Abstract
Aim
Recent work has shown that humans are significantly exposed to isocyanic acid/cyanate, which is generated when coal, biomass, or tobacco is burned. In vivo, cyanate is formed by the phagocyte protein myeloperoxidase and by breakdown of urea. Carbamylation of proteins through cyanate has been demonstrated to predict cardiovascular risk and is thought to promote vascular dysfunction; however, the underlying mechanisms remain unclear. Results: Here, we show that cyanate induces intercellular cell adhesion molecule-1 (ICAM-1) expression with subsequently enhanced neutrophil adhesion in human coronary artery endothelial cells. Cyanate triggers ICAM-1 expression through a mechanism depending on activation of the mitogen-activated protein kinase p38 and nuclear factor-kappaB. Endothelial ICAM-1 expression was not induced when low-molecular-weight substances were removed from cell culture medium, thus ruling out a role of carbamylated (lipo)proteins in ICAM-1 induction. In mice, oral administration of cyanate induced marked endothelial ICAM-1 expression in the aorta. Moreover, in patients with end-stage renal disease, the extent of plasma protein carbamylation (a marker for cyanate exposure) significantly correlated with plasma levels of soluble ICAM-1. Innovation: Here, we demonstrate for the first time that cyanate, rather than carbamylated lipoproteins, induces vascular ICAM-1 expression in vivo. Conclusion: Collectively, our data raise the possibility that cyanate amplifies vascular inflammation, linking inflammation, smoking, and uremia. Antioxid. Redox Signal. 16, 129–137.
Introduction
Post-translational carbamylation of proteins through cyanate is thought to promote vascular dysfunction (41). Cyanate irreversibly transforms lysine to ɛ-amino-carbamyllysine, also known as homocitrulline (HCit) (36). This pathway is of particular relevance, as clinical studies have shown that carbamylated proteins are independent risk factors for development of coronary artery disease and stroke (41). A potential role for protein carbamylation in human disease has been investigated mainly in the context of renal disease. Cyanate is formed in vivo by breakdown of urea, and about 0.8% of urea decomposes to cyanate (11). Since urea levels increase up to 110 mM in patients with chronic renal failure, cyanate concentrations of about 1 mM may be formed (5, 6). In patients who undergo dialysis, cardiovascular disease is the principal cause of morbidity, and cardiac mortality of patients aged 45 years or younger is more than 100-fold increased when compared with the general population (8, 22, 37).
Importantly, isocyanic acid was recently identified as a component of smoke from coal, biomass, or tobacco, thus causing protein carbamylation at physiologically significant levels (31). Moreover, it was recently observed that cyanate is a major product of the phagocyte protein myeloperoxidase (MPO) (3, 41). In human atherosclerotic lesions, MPO selectively carbamylates high-density lipoprotein (HDL), thus rendering HDL dysfunctional (15). Of particular interest, MPO released by degranulation of activated neutrophils avidly associates with endothelial cells and accumulates in the subendothelial matrix of vascular tissues (4). Thus, it can be assumed that vascular endothelial cells might be exposed to high local concentrations of cyanate.
One key event in the development of atherosclerosis is the adhesion of leukocytes to the vascular endothelium. In large part, these processes are mediated by a diverse group of cellular adhesion molecules such as intercellular cell adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and E-selectin, which are expressed on the surface of activated vascular endothelial cells (9, 23). Recent data from patients with renal failure strongly suggest that high serum levels of adhesion molecules may predict future cardiovascular events (29, 38, 39). In the current study, we demonstrate that cyanate induces endothelial ICAM-1 expression in vivo and in vitro. We observed that the carbamyllysine content of plasma, a marker for cyanate formation, significantly correlates with soluble ICAM-1 (sICAM-1) levels in patients with end-stage renal disease. Since ICAM-1 is known for its importance in mediating cell-cell interactions and facilitating leukocyte endothelial transmigration, our results suggest that cyanate promotes vascular inflammation.
Innovation
Humans are exposed to significant amounts of isocyanic acid/cyanate formed by pyrolysis/combustion of coal, biomass, or tobacco. Important endogenous sources of cyanate include the breakdown of urea and myeloperoxidase (MPO)-catalyzed oxidation of thiocyanate. Carbamylation of proteins through cyanate has been demonstrated to predict cardiovascular risk and is thought to promote vascular dysfunction; however, the underlying mechanisms remain unclear.
Here, we show for the first time that cyanate, rather than carbamylated (lipo)proteins, triggers vascular intercellular cell adhesion molecule-1 (ICAM-1) expression. Cyanate induces ICAM-1 expression through a mechanism depending on activation of p38 mitogen-activated protein kinase (MAPK) and nuclear factor-kappaB. In mice, oral administration of cyanate induces marked endothelial ICAM-1 expression in the aortic arch. Most importantly, in patients with end-stage renal disease, the extent of plasma protein carbamylation (a marker for cyanate exposure) significantly correlates with plasma levels of soluble ICAM-1. Since ICAM-1 is known for its importance in mediating cell–cell interactions and facilitating leukocyte-endothelial transmigration, our results provide further insights to explain a part of the underlying mechanisms that contribute to the enhanced cardiovascular risk associated with smoking and chronic renal failure.
Results
Cyanate induces ICAM-1 expression in human coronary artery endothelial cells
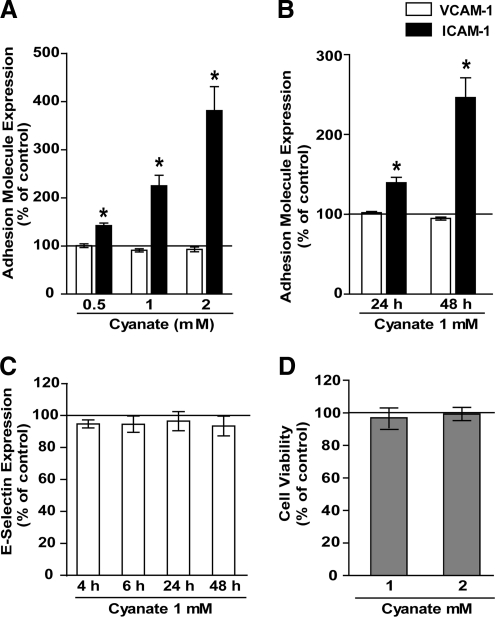
Vascular endothelial cells might be locally exposed to high cyanate concentrations. Treatment of human coronary artery endothelial cells (HCAEC) for 48 h with increasing concentrations of cyanate induced a concentration-dependent expression of ICAM-1, whereas VCAM-1 (Fig. 1A) and E-selectin expression (Fig. 1C) was unaltered. Cyanate-induced ICAM-1 expression was time dependent and substantially increased from 24 to 48 h (Fig. 1B). Cyanate treatment had no effect on viability of endothelial cells (Fig. 1D).
