Abstract
Fragile X-associated tremor/ataxia syndrome (FXTAS) is an adult-onset neurodegenerative disorder generally presenting with intention tremor and gait ataxia, but with a growing list of co-morbid medical conditions including hypothyroidism, hypertension, peripheral neuropathy, and cognitive decline. The pathological hallmark of FXTAS is the presence of intranuclear inclusions in both neurons and astroglia. However, it is unknown to what extent such inclusions are present outside the central nervous system (CNS). To address this issue, we surveyed non-CNS organs in ten human cases with FXTAS and in a CGG repeat knock-in (CGG KI) mouse model known to possess neuronal and astroglial inclusions. We find inclusions in multiple tissues from FXTAS cases and CGG KI mice, including pancreas, thyroid, adrenal gland, gastrointestinal, pituitary gland, pineal gland, heart, and mitral valve, as well as throughout the associated autonomic ganglia. Inclusions were observed in the testes, epididymis, and kidney of FXTAS cases, but were not observed in mice. These observations demonstrate extensive involvement of the peripheral nervous system and systemic organs. The finding of intranuclear inclusions in non-CNS somaticorgan systems, throughout the PNS, and in the enteric nervous system of both FXTAS cases as well as CGG KI mice suggests that these tissues may serve as potential sites to evaluate early intervention strategies or be used as diagnostic factors.
Keywords: FXTAS, Intranuclear inclusions, Fragile X premutation, CGG KI mouse
Introduction
Fragile X-associated tremor/ataxia syndrome (FXTAS) is a progressive neurodegenerative disorder that is characterized by cerebellar gait ataxia and intention tremor. FXTAS affects carriers of a fragile X mental retardation 1 (FMR1) gene containing a CGG trinucleotide repeat expansion in the 5′ untranslated region (UTR) within the premutation range (PM; 55–200 CGG repeats). Premutation length CGG trinucleotide repeat expansions are present in as many as 1:251 males and 1:116 females in the population [4, 24, 56]. Both male and female PM carriers older than 50 years of age may develop FXTAS, although the incidence of FXTAS in female PM carriers is less than half of males (~40% of male PM carriers vs. 8–16% of female PM carriers develop FXTAS [9, 24, 45, 64]), primarily thought due to a protective effect of the non-expanded FMR1 gene on the second X chromosome. Premutation carriers produce elevated levels (2–10 times normal measured in lymphocytes) of PM FMR1 messenger RNA (mRNA) and normal to moderately reduced levels of FMR1 protein (FMRP) in leukocytes [4, 57, 58, 70], fibroblasts [29], and brain tissue [71]. The current hypothesis underlying the pathophysiology of FXTAS focuses on a toxic mRNA gain-of-function mechanism [28].
The onset of FXTAS symptomatology begins at a mean age of 60 years, and penetrance is age-dependent [45, 52]. The core features of FXTAS include progressive intention tremor and cerebellar gait ataxia [8, 10, 11, 35, 44]. Radiologic evaluations using magnetic resonance imaging (MRI) have identified generalized atrophy of the cerebrum, brainstem, and cerebellum in FXTAS [1, 2, 25], and a majority of male FXTAS patients show distinctive, bilateral, white matter signal hyperintensities in the middle cerebellar peduncles on T2-weighted or FLAIR MRI scans [21, 53]. These radiologic features have been histologically confirmed in multiple post mortem cases [31–34, 71].
Associated clinical features of FXTAS include cognitive decline, Parkinsonism, peripheral neuropathy, and autonomic dysfunction [23, 35, 36, 38, 46, 54, 55, 61, 67, 77]. The neuropathological hallmark of FXTAS is the presence of eosinophilic, ubiquitin-positive intranuclear inclusions in both neurons and astroglia throughout brain upon post mortem histological analysis [30–34, 77]. These inclusions appear as eosinophilic, hyaline, refractile, 2–5 μm diameter, round to ovoid bodies that show positive reactivity with antibodies against over 20 different proteins including ubiquitin, αB-crystallin, lamin A/C, hnRNP A2, myelin basic protein, DNA repair-ubiquitin-associated HR23B, and Sam68, among others [6, 27, 43, 59, 65]. The inclusions are PAS, silver, amyloid, and α-synuclein negative [31, 32]. Additionally, the FMR1 mRNA, but not FMRP, has been found contained within intranuclear inclusions [43, 72]. Additional neuropathological features present in FXTAS include reduced Purkinje cell number, axonal torpedoes, and prominent cortical and subcortical white matter pathology [31, 32].
The CGG knock-in (CGG KI) mouse model of the PM has proven to be an invaluable tool for the study of the pathophysiology of FXTAS, including neuropathological events that occur with the onset and progression of the disorder (cf., [7]). The CGG KI mouse model was developed by homologous recombination wherein the endogenous mouse (CGG)8 trinucleotide repeat within the mouse Fmr1 gene was replaced by a (CGG)98 repeat of human origin. As such, expression of the expanded (CGG)98 repeat is on the endogenous mouse Fmr1 promoter [76]. The CGG KI mouse recapitulates many neuropathological features present in FXTAS, including intranuclear inclusions in neurons and astroglia, elevated Fmr1 mRNA levels, and reduced Fmrp levels [17–20, 76] (cf., [26, 59]) for a different knock-in model of the PM demonstrating similar molecular features. In addition, a correlation has been demonstrated between the presence of intranuclear inclusions in brain and phenotypes in CGG KI mice that model the clinical features of FXTAS [18, 40–42,76]. Furthermore, CGG KI mice show an increase in neurocognitive dysfunction both with increasing age and CGG repeat length across a growing battery of behavioral tests [40–42, 73].
It has become apparent that the spectrum of clinical involvement in PM carriers with FXTAS extends beyond symptoms and signs that correspond to pathology in the CNS [10, 11, 14–16, 24, 30, 33, 67]. The increasingly broad clinical spectrum of FXTAS symptomatology seems to encompass a number of medical co-morbidities that include thyroid disease [24, 64], fibromyalgia [24], gastrointestinal symptoms [12, 37] hypertension [24], migraine [3, 12], impotence [33], autoimmune disease [24,51], peripheral neuropathy [36, 67], seizure disorders [5], and cardiac arrhythmia [35, 44]. Intriguingly, evidence for this broadened spectrum of immune mediated disorders [24, 51] arise primarily in women both with and without FXTAS, but many of the medical co-morbidities are more commonly observed in individuals with FXTAS comparedto PM carriers without FXTAS [24, 30, 33, 67]. Furthermore, intranuclear inclusions have been identified in non-CNS tissues, but to date have only been evaluated in a limited number of cases [30, 33, 54]. Here we report the presence of inclusions in autonomic ganglia throughout the peripheral autonomic nervous system, as well as in somatic tissues themselves from ten PM carriers with FXTAS (9 male, 1 female), and compare these findings to homologous pathological features present in the CGG KI mouse model of the fragile X PM.
