Abstract
The eye is the human organ most sensitive to vitamin A deficiency because of vision's absolute and heavy dependence on vitamin A for light perception. Studies of the molecular basis of vision have provided important insights into the intricate mechanistic details of the function, transport and recycling of vitamin A and its derivatives (retinoid). This review focuses on retinoid-related membrane receptors and transporters. Three kinds of mammalian membrane receptors and transporters are discussed: opsins, best known as vitamin A-based light sensors in vision; ABCA4, an ATP-dependent transporter specializes in the transport of vitamin A derivative; and STRA6, a recently identified membrane receptor that mediates cellular uptake of vitamin A. The evolutionary driving forces for their existence and the wide spectrum of human diseases associated with these proteins are discussed. Lessons learned from the study of the visual system might be useful for understanding retinoid biology and retinoid-related diseases in other organ systems as well.
Keywords: vitamin A, retinoid, STRA6, retinol binding protein, opsin, ABCA4
1. Introduction
Vitamins are essential organic molecules that an organism depends on for survival. An essential organic molecule becomes a vitamin if the organism cannot synthesize it and must obtain it from the diet. The principle diet of an organism (or its ancestor) may determine that organism's definition of vitamins. A well-known example is vitamin C, which is not a vitamin for many organisms such as mice [1], but vitamin C intake is essential for humans. Human ancestors likely had easy access to vitamin C-rich food and thus lost the ability (and the need) to synthesize vitamin C. A key feature of vitamin biology is that vitamin status is heavily influenced by the environment. In addition to diet, other environmental factors may also influence vitamin status. For example, human skin can produce vitamin D in the presence of UV light [2] (in this sense, vitamin D is not a true vitamin). Therefore, both light exposure and human skin color influence vitamin D status and diseases related to vitamin D deficiency [3]. Human intestinal microbes can produce vitamin K to alleviate its deficiency [4].
Vitamin A is arguably the most multifunctional vitamin in the human body and is essential for human survival at every point from embryogenesis to adulthood. The range of cellular activities it participates in is mind-boggling and is still being discovered. The molecular mechanism of vitamin A's physiological functions was first elucidated for vision [5, 6]. Since then, biological functions of vitamin A have been discovered in almost every vertebrate organ. Examples include its roles in reproduction, embryonic growth and development, immune competence, maintenance of epithelial surfaces, and proper functioning of the adult brain [7–13]. The most well known example of vitamin A's large impact on human survival is the discovery that administration of vitamin A alone is sufficient to decrease childhood mortality by 20–70% in developing countries [14]. This finding has led to the development of an alternative vitamin A-rich diet to decrease childhood mortality [15]. Except for vision, which depends on the aldehyde form of vitamin A (retinal), most of the physiological functions of vitamin A can be ascribed to the acid form (retinoic acid) acting on nuclear hormone receptors that regulate gene transcription [16, 17]. New biological functions are continuously being discovered for vitamin A derivatives. For example, retinol, the alcohol form of vitamin A, also has distinct biological activities [18–23]. It was recently discovered that retinal inhibits adipogenesis [24, 25] and that retinoic acid regulates synaptic protein translation, in addition to its role in regulating gene transcription [26, 27]. Although many organisms (from unicellular organisms to vertebrates) use vitamin A aldehyde for light detection (vision or the equivalent of vision), the biological functions of vitamin A derivatives other than vitamin A aldehyde (e.g., retinoic acid) have only been well defined in vertebrates. Interestingly, retinoic acid was recently isolated from cyanobacteria, but its biological function has not been identified yet [28].
This review focuses on membrane receptors and transporters involved in the function and transport of vitamin A and its derivatives. Three kinds of membrane receptors and transporters involved in retinoid biology are discussed here: opsins, ABCA4 (ABCR) and STRA6. The opsins and ABCA4 are related to vitamin A aldehyde. Opsins use vitamin A aldehyde as the chromophore for light absorption, and ABCA4's function is to accelerate the elimination of vitamin A aldehyde in light-bleached photoreceptor cells. STRA6 is related to alcohol form of vitamin A, retinol, which is the major transport form of vitamin A in the blood.
2. Retinoid-Related Membrane Receptors and Transporters
2.1. Opsins: Vitamin A-based transmembrane light sensor
2.1.1. Biological functions
Visual pigments are the light-absorbing proteins in vision that use vitamin A-aldehyde as the chromophore. They belong to the seven-transmembrane domain G-protein coupled receptor family. Visual pigments in each organism are uniquely suited to the needs of each organism. Human has three cone visual pigments (the long-wave, medium wave and short wave pigments) responsible for bright light vision and trichromatic color vision [29–31] and one rod visual pigment rhodopsin responsible for dim light vision [32–35] (Figure 1). Mice do not have the long-wave cone pigment but have a medium wave cone pigment [36] and a UV cone pigment [37] responsible for dichromatic vision. The discovery of visual pigments has led to the discovery of many homologous proteins (opsins) that are expressed in rod and cone photoreceptor cells and/or other cell types. For example, chicken has a light-sensitive pinopsin in its pineal gland [38, 39]. In one extreme example of visual adaptation, a fish that has vision both above and below water (Anableps anableps) was recently found to have ten different opsins expressed in its eye [40]. Frog has melanopsin, which is an opsin expressed in its skin melanocytes, eye and brain [41]. Melanopsin is also expressed in light sensitive ganglion cells in the mammalian retina [42, 43] that, together with rod and cone photoreceptor cells, sense light for the circadian clock in the brain [44–47]. Vertebrate ancient (VA) opsin is an opsin expressed in the horizontal and amacrine cells of the retina and also in the brain of salmon [48, 49]. Parietopsin and pinopsin are expressed in the parietal eye of the lizard [50].
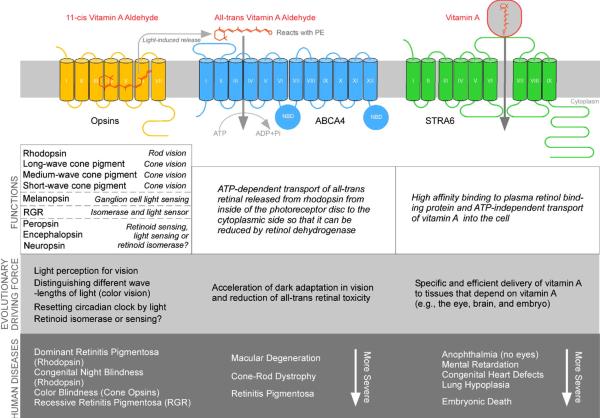
Figure 1.
Human membrane receptors and transporters discussed in this review that mediate the function and transport of vitamin A and its derivatives. The transmembrane topologies of these proteins are depicted in the upper picture. Rhodopsin is depicted in the picture as an example for opsins. Physiological functions, likely evolutionary driving forces, and human diseases associated with these proteins are presented in the lower picture.
There are also opsins that are unlikely to sense light for light perception. Peropsin and retinal G protein-coupled receptor (RGR) are two opsin family members expressed in the retinal pigment epithelium (RPE) cell, a highly pigmented cell type that primarily functions as the “caretaker” of photoreceptor cells in the eye [51, 52]. Peropsin is a plasma membrane protein, expressed specifically on the apical microvilli of the RPE cells (Figure 2) [53]. RGR is expressed on the intracellular membrane of the RPE cells [54] (Figure 2). Both peropsin and RGR share the signature lysine residue in the seventh transmembrane helix that is used by opsins to attach to the chromophore. RGR has been shown to have light-dependent isomerase activity [55, 56] and also regulates the primary retinoid isomerase in a light-independently manner [57]. RGR also mediates light-dependent translocation of retinyl esters [58]. Peropsin homologs have been identified in amphioxus [59] and spider [60]. These peropsins bind to all-trans-retinal as a chromophore and mediate light-dependent isomerization of the chromophore to the 11-cis form [59, 60]. Encephalopsin [61] and neuropsin [62] are two opsins that are expressed in the mammalian brain and other tissues. Although neuropsin's bird homolog is responsible for light sensitivity of the bird brain [63], the roles of these two opsins in the mammalian brain, which is not known to be photosensitive, are unknown. Given the fact that bovine rhodopsin can be functionally expressed in the mouse brain to sense light without exogenous retinal chromophore [64], these native opsins in the brain may have sufficient access to the retinal chromophore. The visual cycle to regenerate 11-cis retinal for vision is not known to be present in the brain. The likely endogenous retinal chromophore in the brain that can regenerate opsins is 9-cis retinal because 9-cis retinal can reconstitute functional rhodopsin [65, 66] and 9-cis retinoid is known to exist in vivo [67–69].
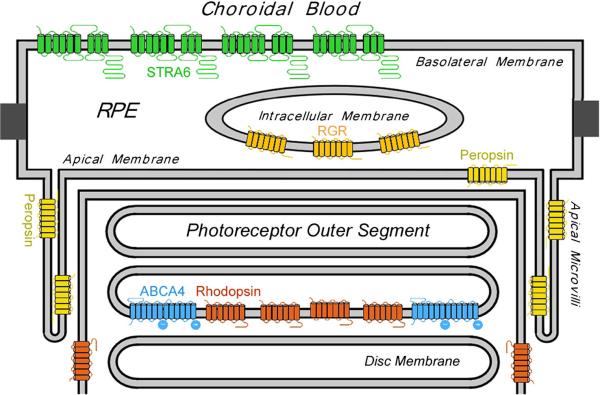
Figure 2.
Retinoid-related membrane receptors and transporters in the RPE and photoreceptor outer segment are localized to highly specific cellular structures. Rhodopsin is localized to both the disc membrane and the plasma membrane of the photoreceptor outer segment. ABCA4 is localized only to the disc membrane of the outer segment. It is unclear why rhodopsin is expressed on the plasma membrane where there is no ABCA4. Peropsin is localized to the apical microvilli of the RPE cell surface. RGR is localized to the internal membrane of the RPE cells. STRA6 is localized to the basolateral membrane of the RPE cells.
2.1.2. Evolutionary roles
Light is not only the ultimate energy source of most living organisms on earth, it is also used throughout evolution as an information source. One definition of “light” is the region of the electromagnetic spectrum to which photoreceptor cells can respond (that's why X-rays are not considered “light”). The use of vitamin A-based chromophore for light detection is extremely ancient and widespread throughout evolution. More than 300 prokaryotic and eukaryotic seven-transmembrane domain proteins are known to use vitamin-A aldehyde as the chromophore for light absorption [70]. Although there are other chromophores in biology [71], two unique properties of vitamin A aldehyde likely explain why it is the most popular chromophore for photoreception in evolution. It combines readily with proteins through its aldehyde end and can create photopigments with absorption maxima in the range of the peak wavelengths of sunlight reaching earth surface. In addition, its long chain of conjugated double bonds allows drastic light-induced geometrical isomerization, which is the basis of initiation of signal transduction. Interestingly, all multicellular organisms that depend on retinal chromophore for light perception use the 11-cis form, despite the existence of other forms (e.g., 9-cis, 13-cis and all-trans forms). Most vertebrate visual pigments use 11-cis retinal, but some fishes and amphibians use 11-cis 3,4-dehydroretinal as a second chromophore [72, 73]. Invertebrates use 11-cis 3-hydroxylretinal, 11-cis 4-hydroxylretinal, 11-cis, 3,4-hydroxylretinal and 11-cis retinal as chromophores [74, 75]. In contrast, all unicellular organisms use all-trans retinal as the chromophore [70].
The evolutionary driving forces for the emergence of vitamin A-based light sensors include not only light detection but also differentiation of different wavelengths of light (color vision). By conjugating covalently to opsins, retinal's absorption maximum can be red-shifted away from the UV range. In addition to avoiding damage from UV light, the red-shift allows the retinal chromophore to have different absorption maxima that are influenced by the opsin proteins [36, 76–79]. Diversification of absorption maxima of visual pigments is required for color vision. The emergence of a new color vision sensation can be driven by the emergence of new visual pigments. It has been demonstrated in animal models that expressing a new cone visual pigment alone is sufficient to drive the new sensation in color space [80–82]. In addition to rod and cone photoreceptor cells in the eye, opsin expression has made other cell types in the eye [41, 42, 58, 83, 84] and extraocular tissues, such as skin and brain, light-sensitive [38, 39, 41, 50, 63].
In addition to light detection, opsins may have other functions. Vertebrate rhodopsin was recently found to function as a lipid flipase [85]. This will be discussed later in the context of ABCA4. Rhodopsin in Drosophila larvae, but not adults, was recently found to function as a thermosensor in addition to its role as a light sensor [86]. Because vitamin A is required for this activity, this is the first time a vitamin A-based protein has been linked to thermosensation. Since the human skin, not the eye, is known to be important in thermo sensation, opsin expression elsewhere in the body may be relevant for the task (Figure 3).
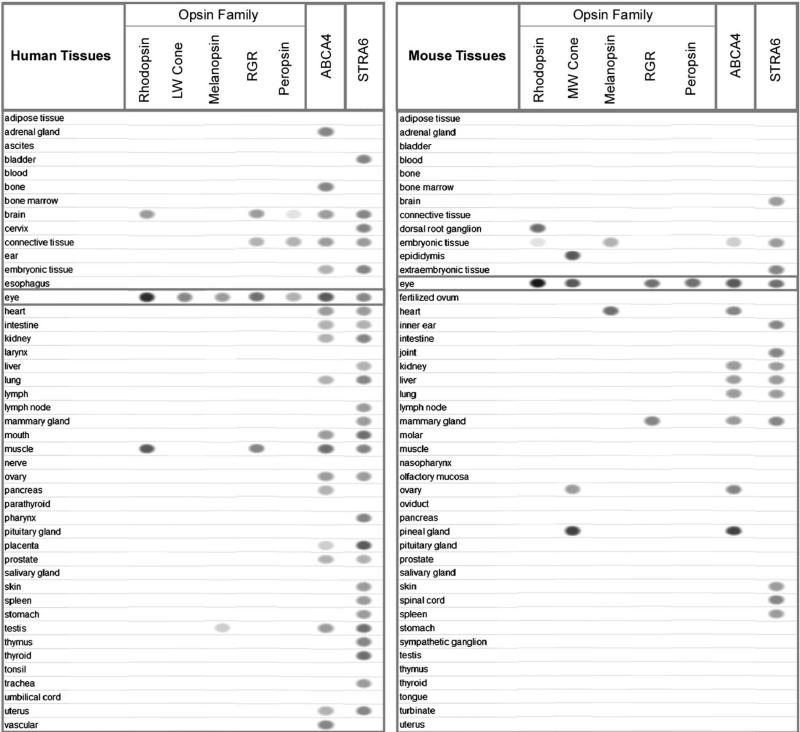
Figure 3.
Distribution of retinoid-related membrane receptors and transporters in human and mouse tissues. The intensity of the ovals indicates the abundance of each message as suggested by EST (Expressed Sequence Tag) counts recorded by NCBI's EST Profile Viewer. The absence of the oval for a particular tissue means no detectable expression. Abundant expression from rare cell types, such as the RPE cells, may be underrepresented in this analysis.
