Abstract
Aims
Heme oxygenase-1 (HMOX1) is a cytoprotective enzyme degrading heme to biliverdin, iron ions, and carbon monoxide, whose expression is induced in response to oxidative stress. Its overexpression has been suggested as a strategy improving survival of transplanted muscle precursors. Results: Here we demonstrated that HMOX1 inhibits differentiation of myoblasts and modulates miRNA processing: downregulates Lin28 and DGCR8, lowers the total pool of cellular miRNAs, and specifically blocks induction of myomirs. Genetic or pharmacological activation of HMOX1 in C2C12 cells reduces the abundance of miR-1, miR-133a, miR-133b, and miR-206, which is accompanied by augmented production of SDF-1 and miR-146a, decreased expression of MyoD, myogenin, and myosin, and disturbed formation of myotubes. Similar relationships between HMOX1 and myomirs were demonstrated in murine primary satellite cells isolated from skeletal muscles of HMOX1+/+, HMOX1+/−, and HMOX1−/− mice or in human rhabdomyosarcoma cell lines. Inhibition of myogenic development is independent of antioxidative properties of HMOX1. Instead it is mediated by CO-dependent inhibition of c/EBPδ binding to myoD promoter, can be imitated by SDF-1, and partially reversed by enforced expression of miR-133b and miR-206. Control C2C12 myoblasts injected to gastrocnemius muscles of NOD-SCID mice contribute to formation of muscle fibers. In contrast, HMOX1 overexpressing C2C12 myoblasts form fast growing, hyperplastic tumors, infiltrating the surrounding tissues, and disseminating to the lungs. Innovation: We evidenced for the first time that HMOX1 inhibits differentiation of myoblasts, affects the miRNA processing enzymes, and modulates the miRNA transcriptome. Conclusion: HMOX1 improves the survival of myoblasts, but concurrently through regulation of myomirs, may act similarly to oncogenes, increasing the risk of hyperplastic growth of myogenic precursors. Antioxid. Redox Signal. 16, 113–127.
Introduction
Growth and regeneration of skeletal muscles are accomplished by satellite cells, located beneath the basal lamina of muscle fibers. Under normal conditions, the satellite cells remain quiescent, but upon muscle damage they convert to proliferating myoblasts, which differentiate, fuse into multinucleated myocytes, and form new muscle fibers or increase the size of preexisting ones (4).
Activation of satellite cells is governed by myogenic determination factor-1 (MyoD), myogenin, myogenic factor-5 (Myf5), and myogenic factor-6 (Myf6), known as the muscle regulatory factors (MRFs). Early stages of development are characterized by induction of Myf5 and MyoD (4). Myf5 leads to rapid myoblast proliferation (32), while upregulation of MyoD results in cell cycle arrest and transition from proliferation to differentiation stage. Together with myocyte enhancer factor-2 (MEF2), MyoD induces expression of myogenin and Myf6, the proteins specific for terminal stages of development (4, 32). Finally, the mature muscles increase the level of myosin heavy chain (MHC) and creatine phosphokinase (CPK) (4).
Innovation
This work demonstrates for the first time that HO-1, a cytoprotective, heme-degrading enzyme, potently inhibits differentiation of myoblasts and can act similarly to oncogenes. These effects depend on HO-1 enzymatic activity and are mediated by HO-1-derived carbon monoxide, which inhibits cEBPδ binding to the MyoD promoter. Induction of HO-1 is also associated with upregulation of SDF-1, and its influence can be mocked by incubation of myoblasts with exogenous SDF-1. Accordingly, after intramuscular transplantation to murine gastrocnemius muscle, the HO-1 overexpressing myoblasts form hyperplastic, undifferentiated tumors, infiltrating healthy tissue, and spreading to the lungs.
Additionally, it demonstrates for the first time that HO-1 affects microRNA transcriptome, downregulating Lin28 and DGCR8, the miRNA processing enzymes, and reducing the total pool of miRNA. Among ∼18% miRNAs differentially expressed, the most profound inhibitory effect was found for miRNA involved in myoblast differentiation: miR-1, miR-133a, miR-133b, and miR-206. Moreover, enforced expression of miR-206 and mir-133b partially reversed the effect of HO-1.
This study not only broadens the understanding of biological significance of HO-1, but also suggests new molecular mechanisms involved in regeneration of muscles and development of rhabdomyosarcoma.
MRFs and MEF2 control the generation of myomirs, a set of conserved microRNAs (miRNAs) specific for skeletal or cardiac muscles, such as miR-1, miR-133a, miR-133b, and miR-206 (34), which function by fine-tuning the output of MRF network. Temporal upregulation of myomirs negatively regulates the target genes, and is necessary for proper muscle development (45). On the other hand, miR-1 and miR-206 attenuate proliferation and promote myoblast differentiation via activation of MRFs (5, 39). Their induction is associated with increased expression of MyoD, myogenin, MHC, or CPK, and with fusion of myoblasts (17), whereas inhibition is related to development of rhabdomyosarcoma (47).
Understanding the mechanisms of myoblast differentiation may help in establishing cell therapies. Although it has been proven that muscle progenitors are able to incorporate into host tissue, the final outcomes of clinical trials have been rather disappointing, mainly because of massive cell death soon after transplantation (40). It appears that genetic modification of progenitor cells can improve their survival (41). Upregulation of heme oxygenase-1 (HMOX1) has been proposed as a potential approach to improve the viability of muscle precursors or cardiac grafts (20, 26, 31, 51).
Enzymatic activity of HMOX1 attenuates oxidative stress, increases cell survival, and influences cell cycle, acting through degradation of pro-oxidative heme to carbon monoxide (CO), ferrous ions, and biliverdin (25). The cytoprotective and antiapoptotic effects of HMOX1 have been demonstrated in different cell types exposed to reactive oxygen species (ROS) or proinflammatory cytokines (12). Furthermore, HMOX1 upregulates expression of vascular endothelial growth factor (VEGF) (8, 15), and is necessary for proper function of stromal cell-derived factor-1 (SDF-1) (7). Beneficial effects of HMOX1 have been shown in cutaneous wounds, tissues subjected to ischemia-reperfusion injury, and in transplanted organs (13, 48). Nothing is known, however, about the role of HMOX1 in differentiation of muscle precursors.
Our aim was to examine whether overexpression of HMOX1 may improve the survival of myoblasts after intramuscular transplantation in mice and to investigate the role of HMOX1 in myoblast differentiation.
Results
Cytoprotective potential of HMOX1
To check whether HMOX1 improves survival of myoblasts, we used C2C12 murine myoblast cell line expressing green fluorescent protein (GFP) and luciferase (C2C12-Luc-GFP), and overexpressing HMOX1 (C2C12-Luc-GFP-HO1). Stable upregulation of HMOX1 was confirmed by mRNA, protein, and enzymatic activity measurements (Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/ars).
As expected, overexpression of HMOX1 led to decreased generation of ROS. The same effect was produced by supplementation of control cells with N-acetylcysteine (NAC) (Supplementary Fig. S2A). HMOX1 was also cytoprotective, improving the survival of cells exposed to H2O2 and enhancing their serum-induced proliferation (Supplementary Figs. S2B and S2C).
Effect of HMOX1 on survival of intramuscularly transplanted cells
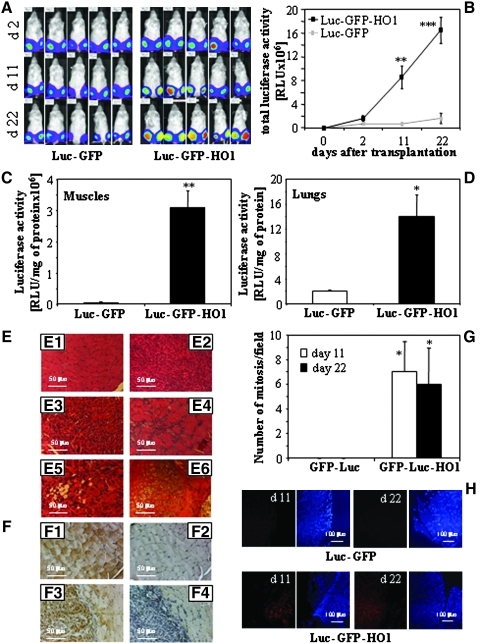
To examine whether HMOX1 overexpression improves cell survival in vivo, we injected C2C12-Luc-GFP and C2C12-Luc-GFP-HO1 myoblasts into gastrocnemius muscles of NOD/SCID mice (250,000 cells per muscle). As shown in Figure 1A, the transplanted cells-derived luciferase activity was easily detectable at the sites of injection. Importantly, in the C2C12-Luc-GFP-injected animals, it remained at a constant level during the experiment, whereas in animals injected with C2C12-Luc-GFP-HO1 cells, it rapidly grew up (Figs. 1A and 1B). As a consequence, 3 weeks after transplantation the legs of all animals injected with HMOX1 overexpressing myoblasts were deformed, rigid, and filled with tumor-like structures. Mice stopped walking and had to be euthanized on day 22. In the same time, all animals injected with C2C12-Luc-GFP cells behaved normally and did not show any deformations.