Materials and methods
FXTAS case autopsies
Clinical history reports for all FXTAS cases included in this report are available in Table 1, along with molecular correlates of the PM. Written informed consent was received for all cases and all experimental protocols conformed to IRB approved protocols. Tissues (pancreas, kidney, brain, thyroid gland, heart, testes, adrenal gland, gastrointestinal, and esophagus) were removed from a subset (cf., Table 1) at autopsy and immersion fixed in 10% formalin, followed by paraffin embedding of representative samples. The brain was also removed from each autopsy case, as was the spinal cord on select cases. In standard fashion, histological sections (5 μm) were stained by hematoxylin and eosin (H&E) for routine histological examination, and immunoperoxidase labeling was performed using rabbit antibodies targeted against ubiquitin (Dako, ZO458, Carpinteria, CA, USA) and counterstained with hematoxylin.
Table 1.
Clinical history and molecular characterizations of all FXTAS cases and tissues samples evaluated from each in the present study
| FXTAS Case |
CGG | FMR1 mRNA |
Tissues sampled |
FXTAS Onset |
Co-morbid diagnoses |
MRI findings | Age at death |
Cause of death |
FMR1 + relatives |
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 80 | 2.59 ± 0.42 | Adrenal Testes Pineal |
69 | Cognitive decline Neuropathy Choking |
Brain atrophy White matter disease |
79 | Aspiration pneumonia |
1 grandchild with FXS PM daughter |
| Case 2 | 160 | Not determined | Colon Testes |
64 | Seizures COPDHistory of smoking Type II diabetes Emphysema Depression Myelodysplastic syndrome with sideroblastic anemia, Cognitive decline Obsessive compulsive disorder Anxiety |
White matter disease | 69 | 4 grandchildren with FXS |
|
| Case 3 | 75 | 2.02 ± 0.27 | Adrenal Colon Testes Pituitary |
69 | Dementia, Herpes zoster |
Not determined | 93 | Aspiration pneumonia Pulmonary edema |
Grandchildren with FXS 3 PM daughters |
| Case 4 | 85 | 4.25 ± 0.21 | Adrenal Colon Heart Pituitary Pancreas |
67 | Hypertension Neuropathy Cognitive decline |
Global brain atrophy MCP sign White matter disease |
78 | ||
| Case 5 | 72 | Not determined | Adrenal Testes Epididymis |
80 | Neuropathy Dementia Cardiac arrhythmia Hypertension Angina Type II diabetes Irritability |
White matter disease in the pons |
87 | Stroke with hemiparesis |
|
| Case 6 | 109 | Not determined | Heart Kidney Pancreas Thyroid |
63 | Hypertension COPD Cardiac arrhythmia Cognitive decline Type II diabetes Pacemaker Syncope |
Global brain atrophy White matter disease |
73 | Postoperative Carotid endarterectomy |
2 grandchildren with FXS Premutation daughter |
| Case 7 | 95 | 3.35 ± 0.27 | Esophagus Heart |
56 | Type II diabetes Cognitive decline Impotence Bladder incontinence Seizures Neuropathy Hypertension Dementia Hallucination Mood lability |
Pituitary cyst Global brain atrophy Diffuse white matter disease |
67 | Myocardial infarction |
Grandchildren with FXS |
| Case 8 | 30, 96 AR = 0.76 |
2.80± 0.12 |
Mitral valve biopsy |
49 | PTSD Depression Hypertension Neuropathy Nonepileptic seizures Conversion disorder MVP Mitral regurgitation Hypothyroidism Anxiety |
Mild brain atrophy, White matter disease |
N/A | N/A | |
| Case 9 | 80 | 3.75 ± 0.51 | Testes | 65 | Constipation Neuropathy Impotence Hypertension Type II diabetes Hyperlipidemia Cardiac arrythmia Pituitary adenoma Adenocarcinoma |
72 | Adenocarcinoma | ||
| Case 10 |
95 | Not determined | Pituitary | 61 | Impotence Hypertension Stroke Sleep apnea Mood lability Neuropathy Anxiety Prostate cancer Chronic pain Choking Irritability |
Global brain atrophy White matter disease |
67 | Pulmonary edema Congestive heart failure |
FMR1 mRNA levels were not determined for Cases 2, 5, 6, 10 due to the unavailability of unfixed tissues. Activation ratio (AR: ratio of cells with the normal X as the active X) is provided for female Case 8
Mitral valve biopsy tissue
A1 x 1 mm biopsy was taken from the mitral valve of Case 8 during surgery, placed in phosphate-buffered saline for 2 h, postfixed in freshly made 4% phosphate-buffered paraformaldehyde for 2 h, and cryoprotected in 30% sucrose. Microtome sections (30 μm) were taken and stained using iron hematoxylin and eosin for histological analysis as well as a modified Van Gieson’s stain (Van Gieson’s stain with the addition of Eosin Y) to further characterize specific cell and tissue types. Immunostains were performed using rabbit antibodies targeted against ubiquitin (DAKO, ZO458; 1:2,000) and counterstained with hematoxylin.
CGG KI mice
The generation of the expanded CGG mice used in this study has been described previously [76]. For the current study, male mice with repeat sizes between 100 and 150 CGG repeats were used (all experiments), as well as 1 female mouse with 8 and 150 CGG repeats (mitral valve to compare with biopsy material from Case 8). All experiments were carried out in accordance with approved animal use protocols (Erasmus MC; University of California, Davis). CGG repeat lengths were determined as described previously [18, 42]. All mice used in this study were between 48 and 90 weeks of age at time of killing.
Tissues (pancreas, pituitary, heart, kidney, brain, thyroid gland, testis, adrenal gland, and intestine) were dissected immediately following cervical dislocation and fixed overnight in 4% paraformaldehyde at 4°C. Subsequently, tissues were embedded in paraffin according to standard protocols. Paraffin sections (7 μm) were cut and mounted on gelatin-coated slides. Immunostains were performed with rabbit antibodies against ubiquitin (Dako, ZO458; 1:500) followed by indirect immunoperoxidase labeling with hematoxylin counterstain. For co-localization studies, antibodies targeted against somatostatin and glucagon were combined with ubiquitin labeling using immunofluorescence techniques and counterstained with DAPI to identify cell nuclei.
For experiments evaluating CGG KI heart, mitral valve, and pineal gland, the heart was dissected from a CGG KI mouse immediately following cervical dislocation and immersion fixed in 4% paraformaldehyde for 2 h at 4°C, then transferred overnight into 30% sucrose at 4°C as a cryoprotectant. Concurrently, the brain was trans-aortically perfused with 12 mL of potassium shifted Ringer’s solution, followed by 60 mL of 4% paraformaldehyde over 20 min via gravity feed, gently removed from the skull so as not to damage the pineal gland, and transferred into 4% paraformaldehyde at 4°C for 1 h postfixation, followed by 10 and 30% sucrose solutions. The heart and brain, including the pineal gland were sagittally sectioned at 30 μm on a freezing stage microtome and a set of every fifth section was mounted on gelatin-coated slides and stained for H&E. Another section set of heart tissue was stained using a modification of Van Gieson’s stain similar to that done for the human aorta tissue. Indirect immunoperoxidase staining was performed on a third set of free floating sections using polyclonal antibodies targeted to ubiquitin (Dako, ZO458; 1:2,000), after which tissues were mounted on gelatin-coated slides, counterstained with hematoxylin, dehydrated, and coverslipped with Permount resinous mounting media.
