Abstract
S-box (SAM-I) riboswitches are a widespread class of riboswitches involved in the regulation of sulfur metabolism in Gram-positive bacteria. We report here the 3.0-Å crystal structure of the aptamer domain of the Bacillus subtilis yitJ S-box (SAM-I) riboswitch bound to S-adenosyl-L-methionine (SAM). The RNA folds into two sets of helical stacks spatially arranged by tertiary interactions including a K-turn and a pseudoknot at a four-way junction. The tertiary structure is further stabilized by metal coordination, extensive ribose zipper interactions, and SAM-mediated tertiary interactions. Despite structural differences in the peripheral regions, the SAM-binding core of the B. subtilis yitJ riboswitch is virtually superimposable with the previously determined Thermoanaerobacter tengcongensis yitJ riboswitch structure, suggesting that a highly conserved ligand-recognition mechanism is utilized by all S-box riboswitches. SHAPE (selective 2′-hydroxyl acylation analyzed by primer extension) chemical probing analysis further revealed that the alternative base-pairing element in the expression platform controls the conformational switching process. In the absence of SAM, the apo yitJ aptamer domain folds predominantly into a pre-binding conformation that resembles, but is not identical with, the SAM-bound state. We propose that SAM enters the ligand-binding site through the “J1/2–J3/4” gate and “locks” down the SAM-bound conformation through an induced-fit mechanism. Temperature-dependent SHAPE revealed that the tertiary interaction-stabilized SAM-binding core is extremely stable, likely due to the cooperative RNA folding behavior. Mutational studies revealed that certain modifications in the SAM-binding region result in loss of SAM binding and constitutive termination, which suggests that these mutations lock the RNA into a form that resembles the SAM-bound form in the absence of SAM.
Keywords: riboswitch, S-box, SAM-I, SHAPE, SAM
Introduction
Riboswitches are short cis-acting RNAs that regulate gene expression in response to environmental cues (reviewed in Refs. 1–10). They usually reside in the 5′ untranslated region of bacterial genes or operons and interact directly with effector molecules to regulate downstream gene expression at either the transcriptional or translational level. Bacteria use riboswitches to control vitamin metabolism, nucleotide and amino acid biosynthesis, sulfur metabolism, and metal transport. Rare examples of riboswitches that regulate alternative splicing or mRNA stability have also been found in eukaryotes.11,12
Among more than a dozen distinct riboswitch classes that respond to various metabolites, the S-adenosylmethionine (SAM)-binding S-box (SAM-I) riboswitch class is arguably the most prevalent class,13 especially in Gram-positive bacterial genomes.14 S-box riboswitches were originally identified and extensively studied in Bacillus subtilis, where they control more than 26 genes involved in the import, synthesis, and recycling pathways for methionine or SAM.15–18 The S-box riboswitch is composed of a SAM-sensing “aptamer domain” followed by an “expression platform” to regulate downstream gene through transcription attenuation.15,16,18,19 Alternative base pairing between the aptamer domain and the expression platform is regulated by the intracellular concentration of SAM.16,20 Binding of SAM to the aptamer domain induces a tightly folded aptamer structure, which in turn allows the downstream formation of a transcription terminator to attenuate transcription in most S-box-regulated genes.2,16,17,19,20 The aptamer domain is destabilized in the absence of SAM, which allows the formation of a downstream antiterminator helix that allows RNA polymerase (RNAP) to read through the termination site and produce full-length transcripts. In addition, the SAM-IV class of riboswitches adopt secondary structures similar to that of the S box, but are proposed to regulate at the level of translation initiation; in this case, the terminator helix is replaced by an element that is predicted to sequester the ribosome binding site.21
S-box riboswitches in general are highly sensitive to and selective for the intracellular SAM pool. For example, the B. subtilis yitJ S-box riboswitch exhibits high affinity for SAM (Kd ~20 nM) and strongly discriminates against closely related natural analogues such as S-adenosyl-L-homocysteine (SAH; 100-fold lower) and S-adenosyl-L-cysteine (nearly 10,000-fold lower).16,18 Different S-box elements in B. subtilis vary widely in their sensitivity for SAM, despite the high conservation of the riboswitch sequence and predicted structure, with a 200-fold range of affinity for SAM; genes that are part of the core biosynthetic pathway generally show higher sensitivity than genes encoding methionine transporters, resulting in a hierarchical response to changing SAM pools.20
Phylogenetic and mutagenesis analyses revealed that the S-box aptamer domain consists of four helical segments organized around a four-way junction, and P2 and P4 were shown to participate in formation of a pseudoknot.15,17,22 The crystal structure of a 94-nt yitJ SAM-I (S box) riboswitch from the thermophilic bacterium Thermoanaerobacter tengcongensis has been reported.23,24 It showed that the phylogenetically conserved helices organize into two sets of coaxial helices packed against each other at a tilted angle and enclosing SAM at the helical packing interface.23,24 Mutagenesis studies on the T. tengcongensis yitJ riboswitch further confirmed that the riboswitch distinguishes SAM from SAH by specifying the positively charged sulfonium ion in SAM.24 Recent structural and biochemical studies on the apo T. tengcongensis yitJ riboswitch aptamer domain revealed a number of closely related, pre-organized conformational states in the presence of magnesium ion,25 suggesting the presence of a “sensing phase” during transcription and riboswitch folding. In this study, we combine crystal structure determination, selective 2′-hydroxyl acylation analyzed by primer extension (SHAPE) chemical probing, and mutagenesis to study the B. subtilis yitJ S-box aptamer RNA, which is the S-box riboswitch that has been most carefully characterized in biochemical and genetic studies.15,16,17,22 Our structure revealed that the ligand-recognition mechanism is highly conserved among the S-box riboswitch family. We further conclude that the alternative base-pairing element in the expression platform controls the conformational switching process, and SAM is recruited to the ligand-binding site through the “J1/2–J3/4” gate and “locks” the S-box SAM-binding core through an induced-fit mechanism.
Results
Comparison of the B. subtilis and T. tengcongensis yitJ riboswitch structures
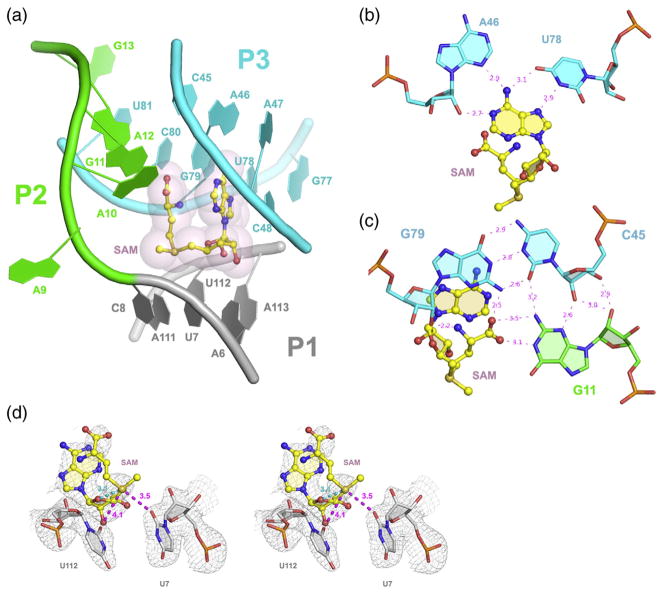
The overall architecture of the 119-nt B. subtilis yitJ S-box RNA is similar to that of the 94-nt T. tengcongensis riboswitch (PDB code: 2GIS, 3GX5),23,24 despite a large deviation in the equivalent phosphorous atoms (r.m.s.d.=2.5 Å; Fig. 1a). Similar to the T. tengcongensis yitJ RNA, the four helices in the B. subtilis yitJ S-box aptamer assemble into two sets of coaxial helices, P1/P3 and P2/P4 (Fig. 1b). These superhelices then pack against each other at an ~70° tilted angle through extensive minor-groove ribose zipper interactions (described below), a K-turn motif in the middle of P2 that bends its distal portion ~120° to wrap around P1/P3, and a pseudoknot in the distal loop of P2 and J3/4 that weaves the P1/P3 and P2/P4 into a rigid conformation. The distal portion of the P3 helix, which is significantly longer in the B. subtilis yitJ structure, packs against the P4 helix at an average distance of 6 Å (Fig. 1b).
