Abstract
Background. Women diagnosed with cervical cancer report longer duration and more recent use of combined oral contraceptives (COCs). It is unclear how COC use impacts risk of cervical carcinogenesis.
Methods. We estimated the risk of new human papillomavirus (HPV) DNA detection and persistence among 1135 human immunodeficiency virus (HIV)–negative women aged 20–37 years from Thailand who were followed for 18 months at 6-month intervals. Type-specific HPV DNA, demographic information, hormonal contraceptive use, sexual behavior, genital tract coinfection, and Papanicolaou test results were assessed at baseline and each follow-up.
Results. Women who reported current COC use during follow-up were less likely to clear HPV infection compared with nonusers, independent of sexual behavior, and Papanicolaou test diagnosis (AHR: 0.67 [95% CI: .49–.93]). Similar associations were not observed among women reporting current use of depomedroxyprogesterone acetate (DMPA). Neither COC nor DMPA use was significantly associated with new HPV DNA detection.
Conclusions. These data do not support the hypothesis that contraceptive use is associated with cervical cancer risk via increased risk of HPV acquisition. The increased risk of HPV persistence observed among current COC users suggests a possible influence of female sex hormones on host response to HPV infection.
The risk of human papillomavirus (HPV)–associated cervical cancer has been shown to be significantly higher among women reporting longer (>5 years) and more recent use of combined oral contraceptives (COCs) [1, 2]. Multiple theories have been advanced to explain the association between long-term oral contraceptive use and cervical cancer risk, including increased risk of high-risk (HR) HPV infection among oral contraceptive users, a direct carcinogenic effect of estrogen and progesterone by upregulation of viral oncogene expression [3–8], and the ability for estrogens to promote tumor growth and persistence [9–12].
We have previously reported that COC use for >6 years was associated with increased HPV prevalence in a cohort of women aged 20–37 years in Thailand [13]. The increased risk associated with prevalent infection suggests that long-duration COC use increases the risk of HPV acquisition, HPV persistence, or both. The current study aims to evaluate these alternatives by estimating the risk of new HPV detection and HPV clearance in the same population of Thai women during 18 months of semiannual follow-up.
METHODS
Study Population and Enrollment
Women attending family planning clinics in northern (Chiang Mai), northeast (Khon Kaen), central (Bangkok) and southern (Songkhla and HatYai) Thailand between 2002 and 2003 were recruited into a prospective study to assess the natural history of HPV and cervical intraepithelial neoplasia grade 2/3 (CIN 2/3), and were between 20 and 37 years of age. These women were originally enrolled in a study designed to evaluate the effects of hormonal contraceptive use on HIV acquisition (HC-HIV). Selection criteria are described in detail elsewhere [14]. Briefly, inclusion criteria for enrollment in the HC-HIV study were as follows: (1) HIV negative, (2) not pregnant, (3) intact uterus, (4) used some form of contraception within 3 months prior to enrollment, and (5) willing to adhere to self-selected contraceptive method for at least 1 year of follow-up. Among women who participated in the HC-HIV study, 79% were reconsented for inclusion in the extension study (n = 1256). The study protocols were reviewed and approved by the committees on human subject research at Johns Hopkins Bloomberg School of Public Health, Baltimore, MD; Merck & Co. Inc., West Point, PA; each participating recruitment site; and the Institutional Review Board of the Thailand Ministry of Public Health (MoPH), Thailand.
At enrollment and each follow-up visit, information on sociodemographic characteristics, sexual risk behavior, reproductive and contraceptive history, current contraceptive use, self-reported medical history, and woman’s report of the sexual behavior of her partner was collected at each study site by trained interviewers using a standardized questionnaire.
Sexual behavior variables measured include age of sexual debut, lifetime number of sexual partners number of recent sexual partners, recently acquired new sexual partners, primary partner sexual risk behaviors, lifetime and recent commercial sex work, frequency of sexual intercourse and frequency of male/female condom use per sexual act. Reproductive information including recent pregnancy and total number of live births was collected. There was little variability in the formulation of hormonal contraception used among the study participants. Therefore, contraceptive use was broadly classified as (1) combined low-dose oral contraceptives (COCs), (2) depomedroxyprogesterone acetate (DMPA), or (3) nonhormonal contraceptive use and nonuse (NHC). Current use and duration of use prior to enrollment for each category of hormonal contraception was assessed.
Laboratory-confirmed sexually transmitted infections (STIs) during the 2 years prior to study enrollment (ie, STIs diagnosed in the HC-HIV parent study) included gonorrhea (GC), chlamydia (CT), syphilis, and trichomoniasis.
Physical Examination and Specimen Collection
At enrollment and at each 6-month follow-up, each participant underwent a pelvic examination. Exfoliated cervical cells were collected using a cytobrush and placed in PreservCyt for Thinprep liquid-based cytology. Bacterial vaginosis was diagnosed based on the Amsel criteria [15]. Cervical ectopy was identified at the 6-, 12-, and 18-month follow-up visit by clinical assessment of the cervical transformation zone after application of acetic acid. Diagnosis of current CT and/or GC from ectocervical mucus specimens was performed using the Roche Amplicor assay per manufacturer’s instructions (Roche Molecular Systems). At enrollment and each 6-month follow-up, an endo-/ectocervical swab specimen was collected for HPV DNA genotyping using a Dacron swab stored in specimen transport medium (STM; Digene), stored at −20°C until testing.