Results
Human FXTAS cases
A summary of the organs in which intranuclear inclusions were identified across all cases of FXTAS is presented in Table 2. A brief summary is presented below focusing on theintranuclear inclusions identified in each organ, as well as rough percentages of cell nuclei in which inclusions were present. Novel pathological findings are illustrated in Fig. 1.
Table 2.
Intranuclear inclusions are present in somatic organs and autonomic ganglia in human FXTAS cases and the CGG KI mouse model of the PM, organized by organ system and cell type
| Human FXTASa |
Cell type(s) with inclusions | CGG KI | Cell type(s) with inclusions | |
|---|---|---|---|---|
| Adrenals | 1, 3, 4, 5 | Chromaffin secreting cells of medulla Steroid secreting cells of cortex |
Yes | Chromaffin secreting cells of medulla |
| Colon | 2, 3, 4 | Ganglion cells of myenteric plexus & submucosal plexus |
Yes | Ganglion cells of myenteric plexus |
| Esophagus | 7 | Ganglion cells of myenteric plexus | Not analyzed |
|
| Heart | 4, 6, 7 | Cardiomyocytes ganglia | Yes | Cardiomyocytes |
| Autonomic ganglia | Intracardiac autonomic ganglia | |||
| Mitral valve | 8 | Ganglion cells | Yes | Ganglion cells |
| Smooth muscle cells | ||||
| Kidney | 1, 6 | Mesangial cells | None detected |
- |
| Peripheral autonomic ganglia |
4 | Ganglion cells | Yes | Ganglion cells |
| Pituitary gland | 3, 10 | Acidophils | Yes | Pars anterior |
| Basophils | Pars intermedia | |||
| Pituicytes | ||||
| Testes | 1, 2, 3, 5, 9 | Leydig cells | None detected |
– |
| Epididymis | 5 | Epithelial cells of distal tubule | Not analyzed |
– |
| Pancreas | 4, 6 | Islets of Langerhans | Yes | Islets of Langerhans |
| A and D cells | ||||
| Thyroid | 1, 6 | Follicular Cells | Yes | Parafollicular cells |
| Parafollicular cells | ||||
| Pineal gland | 1 | Ganglion cells | Yes | Ganglion Cells |
| Pinealocytes | Pinealocytes | |||
| Astroglia | Astroglia |
The number of organ tissues available for this series varied case by case. Intranuclear inclusions were present in all cases where that tissue was received (e.g., for the adrenals, 4 cases had adrenal tissue available, and intranuclear inclusions were seen in each of the 4 cases). See text for percentages of inclusions in each organ system
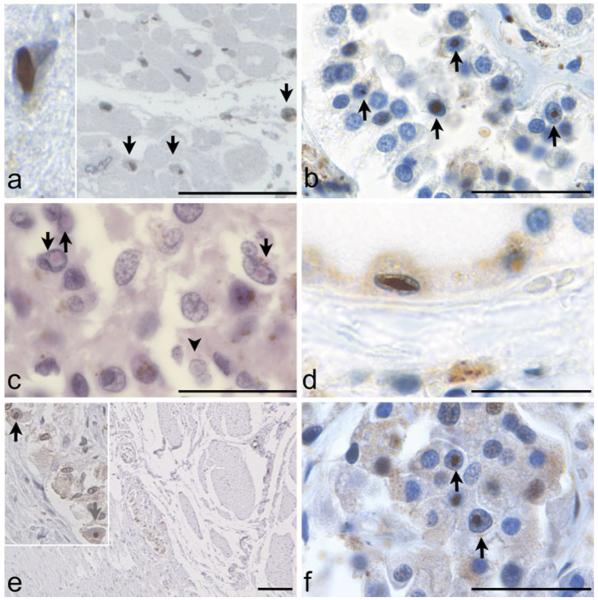
Fig. 1.
a Intranuclear inclusions in cardiomyocytes in Case 7 (x400; Immunoperoxidase (IP) stain). Insert Extremely large, oval-shaped inclusion in a cardiomyocyte from Case 6 (x1,000). b Intranuclear inclusions in pinealocytes and astrocytes in pineal gland of Case 1 (x1,000; H&E stain). c Autonomic ganglion of the myenteric/Auerbach’s plexus is seen between longitudinal and circular muscular layers of the rectosigmoid colon (x40; IP stain) in Case 4. Insert Higher magnification identifies intranuclear inclusions in ganglion cells (x400). d Intranuclear inclusions in cells of the distal tubule of the kidney in Case 6 (x1,000; IP stain). e Intranuclear inclusions in thyroid of Case 6 (x1,000; IP stain). f Intranuclear inclusion in pancreas of Case 6 (x1,000; IP stain). All immunoperoxidase stains counterstained with hematoxylin. All scale bars represent 100 μm
Heart
Intranuclear inclusions were identified in cardiomyocytes in the heart and surrounding autonomic ganglia in Cases 4, 6, and 7 (3–5% of cardiomyocytes), as well as in smooth muscle cells of the mitral valve (~1–2% of cells) and autonomic ganglia in the tunica externa from Case 8 (~5% of cells; Fig. 1a).
Pineal
Intranuclear inclusions were identified in pinealocytes and ganglion cells in the pineal gland of Case 1 (1–2% of cells; Fig. 1b).
Colon
Intranuclear inclusions were identified in smooth muscle cells, as well as in neurons of the submucosal and myenteric plexi of the rectum (1–2% of cells; Fig. 1c), sigmoid colon, and appendix of Cases 2, 3, and 4.
Kidney
Intranuclear inclusions were identified in mesangial cells and epithelial cells of the distal tubules of the kidney in Cases 1 and 6 (3–5% of cells; Fig. 1d).
Thyroid
Intranuclear inclusions were identified in the follicular and parafollicular cells in the thyroid glands of Cases 1 and 6 (~1% of cells; Fig. 1e).
Pancreas
Intranuclear inclusions were identified in Islets of Langerhans cells in the pancreas of Cases 4 and 6. The precise cell type harboring inclusions was not determined in human cases due to autolytic change (5–10% of cells; Fig. 1f).
Adrenal gland
Intranuclear inclusions were identified in the medullary cells of the adrenal gland, as well as in periadrenal ganglia of Cases 1, 3, 4, and 5 (1–2% of cells).
Esophagus
Intranuclear inclusions were identified in neurons of the myenteric plexus of the esophagus of Case 7 (1–2% of cells).
Testes
Intranuclear inclusions were identified in Leydig cells, smooth muscle cells, and nurse cells (1–2% of cells) in the testes of Cases 1, 2, 3, 5, and 9, supporting previous reports of inclusion presence in the testes [33].
Epididymis
Intranuclear inclusions were identified in the epithelial cells of the distal tubule of Case 5 (1–2% of cells).
Pituitary
Intranuclear inclusions were identified in basophiles, chromophobes, and acidophiles of the anterior pituitary, and pituicytes of the posterior pituitary of Cases 3 and 10 (1–2% of cells).
