Abstract
The extent of in vitro formation of the borate-dimeric-rhamnogalacturonan II (RG-II) complex was stimulated by Ca2+. The complex formed in the presence of Ca2+ was more stable than that without Ca2+. A naturally occurring boron (B)-RG-II complex isolated from radish (Raphanus sativus L. cv Aokubi-daikon) root contained equimolar amounts of Ca2+ and B. Removal of the Ca2+ by trans-1,2-diaminocyclohexane-N,N,N′,N′-tetraacetic acid induced cleavage of the complex into monomeric RG-II. These data suggest that Ca2+ is a normal component of the B-RG-II complex. Washing the crude cell walls of radish roots with a 1.5% (w/v) sodium dodecyl sulfate solution, pH 6.5, released 98% of the tissue Ca2+ but only 13% of the B and 22% of the pectic polysaccharides. The remaining Ca2+ was associated with RG-II. Extraction of the sodium dodecyl sulfate-washed cell walls with 50 mm trans-1,2-diaminocyclohexane-N,N,N′,N′-tetraacetic acid, pH 6.5, removed the remaining Ca2+, 78% of B, and 49% of pectic polysaccharides. These results suggest that not only Ca2+ but also borate and Ca2+ cross-linking in the RG-II region retain so-called chelator-soluble pectic polysaccharides in cell walls.
Boron (B) is an essential element for higher plant growth, although its primary function is not known (Loomis and Durst, 1992). Determining the sites of B in cells is required to identify its function. In cultured tobacco cells more than 80% of cellular B is in the cell wall (Matoh et al., 1993), whereas the membrane fraction (Kobayashi et al., 1997) and protoplasts (Matoh et al., 1992) do not contain a significant amount of B. In radish (Raphanus sativus L. cv Aokubi-daikon) root cell walls, B cross-links two RG-II regions of pectic polysaccharides through a borate-diol ester (Kobayashi et al., 1995, 1996). The association of B with RG-II has been confirmed in sugar beet (Ishii and Matsunaga, 1996), bamboo (Kaneko et al., 1997), sycamore and pea (O'Neill et al., 1996), and red wine (Pellerin et al., 1996). In cultured tobacco cells the B associated with RG-II accounts for about 80% of the cell wall B (Kobayashi et al., 1997) and RG-II may be the exclusive carrier of B in higher plant cell walls (Matoh et al., 1996). Germanic acid, which partly substitutes for B in the growth of the B-deprived plants (Skok, 1957), also cross-links two RG-II chains (Kobayashi et al., 1997). These results suggest that the physiological role of B is to cross-link cell wall pectic polysaccharides in the RG-II region and thereby form a pectic network.
It is believed that in the cell wall pectic polysaccharides are cross-linked with Ca2+, which binds to carboxyl groups of the polygalacturonic acid regions (Jarvis, 1984). Thus, the ability of B and Ca2+ to cross-link cell wall pectic polysaccharides needs to be evaluated. In this report we describe the B-RG-II complex of radish root and the role of B-RG-II and Ca2+ in the formation of a pectic network.
MATERIALS AND METHODS
Preparation and Analyses of the Radish B-RG-II Complex
Radish (Raphanus sativus L. cv Aokubi-daikon) B-RG-II was prepared as described previously (Kobayashi et al., 1996). B-RG-II (3 mg) was dissolved in 0.3 mL of 10 mm Tris-HCl, pH 8.0, and passed through a column (1 × 1 cm) containing Chelex 100 resin (Bio-Rad) equilibrated with the same buffer. The effluent was then fractionated on a PD-10 size-exclusion column (Pharmacia) equilibrated with the same buffer. The high-Mr fraction was collected and its mineral composition determined.
Reconstitution of the B-RG-II Complex
Monomeric RG-II was prepared from the radish B-RG-II complex as described previously (Kobayashi et al., 1997). One-millimolar monomeric RG-II (assuming the Mr of the monomeric RG-II as 5000) and 10 mm boric acid were incubated in 50 mm sodium acetate buffer (pH 4.0) with or without 10 mm divalent cation, and an aliquot was subjected to size-exclusion HPLC (YMC-pack Diol 120, 8 × 300 mm, YMC, Karasuma-Oike, Kyoto, Japan) equipped with a refractive index detector (Kobayashi et al., 1997).
