |
|---|---|
| Age (years) | 57 ± 17 |
| Male/female | 72/54 |
| SAPS II | 45 ± 18 |
| Hospitalization before admission | 88 (70.0%) |
| Underlying conditions | 0.7 ± 0.7 |
| immunosuppression | 29 (23.0%) |
| chronic respiratory failure | 24 (19.0%) |
| diabetes | 22 (17.5%) |
| heart disease | 4 (3.2%) |
| renal disease | 4 (3.2%) |
| cirrhosis | 2 (1.6%) |
| Invasive device | |
| Mechanical ventilation (%) | 78 |
| Duration (days) | 6 (2 to 10) |
| Central venous catheter (%) | 65 |
| Duration (days) | 5 (0 to 10) |
| Nasogastric tube (%) | 72 |
| Duration (days) | 6 (0 to 10) |
| Enteral nutrition (%) | 93 |
| Duration (days) | 6 (4 to 9) |
| Foley catheter (%) | 79 |
| Duration (days) | 6 (2 to 11) |
| Length of stay (days) median | 8 (6 to 12) |
| ICU mortality | 29 (23%) |
Values are shown as mean ± SD, n (%), or median (1st to 3rd quartile).
SAPS II: Simplified Acute Physiology Score; ICU: intensive care unit.
Microbiological screening
During the study, microbiological screening yielded 807 samples: 166 sputum or bronchoalveolar cultures, 144 blood cultures, 114 nasal, 111 rectal, 109 throat, 108 urine and 55 miscellaneous cultures. Cultures were not available for 15 patients, accounting for 94 patient-days. Each patient had a median of five cultures (range: two to nine) during their ICU stay. Acquired P. aeruginosa was present in 27 cultures (3.4%): 11 respiratory, 7 rectal, 4 throat and 3 nasal cultures, 1 stool and 1 peritoneal sample.
Acquired colonization/infection
Twenty patients (16%) acquired P. aeruginosa during their ICU stay. P. aeruginosa colonization was present in 11 patients: rectal culture (n = 5), sputum culture (n = 2), rectal and throat or nasal culture (n = 2), sputum culture associated with rectal, nasal and throat colonization (n = 1) and stool culture (n = 1). P. aeruginosa infection was observed in nine other patients (nosocomial pneumonia (n = 8) and nosocomial peritonitis (n = 1)). P. aeruginosa isolation occurred a median of 11 days (range: 8 to 16) after admission.
Antibiotic treatment
During their ICU stay, 106 patients (84%) received a total of 970 antibiotic days with a median of two antibiotics (range: one to three) for a median duration of seven days (range: 3 to 11) per patient. The antibiotics used are described in Table 2. All patients who acquired P. aeruginosa (except one) had received antibiotics before acquisition (median of two antibiotics (two to four) vs. median of two antibiotics (two to three) in the other group; P = 0.09). Among the 106 patients treated with antibiotics, two-thirds (n = 67) received at least one day of antibiotics active against P. aeruginosa whereas one-third (n = 39) did not.
Table 2.
Distribution of antibiotic treatment according to acquisition group*
| P. aeruginosa acquisition n = 20 (%) | No P. aeruginosa acquisition n = 106 (%) | Total n = 126 (%) | |
|---|---|---|---|
| Antibiotics active against P. aeruginosa | 10 (50) | 57 (54) | 67 (53) |
| Aminosides | 6 (30) | 17 (16) | 23 (18) |
| Ureido/carboxypenicillins | 5 (25) | 19 (18) | 24 (19) |
| Piperacillin-tazobactam | 5 (25) | 12 (11) | 17 (13) |
| Ticarcillin-clavulanic acid | 0 (0) | 7 (7) | 7 (6) |
| Antipseudomonal cephalosporins | 3 (15) | 13 (12) | 16 (13) |
| Ceftazidime | 3 (15) | 6 (6) | 9 (7) |
| Cefepime | 0 (0) | 7 (7) | 7 (6) |
| Carbapenems | 4 (20) | 12 (11) | 16 (13) |
| Fluoroquinolones | 7 (35) | 33 (31) | 40 (32) |
| Others | 1 (5) | 3 (3) | 4 (3) |
| Fosfomycin | 0 (0) | 2 (2) | 2 (2) |
| Colomycin | 1 (5) | 1 (1) | 2 (2) |
| Antibiotics not active against P. aeruginosa | 14 (70) | 85 (80) | 99 (79) |
| Glycopeptides | 5 (25) | 30 (28) | 35 (28) |
| Non-antipseudomonal penicillins | 4 (20) | 43 (41) | 47 (37) |
| Penicillin G | 0 (0) | 1 (1) | 1 (1) |
| Penicillin M | 0 (0) | 2 (2) | 2 (2) |
| Amoxicillin | 1 (5) | 3 (3) | 4 (3) |
| Amoxicillin-clavulanic acid | 3 (15) | 37 (35) | 40 (32) |
| Non-antipseudomonal cephalosporins (cefotaxim; cefuroxim; ceftriaxon) | 10 (50) | 23 (22) | 33 (26) |
| Macrolides | 5 (25) | 12 (11) | 17 (13) |
| Other | 2 (10) | 18 (17) | 20 (16) |
| Pristinamycin | 0 (0) | 3 (3) | 3 (2) |
| Metronidazole | 0 (0) | 10 (9) | 10 (8) |
| Cotrimoxazole | 1 (5) | 1 (1) | 2 (2) |
| Rifampicin | 1 (5) | 4 (4) | 5 (4) |
* The data represent the number of patients who received at least one day of antibiotic of each class (percentage of patients in each group).
