Abstract
Three hundred and twenty-two patients (192 male and 130 female) with cystic lesions of the jaw were successfully diagnosed and treated. One hundred and fifty-five (48%) were radicular cysts, 80 (25%) were dentigerous cysts, 23 (7%) were odontogenic keratocyst (=keratocystic odontogenic tumor), 19 (6%) were eruption cysts, 16 (5%) were traumatic bone cysts, and 29 (9%) were non-odontogenic cysts.
There were 95 in the pediatric age group (1 month to 16 years) and 227 in the adult age group (17 years and older). Male to female ratio was 1 in the pediatric age group and 1.7 in the adult age group. The treatment modalities were: marsupialization, enucleation, enucleation with bone grafting, or resection. The distribution and characteristics of jaw cysts in children are different from those in adults. In children there is a relatively high rate of developmental cysts, whereas in adults the inflammatory cysts are more common. Following enucleation of a cystic jaw lesion, the entire surgical specimen and not only a biopsy specimen, should be examined histopathologically to prevent any possibility of an intramural squamous cell carcinoma that may be overlooked. The differences in prevalence of each type of jaw cyst during a lifetime may point toward a multifactorial polygenic pattern rather than a monogenic pattern.
Keywords: cyst, jaw bone, children, adults, genetics
Introduction
Odontogenic cysts are defined as those cysts that arise from odontogenic epithelium and occur in the tooth-bearing regions of the jaws. It is usually considered that proliferation and/or degeneration of this epithelium leads to odontogenic cyst development. Cystic jaw lesions may be epithelial or non-epithelial, odontogenic or non-odontogenic, developmental, or inflammatory in origin. The distribution of jaw cysts according to diagnosis in a general population is: radicular cysts (RC) 56%, dentigerous cysts (DC) 17%, nasopalatine duct cysts (NPDC) 13%, odontogenic keratocysts (OKC) 11%, globulomaxillary cysts 2.3%, traumatic bone cysts (TBC) 1.0%, and eruption cysts (EC) 0.7% 1,2. According to the 2005 WHO Classification of Tumors 3, OKC is currently designated as a keratocystic odontogenic tumor (KCOT) and is defined as a benign uni-or multicystic, intraosseous tumor of odontogenic origin, with a characteristic lining of parakeratinized stratified squamous epithelium with the potential for aggressive, infiltrative behavior. Although they are benign, keratocystic odontogenic tumors (KCOTs) are locally aggressive and have a tendency to recur after treatment; reported recurrence rates range from 3% to 60% 2.
Several long-term developmental processes are taking place in the maxillofacial area during the pediatric age period. These include the three-dimensional growth of the maxillofacial skeleton as well as odontogenesis of the deciduous and permanent dentition, all of which may be associated with cyst formation. During the adult age period the permanent dentition sustains damage originating from caries and / or trauma, both of which may be associated with cyst formation. As most jaw cysts are odontogenic in origin, it would be interesting to look at the distribution of jaw cysts in the different age groups.
The purpose of this article is to follow the distribution, characteristics, histopathologic diagnosis, treatment, and outcome of jaw cysts in a series of 322 patients and review the literature.
Materials and Methods
During a 20-year period, 322 patients (192 male and 130 female) with cystic lesions of the jaws were referred for consultation. All patients underwent clinical examination and plain film radiography (panoramic, periapical, and occlusal views). Some of them were referred to computed tomography (CT) with a multiplanar reconstruction program (MPR) 4. Age, sex, cyst type, and cyst diameter were recorded. Patients were divided into two age groups—pediatric age (range: 1 month to 16 years) and adult age (range: 17 years and older). Depending on the case, surgery was performed under local or general anesthesia and included one of the following treatment modalities: marsupialization, enucleation, enucleation with bone grafting or resection.
Student-Newman-Keuls test was used for statistical analysis at the P = 0.05 level for significant differences.
Results
The types of cysts, mean age of patients, and mean diameter of cysts in the pediatric age group are listed in Table 1. In this group (N=95), the most common cysts were DC (44%), followed by EC (21%), TBC (18%), and RC (17%). The mean ages were 11, 4.3, 14, and 8 years for DC, EC, TBC, and RC, respectively.