FIG. 1.
Effect of HMOX1 overexpression on survival and proliferation of myoblasts transplanted intramuscularly to the gastrocnemius muscle of NOD/SCID mice. (A) Luciferase activity monitored in vivo using IVIS Lumina system. Images show the same individuals analyzed at 2nd, 11th, and 22nd day of experiment (blue: low intensity; red: high intensity). (B) Quantification of IVIS measurements. Total luminescence from each animal is expressed in relative luminescence units (RLU). (C, D) Luciferase activity measured in tissue lysates prepared from gastrocnemius muscles (C) or lungs (D) on 22nd day after injection. No signals were found in heart, liver, and kidney lysates. (E) Histology of gastrocnemius muscle of NOD/SCID mice, 22 days after cell transplantation (paraffin sections stained with H&E): E1: Mice injected with C2C12-Luc-GFP cells. E2–E6: Mice injected with C2C12-Luc-GFP-HO1 cells: hyperplastic tissue (E2), infiltrating the muscle (E3 and E4), with adipogenic (E5) or chondro-osteogenic (E6) accumulations. Paraffin sections stained with H&E. (F) Histochemical detection of GFP in gastrocnemius muscles 22 days after cell transplantation. Representative pictures (F1 and F2) C2C12-Luc-GFP-injected muscle: staining (F1) and negative control (F2). (F3 and F4) C2C12-Luc-GFP-HO1 injected muscle: staining (F3) and negative control (F4). (G) Semi-quantitative assessment of mitotic index. (H) Detection of proliferating cells by immunofluorescent staining of PCNA (red) 11 and 22 days after cell transplantation. Nuclei were visualized with DAPI (blue). Representative pictures. Each point or bar represents mean±SEM of 5–10 animals (B) or 5 animals (C, D, G); *p<0.05, **p<0.01, ***p<0.001 vs. C2C12-Luc-GFP cells.
Measurements of luciferase activity in tissue lysates confirmed a tremendous difference between the growth of C2C12-Luc-GFP and C2C12-Luc-GFP-HO1 cells in gastrocnemius muscles (Fig. 1C). Additionally, in the animals injected with HMOX1 overexpressing myoblasts, we detected a weak but measurable signal in the lungs (Fig. 1D), which suggests that cells can disseminate out of the site of injection.
Histological analysis of paraffin sections prepared from muscles injected with C2C12-Luc-GFP cells showed the normally looking mature myofibers in all specimens on the 11th or 22nd day of experiment (Fig. 1E1). In contrast, muscles injected with C2C12-Luc-GFP-HO1 cells contained also large areas of undifferentiated, hyperplastic tumors (Fig. 1E2), infiltrating healthy tissue (Figs. 1E3 and 1E4). Such tumors were visible in 2 of 5 individuals sacrificed on day 11, and in all 5 individuals sacrificed on day 22. In some samples, the adipogenic (Fig. 1E5) or chondro-osteogenic (Fig. 1E6) accumulations were observed, suggesting that HMOX1 overexpression inhibits myoblast differentiation or directs it toward chondro-osteogenic and adipogenic lineages. Immunohistochemical staining for GFP confirmed that both transplanted cell lines contributed to formation of muscle fibers (Fig. 1F). This process seemed to be less effective in the case of HMOX1 overexpressing cells, which formed the strongly GFP-positive hyperplastic areas (Figs. 1F3 and 1F4). Finally, C2C12-Luc-GFP-HO1 myoblasts proliferated more intensively, as demonstrated by calculating the mitotic index (Fig. 1G) or by detection of proliferating cell nuclear antigen (PCNA)-positive cells (Fig. 1H).
Real-time PCR analysis, carried out on the 22nd day of experiment, confirmed the high level of HMOX1 mRNA in muscles injected with C2C12-Luc-GFP-HO1 myoblasts (Supplementary Fig. S3A). Interestingly, it was accompanied by significant upregulation of SDF-1, and by reduced expression of MyoD (Supplementary Figs. S3B and S3C). Expression of myogenin was similar in muscles injected with control and HMOX1 overexpressing cells (Supplementary Fig. S3D).
Effect of HMOX1 overexpression on cell differentiation
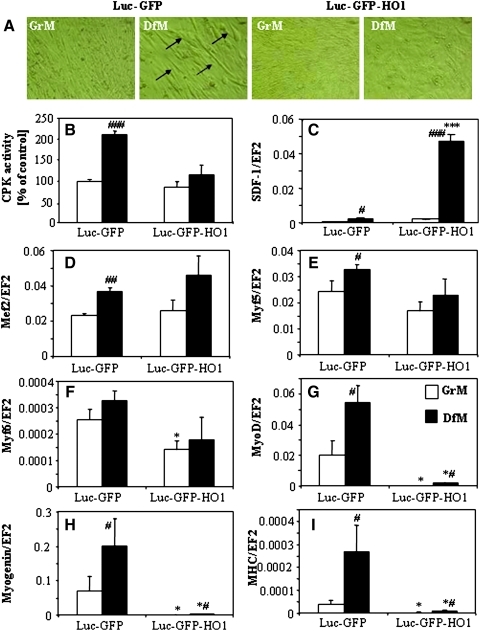
In vivo analysis suggested that HMOX1 overexpression may influence myoblast maturation. Therefore in the next set of experiments, we compared the differentiation process induced in vitro by incubation of cells for 5 days in media supplemented with 2% horse serum (differentiation medium, DfM) instead of 10% fetal calf serum (growth medium, GrM). Indeed, formation of elongated, multinucleated myotubes, well visible in control C2C12-Luc-GFP cell cultures, was completely blocked in HMOX1 overexpressing cells (Fig. 2A). Also DfM-induced upregulation of CPK activity was prohibited by HMOX1 overexpression (Fig. 2B). On the other hand, C2C12-Luc-GFP-HO1 myoblasts produced much more SDF-1, a chemokine involved in regulation of myogenesis. In GrM they displayed a slight (∼30%) increase in SDF-1 mRNA, which became profound (∼19-fold) in cells cultured in DfM (Fig. 2C). These effects were reversed by HMOX1-specific siRNA (data not shown).
FIG. 2.
Effect of HMOX1 overexpression on differentiation of C2C12 cells. Representative photos. (A) Formation of myotubes by C2C12-Luc-GFP and C2C12-Luc-GFP-HO1 cells in DfM (GrM, open bars; DfM, black bars). (B) CPK activity showed as a percentage of C2C12-Luc-GFP control. (C) Expression of SDF-1 mRNA. (D–I) Expression of myogenic differentiation markers: MEF2 mRNA (D), Myf5 mRNA (E), Myf6 mRNA (F), MyoD mRNA (G), myogenin mRNA (H), and MHC mRNA (I). qRT-PCR: EF2 served as a constitutive gene. Each bar represents mean±SEM of 4 experiments. *p<0.05, ***p<0.001 versus C2C12-Luc-GFP cells, #p<0.05, ##p<0.01, ###p<0.001 versus cells cultured in GrM.
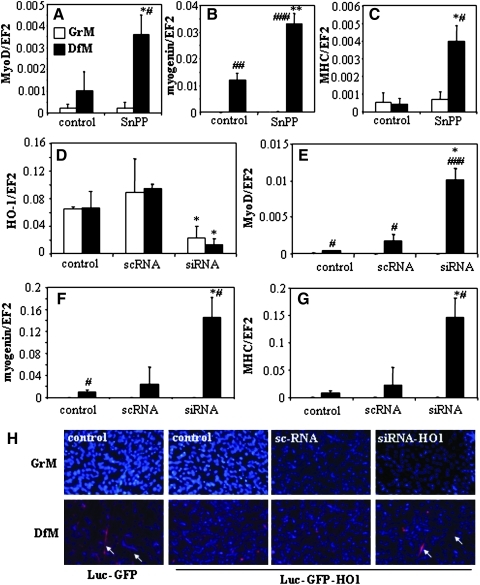
Differentiation of control cells cultured in DfM was confirmed by upregulation of all markers tested: Mef2, Myf5, Myf6, MyoD, myogenin, and MHC (Figs. 2D–2I). Similar levels of gene expressions were observed in C2C12-Luc-GFP-HO1 cells for early markers Mef2 and Myf5 (Figs. 2D and 2E) and for Myf6, the gene forming a cluster with Myf5 (1) (Fig. 2F). In contrast, expression of MyoD, myogenin, and MHC was almost completely abrogated in HMOX1 overexpressing myoblasts cultured either in GrM or in DfM (Figs. 2G–2I). Inhibition of enzymatic activity of HMOX1 in C2C12-Luc-GFP-HO1 cells using tin protoporphyrin-IX (SnPP) increased the DfM-induced upregulation of MyoD, myogenin, and MHC (Figs. 3A–3C). Similarly, suppression of HMOX1 using siRNA, significantly increased the DfM-induced expression of MyoD, myogenin, and MHC mRNAs, or MHC protein (Figs. 3D–3H). Thus, downregulation of differentiation markers in C2C12-Luc-GFP-HO1 cells appears to be a specific effect of HMOX1 expression and enzymatic activity, although their restoration by siRNA or SnPP was only partial, possibly due to not total inhibition of HMOX1.