CGG KI Mouse
A summary of organ sites of intranuclear inclusions were identified in CGG KI mice compared with the findings in FXTAS is presented in Table 2. A brief summary is provided below organized in a similar manner to the human results (Fig. 2).
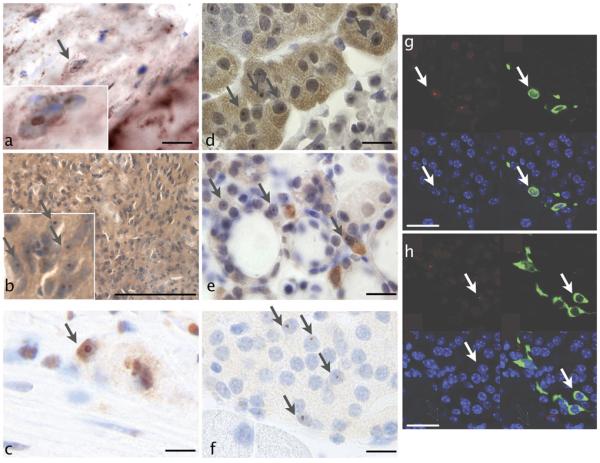
Fig. 2.
a Intranuclear inclusions in cardiomyocytes of CGG KI mice (x1,000; IP stain). b Intranuclear inclusions in pinealocytes of CGG KI mouse pineal gland (x1,000; IP stain). c Intranuclear inclusions in ganglion cells of the myenteric plexus of the colon in CGG KI mice (x1,000; IP stain). d Intranuclear inclusions in adrenal gland of CGG KI mice (x1,000; IP stain). e Intranuclear inclusions in thyroid of CGG KI mice (1,000x; IP stain). f Intranuclear inclusions in pancreas of CGG KI mice (x1,000; IP stain). g Immunofluorescence of intranuclear inclusions (red) and somatostatin (green) in pancreas of CGG KI Mice (nuclei counterstained with DAPI (blue)) (x1,000; IP stain). h Immunofluorescence of intranuclear inclusions (red) and glucagon (green; IP stain) in pancreas of CGG KI mice (nuclei counterstained with DAPI (blue)) (x1,000). All scale bars represent 50 μm
Heart
A considerable number of cardiac muscle cells contained ubiquitin-positive intranuclear inclusions in 48- to 72-week-old CGG KI mice (~2–3% of cells; Fig. 2a) with some being so large as to occupy nearly the entire nucleus of the cell. Intranuclear inclusions were not conclusively identified in the smooth muscle of the mitral valve, but were numerous in the autonomic ganglia in the tunica externa (~5% of cells).
Pineal gland
Intranuclear inclusions were present in pinealocytes, astrocytes, and ganglion cells in the pineal gland (~2–3% of cells; Fig. 2b).
Colon
Intranuclear inclusions were identified in myenteric ganglia in the colon (10% of cells; Fig. 2c).
Adrenal gland
Ubiquitin-positive intranuclear inclusions were present in chromaffin cells of the adrenal gland (5–10% of cells; Fig. 2d).
Thyroid
In thyroid tissue from CGG KI mice, the gland structure was normal in H&E sections. Further examination revealed the presence of ubiquitin-positive intranuclear inclusions in a significant number of parafollicular cells that secrete calcitonin (3–5% of cells; Fig. 2e).
Pancreas
In pancreatic tissue from CGG KI mice, the general histological features on H&E staining were similar to those of normal age-matched controls. However, in sections that were immunostained for ubiquitin we could detect ubiquitin-positive intranuclear inclusions in specific cells of islets of Langerhans (3–5% of cells). To determine which cell types contained intranuclear inclusions (Fig. 2f), costaining for somatostatin and ubiquitin as well as glucagon and ubiquitin were carried out (Fig. 2g). Intranuclear inclusions were found in the somatostatin producing D cells of the islets of Langerhans as well as glucagon producing A cells (Fig. 2h).
Pituitary
Intranuclear inclusions were identified in the anterior and intermediate pituitary in CGG KI mice with more in the pars intermedia (~58% of cells) than the pars anterior (18% of cells). Few inclusions were detected in the pars posterior (<1% of cells) [48]. The precise cell types harboring inclusions were not determined.
Testes
No inclusions were detected in the testes of CGG KI mice.
Kidney
No inclusions were detected in the kidney of CGG KI mice.
Discussion
The present results demonstrate pathological features in broad distribution within the peripheral autonomic nervous system as well as neuroendocrine and somatic organs in PM carriers with FXTAS. These findings are consistent with the expanding range of co-morbid medical features reported in FXTAS that include neuroendocrine dysfunction [24, 68], impotence [33], gastrointestinal symptoms [50], cardiac arrhythmias [35, 44], peripheral neuropathy [36, 66], and bladder dysfunction [12, 24, 33, 37, 44, 54,64, 67]. Type II diabetes has not been formally established as an associated clinical feature of FXTAS; however, there is ample anecdotal evidence from case histories that a substantial number of patients develop type II diabetes during their lifetime (cf., Cases 2, 5, 6, 9). Because the incidence of hypothyroidism and other thyroid disorders (cf., Case 8), hypertension (cf., Cases 4, 5, 6, 7, 8, 9, 10), peripheral neuropathy (cf., Cases 1, 4, 5, 6, 7, 8, 9, 10), and fibromyalgia have been reported to be higher in PM carriers with FXTAS as compared to age-matched non-PM carriers, these diseases may well be part of the syndrome of FXTAS, or at least associated medical features [24, 64]. In addition, cardiac arrhythmias (cf., Cases 5, 6) and gastrointestinal problems including constipation are commonly encountered in carriers with FXTAS [12, 37] (cf., Case 9). Previously, Louis et al. [54] reported inclusions in pituitary tissue (hypophysis) from one patient with FXTAS, and Gokden et al. [30] reported inclusions in a number of peripheral tissues including autonomic ganglia of the mesenteric plexus, pericardial tissue, adrenal tissue and paraspinal ganglia. The present report has expanded upon these findings in a larger group of FXTAS cases. Greco et al. [13, 14, 33] have reported inclusions in the Leydig cells of the testes of men who died of FXTAS and proposed that these inclusions may likely be related to the lowered testosterone levels and impotence seen in these men since the Leydig cells produce testosterone. Impotence is common in PM males (cf., Cases 7, 9), often becoming apparent even before the development of intention tremor or cerebellar gait ataxia related to FXTAS.
Psychiatric problems, particularly anxiety and depression, are CNS-associated disorders that are increased in PM carriers with and without FXTAS compared to controls [14, 16] (cf., Cases 2, 5, 6, 8, 10). These disorders may well be elicited by a combination of stress and environmental factors [13, 14], particularly as these factors affect the hypothalamic–pituitary–adrenal (HPA) axis. In addition to the CNS component, widespread peripheral pathology have been observed in the present study, namely the presence of intranuclear inclusions identified throughout the HPA axis, pineal gland, cardiac conduction system, peripheral nerves and autonomic ganglia, thyroid, digestive system, testes, and pancreas. It is quite likely that the medical co-morbidities in systemic organs and the autonomic nervous system share a common pathogenesis with that observed in the CNS of patients with FXTAS (i.e., mRNA toxicity), and thus may be considered non-CNS-associated features of FXTAS [15, 16, 24, 39, 62–64]. The presence ofintranuclear inclusions in somatic organs as observed in the present study are not the cause of these conditions comorbid with FXTAS, but rather signal organ systems affected by the PM FMR1 mRNA. Specifically, inclusions capture and sequester important proteins necessary for normal function of these cells, including splice factors [66] that may negatively affect organ function.