FIG. 1.
Flow-cytometric quantification of adhesion molecule expression in endothelial cells. (A) Human coronary artery endothelial cells (HCAEC) were treated for 48 h with increasing concentrations of sodium cyanate (0.5 up to 2 mM) added to cell culture medium. Subsequently, expression of cell adhesion molecules was assessed by flow cytometry. (B) HCAEC were treated with 1 mM sodium cyanate for 24 h or 48 h, and intercellular cell adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) expression was determined. (C) HCAEC were treated with sodium cyanate (1 mM) for the indicated time points, and E-selectin expression was determined. (D) HCAEC were treated with increasing concentrations of sodium cyanate for 48 h, and cell viability was assessed by performing an MTT (3-(4, 5-methylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide) reduction assay. All experiments were performed in duplicate. Control was set at 100%, and values are expressed as % of control. Results shown are mean±SEM (n=3–5). *p<0.05 versus control.
Cyanate-induced (lipo)protein carbamylation does not mediate endothelial ICAM-1 expression
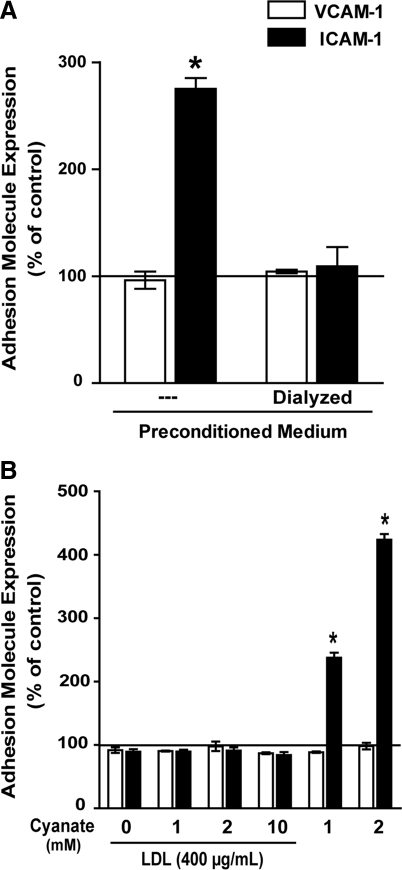
Our experiments on HCAEC were performed in the presence of serum; therefore, cyanate-driven carbamylation of (lipo)proteins may have contributed to ICAM-1 expression. To test whether carbamylated (lipo)proteins are involved in ICAM-1 expression in our experiments, serum-containing cell culture medium was incubated with cyanate (1 mM) for 48 h in the absence of cells to induce protein carbamylation (preconditioned medium). To remove cyanate, an aliquot of the preconditioned medium was dialyzed against fresh cell culture medium. Cells were then treated with preconditioned medium or dialyzed preconditioned medium for 48 h. In contrast to the cyanate-containing preconditioned medium, dialyzed medium failed to induce ICAM-1 expression (Fig. 2A). When cyanate was removed by a different method (gel filtration on Sephadex PD-10 columns), then similar results were observed (Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/ars).
FIG. 2.
Carbamylated lipoproteins do not induce ICAM-1 expression in HCAEC. (A) Growth medium was incubated with sodium cyanate (1 mM) for 48 h (preconditioned medium), and low-molecular-weight substances were subsequently removed by dialysis. HCAEC were then treated for 48 h with preconditioned medium or dialyzed preconditioned medium as described in the Materials and Methods section. (B) Low-density lipoprotein (LDL) was exposed to cyanate (1, 2, or 10 mM) for 48 h to induce protein carbamylation followed by gel filtration to remove residual cyanate. HCAEC were then treated with control LDL, carbamylated LDL (400 μg/ml), or cyanate (1–2 mM) for 48 h. Adhesion molecule expression was determined by flow cytometry. All experiments were performed in duplicate. Control was set at 100%, and values are expressed as % of control. Results shown are mean±SEM (n=3). *p<0.05 versus control.
Previous investigations have shown that carbamylated low-density lipoprotein (LDL) induces adhesion molecule expression in endothelial cells (2). In a further set of experiments, LDL was exposed to cyanate (1, 2, or 10 mM) for 48 h to induce protein carbamylation. The carbamyllysine content of LDL was assessed by mass spectrometry (Supplementary Fig. S2). However, when HCAEC were subsequently exposed to carbamylated LDL, then no change in adhesion molecule expression was observed. This clearly indicates that carbamylated LDL does not contribute to ICAM-1 expression under our experimental conditions (Fig. 2B). Notably, we cannot exclude that more extensively carbamylated LDL, as used in a previous study (2), may trigger adhesion molecule expression.
Cyanate-induced ICAM-1 expression is mediated by the p38 MAPK - NF-κB signaling
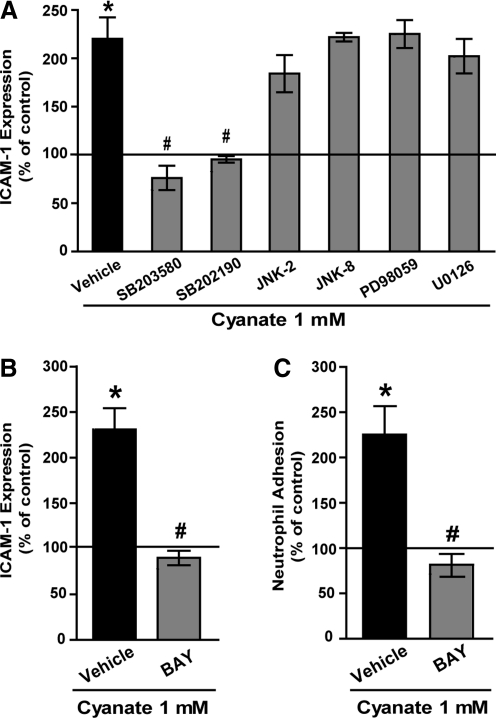
To elucidate the molecular mechanisms involved in endothelial activation, cyanate-induced ICAM-1 expression was examined in the presence of inhibitors of the mitogen-activated protein kinase (MAPK) family members extracellular signal-regulated kinase 1/2 (ERK1/2), p38 MAPK, and c-Jun N-terminal kinase (JNK). As shown in Figure 3A, two different, specific p38 MAPK inhibitors (SB203580 or SB202190) significantly suppressed cyanate-induced ICAM-1 expression in HCAEC, whereas inhibitors of the JNK or ERK1/2 signaling pathways had no effect. Nuclear factor-κB (NF-κB) is a well-characterized transcription factor that is pivotal in ICAM-1 expression. To examine the contribution of NF-κB, experiments were performed in the presence of a specific NF-κB inhibitor (BAY-11 7082). On inhibition of NF-κB, cyanate-induced ICAM-1 expression was completely blocked, thus demonstrating a direct involvement of NF-κB in cyanate-induced ICAM-1 expression (Fig. 3B).