2.1.3. Human diseases
Mutations in cone visual pigments cause color blindness [30, 31, 87]. Red/green color blindness is in fact the most common single-locus genetic disorder in humans. Rhodopsin mutations are implicated in two distinct human diseases: retinitis pigmentosa [88–92], which is a progressive form of retinal degeneration that primarily targets the rod photoreceptor cells, and congenital night blindness [93–96], a non-progressive form of blindness that affects rod vision. Mutations in RGR have also been associated with retinitis pigmentosa [97]. Human diseases associated with other opsins (e.g., melanopsin and peropsin) have not yet been identified.
2.2. ABCA4 (ABCR): ATP-dependent transport of light-bleached chromophore
2.2.1. Biological function
ABCA4 (ABCR) is the only known member of the large ATP-binding cassette (ABC) transporter family that specializes in the transport of vitamin A derivatives [98, 99]. ABCA4 is the only gene associated with recessive Stargardt macular dystrophy, the most common form of early-onset macular degeneration [100]. ABCA4 was also independently identified biochemically as the photoreceptor Rim protein [101, 102]. ABC transporters exist from bacteria to human and are known to transport a wide variety of molecules such as sugars, peptides, metals, lipids, and drugs across membranes. CFTR, a member of the ABC transporter family, even functions as a chloride channel. ABCA4 is most homologous to ABCA1, the Tangier disease protein involved in cholesterol efflux [103, 104], but ABCA4 is unlikely involved in cholesterol transport. What might ABCA4 transport?
Based on the phenotypes of ABCA4 knockout mice [105] and biochemical properties of purified and reconstituted ABCA4 from photoreceptor cells [106–108], it was hypothesized that ABCA4 functions to transport vitamin A aldehyde (retinaldehyde) released from bleached rhodopsin from the disc membrane so that it can be reduced by retinol dehydrogenase in the cytoplasm.
This hypothesis has been confirmed by overwhelming independent evidence. Traditional transport assays that depend on the separation of substrates from liposomes do not apply to retinaldehyde because of its tight association with membranes (more technical challenges discussed below). Since ABC transporters' substrates are known to stimulate their ATP hydrolysis (as their transport consumes ATP), analyzing ATP hydrolysis of purified and reconstituted transporter is another potential method to find the substrate. ABCA4 was purified from bovine eyes and reconstituted into liposomes. Retinaldehyde and a large number of compounds including different retinoids were tested for their ability to stimulate the ATP hydrolysis of ABCA4 [106]. Indeed, retinaldehyde is one of few compounds that stimulate ABCA4's ATP hydrolysis and is the only compound that behaves as expected of a substrate, with a simple Michaelis-Menten behavior indicative of a single-saturable binding site and an interaction with ABCA4 at a rate-limiting step in the ATP hydrolysis cycle [106]. Although ABCA4 does not distinguish different isomers of retinaldehyde [106], its predominant substrate is likely all-trans retinal, not 11-cis retinal, given the rapid binding of 11-cis retinal to apo-opsin. Phenotypes of ABCA4 knockout mice provided independent strong support for the role of ABCA4 in clearing all-trans retinal from disc membranes [105]. ABCA4 knockout mice show delayed dark adaptation, consistent with delayed clearance of all-trans retinal, which likely increases the noise of photoreceptor cells by activating rhodopsin [109, 110]. Interestingly, phosphatidylethanolamine (PE) and the adduct formed between retinal and PE, N-retinylidene-PE are significantly elevated in ABCA4 knockout mice [105]. It was proposed that ABCA4 functions as an N-retinylidene-PE flippase. Solid phase binding assay demonstrated that N-retinylidene-PE, not free retinal, is the preferred substrate for ABCA4 [111, 112]. This is consistent with the propensity of retinaldehyde to form Schiff base with primary amines and the finding that retinaldehyde stimulation of ABCA4's ATP hydrolysis depends on the presence of PE and is absent in phosphatidylcholine vesicles [106, 107]. N-retinylidene-PE binds to ABCA4 at a 1:1 ratio and can be quantitatively released by addition of ATP [111].
There are several interesting puzzles concerning ABCA4's biochemical function. First, ABCA1, the closest homolog of ABCA4, functions in cholesterol efflux from cells [103, 104]. ABCA1 has also recently been shown to be involved in retinol efflux in intestinal cells during intestinal absorption of vitamin A [113]. Surprisingly, ABCA4 functions in the opposite transport direction as ABCA1 (influx not efflux).
Second, due to technical challenges and the fact that retinal diffuses through membranes by itself, ABCA4's transport activity has not been directly demonstrated using purified ABCA4 (despite the efforts of several groups). Retinal's ability to diffuse through membranes without ABCA4's help has been confirmed by three studies that detected little or no role of ABCA4 in all-trans retinal clearance or all-trans retinol formation after light bleaching [105, 114, 115]. Although the fraction of retinal transported to the cytoplasmic side of the disc membrane by ABCA4 may be very small, this fraction is still detrimental in the long term if not removed by ABCA4; this will be discussed later in ABCA4-associated human diseases.
Third, it was recently found using a novel lipid flippase assay that rhodopsin functions as a lipid flippase with high efficiency but little specificity for lipid head groups [85]. Given its high abundance in rod outer segment (90% of total protein) and its extreme high efficiency as a lipid flippase [85], it is hard to image why the much lower-abundance ABCA4 is needed. Since rhodopsin's lipid flippase activity does not depend on ATP, it would be interesting to add ATP to observe ABCA4's activity in the same outer segment preparation that demonstrated the rapid flippase activity of rhodopsin [85].
Fourth, ABCA4 is expressed in other tissues, which are not known to have high flux of retinaldehyde like the photoreceptor cells after light bleaching (Figure 3). For example, in situ hybridization and Western blot analysis showed that ABCA4 is expressed in the choroid plexus [116], which is part of the blood brain barrier. What is the function of ABCA4 in extraocular tissues? It was previously observed that both retinol and retinoic acid (but not retinoid analog □-ionone) can stimulate the ATP hydrolysis of ABCA4, although they are not as potent as retinaldehyde [106]. In addition to a retinoid transporter/lipid flippase, another possibility is that in other tissues, ABCA4 functions similarly to ABCA1, its closest homolog that functions in cholesterol and retinol efflux from cells [103, 104, 113].
2.2.2. Evolutionary roles
The evolutionary driving force for the emergence of a protein may be best understood by comparing a system that depends on it and a similar system that does not depend on it. Why does vertebrate vision depend on ABCA4, while invertebrate vision does not? The ultimate reason may originate from a basic difference in the visual pigments. Invertebrate visual pigments do not release their chromophore after light absorption and can revert the chromophore back to the original state by subsequent light absorption. Therefore, invertebrate visual pigments are called bistable pigments [117]. Invertebrate opsins can also function as light-dependent isomerase to convert retinal from the all-trans form to 11-cis form [118]. In contrast, vertebrate visual pigments release the retinal chromophore after light bleaching. The consequence of this release (or instability after light bleaching) is that there is a need to regenerate the released chromophore after every photoisomerization reaction. For vertebrate visual pigments, the released chromophore goes through a series of complex enzymatic reactions and transport events called the visual cycle before it is converted back to the 11-cis retinal chromophore [119–122]. Although it has generally been assumed that bistable pigments do not need a visual cycle to regenerate, it was recently discovered that Drosophila does have enzymes involved in the regeneration of visual chromophore [123]. Unlike vertebrate rhodopsins, Drosophila rhodopsin can be internalized and degraded in a light-dependent manner. Although a bistable pigment does not need chromophore regeneration after photoisomerization, Drosophila regenerates its chromophore released from degraded rhodopsin, not bleached rhodopsin [123]. This regeneration is important during nutritional deficiency.
Why does vertebrate vision use the kind of pigments that require much higher maintenance, with a complex regeneration cycle after every photoisomerization event? Vertebrates do have bistable opsins, such as parapinopsin in the lamprey pineal gland [124] and melanopsin in the vertebrate retina [125]. Like invertebrate opsins, melanopsin can even form a functional pigment with all-trans retinal due to its intrinsic isomerase activity [125, 126]. What are the advantages of the complex regeneration cycle for vertebrate visual pigments? The first likely advantage is that they can be regenerated in complete darkness, unlike bistable pigment, which depends on light to regenerate. A photoreceptor cell with a visual pigment that can be regenerated in complete darkness can function better and continuously in darkness at night. Invertebrates can also regenerate their pigment in the dark in the initial formation of the bistable pigment [127]. The second likely advantage is spectral discrimination. Although both vertebrates and invertebrates have color vision, a bistable pigment that has two absorption maxima (one for activation and one for regeneration) may be less precise than a pigment that has only one major absorption maximum for distinguishing different wavelength of lights, which is the basis of color vision.
Given its essential role in vision, the vertebrate visual cycle has been the subject of intense investigation. Most, but not all, components of the vertebrate visual cycle have been identified [119]. ABCA4 is part of the vertebrate visual cycle. However, ABCA4 was an unexpected and unknown link in the visual cycle because there was no previous biochemical or physiological evidence for the existence of an ATP-dependent transporter for vitamin A-derivative in vision. Specifically, there was no previous evidence that retinol dehydrogenase needs the assistance from a transporter to provide its all-trans retinal substrate. As mentioned above, even after the discovery of ABCA4, kinetic studies of all-trans retinal clearance or all-trans retinol formation after light bleaching using ABCA4 knockout mice have demonstrated that the vast majority of all-trans retinal released from rhodopsin does not need ABCA4's help to pass through disc membranes to reach retinol dehydrogenase [105, 114, 115]. Consistently, it has been extremely challenging to demonstrate transport or flippase activity using purified ABCA4. What is the evolutionary driving force for the existence of a transporter that is seemingly doing so little biochemically?
Compared to bistable pigments, vertebrate visual pigments have a serious downside. Because the chromophore is released after every photoisomerization event, rhodopsin still keeps on releasing retinal chromophore even in daylight when rod photoreceptors are saturated and not functional for visual perception. Despite the fact that retinal can diffuse through membranes by itself, the high flux of retinal in the vertebrae photoreceptor cells during daytime can constantly generate such a high amount of all-trans retinal that it needs ABCA4's help to completely pass through membrane so that it can be reduced to the less toxic retinol form. All-trans retinal can activate rhodopsin independently of light [109, 110]. Without ABCA4, the accumulated retinal increases the noise of photoreceptor cells and delay dark adaptation. ABCA4's ability to accelerate dark adaptation [105, 128] is obviously of immediate value to vertebrate survival. Dark adaption makes it possible for vision to quickly adjust to much dimmer background light levels [129, 130]. For example, faster dark adaptation can make a life and death difference for a caveman entering a dark cave with a bear inside. Another likely driving force is ABCA4's contribution to reduced all-trans retinal toxicity due to the large amount of all-trans retinal generated by photoreceptor cells in daylight [131, 132].
2.2.3. Human diseases
Human diseases associated with ABCA4 are surprisingly diverse (Figure 1). ABCA4 was first discovered as responsible for Stargardt macular dystrophy, the most common form of early-onset macular degeneration [100]. ABCA4 has also been implicated in retinitis pigmentosa [133–135] and cone rod dystrophy [135–137]. Genetic studies have linked both ABCA4 [138] and its closest homolog ABCA1 [139, 140] to age-related related macular degeneration, the leading cause of blindness in the elderly. Diseased-associated ABCA4 variants have been functionally analyzed [141, 142]. It is generally hypothesized that the degree of loss of ABCA4 function determines the disease association (Stargardt disease being the mildest, cone rod dystrophy a more severe form, and retinitis pigmentosa the most severe form).
ABCA4 is the only gene known to be responsible for autosomal recessive Stargardt disease, which is characterized by the loss of central vision, progressive atrophy of the RPE, appearance of orange-yellow flecks around the macula, and dark adaptation defects [128, 143]. Histopathologically, it is characterized by massive accumulation of lipofuscin-like material in the RPE. Stargardt disease is traumatic because of the suddenness in the loss of central vision, normally around the teenage years. What's responsible for the sudden loss of central vision? The key to this question might be the composition of the lipofuscin. Based on the Stargardt disease phenotypes and the localization of ABCA4 in the photoreceptor outer segment, it was hypothesized that the lipofuscin that accumulates in Stargardt disease patients might be N-retinylidene-N-retinylethanolamine (A2E) [105, 106, 144], which is the Schiff base reaction product of two retinaldehyde molecules and ethanolamine and was first identified as a major component of the fluorophore associated with aging human eyes [145, 146]. This hypothesis is tightly linked to the proposed function of ABCR in eliminating all-trans retinal released by bleached opsins, as described above. A2E formation is indeed dramatically higher in ABCA4 knockout mice than the wild-type [105]. A2E has also been detected in Stargardt disease donor eyes [147]. Visualization of A2E in Stargardt patients using fundus autofluorescence imaging provided a potential clue as to why Stargardt disease is manifested as macular degeneration [148, 149], even though ABCA4 is expressed in both rod and cone photoreceptor cells [144, 150]. A2E formation due to the loss of ABCA4 is another strong piece of evidence supporting its role in eliminating all-trans retinal, the precursor to A2E.
Malfunction of ABCA4 leads to the accumulation of all-trans retinal and N-retinylidene-PE in the photoreceptor outer segment [105]. Further condensation of all-trans retinal with N-retinylidene-PE forms the precursors of A2E [146, 147]. A detailed biogenic pathway for A2E formation has been proposed [146, 147]. Although A2E originates from the photoreceptor outer segments, A2E accumulates in RPE cells through a process of daily phagocytosis of the photoreceptor outer segments by the RPE cells [151, 152]. A2E is structurally so unique that no lysosomal enzymes can degrade it. As a result, it gradually accumulates in the RPE cells and this accumulation is irreversible because RPE cells cannot get rid of it [147]. This gradual accumulation of A2E, a toxin, explains why it takes many years for Stargardt patients to lose their sight and the suddenness of that loss. A2E mediates its toxicity as a photooxidizer [153] and a detergent to destabilize membrane [153–155]. A2E also impairs the phagocytic activity of the RPE cells [156] and inhibits retinoid isomerase activity in the RPE cells as an additional means of toxicity [157]. A2E can induce complement activation on RPE cells [158, 159]. Increased complement activation and complement dysregulation due to A2E accumulation in RPE cells have recently been demonstrated using ABCA4 knockout mice [160]. Given the origin of 2E from vitamin A, A2E may be regarded as a new form of vitamin A-related toxicity: a vitamin A derivative that the cell cannot get rid of. A2E (and its related compounds) is the first and only known vitamin A derivative that has only toxic effects and no beneficial functions. In addition to A2E, it was recently found that all-trans retinal accumulation alone can be sufficiently toxic in the retina independently of A2E formation [131, 132].
