Abstract
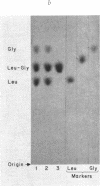
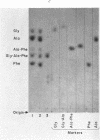
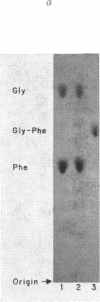
Few studies have been published on peptide hydrolase activities of human small intestine mucosa. We developed methods to screen tissue extracts for such enzymes and to quantitate hydrolase activities for dipeptides containing the aromatic amino acid L-phenylalanine. The screening procedure indicated glycyl-L-proline hydrolase activity was reduced in biopsy specimens from patients with flattened intestinal mucosa. To explore this further, we established optimal assay conditions for hydrolase activities (a) glycyl-L-proline, (b) L-phenylalanyl-L-proline, (c) L-alanyl-L-phenylalanine, and (d) L-phenylalanylglycine. Biopsy specimens from patients with various intestinal disorders, but without flattened mucosa, and from three patients with flattened mucosa, showed a disproportionate reduction in activities (a) and (b), with the reduction being significantly more marked in the latter patients. We suggest that intestinal imidopeptide hydrolase activities, such as (a) and (b), are sensitive to changes in intestinal disease generally, particularly to the altered physiology associated with flattening of the mucosa, and are secondary to, rather than a cause of, the intestinal pathology.
Our finding that intestinal alkaline phosphatase activity tended to parallel imidopeptide hydrolase activity, and that activity (a) was partially localized to the particulate fraction of mucosal homogenate, suggested that imidopeptide hydrolase activities may be located in the microvilli of the intestinal epithelium and that, like alkaline phosphatase activity, they may be reduced in flattened mucosae, in part at least because of the pathologic changes in the microvilli.
In our studies of control subjects we did not detect peptide hydrolase activity deficiency analogous to asymptomatic disaccharidase deficiency.
Full text
PDF


















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATFIELD G. N., MORRIS C. J. Analytical separations by highvoltage paper electrophoresis. Amino acids in protein hydrolysates. Biochem J. 1961 Dec;81:606–614. doi: 10.1042/bj0810606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpers D. H., Isselbacher K. J. Protein synthesis by rat intestinal mucosa. The role of ribonuclease. J Biol Chem. 1967 Dec 10;242(23):5617–5622. [PubMed] [Google Scholar]
- BENSON J. A., Jr, CULVER P. J., RAGLAND S., JONES C. M., DRUMMEY G. D., BOUGAS E. The d-xylose absorption test in malabsorption syndromes. N Engl J Med. 1957 Feb 21;256(8):335–339. doi: 10.1056/NEJM195702212560802. [DOI] [PubMed] [Google Scholar]
- BRANDBORG L. L., RUBIN G. E., QUINTON W. E. A multipurpose instrument for suction biopsy of the esophagus, stomach, small bowel, and colon. Gastroenterology. 1959 Jul;37(1):1–16. [PubMed] [Google Scholar]
- Behal F. J., Asserson B., Dawson F., Hardman J. A study of human tissue aminopeptidase components. Arch Biochem Biophys. 1965 Aug;111(2):335–344. doi: 10.1016/0003-9861(65)90194-3. [DOI] [PubMed] [Google Scholar]
- DAVIS N. C., SMITH E. L. Purification and some properties of prolidase of swine kidney. J Biol Chem. 1957 Jan;224(1):261–275. [PubMed] [Google Scholar]
- Dahlqvist A. Disaccharide intolerance. JAMA. 1966 Jan 17;195(3):225–227. [PubMed] [Google Scholar]
- Friedrich M., Noack R., Schenk G. Zur Lokalisation von peptidatischen und proteolytischen Aktvitäten in isolierten Bürstensäumen aus der Mucosa des Rattendünndarmes. Biochem Z. 1965 Dec 31;343(4):346–353. [PubMed] [Google Scholar]
