Abstract
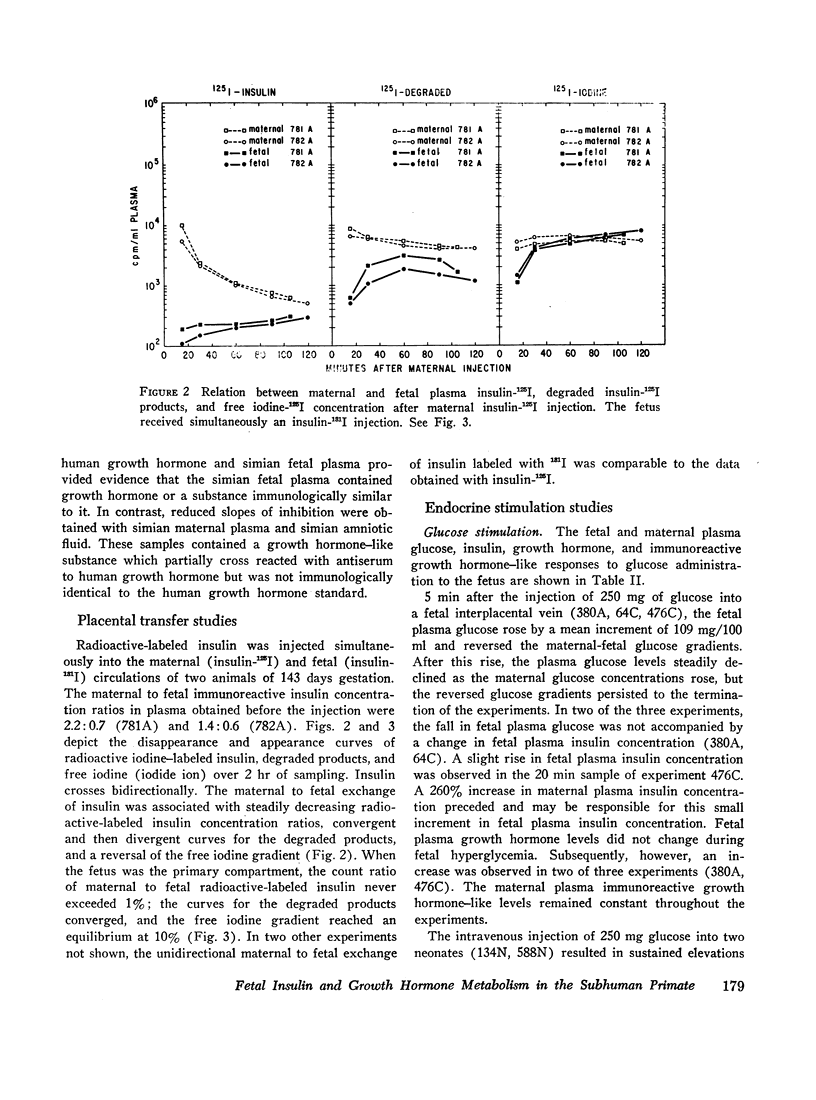
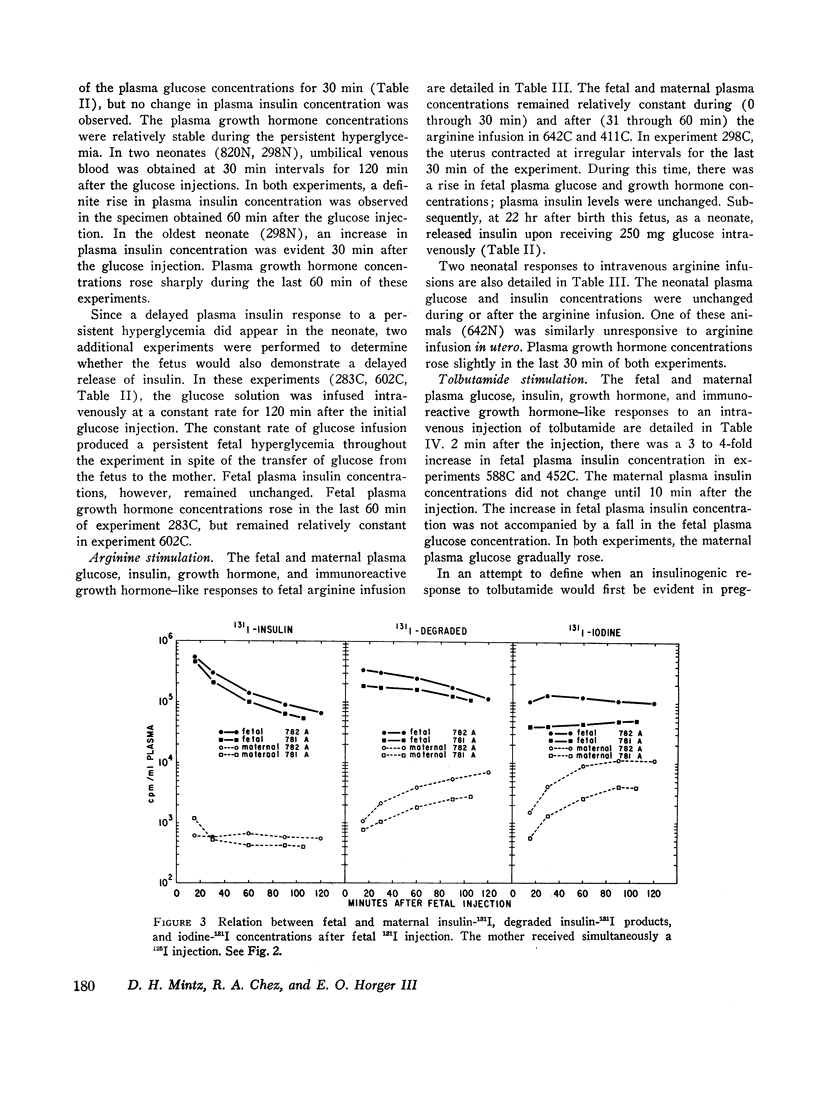
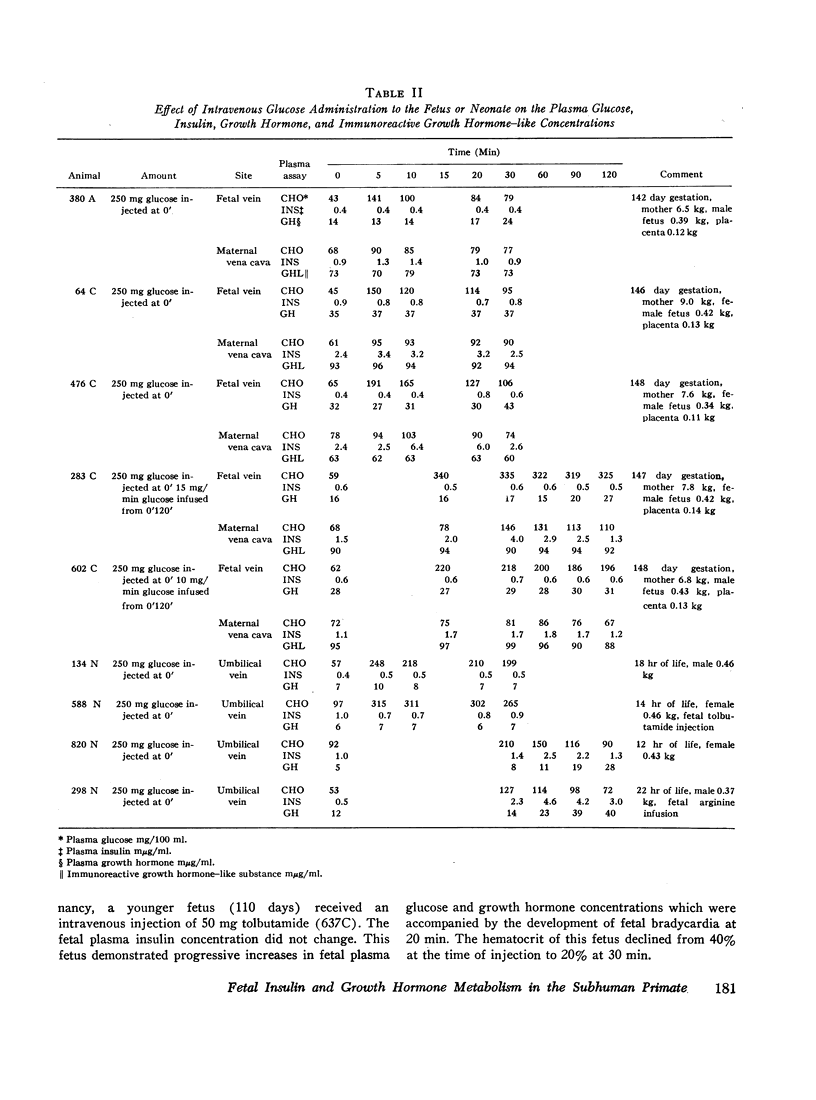
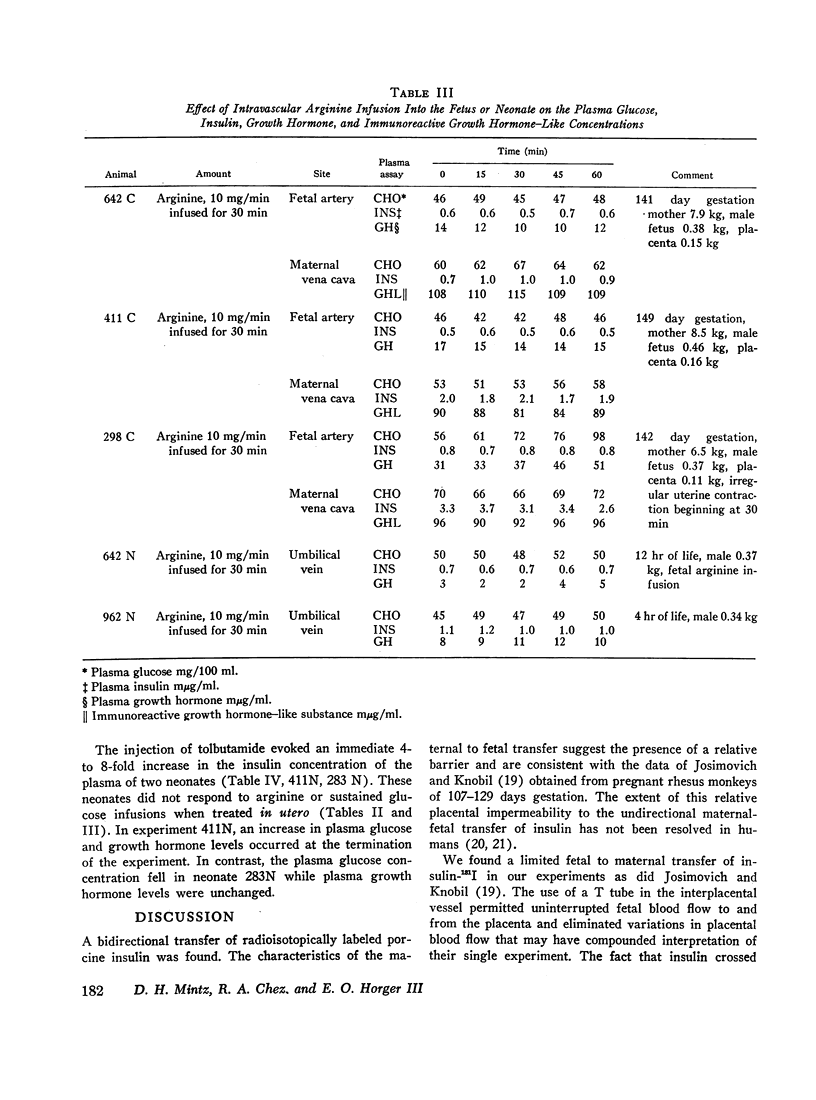
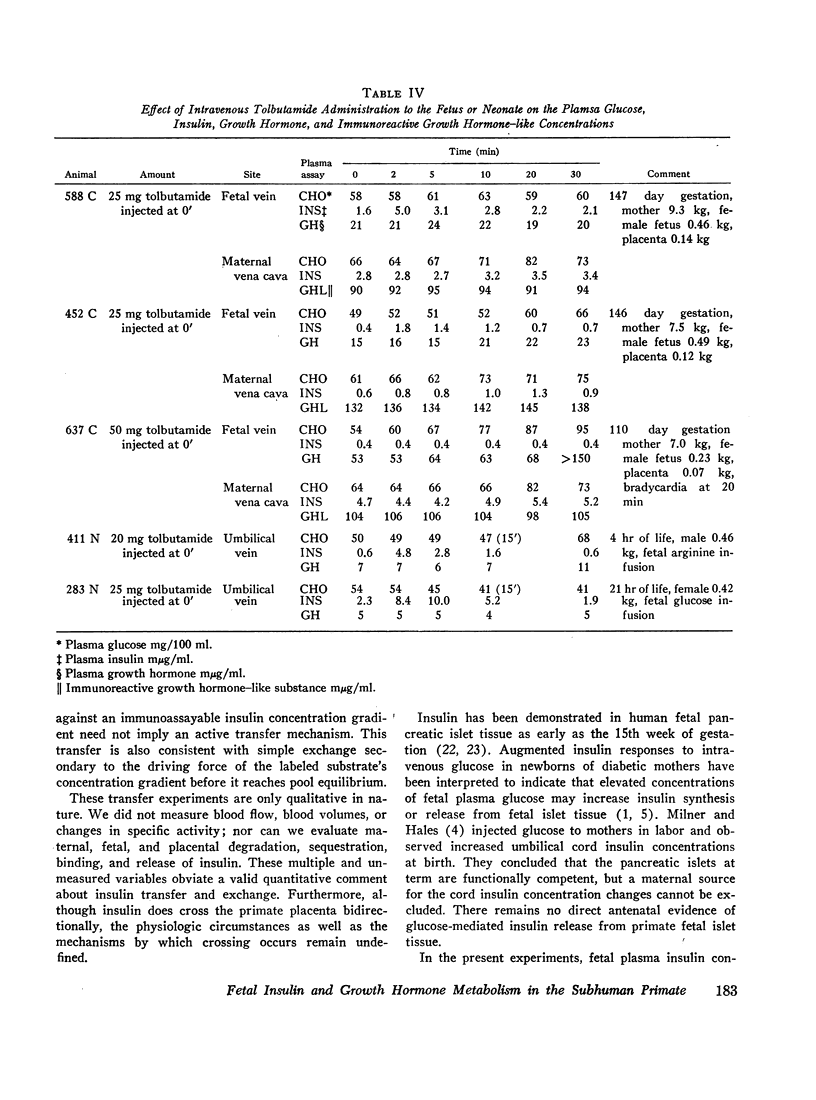
The concentrations of plasma glucose, insulin, growth hormone, and immunoreactive growth hormone-like substance in subhuman primate fetal and maternal plasma were examined after the intravascular administration of glucose, arginine, or tolbutamide to the fetus. Cannulation of interplacental vessels permitted studies on the fetus in utero without disruption of fetal-placental-maternal anatomic integrity. Single glucose injections, glucose infusions, and arginine infusions into the fetus did not alter fetal plasma insulin concentrations. In contrast, tolbutamide injections elicited an immediate 3-4-fold increase in fetal plasma insulin concentrations. A bidirectional placental transfer of insulin was demonstrated with the use of simultaneously injected insulin-125I to the mother and insulin-131I to the fetus.