The broad distribution of intranuclear inclusion formation reported herein further expands the cell types and body systems that may be affected by RNA toxicity and signals new areas for investigation of disease pathology in PM carriers both with and without FXTAS symptomatology. For instance, the prevalence of type II diabetes and hypoglycemic episodes should be investigated in those with the PM and FXTAS compared to the general population, as it appears likely that insulin production may be reduced in FXTAS (cf., Cases 2, 5, 6, 9). The presence of intranuclear inclusions in the Islets of Langerhans may signal disease processes in the pancreas, namely potential RNA toxicity—a potential that warrants further investigation. Such investigations, both in the human and in the CGG KI mouse model, would underscore the value of studying the basic disease mechanisms in non-CNS tissues that are readily available through surgical and biopsy tissues.
The CGG KI mouse was also evaluated for the presence of inclusions in the same organ systems as the cases with FXTAS, and showed a strikingly similar pattern of inclusion formation in somatic organs and the autonomic nervous system. These results verify that CGG KI mouse models the somatic pathologic anatomical features present in FXTAS, in addition to modeling the neuropathological features of FXTAS [19, 20, 40, 75, 76]. This parallel non-CNS pathology underscores the utility of the mouse model for studying the pathogenesis and progression of non-CNS disorders associated with FXTAS.
Our understanding of the molecular mechanisms of RNA toxicity is evolving, and includes dysregulation of a number of proteins such as lamin A/C and heat shock proteins including αß crystallin [22, 28, 60]; sequestration of Sam68 and the dysregulation of the protein products of mRNAs, whose splicing is modulated by Sam68 [65]. Most recently, Ross-Inta et al. [65] demonstrated mitochondrial abnormalities in fibroblasts and brain tissue in PM carriers both with and without FXTAS. It is not clear what cellular processes underlie inclusion formation; and it is not known whether the inclusions are themselves toxic or simply reflect underlying cellular dysfunction. Nevertheless, intranuclear inclusions clearly provide a cellular marker or signal for the disease process underlying FXTAS and associated non-CNS disease. Understanding the breadth of pathological involvement in the peripheral tissues in the PM and FXTAS expands our understanding of the spectrum of medical disease associated with FXTAS.
Recent publications [30, 54] along with our findings place the neurodegenerative condition associated with FXTAS among the neurodegenerative disorders with involvement of the peripheral nervous system [47, 74], and in some cases, the neuroendocrine system and/or visceral organs. Among these disorders are the Lewy body diseases, including Parkinson’s disease and diffuse Lewy body disease [48], multiple system atrophy [56, 69], and neuronal intranuclear inclusion disorder [49]. These disorders are marked by the presence of non-CNS inclusions similar in appearance to those found in the brain and spinal cord. Other polyglutamine disorders showing peripheral inclusions are Machado-Joseph disease/spinocerebellar ataxia type 3 [78] and spinal and bulbar muscular atrophy [74]. The similarity between the presence of inclusions in peripheral and somatic tissues in FXTAS and numerous other inclusion-bearing disorders suggests the inclusions may not cause the medical co-morbidities reported in FXTAS, but rather signal tissues affected by the mRNA toxicity associated with the PM. Furthermore, as the mutation underlying the PM occurs in the 5′ untranslated region of the FMR1 gene, the FMR1 protein (FMRP) is structurally normal, despite the expanded CGG repeats present in the FMR1 mRNA.
Further research is needed to understand the complexity of co-morbid medical problems associated with the PM and how these may be related to RNA toxicity. The finding of intranuclear inclusions in non-CNS somatic organ systems, throughout the PNS, and in the enteric nervous system of both FXTAS cases as well as CGG KI mice suggests that these tissues may serve as potential sites to evaluate early intervention strategies or be used as diagnostic factors. Success in this effort may assist clinicians to confirm a FXTAS diagnosis prior to designing interventions for individuals with the PM, PM-associated diseases, or early FXTAS.
Acknowledgments
This work was supported by National Institute of Health (NIH) grants HD036071, HD056031, NS044299, AG024488, HD02274, MH77554, MH078041, RL1 AG032115, RL1NS062411, and RL1 AG032119; the National Fragile X Foundation; the Circle of Service Foundation; and the M.I.N.D. Institute. This work was also made possible by a grant (UL1 DE019583) from the National Institute of Dental and Craniofacial Research (NIDCR) in support of the NeuroTherapeutics Research Institute (NTRI) consortium; and by a grant (UL1 RR024146) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Footnotes
M. R. Hunsaker and C. M. Greco contributed equally to this manuscript.
Contributor Information
Michael R. Hunsaker, Department of Neurological Surgery, University of California, Davis, Davis, CA, USA
Claudia M. Greco, Department of Pathology, University of California, Davis, Davis, CA, USA; M.I.N.D. Institute, University of California at Davis Medical Center, 2825 50th Street, Sacramento, CA 95817, USA
Marian A. Spath, Department of Human Genetics, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands
Arie P. T. Smits, Department of Human Genetics, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands
Celestine S. Navarro, Department of Pathology, University of California, Davis, Davis, CA, USA
Flora Tassone, Department of Biochemistry and Molecular Medicine, University of California, Davis, Davis, CA, USA; NeuroTherapeutics Research Institute, University of California, Davis, Davis, CA, USA.
Johan M. Kros, Department of Pathology, Erasmus MC, Rotterdam, The Netherlands
Lies-Anne Severijnen, CBG-Department of Clinical Genetics, Erasmus MC, Rotterdam, The Netherlands.
Elizabeth M. Berry-Kravis, Departments of Pediatrics, Neurological Sciences, and Biochemistry, Rush University Medical Center, Chicago, IL, USA
Robert F. Berman, Department of Neurological Surgery, University of California, Davis, Davis, CA, USA; M.I.N.D. Institute, University of California at Davis Medical Center, 2825 50th Street, Sacramento, CA 95817, USA; NeuroTherapeutics Research Institute, University of California, Davis, Davis, CA, USA
Paul J. Hagerman, M.I.N.D. Institute, University of California at Davis Medical Center, 2825 50th Street, Sacramento, CA 95817, USA; Department of Biochemistry and Molecular Medicine, University of California, Davis, Davis, CA, USA; NeuroTherapeutics Research Institute, University of California, Davis, Davis, CA, USA
Rob Willemsen, NeuroTherapeutics Research Institute, University of California, Davis, Davis, CA, USA; CBG-Department of Clinical Genetics, Erasmus MC, Rotterdam, The Netherlands.
