Abstract
The impact of simultaneous environmental stresses on plants and how they respond to combined stresses compared with single stresses is largely unclear. By using a transgene (RD29A-LUC) consisting of the firefly luciferase coding sequence (LUC) driven by the stress-responsive RD29A promoter, we investigated the interactive effects of temperature, osmotic stress, and the phytohormone abscisic acid (ABA) in the regulation of gene expression in Arabidopsis seedlings. Results indicated that both positive and negative interactions exist among the studied stress factors in regulating gene expression. At a normal growth temperature (22°C), osmotic stress and ABA act synergistically to induce the transgene expression. Low temperature inhibits the response to osmotic stress or to combined treatment of osmotic stress and ABA, whereas low temperature and ABA treatments are additive in inducing transgene expression. Although high temperature alone does not activate the transgene, it significantly amplifies the effects of ABA and osmotic stress. The effect of multiple stresses in the regulation of RD29A-LUC expression in signal transduction mutants was also studied. The results are discussed in the context of cold and osmotic stress signal transduction pathways.
Plants grow in an inconstant environment that frequently imposes constraints on growth and development. Among the adverse environmental factors commonly encountered by land plants are extreme temperatures and osmotic stress that results from conditions of high salinity or periods of drought. Much research has been conducted to understand the molecular mechanisms underlying plant responses to these stresses (for review, see Bray, 1993; Guy et al., 1994; Thomashow, 1994; Ingram and Bartels, 1996; Zhu et al., 1997). In most of the laboratory studies a single environmental stress factor is imposed and the consequent responses are analyzed. However, in the natural habitats of plants the adverse environmental factors are almost never present alone. For example, osmotic stress caused by drought in the summer is often accompanied by high-temperature stress, and low temperature may also bring with it osmotic stress because it impairs water absorption and transport. Under conditions of simultaneous stresses, the negative effects on plants and plant adaptive responses may differ from those under a single adverse environmental condition such as that which prevails in laboratory research. It is necessary, therefore, to understand how plants respond to combined stress signals.
The early events of plant adaptation to environmental stress are the sensing and subsequent signal transduction to activate various physiological and metabolic responses, including stress-responsive gene expression. Many genes can be induced by osmotic stress (Bray, 1993; Shinozaki and Yamaguchi-Shinozaki, 1997; Zhu et al., 1997) or low temperature (Thomashow, 1994). Osmotic stress and low temperature increase the cellular level of the phytohormone ABA (Zeevaart and Creelman, 1988; Chandler and Robertson, 1994), and the expression of many osmotic stress-responsive genes can be induced by the application of ABA. It has been established that the expression of some stress genes is mediated by ABA, but that that of other genes is independent of ABA (Gilmour and Thomashow, 1991; Nordin et al., 1991; Gosti et al., 1995). The intricate interplay between ABA and temperature or osmotic stresses in the regulation of gene expression remains unclear.
We used a reporter gene system consisting of the firefly luciferase coding sequence driven by the stress-responsive RD29A promoter (Ishitani et al., 1997) to study the interactive effect of osmotic stress, low-/high-temperature stress, and ABA. The RD29A promoter contains both the ABA-responsive element and the dehydration-responsive element (also termed the C-repeat) and can be activated by osmotic stress, low temperature, or ABA treatment. Transcription of the RD29A gene in response to osmotic and cold stresses is mediated by both ABA-dependent and ABA-independent pathways (Gilmour and Thomashow, 1991; Nordin et al., 1991; Yamaguchi-Shinozaki and Shinozaki, 1994). Genetic evidence indicates extensive interaction between the two pathways (Ishitani et al., 1997). The advantages of the firefly luciferase reporter include the short half-life of its transcript, and the fact that its expression can be quantitatively monitored noninvasively by real-time luminescence imaging.
We present evidence indicating that osmotic stress and ABA are synergistic at normal growth temperatures in activating stress gene transcription, and that low temperature reduces the effect of osmotic stress treatment or the combined treatment with osmotic stress and ABA. ABA induces a higher level of RD29A-LUC expression at low temperature than at room temperature. Although high temperature alone does not activate RD29A-LUC, it enhances the effect of ABA or osmotic stress. These results are discussed in the context of osmotic and cold stress signal transduction pathways.
MATERIALS AND METHODS
Arabidopsis plants (ecotype C24) were transformed with a construct consisting of the RD29A promoter (Yamaguchi-Shinozaki and Shinozaki, 1994) and the firefly luciferase coding sequence (Millar et al., 1992) (LUC) via Agrobacterium tumefaciens infection of the roots (Ishitani et al., 1997). Arabidopsis signal transduction mutants 693 and los1-1 were obtained as described previously (Ishitani et al., 1997).