Fig. 1.
Crystal structure of S-box (SAM-I) RNAs bound to SAM. (a) Phosphorous alignment of the structure between the B. subtilis (gray) and T. tengcongensis yitJ riboswitches (blue).23 (r.m.s.d.=2.5 Å) (b) Secondary structure diagrams of the B. subtilis yitJ riboswitch. Helices P1 through P4 are colored in gray, green, cyan, and yellow, respectively. Red dashes denote pseudoknot base pairs. SAM-contacting bases are labeled in magenta. The sheared A46·U78 base pair that recognizes the adenosine base of SAM is labeled with a red dot. (c) Ribbon representations of the B. subtilis yitJ RNA structure. The two views represent a front view (left) and a 90° rotation (right). The color scheme is the same as that in (b). SAM is shown as a ball-and-stick model overlaid with surface representations in gray. Two assigned magnesium metal ions are shown in pink.
The mesophilic B. subtilis yitJ structure displays a wide distribution of temperature B-factors for each nucleotide, which is a good indicator of local thermal motion/conformational flexibility in the RNA (Supplemental Fig. 1). This is in sharp contrast to the relatively even distribution of B-factors in the thermophilic T. tengcongensis yitJ structure23,24 and likely reflects the adaptation of each riboswitch to its native environment. In the B. subtilis yitJ structure, nucleotides at the SAM-binding core and the SAM molecule itself exhibit a much lower average temperature factor (BAVG =35) than that of the overall structure (BAVG =87; Supplemental Fig. 1). Certain peripheral regions of the B. subtilis yitJ RNA, such as those in the loop of P4 and J2/3, display significantly higher temperature factors (>150; Supplemental Fig. 1). While the absolute temperature factor is impacted by both thermal motion and the order of the crystal lattice, the relative distribution of B-factors is a good indicator of the thermal motions within a macromolecular structure. Therefore, higher overall B-factors in the peripheral regions (including the P4 terminal loop, distal P1, and the K-turn region in P2) suggest that these regions are much more flexible than the SAM-binding core. As shown later, this conclusion is in good agreement with that obtained from a temperature-dependent SHAPE analysis.
The B. subtilis yitJ SAM-binding pocket is almost superimposable with that of the T. tengcongensis yitJ riboswitch
While significant structural differences can be found in the peripheral regions of the two S-box riboswitches, the B. subtilis yitJ RNA geometry at its SAM-binding core displays striking similarity to the T. tengcongensis yitJ RNA (r.m.s.d. of 0.37 Å for alignment of the equivalent phosphorous atoms), suggesting that riboswitches in this family use a conserved mechanism to recognize SAM. Inside the B. subtilis yitJ SAM-binding pocket, SAM adopts the same compact, U-shaped conformation seldom seen in protein-bound structures to allow the parallel stacking of its methionine moiety on top of the adenosine ring (Fig. 2a). The Watson–Crick and Hoogsteen edges of the adenosine base of SAM are collectively recognized by four hydrogen bonds in a universally conserved, sheared A46·U78 pair (Fig. 2b). The methionine moiety of SAM makes four hydrogen bond contacts with the G79-C45·G11 base triple, which is part of the structure responsible for tying together J1/2 and P3 (Fig. 2c).
Fig. 2.
SAM-binding pocket of the B. subtilis yitJ riboswitch. (a) The U-shaped SAM molecule sits at the four-way junction between J2/3 and J1/2. (b) A sheared A46·U78 pair recognizes the Watson–Crick and Hoogsteen faces of the adenosine moiety of SAM. (c) The G79-C45·G11 base triple recognizes the methionine tail of SAM. (d) Stereo view of the electrostatic interactions between U7/U112 with the sulfonium ion. Also note the ~3.0-Å intramolecular interaction between the sulfonium ion and O4′ of the ribose moiety of SAM. All labeling and color schemes are the same as those in Fig. 1b and c. The 3.0-Å simulated annealing omit map is contoured at 1.0 σ. Distances are given in angstrom in magenta. Carbon, oxygen, nitrogen, sulfur, and phosphorus atoms are colored gray, red, blue, gold, and orange, respectively.
The positively charged sulfonium group in SAM is specified by a pair of electrostatic interactions with the O4 carbonyl oxygen of U7 and U112 (3.5and 4.1 Å away, respectively; Fig. 2d, distances shown in magenta). This arrangement is similar to that in the wild-type T. tengcongensis yitJ RNA structure23 and almost identical with that in the A94G/U34C T. tengcongensis yitJ mutant. The importance of this electrostatic interaction has been demonstrated previously by U7C and U112C mutations, which eliminate the two interacting carbonyl groups and severely impaired SAM binding in T. tengcongensis yitJ RNA.24 In addition to the RNA–SAM interactions, the sulfonium ion makes a very strong ~3.0-Å intramolecular electrostatic interaction with the O4′ of the ribose in SAM (Fig. 2d, distance shown in cyan).26,27 The methyl group on SAM does not directly contact the riboswitch and orients toward the solvent. Comparison of the S-box structures to those of the SAM-II and SMK box (SAM-III) SAM-binding riboswitches (Refs. 23,24,26,27 and this study) demonstrates that all three classes of SAM riboswitches use a similar mechanism to selectively recognize the positively charged sulfonium ion in SAM and discriminate against the near-cognate ligand SAH, which bears a neutral sulfide in place of sulfonium ion, via favorable inter- and intra-molecular electrostatic interactions with the sulfonium ion, without direct contact with the S-methyl group. There is no conserved specific contact for the Cβ and Cγ atoms in the methionine moiety of SAM, and their corresponding electron densities are significantly weaker due to thermal motions.
A set of conserved tertiary interactions zip up the SAM-binding site
In addition to the K-turn and pseudoknot interactions previously reported in the T. tengcongensis yitJ structure (and previously predicted by phylogenetic, genetic and biochemical analyses15,17,22), we report additional sets of tertiary interactions that stabilize the B. subtilis yitJ tertiary structure, apparently independent of the SAM molecule (Fig. 3a and b). Surrounding the SAM-binding pocket are several clusters of conserved ribose zippers (Fig. 3a, red bases). One set of these interactions is responsible for adenine Hoogsteen face recognition and defines the back and right side of the SAM-binding pocket, involving the ribose between nucleotides (nt) 112–114 of P1 and nt 77–80 of P3 (Fig. 3b, blue strands). A similar set of ribose zippers are above the methionine portion of the SAM-binding pocket (Fig. 3b, red strands), where interactions between G44, C45, G11, and A12 weave J1/2 together with J3/4 and position G11 for recognition of the carboxyl group in SAM. A magnesium ion, together with J1/2 and J3/4, seals the methionine side of the binding pocket (left side as shown in Fig. 3b), and our SHAPE probing data suggest that this side of the binding pocket is likely to be important for SAM recruitment in the B. subtilis yitJ RNA (see below). Last but not least, the conserved C48 residue makes two hydrogen bonds through its O2 and O2′ with A113, closing off the adenosine side of the binding pocket. This extensive network of ribose-mediated hydrogen bonds seals the back, top, right, and left portions of the binding pocket in an apparently SAM-independent fashion, suggesting that the aptamer domain of the B. subtilis yitJ S-box RNA may pre-assemble into a conformation similar to that observed in the crystal structure in a SAM-independent manner.