All Papanicolaou test specimens were read by trained cytopathologists (Covance). Cytological smears were classified according to the Bethesda system [16]. Participants with a Papanicolaou test of atypical squamous cells of undetermined significance (ASC-US) or more severe diagnosis were referred for colposcopic examination, with biopsy and treatment as indicated.
HPV DNA Testing
All HPV DNA testing was performed on cervical cell samples stored in STM at Johns Hopkins University, Baltimore, Maryland. DNA was extracted using the QIAamp DNA Blood Kit (Qiagen) according to manufacturer’s instructions with modification. After extraction, 8 μL DNA (4% of total volume of extracted DNA) was tested using the Roche HPV Linear Array PCR assay (Roche Diagnostics). The HPV Linear Array is based on the PGMY09/11 primer system that allows for high-efficiency amplification of >40 types of HPV [17, 18]. The quality and validity of the extracted DNA specimen was assessed by inclusion of β-globin gene-specific primers in the polymerase chain reaction (PCR). Only specimens with detectable β-globin were used in this analysis.
HPV types considered to be high risk (HR-HPV) for this analysis included types 16, 18, 31, 33, 35, 39, 45, 51, 52, 55, 56, 58, 59, 68, 73, and 82 [19, 20].
Statistical Analysis
The outcomes of interest for this analysis were new detection and clearance (ie, loss of detection) of type-specific HPV infection. The primary exposure in this analysis was current use of hormonal contraceptive, categorized as either “COC” or “DMPA” compared with NHC during the same interval. Duration of prior use was categorized into “never,” “<4 years,” “4–6 years,” and “>6 years” based on the observed distribution of responses, as well as the previously observed associations of hormonal contraceptive use and cervical cancer [21].
New detection of HPV was defined as the first positive result at a given follow-up visit after a negative result for the same HPV type. Correspondingly, clearance was defined as the first negative PCR result after either prevalent or new HPV infection. In the new detection analysis, the change in HPV PCR status between a given follow-up visit (t) and the previous visit (t – 1) was assessed using visit-pairs as the unit of analysis [22]. In the analysis of clearance, women could contribute 1 or more HPV infections; therefore, HPV type-specific infections instead of individuals were used as the unit of analysis. For both analyses, type-specific infections were analyzed in groups categorized as any HPV and any HR-HPV infection.
Generalized estimating equations for binary outcomes with a logit link were used to estimate odds ratios for the association of current hormonal contraceptive use on new detection of HPV [23]. The potential correlation of repeated measures of HPV status and contraceptive use within the same woman was taken into account by defining an exchangeable correlation structure within the regression model.
Cox proportional hazards models were constructed to evaluate the association of current hormonal contraceptive use on clearance of type-specific HPV infection. The origin of this analysis was the visit of first HPV detection. Women who remained HPV positive at the end of follow-up were censored at that visit. Women with histological diagnosis of CIN 2/3 during follow-up were censored at the visit of diagnosis. Hazard ratios <1.0 indicate a decreased risk for clearance or conversely, an increased risk for viral persistence.
A multivariate model for each analysis was constructed using backward stepwise removal. Covariates having a P value < .1 by univariate analysis were considered for inclusion in the multivariate model. If, upon removal, the covariate showed a >10% change in the effect size of the primary exposure and outcome association or had a P value of < .05, the covariate was retained in the final model. All analyses were conducted using STATA 11 (Stata).
A total of 1256 women were enrolled for follow-up and contributed a total of 4876 visits. Twenty-one women (1.7%) contributed only a single visit and were therefore excluded from the analysis due to being lost to follow-up. There were 11 women with CIN 2/3 at enrollment who were excluded. In order to better control for the potential effects of unmeasured sexual behavior on virologic outcomes, women engaging in commercial sex work in the 6 months prior to enrollment (n = 42) or during follow-up (n = 8) were also excluded from the analyses. Additionally, women with missing parity information (n = 33) and bacterial STI information (n = 1) at enrollment and follow-up (n = 5) were excluded. Therefore, 1135 women contributed a total number of 4327 visits to the analysis of new HPV detection and clearance. The mean follow-up time was 16.6 months (±1.5) with a mean interval of 5.5 months (±0.89) between visits.
RESULTS
Study Population
The mean age at enrollment of the study population was 29.6 years (±4.3). Among women included in the analysis, 376 (33.1%), 331 (29.2%), and 428 (37.7%) reported using COC, DMPA, or NHC, respectively, in the 6 months prior to enrollment (Table 1). Women who reported using DMPA or COC compared with nonhormonal methods or no contraception were more likely to be younger (P < .001), to report a single live birth prior to enrollment (P < .001), to report a single sex partner in the last 6 months (P < .001), and to have used their current hormonal contraceptive method for >6 years (P < .001). Women using COCs were less likely to report >1 sexual partner in the last 6 months (P = .001). Both DMPA (P = .009) and COC (P = .016) users were less likely to report use of male condoms in the 6 months prior to enrollment compared with women reporting NHC. Women who reported using DMPA or COC compared with NHC were less likely to be from the northeast study site (P < .001), and to be diagnosed with bacterial vaginosis at enrollment (P = .007). There was no difference in cytological diagnosis at enrollment, smoking history, or previous history of GC, CT, or syphilis across contraceptive groups. Women who reported DMPA use at enrollment were more likely to have Papanicolaou test results of ASC-US or high-grade squamous intraepithelial lesions (HSIL) compared with women reporting use of nonhormonal methods (P = .016). Women who reported COC use at enrollment were more likely to be diagnosed with cervical ectopy at their 6-month follow-up visit (P = .006).