Plants for assaying the transgene activity were grown at room conditions (22°C ± 2°C) on 0.8% agar plates containing MS salt (JRH Biosciences, Lenexa, KS), 3% Suc, and 30 μg/mL kanamycin. Surface-sterilized seeds were planted in the MS nutrient agar plates and incubated at 4°C for 2 d before being placed at room temperature under constant cool-white light for germination and growth. One-week-old seedlings were used for the experiments.
Stress and ABA Treatments
There were four basic treatments: low-temperature stress, high-temperature stress, osmotic stress, and ABA. At all temperature conditions ABA (mixed isomers dissolved in water) at 100 μm was sprayed directly on leaves and osmotic stress was induced with plants on filter paper saturated with 300 mm NaCl in MS solution. The duration of both ABA and NaCl treatments was 4 h. For the low-temperature treatment, 1-week-old seedlings on agar plates were incubated at 0°C ± 1°C in the dark for 48 h. When cold was combined with other stresses, seedlings on agar plates were first incubated at 0°C ± 1°C for 44 h in the dark, then either sprayed with ABA (cold plus ABA treatment) or transferred to filter paper saturated with NaCl (cold plus NaCl treatment) or transferred to filter paper saturated with NaCl and sprayed with ABA (cold plus NaCl and ABA treatment), and the seedlings were incubated on ice for another 4 h under cool-white light. The control treatment for ABA (without ABA) was to spray with water, and the control for NaCl (without NaCl) was to transfer the seedlings to filter paper saturated with MS salt solution. The results indicated that the transgene was not induced by any of the control treatments.
In addition to the 48-h low-temperature treatments described above, 4.5-h cold-shock treatments were also conducted. When other stresses were combined with cold shock, seedlings on agar plates were incubated at 0°C ± 1°C for 30 min, and then transferred to filter paper saturated with MS salt solution and sprayed with water (control treatment) or with ABA (ABA treatment). Seedlings were either transferred to filter paper saturated with NaCl (NaCl treatment) or were also sprayed with ABA (NaCl plus ABA treatment) and incubated on ice for another 4 h in a cold room under cool-white light before imaging.
For heat treatment at 30°C or 37°C, seedlings in agar plates were first incubated at 30°C or 37°C under white light for 30 min before being sprayed with ABA (heat plus ABA treatment) and then either transferred to NaCl-saturated filter paper (heat plus NaCl treatment) or transferred to filter paper and sprayed with ABA (heat plus NaCl and ABA treatment). The plants were then incubated at either 30°C or 37°C for another 4 h before luciferase imaging. Control treatments with seedlings on filter paper saturated with MS salt solution or on agar plates under the same temperature conditions were also run simultaneously.
Assay of RD29A-LUC Expression
After the treatments described above, plates with seedlings were moved to room temperature, sprayed immediately with Luciferin (Promega), and kept in the dark for 5 min before being placed in the dark camera chamber for luminescence imaging. The exposure time was 5 min at room temperature. The video-imaging system consisted of a high-performance CCD (charge-coupled device) camera (model CCD-512SB, Princeton Instruments, Trenton, NJ), a camera controller, and a computer. Image acquisition, processing, and quantitation were carried out with software provided by the camera manufacturer (WinView, Princeton Instruments).
RNA Analysis
Total RNA from control or stress-treated plants was extracted as described by Liu and Zhu (1997). The RD29A gene-specific probe was from the 3′ noncoding region (Liu and Zhu, 1997).
RESULTS
Selection of Treatment Conditions
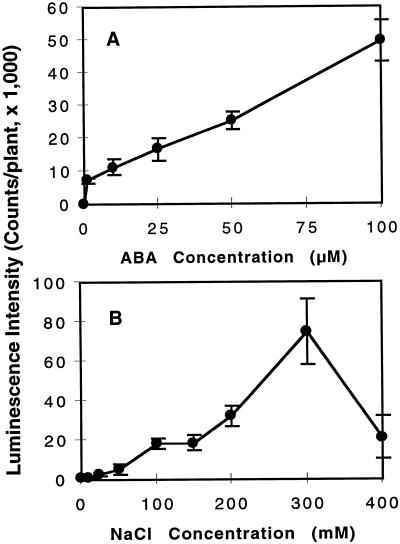
In our previous report (Ishitani et al., 1997) we demonstrated that RD29A-LUC in transgenic Arabidopsis plants is strongly induced by low temperature, exogenous ABA, or osmotic stress. To study the interaction of different stresses in inducing the transgene expression, we first tested the suitable dosages of the inducers that give steady and high-level gene expression but are not lethal to the plants. Figure 1 presents the dose-response curves for ABA and NaCl. Maximum induction of RD29A-LUC for ABA treatment in the tested concentration range was found at 100 μm (Fig. 1A). The RD29A-LUC expression was proportional to the NaCl concentration when it was below 300 mm. Above 300 mm NaCl the expression of RD29A-LUC decreased, indicating that very high levels of NaCl are damaging for the transcriptional apparatus or the luciferase enzyme (Fig. 1B). Therefore, 300 mm NaCl was chosen for the osmotic stress treatment to achieve high levels of induction of RD29A-LUC.