Sequential Extraction from Radish Root Cell Walls
Radish roots purchased at a local market were homogenized in ice-cold water with a kitchen blender and the homogenate was filtered under suction. The filtered cake was washed three times by suspension in 20 volumes (v/w) of ice-cold water and then used as a crude cell wall preparation. A 5-g (wet weight) aliquot of the crude cell wall preparation was put into a column (3.2 cm diameter) and washed with 750 mL of a 1.5% (w/v) SDS solution buffered with 10 mm Mes-NaOH, pH 6.5, and then with 200 mL of water at a flow rate of 4 mL min−1. The SDS-washed cell wall was treated for 18 h at 25°C with 100 mL of 50 mm CDTA, pH 6.5. The residue was further extracted for 18 h at 4°C and then for 2 h at 25°C with 2 mm CDTA dissolved in 50 mm Na2CO3. Aliquots were taken at each step, washed with distilled water, and freeze-dried before the uronic acids were determined. The SDS-washed cell wall was digested with Driselase (Kyowa Hakko, Tokyo) and the digest was chromatographed on a 1- × 20-cm column of DEAE-Sepharose (Cl− form, Pharmacia) as described by Kobayashi et al. (1997).
Assay Methods
B was quantified by the chromotropic acid method (Matoh et al., 1997). Ca2+ was quantified by atomic absorption spectroscopy (AA-640, Shimadzu, Kyoto, Japan). Sr, Br, and Pb were quantified by atomic absorption spectroscopy (AA-660, Shimadzu) using a graphite furnace atomizer (GFA-4A, Shimadzu). Pectic polysaccharides in cell walls or extracted residues were determined as GalUA equivalents according to the method of Ahmed and Labavitch (1977). Wet samples were lyophilized before dissolution in H2SO4. The total sugar content was assayed by the phenol-sulfuric acid method (Dubois et al., 1956), using Glc as a standard. 2-Keto-3-deoxysugars were determined by the modified thiobarbituric acid method (York et al., 1985).
RESULTS
O'Neill et al. (1996) reported that B-RG-II isolated from the walls of sycamore cells and pea stems contains divalent metal cations, such as Mg2+, Ca2+, Sr2+, Ba2+, and Pb2+. These cations and Zn2+ are also present in B-RG-II isolated from beet and bamboo (Matsunaga et al., 1997). B-RG-II isolated from red wine also contains Ca2+ (Pellerin et al., 1996). The radish B-RG-II complex contains Ca2+, Sr2+, Ba2+, and Pb2+ at molar ratios to B of 1.2, 0.1, 0.01, and 0.04, respectively (Table I). The occurrence of Ca2+ at an equimolar concentration to B was also confirmed in the B-RG-II complex isolated from celery, cultured tobacco cells, and cabbage (data not presented).
Table I.
Mineral contents of the radish B-RG-II complex
| Element | Treatment
|
|
|---|---|---|
| Nativea | CDTAb | |
| mol mol−1 complex | ||
| B | 1.8 (1.0)c | 0.78 |
| Ca2+ | 2.2 (1.2) | 0.05 |
| Sr2+ | 0.20 (0.1) | –d |
| Pb2+ | 0.06 (0.04) | – |
| Ba2+ | 0.03 (0.01) | – |
Native radish B-RG-II complex was treated with the Chelex resin before mineral analyses.
Radish B-RG-II complex was incubated with 50 mm CDTA in sodium acetate (pH 6.5) for 3 h and then treated with the Chelex resin before analyses.
Values in parentheses designate mole ratios to B.
–, Not determined.