Table 1.
Baseline Characteristics of Study Participants by Contraceptive Use at Enrollment
| Variable | NHC (n = 428) n (%) | DMPA (n = 331) n (%) | P valuea | COC (n = 376) n (%) | P valueb |
| Age | |||||
| <26 | 36 (8.4) | 90 (27.2) | P < .001 | 80 (21.3) | P < .001 |
| 26–30 | 137 (32.0) | 108 (32.6) | 156 (41.5) | ||
| 31–33 | 110 (25.7) | 73 (22.1) | 84 (22.3) | ||
| 34–38 | 145 (33.9) | 60 (18.1) | 56 (14.9) | ||
| Study site | |||||
| North (Chiang Mai) | 153 (35.8) | 114 (34.4) | P = .003 | 149 (39.6) | P < .001 |
| Northeast (Khon Kaen) | 140 (32.7) | 75 (22.7) | 45 (11.9) | ||
| South (Songkhla and Hat Yai) | 81 (18.9) | 93 (28.1) | 114 (30.3) | ||
| Central (Bangkok) | 54 (12.6) | 49 (14.8) | 68 (18.1) | ||
| Total no. of live births | |||||
| 0 | 7 (1.6) | 1 (0.3) | P < .001 | 14 (3.7) | P < .001 |
| 1 | 107 (25.0) | 212 (64.1) | 247 (65.7) | ||
| >1 | 314 (73.4) | 118 (35.7) | 115 (30.6) | ||
| Duration of COC use prior to enrollment | |||||
| Never | 92 (21.5) | 93 (28.1) | P = .028 | 0 (0) | P < .001 |
| <4 years | 260 (60.8) | 201 (60.7) | 116 (30.9) | ||
| 4–6 years | 67 (15.7) | 34 (10.3) | 189 (50.3) | ||
| >6 years | 9 (2.1) | 3 (0.9) | 71 (18.9) | ||
| Duration of DMPA use prior to enrollment | |||||
| Never | 150 (35.1) | 0 (0) | P < .001 | 105 (27.9) | P < .001 |
| <4 years | 237 (55.4) | 95 (28.7) | 196 (52.1) | ||
| 4–6 years | 38 (8.9) | 188 (56.8) | 71 (18.9) | ||
| >6 years | 3 (0.7) | 48 (14.5) | 4 (1.1) | ||
| Male condom use L6M | |||||
| No | 340 (79.4) | 287 (86.7) | P = .009 | 323 (85.9) | p = .016 |
| Yes | 88 (20.6) | 44 (13.3) | 53 (14.1) | ||
| Age of sexual debut | |||||
| >20 | 175 (40.9) | 129 (38.9) | P = .401 | 140 (37.2) | P = .286 |
| 17–19 | 198 (46.3) | 148 (44.7) | 174 (46.3) | ||
| <17 | 55 (12.9) | 54 (16.3) | 62 (16.5) | ||
| No. of lifetime partners | |||||
| 1 | 318 (74.3) | 255 (77.0) | P = .215 | 262 (69.7) | P = .116 |
| 2 | 55 (12.9) | 47 (14.2) | 70 (18.6) | ||
| 3 | 25 (5.8) | 17 (5.1) | 16 (4.3) | ||
| ≥4 | 30 (7.0) | 12 (3.6) | 28 (7.5) | ||
| No. of partners L6M | |||||
| 0 | 19 (4.4) | 6 (1.8) | P = .129 | 1 (0.3) | P < .001 |
| 1 | 407 (95.1) | 323 (97.6) | 374 (99.5) | ||
| >1 | 2 (0.5) | 2 (0.6) | 1 (0.3) | ||
| New partner L12M | |||||
| No | 406 (99.3) | 319 (98.2) | P = .174 | 372 (99.2) | P = .915 |
| Yes | 3 (0.7) | 6 (1.9) | 3 (0.8) | ||
| Smoked cigarettes L6M | |||||
| No | 418 (97.7) | 317 (95.8) | P = .139 | 366 (97.3) | P = .769 |
| Yes | 10 (2.3) | 14 (4.2) | 10 (2.7) | ||
| Cytology at enrollment | |||||
| Normal | 394 (92.1) | 296 (89.4) | P = .603 | 345 (91.8) | P = .683 |
| Inflammation | 3 (0.7) | 6 (1.8) | 6 (1.6) | ||
| ASC-US | 15 (3.5) | 15 (4.5) | 15 (3.9) | ||
| LSIL | 13 (3.0) | 11 (3.3) | 8 (2.1) | ||
| HSIL | 3 (0.7) | 3 (0.9) | 2 (0.5) | ||
| Worst cytology at follow-up | |||||
| Normal | 349 (81.5) | 239 (72.2) | P = .016 | 286 (76.1) | P = .107 |
| Inflammation | 7 (1.6) | 9 (2.7) | 12 (3.2) | ||
| ASC-US | 36 (8.4) | 47 (14.2) | 42 (11.2) | ||
| LSIL | 26 (6.1) | 20 (6.0) | 19 (5.1) | ||
| HSIL | 10 (2.3) | 16 (4.8) | 17 (4.5) | ||
| Bacterial vaginosis at enrollment | |||||
| Negative | 397 (92.8) | 322 (97.3) | P = .006 | 363 (96.5) | P = .019 |
| Positive | 31 (7.2) | 9 (2.7) | 13 (3.5) | ||
| Prior STI infectionc | |||||
| No | 368 (85.9) | 280 (84.6) | P = .591 | 317 (84.3) | P = .505 |
| Yes | 60 (14.0) | 51 (15.4) | 59 (15.7) | ||
| Cervical ectopyd | |||||
| No | 319 (74.5) | 220 (79.8) | P = .091 | 212 (65.7) | P = .006 |
| Yes | 109 (25.5) | 67 (20.2) | 129 (34.3) |
Abbreviations: ACS-US, atypical squamous cells of undetermined significance; COC, combined oral contraception; DMPA, depomedroxyprogesterone acetate; L6M, last 6 months; L12M, last 12 months; NHC, nonhormonal contraception.