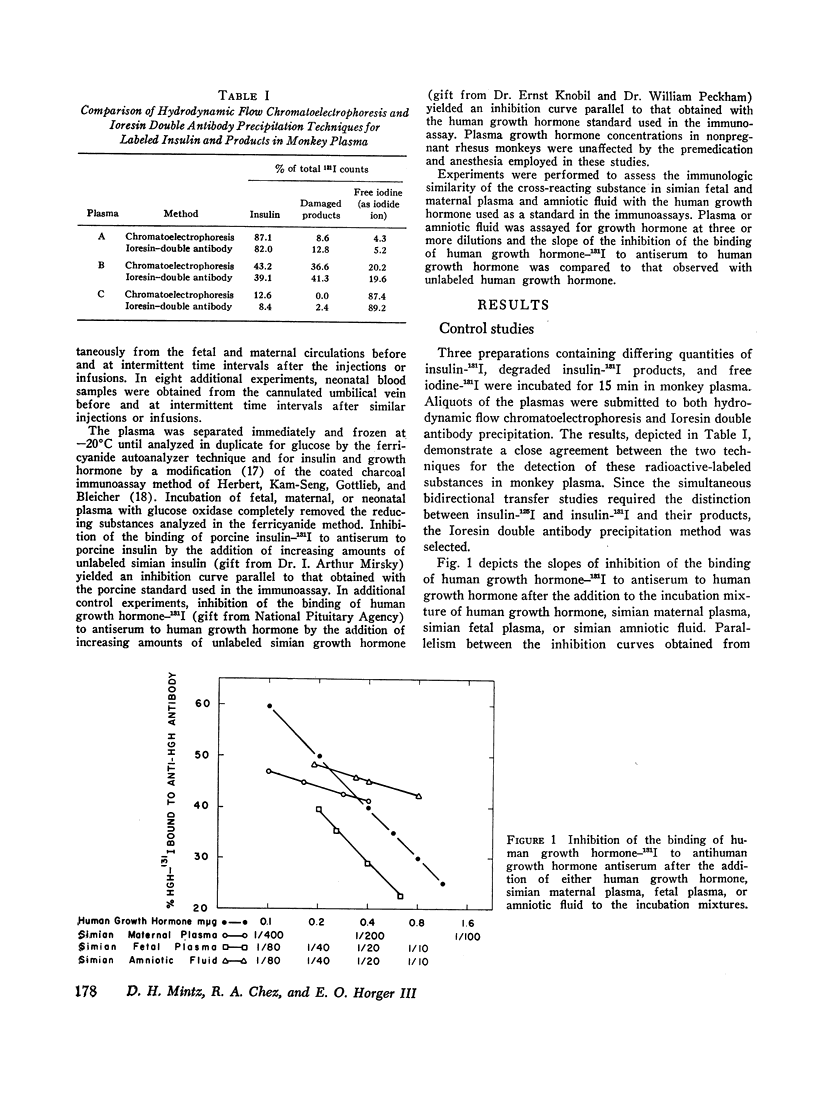
Simian fetal plasma contained a substance which cross-reacted with immunologic identity to human growth hormone. In contrast, simian maternal plasma and amniotic fluid reacted with immunologic nonidentity to human growth hormone. Although glucose administration to the fetus did not suppress nor did arginine infusion consistently augment fetal plasma growth hormone levels, the latter were observed to vary in individual experiments.
The plasma responses to the same stimuli in the neonate were also examined. In contrast to the fetal experiments, glucose injection in the neonate elicited a delayed rise in the concentration of plasma insulin. Similar to the fetus, the plasma concentration of insulin increased after tolbutamide injection and did not change in response to arginine infusion. The initial concentrations of neonatal plasma growth hormone were significantly lower when contrasted with the initial fetal plasma levels. There was no difference in the responsiveness of the fetal and neonatal growth hormone-releasing mechanisms when challenged by glucose or arginine infusion.