FIG. 3.
Cyanate-induced ICAM-1 expression is mediated by p38 mitogen-activated protein kinase (MAPK) and nuclear factor-kappaB (NF-κB) signaling pathway. (A) HCAEC were treated for 48 h with sodium cyanate (1 mM) in the presence of the following MAPK family member inhibitors: p38 MAPK inhibitors (SB203580; 5 μM or SB202190; 2 μM), c-Jun N-terminal kinase (JNK) inhibitors (JNK-2 inhibitor; 5 μM or JNK-8 inhibitor; 5 μM), and extracellular signal-regulated kinase 1/2 inhibitors (PD98059; 10 μM or U0126; 5 μM). (B) The NF-κB inhibitor, BAY-11 7082 (BAY, 5 μM). Subsequently, ICAM-1 expression was determined by flow cytometry. (C) HCAEC monolayers were grown on 96-well plates and treated for 48 h with 1 mM sodium cyanate in the presence or absence of BAY-11 7082 (5 μM). Subsequently, fluorochrome-labeled neutrophils were added to the confluent monolayers, and percentage of adhesion was determined after a 30 min incubation period at 37°C. All experiments were performed in duplicate. Control was set at 100%, and values are expressed as % of control. Results shown are mean±SEM (n=3–5).*p<0.05 versus control; #p<0.05 versus cyanate-treated cells.
Next, we assessed whether cyanate-stimulated ICAM-1 expression induces leukocyte adhesion, a key event in the development of atherosclerosis. For that purpose, human polymorphonuclear leukocytes were isolated and allowed to adhere to cyanate-treated endothelial cells. A significant increase of neutrophil adhesion to cyanate-stimulated HCAEC as compared with control HCAEC was observed (Fig. 3C). Importantly, the increase in neutrophil adhesion was completely reversed in the presence of the NF-κB inhibitor.
Cyanate induces endothelial ICAM-1 expression in vivo
To test the physiologic relevance of our in vitro observations, we examined whether oral administration of cyanate increases ICAM-1 expression in mice. Male C57BL/6 mice were assigned to three groups receiving normal drinking water (control), drinking water containing 0.2 mg/ml sodium cyanate (low-cyanate), and drinking water containing 1 mg/ml sodium cyanate (high-cyanate), respectively, for a period of 9 weeks. General characteristics of mice are shown in Table 1. Mass spectrometry analysis of plasma proteins was performed to assess plasma protein carbamylation as a marker for cyanate exposure. Cyanate-treated mice showed increased carbamyllysine levels compared with controls, whereas plasma total cholesterol and urea values were not altered (Table 1). To investigate the involvement of lipid peroxidation, plasma malondialdehyde levels were measured, but no significant difference was observed between treatment groups (Table 1).
Table 1.
Biochemical Characteristics of Mice Receiving Cyanate in Drinking Water for 9 Weeks
| Characteristic | Control | Low cyanate | High cyanate |
|---|---|---|---|
| Total cholesterol (mg/dl) | 108±4 | 123±10 | 118±14 |
| Urea (mg/dl) | 74±5 | 78±4 | 88±7 |
| Malondialdehyde (nmol/ml) | 28.5±5.5 | 31.0±3.7 | 32.5±4.8 |
| HCit (μmol/mol Lys) | 2.9±0.8 | 276.8±30.8a | 1311.0±128.2a |
Plasma levels of total cholesterol, urea, and malondialdehyde were measured by using commercial kits; carbamyllysine quantification was performed by liquid chromatography tandem mass spectrometry and expressed as HCit μmol/mol Lys. Results are given as mean±SEM (n=5 mice per group).
p<0.05 versus control.
HCit, homocitrulline; Lys, lysine.
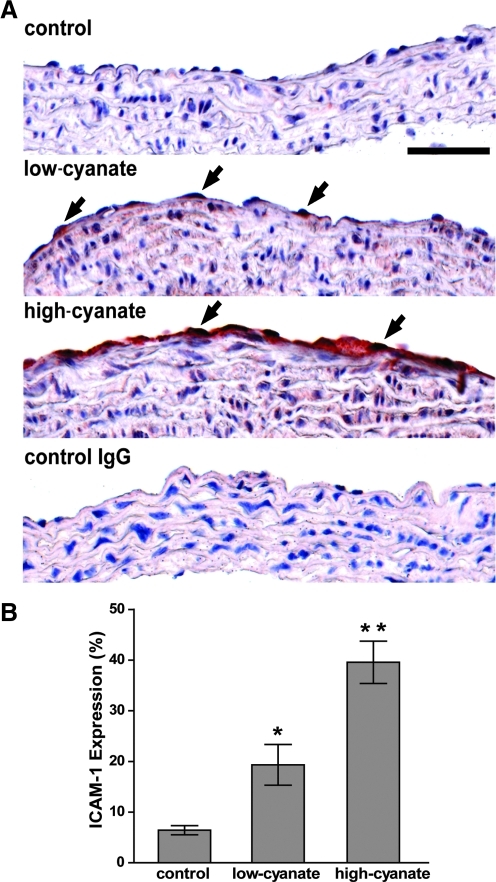
Consistent with our in vitro findings, cyanate treatment significantly increased the expression of ICAM-1 in vascular endothelial cells of the aortic arch in mice (Fig. 4A, 4B).