One hallmark of A2E-related phenotypes is that they can be highly influenced by environmental factors, such as light and retinoid levels. It was first discovered using ABCA4 knockout mice that A2E does not form without light [147]. A2E formation is also influenced by retinoid levels. Vitamin A supplementation worsens A2E related phenotypes [161], while vitamin A deficiency lessens the phenotypes [162]. Based on these findings, two general strategies have been developed to reduce A2E levels: reduction of light exposure and deceleration of the visual cycle. Both strategies have worked effectively in animal models [147, 162, 163]. Other small-molecule based therapies [164] and gene therapy [165, 166] have also been explored to treat ABCA4-associated diseases. Since one of the ultimate reasons that A2E accumulates in RPE is that it cannot be degraded, another treatment strategy is to develop an enzyme that can degrade A2E. It has been demonstrated that A2E can indeed be degraded by an exogenous enzyme (horseradish peroxidase) [167]. Another potential strategy is to grow bacteria on synthesized A2E [146, 168] as the sole carbon source to select a “superbug” that evolves a means to degrade A2E.
2.3. STRA6: cellular uptake of vitamin A from the blood
2.3.1. Biological function
STRA6 was originally identified in cancer cells as a retinoic acid-stimulated gene of unknown function [169, 170]. Through an unbiased strategy of biochemical purification and mass spectrometry, it was identified as the membrane receptor for plasma retinol binding protein (RBP) [171]. RBP is the principle vitamin A carrier protein in the blood [172–178]. STRA6 binds to RBP with high affinity and mediates cellular uptake of vitamin A from the vitamin A/RBP complex (holo-RBP) [171]. Consistent with its role in vitamin A uptake, increasing STRA6 expression in cancer cells by retinoic acid stimulation enhances cellular vitamin A uptake from holo-RBP. Conversely, suppressing STRA6 expression in cancer cells or RPE cells suppresses cellular vitamin A uptake [171], and knocking down STRA6 expression leads to decreased retinoid uptake in the eye [179]. Generation and functional analysis of more than 900 STRA6 mutants have led to the identification of an RBP binding domain in STRA6 [180]. Mutations in any of the three essential residues in this domain are sufficient to abolish the RBP binding and vitamin A uptake activity of STRA6 [180].
STRA6 is expressed in cells or tissues known to depend on vitamin A for proper function. For example, STRA6 is highly expressed in the RPE cells and is specifically localized to the basolateral membrane of the RPE cells [171], the exact cellular location expected of a protein involved in taking up vitamin A from the choroidal blood [181] (Figure 2). Its expression in the immune system is consistent with the role of vitamin A in immune regulation and in preventing infectious diseases [9, 182, 183]. STRA6 is highly expressed in the placenta, which is essential for fetal absorption of vitamin A for embryonic development [178]. STRA6's expression in the brain is consistent with known neuronal functions of vitamin A [10, 11, 184] including recently discovered roles in controlling neuron generation [185] and synaptic protein translation [26, 27]. In general, STRA6 localizes primarily but not exclusively to blood/organ barriers such as blood/retina barrier, blood/brain barrier and maternal/fetal barrier [169, 171]. Both human and animal studies have shown that loss of STRA6 function can lead to severe defects in multiple organs [179, 186, 187]. Human diseases associated with STRA6 mutations will be discussed in detail below.
A number of studies in the past have shown that vitamin A uptake mediated by the RBP receptor does not depend on endocytosis of RBP [181, 188–195]. Consistently, STRA6 has nine transmembrane domains [196], unlike endocytosis receptors which often are single-transmembrane domain proteins. STRA6-mediated vitamin A uptake does not depend on endocytosis of RBP [171]. STRA6's mechanism is highly specific. It mediates retinol uptake only if retinol is bound to RBP, and does not mediate retinol uptake if retinol is bound to BSA or to □-lactoglobulin, a milk retinol binding protein [171].
STRA6 is not homologous to any membrane receptors, transporters or channels of known function, and its sequence provides no clue as to its substrate uptake mechanism. Because STRA6-mediated vitamin A uptake does not depend on cellular energy [171], its substrate uptake mechanism is also not primary or secondary active transport. Because STRA6's substrate, retinol, is not free, has no charge, and can only be provided one molecule at a time by RBP, STRA6's mechanism is also distinct from channels or facilitated transporters, which are driven by an electrochemical gradient of the free substrate. STRA6's mechanism is further complicated by the high affinity interaction between retinol and RBP, its dependence on both RBP binding to deliver retinol and RBP dissociation for the next RBP to bind, and the involvement of intracellular proteins [171]. Therefore, STRA6's mechanism cannot be explained by a known cellular uptake or membrane transport mechanism.
As a new type of multitransmembrane domain protein that has just been studied functionally for a few years, it is not surprising that there are a lot of questions regarding STRA6. The following lists some questions about STRA6's vitamin A uptake mechanism:
2.3.1.1
We [171] and other investigators [179, 197] have found that LRAT stimulates STRA6's vitamin A uptake activity. What is the nature STRA6's vitamin A uptake activity without LRAT? If STRA6 can take up vitamin A without LRAT, why does LRAT stimulate its activity? If STRA6 cannot take up vitamin A (by itself) without LRAT, is STRA6 only a receptor for RBP and does it play any role in vitamin A uptake other than being a receptor for RBP? STRA6 is not an enzyme that can convert retinol to retinyl esters. What is the role of STRA6 in STRA6/LRAT-mediated retinyl ester accumulation? LRAT is localized intracellularly in the ER [198] and cannot physically interact with extracellular RBP.
2.3.1.2
Is LRAT the only protein that can stimulate STRA6's vitamin A uptake activity? If LRAT stimulates retinol uptake because of its ability to store vitamin A, do the cell membranes, which have larger capacity to store retinol, also stimulate retinol uptake? It has been proposed in many previously published hypothetical models of RBP receptor mechanism that cellular retinol binding protein (CRBP) may play an important role in RBP receptor-mediate retinol uptake? This hypothesis is reasonable given the known ability of CRBP-I to supply retinol for LRAT [199, 200]. There are several CRBPs. Can all CRBPs stimulate STRA6's retinol uptake activity? What's the difference between CRBP and LRAT in stimulating vitamin A uptake by STRA6? Why can LRAT stimulate STRA6's vitamin A uptake activity independently of CRBP?
2.3.1.3
Although LRAT can stimulate STRA6's retinol uptake activity, it was discovered previously that STRA6 and LRAT cannot take up retinylamine from the retinylamine/RBP complex [197] or retinoic acid from the retinoic acid/RBP complex [179]. These experiments demonstrated that LRAT is not a general stimulator of STRA6's activity. It is known from previous studies [179, 197, 201, 202] that RBP can bind other retinoids in addition to retinol, although retinol is its natural ligand [172, 173]. Is it still possible for STRA6 to take up other retinoids bound to RBP? Answer to this question can also provide clues to STRA6's mechanism.
2.3.1.4
Without knowing STRA6's mechanism, LRAT's stimulatory effect on STRA6 as observed by us [171] and other investigators [179, 197] suggests that LRAT may “drive” vitamin A uptake into the cell. However, it has been demonstrated that the reverse reaction of vitamin A loading into extracellular apo-RBP still happen in the presence of LRAT [179]. If LRAT drives cellular vitamin A uptake, how does it permit cellular vitamin A loss? One hypothesis to explain retinol loss instead of uptake in the presence of LRAT is that retinyl ester hydrolase [203] present in certain cell types can prevent LRAT from enhancing STRA6's retinol uptake activity to make it possible for partial retinol loss to occur.
2.3.1.5
There are a few interesting questions regarding retinol loading into apo-RBP, since this is a possible mechanism for cells to lose retinoid store. If STRA6 encounters both holo-RBP and apo-RBP, does it mediate cellular vitamin A uptake or cellular vitamin A loss? Does the reverse reaction of vitamin A loading into apo-RBP happen in human blood, which contains mostly holo-RBP and a small fraction of apo-RBP [204] because of the active removal of apo-RBP through kidney filtration? If STRA6 mediates cellular vitamin A loss when it is exposed to the blood, how does it function as the RBP receptor to mediate cellular vitamin A uptake?
2.3.1.6
Scavenger receptor class B, type I (SR-BI) is the receptor for HDL that mediates cellular cholesterol uptake [205]. Mechanistically, STRA6 is perhaps most similar to SR-BI because both membrane proteins take up molecules bound to extracellular carrier proteins (RBP or HDL) without endocytosis. Is STRA6's mechanism the same as SR-BI's mechanism? STAR6 is completely unrelated to SR-BI at the sequence level. STRA6 has 9 transmembrane domains [196], while SR-BI has two [206, 207].
Current research aims to address above questions on STRA6's vitamin A uptake mechanism (unpublished results).
2.3.2. Evolutionary roles
The RBP/STRA6 system of vitamin A delivery is by no means the only possible mechanism for cells or tissues to obtain vitamin A. In some sense, it is surprising that STRA6 (or RBP) exists because there are theoretically much simpler mechanisms to deliver vitamin A or retinoid. First, retinoids clearly have the ability to diffuse systemically by themselves. For example, retinoid-based drugs can be targeted to any organ by random diffusion. Retinoid can “hitchhike” to abundant serum proteins like serum albumin, which binds many small molecules promiscuously, to be transported in the blood. In contrast, each RBP can only bind and deliver one vitamin A molecule and seems to exist primarily for this purpose. Surprisingly, virtually all vitamin A in the blood is bound to RBP under physiological conditions, not to the much more abundant serum albumin. Second, for RBP, why does it need a specific receptor like STRA6 instead of leaking retinol randomly into tissues? Due to its hydrophobicity, retinoid has the ability to pass through membranes without any assistance from a protein.
Evolution came up with the specific vitamin A delivery system (from liver store to RBP to STRA6 on target cells or tissues) for targeted delivery of vitamin A to achieve high specificity and efficiency and to prepare for deficiency. An analogy for the liver/RBP/STRA6 system of vitamin A delivery is water delivery to a house. In this analogy, the liver is the water reservoir and treatment plant. RBP is essential in mobilizing the liver-stored vitamin A [208] and serves as a buffer to maintain stable vitamin A concentration in the blood [175]. The buffering function is important to avoid the adverse effects of both low and high retinoid on the growth and function of diverse organs. Without this buffering function, blood retinoid level would fluctuate dramatically depending on dietary intake. STRA6 binds to RBP with high specificity and affinity [171] and is analogous to the water taps in each house that control water intake. Without water taps that serve to control the outflow, water flowing out randomly would be both wasteful and cause damage. If a nonspecific mechanism were always available (rain or flooding), this specific delivery system would not be essential for survival. However, most, if not all, animals living in natural environments lack continuous access to vitamin A (some don't eat anything for months at a time). In addition to surviving deficiency, the specific mechanism also is much less wasteful and minimizes the damage and side-effects caused by nonspecific mechanism (rain or flooding).
Cellular signaling through retinoids occurs in a precise spatiotemporal manner, and random distribution of retinoids can have toxic effects. The short and long-term toxic side effects caused by random retinoid diffusion are illustrated by toxicity of retinoid drugs such as Accutane that diffuse randomly and are delivered independently of the RBP/STRA6 system [209–211]. Experiments done more than 30 years ago in an animal model [212] and a study of human patients with hypervitaminosis A [213] have shown that more toxicity is associated with vitamin A delivery independent of RBP. An excessive dose of vitamin A is toxic only when the level of vitamin A in the circulation is so high that it is presented to cells in a form other than bound to RBP, such as in retinyl esters [172]. An increase of 10% in retinyl ester is regarded as a sign of vitamin A overload. Toxicity associated with excessive retinoid has also been recently demonstrated using knockout mouse models [131, 132]. Toxicity of excessive endogenous free retinal has also been demonstrated in the Drosophila visual system [214].
STRA6 is a relatively recent “invention” by evolution. Like ABCA4, STRA6 only exists in vertebrates, not in lower organisms. The likely reason to explain the “invention” of STRA6 for vertebrates is that vertebrates use vitamin A for other biological activities such as regulating gene transcription in addition to light sensing. Invertebrates do not need RBP or STRA6 perhaps because precise delivery of vitamin A is unnecessarily complex and costly (in the sense that one RBP only delivers one vitamin A molecule). Random diffusion of retinoid would have much less toxicity if retinoids were not capable of regulating the transcription of hundreds of genes (as in the case of vertebrates). Although invertebrates don't need RBP or STRA6 for vitamin A delivery, they have developed specific mechanism for dietary carotinoids transport. Interestingly the uptake mechanism of □-carotene is shared between the invertebrate and the vertebrate and is mediated by the SR-BI family of receptors [113, 215–218]. One possible explanation is that □-carotene is not as membrane permeable as retinol and requires assistance to efficiently pass through cell membranes.
2.3.3. Human diseases
Consistent with the diverse and critical functions of vitamin A, STRA6 mutations cause a wide spectrum of pathological phenotypes including the absence of eyes (anophthalmia), mental retardation, congenital heart defects, lung hyperplasia, intrauterine growth retardation and embryonic lethality [186, 187, 219–224]. STRA6 is the first example of a retinoid signaling pathway gene whose mutations cause developmental abnormalities in humans [13]. The severe phenotypes caused by STRA6 mutations suggested that there might be less redundancy in vitamin A transport mechanisms than other aspects of retinoid signaling in humans. Redundancy is necessary to ensure that backup mechanisms take over when one mechanism fails. However, the great variability in phenotypes associated STRA6 mutations, as discussed below, suggests that there is redundancy in vitamin A transport as well. Human mutations identified in STRA6 belong to two categories: large deletions and point mutations. All human disease-associated point mutations that have been analyzed abolish STRA6 cell-surface expression, RBP binding and vitamin A uptake activity [180]. Consistently, retinoid analysis showed that knockdown of STRA6 in zebrafish led to decreased tissue retinoid uptake [179].
One hallmark of STRA6 mutations in humans is the tremendous phenotypic variability of pathological phenotypes ranging from embryonic lethality to “mild” eye-specific phenotype, although the eye phenotype is shared by all patients surviving to birth [186, 187, 219–224] (Figure 1). In fact, this is perhaps one of the most extreme examples of phenotypic variability ever known to be caused by mutations of one single human gene. The variability of STRA6 mutation phenotypes in humans is likely caused by variable degrees in the loss of STRA6 function (genetic factor) and the variability in vitamin A intake of the affected individuals (environmental factor). It's well established that vitamin A intake alone (either insufficient or excessive) is sufficient to cause severe developmental defects without any genetic contribution. RBP/STRA6 independent mechanisms of retinoid delivery (e.g., serum albumin bound retinoid independent of liver storage if retinoid intake is sufficiently large) may take over in the absence of RBP or STRA6. But as shown by the extreme severe phenotypes such as embryonic lethality, such a random mechanism often does not work. Without the RBP/STRA6-mediated specific delivery system, an individual is at the “mercy” of the environmental factor by completely depending on constant but not excessive dietary vitamin A intake. As discussed in the above section, problems associated with random mechanisms are what make it necessary to evolve RBP/STRA6 in the first place.