Comparing DMPA users vs NHC users.
Comparing COC users vs NHC users.
Includes laboratory detection of chlamydia, gonorrhea, or syphilis.
Diagnosed at 6-month follow-up.
Hormonal Contraception and New Detection of HPV
The 1135 study participants contributed a total of 3376 visit-pairs to the analysis of new detection of HPV. Among these visits, 269 (8.0%) and 157 (4.7%) contained new detection of any type-specific HPV and any type-specific HR-HPV infection, respectively.
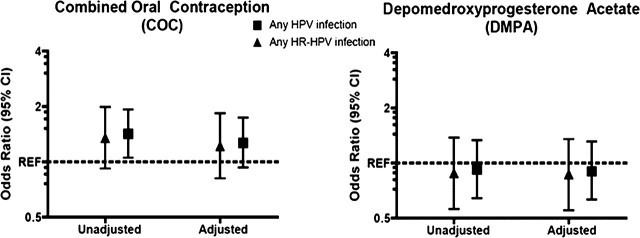
Newly detected HPV was more common among women reporting use of COCs since the last visit compared with NHC users (P = .011) in univariate analysis (Table 2; Figure 1). After adjustment for age, study site, number of lifetime and recent sexual partners, having a new sexual partner, concurrent diagnosis of bacterial vaginosis, and duration of COC and DMPA use, the association between newly detected HPV and COC use was attenuated and lost statistical significance (P = .125). Use of DMPA was not found to be associated with newly detected HPV infections in either univariate or multivariate analysis. Similar observations were observed after restriction to incident HR-HPV infections only.
Table 2.
Association of Demographic, Reproductive History, Contraceptive Use, Sexual Behavior, and Clinical Variables and Incident HPV and HR-HPV Infection Status
| Any HPV infection |
Any HR-HPV infection |
|||
| Variable | OR (95% CI) | aORa (95% CI) | OR (95% CI) | aORa (95% CI) |
| Age (y) | ||||
| <26 | 1.69 (1.13–2.52) | 2.02 (1.31–3.12) | 2.36 (1.43–3.89) | 2.23 (1.25–3.99) |
| 26–30 | 1.67 (1.16–2.39) | 1.56 (1.09–2.22) | 2.06 (1.28–3.30) | 1.90 (1.18–3.06) |
| 31–33 | 2.14 (1.38–3.29) | 1.58 (1.06–2.35) | 2.40 (1.36–4.25) | 2.18 (1.32–3.62) |
| 34–38 | 1.0 | 1.0 | 1.0 | 1.0 |
| Study site | ||||
| North | 1.0 | 1.0 | 1.0 | 1.0 |
| Northeast | 0.85 (.56–1.29) | 0.94 (.61–1.45) | 0.80 (.47–1.37) | 0.91 (.53–1.58) |
| South | 1.20 (.83–1.72) | 1.08 (.75–1.57) | 1.14 (.73–1.77) | 1.05 (.67–1.67) |
| Central | 2.15 (1.47–3.14) | 2.21 (1.52–3.21) | 2.04 (1.29–3.23) | 2.23 (1.41–3.53) |
| Contraceptive use | ||||
| NHC | 1.0 | 1.0 | 1.0 | 1.0 |
| DMPA | 0.92 (.64–1.33) | .90 (.63–1.31) | .88 (.56–1.38) | .87 (.55–1.35) |
| COC | 1.42 (1.05–1.92) | 1.27 (.93–1.74) | 1.35 (.92–1.99) | 1.22 (.81–1.83) |
| No. of lifetime partners | ||||
| 1 | 1.0 | 1.0 | 1.0 | 1.0 |
| 2 | 1.54 (1.08–2.20) | 1.48 (1.04–2.11) | 1.35 (.84–2.18) | 1.26 (.78–2.04) |
| 3 | 3.26 (1.99–5.34) | 3.21 (1.99–5.17) | 3.23 (1.85–5.64) | 3.23 (1.89–5.50) |
| ≥4 | 2.77 (1.82–4.23) | 2.23 (1.47–3.39) | 2.61 (1.53–4.45) | 2.03 (1.18–3.50) |
| No. of partners L6M | ||||
| 1 | 1.0 | 1.0 | 1.0 | 1.0 |
| >1 | 3.60 (1.34–9.71) | 1.41 (.33–6.05) | 4.98 (1.86–13.3) | 1.78 (.32–10.01) |
| New partner L12M | ||||
| No | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 3.31 (1.42–7.70) | 2.44 (.71–8.45) | 4.25 (1.84–9.78) | 2.62 (.64–10.6) |
| Cervical ectopy | ||||
| No | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 1.13 (.85–1.50) | 1.09 (.79–1.49) | 1.11 (.78–1.57) | 1.06 (.71–1.57) |
| Bacterial vaginosis | ||||
| No | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 2.56 (1.59–4.14) | 2.85 (1.74–4.67) | 3.07 (1.74–5.41) | 3.45 (1.96–6.08) |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; COC, combined oral contraception; DMPA, depomedroxyprogesterone acetate; HPV, human papillomavirus; HR-HPV, HPV types considered to be high risk; L6M, last 6 months; L12M, last 12 months; NHC, nonhormonal contraception; OR, odds ratio.