FIG. 4.
Oral administration of cyanate induces ICAM-1 expression in mice. (A) Cyanate induces ICAM-1 expression in aortas of mice. Sections of paraffin-embedded aortic arches stained with polyclonal anti-CD54 (anti-ICAM-1) or rabbit control IgG using immunohistochemistry. In contrast to control mice, mice receiving low cyanate (0.2 mg/ml) and mice receiving high cyanate (1 mg/ml) in drinking water expressed detectable levels of ICAM-1 in vascular endothelial cells (black arrows). Control IgG showed no staining. Positive immunohistochemical staining is indicated by a red immunoreaction product. Images are representatives of the treatment groups (five mice/group). Scale bar indicates 50 μm. (B) Quantification of endothelial ICAM-1 immunostaining in each group expressed as mean±SD. *p<0.05, **p<0.001 versus control.
Increased sICAM-1 in patients with renal failure
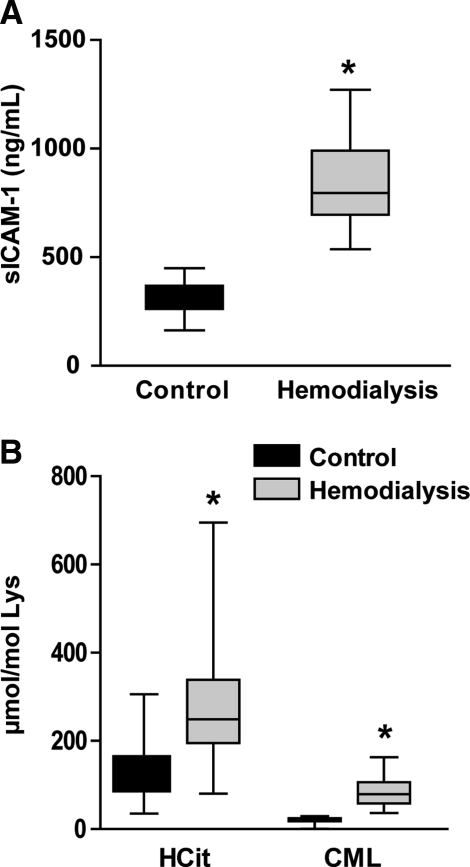
Significantly elevated MPO-activity and high urea concentrations lead to increased cyanate formation in patients with chronic renal failure. Therefore, we next assessed whether plasma carbamyllysine levels in patients with end-stage renal disease correlate with plasma sICAM-1 concentrations, a proteolytic cleavage product of vascular ICAM-1 (21, 42). We measured sICAM-1 concentrations in plasma from patients with end-stage renal disease on maintenance hemodialysis (n=23) and age-matched healthy control subjects (n=19). The general characteristics of both groups are shown in Table 2. In line with others (29, 38, 39), we observed that sICAM-1 concentrations were significantly higher in patients who have undergone hemodialysis compared with control subjects (Fig. 5A). Patients who have undergone hemodialysis showed elevated levels of both HCit and carboxymethyl-lysine (CML) (Fig. 5B). Importantly, sICAM-1 levels significantly correlated with plasma protein carbamylation (HCit content), whereas no correlation was found with plasma levels of the major advanced glycation end product CML, the formation of which is closely linked to increased local oxidative stress (40). In addition, sICAM-1 correlated with C-reactive protein in patients who have undergone hemodialysis, but not with creatinine or uric acid (Table 3).
Table 2.
Clinical Chemistry of Control Subjects and Patients Who Have Undergone Hemodialysis
| Control | Hemodialysis | |
|---|---|---|
| n | 19 | 23 |
| Age (year) | 53 (45–68) | 68 (48–74) |
| Male/female | 9/10 | 13/10 |
| Plasma parameter | ||
| Cholesterol (mg/dl) | 188 (176–195) | 161 (124–196)a |
| Triglycerides (mg/dl) | 122 (83–169) | 147 (90–200) |
| Urea (mg/dl) | 28 (25–31) | 118 (98–143)b |
| CRP (mg/l) | 1 (0–3) | 9 (3–17)b |
| Fibrinogen (mg/dl) | 272 (211–403) | 490 (411–619)b |
| Creatinine (mg/dl) | 0.92 (0.84–1.14) | 6.91 (6.28–9.81)b |
| Uric acid (mg/dl) | 4.8 (4.4–5.4) | 5.8 (5.1–7.1)b |
Results are given as median with the interquartile range. Significance was accepted at ap<0.05; bp<0.01 (Mann–Whitney test).
CRP, C-reactive protein.
FIG. 5.
Increased plasma levels of soluble ICAM-1 (sICAM-1), carbamyllysine (HCit), and carboxymethyl-lysine (CML) in patients who have undergone hemodialysis. (A) Plasma sICAM-1 concentrations of patients who have undergone hemodialysis (n=23) and controls (n=19) were quantified by ELISA. (B) Plasma levels of HCit and CML of patients who have undergone hemodialysis (n=23) and controls (n=19) were quantified by liquid chromatography tandem mass spectrometry. *p<0.001.
Table 3.
Correlation Matrix of Soluble Intercellular Cell Adhesion Molecule-1, HCit, Carboxymethyl-Lysine, C-Reactive Protein, Creatinine, and Uric Acid in Patients Who Have Undergone Hemodialysis
| sICAM-1 | HCit | CML | CRP | Creatinine | |
|---|---|---|---|---|---|
| sICAM-1 | — | — | — | — | — |
| HCit | 0.588a | — | — | — | — |
| CML | −0.026 | −0.143 | — | — | — |
| CRP | 0.609a | 0.360 | −0.296 | — | — |
| Creatinine | −0.304 | −0.090 | 0.421b | −0.531a | — |
| Uric acid | −0.163 | 0.004 | 0.084 | −0.045 | 0.400b |
Spearman rank correlation coefficients are noted at ap<0.01; bp<0.05.
HCit, carbamyllysine; CML, carboxymethyl-lysine; sICAM-1, soluble ICAM-1.