Although currently there is no evidence for its existence, another interesting possibility to account for phenotypic variability are genetic modifiers. For examples, genetic modifiers have been identified to explain the phenotypic variability of cystic fibrosis [225, 226], another human disease that affects the lung (STRA6 mutation can lead to lung hypoplasia). If variability in dietary intake of vitamin A is wholly responsible for the phenotypic variability, constant supplement of the appropriate amount of vitamin A could potentially lessen the severity of the disease. As illustrated by ABCA4, a disease heavily influenced by an environmental factor can be targeted for treatment by changing the environmental factor, which is usually easier to modify than genetic factors.
It is well known that different tissues are very different in their vulnerability to vitamin A deficiency and excess. It is notable that the eye is the organ most sensitive to STRA6 mutations. This is consistent with the fact that the eye is the organ that most depends on vitamin A for both adult physiological function [119, 120, 227] and for embryonic development [228] and therefore is most sensitive to vitamin A deficiency.
3. General lessens learned from the visual cycle
Because of the dependence of human beings on vision, our high sensitivity to vision defects, and vision's absolute dependence on vitamin A, vision research has offered a wealth of information of the mechanisms of vitamin A related proteins, despite the fact that both the photoreceptor cells and the RPE cell are extremely rare cell types in the body as a whole. All three kinds of membrane proteins described here are involved in the visual cycle, which is perhaps the best understood retinoid transport and recycling system. Mechanistic studies of the visual cycle have generated some likely general principles on retinoid transport, storage and usage:
3.1. Storage vs. usage
A cell that needs vitamin A for physiological function is likely not the cell that takes up vitamin A from the blood. This is clearly the case for vision (Figure 2). The RPE cell is known to express STRA6, which takes up vitamin A from the blood, and to store a large quantity of vitamin A as retinyl esters, but ultimately the cell type that needs vitamin A is photoreceptor cell. This principle may also apply to the brain. Blood-brain barriers like the choroid plexus, meninges, and a subset of astrocytes express STRA6 at high levels. It was recently demonstrated that the meninges are the source of retinoic acid for corticogenesis [185]. Despite the many known physiological functions of retinoic acid on neuron [10, 11], STRA6 is not known to be expressed in brain neurons, which, like photoreceptor cells in the retina, are not directly exposed to the blood. There might exist a local delivery system equivalent to the visual cycle to deliver retinoid from cells at the blood brain barrier to neurons.
3.2. Protein bound form vs. free form
Despite the fact that retinoids other than retinyl esters predominantly exist as protein bound forms (e.g., RBP or CRBP), free retinoid does exist under physiological conditions, at least transiently. For example, free retinol is generated by reduction of retinal following light bleaching of rhodopsin at the beginning of the visual cycle. The retinol intermediate of the visual cycle, which eventually moves out of the photoreceptor cell into the RPE cell, was first discovered about 40 years ago [229]. This transient form of free retinol can be visualized in the photoreceptor cell following light bleaching using sensitive fluorescence microscopy [230, 231]. Although the retinal chromophore is supposed to be covalently bound to opsins, biochemical and electrophysiological studies have demonstrated that the free retinal chromophore gets exchanged between opsin molecules [232, 233]. How protein-mediated transport and free diffusion is balanced in each organ or cell context need to be further studied. For example, is the morphogenic gradient of retinoic acid during embryonic development in its free form, the protein bound form (CRABP), or both?
3.3. Environmental factors and human diseases
Retinoid related diseases can be substantially influenced by environmental factors. Both ABCA4 and STRA6-related human diseases can be influenced by environmental factors. In the case of ABCA4, environmental factors such as light can have a huge impact. Without light, the vitamin A-related toxin A2E does not even form in animal model of Stargardt disease, despite the knockout of the gene [147]. Loss of ABCA4 phenotype is also influenced by retinoid levels [162]. This lesson makes it necessary to control environmental factors that influence overall or tissue-specific retinoid levels, especially diet [234], in designing experiments and to understand human disease phenotypes. Phenotypes associated with loss of STRA6 are also highly variable in humans, ranging from embryonic lethality to the “mild” phenotype of anophthalmia [186, 187, 219–223]. As discussed above, environmental factors such as dietary intake of vitamin A likely contribute to the high phenotypic variability.
3.4. Seemingly small contributions can be critical
Despite the overwhelming amount of independent experimental evidence for the biochemical function of ABCA4 in transporting alltrans retinal for its reduction to all-trans retinol, little or no change in either the clearance of alltrans retinal or the production of all-trans retinol was observed in ABCA4 knockout mice [105, 114, 115]. These in vivo experiments demonstrated that ABCA4 contributes little to retinal transport compared to what retinal can do by itself. These surprising findings illustrate an important lesson that a protein might only affect retinoid transport slightly, but this activity can be sufficiently important to justify its existence. Human retinal diseases associated with ABCA4 mutations demonstrate that loss of such as a seemingly “slight” contribution has serious consequences (e.g., delayed dark adaptation and toxic A2E accumulation).
3.5. Passive diffusion vs. active or facilitated transport
Despite its ability to diffuse through membrane, retinoid transporters do exist to facilitate its transport. In addition to ABCA4's function described above, ABCA4's closest homolog, ABCA1, is involved in retinol efflux in small intestine cells [235]. SR-BI, which was first identified as a HDL receptor mediating cholesterol uptake [205], has been shown to mediate beta-carotene uptake in the small intestine [113, 218]. As mentioned above, SR-BI homologs in Drosophila also mediate carotenoid uptake [215–217].
3.6. Need vs. cost
For a biological need (e.g., higher sensitivity or specificity), evolution may come up with a seemingly costly and complex mechanism instead of using a theoretically simpler mechanism. One example in retinoid biology is the vertebrate visual pigment, which is unstable after light absorption and needs to be regenerated after every photoisomerization event. It is amazing to realize that as we see the world, every photon we detect depends on the regeneration of the bleached chromophore through the complex visual cycle consisting of several retinoid-related enzymes, transport proteins and binding proteins. In bright light when rod vision is saturated and not useful, the continuous regeneration of rhodopsin through this complex cycle may even be considered “wasteful”. As discussed above, the choice of this unstable pigment may be advantageous because the pigment can be continuously regenerated in complete darkness and may have better resolution in spectral sensitivity. Another example in retinoid biology is the delivery of vitamin A. Although retinoid can diffuse systemically, evolution came up with a specific vitamin A delivery protein in the blood (RBP) and its receptor (STRA6) for vitamin A uptake. Instead of employing the highly abundant serum albumin, which is capable of binding to vitamin A, virtually all vitamin A in the blood is bound to RBP. Although this choice is seemingly costly because each RBP can only bind one vitamin A molecule, it has the advantages discussed above.
3.7. There exist vitamin A derivatives that have only toxicity and no beneficial function
A2E and related compounds are the only known examples. Most, if not all, other known vitamin A derivatives (e.g., alcohol, aldehyde or ester forms) have beneficial biological functions. The biological functions of less common vitamin A derivatives such as retinoylation of proteins [236] are still not well understood.
4. Unsolved questions
Despite what we have learned, there are still numerous interesting questions that remain unanswered. Addressing these questions will lead to better understanding of retinoid biology and retinoid-related human diseases and potentially lead to the development of new therapies for human diseases. In addition to questions raised in previous sections, other examples are listed below:
-
4.1.
How does the liver sense RBP concentration in the blood to maintain its stable concentration under physiological conditions to prevent excessive secretion or insufficient secretion? This question also applies to the adipose tissue, another major site of vitamin A storage. Excessive secretion of RBP has been linked to insulin resistance [237]. Is there a membrane receptor that senses blood RBP concentration to control its secretion?
-
4.2.
Under physiological conditions, the blood maintains micromolar concentrations of holo-RBP STRA6 can efficiently take up vitamin A from nanomolar holo-RBP concentrations. If STRA6 takes up vitamin A from the blood continuously, a STRA6-expressing cell would become bloated with vitamin A in days if not months. What are the mechanisms that control cellular vitamin A uptake by STRA6 to prevent excessive uptake?
-
4.3.
STRA6 was first identified as a retinoic acid-stimulated gene in cancer cells [169, 170, 238] Certain cancer cells have more than 100 fold higher STRA6 expression levels than normal cells [170]. What is the role of STRA6 in cancer cells? Retinoid can both enhance and suppress cell growth. Is STRA6's role positive or negative for cancer cells? This knowledge may be useful in targeting cancer cells.
-
4.4.
Are there other receptors and transporters for retinoid? There is strong evidence for the existence of a receptor for interphotoreceptor retinoid binding protein on RPE cells that mediates the export of 11-cis retinal produced by the RPE [239, 240]. The identity of this receptor is still unknown.
-
4.5.
There exists a homolog of STRA6, the RBP receptor What is the function of the STRA6 homolog [241]? Is it also involved RBP binding or retinoid uptake?
-
4.6.
Humans also have an orphan GPCR that is rapidly induced by retinoic acid [242]. Is it involved in retinoid biology?
Acknowledgment
I thank Dr. Ouliana Ziouzenkova and Dr. Earl Harrison for the invitation to write this review. Supported by NIH grant 5R01EY018144.
References
- [1].Banhegyi G, Braun L, Csala M, Puskas F, Mandl J. Ascorbate metabolism and its regulation in animals. Free Radic Biol Med. 1997;23:793–803. doi: 10.1016/s0891-5849(97)00062-2. [DOI] [PubMed] [Google Scholar]
- [2].Holick MF. Vitamin D: evolutionary, physiological and health perspectives. Curr Drug Targets. 12:4–18. doi: 10.2174/138945011793591635. [DOI] [PubMed] [Google Scholar]
- [3].Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- [4].Hill MJ. Intestinal flora and endogenous vitamin synthesis. Eur J Cancer Prev. 1997;6(Suppl 1):S43–45. doi: 10.1097/00008469-199703001-00009. [DOI] [PubMed] [Google Scholar]
- [5].Wald G. The molecular basis of visual excitation. Nature. 1968;219:800–807. doi: 10.1038/219800a0. [DOI] [PubMed] [Google Scholar]
- [6].Dowling JE. Night blindness. Sci Am. 1966;215:78–84. doi: 10.1038/scientificamerican1066-78. [DOI] [PubMed] [Google Scholar]
- [7].Ross AC, Gardner EM. The function of vitamin A in cellular growth and differentiation, and its roles during pregnancy and lactation. Adv Exp Med Biol. 1994;352:187–200. doi: 10.1007/978-1-4899-2575-6_15. [DOI] [PubMed] [Google Scholar]
- [8].Napoli JL. Biochemical pathways of retinoid transport, metabolism, and signal transduction. Clin Immunol Immunopathol. 1996;80:S52–62. doi: 10.1006/clin.1996.0142. [DOI] [PubMed] [Google Scholar]
- [9].Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr. 2001;21:167–192. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- [10].Drager UC. Retinoic acid signaling in the functioning brain. Sci STKE. 2006;2006:pe10. doi: 10.1126/stke.3242006pe10. [DOI] [PubMed] [Google Scholar]
- [11].Maden M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci. 2007;8:755–765. doi: 10.1038/nrn2212. [DOI] [PubMed] [Google Scholar]
- [12].Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134:921–931. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Niederreither K, Dolle P. Retinoic acid in development: towards an integrated view. Nat Rev Genet. 2008;9:541–553. doi: 10.1038/nrg2340. [DOI] [PubMed] [Google Scholar]
- [14].Sommer A. Vitamin A deficiency, child health, and survival. Nutrition. 1997;13:484–485. doi: 10.1016/s0899-9007(97)00013-0. [DOI] [PubMed] [Google Scholar]
- [15].Harjes CE, Rocheford TR, Bai L, Brutnell TP, Kandianis CB, Sowinski SG, Stapleton AE, Vallabhaneni R, Williams M, Wurtzel ET, Yan J, Buckler ES. Natural genetic variation in lycopene epsilon cyclase tapped for maize biofortification. Science. 2008;319:330–333. doi: 10.1126/science.1150255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Evans RM. The molecular basis of signaling by vitamin A and its metabolites. Harvey Lect. 1994;90:105–117. [PubMed] [Google Scholar]