Figure 1.
RD29A-LUC expression in response to different ABA and NaCl concentrations at 22°C ± 2°C. A, ABA dose-response curve; B, NaCl dose-response curve. Error bars represent ±sd (n = 15).
Treatments with different low-temperature regimes have shown that 0°C for 48 h resulted in maximal RD29A-LUC expression (Ishitani et al., 1998). Heat-shock treatments were conducted at 30°C and 37°C, representing moderate and extreme heat stresses, respectively, for Arabidopsis seedlings.
Interaction of Temperature and ABA
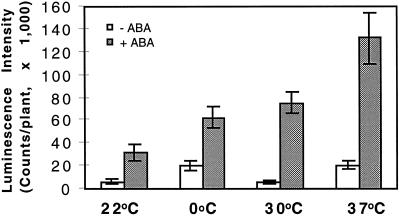
The expression of RD29A-LUC in control (without ABA) and ABA-treated seedlings at various temperature regimes is presented in Figure 2. At room temperature there was essentially no expression in the control. Low-temperature treatment (0°C for 48 h) induced significant expression of RD29A-LUC.
Figure 2.
RD29A-LUC expression induced by ABA treatment at different temperatures. Control plants (−ABA, white bars) at the indicated temperatures were sprayed with water; plants for ABA treatments (+ABA, shaded bars) were sprayed with 100 μm ABA and incubated at the indicated temperatures for 4 h. For the 0°C treatments plants were incubated at 0°C for 44 h before being sprayed with ABA or water and incubated at 0°C for 4 h. Error bars represent ±sd (n = 15).
Heat treatment at 30°C did not induce the transgene expression at all (Fig. 2). This is in agreement with previous studies showing that the steady-state level of RD29A transcript was not up-regulated by high-temperature stress (Yamaguchi-Shinozaki and Shinozaki, 1994). However, heat shock at 37°C did significantly induce the transgene expression, perhaps because of dehydration stress caused by the high-temperature treatment. Although the agar plates were sealed with laboratory film, the high-temperature treatment still resulted in a great deal of water evaporation from the medium, and condensation covered the lids and walls of the plates.
Compared with the control, ABA (100 μm for 4 h) induced significant expression of RD29A-LUC at normal growth temperature (22°C). When the plants were treated with low temperature and ABA at the same time, the resulting RD29A expression level was higher than that achieved by either treatment alone and was approximately equal to the sum of the single treatments. This additive effect suggests, as demonstrated below, that low temperature and ABA signals may be transduced via different signaling pathways.
Although heat treatment at 30°C by itself did not induce obvious expression of the transgene, ABA treatment at 30°C induced a higher level of expression than the same ABA treatment at 22°C. The enhancing effect of high temperature on ABA in RD29A-LUC induction was even greater at 37°C (Fig. 2).
Interaction of Temperature and Osmotic Stress
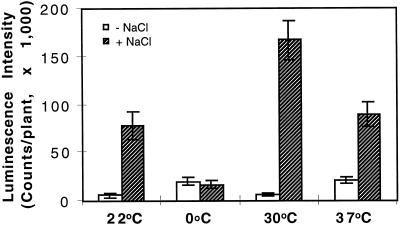
As demonstrated above, at room temperature (22°C ± 2°C) 300 mm NaCl strongly induced RD29A-LUC expression (Fig. 3). Combined treatment with low temperature and 300 mm NaCl did not enhance RD29A-LUC expression compared with low-temperature treatment alone or NaCl treatment at room temperature; in fact, NaCl treatment at 0°C decreased the RD29A-LUC expression compared with cold treatment alone (Fig. 3).
Figure 3.
RD29A-LUC expression in response to NaCl treatment at different temperatures. Control treatments (−NaCl, white bars) at the specified temperatures were carried out with plants on filter paper saturated with MS salt solution; NaCl treatments (+NaCl, hatched bars) were treated with 300 mm NaCl in MS solution. Treatments at the indicated temperatures lasted for 4 h before luciferase imaging. For the 0°C treatments plants were incubated in the cold for 44 h before being transferred to filter paper for treatments with or without NaCl at 0°C for 4 h. Error bars represent ±sd (n = 15).