Table 1.
Types of cysts, ages of patients, and cyst diameters in pediatric age group (N=95).
| Number (%) | Mean age (years) | Mean diameter (cm) | |
|---|---|---|---|
| Dentigerous cyst | 42 (44%) | 11 | 2.1 |
| Eruption cyst | 20 (21%) | 4.3 | |
| Traumatic bone cyst | 17 (18%) | 14 | 1.7 |
| Radicular cyst | 16 (17%) | 8 | 1.4 |
The types of cysts, mean age of patients, and mean diameter of cysts in the adult age group are listed in Table 2. In this group (N=227), the most common cysts were RC (63%), followed by DC (18%), OKCT (10%), and non-odontogenic cyst (9%). The mean age groups were 42, 50, and 46 years for RC, DC, and OKCT, respectively. The difference in distribution of RC and DC between the pediatric and adult age groups was statistically significant (P < 0.05).
Table 2.
Types of cysts, ages of patients, and cyst diameters in adult age group (N=227).
| Number (%) | Mean age (years) | Mean diameter (cm) | |
|---|---|---|---|
| Radicular cyst | 144 (63%) | 42 | 3.0 |
| Dentigerous cyst | 40 (18%) | 50 | 3.2 |
| Odontogenic keratocystic tumor (=Keratocyst, Primordial cyst) |
23 (10%) | 46 | 2.7 |
| Non odontogenic cyst | 20 ( 9%) |
The mean diameters of DCs were 2.1 and 3.2 for the pediatric and adult age groups, respectively. The mean diameters of RCs were 1.4 and 3.0 for the pediatric and adult age groups, respectively. The differences in mean diameters of DC and RC between the age groups were significant (P < 0.05).
The M:F ratio was 1.0 in the pediatric age group and 1.7 in the adult age group. This difference was statistically significant (P < 0.05).
The treatment modalities included marsupialization in 113 (35%) patients, enucleation in 145 (45%), and enucleation and bone grafting in 62 (19%), by intraoral approach. Two cases (0.6%) with recurrent KCOT underwent resection, by extraoral surgical approach.
The follow-up period ranged from a minimum of one year to five years. Such follow-up consisted of an annual examination and periapical or panoramic radiographs. All patients were without evidence of disease during the follow-up period.
Discussion
Cystic lesions of the jaws can be either odontogenic or non-odontogenic, developmental, or inflammatory in origin. In the present study 44% of the cysts were developmental and 48% were inflammatory in origin. This is in general agreement with the type of cyst distribution found in a large series 2. However, when looking separately at the pediatric and adult groups, an overt difference in distribution of RC, an inflammatory cyst (17% and 63%, respectively), and DC, a developmental cyst (44% and 18%, respectively), can be seen. This finding is in agreement with earlier reports 5-10.
The difference in prevalence of developmental cysts is probably related to the fact that during the pediatric age period the jaws are involved in profound developmental processes. These include growth of the maxillofacial skeleton and development of the primary and permanent dentition, all of which can be associated with cyst formation. The difference in distribution of inflammatory cysts may be due to the fact that RCs arising from primary teeth are considered very rare 11. RCs arising from permanent teeth are also infrequent in the pediatric age, because the RC arises from the epithelial residues in the periodontal ligament as a result of inflammation that follows necrosis of the dental pulp. Recently erupted permanent teeth, as in the pediatric age, are usually intact.
Clinical presentation of infection at the cyst area is usually associated with acute or chronic inflammation at the cyst wall. In such cases the epithelial lining of the cyst wall may be destroyed, regardless of cyst origin, leaving the cyst wall with granulation tissue.
Of the 95 pediatric cases in our series, 48 were male and 47 female, providing a M:F ratio of 1. In contrast, of the 227 adult cases 143 were male and 84 were female, with a M:F ratio of 1.70. This in agreement with the reported distribution in the general population, where there is a significant difference in occurrence by sex, with male predominance 12-14.
The greater frequency in adult males may be because they are more likely to neglect their teeth or they are more likely to sustain trauma to their teeth, compared to females, all of which may be the etiology for cyst formation 1,2.