Fig. 3.
Stereo view of important SAM-independent tertiary interactions within the B. subtilis yitJ RNA. The orientation of the S-box RNA structure is the same as that in Fig. 2. In this view, the SAM molecule is overlapped with the letter U, with the adenosine on the right and the methionine on the left. (a) Distribution of ribose zippers in the S-box RNA. Blue, interactions near the binding pocket; pink, interaction stabilizing the K-turn in P2; cyan, interaction orienting the P2 pseudoknot. Boxed interactions around the binding pocket are shown in greater detail in (b). (b) Zoom-in view of the SAM-binding pocket. The interactions are further divided into three groups, colored in slate, orange, and purple to represent their respective locations within the binding pocket. Red dashes are the important interactions involved. The backbone color scheme is the same as that in Fig. 1a and b.
In addition to stabilizing the SAM-binding pocket, ribose-mediated hydrogen bonds also help to stabilize the P2/P4 pseudoknot (Fig. 3b, cyan bases). The A84-C86 turn directly positions A83 and C86 for pseudoknot base paring, while the Watson–Crick face of A82 interacts with O2′ and O2 of C30 in the distal P2 loop. O2′ of A109 interacts with N1 of A24, further stabilizing the pseudoknot. These SAM-independent interactions are also present in the T. tengcongensis yitJ structure, but are not discussed in this article.23,24
A magnesium ion mediates key ligand-induced conformational changes
Two inner-sphere coordinated magnesium ions (Ma and Mb) were assigned with confidence in our 3.0-Å resolution B. subtilis yitJ S-box structure. One of the magnesium ions, Ma, is located near the methionine tail side of the SAM-binding pocket and makes two inner-spherical coordination with phosphoryl oxygen atoms from A10 and U65, as well as four outer-spherical interactions with N7 of A10, O6 and N7 of G11, and the phosphoryl oxygen of A83 (Fig. 4). Ma serves the important function of tying together the J3/4 U-turn and J1/2 to stabilize the coaxial stacking of P1 and P4 and facilitates formation of the pseudoknot by precisely orienting three of the four pseudoknot base pairs. The Ma site is conserved in the two yitJ riboswitch structures23,24; a divalent ion binds to the equivalent location in the T. tengcongensis yitJ riboswitch and makes the identical interactions with surrounding residues including the conserved A10 and G11 residues (Fig. 4). Interestingly, A10 appears to be conserved solely for the purpose of participating in the Ma binding pocket. This magnesium-mediated junction in the B. subtilis yitJ riboswitch also contains an A9/A84 dinucleotide stack, which is not evident in any of the T. tengcongensis yitJ riboswitch structures.23,24 Our SHAPE probing data (see below) suggest that this metal-ion site could potentially serve as a door to the SAM entry site and secure the SAM pocket with J1/2 and J3/4 synergistically as the door is closed. This result is in accordance with recent studies on the apo T. tengcongensis yitJ riboswitch,25 which requires sufficient divalent magnesium ion to fold into ligand-sensing tertiary structures. Such metal-induced tertiary interactions are prevalent in riboswitches, as also recently illustrated in the apo TPP riboswitch.28
Fig. 4.
Magnesium ion Ma mediates tertiary folding of the B. subtilis yitJ RNA. Ma ties the J3/4 U-turn and J1/2 together, sealing the SAM-binding site at the methionine tail end. The labeling and base color scheme is the same as that in Fig. 1b and c. The 3.0-Å simulated annealing omit map is contoured at 1 σ. Distances are given in angstrom in red.
The S-box aptamer adopts a pre-binding conformation that is revealed upon uncoupling from the expression platform
A key open question is the nature of the conformational switching mechanism that is coupled with the ligand-recognition event. To address this, SHAPE analysis, which detects local RNA flexibility through the extent of ribose 2′-OH alkylation, was used to compare the conformation of the B. subtilis yitJ RNA, with or without the 3′ expression platform (i.e., the terminator sequence), in the presence or absence of SAM.
In the first set of experiments, the aptamer domain of the B. subtilis yitJ S box was subjected to SHAPE probing in the presence or absence of SAM. The SHAPE reactivity of the SAM-bound aptamer domain correlates very well with our crystal structure, in that the residues involved in secondary and tertiary structure formations all displayed low SHAPE reactivity, whereas distal loops and single-stranded residues in the P2 K-turn (G19, A20, A33), P3 bulge (G53-A55), and P4 terminal loop (C96) all displayed higher-than-average SHAPE reactivity. These results indicate that our crystal structure is a good depiction of the SAM-bound S-box aptamer domain conformation in solution. Interestingly, SHAPE analysis also revealed that the S-box aptamer domain conformation in the absence of SAM was largely similar to the SAM-bound conformation. This well-folded “pre-binding” conformation, however, contained noticeable differences in discrete regions, most prominent at J1/2, J3/4, and nt A46-A47. These residues are involved in either critical SAM-dependent tertiary contacts (J1/2, J3/4) or direct SAM recognition (nt A46-A47; Fig. 5, right panel). The J1/2 and J3/4 data are particularly interesting, as they suggest the presence of a J1/2–J3/4 gate that is open in the absence of SAM but closes in the presence of SAM. The overall similarity between the pre-binding and liganded aptamer conformations strongly suggests that in the absence of the expression platform, SAM-independent tertiary interactions are strong enough to pre-organize the S-box aptamer domain into a well-folded state largely ready for ligand binding. The discrete conformational differences between the pre-binding and SAM-bound states revealed further conformational changes induced by SAM binding, which suggests that ligand recognition is likely to utilize an induced-fit mechanism. Since the folding of RNA is generally several orders of magnitude faster than transcription (milliseconds versus seconds),29–31 the likely scenario is that the B. subtilis yitJ S-box aptamer domain adopts a pre-binding conformation before the terminator sequence is transcribed, thereby providing a window of opportunity for SAM binding to attenuate transcription (see Discussion).
Fig. 5.
SHAPE mapping of conformational differences between unliganded and liganded B. subtilis yitJ riboswitches. Left panel, profile of the full-length RNA construct containing both the aptamer domain and the expression platform; right panel, B. subtilis yitJ aptamer domain only. In both panels, lane 1, dideoxy sequencing ladder revealing adenosines (runs 1 nt higher than the actual corresponding base position); lane 2, RNA+Mg2+; lane 3, RNA+ Mg2++SAM; lane 4, RNA alone (no 1M7) background. Nucleotide numbers are marked on the left and structural motifs are marked on the right. The color scheme of secondary structure elements is the same as that in Fig. 1. Prominent SHAPE reactivity differences between SAM-bound and unbound aptamer structures are highlighted by asterisks on the right.