Discussion
In the current study, we demonstrate that cyanate exacerbates vascular endothelial inflammation. Cyanate induced a time- and concentration-dependent upregulation of endothelial ICAM-1 expression and subsequently increased neutrophil adhesion. Our results indicate that cyanate-induced ICAM-1 expression in HCAEC is mediated through activation of the stress-activated p38 MAPK and NF-κB signaling pathways. In line with our in vitro results, oral administration of cyanate dose dependently increased ICAM-1 expression in vascular endothelial cells in the aortic arch of mice. Importantly, plasma levels of carbamyllysine in mice of the low-cyanate group reached levels that we observed in patients who have undergone hemodialysis (276±31 vs. 290±30 μmol HCit/mol lysine, respectively), thus indicating that cyanate concentrations used for mouse experiments are biologically relevant. In agreement with our observation, previous studies have shown that intraperitoneal injection of cyanate into rats induces infiltration of mononuclear cells (1, 27).
Several studies have implicated a role of p38 MAPK and NF-κB in the regulation of ICAM-1 expression by various inducers, such as lipopolysaccharide, bile acids, or sphingosine 1-phosphate (30). Recently, it was shown that ketoaldehyde-modified phosphatidylethanolamine induces p38 MAPK and adhesion molecule expression in endothelial cells (13). Of particular interest, we could demonstrate in a previous study that phosphatidylethanolamine is targeted by cyanate (15). Thus, it is tempting to speculate whether phosphatidylethanolamine modified by cyanate could activate endothelial cells.
Many studies have evaluated the impact of protein carbamylation on structure and/or function of proteins, enzymes, or hormones (16, 20, 26). It was long thought that formation of cyanate occurs to a significant extent only during renal dysfunction. Recent studies unambiguously have demonstrated that humans are exposed to significant amounts of isocyanic acid and cyanate formed by pyrolysis/combustion of coal, biomass, or tobacco (31) and that cyanate formation is also catalyzed by the leukocyte heme peroxidase MPO (3, 41). Importantly, in patients who have undergone hemodialysis with high-grade persistent inflammation, significantly elevated MPO activity was recently demonstrated, thus supporting the association between inflammation and cumulative oxidative stress (32). The high carbamyllysine content of HDL in human atherosclerotic lesions correlated with the MPO-specific oxidation product 3-chlorotyrosine, which strongly supports the notion that macrophage-associated MPO generates significant amounts of cyanate (15). Hence, inflammation-driven formation of cyanate is a quantitatively important mechanism for cyanate formation and protein carbamylation.
A most important finding of the current study is that sICAM-1 plasma levels in patients who have undergone hemodialysis significantly correlate with plasma concentrations of carbamyllysine. Notably, the time-average concentration of urea in plasma of patients with renal failure, which correlates with plasma levels of protein-bound carbamyllysine, is associated with an increased odds ratio for death (28). We made the interesting observation that plasma concentrations of the advanced glycation end product CML, the formation of which is closely linked to oxidative stress in patients who have undergone hemodialysis, showed no correlation with sICAM levels (40). In this regard, it needs to be noted that the presence of high serum levels of advanced glycation end products, as measured by CML, may not be linked to increased mortality in patients who have undergone hemodialysis (35).
Circulating adhesion molecules are found to be increased in a variety of inflammatory disorders (12), indicating endothelial activation and enhanced endothelial-leukocyte interaction. Recent data from patients with renal failure strongly suggest that high serum levels of soluble adhesion molecules may predict future cardiovascular events (29, 38, 39). Serum concentrations of adhesion molecules may also increase with the progression of renal dysfunction, thus suggesting that inadequate clearance contributes to elevated serum levels of adhesion molecules in chronic renal failure (7). However, in the current study, no significant relationship was observed between residual renal function and plasma levels of sICAM-1 (Table 3), which is in agreement with a previous study (38).
The localization of phagocytes in the immediate vicinity of endothelial cells at sites of inflammation may contribute to cyanate-induced endothelial activation, as it was previously shown that MPO-containing neutrophils are markedly enriched with carbamylated proteins (19). Therefore, cyanate-induced expression of endothelial ICAM-1 and the subsequently enhanced endothelial-neutrophil interaction might form a vicious circle inducing endothelial dysfunction. In this regard, it was shown that serum MPO levels correlate with levels of inflammatory markers and mortality in patients who have undergone hemodialysis (17).
The present findings provide further insight into the underlying mechanisms that contribute to the enhanced cardiovascular risk associated with smoking and chronic renal failure. Monitoring of plasma carbamyllysine levels may offer a basis for identification of humans at increased risk of cardiovascular disease. Anti-ICAM-1 antibodies and/or interventions aimed at reducing levels of cyanate are potential promising approaches in reducing vascular disease in patients with renal failure.
Materials and Methods
Reagents used are listed in the Supplementary Materials and Methods (available online at www.liebertonline.com/ars).
Blood collection
Blood was taken from patients who have undergone hemodialysis before the dialysis session and from age-matched control subjects at the time of routine laboratory investigations in agreement with the Institutional Review Board of the Medical University of Graz as described (24).
Isolation and carbamylation of LDL
LDL was isolated as described (15) and carbamylated with potassium cyanate (1, 2, or 10 mM) in phosphate buffered saline (pH 7.4) containing 100 μM diethylenetriaminepentaacetic acid for 48 h at 37°C followed by gel filtration on Sephadex PD-10 columns (Amersham Biosciences) to remove residual cyanate. Control LDL was incubated under same conditions in the absence of cyanate.
Cell culture
HCAEC were purchased from Lonza (Verviers, Belgium) and cultured in EGM-2 MV Bullet medium (Lonza) containing FBS (5%). All experiments were performed without serum starvation. Endothelial cells were passaged at 80%–90% confluence and were used within 4 passages for experiments.
Removal of cyanate from preconditioned cell culture medium
Serum containing cell culture medium was incubated with cyanate (1 mM) for 48 h to induce protein carbamylation (preconditioned medium). Subsequently, low-molecular-weight substances (i.e., cyanate) were removed by either dialysis (molecular-weight cut off of 3000 Da) or gel filtration on Sephadex PD-10 columns.
Flow cytometry
The surface expression of ICAM-1, VCAM-1, and E-selectin on HCAEC was assessed by flow cytometry as described (14, 18). HCAEC were harvested by using an EDTA buffer (10 mM) and subsequently incubated with anti-CD54 (PE, 1:40), anti-CD106 (FITC, 1:40), or anti-CD62E (PE, 1:40) at 4°C for 30 min. The negative isotype-matched control was FITC mouse IgG1 or PE mouse IgG1 isotype control. After immunofluorescence staining, cells were rinsed, fixed, and analyzed by flow cytometry.