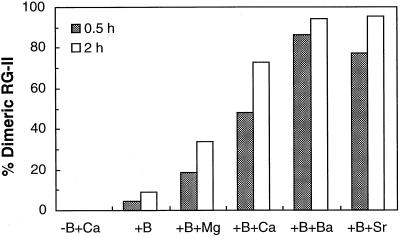
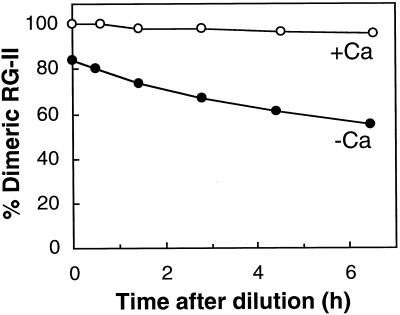
The radish B-RG-II complex was reconstituted in vitro by mixing B and monomeric RG-II at pH 4.0; however, the extent of dimerization was low (Fig. 1; Kobayashi et al., 1997). Following the report by O'Neill et al. (1996), we confirm that Sr2+, Ba2+, and Pb2+ promote B-RG-II complex formation in vitro and that Ca2+ is also effective (Fig. 1). The complex reconstituted in the presence of Ca2+ contained 1.1 to 1.3 mol of Ca2+ per mol of B (data not presented). Divalent cations alone did not bring about dimer formation (Fig. 1), as O'Neill et al. (1996) demonstrated. The B-RG-II complex generated in the absence of Ca2+ gradually decomposed into a monomeric one when the RG-II concentration in the reconstitution solution was reduced by dilution and free B and free Ca2+ were removed by ion-exchange resins (Fig. 2). However, the B-RG-II complex reconstituted in the presence of Ca2+ was stable under the same conditions.
Figure 1.
Effect of divalent cations on the rate of reconstitution of the B-RG-II. Monomeric RG-II (1 mm) was incubated in 50 mm sodium acetate buffer (pH 4.0) with 10 mm H3BO3 and 10 mm metal cations. The treatment is designated on the abscissa. The metal cations were added as chloride salts. At 0.5 and 2 h, an aliquot (10 μL) was subjected to size-exclusion HPLC with a refractive index detector. The figures on the ordinate indicate the mole percentage of RG-II converted into the dimeric form.
Figure 2.
Effect of Ca2+ on the stability of the reconstituted B-RG-II complex. Boric acid (50 mm) and monomeric RG-II (5 mm) were incubated in 50 mm sodium acetate buffer (pH 4.0) with (○) or without (•) 50 mm CaCl2. After 20 h, the reaction mixture was diluted 10 times with the acetate buffer and free boric acid and Ca2+ were removed with Amberlite IRA-743 resin (SO42− form) and Dowex 50W-X8 resin (Na+ form), respectively. At intervals an aliquot (10 μL) was subjected to size-exclusion HPLC with a refractive index detector. The figures on the vertical axis indicate the mole percentage of RG-II converted into the dimeric form.
Removal of the Ca2+ from the native B-RG-II with CDTA induced release of B (Table I) and hence decay of the complex. The spontaneous decomposition of B-RG-II generated without Ca2+ (Fig. 2) suggests that removing Ca2+ from the dimer renders the dimer unstable and allows it to decompose spontaneously. These results are compatible with the notion that Ca2+ is a native constituent of the B-RG-II complex.
To establish the significance of Ca2+ in the B-RG-II formation, the distribution of cell wall Ca2+ was examined by sequential extraction of Ca2+ from radish root cell walls. When freshly prepared crude cell walls were treated with NaCl solutions of various concentrations to solubilize pectic polysaccharides and Ca2+, reproducible results were not obtained. The use of organic solvents such as ethanol, methanol, or a chloroform-methanol mixture was avoided because dehydration may alter the solubility of the polysaccharides. Therefore, a 1.5% (w/v) SDS solution, adjusted to pH 6.5 with 10 mm Mes-NaOH buffer, was used throughout this study to reduce autolytic activities. Pectic polysaccharides were released from crude cell walls more slowly than was Ca2+ (data not presented), perhaps because of a steric factor, as pointed out by Jarvis (1982). Therefore, SDS washing was performed on column-packed crude cell walls.