The data indicate that the fetal plasma concentration of growth hormone is labile, that fetal growth hormone metabolism may differ from that in the neonate, and that pancreatic islet cell responsiveness rapidly changes after delivery.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAIRD J. D., FARQUHAR J. W. Insulin-secreting capacity in newborn infants of normal and diabetic women. Lancet. 1962 Jan 13;1(7220):71–74. doi: 10.1016/s0140-6736(62)91720-8. [DOI] [PubMed] [Google Scholar]
- BUSE M. G., ROBERTS W. J., BUSE J. The role of the human placenta in the transfer and metabolism of insulin. J Clin Invest. 1962 Jan;41:29–41. doi: 10.1172/JCI104464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONKLIN J. L. Cytogenesis of the human fetal pancreas. Am J Anat. 1962 Sep;111:181–193. doi: 10.1002/aja.1001110204. [DOI] [PubMed] [Google Scholar]
- CORNBLATH M., PARKER M. L., REISNER S. H., FORBES A. E., DAUGHADAY W. H. SECRETION AND METABOLISM OF GROWTH HORMONE IN PREMATURE AND FULL-TERM INFANTS. J Clin Endocrinol Metab. 1965 Feb;25:209–218. doi: 10.1210/jcem-25-2-209. [DOI] [PubMed] [Google Scholar]
- Drash A., Field J. B., Garces L. Y., Kenny F. M., Mintz D., Vazquez A. M. Endogenous insulin and growth hormone response in children with newly diagnosed diabetes mellitus. Pediatr Res. 1968 Mar;2(2):94–102. doi: 10.1203/00006450-196803000-00004. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN E. A., GRAY M. J., GRYNFOGEL M., HUTCHINSON D. L., KELLY W. T., PLENTL A. A. The distribution and metabolism of C14-labeled lactic acid and bicarbonate in pregnant primates. J Clin Invest. 1960 Feb;39:227–235. doi: 10.1172/JCI104033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GITLIN D., KUMATE J., MORALES C. ON THE TRANSPORT OF INSULIN ACROSS THE HUMAN PLACENTA. Pediatrics. 1965 Jan;35:65–69. [PubMed] [Google Scholar]
- GLICK S. M., ROTH J., YALOW R. S., BERSON S. A. IMMUNOASSAY OF HUMAN GROWTH HORMONE IN PLASMA. Nature. 1963 Aug 24;199:784–787. doi: 10.1038/199784a0. [DOI] [PubMed] [Google Scholar]
- GLICK S. M., ROTH J., YALOW R. S., BERSON S. A. THE REGULATION OF GROWTH HORMONE SECRETION. Recent Prog Horm Res. 1965;21:241–283. [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., KLOPPER A. ASSAY OF HUMAN GROWTH HORMONE IN PREGNANCY AT PARTURITION AND IN LACTATION. DETECTION OF A GROWTH-HORMONE-LIKE SUBSTANCE FROM THE PLACENTA. Br Med J. 1964 Jan 4;1(5374):22–24. doi: 10.1136/bmj.1.5374.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUIDOTTI G., KANAMEISHI D., FOA P. P. Chick embryo heart as a tool for studying cell permeability and insulin action. Am J Physiol. 1961 Nov;201:863–868. doi: 10.1152/ajplegacy.1961.201.5.863. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- HUTCHINSON D. L., KELLY W. T., FRIEDMAN E. A., PLENTL A. A. The distribution and metabolism of carbon-labeled urea in pregnant primates. J Clin Invest. 1962 Sep;41:1745–1753. doi: 10.1172/JCI104633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- JOSIMOVICH J. B., KNOBIL E. Placental transfer of 1-131-insulin in the rhesus monkey. Am J Physiol. 1961 Mar;200:471–476. doi: 10.1152/ajplegacy.1961.200.3.471. [DOI] [PubMed] [Google Scholar]