FIG. 3.
HO-1 inhibition on differentiation of C2C12 cells. (A–C) Effect of HMOX1 inhibition with SnPP (5 days, 50 μmol/L) on expression of MyoD mRNA (A), myogenin mRNA (B), and MHC mRNA (C) in C2C12-Luc-GFP-HO1 cells cultured in GrM or DfM. (D–G) Effect of scrambled (sc) or HMOX1 siRNA on expression of HMOX1 mRNA (D), MyoD mRNA (E), myogenin mRNA (F), and MHC mRNA (G) in C2C12-Luc-GFP-HO1 cells cultured for 5 days in GrM or DfM. QRT-PCR. EF2 served as a constitutive gene. Each bar represents mean±SEM of 3 experiments. *p<0.05, **p<0.01 versus C2C12-Luc-GFP cells, #p<0.05, ###p<0.001 versus cells cultured in GrM. (H) Immunofluorescent staining of MHC protein (red) in C2C12-Luc-GFP cells and C2C12-Luc-GFP-HO1 cells transfected with sc or HMOX1 siRNA and cultured for 5 days in GrM or DfM (blue: staining of nuclei with DAPI). Representative result.
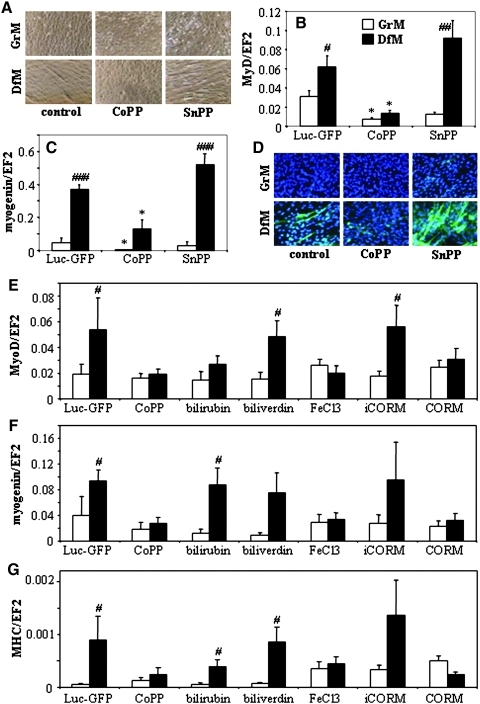
We also checked the differentiation of unmodified C2C12 cells cultured with cobalt protoporphyrin-IX (CoPP, HMOX1 activator) or SnPP. Morphological inspection revealed that activation of HMOX1 with CoPP completely blocked the formation of myotubes, while inhibition of its enzymatic activity augmented the myoblasts differentiation (Fig. 4A). Correspondingly, in cells cultured in DfM the MyoD and myogenin mRNA (Figs. 4B and 4C) together with MHC protein (Fig. 4D) were inhibited by CoPP and augmented by SnPP.
FIG. 4.
Effect of pharmacological activation (with CoPP, 10 μmol/L) or inhibition (with SnPP, 10 μmol/L) of HMOX1 on differentiation of wild-type C2C12 cells cultured for 5 days in GrM or DfM. (A) Formation of myotubes. Contrast phase microscopy. Representative result. Expression of MyoD (B) and myogenin (C) mRNAs. qRT-PCR: EF2 served as a constitutive control. Each bar represents mean±SEM of 3 experiments. *p<0.05 versus unstimulated C2C12 cells, #p<0.05, ##p<0.01, ###p<0.001 versus cells cultured in GrM. (D) Expression of MHC protein (green). Immunofluorescent staining, nuclei labeled with DAPI. Representative result. (E–G) Effect of HMOX1 products on differentiation of C2C12-Luc-GFP line. Cells were incubated for 5 days in GrM or DfM in the presence of 10 μmol/L of CoPP, bilirubin, biliverdin, FeCl3, CORM, or iCORM. Expression of MyoD (A), myogenin (B), or MHC (C) was measured using qRT-PCR. EF2 served as a constitutive control. Each bar represents mean±SEM of 4 experiments. #p<0.05 versus cells cultured in GrM.
Effect of HMOX1 products on differentiation markers
Then we incubated C2C12-Luc-GFP cells with biliverdin, bilirubin, FeCl3, CO-releasing molecule (CORM), inactive CORM (iCORM, negative control), or with CoPP (positive control). It turned out that effects of HMOX1 can be mimicked by two of its potential products, iron ions and CO, but not by biliverdin and bilirubin (Figs. 4E–4G).
Effect of HMOX1 on miRNA transcriptome
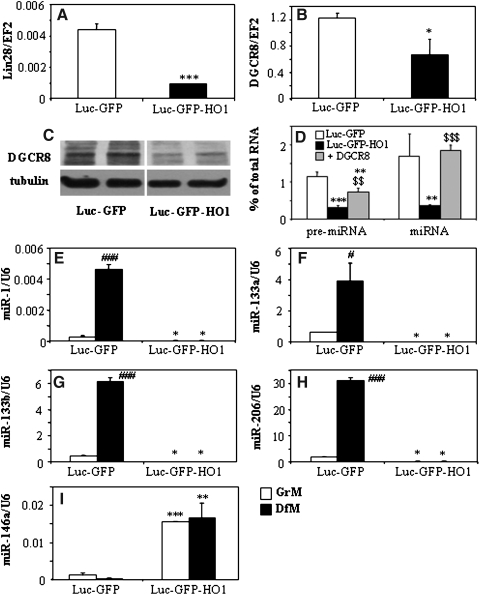
Unexpectedly, we noticed that overexpression of HMOX1 affected the genes involved in regulation of miRNA processing. Namely, it reduced the expression of mRNAs for abnormal cell LINeage (Lin28) (Fig. 5A) and DiGeorge syndrome critical region-8 (DGCR8) (Fig. 5B). Also DGCR8 protein, which acts as a heme-dependent dimer, was decreased in cells with high activity of heme-degrading HMOX1 (Fig. 5C). Furthermore, a total pool of pre-miRNA and miRNA was lower in C2C12-Luc-GFP-HO1 myoblasts, the effect reversed by enforced overexpression of DGCR8 (Fig. 5D). This suggests that some effects of HMOX1 in C2C12-Luc-GFP-HO1 may result from decreased generation of miRNA and disturbed regulation of miRNA-dependent pathways.
FIG. 5.
Effect of HMOX1 overexpression on generation of miRNA and expression of miRNA processing enzymes. Expression of Lin 28 mRNA (A), and DGCR protein (B) in cells cultured in GrM. qRT-PCR: EF2 served as a constitutive control. (C) DGCR8 protein in cell lysates in cells cultured in GrM. Representative Western blot. Tubulin was used as a loading control. (D) Amount of total pre-micro-RNA and micro-RNA pools in C2C12-Luc-GFP, C2C12-Luc-GFP-HO1, and DGCR8 overexpressing C2C12-Luc-GFP-HO1 cells cultured in GrM, expressed as a percentage of total RNA. Measurements used bioanalyser. Each bar represents mean±SEM of 3 experiments. (E–I) Expression of myomirs: miR-1 (E), miR-133a (F), miR-133b (G), miR-206 (H), and miR-146a (I) in C2C12-Luc-GFP and C2C12-Luc-GFP-HO1 cells cultured for 5 days in GrM or DfM. qRT-PCR: U6 served as a constitutive control. Each bar represents mean±SEM of 3 experiments. *p<0.05, **p<0.01, ***p<0.001 versus C2C12-Luc-GFP cells. #p<0.05, ###p<0.001 versus cells cultured in GrM. $$p<0.01, $$$p<0.001 versus Luc-GFP-HO1 cells.
Therefore, we compared a miRNA transcriptome in C2C12-Luc-GFP and C2C12-Luc-GFP-HO1 cells cultured in the GrM or DfM (Supplementary Tables 1 and 2). Importantly, in the C2C12-Luc-GFP cell line, a group of muscle-specific myomirs, namely miRNAs 1, 133a, 133b, and 206, was significantly induced in response to DfM, as shown by transcriptome analysis and confirmed by routine qRT-PCR (Figs. 5E–5H). In sharp contrast, expression of these myomirs was much lower and their upregulation was totally blocked in cells overexpressing HMOX1 (Figs. 5E–5H). On the other hand, miR-146, regarded as an inhibitor of muscle differentiation (18) was strongly increased in C2C12-Luc-GFP-HO1 cells, both in growth and differentiation conditions (Fig. 5I). Partial reversion of these effects by HMOX1 siRNA or SnPP (Supplementary Fig. S4) confirms the specificity of HMOX1 action.