- Gray G. M. Malabsorption of carbohydrate. Fed Proc. 1967 Sep;26(5):1415–1419. [PubMed] [Google Scholar]
- JENCKS W. P., JETTON M. R., DURRUM E. L. Paper electrophoresis as a quantitative method; serum proteins. Biochem J. 1955 Jun;60(2):205–215. doi: 10.1042/bj0600205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefsson L., Lindberg T. Intestinal dipeptidases. I. Spectrophotometric determination and characterization of dipeptidase activity in pig intestinal mucosa. Biochim Biophys Acta. 1965 Jul 29;105(1):149–161. doi: 10.1016/s0926-6593(65)80183-7. [DOI] [PubMed] [Google Scholar]
- Josefsson L., Lindberg T. Intestinal dipeptidases. IX. Studies on dipeptidases of human intestinal mucosa. Acta Chem Scand. 1967;21(7):1965–1966. doi: 10.3891/acta.chem.scand.21-1965. [DOI] [PubMed] [Google Scholar]
- Josefsson L., Sjöström H. Intestinal dipeptidases. IV. Studies on the release and subcellular distribution of intestinal dipeptidases of the mucosa cells of the pig. Acta Physiol Scand. 1966 May;67(1):27–33. doi: 10.1111/j.1748-1716.1966.tb03283.x. [DOI] [PubMed] [Google Scholar]
- Kowlessar O. D. Effect of wheat proteins in celiac disease. Gastroenterology. 1967 May;52(5):893–897. [PubMed] [Google Scholar]
- LA DU B. N., MICHAEL P. J. An enzymatic spectrophotometric method for the determination of phenylalanine in blood. J Lab Clin Med. 1960 Mar;55:491–496. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindberg T. Intestinal dipeptidases. Dipeptidase activity in the mucosa of the gastrointestinal tract of the adult human. Acta Physiol Scand. 1966 Apr;66(4):437–443. doi: 10.1111/j.1748-1716.1966.tb03221.x. [DOI] [PubMed] [Google Scholar]
- Lindberg T. Intestinal dipeptidases: characterization, development and distribution of intestinal dipeptidases of the human foetus. Clin Sci. 1966 Jun;30(3):505–515. [PubMed] [Google Scholar]
- MESSER M., ANDERSON C. M., TOWNLEY R. R. Peptidase activity of biopsies of the duodenal mucosa of children with and without coeliac disease. Clin Chim Acta. 1961 Nov;6:768–775. doi: 10.1016/0009-8981(61)90164-4. [DOI] [PubMed] [Google Scholar]
- Nordström C., Dahlqvist A., Josefsson L. Quantitative determination of enzymes in different parts of the villi and crypts of rat small intestine. Comparison of alkaline phosphatase, disaccharidases and dipepeptidases. J Histochem Cytochem. 1967 Dec;15(12):713–721. doi: 10.1177/15.12.713. [DOI] [PubMed] [Google Scholar]
- PLOTKIN G. R., ISSELBACHER K. J. SECONDARY DISACCHARIDASE DEFICIENCY IN ADULT CELIAC DISEASE (NONTROPICAL SPRUE) AND OTHER MALABSORPTION STATES. N Engl J Med. 1964 Nov 12;271:1033–1037. doi: 10.1056/NEJM196411122712003. [DOI] [PubMed] [Google Scholar]
- Pittman F. E., Pollitt R. J. Studies of jejunal mucosal digestion of peptic-tryptic digests of wheat protein in coeliac disease. Gut. 1966 Aug;7(4):368–371. doi: 10.1136/gut.7.4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J. B., Eichholz A., Crane R. K. Studies on the organization of the brush border in intestinal epithelial cells. IV. Aminopeptidase activity in microvillus membranes of hamster intestinal brush borders. Biochim Biophys Acta. 1967;135(5):959–965. doi: 10.1016/0005-2736(67)90065-x. [DOI] [PubMed] [Google Scholar]
- WELLNER D., MEISTER A. Crystalline L-amino acid oxidase of Crotalus adamanteus. J Biol Chem. 1960 Jul;235:2013–2018. [PubMed] [Google Scholar]