When combined with heat shock at 30°C, 300 mm NaCl induced a level of RD29A-LUC expression approximately twice that of 300 mm NaCl treatment alone (Fig. 3). Because the NaCl treatment was at a saturating concentration (Fig. 1B), and because heat shock (30°C) alone did not induce RD29A-LUC expression, the synergistic effect of the combined treatment is very significant. Combined treatment of 300 mm NaCl and heat shock at 37°C did not significantly increase RD29A-LUC expression compared with treatment of 300 mm NaCl at 22°C. This is in sharp contrast with the combined effect of ABA and 37°C heat shock, which showed significant synergism (Fig. 2).
Interaction of Temperature, Osmotic Stress, and ABA
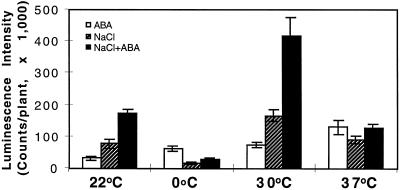
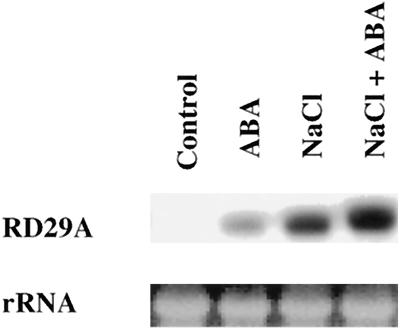
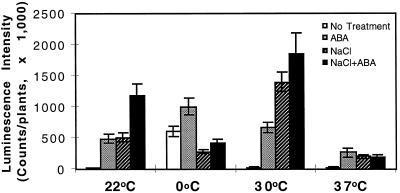
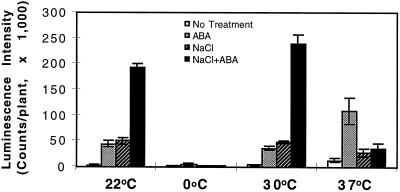
At room temperature, NaCl and ABA had a synergistic effect in the induction of RD29A-LUC (Fig. 4). Northern-blot analysis also demonstrated that ABA and NaCl were synergistic in inducing the expression of the endogenous RD29A gene (Fig. 5). This synergistic effect on RD29A-LUC expression also existed at 30°C. In fact, combined treatment of NaCl and ABA at 30°C yielded the highest RD29A-LUC expression of all of the treatments (Fig. 4). However, at 0°C the presence of 300 mm NaCl reduced RD29A-LUC induction by ABA (Fig. 4). NaCl and ABA were also not synergistic at 37°C (Fig. 4).
Figure 4.
Luminescence intensity of plants treated with ABA, NaCl, or ABA plus NaCl at different temperatures. Plants were placed on filter paper soaked with either MS salt solution (ABA) or 300 mm NaCl in MS solution (NaCl or ABA + NaCl). ABA (100 μm) was sprayed directly on plants. The treatments lasted for 4 h before luciferase imaging. For the 0°C treatments plants were incubated at 0°C for 44 h before being transferred to filter paper for NaCl and/or ABA treatments at 0°C for 4 h. White bars, ABA treatment; hatched bars, NaCl treatment; and black bars, combined NaCl and ABA treatment. Error bars represent ±sd (n = 15).
Figure 5.
Synergistic effect of ABA and NaCl in inducing endogenous RD29A expression at room temperature. Nine-day-old plants on MS agar plates were transferred to filter paper saturated with MS salt solution and sprayed with water (Control) or with 100 μm ABA (ABA), or were treated with 300 mm NaCl in the MS salt background and sprayed with water (NaCl) or 100 μm ABA (NaCl + ABA). The treatment lasted for 4 h under cool-white light at 22°C ± 2°C, and total RNA was extracted immediately after the treatment.
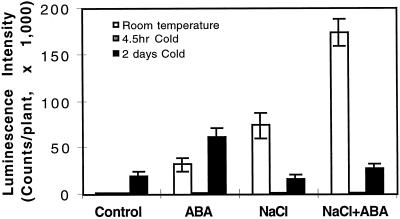
Brief Cold Shock Impairs ABA and Osmotic Stress Response
Our low-temperature treatment typically was 0°C for 48 h because these conditions were found to yield maximal RD29A-LUC induction. During the 48-h cold treatment, many metabolic changes take place and the plants become acclimated (Levitt, 1980). We were interested in determining how a brief cold-shock treatment (0°C for 4.5 h) might interact with osmotic stress and ABA signals in regulating gene expression. Treatment at 0°C for 4.5 h by itself did not induce RD29A-LUC expression (Fig. 6). Furthermore, when the cold shock was combined with ABA, NaCl, or ABA plus NaCl treatment, no RD29A-LUC expression could be detected (Fig. 6). In contrast, ABA and 48 h of cold treatment had an additive effect (Figs. 2 and 6). Cold treatment for 48 h impaired but did not eliminate the effect of NaCl (Figs. 3 and 6).