Interestingly, the generalized downregulation of miRNA biogenesis and specific inhibition of myomirs seem to be separate events. Overexpression of DGCR8 in C2C12-Luc-GFP-HO1 cells fully restored the total pool of miRNA (Fig. 5D), but did not influence the expression of myogenic markers (data not shown). On the other hand, exposure of C2C12-Luc-GFP cells to FeCl3 or CORM mimicked the effect of HMOX1 activity on MRFs (Figs. 4E–4G) but did not affect the total pool of miRNA or pre-miRNA (data not shown).
Mediators of HMOX1 activities
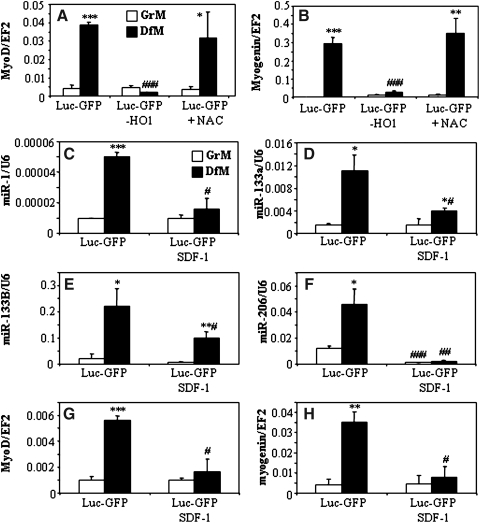
Overexpression of HMOX1 reduced cellular production of ROS and the same effect was observed in NAC-treated control myoblasts (Supplementary Fig. S2A). However, incubation of C2C12-Luc-GFP cells with NAC did not influence the DfM-induced upregulation of MyoD or myogenin (Figs. 6A and 6B). Accordingly, we did not observe any phenotypic differences between cells cultured with or without NAC (not shown). Thus, it appears that inhibition of myoblast differentiation is independent of antioxidative properties of HMOX1. Instead, the influence of HMOX1 on miR-1, miR-133a, miR-133b, and miR-206 was mocked by incubation of myoblasts with SDF-1 protein (Figs. 6C–6F). Such a treatment reduced also the expression of MyoD and myogenin (Figs. 6G and 6H). Thus, SDF-1 might be proposed as one of mediators of HMOX1 activity in C2C12 cells.
FIG. 6.
Potential mediators of HMOX1 activity. (A and B) No effect of NAC on expression of MyoD (A) and myogenin (B) in C2C12-Luc-GFP myoblasts. C2C12-Luc-GFP-HO1 cells were used as positive control. Cells were incubated for 5 days in GrM or DfM with or without NAC (2 mmol/L). qRT-PCR: EF2 served as a constitutive control. Each bar represents mean±SEM of 2 experiments. (C–H) Expression of MyoD (C), myogenin (D), miR-1 (E), miR-133a (F), miR-133b (G), and miR-206 (H) in C2C12-Luc-GFP cultured for 5 days in GrM and DfM in the presence or absence of SDF-1 protein (50 ng/mL). qRT-PCR: EF2 or U6 served as a constitutive controls. Each bar represents mean±SEM of 3 experiments. *p<0.05, **p<0.01, ***p<0.001 versus cells cultured in GrM. #p<0.05, ##p<0.01, ###p<0.001 versus C2C12-Luc-GFP cells.
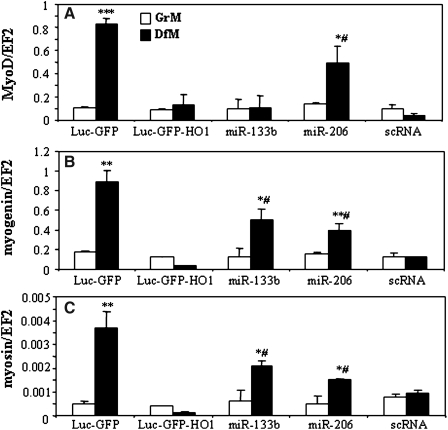
To investigate the role of myomirs in HO-1-induced inhibition of myogenesis, we transfected C2C12-Luc-GFP-HO1 cells with myomirs, and cultured them in GrM or DfM. Enforced expression of miR-133b and miR-206 partially restored the generation of myogenin, and myosin. MiR-206, but not miR-133b, was also able to rescue the MyoD induction in cells cultured in DfM (Fig. 7). This indicates that effect of HMOX1 on myoblast maturation is, in part, mediated by inhibition of miR-133b and miR-206 cluster.
FIG. 7.
Overexpression of myomirs and myoblast differentiation. Effect of miR-133b, miR-206, or scrambled (sc) miR transfection on expression of MyoD (A), myogenin (B), and myosin (C) in C2C12-Luc-GFP-HO1 cells incubated for 5 days in GrM or DfM. qQRT-PCR: EF2 served as a constitutive control. Each bar represents mean±SEM of 3 experiments. *p<0.05, **p<0.01, ***p<0.001 versus cells cultured in GrM. #p<0.05 versus scRNA treated Luc-GFP-HO1 cells.
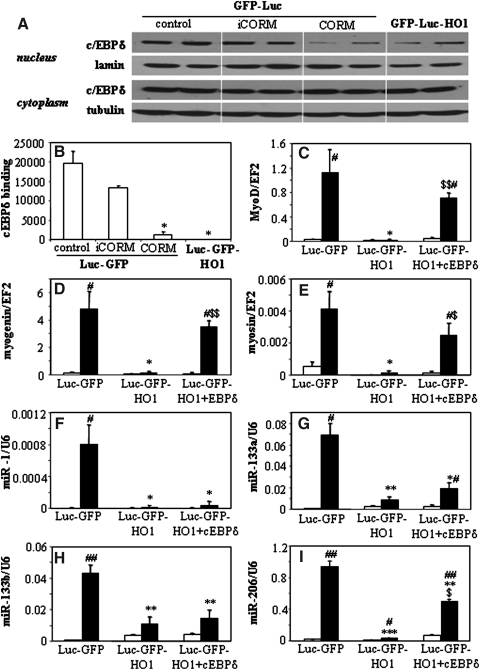
It was reported that CO may inhibit transcriptional activity of CCAAT/Enhancer-binding protein (c/EBPδ) (37), the major transcription factor regulating the myoD expression (38). Therefore, we analyzed the function of c/EBPδ in C2C12-Luc-GFP and C2C12-Luc-GFP-HO1 cells (Fig. 8). The results show that overexpression of HMOX1 or exposure of control cells to CORM inhibits nuclear translocation of c/EBPδ (Fig. 8A) and strongly reduces binding of c/EBPδ to the myoD promoter (Fig. 8B). Importantly, the enforced overexpression of c/EBPδ in C2C12-Luc-GFP-HO-1 myoblasts fully restored the DfM-induced upregulation of myoD, myogenin, and myosin (Figs. 8C–8E), and partially rescued the expression of miR-206 (Fig. 8I). On the other hand, it did not affect the generation of miR-1, miR-133a, and miR-133b (Figs. 8F–8H).
FIG. 8.
Role of c/EBpδ in HO-1-dependent inhibition of myoblast differentiation. (A) Effect of HMOX1 overexpression or treatment of C2C12-Luc-GFP cells with CORM (CO-releasing molecule) and iCORM (inactive CORM) on amount of c/EBPδ protein in the nucleus and cytoplasm. Representative Western blot. Lamin and tubulin were used as loading controls for nuclear and cytoplasmic fractions, respectively. (B) Effect of HMOX1 overexpression or treatment of C2C12-Luc-GFP cells with CORM and iCORM on c/EBPδ binding to the myoD promoter. ChIP assay. (C–I) Effect of c/EBPδ overexpression in C2C12-Luc-GFP-HO1 cells expression of MRFs and myomirs: myoD (C), myogenin (D), myosin (E), miR-1 (F), miR-133a (G), miR-133b (H), and miR-206 (I). qRT-PCR: EF2 and U6 served as constitutive controls. Each bar represents mean±SEM of 2–3 measurements. *p<0.05, **p<0.01, ***p<0.001 versus C2C12-Luc-GFP cells. #p<0.05, ##p<0.01 versus cells cultured in GrM. $$p<0.01 versus Luc-GFP-HO1 cells.
To check whether increase in miR-206 can be modulated by myoD, we overexpressed myoD in C2C12-Luc-GFP-HO1 cells. Such a treatment resulted in total restoration of myogenin and partial rescue of myosin expression (Supplementary Figs. S5A and S5B), but did not show any influence on generation of myomirs, including the miR-206 (Supplementary Figs. S5C–5F). Moreover, myoD overexpression, in contrast to c/EBPδ overexpression, did not promote the myotube formation by C2C12-Luc-GFP-HO1 cells (data not shown).