Environmental screening results
The results of environmental screening are shown in Table 3. In addition to the 20 patients who acquired P. aeruginosa during the study, 27 patients were colonized and/or infected with P. aeruginosa at ICU admission. Thus, 47 patients potentially contributed to the patient colonization pressure. Tap water screening from the patient's rooms yielded 152/464 positive samples (33%). Surveillance of tap water from shared rooms yielded 72 samples, of which 12 were positive for P. aeruginosa (17%). Contaminated tap water was observed four times in the shared toilet, three times in the sterilization room, twice in the night duty bedroom and once in the rest area, office or equipment storage room. The implementation of tap water disinfection at Week 11 of the study should have decreased the patients' environmental pressure. However, no significant interaction was found between tap water colonization and time period (before or after Week 11) (P = 0.69).
Table 3.
Summarization of environmental screening data according to acquisition group
| P. aeruginosa acquisition (n = 20) | No P. aeruginosa acquisition (n = 106) | Total (n = 126) | |
|---|---|---|---|
| Cumulative patient-induced environmental pressure* | |||
| From the same ward | 1.2 (0.6 to 1.8) | 0.8 (0 to 1.7) | 1 (0.1 to 1.8) |
| From the ICU | 4.8 (3.6 to 5.6) | 4.7 (3.3 to 5.6) | 4.7 (3.3 to 5.6) |
| Cumulative tap water-induced environmental pressure* | |||
| From the patients' wards | 0.1 (0 to 0.7) | 0 (0 to 0.6) | 0 (0 to 0.6) |
| From the ICU | 1.9 (1.1 to 2.3) | 1.6 (0 to 3) | 1.8 (0 to 2.9) |
| From shared rooms | 1 (0.7 to 2.3) | 0.8 (0 to 1) | 1 (0 to 1) |
| Patient-induced environmental pressure** | |||
| ≥1 colonized patient on the same ward | |||
| yes | 20 | 79 | 99 |
| no | 0 | 27 | 27 |
| ≥1 colonized patient on the ICU | |||
| yes | 20 | 106 | 126 |
| no | 0 | 0 | 0 |
| Tap water-induced environmental pressure** | |||
| ≥1 colonized tap water on the same ward | |||
| yes | 10 | 51 | 61 |
| no | 10 | 55 | 65 |
| ≥1 colonized tap water on the ICU ¤ | |||
| yes | 18 | 68 | 86 |
| no | 2 | 38 | 40 |
| ≥1 colonized tap water in shared rooms | |||
| yes | 17 | 70 | 87 |
| no | 3 | 36 | 39 |
Values shown are: median (1st to 3rd quartile), or n.
*Cumulative patient/tap water-induced environmental pressure represents the number of contaminated patients/tap water samples since admission.
**Patient/tap water-induced environmental pressure represents the number of patient that were exposed to a contaminated patient/tap water at least one time during their ICU stay.
¤ Excluding tap water in shared rooms.