Jarvis (1982) demonstrated that during preparation of cress, cucumber, and celery cell walls with 0.5% SDS, negligible amounts of pectin are lost. A similar result was obtained for radish roots. The SDS washing of the homogenized root tissue removed 98% of Ca2+ from the tissues (Table II), probably because of an ion-exchange action of Na+, since the SDS solution contains approximately 50 mm Na+. Judging from the Ca2+ concentrations in the crude cell walls and in the fresh material, and the yield of crude cell walls from fresh material, we surmised that at least 70% of Ca2+ of intact radish tissues is localized in the cell walls. A molar ratio of Ca2+ to B of the SDS-washed cell walls was less than 1.5, which was in the same range as that of the purified B-RG-II complex (Table I). Concomitantly, the SDS treatment removed 17% of B and 22% of pectic polysaccharides (Table II). Extraction of the SDS-washed cell walls with CDTA removed all of the remaining Ca2+ and 78% of B, as well as 49% of pectic polysaccharides (Table II). After the CDTA treatment, the walls retained a portion of 29% of pectic polysaccharides, and one-half of the remaining pectic polysaccharides was released with the alkaline-CDTA treatment. SDS-, CDTA-, and alkaline-CDTA solubilized pectic polysaccharides all contain the RG-II region, because 2-keto-3-deoxysugars, which are the diagnostic monosaccharides for RG-II (York et al., 1985), also decreased concomitantly (Table II). RG-II may occur mainly in the CDTA fraction, judging from the contents of 2-keto-3-deoxysugars in the three fractions. Occurrence of the RG-II region in both CDTA- and alkaline-CDTA-solubilized pectic polysaccharides is also demonstrated using an antibody toward RG-II (Matoh et al., 1998).
Table II.
Sequential extraction of Ca2+, B, and pectic polysaccharides from radish root cell walls
| Sample | Ca2+ | B | Pectic Polysaccharidesa | 2-Keto-3-Deoxysugarsb |
|---|---|---|---|---|
| Crude cell walls | 100 (13)c | 100 (0.25) | 100 (25) | 100 (1.0) |
| SDS-treated cell walls | 2 | 83 | 78 ± 0.4 | 90 ± 2.8 |
| SDS- and CDTA-treated cell walls | 0 | 5 | 29 ± 2.1 | 34 ± 0.7 |
| SDS-, CDTA-, and alkaline-CDTA-treated cell walls | –d | – | 14 ± 0.5 | 29 ± 2.8 |
Determined by the m-hydroxydiphenyl method using GalUA as a standard.
Determined by modified thiobarbituric acid method using 3-deoxy-d-manno-2-octulosonic acid as a standard.
Values in the parentheses are μmol Ca2+, μmol B, μmol 2-keto-3-deoxysugars, and mg uronic acid g−1 crude cell walls.
–, Not determined.
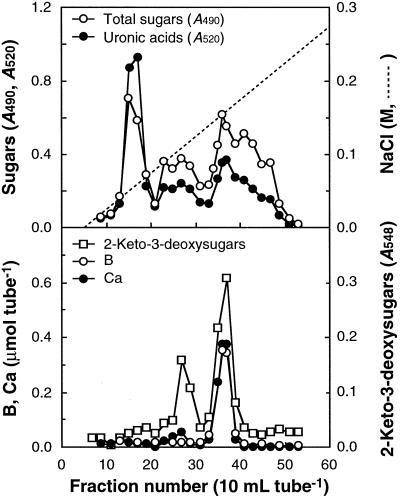
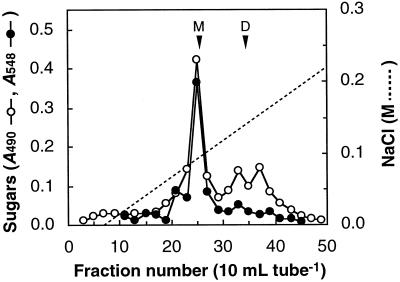
The SDS-washed cell walls were digested with Driselase and the digest was fractionated on DEAE-Sepharose. As presented in Figure 3, Ca2+ comigrated with B and 2-keto-3-deoxysugars. Ca2+ in the RG-II peak accounted for 80% of the Ca2+ present in the SDS-washed cell walls. These results suggest that almost all of the Ca2+ retained in the SDS-washed cell walls occurs in the RG-II region of the pectic polysaccharides. We detected another peak in the chromatogram for 2-keto-3-deoxysugars around fraction no. 27, just before the peak for the B-Ca2+-RG-II complex. The eluting position coincided with that for the monomeric RG-II. The CDTA treatment eliminated not only Ca2+ but also B from the purified RG-II complex (Table I), which may have resulted in decomposition of the complex into monomeric RG-II. Driselase digestion of the CDTA extract of the SDS-washed cell walls yielded only monomeric RG-II, which was free of B (Fig. 4). This is compatible with the result that CDTA released B from cell walls (Table II). The pectic polysaccharides that are not released with SDS but are released with CDTA are probably retained in cell walls through Ca2+-B cross-linking at the RG-II regions.