Figure 6.
Cold shock (4.5 h at 0°C) inhibits RD29A-LUC expression in response to other stress treatments. Experiments were done with plants on filter paper soaked with either MS salt solution (for control or ABA treatments) or 300 mm NaCl in MS solution (for NaCl or ABA plus NaCl treatments). For combined treatments of 4.5 h cold plus other stresses, plants in agar plates were first incubated at 0°C for 30 min before being transferred to filter paper for ABA and/or NaCl treatments for 4 h in the cold. Plants given the normal cold treatment (2 d) were first incubated at 0°C on agar plates for 44 h before being transferred to filter paper for ABA and/or NaCl treatments for 4 h in the cold. Luminescence images were taken immediately after the treatments. White bars, 22°C ± 2°C; hatched bars (not visible), 4.5 h of cold treatment; and black bars, 2 d of cold treatment. Error bars represent ±sd (n = 15).
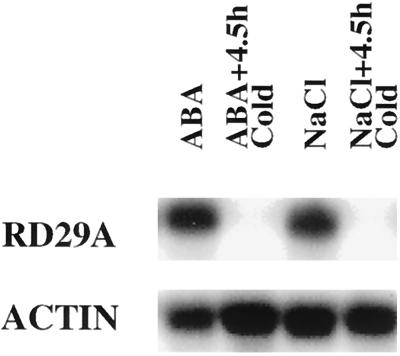
To determine whether the ABA induction of the endogenous RD29A gene was impaired by brief cold-shock treatment, RNA was extracted from plants treated with ABA at 22°C or 0°C for 4.5 h. Northern-blot analysis indicated that whereas ABA alone at room temperature induced significant accumulation of RD29A transcripts, combined treatment with ABA and 4.5 h of cold shock did not induce endogenous RD29A expression at all (Fig. 7). Similarly, combined treatment with NaCl and 4.5 h of cold shock failed to induce the expression of the endogenous RD29A gene (Fig. 7).
Figure 7.
Brief cold shock impaired the induction of endogenous RD29A expression by ABA and NaCl. Nine-day-old plants on MS agar plates were sprayed with 100 μm ABA or transferred to filter paper saturated with 300 mm NaCl in the MS salt background. The treatments lasted for 4 h under light at 22°C ± 2°C. Plants were first incubated on ice in a cold room under light for 30 min and then sprayed with 100 μm ABA (ABA+4.5hCold) or transferred to filter paper saturated with 300 mm NaCl in the MS salt background (NaCl+4.5hCold). The treatments lasted for 4 h under light in the cold. Total RNA was extracted immediately after the end of the treatments.
Interactive Signal Transduction in Mutants with Altered Responses to Low Temperature, Exogenous ABA, or Osmotic Stress
Using the RD29A-LUC reporter and luciferase imaging, several groups of mutants with altered responses to low temperature, osmotic stress, and/or exogenous ABA were selected (Ishitani et al., 1997). Some of the mutants could be defective in the interaction of the different stress signals. To test this hypothesis we chose a recessive hos (high expression of osmotic-responsive genes) mutant (no. 693), which shows enhanced response to all three inducers (i.e. cold, osmotic stress, and ABA), and a recessive los (low expression of osmotic-responsive genes) mutant (los1-1), which shows a reduced response specifically to low-temperature stress.
The levels of RD29A-LUC induction by cold, NaCl, and ABA treatments in the hos mutant (Fig. 8) were 30, 7, and 15 times higher, respectively, than those seen in the wild-type seedlings (Figs. 2 and 3). The interactive effects among low temperature, NaCl, and ABA as seen in the wild-type plants (Figs. 2–4) also exist in the hos mutant. The difference is that the luminescence in the mutant was proportionally higher for the treatments at room temperature or cold. Although NaCl and ABA treatments interacted positively, their combined treatment did not result in more than an additive effect (Fig. 8), possibly because of a saturation in the RD29A-LUC response in the mutant. At 37°C, although luminescence in plants given ABA treatment was still much higher than in wild-type plants, it was lower than at other temperatures. It seems that this hos mutation also affects the responsiveness to ABA at extreme high-temperature conditions.
Figure 8.