Risk factors for P. aeruginosa acquisition
By univariate analysis, the presence of an invasive device (nasogastric tube), previous patient colonization pressure on the same ward and previous tap water colonization pressure from the ICU and shared rooms were significantly associated with P. aeruginosa acquisition (Table 4). Multivariate analysis revealed that the presence of a nasogastric device was independently associated with P. aeruginosa acquisition (OR = 7.72 (95% CI: 2.32 to 25.70); P = 0.001). In addition, the interaction between antibiotics inactive against P. aeruginosa and the patient colonization pressure was also significant (P < 0.03). It means that, in patients receiving equal to or more than three days of antibiotics inactive against P. aeruginosa, the presence of at least one colonized patient on the same ward on the previous day increased the risk of P. aeruginosa acquisition on a given day (OR = 10.26 (95% CI: 1.83 to 57.43); P = 0.01) compared to patients without colonized patient in the same ward. This association was not observed in patients with less than three days of antibiotics inactive against P. aeruginosa.
Table 4.
Risk factors for P. aeruginosa acquisition in the ICU (n = 126)
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Risk factor | OR (95% CI) | P | OR (95% CI) | P |
| SAPS II | ||||
| ≥43 (vs. <43) | 2.54 (0.89 to 7.24) | 0.08 | * | |
| Age | ||||
| ≥70 years (vs. <70) | 4.61 (1.67 to 12.72) | 0.14 | * | |
| Nasogastric tube | ||||
| Equal to or more than nine cumulated days since admission (vs. less than nine days) | 7.66 (2.88 to 20.36) | <0.0001 | 7.72 (2.32 to 25.70) | 0.001 |
| Antibiotic treatment not active against P. aeruginosa | ||||
| More than three days (vs. zero to two days) | 2 (0.76 to 5.27) | 0.16 | *** | |
| Antibiotic treatment active against P. aeruginosa** | ||||
| per cumulated day since admission | 1.02 (0.95 to 1.10) | 0.54 | **** | |
| Previous patient-induced environmental pressure | ||||
| Equal to or more than one colonized patient on the same ward on the previous day (vs. zero) | 4.91 (1.47 to 16.39) | 0.01 | *** | |
| Equal to or more than one colonized patient on the ICU on the previous day (vs. zero) | 1.14 (0.27 to 4.90) | 0.86 | **** | |
| Previous tap water-induced environmental pressure | ||||
| Equal to or more than one colonized tap water on the same ward on the previous day (vs. zero) | 2.37 (0.96 to 5.89) | 0.06 | $ | |
| Equal to or more than one colonized tap water on the ICU on the previous day (vs. zero) | 3.79 (1.26 to 11.44) | 0.02 | 1.99 (0.67 to 5.88) | 0.21 |
| Equal to or more than one colonized tap water in shared rooms on the previous day (vs. zero) | 4.63 (1.37 to 15.65) | 0.01 | 3.07 (0.93 to 10.16) | 0.07 |
| Interaction between previous patient-induced environmental pressure and inactive antibiotics: | 0.03$$ | |||
| If equal to or more than three days of inactive antibiotics | 1 | |||
| - no colonized patient on the same ward on the previous day | 10.26 (1.83 to 57.43) | 0.01 | ||
| - equal to or more than one colonized patient on the same ward on the previous day | ||||
| If zero to two days of inactive antibiotics | ||||
| - no colonized patient on the same ward on the previous day | 1 | |||
| - equal to or more than one colonized patient on the same ward on the previous day | 1.00 (0.26 to 3.87) | 0.99 | ||
*Factors removed by stepwise forward procedure.
**Log-linearity was assumed for this factor.
***Factors included in the interaction.
****Not statistically eligible by univariate analysis (P > 0.20).
$Not included in multivariate analysis for colinearity with tap water in the ICU.
$$P for overall interaction.
Discussion
This study suggests two main conclusions. First, P. aeruginosa acquisition should be related to the proximity of a patient colonized with P. aeruginosa in the area (same room) with a chronological component (the previous day) along with selective antibiotic pressure. Antibiotic selective pressure alone did not influence P. aeruginosa acquisition. The hypothesis of a complex mechanism involving antibiotic selective pressure and patient colonization pressure should be relevant for P. aeruginosa acquisition in an ICU with endemic context. If the interaction of both pressures overriding each pressure taken separately is reviewed, there could be some practical implications. Developing strategies for either decreased antibiotic use for "endogenous-like" acquisition or hygiene improvement in response to environmental contamination in "exogenous-like" acquisition could be insufficient. In an endemic ICU without obvious epidemic acquisition, it is arguable that a reduction in antibiotic selective pressure and improvement in hygiene standards should be combined. The second conclusion is that invasive devices remain an important determinant in P. aeruginosa acquisition. Whether invasive devices are a surrogate of patient's severity (an already known acquisition risk factor) or a step for bacteria in the chain linking the environment to the patients cannot be inferred from the results of this study.