The mean diameter of DC and RC in the adult age group was significantly larger compared to the pediatric age group. Apparently, it does not point toward a more aggressive nature among the cysts in the adult group, but rather a general tendency among adults to postpone medical care, allowing the cyst to increase in size. The normal size difference of the mandible and maxilla between the pediatric and adult groups may also contribute to the cyst diameter.
For most of the cysts, plain film radiography (PFR) was an adequate imaging modality. In some of the cases, CT with MPR program was also performed. The CT with MPR software, originally designed for implant dentistry, has proven to be useful in the evaluation of jaw abnormality and pathology 4, 15-19. Using this software program, anatomic structures, such as the mandibular canal, mental foramen, incisive canal, and maxillary sinus, can be seen in cross-section. CT with MPR has also been shown to be superior to PFR in demonstrating cystic lesions of the jaws and in evaluating bone regeneration following marsupialization of jaw cysts 17-19.
The main advantage of CT with MPR is that it allows planning of the surgical approach with the least morbidity to the adjacent anatomic structures 20. Despite the advantages, CT should not be used routinely, but rather reserved for large lesions, particularly those where extension into the nasal cavity, maxillary antrum, orbit, or pterygomaxillary space must be assessed.
The treatment objective is restoring the morphology and function of the affected area. There are two basic surgical procedures, namely marsupialization (decompression) (Fig.1) and enucleation. Marsupialization, a relatively simple procedure, consists of surgically producing a “window” in the cystic wall to relieve intra-cystic tension. After this, the cystic cavity slowly decreases in size. The cavity is lightly packed with paraffin gauze until the line of junction between the cystic lining and the oral mucosa has healed. Three to six months later, enucleation is performed 16.
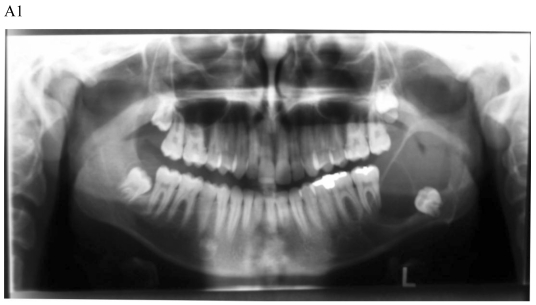
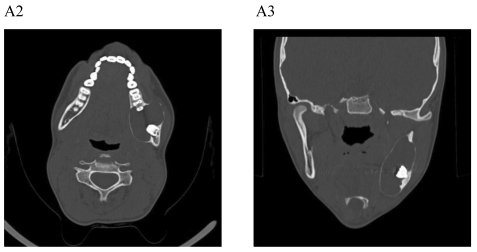
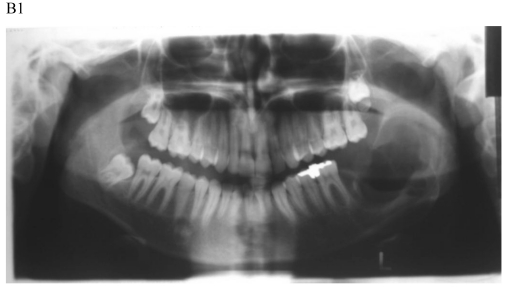
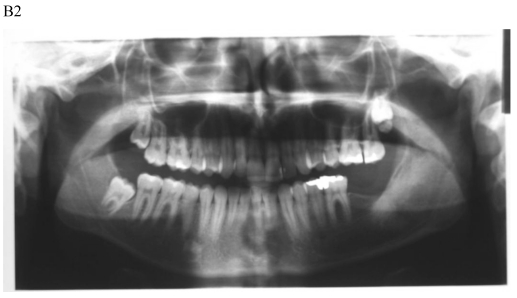
Figure 1.