A parallel set of SHAPE probing experiments was carried out on full-length B. subtilis yitJ riboswitch RNA, including the expression platform. The results suggested that the presence of SAM induces the formation of a well-folded aptamer domain and the terminator stem–loop, consistent with its role in transcription attenuation. In the absence of SAM, however, a drastically less protected reactivity pattern was found in the aptamer domain of the full-length yitJ RNA (Fig. 5, left panel, lane 2). This was especially apparent at the P1 stem, J1/2, P2 K-turn, J2/3, J3/4, part of P3, and P4 pseudoknot. Downstream of the expression platform region, SHAPE revealed that the transcription terminator stem–loop was replaced by a rather flexible structure pattern in the absence of SAM. We could not clearly define the transcription antiterminator stem–loop, but it is likely that the SHAPE reactivity reflects partial formation of this element15 (data not shown). Therefore, we propose that SAM-induced conformational switching is dependent on the presence of the expression platform, which alters the free-energy folding landscape such that in the absence of SAM, an alternatively base-paired conformation involving the participation of the expression platform is significantly more stable than the pre-binding aptamer conformation. As a result, the presence of the expression platform in the RNA strongly influences the folding pattern of the aptamer domain in the absence of SAM.
Mutational analysis of the B. subtilis yitJ riboswitch
Structural predictions were tested by mutation of key residues and testing for effects on SAM binding and transcription termination in vitro. Mutation of residue G11, which participates in the G79-C45·G11 base triple that ties together J1/2 and P3 and contacts the methionine moiety to A, C, or U, resulted in loss of detectable SAM binding (Table 1), consistent with its position in the crystal structure. Surprisingly, each of these mutations resulted in increased transcription termination in the absence of SAM, with the most dramatic effect exhibited by the G11C mutant, which exhibited >80% termination in the presence or absence of high SAM (140 μM; in contrast, the wild-type construct exhibits a maximal response at 0.2 μM SAM). The high termination efficiency in the absence of SAM suggests that alteration of G11 facilitates formation of an RNA structure resembling the SAM-bound form even in the absence of SAM. The G11A substitution had only a modest effect on termination in the absence of SAM, despite complete loss of SAM binding, indicating that the structural change that results in the stabilization of RNA into a form similar to the ligand-bound form is separable from the effects on SAM binding. Mutations of the other residues that participate in the base triple (G79 and C45) had a similar effect, suggesting that any disruption of the base triple causes a surprising stabilization of the aptamer domain structure.
Table 1.
Mutational analysis of the B. subtilis yitJ S-box riboswitch
| yitJ construct | Transcription termination (%)a |
SAM binding (Kd) (μM) | |
|---|---|---|---|
| −SAM | +SAM | ||
| Wild-type | 29 | 99 | 0.019 |
| G11A | 49 | 57 | NDb |
| G11C | 81 | 83 | ND |
| G11U | 70 | 70 | ND |
| G79A | 89 | 90 | ND |
| G79C | 90 | 90 | ND |
| G79U | 89 | 88 | ND |
| C45A | 90 | 95 | ND |
| C45G | 84 | 83 | ND |
| C45U | 99 | 99 | ND |
| A46C | 92 | 100 | ND |
| A46G | 82 | 84 | ND |
| A46U | 90 | 99 | ND |
| U78A | 87 | 96 | |
| U78C | 81 | 92 | |
| U78G | 89 | 89 | ND |
| A47C | 82 | 90 | |
| A47G | 71 | 98 | |
| A47U | 82 | 97 | |
| C48A | 62 | 100 | |
| C48G | 75 | 96 | |
| C48U | 85 | 100 | |
| G77A | 85 | 100 | |
| G77C | 72 | 94 | |
| G77U | 83 | 83 | |
| U85A | 46 | 91 | |
| U85C | 42 | 93 | 4.2 |
| A109C | 19 | 98 | |
| A109G | 19 | 92 | 2.1 |
| U85C/A109G | 27 | 99 | 1.1 |
| A111G | 15 | 43 | 4.2 |
| U7G/A111U | 12 | 68 | 0.56 |
| U7G/A111C | 88 | 97 | 0.41 |
Efficiency of transcription termination in vitro in the absence (−SAM) or presence (+SAM) of SAM (140 μM).
ND, not detectable (Kd >200 μM).
The A46·U78 pair, which forms a base triple with the adenine ring of SAM, was also highly sensitive to mutation and exhibited a pattern similar to that of the G79-C45·G11 region (Table 1). Mutation of the A47·C48-G77 residues that further stabilize the adenine ring of SAM also resulted in high termination in the absence of SAM; however, several substitutions at these positions (notably C48A and C48G) retained a partial response to high SAM, indicating that SAM binding was not affected as strongly. In contrast, mutation of the U85-A109 pair that supports the pseudoknot did not significantly affect termination in the absence of SAM. Combination of the U85C and A138G mutations did not suppress the effect of either single mutation, which shows that these residues exhibit sequence specificity in addition to their ability to base pair, consistent with the role of A139 in the formation of a base triple with A24 adjacent to the pseudoknot.
We also tested the effect of mutations at the U7-A111 pair in the P1 helix, which recognizes the sulfonium ion. An A111G mutation, which places a U-G pair at this position, did not significantly affect termination in the absence of SAM and did not obliterate the response to SAM, although the affinity for SAM was greatly reduced (>200-fold increase in Kd; Table 1). Replacement of the U-A pair with a G-U (U7G/A111U) resulted in a response similar to that of the A111G mutant, although the affinity for SAM was approximately 10-fold higher. In contrast, the U7G/A111C mutant, in which the U-A pair is replaced with a G-C, exhibited high termination in the absence of SAM, but SAM binding was reduced only 20-fold relative to wild-type. In this case, the mutation may simply stabilize the P1 helix, thereby inhibiting antiterminator formation and promoting termination. Stabilization of an RNA conformation similar to the SAM-bound form is therefore separable from the effects on SAM binding, as noted above for the G11A mutation.
Thermal melting of the S-box RNA bound to SAM reveals differential stability of structural elements
The SAM-bound S-box RNA is a compact, well-ordered structure that encompasses many tertiary interactions such as a pseudoknot, ribose zippers, Mg-mediated strand couplings, as well as SAM-dependent tertiary interactions. Therefore, it is likely that the folding/refolding process of the SAM-bound S-box RNA is highly hierarchical. We employed a temperature-dependent SHAPE analysis to obtain thermal stability information for the B. subtilis yitJ riboswitch to study differential stability among the different domains of the RNA.
The B. subtilis yitJ SAM-bound S-box aptamer domain displays remarkable thermal stability, as almost all of the tertiary interactions remain intact even at 50 °C. Toward the higher end of the temperature gradient, about 80% of the structure became more flexible (Fig. 6a). However, in contrast to the extremely stable thermophilic T. tengcongensis yitJ RNA,28 the B. subtilis yitJ riboswitch showed differential melting points among its structural elements. The temperature melting behavior of individual bases was quantified and clustered according to the secondary structures in which they reside. Surprisingly, many tertiary structures appear to be more stable than the secondary structures. For example, helices P1 and P4 melted at intermediate temperatures, with a transition Tm of ~56 °C. In contrast, J3/4 unfolded with a high transition Tm of ~58 °C; the pseudoknot structure unfolded with an even higher transition Tm of 65 °C; and J1/2 and J2/3 did not show signs of unfolding at the higher temperature range of 70 °C (Fig. 6b). The hierarchical melting pathway of the B. subtilis yitJ S-box riboswitch is different from that of tRNA, in which the tertiary interactions disintegrate before the secondary structure.32 Here, the strong tertiary interactions responsible for stabilizing the B. subtilis yitJ aptamer core, both ligand induced (e.g., J1/2 and J2/3) and ligand independent (e.g., the pseudoknot), remain intact before some of the peripheral secondary structure elements (e.g., the P1 helix) disintegrate. Despite the relatively earlier melting of peripheral helices, the SAM-binding core is likely to be held together by several individual bases in P1 and P4 that appeared to unfold at much higher Tm (marked with asterisk in Fig. 6, left panel). This observation is consistent with the structural analysis showing that the nucleotide temperature B-factors in the SAM-binding core are much lower than that in the peripheral region (Figs. 2b and 6b, upper panels). Assuming that folding of the B. subtilis yitJ aptamer domain is thermodynamically reversible, our results also suggest that folding of the B. subtilis yitJ riboswitch is cooperative and that many of the tertiary interactions form first due to higher stability and possibly guide the formation of some of the secondary structures. This folding scheme seems to be consistent with a 2-AP study,33 which showed that the pseudoknot and K-turn structure guide the folding of the B. subtilis yitJ riboswitch.