In our study, the classical binary endogenous/exogenous scheme [12,22] is transcended by the interaction of both factors, which confirms that P. aeruginosa acquisition is complex. In the past, some molecular epidemiology studies have reported a significant role of exogenous colonization [4-7,18], whereas others have predominantly identified the role of endogenous colonization [11,13]. Genotypic methods may detect an epidemic context where exogenous sources are the most important [23] and potentially overestimate its role. Hence, the same group has described two different levels of exogenous P. aeruginosa cross-transmission [9,11]. It is also likely that strains spread rapidly from patients to the environment and vice-versa, complicating environmental and patient screening because screening at distinct time intervals could misclassify some cases of exogenous acquisition [16]. Special attention should also be paid to so-called "endogenous" P. aeruginosa acquisition. P. aeruginosa is not generally considered to be part of the normal human flora [16], and in most patients admitted to hospital for the first time, P. aeruginosa is not usually isolated from bacteriological specimens until the patient has been in the hospital for several days [22,24,25]. In these cases it is unclear if P. aeruginosa is really endogenous (that is, present on admission but undetected by screening and only revealed by antibiotic selective pressure) [17,18]. On the other hand, despite being absent from the flora on admission, P. aeruginosa could be acquired from the environment through repetitive daily healthcare procedures. Sequential cultures with P. aeruginosa isolation from oropharyngeal samples before the gastrointestinal tract support this hypothesis [26]. Moreover, Johnson et al. [22] recently observed that 50% of imipenem-resistant P. aeruginosa acquisition corresponded to neither the classical endogenous nor exogenous route. The question of an undiscovered environmental source was raised. This is the case in some endemic ICU contexts [27]. In our ICU the endemic context was suggested by the fact that one-third of the strains shared the same genotypic profile without an obvious exogenous source of acquisition or epidemic profile [3].
Irrespective of the obvious, undiscovered exogenous or true endogenous source of P. aeruginosa [28], it is likely that acquisition of this microorganism by patients is related to a third factor, namely antibiotic treatment which could interact with the environment to facilitate P. aeruginosa acquisition. Our study confirms this hypothesis. It focused on individual patients with daily recorded antibiotic treatment rather than on a population with collective consumption data [29]. Daily antibiotic recording does not prevent misclassification of antibiotic treatment as active, whereas it was eventually inactive due to poor PK/PD optimization. Even if there is still poor knowledge of the optimal antibiotic dosing strategies to prevent the selection of resistance, an antibiotic stewardship designed to limit insufficient antibiotic doses was set up at the study period, potentially limiting this bias. Besides, all previously known risk factors were adjusted for, as well as widespread and repeated patient and tap water screening (including samples from shared rooms), which have not always been completely (only patient-to-patient transmission) [11,18] or properly (type and frequency of environmental screening) [10,13] assessed. Moreover, active antibiotics were distinguished from inactive antibiotics (selective antibiotic pressure), which could help P. aeruginosa become dominant in the patients' flora.
In our ICU, as potentially in others with the same endemic and antibiotic consumption profiles, the results of this study will lead to the development of coordinated strategies against the use of antibiotics that are inactive against P. aeruginosa (such as a decrease in systematic penicillin or cephalosporin treatment for aspiration pneumonia) and against the environmental spread of bacteria. The latter should include alcohol-based hand-cleaning programmes since cross-contamination between patients and contaminated tap water was suspected in our study. Contaminated tap water and patients' samples were associated with P. aeruginosa acquisition in univariate analysis but only patients' samples were significant in multivariate analysis. Positive cultures from shared rooms were associated with P. aeruginosa acquisition in univariate analysis and should be interpreted as additional to ICU P. aeruginosa colonization pressure.