- [17].Chambon P. A decade of molecular biology of retinoic acid receptors. Faseb J. 1996;10:940–954. [PubMed] [Google Scholar]
- [18].Buck J, Derguini F, Levi E, Nakanishi K, Hammerling U. Intracellular signaling by 14-hydroxy-4,14-retro-retinol. Science. 1991;254:1654–1656. doi: 10.1126/science.1749937. [DOI] [PubMed] [Google Scholar]
- [19].Buck J, Myc A, Garbe A, Cathomas G. Differences in the action and metabolism between retinol and retinoic acid in B lymphocytes. J Cell Biol. 1991;115:851–859. doi: 10.1083/jcb.115.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chen Y, Buck J, Derguini F. Anhydroretinol induces oxidative stress and cell death. Cancer Res. 1999;59:3985–3990. [PubMed] [Google Scholar]
- [21].Chen L, Khillan JS. Promotion of feeder-independent self-renewal of embryonic stem cells by retinol (vitamin A) Stem Cells. 2008;26:1858–1864. doi: 10.1634/stemcells.2008-0050. [DOI] [PubMed] [Google Scholar]
- [22].Chen Y, Derguini F, Buck J. Vitamin A in serum is a survival factor for fibroblasts. Proc Natl Acad Sci U S A. 1997;94:10205–10208. doi: 10.1073/pnas.94.19.10205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hoyos B, Jiang S, Hammerling U. Location and functional significance of retinol-binding sites on the serine/threonine kinase, c-Raf. J Biol Chem. 2005;280:6872–6878. doi: 10.1074/jbc.M412695200. [DOI] [PubMed] [Google Scholar]
- [24].Ziouzenkova O, Orasanu G, Sharlach M, Akiyama TE, Berger JP, Viereck J, Hamilton JA, Tang G, Dolnikowski GG, Vogel S, Duester G, Plutzky J. Retinaldehyde represses adipogenesis and diet-induced obesity. Nat Med. 2007;13:695–702. doi: 10.1038/nm1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ziouzenkova O, Plutzky J. Retinoid metabolism and nuclear receptor responses: New insights into coordinated regulation of the PPAR-RXR complex. FEBS Lett. 2008;582:32–38. doi: 10.1016/j.febslet.2007.11.081. [DOI] [PubMed] [Google Scholar]
- [26].Chen N, Onisko B, Napoli JL. The nuclear transcription factor RARalpha associates with neuronal RNA granules and suppresses translation. J Biol Chem. 2008;283:20841–20847. doi: 10.1074/jbc.M802314200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Aoto J, Nam CI, Poon MM, Ting P, Chen L. Synaptic signaling by all-trans retinoic acid in homeostatic synaptic plasticity. Neuron. 2008;60:308–320. doi: 10.1016/j.neuron.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kaya K, Shiraishi F, Uchida H, Sano T. A novel retinoic acid analogue, 7-hydroxy retinoic acid, isolated from cyanobacteria. Biochim Biophys Acta. 2010;1810:414–419. doi: 10.1016/j.bbagen.2010.11.009. [DOI] [PubMed] [Google Scholar]
- [29].Nathans J, Thomas D, Hogness DS. Molecular genetics of human color vision: the genes encoding blue, green, and red pigments. Science. 1986;232:193–202. doi: 10.1126/science.2937147. [DOI] [PubMed] [Google Scholar]
- [30].Nathans J, Piantanida TP, Eddy RL, Shows TB, Hogness DS. Molecular genetics of inherited variation in human color vision. Science. 1986;232:203–210. doi: 10.1126/science.3485310. [DOI] [PubMed] [Google Scholar]
- [31].Nathans J. The evolution and physiology of human color vision: insights from molecular genetic studies of visual pigments. Neuron. 1999;24:299–312. doi: 10.1016/s0896-6273(00)80845-4. [DOI] [PubMed] [Google Scholar]
- [32].Nathans J. Rhodopsin: structure, function, and genetics. Biochemistry. 1992;31:4923–4931. doi: 10.1021/bi00136a001. [DOI] [PubMed] [Google Scholar]
- [33].Khorana HG. Rhodopsin, photoreceptor of the rod cell. An emerging pattern for structure and function. J Biol Chem. 1992;267:1–4. [PubMed] [Google Scholar]
- [34].Hubbell WL, Altenbach C, Hubbell CM, Khorana HG. Rhodopsin structure, dynamics, and activation: a perspective from crystallography, site-directed spin labeling, sulfhydryl reactivity, and disulfide cross-linking. Adv Protein Chem. 2003;63:243–290. doi: 10.1016/s0065-3233(03)63010-x. [DOI] [PubMed] [Google Scholar]
- [35].Palczewski K. G protein-coupled receptor rhodopsin. Annu Rev Biochem. 2006;75:743–767. doi: 10.1146/annurev.biochem.75.103004.142743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sun H, Macke JP, Nathans J. Mechanisms of spectral tuning in the mouse green cone pigment. Proc Natl Acad Sci U S A. 1997;94:8860–8865. doi: 10.1073/pnas.94.16.8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shi G, Yau KW, Chen J, Kefalov VJ. Signaling properties of a short-wave cone visual pigment and its role in phototransduction. J Neurosci. 2007;27:10084–10093. doi: 10.1523/JNEUROSCI.2211-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Max M, McKinnon PJ, Seidenman KJ, Barrett RK, Applebury ML, Takahashi JS, Margolskee RF. Pineal opsin: a nonvisual opsin expressed in chick pineal. Science. 1995;267:1502–1506. doi: 10.1126/science.7878470. [DOI] [PubMed] [Google Scholar]
- [39].Okano T, Yoshizawa T, Fukada Y. Pinopsin is a chicken pineal photoreceptive molecule. Nature. 1994;372:94–97. doi: 10.1038/372094a0. [DOI] [PubMed] [Google Scholar]
- [40].Owens GL, Windsor DJ, Mui J, Taylor JS. A fish eye out of water: ten visual opsins in the four-eyed fish, Anableps anableps. PLoS One. 2009;4:e5970. doi: 10.1371/journal.pone.0005970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. Melanopsin: An opsin in melanophores, brain, and eye. Proc Natl Acad Sci U S A. 1998;95:340–345. doi: 10.1073/pnas.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Provencio I, Rollag MD, Castrucci AM. Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature. 2002;415:493. doi: 10.1038/415493a. [DOI] [PubMed] [Google Scholar]
- [44].Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, Pletcher MT, Sato TK, Wiltshire T, Andahazy M, Kay SA, Van Gelder RN, Hogenesch JB. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- [46].Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- [47].Do MT, Kang SH, Xue T, Zhong H, Liao HW, Bergles DE, Yau KW. Photon capture and signalling by melanopsin retinal ganglion cells. Nature. 2009;457:281–287. doi: 10.1038/nature07682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Soni BG, Foster RG. A novel and ancient vertebrate opsin. FEBS Lett. 1997;406:279–283. doi: 10.1016/s0014-5793(97)00287-1. [DOI] [PubMed] [Google Scholar]
- [49].Soni BG, Philp AR, Foster RG, Knox BE. Novel retinal photoreceptors. Nature. 1998;394:27–28. doi: 10.1038/27794. [DOI] [PubMed] [Google Scholar]
- [50].Su CY, Luo DG, Terakita A, Shichida Y, Liao HW, Kazmi MA, Sakmar TP, Yau KW. Parietal-eye phototransduction components and their potential evolutionary implications. Science. 2006;311:1617–1621. doi: 10.1126/science.1123802. [DOI] [PubMed] [Google Scholar]
- [51].Bok D. The retinal pigment epithelium: a versatile partner in vision. J Cell Sci Suppl. 1993;17:189–195. doi: 10.1242/jcs.1993.supplement_17.27. [DOI] [PubMed] [Google Scholar]
- [52].Sparrow JR, Hicks D, Hamel CP. The retinal pigment epithelium in health and disease. Curr Mol Med. 2010;10:802–823. doi: 10.2174/156652410793937813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sun H, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. Peropsin, a novel visual pigment-like protein located in the apical microvilli of the retinal pigment epithelium. Proc Natl Acad Sci U S A. 1997;94:9893–9898. doi: 10.1073/pnas.94.18.9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hao W, Fong HK. Blue and ultraviolet light-absorbing opsin from the retinal pigment epithelium. Biochemistry. 1996;35:6251–6256. doi: 10.1021/bi952420k. [DOI] [PubMed] [Google Scholar]
- [55].Hao W, Fong HK. The endogenous chromophore of retinal G protein-coupled receptor opsin from the pigment epithelium. J Biol Chem. 1999;274:6085–6090. doi: 10.1074/jbc.274.10.6085. [DOI] [PubMed] [Google Scholar]
- [56].Chen P, Hao W, Rife L, Wang XP, Shen D, Chen J, Ogden T, Van Boemel GB, Wu L, Yang M, Fong HK. A photic visual cycle of rhodopsin regeneration is dependent on Rgr. Nat Genet. 2001;28:256–260. doi: 10.1038/90089. [DOI] [PubMed] [Google Scholar]
- [57].Wenzel A, Oberhauser V, Pugh EN, Jr., Lamb TD, Grimm C, Samardzija M, Fahl E, Seeliger MW, Reme CE, von Lintig J. The retinal G protein-coupled receptor (RGR) enhances isomerohydrolase activity independent of light. J Biol Chem. 2005;280:29874–29884. doi: 10.1074/jbc.M503603200. [DOI] [PubMed] [Google Scholar]
- [58].Radu RA, Hu J, Peng J, Bok D, Mata NL, Travis GH. Retinal pigment epithelium-retinal G protein receptor-opsin mediates light-dependent translocation of all-trans-retinyl esters for synthesis of visual chromophore in retinal pigment epithelial cells. J Biol Chem. 2008;283:19730–19738. doi: 10.1074/jbc.M801288200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Koyanagi M, Terakita A, Kubokawa K, Shichida Y. Amphioxus homologs of Go-coupled rhodopsin and peropsin having 11-cis- and all-trans-retinals as their chromophores. FEBS Lett. 2002;531:525–528. doi: 10.1016/s0014-5793(02)03616-5. [DOI] [PubMed] [Google Scholar]
- [60].Nagata T, Koyanagi M, Tsukamoto H, Terakita A. Identification and characterization of a protostome homologue of peropsin from a jumping spider. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2010;196:51–59. doi: 10.1007/s00359-009-0493-9. [DOI] [PubMed] [Google Scholar]
- [61].Blackshaw S, Snyder SH. Encephalopsin: a novel mammalian extraretinal opsin discretely localized in the brain. J Neurosci. 1999;19:3681–3690. doi: 10.1523/JNEUROSCI.19-10-03681.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Tarttelin EE, Bellingham J, Hankins MW, Foster RG, Lucas RJ. Neuropsin (Opn5): a novel opsin identified in mammalian neural tissue. FEBS Lett. 2003;554:410–416. doi: 10.1016/s0014-5793(03)01212-2. [DOI] [PubMed] [Google Scholar]
- [63].Nakane Y, Ikegami K, Ono H, Yamamoto N, Yoshida S, Hirunagi K, Ebihara S, Kubo Y, Yoshimura T. A mammalian neural tissue opsin (Opsin 5) is a deep brain photoreceptor in birds. Proc Natl Acad Sci U S A. 2010;107:15264–15268. doi: 10.1073/pnas.1006393107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature. 2009;458:1025–1029. doi: 10.1038/nature07926. [DOI] [PubMed] [Google Scholar]
- [65].Maeda T, Maeda A, Leahy P, Saperstein DA, Palczewski K. Effects of long-term administration of 9-cis-retinyl acetate on visual function in mice. Invest Ophthalmol Vis Sci. 2009;50:322–333. doi: 10.1167/iovs.08-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Tang PH, Fan J, Goletz PW, Wheless L, Crouch RK. Effective and sustained delivery of hydrophobic retinoids to photoreceptors. Invest Ophthalmol Vis Sci. 2010;51:5958–5964. doi: 10.1167/iovs.10-5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Heyman RA, Mangelsdorf DJ, Dyck JA, Stein RB, Eichele G, Evans RM, Thaller C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992;68:397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- [68].Levin AA, Sturzenbecker LJ, Kazmer S, Bosakowski T, Huselton C, Allenby G, Speck J, Kratzeisen C, Rosenberger M, Lovey A, et al. 9-cis retinoic acid stereoisomer binds and activates the nuclear receptor RXR alpha. Nature. 1992;355:359–361. doi: 10.1038/355359a0. [DOI] [PubMed] [Google Scholar]
- [69].Kane MA, Napoli JL. Quantification of endogenous retinoids. Methods Mol Biol. 2010;652:1–54. doi: 10.1007/978-1-60327-325-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Spudich JL, Yang CS, Jung KH, Spudich EN. Retinylidene proteins: structures and functions from archaea to humans. Annu Rev Cell Dev Biol. 2000;16:365–392. doi: 10.1146/annurev.cellbio.16.1.365. [DOI] [PubMed] [Google Scholar]
- [71].Hellingwerf KJ, Hoff WD, Crielaard W. Photobiology of microorganisms: how photosensors catch a photon to initialize signalling. Mol Microbiol. 1996;21:683–693. doi: 10.1046/j.1365-2958.1996.411402.x. [DOI] [PubMed] [Google Scholar]
- [72].Wald G. The distribution and evolution of visual systems. Comparative Biochemistry. 1960;I:311–345. [Google Scholar]
- [73].Ma JX, Kono M, Xu L, Das J, Ryan JC, Hazard ES, 3rd, Oprian DD, Crouch RK. Salamander UV cone pigment: sequence, expression, and spectral properties. Vis Neurosci. 2001;18:393–399. doi: 10.1017/s0952523801183057. [DOI] [PubMed] [Google Scholar]
- [74].Isono K, Tanimura T, Oda Y, Tsukahara Y. Dependency on light and vitamin A derivatives of the biogenesis of 3-hydroxyretinal and visual pigment in the compound eyes of Drosophila melanogaster. J Gen Physiol. 1988;92:587–600. doi: 10.1085/jgp.92.5.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Matsui S, Seidou M, Uchiyama I, Sekiya N, Hiraki K, Yoshihara K, Kito Y. 4-Hydroxyretinal, a new visual pigment chromophore found in the bioluminescent squid, Watasenia scintillans. Biochim Biophys Acta. 1988;966:370–374. doi: 10.1016/0304-4165(88)90087-6. [DOI] [PubMed] [Google Scholar]
- [76].Merbs SL, Nathans J. Role of hydroxyl-bearing amino acids in differentially tuning the absorption spectra of the human red and green cone pigments. Photochem Photobiol. 1993;58:706–710. doi: 10.1111/j.1751-1097.1993.tb04956.x. [DOI] [PubMed] [Google Scholar]
- [77].Lin SW, Sakmar TP. Colour tuning mechanisms of visual pigments. Novartis Found Symp. 1999;224:124–135. doi: 10.1002/9780470515693.ch8. discussion 135–141, 181–190. [DOI] [PubMed] [Google Scholar]
- [78].Fasick JI, Lee N, Oprian DD. Spectral tuning in the human blue cone pigment. Biochemistry. 1999;38:11593–11596. doi: 10.1021/bi991600h. [DOI] [PubMed] [Google Scholar]
- [79].Teller DC, Stenkamp RE, Palczewski K. Evolutionary analysis of rhodopsin and cone pigments: connecting the three-dimensional structure with spectral tuning and signal transfer. FEBS Lett. 2003;555:151–159. doi: 10.1016/s0014-5793(03)01152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Jacobs GH, Williams GA, Cahill H, Nathans J. Emergence of novel color vision in mice engineered to express a human cone photopigment. Science. 2007;315:1723–1725. doi: 10.1126/science.1138838. [DOI] [PubMed] [Google Scholar]