Role of HO-1 in murine satellite cells and human rhabdomyosarcoma cell lines
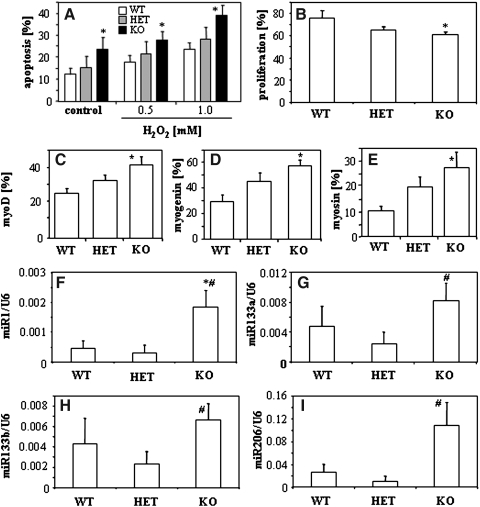
To confirm the importance of HO-1 in muscle differentiation, we analyzed the primary satellite cells isolated from the muscles of HMOX1+/+, HMOX1+/−, and HMOX1−/− mice. We found that, in accordance with results obtained in C2C12 cell lines, expression of HO-1 increased resistance of satellite cells to oxidative stress (Fig. 9A), facilitated the FCS-induced proliferation (Fig. 9B), and decreased the differentiation rate, as demonstrated by lower number of cells expressing the MRFs (Figs. 9C–9E). Importantly, analysis of myomirs in primary satellite cells demonstrated a higher generation of miR-1, miR-133a, miR-133b, and miR-206 in cells isolated from the HMOX1-deficient mice, than in satellite cells possessing at least one functional HMOX1 allele (Figs. 9F–9I). This observation additionally supports the inhibitory effect of HMOX1 on myomir production.
FIG. 9.
Comparison of primary satellite cells isolated from HMOX1+/+ (WT), HMOX1+/− (HET) and HMOX1−/− (KO) mice. (A) Apoptosis of cells cultured for 24 h with H2O2 (0.5 mM and 1.0 mM). Flow cytometric detection of annexin-V positive and PI negative cells. (B) Proliferation of cells after 24 h stimulation with 10% FCS. PCNA staining. (C–E) Proportion of cells expressing MRF proteins: myoD (C), myogenin (D), myosin (E). Immunohistochemical staining. (F–I) Expression of myomirs: miR-1 (F), miR-133a (G), miR-133b (H), and miR-206 (I). qRT-PCR: U6 served as a constitutive control. Each bar represents mean±SEM of 2–6 experiments. *p<0.05, versus WT cells. #p<0.05 versus HET cells.
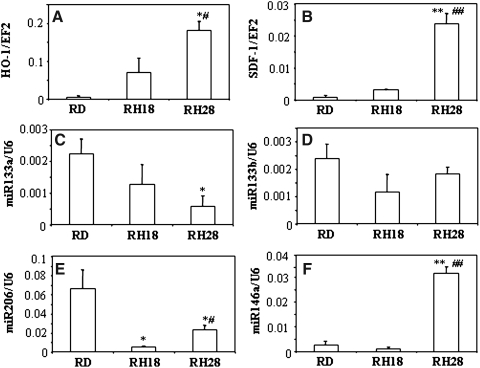
In the last set of experiments, we investigated human rhabdomyosarcoma cell lines: RD (with very low level of HMOX1), RH18 (with moderate level of HMOX1), and RH28 (with high level of HMOX1). Augmented production of HMOX1 was associated with a higher expression of SDF-1 and miR-146a, and lower generation of miR-133a, miR-133b, and miR-206 (miR-1 was undetectable in this model) (Fig. 10). This suggests that the relationship between HMOX1, SDF-1, and myomirs demonstrated here in murine models can occur also in humans.
FIG. 10.
Gene expression profile in human rhabdomyosarcoma. Expression of HMOX1 (A), SDF-1 (B), miR-133a (C), miR-133b (D), and miR-206 (E), and miR-146a (F) in human rhabdomyosarcoma cell lines RD, RH18, and RH28. QRT-PCR. EF-2 and U6 served as constitutive controls. Each bar represents mean±SEM of 2 experiments.
Discussion
The salient finding of our study is that HMOX1, the rate-limiting enzyme in a heme degradation pathway, potently inhibits differentiation of myoblasts in a process dependent on regulation of miRNAs and inhibition of c/EBPδ activity. Pharmacological or genetic activation of HMOX1 attenuates formation of myotubes, decreases expression of MyoD, myogenin, or myosin, and reduces production of myomirs. Inhibition of differentiation is mimicked by SDF-1 and can be partially reversed by miR-133b, miR-206, or myoD overexpression or rescued by enforced upregulation of c/EBPδ.
HMOX1 acts as a cytoprotective enzyme owing to removal of cytotoxic free heme and due to activity of heme degradation products. Namely, biliverdin and bilirubin reduce oxidative stress and inhibit the complement cascade, while CO downregulates caspases, induces antiapoptotic genes, and decreases production of proinflammatory cytokines. Iron ions, the last product of HMOX1, can induce tissue injury via production of free hydroxyl radicals but, at the same time, they stimulate the expression of protective ferritin (12, 25).
Due to antiapoptotic and anti-inflammatory properties, upregulation of HMOX1 has been proposed as a strategy to improve the efficacy of cell therapies by preventing the massive cell death just after transplantation (20). We confirmed that HMOX1 is cytoprotective for C2C12 myoblasts, diminishing production of ROS, improving survival of cells under oxidative stress, and increasing their proliferation. It can be important, as skeletal muscles are subjected to oxidative stress due to a high rate of oxidative metabolism, and increased ROS production impairs regenerative capacity of satellite cells (11).
Possibly both the proproliferative and cytoprotective activities of HMOX1 facilitated growth of C2C12-Luc-GFP-HO1 cells injected intramuscularly into NOD-SCID mice. Similar improvement in cell survival has been already demonstrated in porcine myogenic precursors overexpressing HMOX1 (20). In our model, however, it resulted also in development of large, highly proliferating, hyperplastic tumors. HMOX1 overexpressing cells infiltrated the surrounding tissue and could be detected in lungs, the most frequent site of metastasis in rhabdomyosarcoma patients (6). In contrast, control C2C12-Luc-GFP myoblasts incorporated into muscles, as observed earlier in experiments with C2C12 line (16, 44). This indicates that HMOX1 is an important regulator of muscle maturation. Furthermore, activation of HMOX1 led also to reduced expression of MyoD in the transplanted myoblasts. It has been speculated that satellite cells unable to upregulate MyoD more easily differentiate into other mesenchymal lineages (49). The presence of adipogenic or chondro-osteogenic accumulations in muscles treated with C2C12-Luc-GFP-HO1 cells seems to support such a supposition.
Several studies have indicated the potential significance of HMOX1 in cell maturation. Thus, HMOX1 activation inhibited differentiation of osteoclasts (22), adipocytes (43), monocytes (19), Kupffer cells, and dendritic cells (3), the latter effect dependent on CO (35). It also reduced the maturation of osteoblasts acting through all downstream products: CO, bilirubin, and iron ions (23). On the other hand, HMOX1 facilitated differentiation of hematopoietic precursors (2), enterocytes (42), or odontoblasts (21), again through the CO-dependent pathway (29).
However, effect of HMOX1 on muscle maturation has not been appreciated so far. The sole report describing the role of HMOX1 in porcine myoblasts, did not notice any changes (20). Possibly, the analysis based on cell morphology and expression of single terminal-differentiation marker was not sensitive enough to detect differences after a short-term in vitro incubation, especially when HMOX1 was overexpressed in ∼50% of cells. The myoblasts were then transplanted for 5 days only and their differentiation was not investigated. Similarly as in our model, the cells survived better (20), so it cannot be excluded that a hyperplastic tissue formation would be visible later.
Our in vitro experiments indicate that HMOX1 does not influence the early stages of myoblast maturation characterized by induction of Myf5 and Mef2. Instead it potently inhibits expression of MyoD, the master regulator of muscle differentiation (4), and reduces the downstream MyoD-dependent genes such as myogenin and myosin. Restoration of differentiation of C2C12-Luc-GFP-HO1 cells by SnPP, and results of incubation of C2C12-Luc-GFP cells with HMOX1 products or with NAC indicate that effects of HMOX1 depend on its enzymatic activity, and can be mediated by CO- or iron-regulated pathways, independently of ROS generation. In fact, we demonstrated that HMOX1-derived CO inhibits the nuclear translocation of c/EBPδ and potently decreases its binding to the myoD promoter. The significance of this mechanism is supported by restoration of differentiation in C2C12-Luc-GFP-HO1 engineered to overexpress c/EBPδ. Additionally, inhibitory effects of HMOX1 might be augmented by SDF-1, known regulator of myoblast differentiation (30), which is strongly upregulated in C2C12-Luc-GFP-HO1 cells and can mimic HMOX1 action. This interaction can be, however, cell-type specific, as correlation between HMOX1 and SDF-1 expression in primary satellite cells and rhabdomyosarcoma cell line is not evident.