Randi J. Hagerman, M.I.N.D. Institute, University of California at Davis Medical Center, 2825 50th Street, Sacramento, CA 95817, USA; NeuroTherapeutics Research Institute, University of California, Davis, Davis, CA, USA; Department of Pediatrics, University of California at Davis Medical Center, Sacramento, CA, USA
Renate K. Hukema, CBG-Department of Clinical Genetics, Erasmus MC, Rotterdam, The Netherlands
References
- 1.Adams JS, Adams PE, Nguyen DV, et al. Volumetric brain changes in females with fragile X-associated tremor/ataxia syndrome (FXTAS) Neurology. 2007;69(9):851–859. doi: 10.1212/01.wnl.0000269781.10417.7b. [DOI] [PubMed] [Google Scholar]
- 2.Adams PE, Adams JS, Nguyen DV, et al. Psychological symptoms correlate with reduced hippocampal volume in fragile X premutation carriers. Am J Med Genet B. 2010;153B(3):775–785. doi: 10.1002/ajmg.b.31046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akins RS, Boyd A, Coffey S, et al. High frequency of migraine in FMR1 premutation carriers; 11th international fragile X conference; St. Louis. July 23–27.2008. [Google Scholar]
- 4.Allen EG, He W, Yadav-Shah M, Sherman SL. A study of the distributional characteristics of FMR1 transcript levels in 238 individuals. Hum Genet. 2004;114(5):439–447. doi: 10.1007/s00439-004-1086-x. [DOI] [PubMed] [Google Scholar]
- 5.Bailey DB, Jr, Raspa M, Olmsted M, Holiday DB. Co-occurring conditions associated with FMR1 gene variations: findings from a national parent survey. Am J Med Genet A. 2008;146A(16):2060–2069. doi: 10.1002/ajmg.a.32439. [DOI] [PubMed] [Google Scholar]
- 6.Bergink S, Severijnen LA, Wijgers N, et al. The DNA repair-ubiquitin-associated HR23 proteins are constituents of neuronal inclusions in specific neurodegenerative disorders without hampering DNA repair. Neurobiol Dis. 2006;23(3):708–716. doi: 10.1016/j.nbd.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Berman RF, Willemsen R. Mouse models of fragile x-associated tremor ataxia. J Investig Med. 2009;57(8):837–841. doi: 10.231/JIM.0b013e3181af59d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berry-Kravis E, Lewin F, Wuu J, et al. Tremor and ataxia in fragile X premutation carriers: blinded videotape study. Ann Neurol. 2003;53(5):616–623. doi: 10.1002/ana.10522. [DOI] [PubMed] [Google Scholar]
- 9.Berry-Kravis E, Potanos K, Weinberg D, Zhou L, Goetz CG. Fragile X-associated tremor/ataxia syndrome in sisters related to X-inactivation. Ann Neurol. 2005;57(1):144–147. doi: 10.1002/ana.20360. [DOI] [PubMed] [Google Scholar]
- 10.Berry-Kravis E, Abrams L, Coffey SM, et al. Fragile X-associated tremor/ataxia syndrome: clinical features, genetics, and testing guidelines. Mov Disord. 2007;22(14):2018–2030. doi: 10.1002/mds.21493. [DOI] [PubMed] [Google Scholar]
- 11.Berry-Kravis E, Goetz CG, Leehey MA, et al. Neuropathic features in fragile X premutation carriers. Am J Med Genet A. 2007;143(1):19–26. doi: 10.1002/ajmg.a.31559. [DOI] [PubMed] [Google Scholar]
- 12.Berry-Kravis E, Hall D, Leehey M, Hagerman RJ. Treatment and Management of FXTAS. In: Tassone F, Berry-Kravis E, editors. The fragile X-associated tremor ataxia syndrome (FXTAS) Springer; New York: 2011. pp. 137–154. [Google Scholar]
- 13.Bourgeois JA, Farzin F, Brunberg JA, et al. Dementia with mood symptoms in a fragile X premutation carrier with the fragile X-associated tremor/ataxia syndrome: clinical intervention with donepezil and venlafaxine. J Neuropsychiatry Clin Neurosci. 2006;18(2):171–177. doi: 10.1176/jnp.2006.18.2.171. [DOI] [PubMed] [Google Scholar]
- 14.Bourgeois JA, Cogswell JB, Hessl D, et al. Cognitive, anxiety and mood disorders in the fragile X-associated tremor/ataxia syndrome. Gen Hosp Psychiatry. 2007;29(4):349–356. doi: 10.1016/j.genhosppsych.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bourgeois JA, Coffey SM, Rivera SM, et al. A review of fragile X premutation disorders: expanding the psychiatric perspective. J Clin Psychiatry. 2009;70(6):852–862. doi: 10.4088/JCP.08m04476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bourgeois JA, Seritan AL, Casillas EM, et al. Lifetime prevalence of mood and anxiety disorders in fragile X premutation carriers. J Clin Psychiatry. 2011;72(2):175–182. doi: 10.4088/JCP.09m05407blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brouwer JR, Mientjes EJ, Bakker CE, et al. Elevated Fmr1 mRNA levels and reduced protein expression in a mouse model with an unmethylated Fragile X full mutation. Exp Cell Res. 2007;313(2):244–253. doi: 10.1016/j.yexcr.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brouwer JR, Huizer K, Severijnen IA, et al. CGG-repeat length and neuropathological and molecular correlates in a mouse model for fragile X-associated tremor/ataxia syndrome. J Neurochem. 2008;107(6):1671–1682. doi: 10.1111/j.1471-4159.2008.05747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brouwer JR, Severijnen E, de Jong FH, et al. Altered hypothalamus-pituitary-adrenal gland axis regulation in the expanded CGG-repeat mouse model for fragile X-associated tremor/ataxia syndrome. Psychoneuroendocrinology. 2008;33(6):863–873. doi: 10.1016/j.psyneuen.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brouwer JR, Willemsen R, Oostra BA. The FMR1 gene and fragile X-associated tremor/ataxia syndrome. Am J Med Genet B. 2009;150B(6):782–798. doi: 10.1002/ajmg.b.30910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunberg JA, Jacquemont S, Hagerman RJ, et al. Fragile X premutation carriers: Characteristic MR imaging findings in adult males with progressive cerebellar and cognitive dysfunction. Am J Neuroradiol. 2002;23(10):1757–1766. [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Tassone F, Berman RF, et al. Murine hippocampal neurons expressing Fmr1 gene premutations show early developmental deficits and late degeneration. Hum Mol Genet. 2010;19(1):196–208. doi: 10.1093/hmg/ddp479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cilia R, Kraff J, Canesi M, et al. Screening for the presence of FMR1 premutation alleles in women with parkinsonism. Arch Neurol. 2009;66(2):244–249. doi: 10.1001/archneurol.2008.548. [DOI] [PubMed] [Google Scholar]