Interaction of temperature, osmotic stress, and ABA on RD29A-LUC expression in mutant 693. All treatments were the same as in Figures 2, 3, and 4. Plants for control treatments (white bars) at each temperature regime did not receive NaCl or ABA treatments. Shaded bars, ABA treatment; hatched bars, NaCl treatment; black bars, combined NaCl and ABA treatment. Error bars represent ±sd (n = 15).
At room temperature RD29A-LUC expression in the los1-1 mutant in response to NaCl, ABA, or NaCl combined with ABA was not substantially different from that in the wild type (Fig. 9). Unlike the wild-type plants (Figs. 2 and 3), heat shock at 30°C did not enhance the effect of either ABA or NaCl in the los1-1 mutant (Fig. 9). However, synergism between ABA and NaCl was still seen at 30°C, as well as at room temperature. As seen with the wild-type plants, heat shock at 37°C enhanced the effect of ABA but not NaCl, and NaCl and ABA were not synergistic under this high-temperature condition (Fig. 9). The most striking defect in los1-1 was revealed when the mutant plants were treated at 0°C for 48 h. Unlike the wild-type plants, cold stress even for 48 h did not induce RD29A-LUC expression in the los1-1 mutant. ABA, NaCl, or ABA and NaCl combined also did not induce RD29A-LUC expression when the treatments were carried out at 0°C for 48 h (Fig. 9).
Figure 9.
Interaction of temperature, osmotic stress, and ABA treatments on RD29A-LUC expression in mutant los1-1. All treatments were the same as in Figures 2, 3, and 4. White bars, without ABA or NaCl treatment; shaded bars, ABA treatment; hatched bars, NaCl treatment; and black bars, combined NaCl and ABA treatment. Error bars represent ±sd (n = 15).
DISCUSSION
Genetic and molecular studies have suggested that there is extensive interaction between osmotic stress, temperature stress, and ABA responses. As an initial step toward the characterization of the interplay between the response pathways for these environmental and hormonal signals, the expression of RD29A-LUC in response to various treatment regimes was analyzed. Our results revealed both positive and negative interactions, depending on the nature and duration of the treatments.
Low Temperature Impairs Osmotic Signaling but Moderate Heat Stress Is Synergistic
At room temperature 300 mm NaCl induced a much higher level of RD29A-LUC expression than the same treatment at 0°C (Fig. 3). One explanation for this could be the kinetic effect of low temperature on RNA and protein synthesis. However, this is unlikely because low-temperature and ABA treatments were synergistic (Fig. 2). Another possibility is that low temperature impairs osmotic signal transduction. However, the simplest explanation may be that the activity of one or more components in the osmotic stress pathway was sensitive to low temperature. The component could be a sensor in the plasma membrane. Two sensors for hyperosmotic stress are known in the unicellular eukaryote Saccharomyces cerevisiae and both are membrane proteins (Maeda et al., 1994, 1995). The activity of these membrane-sensor proteins could be impaired directly by low temperature or indirectly by the physical and chemical changes in the membrane lipids that occur at low temperatures (for review, see Murata and Los, 1997). Alternatively, the sensitive component could be an intracellular signaling component.
Extreme high-temperature stress was also not favorable to osmotic signaling. At 37°C, although the control treatment alone induced some expression, NaCl did not induce more expression than at room temperature. Because a significant increase in the expression level of the transgene at 37°C was observed with ABA treatment (Fig. 2), it is unlikely that the lack of synergism between NaCl and 37°C heat shock was caused by inhibition of luciferase enzymatic activity.
In contrast to extreme temperatures, moderate heat stress at 30°C strongly enhanced osmotic stress induction of RD29A-LUC expression. Because the 30°C heat stress by itself cannot activate RD29A-LUC, this synergistic effect suggests a higher efficiency of osmotic signaling at 30°C than at other temperatures. This improved efficiency may be the result of increased enzymatic activity of a rate-limiting signaling protein. The lack of synergism between 37°C heat stress and osmotic stress suggests that a component(s) of osmotic signaling is also sensitive to extremely high temperatures.
Low- and High-Temperature Stresses Are Synergistic with ABA
Whereas extremely low or high temperatures were inhibitory to osmotic stress signaling, the ABA response was enhanced by the temperature stresses (Fig. 2). Components in the ABA signaling pathway are resistant to extreme temperatures, or at least none is temperature labile, so it is possible that the ABA receptor in this response is not a membrane protein. In guard-cell regulation both extracellular and intracellular sensing mechanisms for ABA have been proposed (Hornberg and Weiler, 1984; Allan et al., 1994; Anderson et al., 1994; Schwartz et al., 1994). Nothing is known about the ABA receptor(s) responsible for gene regulation.