- [81].Mancuso K, Hauswirth WW, Li Q, Connor TB, Kuchenbecker JA, Mauck MC, Neitz J, Neitz M. Gene therapy for red-green colour blindness in adult primates. Nature. 2009;461:784–787. doi: 10.1038/nature08401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Jacobs GH, Nathans J. The evolution of Primate color vision. Sci Am. 2009;300:56–63. doi: 10.1038/scientificamerican0409-56. [DOI] [PubMed] [Google Scholar]
- [83].Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J Neurosci. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Cheng N, Tsunenari T, Yau KW. Intrinsic light response of retinal horizontal cells of teleosts. Nature. 2009;460:899–903. doi: 10.1038/nature08175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Menon I, Huber T, Sanyal S, Banerjee S, Barre P, Canis S, Warren JD, Hwa J, Sakmar TP, Menon AK. Opsin is a phospholipid flippase. Curr Biol. 2011;21:149–153. doi: 10.1016/j.cub.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Shen WL, Kwon Y, Adegbola AA, Luo J, Chess A, Montell C. Function of rhodopsin in temperature discrimination in Drosophila. Science. 2011;331:1333–1336. doi: 10.1126/science.1198904. [DOI] [PubMed] [Google Scholar]
- [87].Nathans J, Merbs SL, Sung CH, Weitz CJ, Wang Y. Molecular genetics of human visual pigments. Annu Rev Genet. 1992;26:403–424. doi: 10.1146/annurev.ge.26.120192.002155. [DOI] [PubMed] [Google Scholar]
- [88].Dryja TP, McGee TL, Reichel E, Hahn LB, Cowley GS, Yandell DW, Sandberg MA, Berson EL. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. 1990;343:364–366. doi: 10.1038/343364a0. [DOI] [PubMed] [Google Scholar]
- [89].Sung CH, Davenport CM, Hennessey JC, Maumenee IH, Jacobson SG, Heckenlively JR, Nowakowski R, Fishman G, Gouras P, Nathans J. Rhodopsin mutations in autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci U S A. 1991;88:6481–6485. doi: 10.1073/pnas.88.15.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Dryja TP, Li T. Molecular genetics of retinitis pigmentosa. Hum Mol Genet. 1995;4:1739–1743. doi: 10.1093/hmg/4.suppl_1.1739. Spec No. [DOI] [PubMed] [Google Scholar]
- [91].Nathans J. In the eye of the beholder: visual pigments and inherited variation in human vision. Cell. 1994;78:357–360. doi: 10.1016/0092-8674(94)90414-6. [DOI] [PubMed] [Google Scholar]
- [92].Wilson JH, Wensel TG. The nature of dominant mutations of rhodopsin and implications for gene therapy. Mol Neurobiol. 2003;28:149–158. doi: 10.1385/MN:28:2:149. [DOI] [PubMed] [Google Scholar]
- [93].Dryja TP, Berson EL, Rao VR, Oprian DD. Heterozygous missense mutation in the rhodopsin gene as a cause of congenital stationary night blindness. Nat Genet. 1993;4:280–283. doi: 10.1038/ng0793-280. [DOI] [PubMed] [Google Scholar]
- [94].Sieving PA, Richards JE, Naarendorp F, Bingham EL, Scott K, Alpern M. Dark-light: model for nightblindness from the human rhodopsin Gly-90-->Asp mutation. Proc Natl Acad Sci U S A. 1995;92:880–884. doi: 10.1073/pnas.92.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Dizhoor AM, Woodruff ML, Olshevskaya EV, Cilluffo MC, Cornwall MC, Sieving PA, Fain GL. Night blindness and the mechanism of constitutive signaling of mutant G90D rhodopsin. J Neurosci. 2008;28:11662–11672. doi: 10.1523/JNEUROSCI.4006-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Jin S, Cornwall MC, Oprian DD. Opsin activation as a cause of congenital night blindness. Nat Neurosci. 2003;6:731–735. doi: 10.1038/nn1070. [DOI] [PubMed] [Google Scholar]
- [97].Morimura H, Saindelle-Ribeaudeau F, Berson EL, Dryja TP. Mutations in RGR, encoding a light-sensitive opsin homologue, in patients with retinitis pigmentosa. Nat Genet. 1999;23:393–394. doi: 10.1038/70496. [DOI] [PubMed] [Google Scholar]
- [98].Molday RS, Zhong M, Quazi F. The role of the photoreceptor ABC transporter ABCA4 in lipid transport and Stargardt macular degeneration. Biochim Biophys Acta. 2009;1791:573–583. doi: 10.1016/j.bbalip.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Tsybovsky Y, Molday RS, Palczewski K. The ATP-binding cassette transporter ABCA4: structural and functional properties and role in retinal disease. Adv Exp Med Biol. 2010;703:105–125. doi: 10.1007/978-1-4419-5635-4_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Allikmets R, Singh N, Sun H, Shroyer NF, Hutchinson A, Chidambaram A, Gerrard B, Baird L, Stauffer D, Peiffer A, Rattner A, Smallwood P, Li Y, Anderson KL, Lewis RA, Nathans J, Leppert M, Dean M, Lupski JR. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;15:236–246. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]
- [101].Illing M, Molday LL, Molday RS. The 220-kDa rim protein of retinal rod outer segments is a member of the ABC transporter superfamily. J Biol Chem. 1997;272:10303–10310. doi: 10.1074/jbc.272.15.10303. [DOI] [PubMed] [Google Scholar]
- [102].Azarian SM, Travis GH. The photoreceptor rim protein is an ABC transporter encoded by the gene for recessive Stargardt's disease (ABCR) FEBS Lett. 1997;409:247–252. doi: 10.1016/s0014-5793(97)00517-6. [DOI] [PubMed] [Google Scholar]
- [103].Tang C, Oram JF. The cell cholesterol exporter ABCA1 as a protector from cardiovascular disease and diabetes. Biochim Biophys Acta. 2009;1791:563–572. doi: 10.1016/j.bbalip.2009.03.011. [DOI] [PubMed] [Google Scholar]
- [104].Yvan-Charvet L, Wang N, Tall AR. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler Thromb Vasc Biol. 2010;30:139–143. doi: 10.1161/ATVBAHA.108.179283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt's disease from the phenotype in abcr knockout mice. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- [106].Sun H, Molday RS, Nathans J. Retinal stimulates ATP hydrolysis by purified and reconstituted ABCR, the photoreceptor-specific ATP-binding cassette transporter responsible for Stargardt disease. J Biol Chem. 1999;274:8269–8281. doi: 10.1074/jbc.274.12.8269. [DOI] [PubMed] [Google Scholar]
- [107].Ahn J, Wong JT, Molday RS. The effect of lipid environment and retinoids on the ATPase activity of ABCR, the photoreceptor ABC transporter responsible for Stargardt macular dystrophy. J Biol Chem. 2000;275:20399–20405. doi: 10.1074/jbc.M000555200. [DOI] [PubMed] [Google Scholar]
- [108].Sun H, Nathans J. Mechanistic studies of ABCR, the ABC transporter in photoreceptor outer segments responsible for autosomal recessive Stargardt disease. J Bioenerg Biomembr. 2001;33:523–530. doi: 10.1023/a:1012883306823. [DOI] [PubMed] [Google Scholar]
- [109].Jager S, Palczewski K, Hofmann KP. Opsin/all-trans-retinal complex activates transducin by different mechanisms than photolyzed rhodopsin. Biochemistry. 1996;35:2901–2908. doi: 10.1021/bi9524068. [DOI] [PubMed] [Google Scholar]
- [110].Surya A, Foster KW, Knox BE. Transducin activation by the bovine opsin apoprotein. J Biol Chem. 1995;270:5024–5031. doi: 10.1074/jbc.270.10.5024. [DOI] [PubMed] [Google Scholar]
- [111].Beharry S, Zhong M, Molday RS. N-retinylidene-phosphatidylethanolamine is the preferred retinoid substrate for the photoreceptor-specific ABC transporter ABCA4 (ABCR) J Biol Chem. 2004;279:53972–53979. doi: 10.1074/jbc.M405216200. [DOI] [PubMed] [Google Scholar]
- [112].Zhong M, Molday RS. Binding of retinoids to ABCA4, the photoreceptor ABC transporter associated with Stargardt macular degeneration. Methods Mol Biol. 2010;652:163–176. doi: 10.1007/978-1-60327-325-1_9. [DOI] [PubMed] [Google Scholar]
- [113].During A, Harrison EH. Mechanisms of provitamin A (carotenoid) and vitamin A (retinol) transport into and out of intestinal Caco-2 cells. J Lipid Res. 2007;48:2283–2294. doi: 10.1194/jlr.M700263-JLR200. [DOI] [PubMed] [Google Scholar]
- [114].Maeda A, Golczak M, Maeda T, Palczewski K. Limited roles of Rdh8, Rdh12, and Abca4 in all-trans-retinal clearance in mouse retina. Invest Ophthalmol Vis Sci. 2009;50:5435–5443. doi: 10.1167/iovs.09-3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Blakeley LR, Chen C, Chen CK, Chen J, Crouch RK, Travis GH, Koutalos Y. Rod outer segment retinol formation is independent of Abca4, arrestin, rhodopsin kinase, and rhodopsin palmitylation. Invest Ophthalmol Vis Sci. 2010 doi: 10.1167/iovs.10-6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Bhongsatiern J, Ohtsuki S, Tachikawa M, Hori S, Terasaki T. Retinal-specific ATP-binding cassette transporter (ABCR/ABCA4) is expressed at the choroid plexus in rat brain. J Neurochem. 2005;92:1277–1280. doi: 10.1111/j.1471-4159.2004.02941.x. [DOI] [PubMed] [Google Scholar]
- [117].Tsukamoto H, Terakita A. Diversity and functional properties of bistable pigments. Photochem Photobiol Sci. 2010;9:1435–1443. doi: 10.1039/c0pp00168f. [DOI] [PubMed] [Google Scholar]
- [118].Zemelman BV, Lee GA, Ng M, Miesenbock G. Selective photostimulation of genetically chARGed neurons. Neuron. 2002;33:15–22. doi: 10.1016/s0896-6273(01)00574-8. [DOI] [PubMed] [Google Scholar]
- [119].Travis GH, Golczak M, Moise AR, Palczewski K. Diseases Caused by Defects in the Visual Cycle: Retinoids as Potential Therapeutic Agents. Annu Rev Pharmacol Toxicol. 2006 doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Crouch RK, Chader GJ, Wiggert B, Pepperberg DR. Retinoids and the visual process. Photochem Photobiol. 1996;64:613–621. doi: 10.1111/j.1751-1097.1996.tb03114.x. [DOI] [PubMed] [Google Scholar]
- [121].Mata NL, Radu RA, Clemmons RC, Travis GH. Isomerization and oxidation of vitamin a in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight. Neuron. 2002;36:69–80. doi: 10.1016/s0896-6273(02)00912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Wang JS, Estevez ME, Cornwall MC, Kefalov VJ. Intra-retinal visual cycle required for rapid and complete cone dark adaptation. Nat Neurosci. 2009;12:295–302. doi: 10.1038/nn.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Wang X, Wang T, Jiao Y, von Lintig J, Montell C. Requirement for an enzymatic visual cycle in Drosophila. Curr Biol. 2010;20:93–102. doi: 10.1016/j.cub.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Koyanagi M, Kawano E, Kinugawa Y, Oishi T, Shichida Y, Tamotsu S, Terakita A. Bistable UV pigment in the lamprey pineal. Proc Natl Acad Sci U S A. 2004;101:6687–6691. doi: 10.1073/pnas.0400819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Panda S, Nayak SK, Campo B, Walker JR, Hogenesch JB, Jegla T. Illumination of the melanopsin signaling pathway. Science. 2005;307:600–604. doi: 10.1126/science.1105121. [DOI] [PubMed] [Google Scholar]
- [126].Melyan Z, Tarttelin EE, Bellingham J, Lucas RJ, Hankins MW. Addition of human melanopsin renders mammalian cells photoresponsive. Nature. 2005;433:741–745. doi: 10.1038/nature03344. [DOI] [PubMed] [Google Scholar]
- [127].Oberhauser V, Voolstra O, Bangert A, von Lintig J, Vogt K. NinaB combines carotenoid oxygenase and retinoid isomerase activity in a single polypeptide. Proc Natl Acad Sci U S A. 2008;105:19000–19005. doi: 10.1073/pnas.0807805105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Fishman GA, Farbman JS, Alexander KR. Delayed rod dark adaptation in patients with Stargardt's disease. Ophthalmology. 1991;98:957–962. doi: 10.1016/s0161-6420(91)32196-1. [DOI] [PubMed] [Google Scholar]
- [129].Fain GL. Dark adaptation. Prog Brain Res. 2001;131:383–394. doi: 10.1016/s0079-6123(01)31031-2. [DOI] [PubMed] [Google Scholar]
- [130].Fain GL, Matthews HR, Cornwall MC, Koutalos Y. Adaptation in vertebrate photoreceptors. Physiol Rev. 2001;81:117–151. doi: 10.1152/physrev.2001.81.1.117. [DOI] [PubMed] [Google Scholar]
- [131].Maeda A, Maeda T, Golczak M, Chou S, Desai A, Hoppel CL, Matsuyama S, Palczewski K. Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J Biol Chem. 2009;284:15173–15183. doi: 10.1074/jbc.M900322200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Maeda A, Maeda T, Golczak M, Palczewski K. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J Biol Chem. 2008;283:26684–26693. doi: 10.1074/jbc.M804505200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Martinez-Mir A, Paloma E, Allikmets R, Ayuso C, del Rio T, Dean M, Vilageliu L, Gonzalez-Duarte R, Balcells S. Retinitis pigmentosa caused by a homozygous mutation in the Stargardt disease gene ABCR. Nat Genet. 1998;18:11–12. doi: 10.1038/ng0198-11. [DOI] [PubMed] [Google Scholar]
- [134].Shroyer NF, Lewis RA, Yatsenko AN, Lupski JR. Null missense ABCR (ABCA4) mutations in a family with stargardt disease and retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2001;42:2757–2761. [PubMed] [Google Scholar]
- [135].Cremers FP, van de Pol DJ, van Driel M, den Hollander AI, van Haren FJ, Knoers NV, Tijmes N, Bergen AA, Rohrschneider K, Blankenagel A, Pinckers AJ, Deutman AF, Hoyng CB. Autosomal recessive retinitis pigmentosa and cone-rod dystrophy caused by splice site mutations in the Stargardt's disease gene ABCR. Hum Mol Genet. 1998;7:355–362. doi: 10.1093/hmg/7.3.355. [DOI] [PubMed] [Google Scholar]
- [136].Birch DG, Peters AY, Locke KL, Spencer R, Megarity CF, Travis GH. Visual function in patients with cone-rod dystrophy (CRD) associated with mutations in the ABCA4(ABCR) gene. Exp Eye Res. 2001;73:877–886. doi: 10.1006/exer.2001.1093. [DOI] [PubMed] [Google Scholar]
- [137].Klevering BJ, Blankenagel A, Maugeri A, Cremers FP, Hoyng CB, Rohrschneider K. Phenotypic spectrum of autosomal recessive cone-rod dystrophies caused by mutations in the ABCA4 (ABCR) gene. Invest Ophthalmol Vis Sci. 2002;43:1980–1985. [PubMed] [Google Scholar]