All variables mutually adjusted for.
Figure 1.
Odds ratios of the association of current hormonal contraceptive use and new detection of any and any HR-HPV type. Multivariate models are adjusted for age, study site, number of lifetime and recent sexual partners, having a new sexual partner, concurrent diagnosis of bacterial vaginosis, and presence of cervical ectopy. Reference category is women who report use of nonhormonal contraceptive methods or report nonuse.
Hormonal Contraception and HPV Clearance
Three hundred seventy-nine women contributed a total of 544 infections among which 346 (63.6%) cleared (lost detection) during follow-up. Among all infections, 273 (50.2%) were HR and 174 (63.7%) HR infections cleared during follow-up.
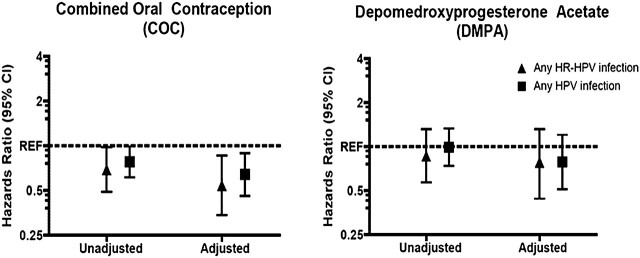
In univariate analysis of all HPV infections, women using COC were less likely to clear their infections as compared with NCH users (P = .048; Table 3; Figure 2). The direction, strength, and significance of the association remained when the analysis was restricted to oncogenic HPV infections only (P = .043). After adjustment for age, study site, number of lifetime sexual partners, cytology, incident vs prevalent HPV infection, and duration of COC and DMPA use, current COC use remained significantly associated with a reduced risk of any HPV clearance (P = .008) as well as oncogenic HPV clearance (P = .002). Current DMPA users were found to have a nonstatistically significant lower risk of clearance in both univariate and multivariate analysis.
Table 3.
Association of Demographic, Reproductive History, Contraceptive Use, Sexual Behavior, and Clinical Variables and Clearance of Any HPV and HR-HPV Infection
| Any HPV infection |
Any HR-HPV infection |
|||
| Variable | HR (95% CI) | aHRa (95% CI) | HR (95% CI) | aHRa (95% CI) |
| Age (y) | ||||
| <26 | 1.0 | 1.0 | 1.0 | 1.0 |
| 26–30 | 0.78 (.57–1.06) | 0.79 (.58–1.07) | 0.92 (.59–1.40) | 1.08 (.69–1.68) |
| 31–33 | 0.87 (.64–1.18) | 0.88 (.65–1.20) | 0.92 (.59–1.42) | 0.98 (.64–1.50) |
| 34–38 | 0.79 (.57–1.09) | 0.86 (.62–1.18) | 0.71 (.44–1.15) | 0.83 (.52–1.33) |
| Study site | ||||
| North | 1.0 | 1.0 | 1.0 | 1.0 |
| Northeast | 0.73 (.54–1.00) | 0.86 (.62–1.18) | 0.62 (.39–.98) | 0.92 (.56–1.50) |
| South | 0.94 (.72–1.24) | 0.99 (.75–1.31) | 0.97 (.66–1.44) | 1.15 (.78–1.70) |
| Central | 0.72 (.54–.95) | 0.73 (.54–.98) | 0.65 (.44–.98) | 0.87 (.57–1.34) |
| Contraceptive use | ||||
| NHC | 1.0 | 1.0 | 1.0 | 1.0 |
| DMPA | 0.99 (.74–1.33) | 0.75 (.50–1.13) | 0.86 (.57–1.31) | 0.78 (.45–1.37) |
| COC | 0.78 (.61–.99) | 0.67 (.49–.93) | 0.69 (.49–.98) | 0.58 (.37–.94) |
| No. of partners L6M | ||||
| 0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 1 | 0.78 (.43–1.42) | 1.12 (.48–2.58) | 0.52 (.13–2.10) | 2.45 (.11–53.0) |
| >1 | 1.46 (.56–3.82) | 1.02 (.37–2.85) | 0.70 (.13–3.70) | 3.13 (.13–75.0) |
| No. of lifetime partners | ||||
| 1 | 1.0 | 1.0 | 1.0 | 1.0 |
| 2 | 0.65 (.48–.87) | 0.61 (.45–.82) | 0.82 (.55–1.22) | 0.86 (.56–1.31) |
| 3 | 0.91 (.64–1.31) | 0.84 (.58–1.21) | 0.93 (.57–1.53) | 0.99 (.59–1.66) |
| ≥4 | 1.11 (.84–1.48) | 1.06 (.80–1.39) | 1.09 (.77–1.53) | 1.04 (.69–1.57) |
| Cervical ectopy | ||||
| No | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 0.88 (.70–1.11) | 0.85 (.62–1.17) | 0.78 (.56–1.09) | 0.76 (.49–1.18) |
| HPV infection type | ||||
| Prevalent | 1.0 | 1.0 | 1.0 | 1.0 |
| Incident | 2.86 (2.24–3.65) | 3.25 (2.55–4.15) | 2.78 (1.93–3.90) | 3.01 (2.08–4.34) |
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; COC, combined oral contraception; DMPA, depomedroxyprogesterone acetate; HPV, human papillomavirus; HR, hazard ratio; HR-HPV, HPV types considered to be high risk; L6M, last 6 months; NHC, nonhormonal contraception.