Dentigerous cyst affecting the left mandible of a 15-year-old boy treated by marsupiallization. At presentation, there was an acute infection in the area. A) Pre-operative radiographs: 1) Panoramic radiograph demonstrating a tooth within a large radiolucent area in the left mandible; 2) Axial CT scan demonstrating expansion of the body of the left mandible with very thin buccal and lingual cortices. A tooth is within the lumen; 3) Coronal CT of the mandible demonstrates an expansion of the ascending ramus of the left mandible with very thin cortical borders. A tooth is within the lumen. B. Postoperative radiographs: 1) Panoramic radiograph shortly post-marsupialization, extraction of tooth 37 that was adjacent the cyst as well as tooth 38 that was within the cyst; 2) Panoramic radiograph one year post-treatment. The radiodensity of the cystic area is similar to normal bone, indicating complete bone regeneration.
The notable disadvantages of the technique are: (a) it is a two-stage surgical procedure, (b) pathological tissue is left behind and a more sinister pathological process (i.e., squamous cell carcinoma) may be overlooked 21, and (c) in a large cystic cavity it takes a long period of time for the bone to regenerate.
Enucleation with primary closure is the treatment of choice 22. It is a one-stage surgical treatment followed by periodic radiographic examinations at regular intervals to observe the progress of bone regeneration of the defect. It also allows pathologic examination of the entire specimen for histopathologic diagnosis. Enucleation can be done only when the jaw bone adjacent to the cyst is intact. If CT demonstrates erosions in the buccal or lingual cortices, marsupialization should be the treatment of choice (Fig. 1) 4.
Marsupialization was performed in all patients with eruption cysts and for a few patients with sizeable cysts. Enucleation without bone grafting was done with patients who had smaller diameter cysts. Enucleation with bone grafting was performed with large cystic lesions. Allogeneic or xenogeneic demineralized freeze-dried bone was used for grafting with satisfactory results 23-25. Autogenous cancellous bone is considered the best grafting material and has been used with clinical success for treatment of cystic lesions for many years 7. However, donor site morbidity, either inta-oral or extra-oral, is a factor when obtaining a bone graft and it is an added morbidity regardless of site 26. Its use for grafting of cystic lesions should be restricted if bone substitutes are available 27. Two cases of recurrent lesion of KCOT treated initially in the community and referred to the hospital following recurrence, underwent resection. The traditional method for treatment of most KCOTs is enucleation. However, due to the lining of the cyst being delicate and the fact that they frequently recur, this method alone may not be sufficient.
The surgical treatment method for KCOT was categorized as conservative or aggressive. Conservative treatment is “cyst-oriented” and includes marsupialization or enucleation with or without curettage. Its advantage is preservation of anatomical structures, including teeth, which is advocated because KCOTs commonly present in younger patients. It has been asserted that a conservative approach is applicable to all age groups as well as to patients with nevoid basal cell carcinoma syndrome (NBCCS). Aggressive treatment addresses the “neoplastic nature” of KCOT and includes peripheral ostectomy, chemical curettage with Carnoy's solution or en bloc resection. The aggressive modalities have been recommended for NBCCS cases, large KOCTs and recurrent lesion 28.
The follow up for patients with cystic lesions of the jaws following surgery is yearly panoramic radiograph, at least up to full bony regeneration of the affected area. KCOT should be followed for a 5-year period, due to the higher recurrence rate 28,29.
The proliferative activity of odontogenic epithelium in cystic lesions was examined by immunolabeling of Ki-67, EGF, and Survinin 30. It was found that the proliferation rate of KCOT was greater than that of DC. In KCOT, the epithelial cells showed neoplastic proliferative characteristics, suggesting the presence of a suprabasal proliferative compartment, maintained by inhibition of apopotosis. The angiogenesis was assessed in KCOT, DC, and normal oral mucosa using CD-105 antigen 31. It was demonstrated that CD-105 antigen is strongly expressed in microvessels of KCOT compared to DC and normal oral mucosa, suggesting that the cyst wall of KCOT plays a role in the neoplastic behavior of the lesion. These finding can further support the WHO decision 3 recommending the term KCOT, as it better reflects its neoplastic nature.
The fact that DC is more common in childhood, whereas RC is more common in adults, may point toward more genetic involvement in the etiology of DC, compared to RC. It is obvious that among the elderly, there is a long-term accumulation of environmental influences, compared to the pediatric age period.