Cell viability
To assess effects on cell viability, an MTT (3-(4, 5-methylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide) reduction assay was performed. After treatment, cells were incubated with fresh medium containing MTT (0.5 mg/ml) for 4 h at 37°C. Acid-isopropanol (100 μL of 0.04 N HCI in isopropanol) was then added to dissolve the formazan crystals. Absorbance was evaluated at 560 nm by using a microplate spectrophotometer (xMark™; BioRAD).
Preparation of polymorphonuclear leukocytes
Blood was sampled from healthy volunteers after an informed consent, according to a protocol approved by the Institutional Review Board of the Medical University of Graz. Polymorphonuclear leukocytes (containing eosinophils and neutrophils) were prepared as previously described (33, 34). The resulting purity and viability of neutrophils (i.e., polymorphonuclear leukocytes) preparation was typically greater than 95%.
Leukocyte adhesion assay
HCAEC monolayers grown on 96-well plates were treated for 48 h with cyanate (1 mM) in the presence or absence of an NF-κB inhibitor BAY-11 7082. The fluorescent dye, calcein-AM, was used to stain freshly isolated neutrophils. Fluorochrome-labeled neutrophils were then added to the confluent monolayers, and percentage of adhesion was determined after a 30 min incubation period at 37°C (FlexStation® II; Molecular Devices).
In vivo study
C57BL/6 mice (males, 20–22 g, 5 weeks old) were purchased from Charles River (Sulzfeld) and housed in plastic sawdust floor cages at constant temperature (22°C) and a 12:12-h light–dark cycle with free access to standard laboratory chow and tap water. Experimental protocols were approved by the Animal Care Committee of the Austrian State Department of Science and Research. Mice (n=15) were equally assigned to three groups and received normal drinking water (control group), drinking water containing 0.2 mg/ml sodium cyanate (low-cyanate group), or drinking water containing 1 mg/ml sodium cyanate (high-cyanate group). Treatment continued for a period of 9 weeks, after which mice were deeply anesthetized with isoflurane, and 0.5 ml blood was collected by cardiac puncture using citrate (3.8%) as an anticoagulant. Plasma was stored at −70°C for further analysis. After blood collection, mice were killed by cervical dislocation. The ascending part of the aortic arch, closer to the lesser curvature, was removed, cleaned of adipose and connective tissue under a dissection microscope, and immediately fixed in 4% paraformaldehyde.
Plasma parameters
Malondialdehyde, total cholesterol, and urea concentrations were measured by commercial assay kits obtained from Cayman (Ann Arbor) and BioVision (Mountain View), respectively. For determination of sICAM-1 plasma concentration, a platinum ELISA kit for human sICAM-1 was used (eBioscience).
Carbamyllysine and CML quantification
Proteins were hydrolyzed with a fast, low-volume hydrolysis method as described (10).
To quantify LDL and plasma protein carbamylation, electrospray ionization liquid chromatography tandem mass spectrometry was used for HCit and CML quantification as previously described (15).
Immunohistochemistry
Aortic arches fixed in 4% paraformaldehyde were embedded in paraffin. Serial cross sections (5 μm) of the aortic arch, proximal to the origin of innominate artery, were generated on a microtome and processed by standard technique for all mice. Briefly, endogenous peroxidase was blocked with 3% H2O2, and immunolocalization at the inner curvature of the cross sections was visualized by using the Ultravision-labeled polymer-horseradish peroxidase detection system specific for rabbit antibodies from Lab Vision according to the manufacturer's protocol. Rabbit anti-CD54 to detect ICAM-1 (1:100) and nonimmune rabbit IgG (isotype control; 1:100) were diluted in a protein-protecting diluent buffer (Lab Vision). Between incubation steps, slides were washed in Tris-buffered saline containing 0.05% (vol/vol) Tween 20. Slides were counterstained with Mayer's hemalaun from Merck.
Cross sections were studied to quantify endothelial ICAM-1 immunostaining at the luminal surface. Percentage coverage with ICAM-1 was measured by using Xcellence imaging software Version 1.1 from Olympus soft imaging solutions (Munich).
Statistical analyses
Data are shown as mean±SEM for n observations unless stated otherwise. Comparison of groups was performed by using one-way ANOVA with Tukey's multiple-comparison post hoc test. Data from hemodialysis and control subjects are shown as median with the interquartile range, and Mann–Whitney test was used to test for differences. Correlations were determined by using Spearman rank correlation. Significance was accepted at p<0.05. Statistical analyses were performed with GraphPad Prism Version 4.03.