All variables mutually adjusted for.
Figure 2.
Hazard ratios for the association of current hormonal contraceptive use on risk of clearance of any HPV and any HR-HPV infection. Multivariate models are adjusted for age, study site, number of lifetime sexual partners, cytology, presence of cervical ectopy, incident vs prevalent HPV infection, and Papanicolaou test status at last visit. Reference category is women who report use of nonhormonal contraceptive methods or report nonuse.
CONCLUSIONS
We have previously reported an increase in HPV prevalence among women with long-duration (>6 years) COC use in a cohort of long-term hormonal contraceptive users from Thailand. During prospective follow-up of the same cohort, we show that current COC users had only a minor and nonstatistically significant 22% increased risk of having a new HPV DNA type detected during follow-up. In contrast, current COC users were 85% less likely to clear HR-HPV infection.
Inferring causal associations between COC use and HPV infection are difficult. Even when adjusting for sexual behavior, unmeasured behaviors (eg, underreporting or unknown risk from male sex partners) may result in residual confounding, and this could explain the residual increased risk of new HPV DNA detection observed in our study. Alternatively, a more biologically causal model has been hypothesized, where estrogen increases susceptibility to viral infection through maintenance of cervical ectopy [24–26]. We attempted to directly explore this hypothesis by measuring the presence of ectopy in this study using acetic acid application. While we did see that COC use was associated with a diagnosis of cervical ectopy at 6-month follow-up, ectopy was not associated with HPV clearance or new HPV detection in this study.
The association between HPV clearance and hormonal contraceptive use has not been consistent across studies. Studies of college-aged women [27, 28] and high-risk adolescent women [29] which had limited sample sizes (N < 400) have not found an association between HPV persistence and current COC use, whereas a larger study (N = 1166) conducted among women 20–29 years of age in Denmark with a longer history of exposure to COCs did report an association of current COC use with a reduced risk of viral clearance [30]. Only the latter study used a population-based sample; it is possible that selection bias in studies recruiting from college campuses and high-risk adolescents may explain some of the discrepancy. Another large population-based study [31] did not show an association between oral contraceptive use and HPV persistence; however, COC use was evaluated only as “ever/never” at baseline. Because both the association of COC use between both HPV and cervical cancer outcomes has been shown to be specific to timing and duration of use, misclassification of the relevant exposure in this study may have attenuated the results to the null.
Hormonal contraception has been hypothesized to facilitate HPV-mediated cervical carcinogenesis through a variety of pathways such as increased expression of viral oncogenes [3–8] and tumor promotion and persistence [9–11]. We have recently shown that human peripheral blood mononuclear cells stimulated with HPV 16 virus–like particles and treated with estradiol and progesterone have lower concentrations of interferon-γ, interleukin-12p70, and tumor necrosis factor–α production and increased concentrations of interleukin-10, transforming growth factor–β, and forkhead box p3 gene expression compared with nonhormone-treated cells [32]. These findings, taken together with the current observation of a decreased risk of HPV clearance among COC users, suggest that estrogen/progesterone may also increase the risk of invasive cervical cancer by suppressing the host immunologic response to infection. It will be important to consider this hypothesis in addition to more direct carcinogenic effects of sex hormones when developing and evaluating in vitro and animal models of hormone-mediated cancers.
Our study has several limitations that need to be considered in drawing inferences from our findings. First, this study had a sampling interval of 6 months; thus, some women may have acquired or cleared infections between the study visits which could result in an underestimation of the number of incident infections and an overestimation of viral persistence. However, the goal of this study was to assess the role of a specific exposure (hormonal contraceptive use) on the relative, not absolute, risk of clearance and new detection. Assuming that the overestimation in duration of infection is nondifferential with respect to hormonal contraceptive use categories, there should be minimal impact of this bias on estimates of relative risk. Second, given the relatively short follow-up (18 months) in this study, we had little power to detect a difference in HPV clearance among incident versus prevalent HPV infections. While the adjusted hazard ratio for HPV clearance among COC users was strengthened, not attenuated, after adjustment for infection status (ie, prevalent vs incident), it will be important to estimate risk of clearance among COC users in a larger study following initial detection. Third, even though every effort was made to control for the potential effects of sexual behavior (eg, excluding women who report commercial sex work), unmeasured sexual behaviors may still be confounding the observed associations. Fourth, condom use was significantly higher among the NHC use group compared with users of COC or DMPA. Because condom users in this study were more likely to report >4 lifetime sexual partners (11.4% vs 5.2%; P < .001) inclusion of condom users within the NHC use category may have led to an underestimation of the association of hormonal contraceptive use with new detection of HPV. However, when we excluded condom users from both the nonhormonal and hormonal contraceptive groups, the point estimates and significance of the association of hormonal contraceptive use with longitudinal endpoints of HPV infection did not change (data not shown).