- Jorgensen K. R., Deckert T., Pedersen L. M., Pedersen J. Insulin, insulin antibody and glucose in plasma of newborn infants of diabetic women. Acta Endocrinol (Copenh) 1966 May;52(1):154–167. doi: 10.1530/acta.0.0520154. [DOI] [PubMed] [Google Scholar]
- KNOPF R. F., CONN J. W., FAJANS S. S., FLOYD J. C., GUNTSCHE E. M., RULL J. A. PLASMA GROWTH HORMONE RESPONSE TO INTRAVENOUS ADMINISTRATION OF AMINO ACIDS. J Clin Endocrinol Metab. 1965 Aug;25:1140–1144. doi: 10.1210/jcem-25-8-1140. [DOI] [PubMed] [Google Scholar]
- Kaplan S. L., Grumbach M. M. Serum chorionic "growth hormone-prolactin" and serum pituitary growth hormone in mother and fetus at term. J Clin Endocrinol Metab. 1965 Oct;25(10):1370–1374. doi: 10.1210/jcem-25-10-1370. [DOI] [PubMed] [Google Scholar]
- MILNER R. D., HALES C. N. EFFECT OF INTRAVENOUS GLUCOSE ON CONCENTRATION OF INSULIN IN MATERNAL AND UMBILICAL-CORD PLASMA. Br Med J. 1965 Jan 30;1(5430):284–286. doi: 10.1136/bmj.1.5430.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer V., Knobil E. Growth hormone secretion in the unanesthetized rhesus monkey in response to noxious stimuli. Endocrinology. 1967 Jan;80(1):163–171. doi: 10.1210/endo-80-1-163. [DOI] [PubMed] [Google Scholar]
- PLENTL A. A., FRIEDMAN E. A. Isotope tracer studies on the carbon dioxide exchange in pregnant primates. Am J Obstet Gynecol. 1962 Nov 1;84:1242–1252. doi: 10.1016/0002-9378(62)90517-3. [DOI] [PubMed] [Google Scholar]
- REYNOLDS S. R., PAUL W. M., HUGGETT A. S. Physiological study of the monkey fetus in utero: a procedure for blood pressure recording, blood sampling, and injection of the fetus under normal conditions. Bull Johns Hopkins Hosp. 1954 Nov;95(5):256–268. [PubMed] [Google Scholar]
- Rabinowitz D., Merimee T. J., Burgess J. A., Riggs L. Growth hormone and insulin release after arginine: indifference to hyperglycemia and epinephrine. J Clin Endocrinol Metab. 1966 Oct;26(10):1170–1172. doi: 10.1210/jcem-26-10-1170. [DOI] [PubMed] [Google Scholar]
- SELTZER H. S. Quantitative effects of glucose, sulfonylureas, salicylate, and indole-3-acetic acid on the secretion of insulin activity into pancreatic venous blood. J Clin Invest. 1962 Feb;41:289–300. doi: 10.1172/JCI104482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPELLACY W. N., GOETZ F. C., GREENBERG B. Z., ELLS J. THE HUMAN PLACENTAL GRADIENT FOR PLASMA INSULIN AND BLOOD GLUCOSE. Am J Obstet Gynecol. 1964 Nov 15;90:753–757. doi: 10.1016/0002-9378(64)90938-x. [DOI] [PubMed] [Google Scholar]
- STIMMLER L., BRAZIE J. V., O'BRIEN D. PLASMA-INSULIN LEVELS IN THE NEWBORN INFANTS OF NORMAL AND DIABETIC MOTHERS. Lancet. 1964 Jan 18;1(7325):137–138. doi: 10.1016/s0140-6736(64)92223-8. [DOI] [PubMed] [Google Scholar]
- Spellacy W. N., Gall S. A., Carlson K. L. Carbohydrate metabolism of the normal term newborn: plasma insulin and blood glucose levels during an intravenous glucose tolerance test. Obstet Gynecol. 1967 Oct;30(4):580–583. [PubMed] [Google Scholar]
- Steinke J., Driscoll S. G. The extractable insulin content of pancreas from fetuses and infants of diabetic and control mothers. Diabetes. 1965 Sep;14(9):573–578. doi: 10.2337/diab.14.9.573. [DOI] [PubMed] [Google Scholar]
- YALOW R. S., BERSON S. A. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960 Jul;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]