Nowadays it is well known that there is a genetic basis for any kind of disease. Some of them are one gene dependent, while most are polygenic and multifactor dependent. The genetic basis might explain the wide range of different host responses to various environmental factors (viruses, bacteria, poisons, etc.) and thus the formation of odontogenic cysts.
Our understanding of the genetic basis of odontogenic cysts is limited. Most reports are on KCOT (odontogenic keratocysts or primordial cysts). It has been found that point mutation occurs in the suppressor gene PTCH mapped onto chromosome 9(q22.3-q31). Also, several genes were over-expressed in 12q13, including KRT6B, ERBB3, and GLT1. Deletions were also found in 3q13.1, 5q14.3, and 7q31.3 including CDH18 and ALCAM, and MEMD. KCOT shows a high expression of bcl-2, p53, p63 32-34.
Shear 35 has discussed the possibility that Knudson's "two hit" hypothesis of cancer development 36 may also explain the transformation of KCOT into neoplasia. However, it cannot explain the predisposition for KCOT formation. Recent advances in genetic and molecular research, i.e., PTCH mutations, have led to increased knowledge of KCOT pathogenesis, which hints at potential new treatment options, although the question of whether the KCOT is a cyst or a cystic neoplasm is yet to be answered with certainty. Since some advocate a more conservative treatment for KCOT, notably marsupialization, future treatment strategies may focus on molecular approaches that will reduce or eliminate the need for aggressive surgery 37,38.
The differences in prevalence of each type of jaw cyst during a lifetime may point toward a multifactorial polygenic pattern rather than a monogenic pattern. Since jaw cysts are common, and some of them carry tumorigenic potential, there is a need for an in-depth genetic study to determine the possible role of genetics in the etiology and prevalence of different types of jaw cysts.
Conclusions
The DC is most common in the pediatric age group, whereas the RC is most common in the adult age group. The treatment modalities in both groups were: marsupialization, enucleation, and enucleation with allogeneic or xenogeneic bone grafting. Harvesting of autogenous bone for grafting should be restricted to cases with large cysts and where bone substitute is not available. In recurrent KCOT lesion resection is the treatment of choice. Following enucleation of a cystic jaw lesion, the entire surgical specimen and not only a biopsy specimen, should be examined histopathologically to prevent any possibility of an intramural squamous cell carcinoma that may be overlooked. An infected cyst may lose its epithelial lining. Cytogenetic studies are useful and should be performed in KCOT.
References
- 1.Killy HC, Kay LW. An analysis of 471 benign cystic lesions of the jaws. Int Surg. 1966;46:540–5. [PubMed] [Google Scholar]
- 2.Shear M, Speight PM. Cysts of the oral and maxillofacial regions; 4th edition. Oxford: Blackwell Munksgaard; 2007. [Google Scholar]
- 3.Barnes L, Eveson JW, Reichart P, World Health Organization Classification of Tumors. Pathology and Genetics of Head and Neck Tumors. Lyon: IARC Press; 2005. [Google Scholar]
- 4.Bodner L, Bar-Ziv J, Kaffe I. Computed tomography of cystic jaw lesions. J Comput Assist Tomogr. 1994;18:22–6. doi: 10.1097/00004728-199401000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Radden BG, Reade PC. Odontogenic cysts. A review and clinicopathological study of 368 odontogenic cysts. Aust Dent J. 1973;18:218–25. doi: 10.1111/j.1834-7819.1973.tb03470.x. [DOI] [PubMed] [Google Scholar]