The generalizability of these study findings may be limited. The number of prevalent HPV infections and cervical precancer in this study is higher than previously reported in Thailand [33]. Given the observed consistent association of COC use on cervical cancer risk, this study may have oversampled women at elevated risk for development of precancerous lesions, thereby resulting in a higher prevalence of HPV. However, restricting the study population to women with normal cytology did not significantly affect the association (data not shown). Alternatively, the current study sampled a more narrow age distribution and a broader geographic area (including the capital Bangkok), and employed more sensitive genotyping methodologies. These differences in study design may also contribute to the higher prevalence of HPV observed in our study.
In summary, the current study identified current COC use to be more strongly associated with a reduced risk of HPV clearance, rather than new HPV detection, in a group of Thai women aged 20–37 years who were long-term hormonal contraceptive users. These results suggest that increased sexual risk among COC users may not fully explain the association between oral contraceptive use and HPV/cervical cancer. Additional work on the epidemiologic and biological relationship of female reproductive hormones on the virus/host interaction will be critical to identify strategies for translation of this association to disease prevention and treatment.
Notes
Financial support.
This work was supported in part by the National Cancer Institute (NCI, at the National Institutes of Health) institutional postdoctoral training fellowship in Cancer Prevention, Etiology, and Control (5T32CA009314-28). This study was funded by Merck.
Potential conflicts of interest.
S. G., K.-L. L., and A. T. are employees of Merck Research Laboratories or Merck, Sharp and Dohme who funded research and manufactures the quadrivalent HPV vaccine. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Appleby P, Beral V, Berrington de Gonzalez A, et al. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370:1609–21. doi: 10.1016/S0140-6736(07)61684-5. [DOI] [PubMed] [Google Scholar]
- 2.Moreno V, Bosch FX, Munoz N, et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study. Lancet. 2002;359:1085–92. doi: 10.1016/S0140-6736(02)08150-3. [DOI] [PubMed] [Google Scholar]
- 3.Chen YH, Huang LH, Chen TM. Differential effects of progestins and estrogens on long control regions of human papillomavirus types 16 and 18. Biochem Biophys Res Commun. 1996;224:651–9. doi: 10.1006/bbrc.1996.1080. [DOI] [PubMed] [Google Scholar]
- 4.Kim CJ, Um SJ, Kim TY, et al. Regulation of cell growth and HPV genes by exogenous estrogen in cervical cancer cells. Int J Gynecol Cancer. 2000;10:157–64. doi: 10.1046/j.1525-1438.2000.00016.x. [DOI] [PubMed] [Google Scholar]
- 5.Mitrani-Rosenbaum S, Tsvieli R, Tur-Kaspa R. Oestrogen stimulates differential transcription of human papillomavirus type 16 in SiHa cervical carcinoma cells. J Gen Virol. 1989;70:2227–32. doi: 10.1099/0022-1317-70-8-2227. [DOI] [PubMed] [Google Scholar]
- 6.Mittal R, Tsutsumi K, Pater A, Pater MM. Human papillomavirus type 16 expression in cervical keratinocytes: role of progesterone and glucocorticoid hormones. Obstet Gynecol. 1993;81:5–12. [PubMed] [Google Scholar]
- 7.Pater MM, Mittal R, Pater A. Role of steroid hormones in potentiating transformation of cervical cells by human papillomaviruses. Trends Microbiol. 1994;2:229–34. doi: 10.1016/0966-842x(94)90627-0. [DOI] [PubMed] [Google Scholar]
- 8.Ruutu M, Wahlroos N, Syrjanen K, Johansson B, Syrjanen S. Effects of 17beta-estradiol and progesterone on transcription of human papillomavirus 16 E6/E7 oncogenes in CaSki and SiHa cell lines. Int J Gynecol Cancer. 2006;16:1261–8. doi: 10.1111/j.1525-1438.2006.00563.x. [DOI] [PubMed] [Google Scholar]
- 9.Brake T, Lambert PF. Estrogen contributes to the onset, persistence, and malignant progression of cervical cancer in a human papillomavirus-transgenic mouse model. Proc Natl Acad Sci USA. 2005;102:2490–5. doi: 10.1073/pnas.0409883102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambert KC, Curran EM, Judy BM, Milligan GN, Lubahn DB, Estes DM. Estrogen receptor alpha (ERalpha) deficiency in macrophages results in increased stimulation of CD4+ T cells while 17beta-estradiol acts through ERalpha to increase IL-4 and GATA-3 expression in CD4+ T cells independent of antigen presentation. J Immunol. 2005;175:5716–23. doi: 10.4049/jimmunol.175.9.5716. [DOI] [PubMed] [Google Scholar]