Figure 3.
Chromatogram of a Driselase digest of the SDS-washed cell walls on DEAE-Sepharose. About 10 g of the SDS-washed cell walls was hydrolyzed with Driselase and the digest was chromatographed on a DEAE-Sepharose column (1.6 × 17 cm), that had been equilibrated with 20 mm Tris-HCl (pH 8.0). The pectic fragments were eluted with a 1-L gradient of 0 to 0.5 m NaCl in 20 mm Tris-HCl (pH 8.0).
Figure 4.
Chromatogram of a Driselase digest of the CDTA extract of the SDS-washed residues on DEAE-Sepharose. About 15 g of the SDS-washed cell walls was extracted with 100 mL of 50 mm CDTA, pH 6.5, at 25°C for 18 h. The extract was dialyzed against distilled water and subjected to hydrolysis with Driselase. The column (1.6 × 17 cm) was equilibrated with 20 mm Tris-HCl (pH 8.0) and eluted with a 1-L gradient of 0 to 0.5 m NaCl in 20 mm Tris-HCl (pH 8.0). The arrowheads indicate the elution position of the radish monomeric (M) and dimeric (D) RG-II on the same column.
DISCUSSION
In a previous report (Kobayashi et al., 1997), we suggested that in vitro formation of B-RG-II from B and monomeric RG-II may not reflect the mode of the B-RG-II bonding in vivo, and we pointed out that another factor(s) may be involved. We now suggest that Ca2+ is a constituent of the naturally occurring B-RG-II complex and promotes the rate of dimer formation in vitro. O'Neill et al. (1996) reported that low concentrations (0.5 mm) of Ca2+ did not stimulate dimer formation effectively, that Mg2+ and Cu2+ were inhibitory, and that monovalent cations had no discernible effect. We have shown, however, that higher concentrations (10 mm) of Ca2+ do stimulate dimer formation and that Mg2+ and Cu2+ are also effective, although the effects were small. We observed that high concentrations (about 100 mm) of monovalent cations such as Na+, K+, and NH4+ also promoted, to a certain extent, reconstitution under our conditions (data not shown). The apparent discrepancies regarding the effects of Ca2+ and other cations may be due to different experimental conditions. In our experimental conditions, monomeric RG-II and boric acid were mixed at a final concentration of 1 and 10 mm, respectively, and divalent cations were added at 10 mm. These concentrations are higher than those used by O'Neill et al. (1996) (0.5 mm monomeric RG-II, 1.2 mm boric acid, and 0.5 mm divalent cation). Thus, the reactivity and effectiveness of coexisting cations may vary significantly according to the concentrations used.
The effect of monovalent cations on reconstitution may be due to the masking of the negative charge owing to GalUA residues of the RG-II backbone (see discussion in Kobayashi et al. [1997]). Although some part of the effect of divalent metal ions may be due to the cationic nature of the elements, the effects of divalent cations such as Sr2+, Ba2+, Pb2+, and Ca2+ are superior to the monovalent ones. The ratio of the dimer and the monomer is under equilibrium in the reconstituting solution; therefore, the divalent metal cations may stabilize the formed dimeric B-RG-II complex and hence prevent the breakdown of the dimer. O'Neill et al. (1996) suggested that divalent metal cations might form metal coordination complexes with B-RG-II, because the cations were not removed by treatment with Chelex resin and they were not bound to monomeric RG-II. Smaller cations such as Ca2+ may not interact strongly enough with the ligands on the two chains to stabilize the complex. However, higher concentrations of the smaller cations, such as Ca2+, may compensate for their lower affinity. The Chelex and the Dowex 50 resins do not remove the Ca2+ in the naturally occurring and reconstituted B-RG-II complex. Therefore, the Ca2+ may not only bind to the carboxyl groups ionically but may also form coordinate bonds, as has been suggested for Sr2+. Van Duin et al. (1987) reported that Ca2+ forms coordination complexes with borate diesters of polyhydroxy carboxylates. In their model the Ca2+ is thought to interact directly with the borate anion. However, O'Neill et al. (1996) suggested that the metal cations in B-RG-II do not interact with the borate anion, because the cations do not affect the 11B-NMR spectrum of the complex. Some carboxyl or hydroxyl groups along the RG-II sugar moiety may provide sites that are favorable to coordinate the cations.