- 6.Pechalova PF, Bakardjiv AG, Beltcheva AB. Jaw cysts at children and adolescence: A single-center retrospective study of 152 cases in southern Bulgaria. Med Oral Pathol Oral Cir Bucal. 2011 doi: 10.4317/medoral.16849. in press. [DOI] [PubMed] [Google Scholar]
- 7.Kreidler JF, Raubenheimer EJ, van Heerden WF. A retrospective analysis of 367 cystic lesions of the jaws- the Ulm experience. J Craniomaxillofac Surg. 1993;21:339–41. doi: 10.1016/s1010-5182(05)80494-9. [DOI] [PubMed] [Google Scholar]
- 8.Daley TD, Wysocky GP, Pringle GA. Relative incidence of odontogenic tumors and oral and jaw cysts in a Canadian population. Oral Surg Oral Med Oral Pathol. 1994;77:276–80. doi: 10.1016/0030-4220(94)90299-2. [DOI] [PubMed] [Google Scholar]
- 9.Jones AV, Craig GT, Franklin CD. Range and demographics of odontogenic cysts diagnosed in UK population over 30-year period. J Oral Pathol Med. 2006;35:500–7. doi: 10.1111/j.1600-0714.2006.00455.x. [DOI] [PubMed] [Google Scholar]
- 10.Iatro I, Theologie-Lygidakis N, Leventis M. Intraosseous cystic lesions of the jaws in children: A retrospective analysis of 47 consecutive cases. Oral Surg Oral Med Oral Pathol Radiol Endod. 2009;107:485–92. doi: 10.1016/j.tripleo.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Lustmann J, Shear M. Radicular cysts arising from deciduous teeth. Int J Oral Surg. 1985;14:153–61. doi: 10.1016/s0300-9785(85)80087-9. [DOI] [PubMed] [Google Scholar]
- 12.Meningaud JP, Oprean N, Pitak-Arnnop P, Bertrand JC. Odontogenic cysts: a clinical study of 695 cases. J Oral Sci. 2006;48:59–62. doi: 10.2334/josnusd.48.59. [DOI] [PubMed] [Google Scholar]
- 13.Jones AV, Franklin CD. An analysis of oral and maxillofacial pathology found in children over a 30-year period. Int J Paed Dent. 2006;16:19–30. doi: 10.1111/j.1365-263X.2006.00683.x. [DOI] [PubMed] [Google Scholar]
- 14.Tortorici S, Amodio E, Massenti M, Buzzanca ML, Burruano F, Vitale F. Prevalence and distribution of odontogenic cysts in Sicily: 1986-2005. J Oral Sci. 2008;50:15–8. doi: 10.2334/josnusd.50.15. [DOI] [PubMed] [Google Scholar]
- 15.Abrahams JJ, Oliverio PJ. Odontogenic cysts: improved imaging with a dental CT software program. Am J Neuroradiol. 1993;14:367–74. [PMC free article] [PubMed] [Google Scholar]
- 16.Bodner L, Bar-Ziv J. Characteristics of bone formation following marsupialization of jaw cyst. Dentomaxillofac Radiol. 1998;27:166–71. doi: 10.1038/sj/dmfr/4600344. [DOI] [PubMed] [Google Scholar]
- 17.Bodner L, Woldenberg Y, Bar-Ziv J. Radiographic features of large cystic lesions of the jaws in children. Pediatr Radiol. 2003;33:3–6. doi: 10.1007/s00247-002-0816-2. [DOI] [PubMed] [Google Scholar]
- 18.Hisatomi M, Asaumi J, Konouchi H, Shigehara H, Yangai Y, Kishi K. MR imaging of epithelial cysts of the oral and maxillofacial region. Eur J Radiol. 2003;48:178–82. doi: 10.1016/S0720-048X(02)00218-8. [DOI] [PubMed] [Google Scholar]
- 19.Krennmair G, Lenglinger F. Imaging of the mandibular cysts with a dental compute tomography software program. Int J Oral Maxillofac Surg. 1995;24:48–52. doi: 10.1016/s0901-5027(05)80856-2. [DOI] [PubMed] [Google Scholar]
- 20.Bodner L, Manor E, Glazer M, Brennan PA. Cystic lesions of the jaws in edentulous patients - Analysis of 27 cases. Brit J Oral Maxillofac Surg. 2010 doi: 10.1016/j.bjoms.2010.10.009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Bodner L, Manor E, Shear M, van der Wall I. Primary intraosseous squamous cell carcinoma arising in an odontogenic cyst- A clinicopathologic analysis of 116 reported cases. J Oral Pathol Med. 2011;40:733–738. doi: 10.1111/j.1600-0714.2011.01058.x. [DOI] [PubMed] [Google Scholar]