Combined treatment with ABA and low temperature gave a slightly more than additive effect (Fig. 2). Although low temperature is known to induce ABA accumulation, low-temperature induction of several cold-responsive genes including RD29A is independent of ABA. The strongest evidence for this statement is perhaps that an ABA deficiency in the Arabidopsis aba1 mutant does not reduce cold induction of RD29A expression (Gilmour and Thomashow, 1991; Nordin et al., 1991). The additive interaction between cold stress and exogenous ABA treatment is consistent with the notion of two separate signaling pathways.
It should be noted that a positive interaction between ABA and cold stress did not occur when the low-temperature treatment was brief (4.5 h) (Figs. 6 and 7). The inhibitory effect of 4.5 h of cold shock on ABA and NaCl responses (Figs. 6 and 7) was likely attributable to the rate-limiting effect of low temperature. Alternatively, it could be that change(s) occurred during the longer (48 h) cold treatment that was necessary for the positive interaction between cold and ABA.
High-temperature stress and ABA are clearly synergistic, and this synergism exists at 37°C and 30°C. The physiological relevance, if any, of this observation is unclear at present. Previous studies indicated that at high temperatures ABA concentrations in plant leaves are very low because of the accelerated conversion of ABA to its metabolites, primarily phaseic acid (Zeevaart and Creelman, 1988; Radin, 1992). It is possible that reduced levels of endogenous ABA lead to increased ABA sensitivity as a result of less cellular desensitization. We have observed that the Arabidopsis aba1 mutants display an enhanced response to exogenous ABA in the induction of RD29A-LUC (L. Xiong, M. Ishitani, and J.-K. Zhu, unpublished observations).
Synergism between Osmotic Stress and ABA
The results presented in Figures 4 and 5 indicate that osmotic stress and ABA are synergistic in inducing the transgene and the endogenous gene expression. Similar results were reported by Bostock and Quatrano (1992), who found that osmotic stress and ABA act synergistically in inducing maize Em gene expression. We isolated a group of Arabidopsis single-gene mutations that confer enhanced responses to both osmotic stress and ABA (Ishitani et al., 1997). Recovery of these mutants suggested that although separate signaling pathways exist for osmotic stress and ABA, the pathways do share certain components. Perhaps the common components mediate the synergistic interaction between osmotic stress and ABA.
The synergy between ABA and osmotic stress did not occur at 0°C or 37°C under the conditions used in this study. At these extreme temperatures, osmotic signaling was impaired.
Why Did the los1-1 Mutation Block RD29A-LUC Induction after All Treatments in the Cold?
Although hos mutant 693 showed RD29A-LUC expression patterns largely similar to those of the wild-type plants in response to multiple stress signals (Fig. 8), the los1-1 mutant plants behaved quite differently. The synergistic effect between moderate high temperature (30°C) and ABA or osmotic stress seen in the wild-type plants (Figs. 2 and 3) was not present in los1-1 (Fig. 9). The synergistic interaction between ABA and NaCl can still be seen clearly at either normal temperature or at 30°C (Fig. 9), suggesting that the mutation does not affect the components responsible for the synergism between ABA and osmotic stress signaling.
The induction of RD29A-LUC by ABA and osmotic stress and by the combined stress was blocked in los1-1 under low temperatures. This is very similar to the results obtained with a brief cold treatment of wild-type plants (Fig. 6), in which the stress-induced luminescence was blocked by the short-term low-temperature treatment. However, longer periods (2 d) of cold treatment brought about significant induction of the transgene and interactions among the signals in the wild-type plants (Figs. 2–4). Apparently, longer cold treatment leads to changes that are essential for the regulation of gene expression by ABA and osmotic stress in the cold. Perhaps the los1-1 mutation blocks cold activation of these changes, thereby preventing the potentiation of ABA and osmotic stress regulation of gene expression.