There are several limitations to our study. It was a single-centre study and the limited observations may give reduced power to detect other contributing risk factors. These limitations prevent its application to other ICUs where the patient case mix, prevalence of P. aeruginosa colonization at admission and antibiotic consumption are different. Antibiotic selective pressure could have played a role in revealing a pre-existing P. aeruginosa flora shared with the patient's environment without a cause-and-effect relationship (which would only have been demonstrated by chronological acquisition of the same genotypic strain) or in rendering the patient susceptible to P. aeruginosa acquisition from the environment. Other limitations include the fact that adherence to hygiene rules was not assessed, antibiotic consumption before admission was not recorded and P. aeruginosa screening was not performed at the end of the ICU stay. Moreover, the environment (patients and tap water) was screened by intermittent samples. However, the inclusion in the model of the most recent sample provided a closer analysis of the time-dependent process of acquisition. Finally, routine surveillance cultures were not obtained from 15 patients with a short stay, although this probably did not significantly influence our findings as they accounted for only 7% of total patient-days.
Conclusions
In conclusion, this study adds further support for an interaction between the patient colonization pressure and antibiotic selective pressure in the process of P. aeruginosa acquisition in the ICU. These results should be confirmed in a larger study in order to generalize their potential implications (that is, target strategies aimed at decreasing antibiotic treatment, where possible, and improving hygiene protocols).
Key messages
• Pseudomonas aeruginosa is still a leading cause of nosocomial infections, yet its mode of acquisition remains the subject of debate.
• In a given patient, the interaction between the environment and the selective antibiotic treatment he (she) just received deserves more study.
• This single-centre ICU-based study shows that a specific interaction between both patient colonization pressure and selective antibiotic pressure is the most relevant factor for P. aeruginosa acquisition.
• Prevention of acquisition in a given patient should include both antibiotic stewardship and cross-transmission prevention.
Abbreviations
AIDS: Acquired Immunodeficiency Syndrome; CFU: colony-forming units; CI: confidence interval; ICU: intensive care unit; OR: odds ratio; P. aeruginosa: Pseudomonas aeruginosa; PK/PD: pharmacokinetic/pharmacodynamic; SAPS II: Simplified Acute Physiology Score.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AB conceived the study, participated in its design and in acquisition of data, coordinated the study and wrote the article. AD participated in the design of the study, performed the statistical analysis, participated in the article redaction, and contributed to this study equally with AB. RT participated in the design of the study and coordinated the statistical analysis. AGV participated in the design of the study. VT carried out the acquisition of data. HB participated in the environmental acquisition of data. CB coordinated the bacteriological study. FV participated in the acquisition of patients' data and in the conception of the study. GH participated in the conception of the study. DG conceived the study, participated in its design and in the article redaction. AMR conceived the study, participated in the environmental acquisition of data, in its design and in the article redaction.
Contributor Information
Alexandre Boyer, Email: alexandre.boyer@chu-bordeaux.fr.
Adélaïde Doussau, Email: Adelaide.Doussau@isped.u-bordeaux2.fr.
Rodolphe Thiébault, Email: Rodolphe.Thiebaut@isped.u-bordeaux2.fr.
Anne Gaëlle Venier, Email: anne-gaelle.venier@chu-bordeaux.fr.
Van Tran, Email: vantran0706@yahoo.com.
Hélène Boulestreau, Email: helene.boulestreau@chu-bordeaux.fr.
Cécile Bébéar, Email: cecile.bebear@chu-bordeaux.fr.
Frédéric Vargas, Email: frederic.vargas@chu-bordeaux.fr.
Gilles Hilbert, Email: gilles.hilbert@chu-bordeaux.Fr.
Didier Gruson, Email: didier.gruson@chu-bordeaux.fr.
Anne Marie Rogues, Email: anne-Marie.rogues@chu-bordeaux.fr.