We showed for the first time that HMOX1 regulates Lin28 and DGCR8, and influences the miRNA expression profile. Because DGCR8, the protein involved in miRNA processing, requires heme (10), one could expect that removal of heme in HMOX1 overexpressing cells may lead to insufficient formation of DGCR8 dimers, and to disturbed miRNA production. Indeed, in C2C12-Luc-GFP-HO1 cells, the DGCR8 protein was lowered, and this effect could not be mocked by any of the HO-1 products. Inhibition of DGCR8 was accompanied by decrease in total pools of pre-miRNA and miRNA, the feature characteristic also for malignant tumor cells (28). Again, restoration of miRNA biogenesis by enforced overexpression of DGCR8 in C2C12-Luc-GFP-HO1 cells confirms role of this pathway in HMOX1-dependent regulation of miRNA transcriptome.
Importantly, activation of HMOX1 not only inhibits generalized miRNA biogenesis, but also specifically blocks the generation of myomirs, which are both the markers and key regulators of muscle differentiation (5). Among their targets are polymerase II (necessary for proliferation, (17), histone deacetylase 4 (HDAC4, transcriptional repressor of muscle genes (5), serum responsive factor (SRF, enabling proliferation, (5), c-met (receptor for hepatocyte growth factor, the myoblast activator (39, 47), fibroblast growth factor binding protein (FGF-BP), augmenting the pro-proliferative activity of FGF (45), inhibitor of differentiation (Id) 1-3 and musculin (repressors of MRFs (17)). Myomir-mediated downregulation of those genes leads to muscle differentiation and cell-cycle withdrawal (5, 17).
Synthesis of myomirs can be upregulated by MRFs (5, 34): Mef2 and MyoD have been reported to control the expression of miR-1 and miR-133a (24) or, together with myogenin, to regulate miR-206 and miR-133b (45). Thus, inhibition of myomirs in our model could be both a direct action of HMOX1 or indirect result of decrease in MyoD and myogenin. Restoration of differentiation capacities in cells transfected to overexpress miR-133b and miR-206 indicates that inhibition of myomirs mediates, at least in part, the HMOX1 activity. On the other hand, overexpression of myoD does not influence myomirs. Thus, miR-206 and miR-133b act upstream but not downstream of myoD in HMOX1 overexpressing myoblasts.
MiR-206 is a known inducer of myogenic pathway (47). Its forced upregulation is sufficient to cause a major switch in the global expression profile toward mature muscle, promote differentiation of neoplastic rhabdomyosarcoma cells, and block rhabdomyosarcoma tumor growth (39). Also the expression of miR-133 is elevated during myoblast differentiation (5, 34). There are, however, some discrepancies regarding its function. Some studies indicate that miR-133 inhibits myoblast proliferation, albeit less potently as miR-1 or miR-206 (17). Some others describe the increased proliferation and attenuated differentiation in response to miR-133, suggesting the inhibition of MyoD and MEF2 as a mechanism responsible for the observed effects (5, 34). Our study, where transfection with miR-133b upregulated expression of myogenin and MHC, supports its pro-differentiation activity in C2C12 myoblasts.
Regulation of miRNAs may become a powerful diagnostic and therapeutic approach as particular miRNAs can modulate dozens of targets, leading to potent phenotypic effects (45). For example, upregulation of miR-206 probably slows progression of amyotrophic lateral sclerosis (ALS) (46), and blocks rhabdomyosarcoma tumor growth (39). Interestingly, we showed that increased production of HMOX1 was associated with a lower generation of myomirs also in human rhabdomyosarcoma cell lines. Our results suggest that HMOX1 can be considered as a potential tool to modulate miRNAs.
We confirmed also the earlier suppositions that pharmacological or genetic activation of HMOX1 may improve the survival of transplanted myoblasts (20, 26, 31). Such improvement has been demonstrated in a pig model of myoblast transplantation (20). Better survival and enhanced engraftment of myoblasts were also observed after a heat shock treatment, a possible inducer of HMOX1 (9, 27, 37). On the other hand, our findings highlight a potential limitation of such strategy—the risk of hyperplastic growth and attenuated differentiation of myogenic precursors. Tumorogenic potential of C2C12 cells has been noticed before (e.g., after a 5-week observation period and transplantation of a high number of cells) (44). In our model, the formation of hyperplastic tissue was not spontaneous, but resulted from overexpression of HMOX1, as it was completely absent in animals treated with a control C2C12-Luc-GFP cell line. Noteworthy, Jia and coworkers (16) used a very similar model of C2C12 cells transduced retrovirally to express luciferase and GFP reporter genes with or without erythopoietin receptor (EpoR). They demonstrated the improvement in cell differentiation, which can further confirm that observed effects are dependent on the transgene, not on the cell line or viral vector.
We do not exclude that risk of uncontrolled proliferation will be lower or even negligible in primary satellite cells transplanted to the immunocompetent hosts. Moreover, among different myogenic subpopulations tested, the activated, highly proliferating, and less differentiated muscle precursors gave the best clinical outcomes in tissue regeneration (14). We postulate, however, that such a potential risk should be taken into consideration and should be excluded before a use of HMOX1 as a cytoprotective factor in clinical applications of muscle precursors.
To sum up, pharmacological or genetic activation of HMOX1 potently inhibits differentiation of myoblasts through a mechanism(s) dependent on HMOX1 enzymatic activity, CO-mediated inhibition of c/EBPδ binding to the myoD promoter, and changes in miRNA profile. This suggests that inhibition of HMOX1 might be proposed as a potential strategy to augment the maturation of myoblast precursors or to induce the differentiation some rhabdomyosarcomas.
Materials and Methods
Cell culture
The C2C12 cell line, a subclone of C3H cells derived from mouse limb muscle, is a model of activated satellite cells (4). In response to low serum conditions, they differentiate in vitro into multinuclear, spontaneously contracting myotubes. Similarly as satellite cells they are able to develop into adipocytes or osteoblasts (49).
C2C12-Luc-GFP control cells were obtained by transduction of wild-type cells with the retroviral vector containing a luciferase-IRES-GFP construct driven by CMV promoter. The cell line was purified by cell sorting for GFP expression using a MoFlo High-Performance Cell Sorter (Dako, Carpinteria, CA). C2C12-Luc-GFP-HO1 cells were produced after second transduction of C2C12-Luc-GFP cells with the retroviral vector harboring a human HMOX1 cDNA under control of CMV promoter, purified by selection on G418 antibiotic. For a routine culture, the Dulbecco's modified Eagle's medium (DMEM) supplemented with glucose (25 mmol/L), 10% of FCS, penicillin (100 U/mL), and streptomycin (100 μg/mL) was used (Growth medium, GrM). C2C12-Luc-GFP-HO1 cells were additionally supplemented with G418 (0.4 mg/mL).
To induce differentiation, cells were seeded in GrM (100,000 cells/well in 24-well plate). Next day, GrM was replaced with differentiation medium (DfM), the DMEM, supplemented with glucose (25 mmol/L), 2% of horse serum, penicillin (100 U/mL), and streptomycin (100 μg/mL), and cells were further incubated for 5 days. Primary muscle satellite cells were isolated according to a pre-plate technique described elsewhere (33). Experiments were performed on pre-plate no. 6 (PP6) which represented a population of muscle-derived cells enriched in satellite cells.
Human rhabdomyosarcoma (RMS) cell lines RD (embryonic RMS, with very low level of HMOX1), RH18 (alveolar RMS, with moderate level of HMOX1), and RH28 (alveolar RMS, with high level of HMOX1) were cultured in RPMI-1640 medium supplemented with penicillin (100 IU/mL), streptomycin (10 μg/mL), and 10% FCS.
Animal models
All procedures were performed in accordance with national and European legislations, after approval by the Local Ethical Committee for Animal Experimentation in Krakow. Animals were kept in standard conditions with water and food available ad libitum.
NOD/SCID mice (8–10-week-old) were subjected to C2C12-Luc-GFP or C2-C12-Luc-GFP-HO1 cell transplantation (10 animals per group). First, the cells were trypsinized, centrifuged (1000 g, 5 min, 4°C), and resuspended in sterile PBS. Each mouse received 2.5×105 of cells in 25 μL of PBS injected into gastrocnemius muscles of both legs. All mice were provided with analgesia (Buprenorphine, 0.5 mg/kg every second day), and sacrificed on day 11 (5 animals) and 22 (5 animals).