Supplementary Material
Abbreviations Used
- BAY
BAY-11 7082
- CML
carboxymethyl-lysine
- CRP
C-reactive protein
- ERK1/2
extracellular signal-regulated kinase 1/2
- H2O2
hydrogen peroxide
- HCAEC
human coronary artery endothelial cells
- HCit
homocitrulline, carbamyllysine
- HDL
high-density lipoprotein
- ICAM-1
intercellular cell adhesion molecule-1
- JNK
c-Jun N-terminal kinase
- LDL
low-density lipoprotein
- Lys
lysine
- MAPK
mitogen-activated protein kinase
- MPO
myeloperoxidase
- MTT
3-(4, 5-methylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide
- NF-κB
nuclear factor-kappaB
- sICAM-1
soluble intercellular cell adhesion molecule-1
- VCAM-1
vascular cell adhesion molecule-1
Acknowledgments
M.H. was funded by the PhD Program Molecular Medicine of the Medical University of Graz. This work was supported by the Austrian Science Fund FWF (Grants P21004-B02, P-22521-B18, P 22771-B18, and P22976-B18).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Apostolov EO. Ray D. Savenka AV. Shah SV. Basnakian AG. Chronic uremia stimulates LDL carbamylation and atherosclerosis. J Am Soc Nephrol. 2010;21:1852–1857. doi: 10.1681/ASN.2010040365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apostolov EO. Shah SV. Ok E. Basnakian AG. Carbamylated low-density lipoprotein induces monocyte adhesion to endothelial cells through intercellular adhesion molecule-1 and vascular cell adhesion molecule-1. Arterioscler Thromb Vasc Biol. 2007;27:826–832. doi: 10.1161/01.ATV.0000258795.75121.8a. [DOI] [PubMed] [Google Scholar]
- 3.Arlandson M. Decker T. Roongta VA. Bonilla L. Mayo KH. MacPherson JC. Hazen SL. Slungaard A. Eosinophil peroxidase oxidation of thiocyanate. Characterization of major reaction products and a potential sulfhydryl-targeted cytotoxicity system. J Biol Chem. 2001;276:215–224. doi: 10.1074/jbc.M004881200. [DOI] [PubMed] [Google Scholar]
- 4.Baldus S. Eiserich JP. Mani A. Castro L. Figueroa M. Chumley P. Ma W. Tousson A. White CR. Bullard DC. Brennan ML. Lusis AJ. Moore KP. Freeman BA. Endothelial transcytosis of myeloperoxidase confers specificity to vascular ECM proteins as targets of tyrosine nitration. J Clin Invest. 2001;108:1759–1770. doi: 10.1172/JCI12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell JD. Lee JA. Lee HA. Sadler PJ. Wilkie DR. Woodham RH. Nuclear magnetic resonance studies of blood plasma and urine from subjects with chronic renal failure: identification of trimethylamine-N-oxide. Biochim Biophys Acta. 1991;1096:101–107. doi: 10.1016/0925-4439(91)90046-c. [DOI] [PubMed] [Google Scholar]
- 6.Blackmore DJ. Elder WJ. Bowden CH. Urea distribution in renal failure. J Clin Pathol. 1963;16:235–243. doi: 10.1136/jcp.16.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonomini M. Reale M. Santarelli P. Stuard S. Settefrati N. Albertazzi A. Serum levels of soluble adhesion molecules in chronic renal failure and dialysis patients. Nephron. 1998;79:399–407. doi: 10.1159/000045084. [DOI] [PubMed] [Google Scholar]
- 8.Cheung AK. Sarnak MJ. Yan G. Dwyer JT. Heyka RJ. Rocco MV. Teehan BP. Levey AS. Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int. 2000;58:353–362. doi: 10.1046/j.1523-1755.2000.00173.x. [DOI] [PubMed] [Google Scholar]
- 9.Cybulsky MI. Gimbrone MA., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251:788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- 10.Damm M. Holzer M. Radspieler G. Marsche G. Kappe CO. Microwave-assisted high-throughput acid hydrolysis in silicon carbide microtiter platforms—a rapid and low volume sample preparation technique for total amino acid analysis in proteins and peptides. J Chromatogr A. 2011;1217:7826–7832. doi: 10.1016/j.chroma.2010.10.062. [DOI] [PubMed] [Google Scholar]
- 11.Dirnhuber P. Schutz F. The isomeric transformation of urea into ammonium cyanate in aqueous solutions. Biochem J. 1948;42:628–632. [PubMed] [Google Scholar]
- 12.Gearing AJ. Newman W. Circulating adhesion molecules in disease. Immunol Today. 1993;14:506–512. doi: 10.1016/0167-5699(93)90267-O. [DOI] [PubMed] [Google Scholar]
- 13.Guo L. Chen Z. Cox BE. Amarnath V. Epand RF. Epand RM. Davies SS. Phosphatidylethanolamines modified by gamma-ketoaldehyde (gammaKA) induce endoplasmic reticulum stress and endothelial activation. J Biol Chem. 2011;286:18170–18180. doi: 10.1074/jbc.M110.213470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hadad N. Tuval L. Elgazar-Carmom V. Levy R. Levy R. Endothelial ICAM-1 protein induction is regulated by cytosolic phospholipase A2alpha via both NF-kappaB and CREB transcription factors. J Immunol. 2011;186:1816–1827. doi: 10.4049/jimmunol.1000193. [DOI] [PubMed] [Google Scholar]
- 15.Holzer M. Gauster M. Pfeifer T. Wadsack C. Fauler G. Stiegler P. Koefeler H. Beubler E. Schuligoi R. Heinemann A. Marsche G. Protein carbamylation renders high-density lipoprotein dysfunctional. Antioxid Redox Signal. 2011;14:2337–2346. doi: 10.1089/ars.2010.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaisson S. Delevallee-Forte C. Toure F. Rieu P. Garnotel R. Gillery P. Carbamylated albumin is a potent inhibitor of polymorphonuclear neutrophil respiratory burst. FEBS Lett. 2007;581:1509–1513. doi: 10.1016/j.febslet.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Kalantar-Zadeh K. Brennan ML. Hazen SL. Serum myeloperoxidase and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2006;48:59–68. doi: 10.1053/j.ajkd.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 18.Konya V. Sturm EM. Schratl P. Beubler E. Marsche G. Schuligoi R. Lippe IT. Peskar BA. Heinemann A. Endothelium-derived prostaglandin I(2) controls the migration of eosinophils. J Allergy Clin Immunol. 2010;125:1105–1113. doi: 10.1016/j.jaci.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Kraus LM. Elberger AJ. Handorf CR. Pabst MJ. Kraus AP., Jr Urea-derived cyanate forms epsilon-amino-carbamoyl-lysine (homocitrulline) in leukocyte proteins in patients with end-stage renal disease on peritoneal dialysis. J Lab Clin Med. 1994;123:882–891. [PubMed] [Google Scholar]