Abbreviation:
- MS
Murashige-Skoog
LITERATURE CITED
- Allan AC, Fricker MD, Ward JL, Beale MH, Trewavas AJ. Two transduction pathways mediate rapid effects of abscisic acid in Commelina guard cells. Plant Cell. 1994;6:1319–1328. doi: 10.1105/tpc.6.9.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BE, Ward JM, Schroeder JI. Evidence for an extracellular reception site for abscisic acid in Commelina guard cells. Plant Physiol. 1994;104:1177–1183. doi: 10.1104/pp.104.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock RM, Quatrano RS. Regulation of Em gene expression in rice, interaction between osmotic stress and abscisic acid. Plant Physiol. 1992;98:1356–1363. doi: 10.1104/pp.98.4.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray EA. Molecular responses to water deficit. Plant Physiol. 1993;103:1035–1040. doi: 10.1104/pp.103.4.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler PM, Robertson M. Gene expression regulated by abscisic acid and its relation to stress tolerance. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:113–141. [Google Scholar]
- Gilmour SJ, Thomashow MF. Cold acclimation and cold-regulated gene expression in ABA mutants of Arabidopsis thaliana. Plant Mol Biol. 1991;17:1233–1240. doi: 10.1007/BF00028738. [DOI] [PubMed] [Google Scholar]
- Gosti F, Bertauche N, Vartanian N, Giraudat J. Abscisic acid-dependent and -independent regulation of gene expression by progressive drought in Arabidopsis thaliana. Mol Gen Genet. 1995;246:10–18. doi: 10.1007/BF00290128. [DOI] [PubMed] [Google Scholar]
- Guy CL, Anderson JV, Haskell DW, Li QB. Caps, cors, dehydrins, and molecular chaperones: their relationship with low temperature responses in spinach. In: Cherry JH, editor. Biochemical and Cellular Mechanisms of Stress Tolerance in Plants. Berlin: Springer-Verlag; 1994. pp. 479–499. [Google Scholar]
- Hornberg C, Weiler EW. High-affinity binding sites for abscisic acid on the plasmalemma of Vicia faba guard cells. Nature. 1984;310:321–324. [Google Scholar]
- Ingram J, Bartels D. The molecular basis of dehydration tolerance in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:377–403. doi: 10.1146/annurev.arplant.47.1.377. [DOI] [PubMed] [Google Scholar]
- Ishitani M, Xiong L, Lee H, Stevenson B, Zhu JK. HOS1, a genetic locus involved in cold-responsive gene expression in Arabidopsis. Plant Cell. 1998;10:1151–1162. doi: 10.1105/tpc.10.7.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani M, Xiong L, Stevenson B, Zhu JK. Genetic analysis of osmotic and cold stress signal transduction in Arabidopsis: interaction and convergence of abscisic acid-dependent and abscisic acid-independent pathways. Plant Cell. 1997;9:1935–1949. doi: 10.1105/tpc.9.11.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt J (1980) Response of Plants to Environmental Stresses, Vol 1, Ed 2. Academic Press, New York
- Liu J, Zhu JK. Proline accumulation and salt-induced gene expression in a salt-hypersensitive mutant of Arabidopsis. Plant Physiol. 1997;114:591–596. doi: 10.1104/pp.114.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Takehara M, Saito H. Activation of yeast PBS2 MAPKK by MAPKKKs or by binding of an SH3-containing osmosensor. Science. 1995;269:554–559. doi: 10.1126/science.7624781. [DOI] [PubMed] [Google Scholar]
- Maeda T, Wurgler-Murphy SM, Saito H. A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature. 1994;396:242–245. doi: 10.1038/369242a0. [DOI] [PubMed] [Google Scholar]
- Millar AJ, Short SR, Chua NH, Kay SA. A novel circadian phenotype based on firefly luciferase expression in transgenic plants. Plant Cell. 1992;4:1075–1089. doi: 10.1105/tpc.4.9.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N, Los DA. Membrane fluidity and temperature perception. Plant Physiol. 1997;115:875–879. doi: 10.1104/pp.115.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordin K, Heino P, Palva ET. Separate signal pathways regulate the expression of a low-temperature-induced gene in Arabidopsis thaliana (L.) Heynh. Plant Mol Biol. 1991;16:1061–1071. doi: 10.1007/BF00016077. [DOI] [PubMed] [Google Scholar]
- Radin JW. Reconciling water-use efficiencies of cotton in field and laboratory. Crop Sci. 1992;32:13–18. [Google Scholar]
- Schwartz A, Wu WH, Tucker EB, Assmann SM. Inhibition of inward K+ channels and stomatal response by abscisic acid: an intracellular locus of phytohormone action. Proc Natl Acad Sci USA. 1994;91:4019–4023. doi: 10.1073/pnas.91.9.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K. Gene expression and signal transduction in water-stress response. Plant Physiol. 1997;115:327–334. doi: 10.1104/pp.115.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow MF. Arabidopsis thaliana as a model for studying mechanisms of plant cold tolerance. In: Somerville C, Meyerowitz EM, editors. Arabidopsis. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. pp. 807–834. [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K. A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell. 1994;6:251–264. doi: 10.1105/tpc.6.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart JAD, Creelman RA. Metabolism and physiology of abscisic acid. Annu Rev Plant Physiol Plant Mol Biol. 1988;39:439–473. [Google Scholar]
- Zhu JK, Hasegawa PM, Bressan RA. Molecular aspects of osmotic stress in plants. Crit Rev Plant Sci. 1997;16:253–277. [Google Scholar]