Fig. 6.
Hierarchical temperature induces unfolding of the B. subtilis yitJ aptamer domain as revealed by SHAPE. (a) Temperature-dependent SHAPE analysis of the B. subtilis yitJ aptamer domain in the presence of SAM. RNA structures are indicated on the right. Lane 1, dideoxy sequencing ladder revealing adenosine positions. Temperatures are indicated on top. (b) Quantified unfolding profiles grouped by domains. Profiles were computed by quantifying the intensities shown in (a) by fragment analysis and processing by ShapeFinder program.45 Data are plotted on a linear scale; continuous blue line indicates average behavior. Nucleotides at pseudoknot, J3/4, and P1+P4 were fit to a unimolecular transition equation.
Discussion
Through comparison of B. subtilis and T. tengcongensis yitJ riboswitch structures, we conclude that despite the differences in their surface and peripheral regions, they utilize a virtually identical mechanism to recognize the SAM molecule because the conformations of SAM and surrounding RNA residues involved in SAM recognition are virtually superimposable. We also revealed that conserved SAM-dependent and SAM-independent tertiary interactions are likely to play important roles in stabilizing the structural integrity as well as the conformational dynamics. The full-length riboswitch, containing the aptamer domain and the expression platform, clearly switches its conformation in a SAM-dependent fashion. In contrast, in the absence of the expression platform, the aptamer domain of the B. subtilis yitJ riboswitch folds into a SAM-bound-like conformation even without SAM. A similar result was observed from the apo T. tengcongensis yitJ aptamer domain, which samples several defined tertiary structures similar to that of the SAM-bound state.25 This suggests that the SAM-independent interactions noted above are strong enough to direct the folding of the aptamer domain into a pre-binding conformation. We propose that this pre-binding conformation has a pre-organized binding pocket, with an open gate at the J1/2–J3/4 region near the methionine side of the SAM-binding pocket. SAM entry is coupled with closure of the J1/2–J3/4 gate, which induces the SAM-bound conformation by chelating a divalent ion (Ma) and positioning G11 in J1/2 for SAM recognition. This hypothesis is supported not only by the structural identification of SAM-independent tertiary interactions but also by the SHAPE analysis revealing localized conformational movement in J1/2–J3/4 in the B. subtilis yitJ aptamer domain. In addition, formation of the pre-binding state is consistent with molecular dynamics simulations34 and structural and biochemical studies28 performed on the aptamer domain of the T. tengcongensis and B. subtilis yitJ riboswitch, which did not detect a globally unstructured yitJ aptamer domain conformation in the absence of SAM.
We also characterized a set of mutations within the SAM-binding domain. Most of these mutations disrupted SAM binding, as expected for mutations of residues that make important contacts with SAM or that are important for stabilization of crucial structural domains that form the SAM-binding pocket. The more surprising mutations were those that resulted in high transcription termination in the absence of SAM, suggesting that they may stabilize a structural arrangement in the aptamer domain similar to the SAM-bound form. Several of these mutants retained the ability to respond to SAM, indicating that formation of this conformation in the absence of SAM is separable from the ability to bind SAM. A better understanding of how these mutations preferentially stabilize the pre-binding state awaits further structural analysis.
In summary, our chemical probing data are consistent with a transcription-dependent conformational switching model for the B. subtilis yitJ S-box riboswitch (Supplemental Fig. 2). RNAP first transcribes the aptamer domain of the S-box RNA, which immediately folds into the pre-binding conformation before transcription of the downstream terminator sequence. It was estimated that it takes less than 100 ms for the riboswitch to fold,29,30 compared to the transcription rate of ~40 nt/s by bacterial RNAP.31 Therefore, the aptamer domain of yitJ has ample time to fold into the pre-binding conformation before RNAP transcribes the expression platform. This pre-binding aptamer conformation has two alternative fates: (1) If the intracellular SAM level is high, the aptamer structure is greatly stabilized by SAM-mediated tertiary interactions and cannot be competed away by the subsequent emergence from RNAP of the antiterminator sequence, leading to terminator hairpin formation and transcription termination. (2) If the intracellular SAM level is low, the aptamer structure collapses owing to competing base-pair formation from the expression platform; the antiterminator hairpin forms, which sequesters sequences that would otherwise participate in the formation of the terminator helix; and transcription continues to produce the full-length transcript (Supplemental Fig. 2). Either decision by the RNAP is irreversible; therefore, S-box transcription riboswitches are not under selection pressure to be able to switch from the SAM-bound to the unfolded conformation. The B. subtilis yitJ S-box RNA–SAM complex is very stable in vitro, with a half-life of >7 min,20 consistent with the model that folding of the RNA into the SAM-free versus SAM-bound form as it emerges from RNAP represents a brief opportunity for the ligand to bind and dictate whether RNAP will terminate or continue past the termination site. This “window-of-opportunity” model would not work if the rate of transcription is faster than that of aptamer domain folding.
The observation that certain sequence changes within the aptamer domain not only block SAM binding but also promote formation of a structure that resembles the SAM bound form is similar to previous observations with the Thi-box riboswitch.35 The identification of mutations of this type indicates that the evolution of a riboswitch capable of an effective regulatory response is subject to multiple constraints. The aptamer domain must have binding affinity appropriate to the physiological pools of the ligand that is detected and selectivity against biologically relevant analogues. Regulatory function also requires that the aptamer domain folds into a conformation in the absence of ligand that is capable of ligand recognition but fails to completely mimic the ligand-bound form, allowing a ligand-dependent structural rearrangement that can be coupled to gene regulation. Subtle sequence changes that cause the RNA to shift into a conformation resembling the ligand-bound form will inappropriately trigger the ligand-dependent gene regulation response.
Materials and Methods
RNA preparation for crystallization
The aptamer domain of the B. subtilis yitJ S-box riboswitch (nt 29–146) was inserted into a pUC19 plasmid under the control of a T7 RNAP promoter; the hepatitis delta virus ribozyme was positioned at the 3′-end to ensure a homogeneous 3′-endpoint after ribozyme cleavage. RNA was prepared by a standard T7 RNAP run-off transcription reaction using linearized plasmid DNA as template and purified by denaturing gel electrophoresis as described.36 SAM was purchased from Sigma and stored as a 100 mM stock solution at pH 6.0 at −80 °C until immediately before use.