Statistical analysis
Unpaired or paired Student's t-tests, α ≤0.05, were used to assess whether the means of two normally distributed groups differed significantly. One-way ANOVA analysis with Bonferroni's multiple comparison post-test was employed to compare multiple groups. Significance is indicated as *p<0.05, **p<0.01, ***p<0.001. All error bars represent the standard mean errors.
Supplementary Material
Abbreviations Used
- ALS
amyotrophic lateral sclerosis
- C/EBP
CCAAT/Enhancer-binding protein
- CoPP
cobalt protoporphyrin-IX
- CORM
carbon monoxide releasing molecule
- CPK
creatinine phosphokinase
- DfM
differentiation medium
- DGCR8
DiGeorge syndrome critical region-8
- EpoR
erythropoietin receptor
- FCS
fetal calf serum
- FGF-BP
fibroblast growth factor binding protein
- GFP
green fluorescence protein
- GrM
growth medium
- HDAC4
histone deacetylase 4
- HMOX1
heme oxygenase-1
- HS
horse serum
- iCORM
inactive form of CORM
- Lin28
abnormal cell LINage
- MEF2
myocyte enhancer factor-2
- MHC
myosin heavy chain
- miRNA
microRNA
- MRFs
muscle regulatory factors
- Myf5
myogenic factor-5
- Myf6
myogenic factor-6
- MyoD
myogenic determination factor-1
- NAC
N-acetylcysteine
- RMS
human rhabdomyosarcoma
- ROS
reactive oxygen species
- SDF-1
stromal cell-derived factor
- SnPP
tin protioporphyrin-IX
- SRF
serum responsive factor
- VEGF
vascular endothelial growth factor
Acknowledgments
The rhabdomyosarcoma cell lines were a gift from Dr. Peter Houghton, St Jude Children's Research Hospital, Memphis, TN. Breeding pairs of HMOX1 +/− mice were kindly gifted by Dr. Anupam Agarwal, University of Alabama, Birmingham, AL. Access to a confocal microscope was kindly provided by Dr. Jerzy Dobrucki. This work was supported by Grants N301 08032/3156, N301 144336, 347/N-INCA/2008, 311/N-COST/2008/0 from Ministry of Science and Higher Education, POIG 01 01.02.00-109/09 and 01 01.02.00-069/09 from the EU Structural Funds, and by PhD grant from Adamed Ltd. AJ was a recipient of the Wellcome Trust Senior Research Fellowship in Biomedical Science. HW is a recipient of START fellowship from Foundation for Polish Science. AL is a recipient of Fellowship for Young Scientists funded by Ministry of Science and Higher Education. The Faculty of Biochemistry, Biophysics and Biotechnology of the Jagiellonian University is a beneficiary of the structural funds from the European Union (Grant No: POIG.02.01.00-12-064/08, 02.02.00-00-014/08).
Author Disclosure Statement
All authors declare no competing financial interest.
References
- 1.Braun T. Rudnicki MA. Arnold HH. Jaenisch R. Targeted inactivation of the muscle regulatory gene Myf-5 results in abnormal rib development and perinatal death. Cell. 1992;71:369–382. doi: 10.1016/0092-8674(92)90507-9. [DOI] [PubMed] [Google Scholar]
- 2.Cao YA. Wagers AJ. Karsunky H. Zhao H. Reeves R. Wong RJ. Stevenson DK. Weissman IL. Contag CH. Heme oxygenase-1 deficiency leads to disrupted response to acute stress in stem cells and progenitors. Blood. 2008;112:4494–4502. doi: 10.1182/blood-2007-12-127621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chauveau C. Remy S. Royer PJ. Hill M. Tanguy-Royer S. Hubert FX. Tesson L. Brion R. Beriou G. Gregoire M. Josien R. Cuturi MC. Anegon I. Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood. 2005;106:1694–1702. doi: 10.1182/blood-2005-02-0494. [DOI] [PubMed] [Google Scholar]
- 4.Charge SB. Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 5.Chen JF. Mandel EM. Thomson JM. Wu Q. Callis TE. Hammond SM. Conlon FL. Wang DZ. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38:228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dagner R. Helman L. Rhabdomyosarcoma: An overview. The Oncologist. 1999;4:34–44. [PubMed] [Google Scholar]
- 7.Deshane J. Chen S. Caballero S. Grochot-Przeczek A. Was H. Li Calzi S. Lach R. Hock TD. Chen B. Hill-Kapturczak N. Dulak J. Jozkowicz A. Grant M. Agarwal A. Stromal cell-derived factor 1 promotes angiogenesis via a heme oxygenase 1-dependent mechanism. J Exp Med. 2007;204:605–618. doi: 10.1084/jem.20061609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dulak J. Jozkowicz A. Foresti R. Kasza A. Frick M. Huk I. Green CJ. Pachinger O. Weidinger F. Motterlini R. Heme oxygenase-1 activity modulates vascular endothelial growth factor synthesis in vascular smooth muscle cells. Antioxid Redox Signal. 2002;4:229–240. doi: 10.1089/152308602753666280. [DOI] [PubMed] [Google Scholar]
- 9.Essig DA. Borger DR. Jackson DA. Induction of heme oxygenase-1 (HSP32) mRNA in skeletal muscle following contractions. Am J Physiol. 1997;272:C59–67. doi: 10.1152/ajpcell.1997.272.1.C59. [DOI] [PubMed] [Google Scholar]
- 10.Faller M. Matsunaga M. Yin S. Loo JA. Guo F. Heme is involved in microRNA processing. Nat Struct Mol Biol. 2007;14:23–29. doi: 10.1038/nsmb1182. [DOI] [PubMed] [Google Scholar]
- 11.Fulle S. Protasi F. Di Tano G. Pietrangelo T. Beltramin A. Boncompagni S. Vecchiet L. Fano G. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp Gerontol. 2004;39:17–24. doi: 10.1016/j.exger.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Gozzelino R. Jeney V. Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323–354. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- 13.Grochot-Przeczek A. Lach R. Mis J. Skrzypek K. Gozdecka M. Sroczynska P. Dubiel M. Rutkowski A. Kozakowska M. Zagorska A. Dulak J. Jozkowicz A. Heme oxygenase-1 accelerates cutaneous wound healing in mice. PLoS One. 2009;4:e5803. doi: 10.1371/journal.pone.0005803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashimoto N. Murase T. Kondo S. Okuda A. Inagawa-Ogashiwa M. Muscle reconstitution by muscle satellite cell descendants with stem cell-like properties. Development. 2004;131:5481–5490. doi: 10.1242/dev.01395. [DOI] [PubMed] [Google Scholar]
- 15.Jazwa A. Loboda A. Golda S. Cisowski J. Szelag M. Zagorska A. Sroczynska P. Drukala J. Jozkowicz A. Dulak J. Effect of heme and heme oxygenase-1 on vascular endothelial growth factor synthesis and angiogenic potency of human keratinocytes. Free Radic Biol Med. 2006;40:1250–1263. doi: 10.1016/j.freeradbiomed.2005.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia Y. Warin R. Yu X. Epstein R. Noguchi CT. Erythropoietin signaling promotes transplanted progenitor cell survival. FASEB J. 2009;23:3089–3099. doi: 10.1096/fj.09-130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim HK. Lee YS. Sivaprasad U. Malhotra A. Dutta A. Muscle-specific microRNA miR-206 promotes muscle differentiation. J Cell Biol. 2006;174:677–687. doi: 10.1083/jcb.200603008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuang W. Kuroda K. Le Grand F. Rudnicki MA. Cyclic stretch induced miR-146a upregulation delays C2C12 myogenic differentiation through inhibition of Numb. Biochem Biophys Res Commun. 2009;378:259–263. doi: 10.1016/j.bbrc.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 19.Lang D. Reuter S. Buzescu T. August C. Heidenreich S. Heme-induced heme oxygenase-1 (HMOX1) in human monocytes inhibits apoptosis despite caspase-3 up-regulation. Int Immunol. 2005;17:155–165. doi: 10.1093/intimm/dxh196. [DOI] [PubMed] [Google Scholar]
- 20.Laumonier T. Yang S. Konig S. Chauveau C. Anegon I. Hoffmeyer P. Menetrey J. Lentivirus mediated HMOX1 gene transfer enhances myogenic precursor cell survival after autologous transplantation in pig. Mol Ther. 2008;16:404–410. doi: 10.1038/sj.mt.6300354. [DOI] [PubMed] [Google Scholar]