- [138].Allikmets R. Further evidence for an association of ABCR alleles with age-related macular degeneration. The International ABCR Screening Consortium. Am J Hum Genet. 2000;67:487–491. doi: 10.1086/303018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Chen W, Stambolian D, Edwards AO, Branham KE, Othman M, Jakobsdottir J, Tosakulwong N, Pericak-Vance MA, Campochiaro PA, Klein ML, Tan PL, Conley YP, Kanda A, Kopplin L, Li Y, Augustaitis KJ, Karoukis AJ, Scott WK, Agarwal A, Kovach JL, Schwartz SG, Postel EA, Brooks M, Baratz KH, Brown WL, Brucker AJ, Orlin A, Brown G, Ho A, Regillo C, Donoso L, Tian L, Kaderli B, Hadley D, Hagstrom SA, Peachey NS, Klein R, Klein BE, Gotoh N, Yamashiro K, Ferris Iii F, Fagerness JA, Reynolds R, Farrer LA, Kim IK, Miller JW, Corton M, Carracedo A, Sanchez-Salorio M, Pugh EW, Doheny KF, Brion M, Deangelis MM, Weeks DE, Zack DJ, Chew EY, Heckenlively JR, Yoshimura N, Iyengar SK, Francis PJ, Katsanis N, Seddon JM, Haines JL, Gorin MB, Abecasis GR, Swaroop A. Genetic variants near TIMP3 and high-density lipoprotein-associated loci influence susceptibility to age-related macular degeneration. Proc Natl Acad Sci U S A. 2010;107:7401–7406. doi: 10.1073/pnas.0912702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Neale BM, Fagerness J, Reynolds R, Sobrin L, Parker M, Raychaudhuri S, Tan PL, Oh EC, Merriam JE, Souied E, Bernstein PS, Li B, Frederick JM, Zhang K, Brantley MA, Jr., Lee AY, Zack DJ, Campochiaro B, Campochiaro P, Ripke S, Smith RT, Barile GR, Katsanis N, Allikmets R, Daly MJ, Seddon JM. Genome-wide association study of advanced age-related macular degeneration identifies a role of the hepatic lipase gene (LIPC) Proc Natl Acad Sci U S A. 2010;107:7395–7400. doi: 10.1073/pnas.0912019107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Sun H, Smallwood PM, Nathans J. Biochemical defects in ABCR protein variants associated with human retinopathies. Nat Genet. 2000;26:242–246. doi: 10.1038/79994. [DOI] [PubMed] [Google Scholar]
- [142].Zhong M, Molday LL, Molday RS. Role of the C terminus of the photoreceptor ABCA4 transporter in protein folding, function, and retinal degenerative diseases. J Biol Chem. 2009;284:3640–3649. doi: 10.1074/jbc.M806580200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Bither PP, Berns LA. Stargardt's disease: a review of the literature. J Am Optom Assoc. 1988;59:106–111. [PubMed] [Google Scholar]
- [144].Sun H, Nathans J. Stargardt's ABCR is localized to the disc membrane of retinal rod outer segments. Nat Genet. 1997;17:15–16. doi: 10.1038/ng0997-15. [DOI] [PubMed] [Google Scholar]
- [145].Eldred GE, Lasky MR. Retinal age pigments generated by self-assembling lysosomotropic detergents. Nature. 1993;361:724–726. doi: 10.1038/361724a0. [DOI] [PubMed] [Google Scholar]
- [146].Parish CA, Hashimoto M, Nakanishi K, Dillon J, Sparrow J. Isolation and one-step preparation of A2E and iso-A2E, fluorophores from human retinal pigment epithelium. Proc Natl Acad Sci U S A. 1998;95:14609–14613. doi: 10.1073/pnas.95.25.14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Mata NL, Weng J, Travis GH. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc Natl Acad Sci U S A. 2000;97:7154–7159. doi: 10.1073/pnas.130110497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Cideciyan AV, Aleman TS, Swider M, Schwartz SB, Steinberg JD, Brucker AJ, Maguire AM, Bennett J, Stone EM, Jacobson SG. Mutations in ABCA4 result in accumulation of lipofuscin before slowing of the retinoid cycle: a reappraisal of the human disease sequence. Hum Mol Genet. 2004;13:525–534. doi: 10.1093/hmg/ddh048. [DOI] [PubMed] [Google Scholar]
- [149].Sparrow JR, Yoon KD, Wu Y, Yamamoto K. Interpretations of fundus autofluorescence from studies of the bisretinoids of the retina. Invest Ophthalmol Vis Sci. 2010;51:4351–4357. doi: 10.1167/iovs.10-5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Molday LL, Rabin AR, Molday RS. ABCR expression in foveal cone photoreceptors and its role in Stargardt macular dystrophy. Nat Genet. 2000;25:257–258. doi: 10.1038/77004. [DOI] [PubMed] [Google Scholar]
- [151].Young RW, Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol. 1969;42:392–403. doi: 10.1083/jcb.42.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Bok D, Young RW. The renewal of diffusely distributed protein in the outer segments of rods and cones. Vision Res. 1972;12:161–168. doi: 10.1016/0042-6989(72)90108-3. [DOI] [PubMed] [Google Scholar]
- [153].Sparrow JR, Boulton M. RPE lipofuscin and its role in retinal pathobiology. Exp Eye Res. 2005;80:595–606. doi: 10.1016/j.exer.2005.01.007. [DOI] [PubMed] [Google Scholar]
- [154].Sparrow JR, Cai B, Jang YP, Zhou J, Nakanishi K. A2E, a fluorophore of RPE lipofuscin, can destabilize membrane. Adv Exp Med Biol. 2006;572:63–68. doi: 10.1007/0-387-32442-9_10. [DOI] [PubMed] [Google Scholar]
- [155].De S, Sakmar TP. Interaction of A2E with model membranes. Implications to the pathogenesis of age-related macular degeneration. J Gen Physiol. 2002;120:147–157. doi: 10.1085/jgp.20028566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Vives-Bauza C, Anand M, Shirazi AK, Magrane J, Gao J, Vollmer-Snarr HR, Manfredi G, Finnemann SC. The age lipid A2E and mitochondrial dysfunction synergistically impair phagocytosis by retinal pigment epithelial cells. J Biol Chem. 2008;283:24770–24780. doi: 10.1074/jbc.M800706200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Moiseyev G, Nikolaeva O, Chen Y, Farjo K, Takahashi Y, Ma JX. Inhibition of the visual cycle by A2E through direct interaction with RPE65 and implications in Stargardt disease. Proc Natl Acad Sci U S A. 2010;107:17551–17556. doi: 10.1073/pnas.1008769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Zhou J, Jang YP, Kim SR, Sparrow JR. Complement activation by photooxidation products of A2E, a lipofuscin constituent of the retinal pigment epithelium. Proc Natl Acad Sci U S A. 2006;103:16182–16187. doi: 10.1073/pnas.0604255103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Zhou J, Kim SR, Westlund BS, Sparrow JR. Complement activation by bisretinoid constituents of RPE lipofuscin. Invest Ophthalmol Vis Sci. 2009;50:1392–1399. doi: 10.1167/iovs.08-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Radu RA, Hu J, Yuan Q, Welch DL, Makshanoff J, Lloyd M, McMullen S, Travis GH, Bok D. Complement system dysregulation and inflammation in the retinal pigment epithelium of a mouse model for stargardt macular degeneration. J Biol Chem. 2011 doi: 10.1074/jbc.M110.191866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Radu RA, Yuan Q, Hu J, Peng JH, Lloyd M, Nusinowitz S, Bok D, Travis GH. Accelerated accumulation of lipofuscin pigments in the RPE of a mouse model for ABCA4-mediated retinal dystrophies following Vitamin A supplementation. Invest Ophthalmol Vis Sci. 2008;49:3821–3829. doi: 10.1167/iovs.07-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Radu RA, Han Y, Bui TV, Nusinowitz S, Bok D, Lichter J, Widder K, Travis GH, Mata NL. Reductions in serum vitamin A arrest accumulation of toxic retinal fluorophores: a potential therapy for treatment of lipofuscin-based retinal diseases. Invest Ophthalmol Vis Sci. 2005;46:4393–4401. doi: 10.1167/iovs.05-0820. [DOI] [PubMed] [Google Scholar]
- [163].Radu RA, Mata NL, Nusinowitz S, Liu X, Travis GH. Isotretinoin treatment inhibits lipofuscin accumulation in a mouse model of recessive Stargardt's macular degeneration. Novartis Found Symp. 2004;255:51–63. discussion 63–57, 177–178. [PubMed] [Google Scholar]
- [164].Maeda T, Maeda A, Matosky M, Okano K, Roos S, Tang J, Palczewski K. Evaluation of potential therapies for a mouse model of human age-related macular degeneration caused by delayed all-trans-retinal clearance. Invest Ophthalmol Vis Sci. 2009;50:4917–4925. doi: 10.1167/iovs.09-3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Kong J, Kim SR, Binley K, Pata I, Doi K, Mannik J, Zernant-Rajang J, Kan O, Iqball S, Naylor S, Sparrow JR, Gouras P, Allikmets R. Correction of the disease phenotype in the mouse model of Stargardt disease by lentiviral gene therapy. Gene Ther. 2008;15:1311–1320. doi: 10.1038/gt.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Cideciyan AV, Swider M, Aleman TS, Tsybovsky Y, Schwartz SB, Windsor EA, Roman AJ, Sumaroka A, Steinberg JD, Jacobson SG, Stone EM, Palczewski K. ABCA4 disease progression and a proposed strategy for gene therapy. Hum Mol Genet. 2009;18:931–941. doi: 10.1093/hmg/ddn421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Wu Y, Zhou J, Fishkin N, Rittmann BE, Sparrow JR. Enzymatic degradation of A2E, a retinal pigment epithelial lipofuscin bisretinoid. J Am Chem Soc. 2011;133:849–857. doi: 10.1021/ja107195u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Sparrow JR, Kim SR, Wu Y. Experimental approaches to the study of A2E, a bisretinoid lipofuscin chromophore of retinal pigment epithelium. Methods Mol Biol. 2010;652:315–327. doi: 10.1007/978-1-60327-325-1_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Bouillet P, Sapin V, Chazaud C, Messaddeq N, Decimo D, Dolle P, Chambon P. Developmental expression pattern of Stra6, a retinoic acid-responsive gene encoding a new type of membrane protein. Mech Dev. 1997;63:173–186. doi: 10.1016/s0925-4773(97)00039-7. [DOI] [PubMed] [Google Scholar]
- [170].Szeto W, Jiang W, Tice DA, Rubinfeld B, Hollingshead PG, Fong SE, Dugger DL, Pham T, Yansura DG, Wong TA, Grimaldi JC, Corpuz RT, Singh JS, Frantz GD, Devaux B, Crowley CW, Schwall RH, Eberhard DA, Rastelli L, Polakis P, Pennica D. Overexpression of the retinoic acid-responsive gene Stra6 in human cancers and its synergistic induction by Wnt-1 and retinoic acid. Cancer Res. 2001;61:4197–4205. [PubMed] [Google Scholar]
- [171].Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315:820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- [172].Goodman DS. Plasma Retinol-Binding Protein. In: Sporn MB, Boberts AB, Goodman DS, editors. The Retinoids. vol. 2. Academic Press, Inc.; 1984. pp. 41–88. [Google Scholar]
- [173].Rask L, Anundi H, Bohme J, Eriksson U, Fredriksson A, Nilsson SF, Ronne H, Vahlquist A, Peterson PA. The retinol-binding protein. Scand J Clin Lab Invest Suppl. 1980;154:45–61. [PubMed] [Google Scholar]
- [174].Blaner WS. Retinol-binding protein: the serum transport protein for vitamin A. Endocr Rev. 1989;10:308–316. doi: 10.1210/edrv-10-3-308. [DOI] [PubMed] [Google Scholar]
- [175].Blomhoff R, Green MH, Berg T, Norum KR. Transport and storage of vitamin A. Science. 1990;250:399–404. doi: 10.1126/science.2218545. [DOI] [PubMed] [Google Scholar]
- [176].Newcomer ME, Ong DE. Plasma retinol binding protein: structure and function of the prototypic lipocalin. Biochim Biophys Acta. 2000;1482:57–64. doi: 10.1016/s0167-4838(00)00150-3. [DOI] [PubMed] [Google Scholar]
- [177].Zanotti G, Berni R. Plasma retinol-binding protein: structure and interactions with retinol, retinoids, and transthyretin. Vitam Horm. 2004;69:271–295. doi: 10.1016/S0083-6729(04)69010-8. [DOI] [PubMed] [Google Scholar]
- [178].Quadro L, Hamberger L, Gottesman ME, Wang F, Colantuoni V, Blaner WS, Mendelsohn CL. Pathways of vitamin A delivery to the embryo: insights from a new tunable model of embryonic vitamin A deficiency. Endocrinology. 2005;146:4479–4490. doi: 10.1210/en.2005-0158. [DOI] [PubMed] [Google Scholar]
- [179].Isken A, Golczak M, Oberhauser V, Hunzelmann S, Driever W, Imanishi Y, Palczewski K, von Lintig J. RBP4 Disrupts Vitamin A Uptake Homeostasis in a STRA6-Deficient Animal Model for Matthew-Wood Syndrome. Cell Metab. 2008;7:258–268. doi: 10.1016/j.cmet.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Kawaguchi R, Yu J, Wiita P, Honda J, Sun H. An essential ligand-binding domain in the membrane receptor for retinol-binding protein revealed by large-scale mutagenesis and a human polymorphism. J Biol Chem. 2008;283:15160–15168. doi: 10.1074/jbc.M801060200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Bok D, Heller J. Transport of retinol from the blood to the retina: an autoradiographic study of the pigment epithelial cell surface receptor for plasma retinol-binding protein. Exp Eye Res. 1976;22:395–402. doi: 10.1016/0014-4835(76)90177-9. [DOI] [PubMed] [Google Scholar]
- [182].Sommer A. Vitamin A status, resistance to infection, and childhood mortality. Ann N Y Acad Sci. 1990;587:17–23. doi: 10.1111/j.1749-6632.1990.tb00129.x. [DOI] [PubMed] [Google Scholar]
- [183].Ross AC. Vitamin A status: relationship to immunity and the antibody response. Proc Soc Exp Biol Med. 1992;200:303–320. doi: 10.3181/00379727-200-43436a. [DOI] [PubMed] [Google Scholar]
- [184].Chiang MY, Misner D, Kempermann G, Schikorski T, Giguere V, Sucov HM, Gage FH, Stevens CF, Evans RM. An essential role for retinoid receptors RARbeta and RXRgamma in long-term potentiation and depression. Neuron. 1998;21:1353–1361. doi: 10.1016/s0896-6273(00)80654-6. [DOI] [PubMed] [Google Scholar]
- [185].Siegenthaler JA, Ashique AM, Zarbalis K, Patterson KP, Hecht JH, Kane MA, Folias AE, Choe Y, May SR, Kume T, Napoli JL, Peterson AS, Pleasure SJ. Retinoic acid from the meninges regulates cortical neuron generation. Cell. 2009;139:597–609. doi: 10.1016/j.cell.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Pasutto F, Sticht H, Hammersen G, Gillessen-Kaesbach G, Fitzpatrick DR, Nurnberg G, Brasch F, Schirmer-Zimmermann H, Tolmie JL, Chitayat D, Houge G, Fernandez-Martinez L, Keating S, Mortier G, Hennekam RC, von der Wense A, Slavotinek A, Meinecke P, Bitoun P, Becker C, Nurnberg P, Reis A, Rauch A. Mutations in STRA6 Cause a Broad Spectrum of Malformations Including Anophthalmia, Congenital Heart Defects, Diaphragmatic Hernia, Alveolar Capillary Dysplasia, Lung Hypoplasia, and Mental Retardation. Am J Hum Genet. 2007;80:550–560. doi: 10.1086/512203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Golzio C, Martinovic-Bouriel J, Thomas S, Mougou-Zrelli S, Grattagliano-Bessieres B, Bonniere M, Delahaye S, Munnich A, Encha-Razavi F, Lyonnet S, Vekemans M, Attie-Bitach T, Etchevers HC. Matthew-Wood Syndrome Is Caused by Truncating Mutations in the Retinol-Binding Protein Receptor Gene STRA6. Am J Hum Genet. 2007;80:1179–1187. doi: 10.1086/518177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Heller J. Interactions of plasma retinol-binding protein with its receptor. Specific binding of bovine and human retinol-binding protein to pigment epithelium cells from bovine eyes. J Biol Chem. 1975;250:3613–3619. [PubMed] [Google Scholar]