References
- Souli M, Galani I, Giamarellou H. Emergence of extensively drug-resistant and pandrug-resistant gram negative bacilli in Europe. Euro Surveill. 2008;13:pii: 19045. [PubMed] [Google Scholar]
- Bonten MJ, Bergmans DC, Ambergen AW, De Leeuw PW, Van der Geest S, Stobberingh EE, Gaillard CA. Risk factors for pneumonia, and colonization of respiratory tract and stomach in mechanically ventilated ICU patients. Am J Respir Crit Care Med. 1996;154:1339–1346. doi: 10.1164/ajrccm.154.5.8912745. [DOI] [PubMed] [Google Scholar]
- Rogues AM, Boulestreau H, Lasheras A, Boyer A, Gruson D, Merle C, Castaing Y, Bébéar CM, Gachie JP. Contribution of tap water to patient colonisation with Pseudomonas aeruginosa in a medical intensive care unit. J Hosp Infect. 2007;67:72–78. doi: 10.1016/j.jhin.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Bertrand X, Thouverez M, Talon D, Boillot A, Capellier G, Floriot C, Hélias JP. Endemicity, molecular diversity and colonization routes of Pseudomonas aeruginosa in intensive care units. Intensive Care Med. 2001;27:1263–1268. doi: 10.1007/s001340100979. [DOI] [PubMed] [Google Scholar]
- Talon D, Mulin B, Rouget C, Bailly P, Thouverez M, Viel JF. Risk and routes for ventilator-associated pneumonia with Pseudomonas aeruginosa. Am J Respir Crit Care Med. 1998;157:978–984. doi: 10.1164/ajrccm.157.3.9702096. [DOI] [PubMed] [Google Scholar]
- Agodi A, Barchitta M, Cipresso R, Giaquinta L, Romeo MA, Denaro C. Pseudomonas aeruginosa carriage, colonization, and infection in ICU patients. Intensive Care Med. 2007;33:1155–1161. doi: 10.1007/s00134-007-0671-6. [DOI] [PubMed] [Google Scholar]
- Blanc DS, Nahimana I, Petignat C, Wenger A, Bille J, Francioli P. Faucets as a reservoir of endemic Pseudomonas aeruginosa colonization/infections in intensive care units. Intensive Care Med. 2004;30:1964–1968. doi: 10.1007/s00134-004-2389-z. [DOI] [PubMed] [Google Scholar]
- Valles J, Mariscal D, Cortes P, Coll P, Villagrá A, Díaz E, Artigas A, Rello J. Patterns of colonization by Pseudomonas aeruginosa in intubated patients: a 3-year prospective study of 1,607 isolates using pulsed-field gel electrophoresis with implications for prevention of ventilator-associated pneumonia. Intensive Care Med. 2004;30:1768–1775. doi: 10.1007/s00134-004-2382-6. [DOI] [PubMed] [Google Scholar]
- Bergmans DC, Bonten MJ, Stobberingh EE, Van Tiel FH, Van der Geest S, De Leeuw PW, Gaillard CA. Colonization with Pseudomonas aeruginosa in patients developing ventilator-associated pneumonia. Infect Control Hosp Epidemiol. 1998;19:853–855. doi: 10.1086/647745. [DOI] [PubMed] [Google Scholar]
- Blanc DS, Petignat C, Janin B, Bille J, Francioli P. Frequency and molecular diversity of Pseudomonas aeruginosa upon admission and during hospitalization: a prospective epidemiologic study. Clin Microbiol Infect. 1998;4:242–247. doi: 10.1111/j.1469-0691.1998.tb00356.x. [DOI] [PubMed] [Google Scholar]
- Bonten MJM, Bergmans DC, Speijer H, Stobberingh EE. Characteristics of polyclonal endemicity of colonization in intensive care units. Implications for infection control. Am J Respir Crit Care Med. 1999;160:1212–1219. doi: 10.1164/ajrccm.160.4.9809031. [DOI] [PubMed] [Google Scholar]
- Cholley P, Thouverez M, Floret N, Bertrand X, Talon D. The role of water fittings in intensive care rooms as reservoirs for the colonization of patients with Pseudomonas aeruginosa. Intensive Care Med. 2008;34:1428–1433. doi: 10.1007/s00134-008-1110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthelot P, Grattard F, Mahul P, Pain P, Jospé R, Venet C, Carricajo A, Aubert G, Ros A, Dumont A, Lucht F, Zéni F, Auboyer C, Bertrand JC, Pozzetto B. Prospective study of nosocomial colonization and infection due to Pseudomonas aeruginosa in mechanically ventilated patients. Intensive Care Med. 2001;27:503–512. doi: 10.1007/s001340100870. [DOI] [PubMed] [Google Scholar]
- Hota S, Hirji Z, Stockton K, Lemieux C, Dedier H, Wolfaardt G, Gardam MA. Outbreak of multidrug-resistant Pseudomonas aeruginosa colonization and infection secondary to imperfect intensive care unit room design. Infect Control Hosp Epidemiol. 2009;30:25–33. doi: 10.1086/592700. [DOI] [PubMed] [Google Scholar]