- 20.Kraus LM. Kraus AP., Jr Carbamoylation of amino acids and proteins in uremia. Kidney Int Suppl. 2001;78:S102–S107. doi: 10.1046/j.1523-1755.2001.59780102.x. [DOI] [PubMed] [Google Scholar]
- 21.Leeuwenberg JF. Smeets EF. Neefjes JJ. Shaffer MA. Cinek T. Jeunhomme TM. Ahern TJ. Buurman WA. E-selectin and intercellular adhesion molecule-1 are released by activated human endothelial cells in vitro. Immunology. 1992;77:543–549. [PMC free article] [PubMed] [Google Scholar]
- 22.Lindner A. Charra B. Sherrard DJ. Scribner BH. Accelerated atherosclerosis in prolonged maintenance hemodialysis. N Engl J Med. 1974;290:697–701. doi: 10.1056/NEJM197403282901301. [DOI] [PubMed] [Google Scholar]
- 23.Luscinskas FW. Cybulsky MI. Kiely JM. Peckins CS. Davis VM. Gimbrone MA., Jr Cytokine-activated human endothelial monolayers support enhanced neutrophil transmigration via a mechanism involving both endothelial-leukocyte adhesion molecule-1 and intercellular adhesion molecule-1. J Immunol. 1991;146:1617–1625. [PubMed] [Google Scholar]
- 24.Marsche G. Frank S. Hrzenjak A. Holzer M. Dirnberger S. Wadsack C. Scharnagl H. Stojakovic T. Heinemann A. Oettl K. Plasma-advanced oxidation protein products are potent high-density lipoprotein receptor antagonists in vivo. Circ Res. 2009;104:750–757. doi: 10.1161/CIRCRESAHA.108.193169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marsche G. Semlitsch M. Hammer A. Frank S. Weigle B. Demling N. Schmidt K. Windischhofer W. Waeg G. Sattler W. Malle E. Hypochlorite-modified albumin colocalizes with RAGE in the artery wall and promotes MCP-1 expression via the RAGE-Erk1/2 MAP-kinase pathway. FASEB J. 2007;21:1145–1152. doi: 10.1096/fj.06-7439com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mun KC. Golper TA. Impaired biological activity of erythropoietin by cyanate carbamylation. Blood Purif. 2000;18:13–17. doi: 10.1159/000014403. [DOI] [PubMed] [Google Scholar]
- 27.Mun KC. Yeo MY. Kim SP. Kim HC. Kwak CS. Chronic peritoneal inflammation by cyanate in rats. Perit Dial Int. 2000;20:699–702. [PubMed] [Google Scholar]
- 28.Owen WF., Jr. Lew NL. Liu Y. Lowrie EG. Lazarus JM. The urea reduction ratio and serum albumin concentration as predictors of mortality in patients undergoing hemodialysis. N Engl J Med. 1993;329:1001–1006. doi: 10.1056/NEJM199309303291404. [DOI] [PubMed] [Google Scholar]
- 29.Rabb H. Calderon E. Bittle PA. Ramirez G. Alterations in soluble intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in hemodialysis patients. Am J Kidney Dis. 1996;27:239–243. doi: 10.1016/s0272-6386(96)90547-8. [DOI] [PubMed] [Google Scholar]
- 30.Rahman A. Fazal F. Hug tightly and say goodbye: role of endothelial ICAM-1 in leukocyte transmigration. Antioxid Redox Signal. 2009;11:823–839. doi: 10.1089/ars.2008.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts JM. Veres PR. Cochran AK. Warneke C. Burling IR. Yokelson RJ. Lerner B. Gilman JB. Kuster WC. Fall R. de Gouw J. Isocyanic acid in the atmosphere and its possible link to smoke-related health effects. Proc Natl Acad Sci U S A. 2011;108:8966–8971. doi: 10.1073/pnas.1103352108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez-Ayala E. Anderstam B. Suliman ME. Seeberger A. Heimburger O. Lindholm B. Stenvinkel P. Enhanced RAGE-mediated NFkappaB stimulation in inflamed hemodialysis patients. Atherosclerosis. 2005;180:333–340. doi: 10.1016/j.atherosclerosis.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Schratl P. Royer JF. Kostenis E. Ulven T. Sturm EM. Waldhoer M. Hoefler G. Schuligoi R. Lippe IT. Peskar BA. Heinemann A. The role of the prostaglandin D2 receptor, DP, in eosinophil trafficking. J Immunol. 2007;179:4792–4799. doi: 10.4049/jimmunol.179.7.4792. [DOI] [PubMed] [Google Scholar]
- 34.Schratl P. Sturm EM. Royer JF. Sturm GJ. Lippe IT. Peskar BA. Heinemann A. Hierarchy of eosinophil chemoattractants: role of p38 mitogen-activated protein kinase. Eur J Immunol. 2006;36:2401–2409. doi: 10.1002/eji.200535672. [DOI] [PubMed] [Google Scholar]
- 35.Schwedler SB. Metzger T. Schinzel R. Wanner C. Advanced glycation end products and mortality in hemodialysis patients. Kidney Int. 2002;62:301–310. doi: 10.1046/j.1523-1755.2002.00423.x. [DOI] [PubMed] [Google Scholar]
- 36.Stark GR. Reactions of cyanate with functional groups of proteins. 3. Reactions with amino and carboxyl groups. Biochemistry. 1965;4:1030–1036. doi: 10.1021/bi00882a008. [DOI] [PubMed] [Google Scholar]
- 37.Stenvinkel P. Interactions between inflammation, oxidative stress, and endothelial dysfunction in end-stage renal disease. J Ren Nutr. 2003;13:144–148. doi: 10.1053/jren.2003.50018. [DOI] [PubMed] [Google Scholar]
- 38.Stenvinkel P. Lindholm B. Heimburger M. Heimburger O. Elevated serum levels of soluble adhesion molecules predict death in pre-dialysis patients: association with malnutrition, inflammation, and cardiovascular disease. Nephrol Dial Transplant. 2000;15:1624–1630. doi: 10.1093/ndt/15.10.1624. [DOI] [PubMed] [Google Scholar]
- 39.Suliman ME. Qureshi AR. Heimburger O. Lindholm B. Stenvinkel P. Soluble adhesion molecules in end-stage renal disease: a predictor of outcome. Nephrol Dial Transplant. 2006;21:1603–1610. doi: 10.1093/ndt/gfl005. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki D. Miyata T. Saotome N. Horie K. Inagi R. Yasuda Y. Uchida K. Izuhara Y. Yagame M. Sakai H. Kurokawa K. Immunohistochemical evidence for an increased oxidative stress and carbonyl modification of proteins in diabetic glomerular lesions. J Am Soc Nephrol. 1999;10:822–832. doi: 10.1681/ASN.V104822. [DOI] [PubMed] [Google Scholar]
- 41.Wang Z. Nicholls SJ. Rodriguez ER. Kummu O. Horkko S. Barnard J. Reynolds WF. Topol EJ. DiDonato JA. Hazen SL. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med. 2007;13:1176–1184. doi: 10.1038/nm1637. [DOI] [PubMed] [Google Scholar]
- 42.Witkowska AM. Soluble ICAM-1: a marker of vascular inflammation and lifestyle. Cytokine. 2005;31:127–134. doi: 10.1016/j.cyto.2005.04.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.