Before crystallization, the RNA was refolded by heating up to 65 °C for 10 min in a buffer containing 25 mM Tris–HCl (pH 7.5), 40 mM NaCl, and 5 mM MgCl2, at which point SAM was added to a final concentration of 1 mM. The solution was allowed to cool gradually to 20 °C over a course of 1 h and stored frozen at −80 °C. The wild-type S-box aptamer domain constructs produced crystals that diffracted only to ~6 Å resolution. The distal P3 loop was found to have undergone spontaneous cleavage during crystallization (data not shown). This observation led to the design of three constructs in which the flexible stem–loop was replaced with a stable GAAA tetraloop and the base pairs below the tetraloop were systematically shortened; these constructs retained wild-type SAM binding activity. One of the resulting constructs, designated TL5, produced a rare crystal form [in 40 mM sodium cacodylate (pH 7.0), 80 mM strontium chloride, 15% methyl-2,4-pentanediol, and 2 mM spermine] after prolonged incubation, which diffracted X-rays to a resolution of ~3 Å. The crystals were soaked in increasing concentrations of methyl-2,4-pentanediol (up to 25%) in the presence of 1 mM freshly prepared SAM and then flash-frozen in liquid nitrogen.
Molecular replacement and structure refinement
A 3-Å data set was collected from a TL5 crystal at beamline F1 at Cornell High Energy Synchrotron Source (CHESS). We solved the structure with the molecular replacement program PHASER,37 using the core T. tengcongensis yitJ structure23 (PDB code: 2GIS), excluding SAM and the distal portion of the P3 helix, as the search model. The P3 helix was later located by PHASER37 using a 17-bp ideal A-form helix capped by a GAAA tetraloop generated by COOT.38 Repeated rounds of density modification with model-phase inputs, rigid-body refinement, and harmonically restrained simulated annealing refinement procedures in CNS39,40 reduced the initial-phase errors and allowed tracing of most of the sugar–phosphate backbone (Table 2). Phylogenetically conserved base pairing was reinforced by specifying Watson–Crick base pairs throughout CNS refinement.39,40 To further reduce the model bias and phase errors, the original search model was refined for several rounds as a poly-cytosine model, which produced an electron density map of sufficient quality to distinguish purines from pyrimidines. This map allowed accurate modeling of bases in COOT38 and restrained refinement in Refmac541 until the model was ~93% complete and Rfree was below 35%. The complete model was generated after iterative cycles of group B-factor refinement, energy minimization, and simulated annealing in CNS.39,40 The SAM molecule, two divalent metal ions, and eight water molecules were identified in the Fo −Fc difference density map and refined using real-space fitting in COOT.38 The entire structure was verified using simulated annealing composite-omit maps by excluding 5% of the model in each calculation.39,40 The final model includes all but four flipped out nucleotides in the P3 stem and the P4 loop with a Rwork/Rfree of 0.236/0.286.
Table 2.
Data collection and refinement statistics
| Data collection | CHESS F1 |
|---|---|
| Space group | P3121 |
| Unit cell parameters | |
| a, b, c (Å) | a=58.732, b=58.732, c=204.091 |
| α, β, γ (°) | α=90, β=90, γ=120 |
| Wavelength (Å) | 0.9181 |
| Resolution (Å) | 20–3.0 |
| Redundancy [overall (outer shell)] | 4.0 (2.5) |
| Average I/σ(I) | 8.0 (1.9) |
| Reflections | 8152 (931) |
| Completeness (%) | 94.4 (89.4) |
| Rsyma | 0.12 (0.43) |
| Refinement | |
| Resolution (Å) | 20–3.0 |
| No. of reflections used in the working set | 7350 |
| No. of reflections used in the testing set | 802 |
| Average B-factor (Å2) | 72.4 |
| Rwork (%)b | 23.6 |
| Rfree (%)b | 28.6 |
| r.m.s.d. bond lengths (Å) | 0.007 |
| r.m.s.d. bond angles (°) | 1.57 |
Rsym = Σ|I−〈I〉|/ΣI, where I is the observed intensity and 〈I〉 is the statistically weighted absolute intensity of multiple measurements of symmetry-related reflections. ‡‡§
R=Σ|Fo −k|Fc||/Σ|Fo|, Rwork from the working set and Rfree from the test set.
RNA preparation for SHAPE analysis
The B. subtilis yitJ S-box RNA with or without the 3′ expression platform (containing the complete antiterminator and terminator sequences) was inserted into a SHAPE cassette containing a 5′ T7 RNAP promoter and a 3′-reverse transcription primer site by overlapping PCR and then cloned into a pJet cloning vector (from Fermentas). After sequence verification, the SHAPE cassette DNA templates were PCR amplified using terminal primers and used directly to produce RNAs for SHAPE analysis by in vitro transcription as described.27 The RNA product was purified and recovered by denaturing polyacrylamide gel electrophoresis as described.36 All RNAs were subsequently diluted into RNA folding buffer [111 mM Hepes (pH 8), 6.7 mM MgCl2, 111 mM NaCl] to ~0.5 μM. Immediately prior to chemical probing, the diluted RNA was refolded as previously described27 in the presence or absence of SAM during the annealing process.
SHAPE chemical probing analysis
SHAPE chemical probing was performed as described previously.32,42,43 Using temperature-dependent SHAPE analysis as an example, 15 μL of the S-box RNA (0.3–1.0 μM, refolded ±SAM) was pre-equilibrated using a gradient PCR machine to the desired temperature (20, 30.0, 33.8, 43.9, 48.4, 50.0, 52.0, 53.3, 58.7, 58.9, 63.0, 66.4, or 68.6 °C) for 1 min and subsequently mixed with 1.5 μL of 100 mM electrophile 1M7 to initiate 2′-ribose hydroxyl alkylation. The reactions were allowed to proceed to completion at each temperature point by incubating for five half-lives of 1M732,44; the reaction was then quenched with 900 μL of precipitation buffer containing 80% EtOH, 45 μM NaCl, 0.45 μM EDTA (ethylenediaminetetraacetic acid), and 2 μL glycoblue (15 mg/mL; Ambion, Inc). The RNA was precipitated by incubation at −80 °C for 30 min, followed by centrifugation at 16,000g at 4 °C for 45 min. The pellet was Air-dried and resuspended in 12 μL of 0.5× TE buffer.
The primer extension protocol was similar to that described previously.42 A fluorescently labeled DNA primer (HEX-GAACCGGACCGAAGCCCG; 1 μL, 50 μM) was annealed to the recovered RNA (12 μL) by incubation at 65 °C for 2 min, then 35 °C for 5 min, followed by snap cooling to 4 °C for another 2 min. The resulting 13 μL of RNA–primer mixture was mixed with 6 μL of reverse transcription buffer [167 mM Tris (pH 8.3), 250 mM KCl, 10 mM MgCl2, 1.67 mM of each dNTP], preheated to 52 °C for 45 s, and then incubated with 1 μL of Superscript III (Invitrogen, 200 units) at 52 °C for 20 min. The reactions were quenched, and the RNA template was destroyed by addition of 0.8 μL of 5 M NaOH, followed by heating to 90 °C for 4 min. Acid stop mix [4:25 (v/v) mixture of 1 M unbuffered Tris–HCl and stop solution (85% formamide, 0.5× TBE, 50 mM EDTA, pH 8.0); 29 μL] was added to each reaction; the reaction was incubated for an additional 4 min at 90 °C and then cooled to −20 °C. No internal tracking dye was used in the reaction to avoid interference with fragment analysis.
SHAPE data analysis
Each set of primer extension reactions was evaluated by polyacrylamide sequencing gel electrophoresis and capillary electrophoresis/fragment analysis. To visualize band intensities, 5–10 μL of each reverse transcription reaction was separately loaded onto lanes of 10% denaturing sequencing gels (10% 29:1 acrylamide/bis acrylamide, 1× TBE, 8 M urea) and run at a constant voltage of 55 W. In order to obtain optimal separation for both high and low molecular weight fragments, we performed two scans from a Typhoon phosphor-imager (Molecular Dynamics) for every gel image. The first image for low molecular weight bands was scanned after 2.5 h of electrophoresis, and a second image for higher molecular weight fragments was obtained after 5 h of electrophoresis.