- [189].Chen CC, Heller J. Uptake of retinol and retinoic acid from serum retinol-binding protein by retinal pigment epithelial cells. J Biol Chem. 1977;252:5216–5221. [PubMed] [Google Scholar]
- [190].Rask L, Peterson PA. In vitro uptake of vitamin A from the retinol-binding plasma protein to mucosal epithelial cells from the monkey's small intestine. J Biol Chem. 1976;251:6360–6366. [PubMed] [Google Scholar]
- [191].Ottonello S, Petrucco S, Maraini G. Vitamin A uptake from retinol-binding protein in a cell-free system from pigment epithelial cells of bovine retina. Retinol transfer from plasma retinol-binding protein to cytoplasmic retinol-binding protein with retinyl-ester formation as the intermediate step. J Biol Chem. 1987;262:3975–3981. [PubMed] [Google Scholar]
- [192].Sivaprasadarao A, Findlay JB. The mechanism of uptake of retinol by plasma-membrane vesicles. Biochem J. 1988;255:571–579. [PMC free article] [PubMed] [Google Scholar]
- [193].Shingleton JL, Skinner MK, Ong DE. Characteristics of retinol accumulation from serum retinol-binding protein by cultured Sertoli cells. Biochemistry. 1989;28:9641–9647. doi: 10.1021/bi00451a015. [DOI] [PubMed] [Google Scholar]
- [194].Sundaram M, Sivaprasadarao A, DeSousa MM, Findlay JB. The transfer of retinol from serum retinol-binding protein to cellular retinol-binding protein is mediated by a membrane receptor. J Biol Chem. 1998;273:3336–3342. doi: 10.1074/jbc.273.6.3336. [DOI] [PubMed] [Google Scholar]
- [195].Quadro L, Blaner WS, Hamberger L, Van Gelder RN, Vogel S, Piantedosi R, Gouras P, Colantuoni V, Gottesman ME. Muscle expression of human retinol-binding protein (RBP). Suppression of the visual defect of RBP knockout mice. J Biol Chem. 2002;277:30191–30197. doi: 10.1074/jbc.M205046200. [DOI] [PubMed] [Google Scholar]
- [196].Kawaguchi R, Yu J, Wiita P, Ter-Stepanian M, Sun H. Mapping the membrane topology and extracellular ligand binding domains of the retinol binding protein receptor. Biochemistry. 2008;47:5387–5395. doi: 10.1021/bi8002082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Golczak M, Maeda A, Bereta G, Maeda T, Kiser PD, Hunzelmann S, von Lintig J, Blaner WS, Palczewski K. Metabolic basis of visual cycle inhibition by retinoid and nonretinoid compounds in the vertebrate retina. J Biol Chem. 2008;283:9543–9554. doi: 10.1074/jbc.M708982200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Imanishi Y, Batten ML, Piston DW, Baehr W, Palczewski K. Noninvasive two-photon imaging reveals retinyl ester storage structures in the eye. J Cell Biol. 2004;164:373–383. doi: 10.1083/jcb.200311079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Yost RW, Harrison EH, Ross AC. Esterification by rat liver microsomes of retinol bound to cellular retinol-binding protein. J Biol Chem. 1988;263:18693–18701. [PubMed] [Google Scholar]
- [200].Herr FM, Ong DE. Differential interaction of lecithin-retinol acyltransferase with cellular retinol binding proteins. Biochemistry. 1992;31:6748–6755. doi: 10.1021/bi00144a014. [DOI] [PubMed] [Google Scholar]
- [201].Horwitz J, Heller J. Interactions of all-trans, 9-, 11-, and 13-cis-retinal, all-trans-retinyl acetate, and retinoic acid with human retinol-binding protein and prealbumin. J Biol Chem. 1973;248:6317–6324. [PubMed] [Google Scholar]
- [202].Berni R, Clerici M, Malpeli G, Cleris L, Formelli F. Retinoids: in vitro interaction with retinol-binding protein and influence on plasma retinol. FASEB J. 1993;7:1179–1184. doi: 10.1096/fasebj.7.12.8375617. [DOI] [PubMed] [Google Scholar]
- [203].Harrison EH, Gad MZ. Hydrolysis of retinyl palmitate by enzymes of rat pancreas and liver. Differentiation of bile salt-dependent and bile salt-independent, neutral retinyl ester hydrolases in rat liver. J Biol Chem. 1989;264:17142–17147. [PubMed] [Google Scholar]
- [204].Mills JP, Furr HC, Tanumihardjo SA. Retinol to retinol-binding protein (RBP) is low in obese adults due to elevated apo-RBP. Exp Biol Med (Maywood) 2008;233:1255–1261. doi: 10.3181/0803-RM-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- [206].Babitt J, Trigatti B, Rigotti A, Smart EJ, Anderson RG, Xu S, Krieger M. Murine SR-BI, a high density lipoprotein receptor that mediates selective lipid uptake, is N-glycosylated and fatty acylated and colocalizes with plasma membrane caveolae. J Biol Chem. 1997;272:13242–13249. doi: 10.1074/jbc.272.20.13242. [DOI] [PubMed] [Google Scholar]
- [207].Connelly MA, de la Llera-Moya M, Monzo P, Yancey PG, Drazul D, Stoudt G, Fournier N, Klein SM, Rothblat GH, Williams DL. Analysis of chimeric receptors shows that multiple distinct functional activities of scavenger receptor, class B, type I (SR-BI), are localized to the extracellular receptor domain. Biochemistry. 2001;40:5249–5259. doi: 10.1021/bi002825r. [DOI] [PubMed] [Google Scholar]
- [208].Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, Freeman S, Cosma MP, Colantuoni V, Gottesman ME. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. Embo J. 1999;18:4633–4644. doi: 10.1093/emboj/18.17.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Adams J. Structure-activity and dose-response relationships in the neural and behavioral teratogenesis of retinoids. Neurotoxicol Teratol. 1993;15:193–202. doi: 10.1016/0892-0362(93)90015-g. [DOI] [PubMed] [Google Scholar]
- [210].Nau H. Teratogenicity of isotretinoin revisited: species variation and the role of all-transretinoic acid. J Am Acad Dermatol. 2001;45:S183–187. doi: 10.1067/mjd.2001.113720. [DOI] [PubMed] [Google Scholar]
- [211].Nau H, Chahoud I, Dencker L, Lammer EJ, Scott WJ. Vitamin A in Health and Disease. Marcel Dekker, Inc.; 1994. Teratogenicity of Vitamin A and Retinoids; pp. 615–664. [Google Scholar]
- [212].Mallia AK, Smith JE, Goodman DW. Metabolism of retinol-binding protein and vitamin A during hypervitaminosis A in the rat. J Lipid Res. 1975;16:180–188. [PubMed] [Google Scholar]
- [213].Smith FR, Goodman DS. Vitamin A transport in human vitamin A toxicity. N Engl J Med. 1976;294:805–808. doi: 10.1056/NEJM197604082941503. [DOI] [PubMed] [Google Scholar]
- [214].Voolstra O, Oberhauser V, Sumser E, Meyer NE, Maguire ME, Huber A, von Lintig J. NinaB is essential for Drosophila vision but induces retinal degeneration in opsin-deficient photoreceptors. J Biol Chem. 2010;285:2130–2139. doi: 10.1074/jbc.M109.056101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Kiefer C, Sumser E, Wernet MF, Von Lintig J. A class B scavenger receptor mediates the cellular uptake of carotenoids in Drosophila. Proc Natl Acad Sci U S A. 2002;99:10581–10586. doi: 10.1073/pnas.162182899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Voolstra O, Kiefer C, Hoehne M, Welsch R, Vogt K, von Lintig J. The Drosophila class B scavenger receptor NinaD-I is a cell surface receptor mediating carotenoid transport for visual chromophore synthesis. Biochemistry. 2006;45:13429–13437. doi: 10.1021/bi060701u. [DOI] [PubMed] [Google Scholar]
- [217].Wang T, Jiao Y, Montell C. Dissection of the pathway required for generation of vitamin A and for Drosophila phototransduction. J Cell Biol. 2007;177:305–316. doi: 10.1083/jcb.200610081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Lobo GP, Hessel S, Eichinger A, Noy N, Moise AR, Wyss A, Palczewski K, von Lintig J. ISX is a retinoic acid-sensitive gatekeeper that controls intestinal beta,beta-carotene absorption and vitamin A production. FASEB J. 2010;24:1656–1666. doi: 10.1096/fj.09-150995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].White T, Lu T, Metlapally R, Katowitz J, Kherani F, Wang TY, Tran-Viet KN, Young TL. Identification of STRA6 and SKI sequence variants in patients with anophthalmia/microphthalmia. Mol Vis. 2008;14:2458–2465. [PMC free article] [PubMed] [Google Scholar]
- [220].Chassaing N, Golzio C, Odent S, Lequeux L, Vigouroux A, Martinovic-Bouriel J, Tiziano FD, Masini L, Piro F, Maragliano G, Delezoide AL, Attie-Bitach T, Manouvrier-Hanu S, Etchevers HC, Calvas P. Phenotypic spectrum of STRA6 mutations: from Matthew-Wood syndrome to non-lethal anophthalmia. Hum Mutat. 2009;30:E673–681. doi: 10.1002/humu.21023. [DOI] [PubMed] [Google Scholar]
- [221].Gavrilova R, Babovic N, Lteif A, Eidem B, Kirmani S, Olson T, Babovic-Vuksanovic D. Vitamin A deficiency in an infant with PAGOD syndrome. Am J Med Genet A. 2009;149A:2241–2247. doi: 10.1002/ajmg.a.32998. [DOI] [PubMed] [Google Scholar]
- [222].Segel R, Levy-Lahad E, Pasutto F, Picard E, Rauch A, Alterescu G, Schimmel MS. Pulmonary hypoplasia-diaphragmatic hernia-anophthalmia-cardiac defect (PDAC) syndrome due to STRA6 mutations--what are the minimal criteria? Am J Med Genet A. 2009;149A:2457–2463. doi: 10.1002/ajmg.a.33038. [DOI] [PubMed] [Google Scholar]
- [223].West B, Bove KE, Slavotinek AM. Two novel STRA6 mutations in a patient with anophthalmia and diaphragmatic eventration. Am J Med Genet A. 2009;149A:539–542. doi: 10.1002/ajmg.a.32682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Casey J, Kawaguchi R, McGettigan P, Sun H, Morrissey M, Nielsen J, Conroy J, Regan R, Tormey P, Ni Chroinin M, Kennedy BN, Lynch S, Green A, Ennis S. First implication of STRA6 mutations in isolated anophthalmia, microphthalmia and coloboma: adding a new dimension to the STRA6 phenotype. Human Mutation. 2011 doi: 10.1002/humu.21590. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Cutting GR. Modifier genes in Mendelian disorders: the example of cystic fibrosis. Ann N Y Acad Sci. 2010;1214:57–69. doi: 10.1111/j.1749-6632.2010.05879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Gu Y, Harley IT, Henderson LB, Aronow BJ, Vietor I, Huber LA, Harley JB, Kilpatrick JR, Langefeld CD, Williams AH, Jegga AG, Chen J, Wills-Karp M, Arshad SH, Ewart SL, Thio CL, Flick LM, Filippi MD, Grimes HL, Drumm ML, Cutting GR, Knowles MR, Karp CL. Identification of IFRD1 as a modifier gene for cystic fibrosis lung disease. Nature. 2009;458:1039–1042. doi: 10.1038/nature07811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Dowling JE, Wald G. VITAMIN A DEFICIENCY AND NIGHT BLINDNESS. Proc Natl Acad Sci U S A. 1958;44:648–661. doi: 10.1073/pnas.44.7.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Hyatt GA, Dowling JE. Retinoic acid. A key molecule for eye and photoreceptor development. Invest Ophthalmol Vis Sci. 1997;38:1471–1475. [PubMed] [Google Scholar]
- [229].Dowling JE. Chemistry of visual adaptation in the rat. Nature. 1960;188:114–118. doi: 10.1038/188114a0. [DOI] [PubMed] [Google Scholar]
- [230].Chen C, Tsina E, Cornwall MC, Crouch RK, Vijayaraghavan S, Koutalos Y. Reduction of all-trans retinal to all-trans retinol in the outer segments of frog and mouse rod photoreceptors. Biophys J. 2005;88:2278–2287. doi: 10.1529/biophysj.104.054254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Chen C, Koutalos Y. Rapid formation of all-trans retinol after bleaching in frog and mouse rod photoreceptor outer segments. Photochem Photobiol Sci. 2010;9:1475–1479. doi: 10.1039/c0pp00124d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Defoe DM, Bok D. Rhodopsin chromophore exchanges among opsin molecules in the dark. Invest Ophthalmol Vis Sci. 1983;24:1211–1226. [PubMed] [Google Scholar]
- [233].Kefalov VJ, Estevez ME, Kono M, Goletz PW, Crouch RK, Cornwall MC, Yau KW. Breaking the covalent bond--a pigment property that contributes to desensitization in cones. Neuron. 2005;46:879–890. doi: 10.1016/j.neuron.2005.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Ross AC. Diet in vitamin A research. Methods Mol Biol. 2010;652:295–313. doi: 10.1007/978-1-60327-325-1_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].During A, Dawson HD, Harrison EH. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe. J Nutr. 2005;135:2305–2312. doi: 10.1093/jn/135.10.2305. [DOI] [PubMed] [Google Scholar]
- [236].Takahashi N, Breitman TR. Retinoylation of Proteins in Mammalian Cells. In: Blomhoff R, editor. Vitamin A in Health and Disease. Marcel Dekker, Inc.; 1994. pp. 257–273. [Google Scholar]
- [237].Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- [238].Huang S, Li Y, Chen Y, Podsypanina K, Chamorro M, Olshen AB, Desai KV, Tann A, Petersen D, Green JE, Varmus HE. Changes in gene expression during the development of mammary tumors in MMTV-Wnt-1 transgenic mice. Genome Biol. 2005;6:R84. doi: 10.1186/gb-2005-6-10-r84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Carlson A, Bok D. Promotion of the release of 11-cis-retinal from cultured retinal pigment epithelium by interphotoreceptor retinoid-binding protein. Biochemistry. 1992;31:9056–9062. doi: 10.1021/bi00152a049. [DOI] [PubMed] [Google Scholar]
- [240].Carlson A, Bok D. Polarity of 11-cis retinal release from cultured retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1999;40:533–537. [PubMed] [Google Scholar]
- [241].Wyatt N, Ponting C, Dorin J, Fitzpatrick D, Hill R. Stra6.2: A novel member of the STRA6 gene family. MECHANISMS OF DEVELOPMENT. 2009;126:S259–S259. [Google Scholar]
- [242].Cheng Y, Lotan R. Molecular cloning and characterization of a novel retinoic acid-inducible gene that encodes a putative G protein-coupled receptor. J Biol Chem. 1998;273:35008–35015. doi: 10.1074/jbc.273.52.35008. [DOI] [PubMed] [Google Scholar]