- 11.Shai A, Brake T, Somoza C, Lambert PF. The human papillomavirus E6 oncogene dysregulates the cell cycle and contributes to cervical carcinogenesis through two independent activities. Cancer Res. 2007;67:1626–35. doi: 10.1158/0008-5472.CAN-06-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung SH, Wiedmeyer K, Shai A, Korach KS, Lambert PF. Requirement for estrogen receptor alpha in a mouse model for human papillomavirus-associated cervical cancer. Cancer Res. 2008;68:9928–34. doi: 10.1158/0008-5472.CAN-08-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marks M, Gravitt PE, Gupta SB, et al. The association of hormonal contraceptive use and HPV prevalence. Int J Cancer. 2010;128:2962–70. doi: 10.1002/ijc.25628. [DOI] [PubMed] [Google Scholar]
- 14.Morrison CS, Richardson BA, Mmiro F, et al. Hormonal contraception and the risk of HIV acquisition. AIDS. 2007;21:85–95. doi: 10.1097/QAD.0b013e3280117c8b. [DOI] [PubMed] [Google Scholar]
- 15.Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis: diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 16.Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114–9. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 17.Gravitt PE, Peyton CL, Alessi TQ, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–61. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coutlee F, Gravitt P, Kornegay J, et al. Use of PGMY primers in L1 consensus PCR improves detection of human papillomavirus DNA in genital samples. J Clin Microbiol. 2002;40:902–7. doi: 10.1128/JCM.40.3.902-907.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munoz N, Castellsague X, de Gonzalez AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine. 2006;24(Suppl 3):S1–S10. doi: 10.1016/j.vaccine.2006.05.115. [DOI] [PubMed] [Google Scholar]
- 20.Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 21.Green J, Berrington de Gonzalez A, Smith JS, et al. Human papillomavirus infection and use of oral contraceptives. Br J Cancer. 2003;88:1713–20. doi: 10.1038/sj.bjc.6600971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phelan DF, Gange SJ, Ahdieh-Grant L, et al. Determinants of newly detected human papillomavirus infection in HIV-infected and HIV-uninfected injection drug using women. Sex Transm Dis. 2009;36:149–56. doi: 10.1097/OLQ.0b013e31818d3df3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 24.Jacobson DL, Peralta L, Farmer M, Graham NM, Gaydos C, Zenilman J. Relationship of hormonal contraception and cervical ectopy as measured by computerized planimetry to chlamydial infection in adolescents. Sex Transm Dis. 2000;27:313–9. doi: 10.1097/00007435-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Veress G, Csiky-Meszaros T, Czegledy J, Gergely L. Oral contraceptive use and human papillomavirus infection in women without abnormal cytological results. Med Microbiol Immunol. 1992;181:181–9. doi: 10.1007/BF00215764. [DOI] [PubMed] [Google Scholar]
- 26.Rocha-Zavaleta L, Yescas G, Cruz RM, Cruz-Talonia F. Human papillomavirus infection and cervical ectopy. Int J Gynaecol Obstet. 2004;85:259–66. doi: 10.1016/j.ijgo.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Sycuro LK, Xi LF, Hughes JP, et al. Persistence of genital human papillomavirus infection in a long-term follow-up study of female university students. J Infect Dis. 2008;198:971–8. doi: 10.1086/591625. [DOI] [PubMed] [Google Scholar]
- 28.Richardson H, Abrahamowicz M, Tellier PP, et al. Modifiable risk factors associated with clearance of type-specific cervical human papillomavirus infections in a cohort of university students. Cancer Epidem Biomar. 2005;14:1149–56. doi: 10.1158/1055-9965.EPI-04-0230. [DOI] [PubMed] [Google Scholar]
- 29.Shew ML, Fortenberry JD, Tu W, et al. Association of condom use, sexual behaviors, and sexually transmitted infections with the duration of genital human papillomavirus infection among adolescent women. Arch Pediatr Adolesc Med. 2006;160:151–6. doi: 10.1001/archpedi.160.2.151. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen A, Kjaer SK, Munk C, Osler M, Iftner T. Persistence of high-risk human papillomavirus infection in a population-based cohort of Danish women. J Med Virol. 2010;82:616–23. doi: 10.1002/jmv.21750. [DOI] [PubMed] [Google Scholar]
- 31.Munoz N, Hernandez-Suarez G, Mendez F, et al. Persistence of HPV infection and risk of high-grade cervical intraepithelial neoplasia in a cohort of Colombian women. Br J Cancer. 2009;100:1184–90. doi: 10.1038/sj.bjc.6604972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marks MA, Gravitt PE, Burk RD, Studentsov Y, Farzadegan H, Klein SL. Progesterone and 17beta-estradiol enhance regulatory responses to human papillomavirus type 16 virus-like particles in peripheral blood mononuclear cells from healthy women. Clin Vaccine Immunol. 2010;17:609–17. doi: 10.1128/CVI.00441-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sukvirach S, Smith JS, Tunsakul S, et al. Population-based human papillomavirus prevalence in Lampang and Songkla, Thailand. J Infect Dis. 2003;187:1246–56. doi: 10.1086/373901. [DOI] [PubMed] [Google Scholar]