- 22.van Doorm ME. Enucleation and primary closure of jaw cysts. Int J Oral Surg. 1972;1:17–25. doi: 10.1016/s0300-9785(72)80032-2. [DOI] [PubMed] [Google Scholar]
- 23.Bodner L. Effect of decalcified freeze-dried bone allograft on the healing of jaw defects following cyst enucleation. J Oral Maxillofac Surg. 1996;54:1282–6. doi: 10.1016/s0278-2391(96)90482-6. [DOI] [PubMed] [Google Scholar]
- 24.Bodner L. Osseous regeneration in the jaws using demineralized allogeneic bone implants. J Cranio Maxillofac Surg. 1998;26:116–20. doi: 10.1016/s1010-5182(98)80051-6. [DOI] [PubMed] [Google Scholar]
- 25.Horowitz I, Bodner L. The use of xenograft bone in combination with aspirated bone marrow for treatment of cystic defects of the jaws. Head & Neck. 1989;11:516–23. doi: 10.1002/hed.2880110608. [DOI] [PubMed] [Google Scholar]
- 26.Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192–5. doi: 10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Habal MB, Reddi AH. Bone grafts and bone induction substitutes. Clin Plast Surg. 1994;21:525–52. [PubMed] [Google Scholar]
- 28.Morgan TA, Burton CC, Qian FA. A retrospective review of treatment of odontogenic keratocyst. J Oral M axillofac Surg. 2005;63:960–3. doi: 10.1016/j.joms.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 29.Zecha JA, Mendes RA, Lindeboom VB, van der Waal I. Recurrent rate of keratocystic odontogenic tumorafter conservative surgical treatment without adjunctive therapies-A 35-years single institution experience. Oral Oncol. 2010;46:740–2. doi: 10.1016/j.oraloncology.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 30.de Oliveira MG, da Silva Lauxen I, Chaves AC. et al. Odontogenic epithelium: Immunolabeling of Ki-67, EGFR and Survinin in pericoronal follicles, dentigerous cysts and keratocystic odontogenic tumors. Head Neck Pathol. 2011;5:1–7. doi: 10.1007/s12105-010-0216-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gadbail AR, Hande A, Chaudhary M, Nikam A, Gawande M, Patil S, Tekade S, Gondivkar S. Tumor angiogenesis in keratinocystic odontogenic tumor assessed by using CD-105 antigen. J Oral Pathol Med. 2010 doi: 10.1111/j.1600-0714.2010.00962.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Frandon PA, Del Mastro RG, Evans DG. et al. Location of gene for Gorlin syndrome. Lancet. 1992;339:581–2. doi: 10.1016/0140-6736(92)90868-4. [DOI] [PubMed] [Google Scholar]
- 33.Li TJ, Browne RM, Prime SS, Paterson IC, Matthews JB. p53 expression in odontogenic keratocyst epithelium. J Oral Pathol Med. 1996;25:245–55. doi: 10.1111/j.1600-0714.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- 34.Lench NJ, Telford EAR, High AS, Markham AF, Wicking C, Wainwright BJ. Characterisation of human patched germ line mutations in naevoid basal cell carcinoma syndrome. Hum Genet. 1997;100:497–502. doi: 10.1007/s004390050541. [DOI] [PubMed] [Google Scholar]
- 35.Shear M. The aggressive nature of the odontogenic keratocyst is it a benign cystic neoplasm? Part 2. Proliferation and genetic studies. Oral Oncol. 2002;38:323–31. doi: 10.1016/s1368-8375(01)00066-5. [DOI] [PubMed] [Google Scholar]
- 36.Knudson AG. Mutation and cancer; statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971;68:823–30. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li TJ. The odontogenic keratocyst: a cyst, or a cystic neoplasm? J Dent Res. 2011;90:133–42. doi: 10.1177/0022034510379016. [DOI] [PubMed] [Google Scholar]
- 38.Mendes RA, Carvalho JF, van der Waal I. Biological pathways involved inaggressive behavior of the keratinocystic odontogenic tumor and possible implication for molecular oriented treatment-An overview. Oral Oncol. 2010;46:19–24. doi: 10.1016/j.oraloncology.2009.10.009. [DOI] [PubMed] [Google Scholar]