- 24.Coffey SM, Cook K, Tartaglia N, et al. Expanded clinical phenotype of women with the FMR1 premutation. Am J Med Genet A. 2008;146A(8):1009–1016. doi: 10.1002/ajmg.a.32060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen S, Masyn K, Adams J, et al. Molecular and imaging correlates of the fragile X-associated tremor/ataxia syndrome. Neurology. 2006;67(8):1426–1431. doi: 10.1212/01.wnl.0000239837.57475.3a. [DOI] [PubMed] [Google Scholar]
- 26.Entezam A, Biacsi R, Orrison B, et al. Regional FMRP deficits and large repeat expansions into the full mutation range in a new Fragile X premutation mouse model. Gene. 2007;395(1–2):125–134. doi: 10.1016/j.gene.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez-Carvajal I, Posadas B Lopez, Pan R, Raske C, Hagerman PJ, Tassone F. Expansion of an FMR1 grey-zone allele to a full mutation in two generations. J Mol Diagn. 2009;11(4):306–310. doi: 10.2353/jmoldx.2009.080174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Arocena D, Hagerman PJ. Advances in understanding the molecular basis of FXTAS. Hum Mol Genet. 2010;19(R1):R83–R89. doi: 10.1093/hmg/ddq166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Arocena D, Yang JE, Brouwer JR, et al. Fibroblast phenotype in male carriers of FMR1 premutation alleles. Hum Mol Genet. 2010;19(2):299–312. doi: 10.1093/hmg/ddp497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gokden M, Al-Hinti JT, Harik SI. Peripheral nervous system pathology in fragile X tremor/ataxia syndrome (FXTAS) Neuropathology. 2009;29(3):280–284. doi: 10.1111/j.1440-1789.2008.00948.x. [DOI] [PubMed] [Google Scholar]
- 31.Greco CM, Hagerman RJ, Tassone F, et al. Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain. 2002;125(8):1760–1771. doi: 10.1093/brain/awf184. [DOI] [PubMed] [Google Scholar]
- 32.Greco CM, Berman RF, Martin M, et al. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) Brain. 2006;129(Pt 1):243–255. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]
- 33.Greco CM, Soontarapornchai K, Wirojanan J, Gould JE, Hagerman PJ, Hagerman RJ. Testicular and pituitary inclusion formation in fragile X associated tremor/ataxia syndrome. J Urol. 2007;177(4):1434–1437. doi: 10.1016/j.juro.2006.11.097. [DOI] [PubMed] [Google Scholar]
- 34.Greco CM, Tassone F, Garcia Arocena D, et al. Clinical and neuropathologic findings in a woman with the FMR1 premutation and multiple sclerosis. Arch Neurol. 2008;65(8):1114–1116. doi: 10.1001/archneur.65.8.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hagerman RJ, Leehey M, Heinrichs W, et al. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001;57(1):127–130. doi: 10.1212/wnl.57.1.127. [DOI] [PubMed] [Google Scholar]
- 36.Hagerman RJ, Coffey SM, Maselli R, et al. Neuropathy as a presenting feature in fragile X-associated tremor/ataxia syndrome. Am J Med Genet A. 2007;143(19):2256–2260. doi: 10.1002/ajmg.a.31920. [DOI] [PubMed] [Google Scholar]
- 37.Hagerman RJ, Hall D, Coffey S, et al. Treatment of fragile X-associated tremor ataxia syndrome (FXTAS) and related neurological problems. Clin Interv Aging. 2008;3(2):251–262. doi: 10.2147/cia.s1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hall D, Pickler L, Riley K, Tassone F, Hagerman RJ. Parkinsonism and cognitive decline in a fragile X mosaic male. Mov Disord. 2010;25(10):1523–1524. doi: 10.1002/mds.23150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hessl D, Tassone F, Loesch DZ, et al. Abnormal elevation of FMR1 mRNA is associated with psychological symptoms in individuals with the fragile X premutation. Am J Med Genet B. 2005;139B(1):115–121. doi: 10.1002/ajmg.b.30241. [DOI] [PubMed] [Google Scholar]
- 40.Hunsaker MR, Wenzel HJ, Willemsen R, Berman RF. Progressive spatial processing deficits in a mouse model of the fragile X premutation. Behav Neurosci. 2009;123(6):1315–1324. doi: 10.1037/a0017616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hunsaker MR, Goodrich-Hunsaker NJ, Willemsen R, Berman RF. Temporal ordering deficits in female CGG KI miceheterozygous for the fragile X premutation. Behav Brain Res. 2010;213(2):263–268. doi: 10.1016/j.bbr.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hunsaker MR, von Leden RE, Ta BT, et al. Motor deficits on a ladder rung task in male and female adolescent and adult CGG knock-in mice. Behav Brain Res. 2011;222(1):117–121. doi: 10.1016/j.bbr.2011.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iwahashi CK, Yasui DH, An HJ, et al. Protein composition of the intranuclear inclusions of FXTAS. Brain. 2006;129(Pt 1):256–271. doi: 10.1093/brain/awh650. [DOI] [PubMed] [Google Scholar]
- 44.Jacquemont S, Hagerman RJ, Leehey M, et al. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am J Hum Genet. 2003;72(4):869–878. doi: 10.1086/374321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jacquemont S, Hagerman RJ, Leehey MA, et al. Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. JAMA. 2004;291(4):460–469. doi: 10.1001/jama.291.4.460. [DOI] [PubMed] [Google Scholar]
- 46.Jacquemont S, Hagerman RJ, Hagerman PJ, Leehey MA. Fragile-X syndrome and fragile X-associated tremor/ataxia syndrome: two faces of FMR1. Lancet Neurol. 2007;6(1):45–55. doi: 10.1016/S1474-4422(06)70676-7. [DOI] [PubMed] [Google Scholar]
- 47.Kaufmann H, Biaggioni I. Autonomic failure in neurodegenerative disorders. Semin Neurol. 2003;23(4):351–363. doi: 10.1055/s-2004-817719. [DOI] [PubMed] [Google Scholar]
- 48.Kovari E, Horvath J, Bouras C. Neuropathology of Lewy body disorders. Brain Res Bull. 2009;80(4–5):203–210. doi: 10.1016/j.brainresbull.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 49.Kulikova-Schupak R, Knupp KG, Pascual JM, Chin SS, Kairam R, Patterson MC. Rectal biopsy in the diagnosis of neuronal intranuclear hyaline inclusion disease. J Child Neurol. 2004;19(1):59–62. doi: 10.1177/08830738040190010707. [DOI] [PubMed] [Google Scholar]
- 50.Leehey M, Berry-Kravis E, Goetz CG, Hagerman R. Clinical neurological phenotype of FXTAS. In: Tassone F, Berry-Kravis E, editors. The fragile X-associated tremor ataxia syndrome (FXTAS) New York; Springer: 2011. pp. 1–16. [Google Scholar]
- 51.Leehey M, Legg W, Tassone F, Hagerman R. Fibromyalgia in fragile X mental retardation 1 gene premutation carriers. Rheumatology, Oxford. 2011 doi: 10.1093/rheumatology/ker273. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leehey MA, Berry-Kravis E, Min SJ, et al. Progression of tremor and ataxia in male carriers of the FMR1 premutation. Mov Disord. 2007;22(2):203–206. doi: 10.1002/mds.21252. [DOI] [PubMed] [Google Scholar]