The conditions that promote dimer formation in the cell wall are not known. However, we suggest that Ca2+ stabilizes the B-RG-II complex in vivo, based on the following considerations. The concentration of water-soluble Ca2+ in root tip cell walls is approximately 2.5 mm (Bjorkman and Cleland, 1991), whereas the content of Sr2+ in angiosperm is three orders lower than that of Ca2+ (Bowen, 1966) , even though the effect of Ca2+ on the stabilization is not as high as Sr2+; Ca2+ is more abundant in soil than Sr2+; the nutritional requirement of Sr2+, Ba2+, and Pb2+ has not been reported unequivocally for higher plants (Bowen, 1966), except for the substitution effect of Sr2+ under Ca2+-deficient conditions (Da Silva, 1962); and, finally, Sr2+, Pb2+, and Ba2+ account for only 0.15 mol per mol of B in the native radish B-RG-II (Table I) and less than 0.21 mol per mol of B-RG-II (O'Neill et al., 1996), in contrast to the higher content of Ca2+ (1.2 mol Ca2+ to 1.0 mol B) in native B-RG-II.
In cell walls, pectic polysaccharides are believed to be cross-linked by Ca2+ bridges, phenolic coupling, galacturonate ester bonds (Fry, 1986), and borate-diester bonds (Kobayashi et al., 1996). Jarvis (1982) demonstrated that CDTA solubilizes Ca2+-bound pectic polysaccharides. However, the data presented in this report suggest that the RG-II regions provide the sites for linkages through borate-diester and Ca2+ bonding and the linkages help to retain so-called chelator-soluble pectic polysaccharides in cell walls. During this series of experiments we noticed that SDS washing increased the packed volume of cell wall preparations, that the apparent volume of cell walls increased 2-fold after washing with SDS, and that the change was reversed by incubation of the swollen cell walls with 50 mm CaCl2 (data not presented). This suggests that the SDS-extractable Ca2+, which accounts for the 98% of the cell wall Ca2+ that is probably associated with the polygalacturonic acid regions, may control the properties of the pectic polysaccharides in cell walls.
The RG-II regions provide cross-linking sites for pectic polysaccharides through borate-diester bonding, and the residue responsible for the bonding is believed to be the apiosyl (O'Neill et al., 1996). Ca2+ also cross-links pectic chains through ionic and coordinate bonding in the polygalacturonic acid region. Although formation of the Ca2+ cross-linking is controlled by the esterification/ de-esterification of the carboxyl groups on the uronic residues (Fry, 1986), the Ca2+ bridge may be more arbitrary than the B bridge; the latter may be more site specific. This may give a clue for elucidating the physiological significance of B as an essential element in plants.
Abbreviations:
- CDTA
trans-1,2-diaminocyclohexane-N,N,N′,N′-tetraacetic acid
- RG-II
rhamnogalacturonan II
Footnotes
This work was financially supported in part by a grant-in-aid (no. 09660063) from the Ministry of Education, Science and Culture of Japan to T.M.