- 21.Lee HJ. Jeong GS. Pi SH. Lee SI. Bae WJ. Kim SJ. Lee SK. Kim EC. Heme oxygenase-1 protects human periodontal ligament cells against substance P-induced RANKL expression. J Periodontal Res. 2010;45:367–374. doi: 10.1111/j.1600-0765.2009.01247.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee SK. Lee CY. Kook YA. Lee SK. Kim EC. Mechanical stress promotes odontoblastic differentiation via the heme oxygenase-1 pathway in human dental pulp cell line. Life Sci. 2010;86:107–114. doi: 10.1016/j.lfs.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Lin TH. Tang CH. Hung SY. Liu SH. Lin YM. Fu WM. Yang RS. Upregulation of heme oxygenase-1 inhibits the maturation and mineralization of osteoblasts. J Cell Physiol. 2010;222:757–768. doi: 10.1002/jcp.22008. [DOI] [PubMed] [Google Scholar]
- 24.Liu N. Williams AH. Kim Y. McAnally J. Bezprozvannaya S. Sutherland LB. Richardson JA. Bassel-Duby R. Olson EN. An intragenic MEF2-dependent enhancer directs muscle-specific expression of microRNAs 1 and 133. Proc Natl Acad Sci USA. 2007;104:20844–20849. doi: 10.1073/pnas.0710558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loboda A. Jazwa A. Grochot-Przeczek A. Rutkowski AJ. Cisowski J. Agarwal A. Jozkowicz A. Dulak J. Heme oxygenase-1 and the vascular bed: From molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2008;10:1767–1812. doi: 10.1089/ars.2008.2043. [DOI] [PubMed] [Google Scholar]
- 26.Ma J. Lau CK. Obed A. Dada A. Doenecke A. Fan ST. Schlitt HJ. Tsui TY. Cell penetrating heme oxygenase protein protects heart graft against ischemia/reperfusion injury. Gene Ther. 2009;16:320–328. doi: 10.1038/gt.2008.162. [DOI] [PubMed] [Google Scholar]
- 27.Maglara AA. Vasilaki A. Jackson MJ. McArdle A. Damage to developing mouse skeletal muscle myotubes in culture: Protective effect of heat shock proteins. J Physiol. 2003;548:837–846. doi: 10.1113/jphysiol.2002.034520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martello G. Rosato A. Ferrari F. Manfrin A. Cordenonsi M. Dupont S. Enzo E. Guzzardo V. Rondina M. Spruce T. Parenti AR. Daidone MG. Bicciato S. Piccolo S. A microRNA targeting dicer for metastatic control. Cell. 2010;141:1195–1207. doi: 10.1016/j.cell.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Min KS. Lee YM. Hong SO. Kim EC. Simvastatin promotes odontoblastic differentiation and expression of angiogenic factors via heme oxygenase-1 in primary cultured human dental pulp cells. J Endod. 2010;36:447–452. doi: 10.1016/j.joen.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 30.Odemis V. Boosmann K. Dieterlen MT. Engele J. The chemokine SDF1 controls multiple steps of myogenesis through atypical PKCzeta. J Cell Sci. 2007;120:4050–4059. doi: 10.1242/jcs.010009. [DOI] [PubMed] [Google Scholar]
- 31.Ollinger R. Pratschke J. Role of heme oxygenase-1 in transplantation. Transpl Int. 2010;23:1071–1081. doi: 10.1111/j.1432-2277.2010.01158.x. [DOI] [PubMed] [Google Scholar]
- 32.Parise G. O'Reilly CE. Rudnicki MA. Molecular regulation of myogenic progenitor populations. Appl Physiol Nutr Metab. 2006;31:773–781. doi: 10.1139/h06-055. [DOI] [PubMed] [Google Scholar]
- 33.Qu Z. Huard J. Matching host muscle and donor myoblasts for myosin heavy chain improves myoblast transfer therapy. Gene Ther. 2000;7:428–437. doi: 10.1038/sj.gt.3301103. [DOI] [PubMed] [Google Scholar]
- 34.Rao PK. Kumar RM. Farkhondeh M. Baskerville S. Lodish HF. Myogenic factors that regulate expression of muscle-specific microRNAs. Proc Natl Acad Sci USA. 2006;103:8721–8726. doi: 10.1073/pnas.0602831103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Remy S. Blancou P. Tesson L. Tardif V. Brion R. Royer PJ. Motterlini R. Foresti R. Painchaut M. Pogu S. Gregoire M. Bach JM. Anegon I. Chauveau C. Carbon monoxide inhibits TLR-induced dendritic cell immunogenicity. J Immunol. 2009;182:1877–1884. doi: 10.4049/jimmunol.0802436. [DOI] [PubMed] [Google Scholar]
- 36.Riederer I. Negroni E. Bigot A. Bencze M. Di Santo J. Aamiri A. Butler-Browne G. Mouly V. Heat shock treatment increases engraftment of transplanted human myoblasts into immunodeficient mice. Transplant Proc. 2008;40:624–630. doi: 10.1016/j.transproceed.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 37.Suh GY. Jin Y. Wang XM. Choi AM. CCAAT/enhancer-binding protein mediates carbon monoxide-induced suppression of cyclooxygenase-2. Am J Respir Cell Mol Biol. 2006;35:220–226. doi: 10.1165/rcmb.2005-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tapscott SJ. Lassar AB. Weintraub H. A novel myoblast enhancer element mediates myoD transcription. Mol Cell Biol. 1992;12:4994–5003. doi: 10.1128/mcb.12.11.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taulli R. Bersani F. Foglizzo V. Linari A. Vigna E. Ladanyi M. Tuschl T. Ponzetto C. The muscle-specific microRNA miR-206 blocks human rhabdomyosarcoma growth in xenotransplanted mice by promoting myogenic differentiation. J Clin Invest. 2009;119:2366–2378. doi: 10.1172/JCI38075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tedesco FS. Dellavalle A. Diaz-Manera J. Messina G. Cossu G. Repairing skeletal muscle: Regenerative potential of skeletal muscle stem cells. J Clin Invest. 2010;120:11–19. doi: 10.1172/JCI40373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Degenfeld G. Banfi A. Springer ML. Blau HM. Myoblast-mediated gene transfer for therapeutic angiogenesis and arteriogenesis. Br J Pharmacol. 2003;140:620–626. doi: 10.1038/sj.bjp.0705492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uc A. Britigan BE. Does heme oxygenase-1 have a role in Caco-2 cell cycle progression? Exp Biol Med. 2003;228:590–595. doi: 10.1177/15353702-0322805-52. [DOI] [PubMed] [Google Scholar]
- 43.Vanella L. Kim DH. Asprinio D. Peterson SJ. Barbagallo I. Vanella A. Goldstein D. Ikehara S. Kappas A. Abraham NG. HMOX1 expression increases mesenchymal stem cell-derived osteoblasts but decreases adipocyte lineage. Bone. 2010;46:236–243. doi: 10.1016/j.bone.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wernig A. Irintchev A. Hartling A. Stephan G. Zimmermann K. Starzinski-Powitz A. Formation of new muscle fibres and tumours after injection of cultured myogenic cells. J Neurocytol. 1991;20:982–997. doi: 10.1007/BF01187916. [DOI] [PubMed] [Google Scholar]
- 45.Williams AH. Ning L. van Rooij E. Olson EN. MicroRNA control of muscle development and disease. Curr Opin Cell Biol. 2009;21:461–469. doi: 10.1016/j.ceb.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams AH. Valdez G. Moresi V. Qi X. McAnally J. Elliott JL. Bassel-Duby R. Sanes JR. Olson EN. MicroRNA-206 delays ALS progression and promotes regeneration of neuromuscular synapses in mice. Science. 2009;326:1549–1554. doi: 10.1126/science.1181046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan D. Xda E. Chen X. Wang L. Lu C. Wang J. Qu J. Tu L. MicroRNA-1/206 targets c-Met and inhibits rhabdomyosarcoma development. J Biol Chem. 2009;284:29596–29604. doi: 10.1074/jbc.M109.020511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamashita K. Ollinger R. McDaid J. Sakahama H. Wang H. Tyagi S. Csizmadia E. Smith NR. Soares MP. Bach FH. Heme oxygenase-1 is essential for and promotes tolerance to transplanted organs. FASEB J. 2006;20:776–778. doi: 10.1096/fj.05-4791fje. [DOI] [PubMed] [Google Scholar]
- 49.Yeow K. Phillips B. Dani C. Cabane C. Amri EZ. Derijard B. Inhibition of myogenesis enables adipogenic trans-differentiation in the C2C12 myogenic cell line. FEBS Lett. 2001;506:157–162. doi: 10.1016/s0014-5793(01)02900-3. [DOI] [PubMed] [Google Scholar]
- 50.Zammit PS. Partidge TA. Yablonka-Reuvenim Z. The skeletal muscle satellite cell: The stem cell that came in from the cold. J Histochem Cytochem. 2006;54:1177–1191. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]
- 51.Zhen-Wei X. Jian-Le S. Qi Q. Wen-Wei Z. Xue-Hong Z. Li Z. Heme oxygenase-1 improves the survival of discordant cardiac xenograft through its anti-inflammatory and anti-apoptotic effects. Pediatr Transplant. 2007;11:850–859. doi: 10.1111/j.1399-3046.2007.00701.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.