- Aumeran C, Paillard C, Robin F, Kanold J, Baud O, Bonnet R, Souweine B, Traore O. Pseudomonas aeruginosa and Pseudomonas putida outbreak associated with contaminated water outlets in an oncohaematology paediatric unit. J Hosp Infect. 2007;65:47–53. doi: 10.1016/j.jhin.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Kerr KG, Snelling AM. Pseudomonas aeruginosa: a formidable and ever-present adversary. J Hosp Infect. 2009;73:338–344. doi: 10.1016/j.jhin.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Bertrand X, Bailly P, Blasco G, Balvay P, Boillot A, Talon D. Large outbreak in an intensive care unit of colonization or infection with Pseudomonas aeruginosa that overexpressed an active efflux pump. Clin Infect Dis. 2000;31:E9–E14. doi: 10.1086/318117. [DOI] [PubMed] [Google Scholar]
- Thuong M, Arvaniti K, Ruimy R, de la Salmonière P, Scanvic-Hameg A, Lucet JC, Régnier B. Epidemiology of Pseudomonas aeruginosa and risk factors for carriage acquisition in an intensive care unit. J Hosp Infect. 2003;53:274–282. doi: 10.1053/jhin.2002.1370. [DOI] [PubMed] [Google Scholar]
- Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. doi: 10.1093/biomet/73.1.13. [DOI] [Google Scholar]
- Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. doi: 10.1109/TAC.1974.1100705. [DOI] [Google Scholar]
- Johnson JK, Smith G, Lee MS, Venezia RA, Stine OC, Nataro JP, Hsiao W, Harris AD. The role of patient-to-patient transmission in the acquisition of imipenem-resistant Pseudomonas aeruginosa colonization in the intensive care unit. J Infect Dis. 2009;200:900–905. doi: 10.1086/605408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershman MD, Kennedy DJ, Noble-Wang J, Kim C, Gullion J, Kacica M, Jensen B, Pascoe N, Saiman L, McHale J, Wilkins M, Schoonmaker-Bopp D, Clayton J, Arduino M, Srinivasan A. Multistate outbreak of Pseudomonas fluorescens bloodstream infection after exposure to contaminated heparinized saline flush prepared by a compounding pharmacy. Clin Infect Dis. 2008;47:1372–1379. doi: 10.1086/592968. [DOI] [PubMed] [Google Scholar]
- Fourrier F, Cau-Pottier E, Boutigny H, Roussel-Delvallez M, Jourdain M, Chopin C. Effects of dental plaque antiseptic decontamination on bacterial colonization and nosocomial infections in critically ill patients. Intensive Care Med. 2000;26:1239–1247. doi: 10.1007/s001340000585. [DOI] [PubMed] [Google Scholar]
- Ewig S, Torres A, El-Ebiary M, Fábregas N, Hernández C, González J, Nicolás JM, Soto L. Bacterial colonization patterns in mechanically ventilated patients with traumatic and medical head injury. Incidence, risk factors, and association with ventilator-associated pneumonia. Am J Respir Crit Care Med. 1999;159:188–198. doi: 10.1164/ajrccm.159.1.9803097. [DOI] [PubMed] [Google Scholar]
- Bonten MJ, Gaillard CA, van Tiel FH, Smeets HG, van der Geest S, Stobberingh EE. The stomach is not a source for colonization of the upper respiratory tract and pneumonia in ICU patients. Chest. 1994;105:878–884. doi: 10.1378/chest.105.3.878. [DOI] [PubMed] [Google Scholar]
- Jonas D, Meyer E, Schwab F, Grundmann H. Genodiversity of resistant Pseudomonas aeruginosa isolates in relation to antimicrobial usage density and resistance rates in intensive care units. Infect Control Hosp Epidemiol. 2008;29:350–357. doi: 10.1086/528811. [DOI] [PubMed] [Google Scholar]
- Kolla A, Schwab F, Bärwolff S, Eckmanns T, Weist K, Dinger E, Klare I, Witte W, Ruden H, Gastmeier P. Is there an association between nosocomial infection rates and bacterial cross transmissions? Crit Care Med. 2010;38:46–50. doi: 10.1097/CCM.0b013e3181b42a9b. [DOI] [PubMed] [Google Scholar]
- Kaier K, Frank U, Hagist C, Conrad A, Meyer E. The impact of antimicrobial drug consumption and alcohol-based hand rub use on the emergence and spread of extended-spectrum βlactamase-producing strains: a time-series analysis. J Antimicrob Chemother. 2009;63:609–614. doi: 10.1093/jac/dkn534. [DOI] [PubMed] [Google Scholar]