LITERATURE CITED
- Ahmed AER, Labavitch JM. A simplified method for accurate determination of cell wall uronide content. J Food Biochem. 1977;1:361–365. [Google Scholar]
- Bjorkman T, Cleland RE. The role of extracellular free-calcium gradients in gravitropic signaling in maize roots. Planta. 1991;185:379–384. [PubMed] [Google Scholar]
- Bowen HJM (1966) Trace Elements in Biochemistry. Academic Press, London, pp 61–84
- Da Silva PGP. On the possibility of substitution of strontium for calcium in maize plants. Agron Lusit. 1962;24:133–164. [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- Fry SC. Cross-linking of matrix polymers in the growing cell walls of angiosperms. Annu Rev Plant Physiol. 1986;37:165–186. [Google Scholar]
- Ishii T, Matsunaga T. Isolation and characterization of a boron-rhamnogalacturonan-II complex from cell walls of sugar beet pulp. Carbohydr Res. 1996;284:1–9. [Google Scholar]
- Jarvis MC. The proportion of calcium-bound pectin in plant cell walls. Planta. 1982;154:344–346. doi: 10.1007/BF00393913. [DOI] [PubMed] [Google Scholar]
- Jarvis MC. Structure and properties of pectin gels in plant cell walls. Plant Cell Environ. 1984;7:153–164. [Google Scholar]
- Kaneko S, Ishii T, Matsunaga T. A boron-rhamnogalacturonan-II complex from bamboo shoot cell walls. Phytochemistry. 1997;44:243–248. [Google Scholar]
- Kobayashi M, Matoh T, Azuma J. Structure and glycosyl composition of the boron-polysaccharide complex of radish roots. Plant Cell Physiol Suppl. 1995;36:S-139. [Google Scholar]
- Kobayashi M, Matoh T, Azuma J. Two chains of rhamnogalacturonan II are cross-linked by borate-diol ester bonds in higher plant cell walls. Plant Physiol. 1996;110:1017–1020. doi: 10.1104/pp.110.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Ohno K, Matoh T. Boron nutrition of cultured tobacco BY-2 cells. II. Characterization of the boron-polysaccharide complex. Plant Cell Physiol. 1997;38:676–683. [Google Scholar]
- Loomis WD, Durst RW. Chemistry and biology of boron. Biofactors. 1992;3:229–239. [PubMed] [Google Scholar]
- Matoh T, Akaike R, Kobayashi M. A sensitive and convenient assay for boron in plant using chromotropic acid and HPLC. Plant Soil. 1997;192:115–118. [Google Scholar]
- Matoh T, Ishigaki K, Mizutani M, Matsunaga W, Takabe K. Boron nutrition of cultured tobacco BY-2 cells. I. Requirement for and intracellular localization of boron and selection of cells that tolerate low levels of boron. Plant Cell Physiol. 1992;33:1135–1141. [Google Scholar]
- Matoh T, Ishigaki K, Ohno K, Azuma J. Isolation and characterization of a boron-polysaccharide complex from radish roots. Plant Cell Physiol. 1993;34:639–642. [Google Scholar]
- Matoh T, Kawaguchi S, Kobayashi M. Ubiquity of a borate-rhamnogalacturonan II complex in the cell walls of higher plants. Plant Cell Physiol. 1996;37:636–640. [Google Scholar]
- Matoh T, Takasaki M, Takabe K, Kobayashi M. Immunocytochemistry of rhamnogalacturonan II in cell walls of higher plants. Plant Cell Physiol. 1998;39:483–491. [Google Scholar]
- Matsunaga T, Ishii T, Watanabe-Oda H. HPLC/ICP-MS study of metals bound to borate-rhamnogalacturonan-II from plant cell walls. In: Ando T, Fujita K, Mae T, Matsumoto H, Mori S, Sekiya J, editors. Plant Nutrition for Sustainable Food Production and Environment. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1997. pp. 81–82. [Google Scholar]
- O'Neill MA, Warrenfeltz D, Kates K, Pellerin P, Doco T, Darvill AG, Albersheim P. Rhamnogalacturonan-II, a pectic polysaccharide in the walls of growing plant cell, forms a dimer that is covalently cross-linked by a borate ester. In vitro conditions for the formation and hydrolysis of the dimer. J Biol Chem. 1996;271:22923–22930. doi: 10.1074/jbc.271.37.22923. [DOI] [PubMed] [Google Scholar]
- Pellerin P, Doco T, Vidal S, Williams P, Brillouet JM, O'Neill MA. Structural characterization of red wine rhamnogalacturonan II. Carbohydr Res. 1996;290:183–197. doi: 10.1016/0008-6215(96)00139-5. [DOI] [PubMed] [Google Scholar]
- Skok J. The substitution of complexing substances for boron in plant growth. Plant Physiol. 1957;32:308–312. doi: 10.1104/pp.32.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duin M, Peters JA, Kieboom APG, Van Bekkum H. Synergic coordination of calcium in borate-polyhydroxycarboxylate systems. Carbohydr Res. 1987;162:65–78. [Google Scholar]
- York WS, Darvill AG, McNeil M, Albersheim P. 3-Deoxy-d-manno-2-octulosonic acid (KDO) is a component of rhamnogalacturonan II, a pectic polysaccharide in the primary cell walls of plants. Carbohydr Res. 1985;138:109–126. [Google Scholar]