Our quantification analysis used results from fragment analysis (Applied BioSystems 3730xl DNA Analyzer) to determine intensity differences at the regions of interest. Traces from fragment analysis were quantified by the program ShapeFinder.45 Nucleotide reactivities were scaled to uniformly reactive nucleotides at loop positions (G61-A63 for nt U1-C66; A96-C100 for A68-C118). Strong signals possibly caused by pausing were manually eliminated from the data. For temperature melting data, intensities from several key regions (J1/2, J2/3, pseudoknot, J3/4, and P1+P4) were rescaled to a unit (0 to 1) and fitted to the following equation, assuming a unimolecular transition,46 using the program Origin (Origin Lab).
where I is SHAPE activity at a given temperature (T); R is the gas constant; and A and b are the maximum and initial intensity, respectively. Since it is difficult to get a consistent behavior from a single base, the resulting melting curve is an averaged melting profile for a domain of interest.
Mutational analysis
Mutations in the B. subtilis yitJ S-box riboswitch were generated by oligonucleotide-directed mutagenesis as previously described.16 Templates for in vitro transcription assay were PCR fragments in which a B. subtilis glyQS promoter was positioned upstream of the B. subtilis yitJ leader sequence; template DNA extended past the termination site so that terminated and readthrough transcription products could be resolved easily on a 6% denaturing polyacrylamide gel. Templates were transcribed using B. subtilis RNAP in the presence or absence of SAM (140 μM) as previously described.16,20 Percent termination was calculated as the amount of terminated product relative to the total of terminated and readthrough products after Phosphor-Imager analysis. All assays were carried out in triplicate, and values shown are the average of three independent trials; variance was ±5%.
RNA was prepared for SAM-binding assays by positioning the yitJ leader sequence downstream of a T7 RNAP promoter and carrying out T7 RNAP transcription and RNA purification as described above. RNAs were heated and slow-cooled in 1× transcription buffer containing 10 mM MgCl2, in the presence or absence of 3H-SAM; free SAM was separated from RNA-bound SAM by filtration and quantified for Kd determination as previously described.20 All binding assays were carried out in triplicate, and error was ±5%.
Acknowledgments
We thank the beam line staff at MACCHESS for assistance in data collection. Work in the Henkin laboratory and Ke laboratory was supported by Public Health Service grants from NIH (GM63615 and GM086766, respectively). We thank Kevin Weeks for the generous gift of SHAPE reagent 1M7 and Jennifer Doudna for helpful comments and suggestions. The authors declare no financial conflict of interest. This work is based on a research conducted at the Cornell High Energy Synchrotron Source (CHESS), which is supported by NSF award DMR 0225180 and NCI award RR-01646.
Abbreviations used
- SAM
S-adenosyl-L-methionine
- RNAP
RNA polymerase
- SAH
S-adenosyl-L-homocysteine
- CHESS
Cornell High Energy Synchrotron Source
- SHAPE
selective 2′-hydroxyl acylation analyzed by primer extension
Footnotes
Accession codes
Protein Data Bank: The coordinates for the B. subtilis yitJ riboswitch structures have been deposited in the Protein Data Bank with accession number 3NPB.
Supplementary materials related to this article can be found online at doi:10.1016/j.jmb.2010.09.059
References
- 1.Tucker BJ, Breaker RR. Riboswitches as versatile gene control elements. Curr Opin Struct Biol. 2005;15:342–348. doi: 10.1016/j.sbi.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Grundy FJ, Henkin TM. From ribosome to riboswitch: control of gene expression in bacteria by RNA structural rearrangements. Crit Rev Biochem Mol Biol. 2006;41:329–338. doi: 10.1080/10409230600914294. [DOI] [PubMed] [Google Scholar]
- 3.Smith AM, Fuchs RT, Grundy FJ, Henkin TM. Riboswitch RNAs: Regulation of gene expression by direct monitoring of a physiological signal. RNA Biol. 7 doi: 10.4161/rna.7.1.10757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henkin TM, Grundy FJ. Sensing metabolic signals with nascent RNA transcripts: the T box and S box riboswitches as paradigms. Cold Spring Harb Symp Quant Biol. 2006;71:231–237. doi: 10.1101/sqb.2006.71.020. [DOI] [PubMed] [Google Scholar]
- 5.Serganov A, Patel DJ. Amino acid recognition and gene regulation by riboswitches. Biochim Biophys Acta. 2009;1789:592–611. doi: 10.1016/j.bbagrm.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards TE, Klein DJ, Ferre-D’Amare AR. Riboswitches: small-molecule recognition by gene regulatory RNAs. Curr Opin Struct Biol. 2007;17:273–279. doi: 10.1016/j.sbi.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Montange RK, Batey RT. Riboswitches: emerging themes in RNA structure and function. Annu Rev Biophys. 2008;37:117–133. doi: 10.1146/annurev.biophys.37.032807.130000. [DOI] [PubMed] [Google Scholar]
- 8.Sashital DG, Butcher SE. Flipping off the riboswitch: RNA structures that control gene expression. ACS Chem Biol. 2006;1:341–345. doi: 10.1021/cb6002465. [DOI] [PubMed] [Google Scholar]
- 9.Dambach MD, Winkler WC. Expanding roles for metabolite-sensing regulatory RNAs. Curr Opin Microbiol. 2009;12:161–169. doi: 10.1016/j.mib.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wakeman CA, Winkler WC, Dann CE., 3rd Structural features of metabolite-sensing riboswitches. Trends Biochem Sci. 2007;32:415–424. doi: 10.1016/j.tibs.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheah MT, Wachter A, Sudarsan N, Breaker RR. Control of alternative RNA splicing and gene expression by eukaryotic riboswitches. Nature. 2007;447:497–500. doi: 10.1038/nature05769. [DOI] [PubMed] [Google Scholar]
- 12.Wachter A, Tunc-Ozdemir M, Grove BC, Green PJ, Shintani DK, Breaker RR. Riboswitch control of gene expression in plants by splicing and alternative 3′ end processing of mRNAs. Plant Cell. 2007;19:3437–3450. doi: 10.1105/tpc.107.053645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barrick JE, Breaker RR. The distributions, mechanisms, and structures of metabolite-binding riboswitches. Genome Biol. 2007;8:R239. doi: 10.1186/gb-2007-8-11-r239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodionov DA, Vitreschak AG, Mironov AA, Gelfand MS. Regulation of lysine biosynthesis and transport genes in bacteria: yet another RNA riboswitch? Nucleic Acids Res. 2003;31:6748–6757. doi: 10.1093/nar/gkg900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grundy FJ, Henkin TM. The S box regulon: a new global transcription termination control system for methionine and cysteine biosynthesis genes in gram-positive bacteria. Mol Microbiol. 1998;30:737–749. doi: 10.1046/j.1365-2958.1998.01105.x. [DOI] [PubMed] [Google Scholar]