- 53.Loesch DZ, Litewka L, Brotchie P, Huggins RM, Tassone F, Cook M. Magnetic resonance imaging study in older fragile X premutation male carriers. Ann Neurol. 2005;58(2):326–330. doi: 10.1002/ana.20542. [DOI] [PubMed] [Google Scholar]
- 54.Louis E, Moskowitz C, Friez M, Amaya M, Vonsattel JP. Parkinsonism, dysautonomia, and intranuclear inclusions in a fragile X carrier: a clinical-pathological study. Mov Disord. 2006;21(3):420–425. doi: 10.1002/mds.20753. [DOI] [PubMed] [Google Scholar]
- 55.Munoz DG. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2002;58(6):987. doi: 10.1212/wnl.58.6.987-a. (author reply 987–988) [DOI] [PubMed] [Google Scholar]
- 56.Nishie M, Mori F, Fujiwara H, et al. Accumulation of phosphorylated alpha-synuclein in the brain and peripheral ganglia of patients with multiple system atrophy. Acta Neuropathol. 2004;107(4):292–298. doi: 10.1007/s00401-003-0811-1. [DOI] [PubMed] [Google Scholar]
- 57.Peprah E, He W, Allen E, Oliver T, Boyne A, Sherman SL. Examination of FMR1 transcript and protein levels among 74 premutation carriers. J Hum Genet. 2010;55(1):66–68. doi: 10.1038/jhg.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peprah EK, Allen EG, Williams SM, Woodard LM, Sherman SL. Genetic diversity of the fragile X syndrome gene (FMR1) in a large Sub-Saharan West African population. Ann Hum Genet. 2010;74(4):316–325. doi: 10.1111/j.1469-1809.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qin M, Entezam A, Usdin K, et al. A mouse model of the fragile X premutation: Effects on behavior, dendrite morphology, and regional rates of cerebral protein synthesis. Neurobiol Dis. 2011;42(1):85–98. doi: 10.1016/j.nbd.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raske C, Hagerman PJ. Molecular pathogenesis of fragile X-associated tremor/ataxia syndrome. J Investig Med. 2009;57(8):825–829. doi: 10.231/JIM.0b013e3181be329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reis AH, Ferreira AC, Gomes KC, et al. Frequency of FMR1 premutation in individuals with ataxia and/or tremor and/or parkinsonism. Genet Mol Res. 2008;7(1):74–84. doi: 10.4238/vol7-1gmr357. [DOI] [PubMed] [Google Scholar]
- 62.Roberts J, Mazzocco MM, Murphy MM, Hoehn-Saric R. Arousal modulation in females with fragile X or Turner syndrome. J Autism Dev Disord. 2008;38(1):20–27. doi: 10.1007/s10803-007-0356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roberts JE, Bailey DB, Jr, Mankowski J, et al. Mood and anxiety disorders in females with the FMR1 premutation. Am J Med Genet B. 2009;150B(1):130–139. doi: 10.1002/ajmg.b.30786. [DOI] [PubMed] [Google Scholar]
- 64.Rodriguez-Revenga L, Madrigal I, Pagonabarraga J, et al. Penetrance of FMR1 premutation associated pathologies in fragile X syndrome families. Eur J Hum Genet. 2009;17(10):1359–1362. doi: 10.1038/ejhg.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ross-Inta C, Omanska-Klusek A, Wong S, et al. Evidence of mitochondrial dysfunction in fragile X-associated tremor/ataxia syndrome. Biochem J. 2010;429(3):545–552. doi: 10.1042/BJ20091960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sellier C, Rau F, Liu Y, et al. Sam68 sequestration and partial loss of function are associated with splicing alterations in FXTAS patients. EMBO J. 2010;29(7):1248–1261. doi: 10.1038/emboj.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Soontarapornchai K, Maselli R, Fenton-Farrell G, et al. Abnormal nerve conduction features in fragile X premutation carriers. Arch Neurol. 2008;65(4):495–498. doi: 10.1001/archneur.65.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sullivan AK, Marcus M, Epstein MP, et al. Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod. 2005;20(2):402–412. doi: 10.1093/humrep/deh635. [DOI] [PubMed] [Google Scholar]
- 69.Takahashi-Fujigasaki J. Neuronal intranuclear hyaline inclusion disease. Neuropathology. 2003;23(4):351–359. doi: 10.1046/j.1440-1789.2003.00524.x. [DOI] [PubMed] [Google Scholar]
- 70.Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TW, Hagerman PJ. Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am J Hum Genet. 2000;66(1):6–15. doi: 10.1086/302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tassone F, Hagerman RJ, Garcia-Arocena D, Khandjian EW, Greco CM, Hagerman PJ. Intranuclear inclusions in neural cells with premutation alleles in fragile X associated tremor/ataxia syndrome. J Med Genet. 2004;41(4):e43. doi: 10.1136/jmg.2003.012518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tassone F, Iwahashi C, Hagerman PJ. FMR1 RNA within the intranuclear inclusions of fragile X-associated tremor/ataxia syndrome (FXTAS) RNA Biol. 2004;1(2):103–105. doi: 10.4161/rna.1.2.1035. [DOI] [PubMed] [Google Scholar]
- 73.Van Dam D, Errijgers V, Kooy RF, et al. Cognitive decline, neuromotor and behavioural disturbances in a mouse model for fragile-X-associated tremor/ataxia syndrome (FXTAS) Behav Brain Res. 2005;162(2):233–239. doi: 10.1016/j.bbr.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 74.Wakabayashi K, Mori F, Tanji K, Orimo S, Takahashi H. Involvement of the peripheral nervous system in synucleinopathies, tauopathies and other neurodegenerative proteinopathies of the brain. Acta Neuropathol. 2010;120(1):1–12. doi: 10.1007/s00401-010-0706-x. [DOI] [PubMed] [Google Scholar]
- 75.Wenzel HJ, Hunsaker MR, Greco CM, Willemsen R, Berman RF. Ubiquitin-positive intranuclear inclusions in neuronal and glial cells in a mouse model of the fragile X premutation. Brain Res. 2010;1318:155–166. doi: 10.1016/j.brainres.2009.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Willemsen R, Hoogeveen-Westerveld M, Reis S, et al. The FMR1 CGG repeat mouse displays ubiquitin-positive intranuclear neuronal inclusions; implications for the cerebellar tremor/ataxia syndrome. Hum Mol Genet. 2003;12(9):949–959. doi: 10.1093/hmg/ddg114. [DOI] [PubMed] [Google Scholar]
- 77.Yachnis AT, Roth HL, Heilman KM. Fragile X dementia Parkinsonism syndrome (FXDPS) Cogn Behav Neurol. 2010;23(1):39–43. doi: 10.1097/WNN.0b013e3181b6e1b9. [DOI] [PubMed] [Google Scholar]
- 78.Yamada M, Hayashi S, Tsuji S, Takahashi H. Involvement of the cerebral cortex and autonomic ganglia in Machado-Joseph disease. Acta Neuropathol. 2001;101(2):140–144. doi: 10.1007/s004010000277. [DOI] [PubMed] [Google Scholar]