- 16.McDaniel BA, Grundy FJ, Artsimovitch I, Henkin TM. Transcription termination control of the S box system: direct measurement of S-adenosylmethionine by the leader RNA. Proc Natl Acad Sci USA. 2003;100:3083–3088. doi: 10.1073/pnas.0630422100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDaniel BA, Grundy FJ, Henkin TM. A tertiary structural element in S box leader RNAs is required for S-adenosylmethionine-directed transcription termination. Mol Microbiol. 2005;57:1008–1021. doi: 10.1111/j.1365-2958.2005.04740.x. [DOI] [PubMed] [Google Scholar]
- 18.Winkler WC, Nahvi A, Sudarsan N, Barrick JE, Breaker RR. An mRNA structure that controls gene expression by binding S-adenosyl-methionine. Nat Struct Biol. 2003;10:701–707. doi: 10.1038/nsb967. [DOI] [PubMed] [Google Scholar]
- 19.Epshtein V, Mironov AS, Nudler E. The riboswitch-mediated control of sulfur metabolism in bacteria. Proc Natl Acad Sci USA. 2003;100:5052–5056. doi: 10.1073/pnas.0531307100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomsic J, McDaniel BA, Grundy FJ, Henkin TM. Natural variability in S-adenosylmethionine (SAM)-dependent riboswitches: S-box elements in bacillus subtilis exhibit differential sensitivity to SAM In vivo and in vitro. J Bacteriol. 2008;190:823–833. doi: 10.1128/JB.01034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poiata E, Meyer MM, Ames TD, Breaker RR. A variant riboswitch aptamer class for S-adenosylmethionine common in marine bacteria. RNA. 2009;15:2046–2056. doi: 10.1261/rna.1824209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winkler WC, Grundy FJ, Murphy BA, Henkin TM. The GA motif: an RNA element common to bacterial antitermination systems, rRNA, and eukaryotic RNAs. RNA. 2001;7:1165–1172. doi: 10.1017/s1355838201002370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montange RK, Batey RT. Structure of the S-adenosylmethionine riboswitch regulatory mRNA element. Nature. 2006;441:1172–1175. doi: 10.1038/nature04819. [DOI] [PubMed] [Google Scholar]
- 24.Montange RK, Mondragon E, van Tyne D, Garst AD, Ceres P, Batey RT. Discrimination between closely related cellular metabolites by the SAM-I riboswitch. J Mol Biol. 2010;396:761–772. doi: 10.1016/j.jmb.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoddard CD, Montange RK, Hennelly SP, Rambo RP, Sanbonmatsu KY, Batey RT. Free state conformational sampling of the SAM-I riboswitch aptamer domain. Structure. 2010;18:787–797. doi: 10.1016/j.str.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilbert SD, Rambo RP, Van Tyne D, Batey RT. Structure of the SAM-II riboswitch bound to S-adenosylmethionine. Nat Struct Mol Biol. 2008;15:177–182. doi: 10.1038/nsmb.1371. [DOI] [PubMed] [Google Scholar]
- 27.Lu C, Smith AM, Fuchs RT, Ding F, Rajashankar K, Henkin TM, Ke A. Crystal structures of the SAM-III/S(MK) riboswitch reveal the SAM-dependent translation inhibition mechanism. Nat Struct Mol Biol. 2008;15:1076–1083. doi: 10.1038/nsmb.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kulshina N, Edwards TE, Ferre-D’Amare AR. Thermodynamic analysis of ligand binding and ligand binding-induced tertiary structure formation by the thiamine pyrophosphate riboswitch. RNA. 2010;16:186–196. doi: 10.1261/rna.1847310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlatterer JC, Kwok LW, Lamb JS, Park HY, Andresen K, Brenowitz M, Pollack L. Hinge stiffness is a barrier to RNA folding. J Mol Biol. 2008;379:859–870. doi: 10.1016/j.jmb.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwok LW, Shcherbakova I, Lamb JS, Park HY, Andresen K, Smith H, et al. Concordant exploration of the kinetics of RNA folding from global and local perspectives. J Mol Biol. 2006;355:282–293. doi: 10.1016/j.jmb.2005.10.070. [DOI] [PubMed] [Google Scholar]
- 31.Gotta SL, Miller OL, Jr, French SL. rRNA transcription rate in Escherichia coli. J Bacteriol. 1991;173:6647–6649. doi: 10.1128/jb.173.20.6647-6649.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilkinson KA, Merino EJ, Weeks KM. RNA SHAPE chemistry reveals nonhierarchical interactions dominate equilibrium structural transitions in tRNA(Asp) transcripts. J Am Chem Soc. 2005;127:4659–4667. doi: 10.1021/ja0436749. [DOI] [PubMed] [Google Scholar]
- 33.Heppell B, Lafontaine DA. Folding of the SAM aptamer is determined by the formation of a K-turn-dependent pseudoknot. Biochemistry. 2008;47:1490–1499. doi: 10.1021/bi701164y. [DOI] [PubMed] [Google Scholar]
- 34.Huang W, Kim J, Jha S, Aboulela F. A mechanism for S-adenosyl methionine assisted formation of a riboswitch conformation: a small molecule with a strong arm. Nucleic Acids Res. 2009;37:6528–6539. doi: 10.1093/nar/gkp664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ontiveros-Palacios N, Smith AM, Grundy FJ, Soberon M, Henkin TM, Miranda-Rios J. Molecular basis of gene regulation by the THI-box riboswitch. Mol Microbiol. 2008;67:793–803. doi: 10.1111/j.1365-2958.2007.06088.x. [DOI] [PubMed] [Google Scholar]
- 36.Ke A, Doudna JA. Crystallization of RNA and RNA–protein complexes. Methods. 2004;34:408–414. doi: 10.1016/j.ymeth.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 37.Storoni LC, McCoy AJ, Read RJ. Likelihood-enhanced fast rotation functions. Acta Crystallogr Sect D: Biol Crystallogr. 2004;60:432–438. doi: 10.1107/S0907444903028956. [DOI] [PubMed] [Google Scholar]
- 38.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr Sect D: Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 39.Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr Sect D: Biol Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 40.Brunger AT. Version 1.2 of the crystallography and NMR system. Nat Protoc. 2007;2:2728–2733. doi: 10.1038/nprot.2007.406. [DOI] [PubMed] [Google Scholar]
- 41.Collaborative Computational Project, N. The CCP4 suite: programs for protein crystallography. Acta Crystallogr Sect D: Biol Crystallogr. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 42.Wilkinson KA, Merino EJ, Weeks KM. Selective 2′-hydroxyl acylation analyzed by primer extension (SHAPE): quantitative RNA structure analysis at single nucleotide resolution. Nat Protoc. 2006;1:1610–1616. doi: 10.1038/nprot.2006.249. [DOI] [PubMed] [Google Scholar]
- 43.Merino EJ, Wilkinson KA, Coughlan JL, Weeks KM. RNA structure analysis at single nucleotide resolution by selective 2′-hydroxyl acylation and primer extension (SHAPE) J Am Chem Soc. 2005;127:4223–4231. doi: 10.1021/ja043822v. [DOI] [PubMed] [Google Scholar]
- 44.Wilkinson KA, Vasa SM, Deigan KE, Mortimer SA, Giddings MC, Weeks KM. Influence of nucleotide identity on ribose 2′-hydroxyl reactivity in RNA. RNA. 2009;15:1314–1321. doi: 10.1261/rna.1536209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vasa SM, Guex N, Wilkinson KA, Weeks KM, Giddings MC. ShapeFinder: a software system for high-throughput quantitative analysis of nucleic acid reactivity information resolved by capillary electrophoresis. RNA. 2008;14:1979–1990. doi: 10.1261/rna.1166808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.John DM, Weeks KM. van’t Hoff enthalpies without baselines. Protein Sci. 2000;9:1416–1419. doi: 10.1110/ps.9.7.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]